Abstract
Study Objectives:
Patients with obstructive sleep apnea (OSA) and coronary artery disease have a poor long-term prognosis. It is unknown whether the coronary blood flow (CBF) response to OSA is appropriate for myocardial metabolic requirements. Therefore, CBF was assessed during OSA, before and after the development of coronary artery endothelial dysfunction.
Setting:
University Hospital Animal Laboratory.
Patients or Participants:
Newborn lambs.
Interventions:
Lambs were surgically instrumented for invasive hemodynamic monitoring and sleep-wake EEG recordings. A tracheostomy was inserted to control the upper airway and model OSA during sleep. Coronary artery endothelial dysfunction was created using infusions of lipopolysaccharide (LPS). The CBF response during OSA was assessed and compared to changes in myocardial work (rate-pressure product [RPP]), O2 saturation, and cortical arousal, before and after the LPS infusions.
Measurements and Results:
During OSA, CBF increased by 8.6% ± 2.4% above baseline in the pre-LPS condition and 8.8% ± 1.9% post-LPS, peaking following termination of the respiratory event. Pre-LPS, change in CBF post-apnea was independently correlated with change in RPP (R2 = 0.50), minimum SpO2 (R2 = 0.11) and the presence of cortical arousal (R2 = 0.04) (P < 0.01, forward stepwise regression analysis). Following LPS, the only predictor of CBF was degree of O2 desaturation (R2 = 0.14, P < 0.05).
Conclusion:
Under baseline conditions, CBF correlates well with myocardial work following the termination of apnea in lambs. After the creation of coronary artery endothelial dysfunction with LPS, there is uncoupling of the normal CBF-myocardial work relationship.
Citation:
Hamilton GS; Solin P; Walker A. Coronary blood flow becomes uncoupled from myocardial work during obstructive sleep apnea in the presence of endothelial dysfunction. SLEEP 2008;31(6):809-816.
Keywords: Sleep apnea, obstructive, coronary circulation, endothelium, vascular
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY REPETITIVE COLLAPSE OF THE UPPER AIRWAY DURING SLEEP. THIS COLLAPSE MAY BE COMPLETE, leading to apnea, or partial, leading to hypopnea. It is a common condition affecting 24% of men and 9% of women aged 30 to 60 years1 and has been associated with a variety of cardiovascular disorders, including coronary artery disease (CAD).2 There is clinical evidence suggesting that patients with both OSA and CAD have a worse prognosis than those with either disorder alone. In patients with CAD, the presence of untreated OSA is an independent predictor of mortality during prospective follow-up,3,4 and nocturnal ischemia is common if OSA and CAD coexist.5 Furthermore, it has recently been reported that patients with OSA have a greater likelihood of nocturnal sudden cardiac death than a control population.6
The mechanisms underlying this poor cardiac prognosis are incompletely understood, but abnormalities of coronary blood flow during OSA are likely be involved. The myocardium has limited anaerobic capacity, and under resting conditions a high proportion of delivered O2 is extracted from the coronary blood. Therefore, whenever there is elevated myocardial O2 consumption, such as from rises in heart rate or blood pressure, myocardial O2 requirements are largely met via increases in coronary blood flow.7 In OSA there are repetitive increases in myocardial O2 utilization (commonly referred to as myocardial work), with rises in heart rate and blood pressure occurring predominantly following termination of the apnea.5,8,9 Therefore, this post-apneic period may represent a time of elevated risk for patients if coronary blood flow (CBF) does not increase adequately to match myocardial work.
Whether CBF increases appropriately to match myocardial work during OSA is unclear, as is the role of the coronary endothelium in this setting. Patients with CAD (or risk factors such as diabetes and hypertension) have coronary artery endothelial dysfunction,10–12 and the effect of this on the CBF response in OSA is unknown. The coronary endothelium is a source of vasoactive mediators and is important in regulating CBF at rest and following metabolic stimulation.13 Endothelial dysfunction results in impaired release of vasodilator substances, such as nitric oxide, and may therefore potentially affect CBF regulation during OSA. This may result in patients with CAD and endothelial dysfunction being more at risk of adverse outcomes from OSA than those who have normal coronary arteries.
The roles played by cortical arousal and, particularly, hypoxia in driving the hemodynamic responses to OSA are also uncertain. Prior animal models have shown conflicting results as to the most important mediator of CBF change with OSA – hypoxia or activation of the sympathetic nervous system (with rises in heart rate and blood pressure).14,15 Clarifying this area is important, particularly given that the bulk of clinical disease is mild to moderate in severity, and only associated with minimal O2 desaturation.1 If the coronary hemodynamic changes of OSA occur independently of the associated hypoxia, then milder forms of the disease potentially take on more significance, particularly in patients with underlying cardiac disease.
To evaluate these questions we designed a study to assess CBF during OSA in lambs before and after the development of coronary artery endothelial dysfunction. We examined the relationship of CBF to myocardial work, hypoxia, and arousal during apneas and hypopneas of varying severity, and thereafter, repeated these measurements following the creation of endothelial dysfunction. This was performed with a recently developed model of coronary artery endothelial dysfunction in sleeping lambs, using treatment with bacterial lipopolysaccharide (LPS).16
MATERIALS AND METHODS
Five newborn lambs (Merino/Border-Leicester cross) were separated from their ewes within 24-48 h of birth and housed in a Plexiglas cage. They were then taught to feed independently from a nipple connected to a continuous supply of lamb milk replacer (Veanaivite Pty. Ltd., Shepparton, Australia). Once the lambs had learned to feed and were gaining weight normally, they were surgically prepared for chronic study. All surgical and experimental procedures were performed in accordance with the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, established by the National Health and Medical Research Council of Australia, and were approved by the Monash Medical Centre's Committee on Ethics in Animal Experimentation.
Surgical Preparation and Instrumentation
Each lamb was anesthetized (2% Halothane, 50% O2, balance N2O), intubated, and then ventilated. Using sterile surgical techniques, we instrumented each lamb to record coronary blood flow (CBF). An incision was made through the fourth intercostal space, followed by blunt dissection through the intercostal muscles down to the parietal pleura. After breaching the pleura, the lungs were then retracted to expose the pericardium, which was opened with forceps and surgical scissors. The left circumflex coronary artery was identified and subsequently blunt dissected partially away from the myocardium, to expose the outer wall circumferentially. A transit-time ultrasonic flow probe (2-mm diameter, Transonic Systems Inc., Ithaca, NY) was positioned around the exposed section of the left circumflex coronary artery. This flow probe provides a quantitative beat-by-beat measurement of CBF in lambs.17 A fluid-filled manometer was left inside the thoracic cavity for subsequent monitoring of intrathoracic pressure (ITP), and the thoracic cage was then sutured closed. We also inserted nonocclusive saline-filled catheters (0.86 mm id, 1.52 mm od) into the left atrium, the carotid artery (to record arterial blood pressure [ABP]), and into the jugular vein (to record central venous pressure [JVP]).
Following 48 h recovery, a second operation was performed. The lambs were again anesthetized (2% Halothane, 50% O2, balance N2O), intubated and then ventilated. In order to measure sleep-wake state, pairs of Teflon-coated stainless steel wires were surgically implanted on the parietal cortex (electrocorticogram, ECoG), at the inner and outer canthus of the left eye (electrooculogram, EOG), and in the dorsal musculature of the neck (nuchal electromyogram, EMG). An incision was then made in the mid trachea, and a cuffed, fenestrated tracheostomy tube was inserted and sutured into place. An extra piece of tubing was added over the tracheostomy fenestration to ensure it would remain patent and help anchor the tracheostomy within the trachea. Following surgery, the tracheostomy opening was covered with a removable cap and the cuff was deflated, so the lamb could breathe via its own upper airway until the time of the chronic study.
Experimental Protocol
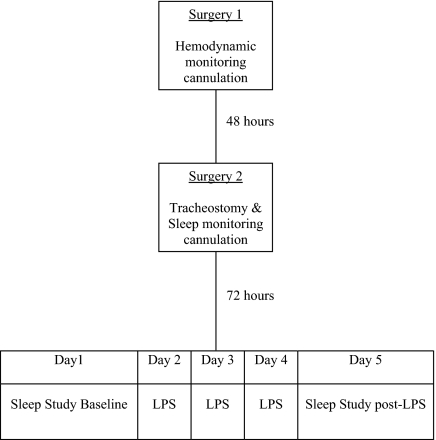
Each lamb was allowed a minimum of 72 h to recover from the second surgery and then studied over 5 consecutive days. A flow chart summarizing the experimental protocol is shown in figure 1. During the study period, the lambs' cages were partitioned to prevent them from turning around, while still allowing freedom to move forward and backward and to stand up and lie down. All studies took place between 09:00 and 17:00 and room temperature was maintained between 22°C and 25°C. Food was available ad libitum throughout the study.
Figure 1.
Schematic diagram illustrating the experimental protocol.
Studies were performed over 5 consecutive days. On day 1, a sleep study was performed and OSA was modelled. Apneas and hypopneas were created by manually occluding the tracheostomy during sleep. On days 2-4, endothelial damage and dysfunction was created using a daily infusion of LPS (2 μg/kg) over 30 min. This protocol of LPS infusion in lambs has been shown in our lab to lead to coronary artery endothelial dysfunction.16 On day 5 (one day after the final LPS infusion), a second sleep study was performed under the same conditions as the first, with repeated modelling of OSA.
Sleep Study with Obstructive Sleep Apnea Modelling
Data were acquired at 400 Hz, converted from analogue to digital signals and stored on a personal computer using Chart5 acquisition software (ADInstruments, Sydney, Australia). Continuous measurements of CBF, ABP, JVP, ITP, and pulse rate (PR, calculated from the blood pressure tracing) were recorded, as well as electrophysiological signals from the sleep electrodes. Quiet wakefulness (QW) was defined as periods where the lamb was lying down, when the ECoG displayed a pattern of low-voltage and high-frequency activity and when eye movements and EMG tone were present. Quiet (or NREM) sleep was defined when the ECoG displayed a pattern of high-voltage and low-frequency activity, eye movements were absent, and EMG tone was reduced compared with that in QW. Active (or REM) sleep was defined as a pattern of low-voltage and high-frequency activity, with absent EMG tone and intermittent phasic eye movements.18
During the sleep study, O2 saturation was monitored with an oximetry probe situated on the tail. The tracheostomy cuff was inflated and the external cap removed. A rubber disc, attached around a thin wire, was inserted into the internal catheter of the tracheostomy. The disc was the same diameter as the tracheostomy lumen and thus occluded it when sitting inside. Throughout most of the sleep study the disc was situated proximal to the fenestration of the tracheostomy, thus the lamb was able to breathe via its own upper airway, with air traversing the fenestration. During sleep and at times of hemodynamic stability, the disc was intermittently moved distally to create upper airway obstruction. This was confirmed by the presence of a more negative ITP than baseline. The degree of obstruction depended on whether the rubber disc lay completely distal to the fenestration (creating an apnea) or whether the disc traversed the fenestration, allowing some residual airflow (creating a hypopnea). This was randomly determined. If the lamb aroused, then the obstruction was immediately relieved by moving the rubber disc proximally again. Other apneas and hypopneas were randomly terminated prior to arousal to allow an assessment of the differential effect of cortical arousal and hypoxia on the hemodynamic responses to OSA.
At the end of all experimental procedures, lambs were killed using a lethal dose of anaesthetic (150 mg/kg sodium pentobarbitone intravenously).
Data Analysis
Values for mean CBF, mean arterial blood pressure (MAP), JVP, and PR were calculated using the internal analysis software of Chart5. Two further hemodynamic parameters were calculated offline: rate-pressure product (RPP) and coronary vascular resistance (CVR). RPP was calculated as the product of systolic arterial blood pressure and heart rate. This has been validated as an excellent noninvasive correlate of myocardial work.19,20 CVR was calculated by the formula: (MAP − JVP)/CBF.
During the periods of airway occlusion, data were averaged over the following 5 time periods for each obstructive event: baseline (5 sec prior to onset of obstruction), early apnea (first 5 sec of the obstruction), late apnea (last 5 sec of the obstruction), post-apnea1 (first 5 sec following end of obstruction) and post-apnea2 (subsequent 5 sec following end of obstruction). Data points for each of these epochs were expressed as percentage change from baseline. Almost all obstructive events occurred during quiet (NREM) sleep. The rare obstructive events occurring during active (REM) sleep were excluded from the analysis to avoid any confounding effect of sleep stage on the results.
Statistical Analysis
Data summarizing the characteristics of the obstructive events are expressed as mean ± SD. Paired t tests were used to compare these characteristics before and after LPS administration. Haemodynamic data are expressed as mean ± SEM. Baseline mean values during wake and sleep, both pre and post-LPS, were compared using 2-way repeated measures analysis of variance. Two-way repeated measures analysis of variance was also used to assess changes over time in CBF through the obstructive event, both at baseline and following LPS infusion. If significant differences were found, analysis of multiple pairwise comparisons were performed using the Student-Newman-Keuls method. In the post-apnea1 period, the mean CBF responses with and without a cortical arousal were compared with a student's t test, for both pre and post-LPS. Simple linear regression was used to demonstrate the univariate relationships between CBF and myocardial work (RPP) and hypoxia in the post-apnea1 period. Forward stepwise multilinear regression analysis was used to determine the multivariate predictors of CBF. Statistical testing was performed using SigmaStat software version 3.0 (Systat Software Inc, www.systat.com), and a P value of < 0.05 was considered to be statistically significant.
RESULTS
The characteristics of the obstructive events are summarized in table 1. Minimum SpO2 was 91% ± 5% pre-LPS and 92% ± 3% post-LPS. Degree of O2 desaturation with respiratory events was 7% ± 4% pre-LPS and 6% ± 2% post-LPS. Proportion of events terminating in arousal was 54% pre-LPS and 52% post-LPS. There were no significant differences between the pre and post-LPS conditions for any of the parameters.
Table 1.
Characteristics of the Obstructive Events
| Pre-LPS | Post-LPS | P value | |
|---|---|---|---|
| Obstructions per lamb | 14 ± 4.8 | 11 ± 3.4 | 0.26 |
| Mean duration (s) | 41 ± 12 | 45 ± 22 | 0.74 |
| % with arousal | 54 | 52 | 0.81 |
| Mean SpO2 min (%) | 91 ± 5 | 92 ± 3 | 0.29 |
| Mean O2 desaturation (%) | 7 ± 4 | 6 ± 2 | 0.37 |
Table showing the characteristics of the obstructive events during the sleep studies performed at baseline and following LPS infusion. Values are mean ± SD. There was no significant difference between the baseline and post LPS conditions for any parameter.
Table 2 summarizes the baseline hemodynamic parameters during the different sleep stages (both before and after the administration of LPS), prior to any upper airway obstruction. Following LPS, baseline systolic blood pressure and RPP were significantly lower than the pre-LPS values (P < 0.05). There was a trend to lower MAP post LPS (P = 0.1), but a nonsignificant difference in CBF pre and post-LPS (P = 0.22). CVR and PR were not different pre- and post LPS (P = ns). There were no significant differences between wake and NREM sleep for any hemodynamic parameter, either before or after the administration of LPS.
Table 2.
Baseline Hemodynamic Data in Pre-LPS Sleep Study
| MAP (mm Hg) | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | HR (bpm) | CBF (mL/min) | CVR (mg mL min−1) | SpO2 (%) | RPP (mm Hg bpm) | |
|---|---|---|---|---|---|---|---|---|
| Wake pre-LPS | 84.0 ± 4.7 | 104.8 ± 5.2 | 66.8 ± 4.6 | 153 ± 14 | 21.2 ± 2.3 | 4.04 ± 0.4 | 98 ± 0.9 | 16116 ± 1697 |
| Wake post-LPS | 73.3 ± 1.3 | 88.7 ± 1.4 | 60.7 ± 1.5 | 151 ± 9 | 19.2 ± 2.8 | 4.03 ± 0.7 | 98 ± 0.5 | 13367 ± 989 |
| Sleep pre-LPS | 83.6 ± 4.6 | 104.3 ± 5.5 | 66.6 ± 4.6 | 154 ± 14 | 21.0 ± 2.3 | 4.03 ± 0.4 | 98 ± 0.6 | 16103 ± 1712 |
| Sleep post-LPS | 76.2 ± 2.9 | 91.3 ± 3.4 | 63.3 ± 2.9 | 147 ± 7 | 19.2 ± 2.8 | 4.18 ± 0.7 | 97 ± 0.2 | 13510 ± 1068 |
Table showing hemodynamic data (prior to the modelling of obstructive sleep apnea) in the baseline (pre-LPS) sleep study and post-LPS sleep study. MAP = mean arterial pressure; HR = heart rate; CBF = coronary blood flow; CVR = coronary vascular resistance; RPP = rate-pressure product; Sleep refers to NREM sleep. Values are mean ± SEM.
Coronary Blood Flow
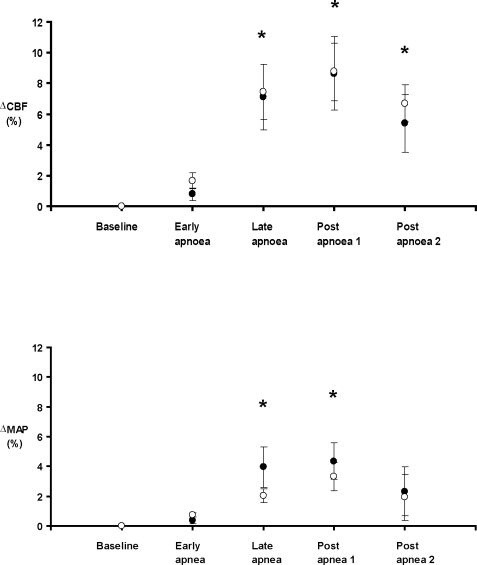
The mean CBF and mean arterial blood pressure changes during the obstructive events are summarized in Figure 2. Following termination of the apnea (post-apnea1 phase) the mean CBF peaked 8.6% ± 2.4% above baseline in the pre-LPS condition and 8.8% ± 1.9% above baseline in the post-LPS condition (P = ns). In both pre and post-LPS conditions, CBF was significantly greater in the late apnea, post-apnea1 and post-apnea2 periods when compared to baseline and the early apnea period (P < 0.05). There was a nonsignificant trend for CBF to be maximal in the post apnea1 period. There was no difference in the CBF response to upper airway obstruction between the pre- and post-LPS conditions.
Figure 2.
Mean (± SEM) change in coronary blood flow (CBF) and mean arterial blood pressure (MAP) from baseline over the course of the apnea cycle, at baseline (•) and post treatment (○) with lipopolysaccharide (LPS). * P < 0.05 compared to baseline for each condition. No difference between pre-LPS and post-LPS (2-way repeated measures ANOVA).
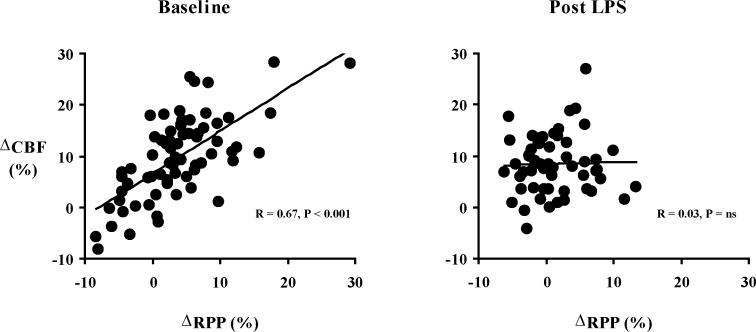
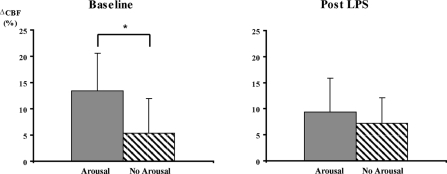
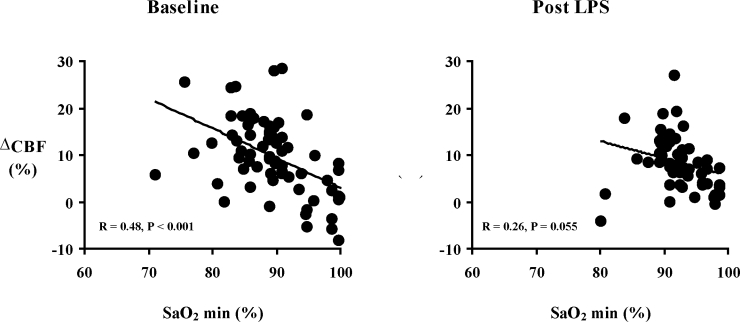
In the post-apnea1 period, the univariate relationships between CBF and myocardial work (RPP), hypoxia (SpO2 min), and arousal are shown in Figures 3–5. Before the administration of LPS, change in CBF with obstructive events correlated closely with change in RPP (R = 0.67, P < 0.001) and also SpO2 min (R = 0.48, P < 0.001). The presence of arousal with the event was associated with a greater CBF response than if no arousal occurred (13% ± 7% v 5% ± 7%, P < 0.05). Following LPS, with obstructive events there were no longer significant relationships between change in CBF and RPP (R = 0.03, P = ns) nor SpO2 min (R = 0.26, P = 0.055). The presence or absence of arousal did not affect the CBF response (9% ± 7% v 7% ± 5%, P = ns). When lambs were analyzed individually, the results were similar. Pre LPS, there was a significant correlation between CBF and RRP for each lamb, with R values ranging from 0.96 to 0.64 (average 0.80). Post LPS, there were significant correlations for only 2 out of 5 lambs.
Figure 3.
Correlation (simple linear regression) between change in coronary blood flow (CBF) and rate-pressure product (RPP) during the post-apnea 1 period, both at baseline and post LPS.
Figure 5.
Mean (± SEM) change in coronary blood flow (CBF) from baseline during the post-apnea 1 period, in the presence (grey) and absence (hashed) of cortical arousal. During baseline sleep study, * P < 0.05, t test.
Figure 4.
Correlation (simple linear regression) between change in coronary blood flow (CBF) and minimum oxygen saturation reached (SpO2 min) during the post-apnea 1 period, both at baseline and post LPS.
The multivariate relationships between change in CBF (dependent variable) and RPP, SpO2 min, extent of O2 desaturation, and arousal were assessed with a forward stepwise regression analysis. The identity of each lamb was forced into the model to take account for any individual animal effects. Before LPS, the change in CBF with obstructive events can be predicted by a linear regression model (R = 0.81, R2 = 0.64, F = 30.04, P < 0.001). Independent predictors of CBF were RPP (R = 0.71, R2 = 0.50, P < 0.001), SpO2 min (R = 0.33, R2 = 0.11, P < 0.01) and the presence of arousal (R = 0.20, R2 = 0.04, P < 0.01). Following LPS the predictors of change in CBF were different. The only significant predictor of CBF was extent of O2 desaturation with obstructive events (R = 0.38, R2 = 0.14, P < 0.05). Change in RPP or presence of arousal were not independent predictors of CBF after LPS administration.
DISCUSSION
There are two major new findings in this study. Firstly, we have demonstrated that in normal lambs, CBF increases modestly with obstructive sleep apnea hypopnea syndrome and is closely matched to changes in myocardial work. We demonstrated an increase in CBF of 8.6% ± 2.4% over baseline in the early post-apneic period. Forward stepwise regression analysis showed this rise in CBF was predominantly dependent upon the degree of increase in myocardial work (RPP), but also, to a smaller degree, correlated with the minimum SpO2 reached and the presence of cortical arousal. Secondly, following the creation of coronary artery endothelial dysfunction, the increase in CBF with obstructive respiratory events no longer correlated with myocardial work, and the degree of hypoxia associated with the obstruction was the only predictor of CBF change.
The apneas and hypopneas that characterize obstructive sleep apnea are associated with significant effects on the cardiovascular system. Towards the end of an obstructive event, there is an increase in heart rate and blood pressure and usually a decrease in arterial O2 saturation.9 These changes peak in the period following termination of the apnea, when there is a rapid resumption of ventilation, and result in increases in myocardial work.8,9 As the myocardium has limited anaerobic capacity,7 an increase in CBF is required to maintain adequate O2 delivery as myocardial work increases, and any situation that impairs CBF may lead to relative ischemia. This has clinical relevance in OSA, as it has been shown that when there is nocturnal ischemia in the presence of OSA, the ischemia occurs predominantly during the postobstructive hyperventilation phase of the apnea cycle.5 Because of this, we specifically looked at this time to determine if CBF responds appropriately to rises in myocardial work, and also to determine the relative influences of hypoxia and cortical arousal on the CBF change.
Previous animal studies have assessed the effect of obstructive apneas on CBF. In a naturally sleeping tracheostomized pig model, the effect of apnea with arousal on heart rate, blood pressure, and CBF was measured.14,21 The influence of hypoxia was not assessed in these studies. In NREM sleep, CBF increased by 12%21 and 13%14 following the termination of obstructive apnea with cortical arousal. These changes are in keeping with the rise in CBF demonstrated in our study. We demonstrated a mean increase in CBF of 8.6%, but notably only 54% of events ended in arousal. In those events where arousal was present, the mean increase in CBF in our study was greater, averaging 13%. The studies by Pinto21 and Kirby14 assessed the role of the sympathetic nervous system in mediating these hemodynamic changes. α-Adrenoreceptor blockade prevented any change in blood pressure21 with obstructive apnea, and β-adrenoreceptor blockade prevented any increase in heart rate or CBF.14 These results indicated that activation of the sympathetic nervous system was the major mediator of hemodynamic change during obstructive apneas. This conclusion is supported by other studies that demonstrate no significant effect of supplemental oxygen (adequate to prevent hypoxia) at inhibiting the blood pressure increases seen in OSA.9,22
However, other data show some conflicting results to those discussed above. Chen et al studied the effect of obstructive apneas on CBF in anaesthetized pigs.15,23 In this study CBF increased at the end of and immediately following the termination of apnea, in association with significant hypoxia (mean PaO2 46.7 mm Hg). Blocking the sympathetic nervous system had no effect on CBF, whereas the administration of supplemental O2 prevented any increase in CBF. The conclusion was therefore that hypoxia is the major mediator of CBF with obstructive apneas. This study was unable to assess the effect of cortical arousal due to the use of an anesthetized pig model.
Given that these studies discussed above show conflicting results, our data provide important clarification. Due to our study design, we were able to assess the independent effects of myocardial work (driven by sympathetically mediated increases in heart rate and blood pressure), cortical arousal, and hypoxia on CBF during obstructive apneas and hypopneas. Using forward stepwise regression, we showed that increase in myocardial work is the major mediator of the CBF response, with 50% of the variance in CBF being explained by changes in RPP. Once this is taken into account, the effects of hypoxia and arousal are relatively small, explaining only 11% and 4% respectively of the variance in CBF. The main caveat of this is that the degree of O2 desaturation in our study was mild, and we cannot exclude that hypoxia plays a more prominent role in the CBF response with more severe degrees of O2 desaturation. In order to more precisely define the relationship between hypoxia and CBF in OSA, additional studies that administer varying degrees of hypoxia and normoxia during upper airway obstruction would be required. Nevertheless, our finding is of clinical importance, as it underlines that substantial hypoxia with OSA is not necessary in order to have significant cardiovascular effects. Obstructive events with only mild O2 desaturation will still lead to increases in heart rate, blood pressure,9 and subsequently, CBF. If there are impediments to CBF increase, such as a coronary artery stenosis, then myocardial ischemia may result from OSA.
A novel aspect of our study is the investigation of CBF during upper airway obstruction following the creation of coronary artery endothelial dysfunction. We used a model of LPS infusion that has been previously shown to lead to coronary artery endothelial dysfunction in sleeping lambs.16 The overall magnitude of CBF change post-LPS was the same as in our pre-LPS study, with CBF peaking at 8.8% ± 1.9% above baseline in the early post apneic period (post-apnea1). There was no difference in the nature of the obstructive events following the creation of endothelial dysfunction (see Table 1), but importantly, the hemodynamic response was significantly altered. The strong correlation between CBF and RPP was no longer present, either on univariate (see Figure 3) or multivariate analysis. On forward stepwise regression analysis the only significant predictor of CBF increase was the extent of O2 desaturation with the event, and this effect of hypoxia was modest, explaining only 14% of the variance in CBF. Although the range of RRP change post LPS is slightly smaller than that pre LPS (see Figure 3), this does not influence the results or conclusions. When the pre LPS analysis is repeated after removing outlying values (such that the range of RPP is identical to that seen post LPS), the significant correlation between CBF and RPP persists, with an R value of 0.55.
CBF is normally closely matched to myocardial energy requirements and the coronary endothelium plays a vital role in this regulation. In normal coronary arteries, sympathetic stimulation (with a rise in heart rate and blood pressure) leads to an overall increase in CBF with vasodilatation of both conduit and resistance coronary vessels.24,25 Endothelial derived mediators, such as nitric oxide, are crucially involved in both the flow-mediated dilatation of large arteries and the metabolic vasodilatation of smaller resistance vessels.13,24,26 In the presence of endothelial dysfunction, such as with atherosclerosis, there is reduced production of these vasodilating substances and evidence for impaired CBF regulation—vasoconstriction of large epicardial arteries and attenuated vasodilatation in the resistance vessels.13,25,27,28 The precise role the endothelium plays in CBF regulation remains controversial, however, as there are also data suggesting that pharmacologically blocking nitric oxide does not change the CBF response to increased myocardial metabolic demand.29,30 Whether the presence of coronary artery endothelial dysfunction affects CBF regulation during upper airway obstruction with apnea has been unknown until now. The results from our study demonstrate uncoupling of the normal CBF − metabolic work relationship following the creation of endothelial dysfunction. We hypothesize that this may contribute to the poor prognosis seen in patients with both OSA and coronary artery disease by contributing to the progression of atherosclerosis (via increased oxidative stress) or increasing the risk of cardiac ischemia, particularly if there is a coronary stenosis and marginal coronary flow reserve. In support of this hypothesis is that the presence of endothelial dysfunction in non–sleep apneic populations is an independent risk factor for cardiovascular events.10,31,32
Lambs are well validated for the measurement of hemodynamic parameters during sleep,17,33,34 however, there are a number of potential limitations to our study. The main limitation is that we used LPS to create the endothelial dysfunction, rather than atherosclerosis and were unable to assess the endothelial function directly on the animals studied. However, LPS is well validated as a cause of endothelial dysfunction, including of the coronary arteries, with a similar functional impact as is seen in atherosclerosis.35–37 Moreover, we have previously demonstrated that our protocol of LPS infusion leads to a functionally significant reduction in coronary artery endothelial function, similar to that seen in disease states such as atherosclerosis.16 The fact that we demonstrated differences in CBF regulation pre- and post-LPS administration also supports the concept that we effectively created coronary artery endothelial dysfunction. A second potential limitation is that baseline systolic blood pressure and RPP (measured prior to upper airway obstruction) were slightly lower in the post-LPS sleep study than at baseline. This is unlikely to have influenced our results for the following reasons. In this study we were interested in the way CBF was regulated during the changing hemodynamic responses seen during upper airway obstruction, rather than the absolute baseline values during stable sleep. A slightly lower baseline blood pressure would not influence our results, as there is evidence that vascular mechanisms regulating coronary blood flow remain intact until the coronary perfusion pressure reaches critically low levels (<45 mm Hg)—pressures far lower than those seen in our study.38,39 This process of maintaining metabolically appropriate CBF despite changes in coronary perfusion pressure is known as autoregulation. In addition, the CVR was no different pre-LPS compared to post-LPS, confirming a stable balance between coronary artery vasodilators and vasoconstrictors. A final point to emphasize with our study is that all results are from NREM sleep. Although there is no reason to suspect CBF would be any different during REM sleep, this point cannot be assumed, and specific studies would be required in REM to address that question.
In summary, we have demonstrated that in normal lambs, CBF during OSA increases in proportion to myocardial metabolic demands. Moreover, CBF is tightly matched to increases in myocardial work. However, in the presence of coronary artery endothelial dysfunction there is uncoupling of the normal CBF – myocardial work relationship. Furthermore, substantial hypoxia with obstructive apneas and hypopneas is not necessary in order to have significant cardiovascular effects. We conclude that an intact coronary endothelium is important in regulating CBF during OSA, which supports the concept that abnormalities of CBF associated with endothelial dysfunction contribute to the poor cardiovascular prognosis seen in patients with both atherosclerosis and OSA.
ACKNOWLEDGMENTS
The National Health and Medical Research Council of Australia provided experimental funding and a Postgraduate Medical Research Scholarship for Dr Hamilton.
Site of Study: Monash Institute of Medical Research, Monash University, Clayton, Victoria, Australia
Funding source: National Health and Medical Research Council of Australia
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–6. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Peled N, Abinader EG, Pillar G, Sharif D, Lavie P. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol. 1999;34:1744–9. doi: 10.1016/s0735-1097(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 7.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–15. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 8.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringler J, Basner RC, Shannon R, et al. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol. 1990;69:2143–8. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 10.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 11.Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–43. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 12.Friedman PL, Brown EJ, Jr, Gunther S, et al. Coronary vasoconstrictor effect of indomethacin in patients with coronary-artery disease. N Engl J Med. 1981;305:1171–5. doi: 10.1056/NEJM198111123052002. [DOI] [PubMed] [Google Scholar]
- 13.Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation. 1999;100:1951–7. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- 14.Kirby DA, Pinto JM, Weiss JW, Garpestad E, Zinkovska S. Effects of beta adrenergic receptor blockade on hemodynamic changes associated with obstructive sleep apnea. Physiol Behav. 1995;58:919–23. doi: 10.1016/0031-9384(95)00150-h. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Scharf SM. Systemic and myocardial hemodynamics during periodic obstructive apneas in sedated pigs. J Appl Physiol. 1998;84:1289–98. doi: 10.1152/jappl.1998.84.4.1289. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton GS, Edwards B, Solin P, Walker AM. A model of coronary artery endothelial dysfunction in the sleeping lamb. Sleep Med. 2006;7:573–9. doi: 10.1016/j.sleep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Fewell JE. Influence of sleep on systemic and coronary hemodynamics in lambs. J Dev Physiol. 1993;19:71–6. [PubMed] [Google Scholar]
- 18.Silvani A, Bojic T, Franzini C, et al. Sleep-related changes in the regulation of cerebral blood flow in newborn lambs. Sleep. 2004;27:36–41. doi: 10.1093/sleep/27.1.36. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972;32:516–22. doi: 10.1152/jappl.1972.32.4.516. [DOI] [PubMed] [Google Scholar]
- 20.Baller D, Bretschneider HJ, Hellige G. Validity of myocardial oxygen consumption parameters. Clin Cardiol. 1979;2:317–27. doi: 10.1002/clc.4960020502. [DOI] [PubMed] [Google Scholar]
- 21.Pinto JM, Garpestad E, Weiss JW, Bergau DM, Kirby DA. Hemodynamic changes associated with obstructive sleep apnea followed by arousal in a porcine model. J Appl Physiol. 1993;75:1439–43. doi: 10.1152/jappl.1993.75.4.1439. [DOI] [PubMed] [Google Scholar]
- 22.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526–32. doi: 10.1378/chest.101.6.1526. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Sica AL, Scharf SM. Mechanisms of acute cardiovascular response to periodic apneas in sedated pigs. J Appl Physiol. 1999;86:1236–46. doi: 10.1152/jappl.1999.86.4.1236. [DOI] [PubMed] [Google Scholar]
- 24.Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989;14:1181–90. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- 25.Zeiher AM, Drexler H. Coronary hemodynamic determinants of epicardial artery vasomotor responses during sympathetic stimulation in humans. Basic Res Cardiol. 1991;86(Suppl 2):203–13. doi: 10.1007/978-3-642-72461-9_20. [DOI] [PubMed] [Google Scholar]
- 26.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation. 2000;101:2942–8. doi: 10.1161/01.cir.101.25.2942. [DOI] [PubMed] [Google Scholar]
- 27.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 28.Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–52. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 29.Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res. 1994;28:119–24. doi: 10.1093/cvr/28.1.119. [DOI] [PubMed] [Google Scholar]
- 30.Egashira K, Katsuda Y, Mohri M, et al. Role of endothelium-derived nitric oxide in coronary vasodilatation induced by pacing tachycardia in humans. Circ Res. 1996;79:331–5. doi: 10.1161/01.res.79.2.331. [DOI] [PubMed] [Google Scholar]
- 31.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 32.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 33.Loos N, Grant DA, Wild J, et al. Sympathetic nervous control of the cerebral circulation in sleep. J Sleep Res. 2005;14:275–83. doi: 10.1111/j.1365-2869.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RV, Grant DA, Wilkinson MH, Walker AM. The effects of repeated exposure to hypercapnia on arousal and cardiorespiratory responses during sleep in lambs. J Physiol. 2007;582(Pt 1):369–78. doi: 10.1113/jphysiol.2007.132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piepot HA, Groeneveld AB, van Lambalgen AA, Sipkema P. Endotoxin impairs endothelium-dependent vasodilation more in the coronary and renal arteries than in other arteries of the rat. J Surg Res. 2003;110:413–8. doi: 10.1016/s0022-4804(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 36.Leclerc J, Pu Q, Corseaux D, et al. A single endotoxin injection in the rabbit causes prolonged blood vessel dysfunction and a procoagulant state. Crit Care Med. 2000;28:3672–8. doi: 10.1097/00003246-200011000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Bogle RG, McLean PG, Ahluwalia A, Vallance P. Impaired vascular sensitivity to nitric oxide in the coronary microvasculature after endotoxaemia. Br J Pharmacol. 2000;130:118–24. doi: 10.1038/sj.bjp.0703267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith TP, Jr, Canty JM., Jr Modulation of coronary autoregulatory responses by nitric oxide. Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res. 1993;73:232–40. doi: 10.1161/01.res.73.2.232. [DOI] [PubMed] [Google Scholar]
- 39.Rouleau JR, Simard D, Kingma JG., Jr Myocardial blood flow regulation relative to left ventricle pressure and volume in anesthetized dogs. Can J Physiol Pharmacol. 1999;77:902–8. [PubMed] [Google Scholar]