Abstract
Objective:
Mirtazapine is an α2A antagonist and mixed 5-HT2/5-HT3 antagonist that has been proposed as a potential treatment for obstructive sleep apnea (OSA). A small, randomized, controlled trial has previously found an approximate halving in the severity of OSA with daily doses of 4.5 and 15 mg. We aimed to confirm and extend these findings in 2 randomized placebo-controlled, proof-of-concept trials.
Methods:
Two randomized, double-blind, placebo-controlled trials of mirtazapine for OSA (apnea-hypopnea index 10–40/h). Study 1: 3-way crossover, dose-finding study testing the self-administration of mirtazapine (7.5, 15, 30, and/or 45 mg) or placebo 30 minutes prior to bedtime for 2 weeks at each dose. Twenty patients were randomly assigned to 1 of 6 different dose-sequence groups, with each patient exposed to a maximum of 3 doses. Study 2: 3-arm, randomized, parallel-group trial of mirtazapine at 15 mg or mirtazapine 15mg + Compound CD0012 or placebo for 4 weeks in 65 patients with OSA.
Results:
Two patients withdrew from Study 1 after complaints of unacceptable lethargy. Fifteen patients were withdrawn from study 2, 7 after complaints of unacceptable lethargy or other side-effects. No measurement of sleep apnea improved due to mirtazapine in either study. Weight gain was significantly greater on mirtazapine than on placebo in both trials.
Conclusions:
Mirtazapine did not improve sleep apnea in either trial. Mirtazapine caused weight gain, which may further worsen OSA. Therefore, mirtazapine is not recommended for the treatment of OSA.
Citation:
Marshall NS; Yee BJ; Desai AV; Buchanan PR; Wong KKH; Crompton R; Melehan KL; Zack N; Rao SG; Gendreau RM; Kranzler J; Grunstein RR. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apnea. SLEEP 2008;31(6):824–831.
Keywords: Apnea, drug treatment, clinical trial, randomized, placebo-controlled, mirtazapine, serotonin
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON SLEEP DISORDER1 THAT HAS BEEN PROSPECTIVELY ASSOCIATED WITH HYPERTENSION AND DEPRESSION in community-based cohorts2,3 and with mortality in patient cohorts and case-control studies.4–6 Continuous positive airway pressure is the most efficacious treatment for severe OSA.7,8 However, effectiveness is limited in the mild to moderate end of the OSA spectrum9 and by poor compliance generally. Moreover, other nonpharmacologic treatment methods (e.g., mandibular advancement splints and upper airway surgery) can be unacceptable to patients or have limited or questionable efficacy.10,11 As a result, there is strong interest in the development of pharmacotherapy for OSA, which might better balance efficacy and tolerability, particularly for certain patient subsets.
Despite many attempts, there has been a general lack of success with pharmacotherapy.12,13 Strategies have included drugs that putatively alter the physiology of sleep apnea by increasing upper airway muscle tone during sleep, increasing respiratory drive, or altering sleep architecture (either increasing slow-wave sleep or decreasing REM sleep). Most trials of these medications have shown no benefit or limited benefits of questionable clinical importance.11 However, some medications have shown clinically relevant effects in placebo-controlled studies. For example, in 1 study, acetazolamide, a weak diuretic with carbonic anhydrase inhibitory activity, reduced the apnea-hypopnea index (AHI) from about 50 to 25 events per hour in 10 patients.14 However, as a result of related side effects, its posttrial use was reported in only 1 patient. There also remains the potential for pharmacotherapy for sleep apnea via drugs that result in weight reduction.15
Recently it was reported that daily administration of the antidepressant mirtazapine, (Remeron in the US and Avanza in Australia) at doses of 4.5 or 15 mg for 1 week, reduced AHI from a mean 22 events per hour to 11 events per hour in a placebo-controlled, 3-way, crossover trial.16 This report stated that “this represents the largest and most consistent drug-treatment effect demonstrated to date in a controlled trial.” Such data, if confirmed, would hold great promise for providing a new treatment alternative for some patients with OSA. This easily available drug might offer a particularly attractive treatment option for patients with mild to moderate OSA, the largest group of patients and the group in which other established treatment options are of questionable effectiveness.
Therefore, 2 proof-of-concept trials were designed to assess if different and higher doses of mirtazapine are associated with a superior risk-benefit profile than has been previously observed.16 For the purposes of comparison, 15 mg, the highest dose tested in the previous study, is a typical starting dose for the antidepressant indication. The goal of the present studies was to confirm and extend the results of the previous trial.16 Study 1 was a triple-arm, randomized, crossover, dose-finding study (mirtazapine doses 0, 7.5, 15, 30, and 45mg/day) for 2 weeks per dose. Study 2 was a 3-arm parallel-group, 2:2:1, randomized, controlled trial that compared mirtazapine 15 mg versus mirtazapine + another compound (CD0012, a dopaminergic and serotinergic agent undergoing evaluation for efficacy in sleep apnea), versus placebo for 4 weeks. The 2 studies were run simultaneously.
METHODS
Study 1
Study 1 was a 2-week per arm, double-blind, 3-way crossover trial of mirtazapine in 4 doses for the treatment of sleep apnea (Australian and New Zealand Clinical Trials Registry #12605000687695). The doses of mirtazapine used were 0 (i.e., placebo), 7.5, 15, 30, and 45 mg taken 30 minutes before bedtime. Patients were randomly assigned to 1 of 6 dose-sequence groups stratified by their baseline AHI score. The dose sequence groups are listed in Table 4. Dosing sequence groups were pre-selected to ensure that all patients were started at or below 15 mg, that no patients went from active drug to placebo and back to active drug, and that there was equal distribution of dosing options. The study initially aimed to recruit 42 patients; however, the decision to discontinue enrollment was made midway through recruitment due to an interim analysis that was initiated because of accumulated safety concerns that accrued as part of the data quality management process. Moreover, this interim analysis demonstrated trial failure, a fact that further supported the decision to stop the study.
Table 4.
Study 1, Order in Which Dose of Mirtazapine Was Given
| Sequence group | Dose of mirtazapine, mg | Patients in group, no. | ||
|---|---|---|---|---|
| 1st | 2nd | 3rd | ||
| 1 | 0 | 15 | 45 | 3 |
| 2 | 15 | 45 | 7.5 | 4 |
| 3 | 7.5 | 30 | 15 | 3 |
| 4 | 15 | 30 | 0 | 2 |
| 5 | 0 | 7.5 | 30 | 3 |
| 6 | 7.5 | 45 | 30 | 3 |
Each subject had general demographics and a diagnostic polysomnography administered at baseline and after each 2-week treatment period.
Study 2
Study 2 was a double-blind, 3-arm, parallel-group study of 4 weeks' treatment duration. The 3 treatment arms were placebo + placebo, mirtazapine 15 mg + placebo, and mirtazapine in combination with another compound (CD0012; Australian and New Zealand Clinical Trials Registry #12605000688684).
Randomization and Allocation Concealment: Study 1 and 2
The pharmacy at Royal Prince Alfred Hospital (Sydney, Australia) facilitated the over-encapsulation of all study drug to ensure that proper blinding was maintained. Randomization was also facilitated through the onsite pharmacy and was conducted manually using a randomized lists generated by an independent statistician contracted by Cypress Bioscience, Inc., who was not known to onsite study investigators and who never met any of the patients. Randomization to treatment in Study 1 gave equal chance of entry into each of the 6 treatment sequence groups. Randomization in Study 2 was undertaken using a 2:2:1 weighting, in which the placebo group was the underrepresented group. No study investigators in either study who were aware of dose allocation met any patient who was being treated, and no patient was aware of the dose they were receiving.
Inclusion and Exclusion Criteria: Study 1 and 2
Patients were eligible for inclusion if they could give informed consent, were at least 21 years old, had an AHI of 10 to 40, had a body mass index of 40 kg/m2 or less (Study 1) or had a body mass index of 34 kg/m2 of less (Study 2), either had never used continuous positive airway pressure or had used it for less than 4 days within the past year, were nonsmokers or had no history of smoking for at least 2 years, and had a baseline Epworth Sleepiness Scale17 score higher than 10. Women were eligible if postmenopausal; status post hysterectomy or bilateral oophorectomy; or, if of childbearing potential, had to have a negative urine pregnancy test prior to randomization and use a medically acceptable form of contraception throughout the trial.
Patients were excluded from the study if they had any clinically significant comorbidity, including any unstable cardiovascular, gastrointestinal, metabolic, pulmonary, renal, neurologic, hepatic, hematologic, immunologic, endocrine, or neoplastic disease; had uncontrolled hypertension; had a current diagnosis of any psychiatric illness or substance abuse disorder according to DSM IV criteria; were pregnant or lactating; or had severe craniofacial abnormalities.
Concomitant use of psychoactive drugs (e.g., stimulants, sedative hypnotics, tranquilizers, sedating antihistamines, benzodiazepines, anticonvulsants, or clonidine) was not allowed. Patients who were receiving therapy with MAO-A or -B inhibitors, tricyclics, tetracyclics, SSRI agents, NARI agents, SNRI agents, or α-agonists were excluded. Concomitant use of drugs having known cytochrome P450 induction or inhibition properties was prohibited.
Patients were instructed to take the daily mirtazapine doses 30 minutes prior to bed time. Compliance was assessed during clinic visits and by reviewing all returned treatment blister packs.
Outcome Measurements
Full overnight polysomnography was performed in the Royal Prince Alfred Hospital, the Royal North Shore Hospital, or the St Vincents Hospital sleep laboratories. Continuous recordings were made on a computerized system (Alice 4, Respironics, Pittsburgh, PA), and sleep variables recorded included 4 electroencephalograms channels (C3/A2,C4/A1, O1/A2 and O2/A1), 1 channel recording the mentalis/submentalis muscle activity, and 2 ocular channels recording from the left and right outer canthus (LOC/A2, ROC/A1). Additionally, bilateral anterior tibialis electromyogram leads were used to assess for periodic leg movement, bipolar electrocardiogram leads recorded heart rate, thoracic and abdominal movement were monitored via piezoelectric effort sensors, and a mercury gauge sensor detected body position. Oxyhemoglobin saturations (SaO2) were recorded using the Alice 4 internal oximeter, and nasal airflow was recorded via nasal prongs attached to a pressure transducer (Rescontrol™, ResMed Inc., Sydney, Australia). Periods of artifact, including those due to body movements or loss of contact of oximetry, were manually excluded from analysis. Such exclusions accounted for less than 1% of the overall recordings.
Sleep studies were scored in 30-second epochs according to standard criteria.18 All studies were scored by a single registered polysomnographic technician who was blinded to the study medication and dose sequencing. Arousals were scored according to American Sleep Disorders Association criteria.19
Apneas and hypopneas had to be of at least 10 seconds in duration. Obstructive apneas were scored when airflow ceased in the presence of continued thoracoabdominal movement. A hypopnea was defined as a clear reduction (compared with the baseline over the preceding 2 minutes) in 1 of the measures of breathing during sleep in association with at least a 3% oxygen desaturation, an electroencephalographic arousal, or both or a reduction in 1 of the measures of breathing by more than 50%. When there was no airflow and an absence of thoracoabdominal movement, the event was scored as a central apnea.20 The total AHI was calculated as the number of apneas and hypopneas that occurred per hour of sleep and was subdivided into non-rapid eye movement (NREM) AHI and rapid eye movement (REM) AHI according to appropriate sleep stage. Sleep efficiency (the proportion of time slept while in bed), apnea duration, and nadir SaO2 during sleep were also determined.
The Epworth Sleepiness Scale17 was used to measure daytime sleep propensity. Blood pressure and pulse were measured 3 times in the seated position after 15 minutes of rest. Pressures were determined via a sphygmomanometer or via an automated oscillometric device (Omron T5 BP monitor, Omron Healthcare Inc, Kyoto, Japan) with appropriately sized cuffs. Pulse was measured with the automated device.
Data Handling and Statistical Analyses
The primary outcome of these studies was the comparison between placebo and mirtazapine of the changes in the AHI between baseline and the end of treatment.
Study 1
Significance and size of treatment effects in both Studies 1 and 2 were tested in SAS (SAS institute v.9, SAS Institute, Inc., Cary, NC) by a study investigator who did not meet any of the patients and who was completely independent of the industry sponsor (NSM). Mixed model analyses of variance were employed to investigate treatment-induced improvements in Study 1. This is an accepted approach when a crossover trial such as this has been designed to have more treatments than each individual patient will receive.21 Treatment (5 levels) and order of treatment (3 levels) were fixed effects; individuals were random effects. When order was not statistically significant, it was dropped from the model. In all models, the difference in least square means option was used to test for any differences between any treatment dose and placebo. Main effects and tests for differences between doses were regarded as statistically significant when P < 0.05. Treatment-by-order interactions were not testable because not all doses were available for each order block. The figures given in the tables and figures are based on estimated means and 95% confidence intervals from mixed models that used end-of-treatment raw figures for data rather than net effects during each treatment arm as data. The net-effects data analyses are relied upon for the determination of statistical difference between doses.
Study 2
Study 2 data were analyzed similarly to Study 1 except that differences in treatment effect were tested for using analysis of variance or Kruskal-Wallis tests, where appropriate.
RESULTS
Patient Characteristics and Retention
Study 1
Forty-eight patients initially volunteered for the study, but 23 of these did not meet the screening criteria, 4 decided not to enter the study after having the trial explained to them, and 1 withdrew consent before randomization. The 20 remaining patients who met eligibility criteria were randomly assigned into 1 of the 6 treatment sequences. Two of the remaining 20 patients dropped out after randomization due to adverse events. In both cases, patients complained of excessive lethargy and resultant difficulty driving. Patients randomly assigned to treatment were, on average, obese (mean body mass index: 34.9 kg/m [SD: 4.4 kg/m2], mean weight: 106 kg [14.3 kg]), middle aged (mean: 47 years, range: 28–64 years) and predominantly men (n: 15, 83%). Other patient characteristics are detailed in Table 1. Over the course of the trial, across all treatments, the patients gained a mean 1.06 kg (1.8 kg). Table 2 describes the significant differences between treatment and placebo in terms of 2-week weight gain or loss.
Table 1.
Effects of 2 Weeks of Mirtazapine at Various Doses on Polysomnographic Variables and Daytime Sleepiness: Study 1
| Outcome variable | Baselinea | Placebob | Mirtazapine dose, mgb |
|||
|---|---|---|---|---|---|---|
| AHI, no./h | 7.5 | 15 | 30 | 45 | ||
| Total | 24.1 (8.0) | 25.0 (13.1–37.0) | 30.5 (20.1–41.0) | 37.3 (26.6–47.9)c | 39.2 (28.3–50.1)c | 26.7 (15.5–37.8) |
| Apneas only | 6.3 (6.8) | 13.2 (3.3–23.2) | 9.7 (0.8–18.6) | 12.9 (3.9–21.9) | 16.8 (7.7–26.0) | 9.9 (0.5–19.3) |
| Hypopneas only | 17.8 (6.3) | 12.4 (5.0–19.8) | 21.9 (16.0–27.9)c | 25.4 (19.3–31.6)d | 22.9 (16.6–29.2)c | 18.0 (11.1–24.8) |
| in REM | 36.6 (20.4) | 35.4 (21.6–49.1) | 39.7 (27.4–52.0) | 43.5 (31.0–55.9) | 46.4 (33.7–59.1) | 34.0 (21.0–47.0) |
| in NREM | 21.7 (9.2) | 23.3 (10.8–35.7) | 29.7 (18.7–40.6) | 37.4 (26.2–48.6)c | 38.8 (27.4–50.2)c | 24.9 (13.2–36.6) |
| AI, no./h | 23.6 (6.4) | 22.5 (13.6–31.4) | 29.5 (21.9–37.2) | 33.7 (25.9–41.5)c | 37.0 (29.0–45.1)c | 27.4 (19.2–35.7) |
| Sleep efficiency, % | 76.9 (9.4) | 82.6 (77.2–88.1) | 84.6 (80.2–89.1) | 83.9 (79.3–88.5) | 85.8 (81.0–90.6) | 85.1 (80.2–90.1) |
| Sleep stage, min | ||||||
| SWS | 51.8 (22.1) | 61.0 (33.9–88.2) | 62.3 (39.1–85.4) | 53.9 (30.2–77.6) | 54.4 (30.0–78.8) | 63.9 (38.7–89.0) |
| REM | 61.3 (21.1) | 72.5 (56.1–88.9) | 60.2 (46.7–73.8) | 72.4 (58.4–86.3) | 57.8 (43.3–72.2) | 67.3 (52.3–82.3) |
| 1&2 | 242.5 (47.4) | 250.0 (215.0–285.0) | 274.0 (245.7–302.3) | 260.0 (230.6–289.1) | 279.7 (249.3–310.1) | 259.9 (228.2–291.6) |
| ESS score | 13.6 (2.6) | 11.2 (8.5–13.8) | 10.6 (8.3–13.0) | 12.2 (9.8–14.6) | 12.3 (9.8–14.8) | 10.9 (8.4–13.4) |
Data are presented as the simple arithmetic mean (SD).
Data are presented as estimated means (95% confidence interval) using mixed-model estimates at the end of each treatment arm (with random patient effects and a fixed treatment effect).
P < 0.05 significantly worse than placebo.
P < 0.01 significantly worse than placebo. There were no significant order effects.
AHI refers to apnea-hypopnea index; REM, rapid eye movement sleep; NREM, non-rapid eye movement sleep; AI, Arousal Index; SWS, slow wave sleep; ESS, Epworth Sleepiness Scale.
Table 2.
Effects of 2 Weeks of Mirtazapine at Various Doses on Safety Variables: Study 1
| Safety variable | Baselinea | Placebob | Mirtazapine dose, mgb |
|||
|---|---|---|---|---|---|---|
| 7.5 | 15 | 30 | 45 | |||
| Weight, kg | 106 (14.3) | −0.80 (−1.63–0.03) | 0.55 (−0.06–1.16)c | 0.42 (−0.22–1.05)c | 0.60 (−0.06–1.26)c | 0.60 (−0.09–1.30)c |
| Blood pressure, mm Hg | ||||||
| Sitting systolic | 128.5 (8.5) | 128.0 (122.2–133.8) | 130.4 (125.5–135.2) | 123.5 (118.5–128.5) | 126.7 (121.6–131.9) | 125.6 (120.3–131.0) |
| Sitting diastolic | 85.2 (7.1) | 82.8 (76.7–88.8) | 86.2 (81.3–91.1) | 85.6 (80.5–90.6) | 85.1 (79.9–90.4) | 85.2 (79.7–90.7) |
| Standing systolic | 125.3 (7.8) | 123.9 (117.5–130.4) | 129.2 (123.6–134.7) | 126.4 (120.9–132.0) | 124.5 (118.8–130.2) | 126.5 (120.6–132.4) |
| Standing diastolic | 88.6 (6.2) | 82.4 (75.9–89.0) | 88.5 (83.1–93.9) | 88.4 (83.0–93.8) | 87.1 (81.5–92.8) | 89.2 (83.3–95.0) |
| Pulse, bpm | 75.9 (9.9) | 74.1 (65.2–83.0) | 83.8 (76.5–91.2) | 75.2 (67.9–82.5) | 85.9 (78.3–93.6)c | 85.0 (77.0–93.0)e |
Data are presented as the simple arithmetic mean (SD). Weight is shown as total weight at baseline.
Data are presented as estimated means (95% confidence interval) using mixed-model analyses of variance with random patient effects. Weight-loss analyses compare the measured weight at the end of each treatment arm with the weight at the end of the treatment arm immediately before (i.e., usually not with the baseline visit because weight is carried over from the previous treatment arm).
P < 0.05 significantly worse than placebo.
P < 0.01 significantly worse than placebo.
P = 0.065.
There were no significant order effects.
Pulse and blood pressure statistical analyses compare the end-of-treatment with the baseline-visit measurement.
Study 2
Sixty-four patients were randomly assigned to treatment, with 15 patients not completing the trial (6 from mirtazapine arm, 5 from mirtazapine+CD0012 arm, and 2 from placebo), 7 due to unacceptable lethargy (6 in mirtazapine arm and 1 in the mirtazapine+CD0012 arm). A further 3 patients (1 in each arm) had technically unsatisfactory polysomnograms either at baseline or at the end of the study, meaning that we could not include them in analyses of the primary outcome.
Patients randomly assigned to treatment were on average overweight (mean body mass index: 28.2 [SD: 2.9], mean weight: 87.1 kg [13.6 kg]), middle aged (mean: 52 years, range: 30–74 years), and predominantly men (n: 56, 86%). Other patient characteristics are detailed in Table 3.
Table 3.
Effects of 4 Weeks of Mirtazapine, 15 mg, on Polysomnographic Indexes and Sleepiness, Compared with Placebo: Study 2
| Outcome Variable | Baselinea |
Change while on treatmentb |
||||
|---|---|---|---|---|---|---|
| Placebo | Mirtazapine | Mirtazapine + CD0012 | Placebo | Mirtazapine | Mirtazapine + CD0012 | |
| AHI, no./h | ||||||
| Total | 27.4 (11.1) | 23.7 (9.8) | 23.6 (10.8) | −3.7 (5.4) | −5.0 (3.2) | −4.3 (2.2) |
| Apneas | 7.4 (7.0) | 5.6 (6.2) | 8.1 (10.4) | −3.5 (3.2) | −5.5 (2.2) | −3.5 (1.5) |
| Hypopneas | 19.1 (9.5) | 17.8 (7.7) | 15.1 (8.3) | −0.4 (5.1) | 0.6 (2.1) | −1.1 (2.2) |
| In REM sleep | 28.1 (19.7) | 25.9 (19.0) | 34.6 (20.1) | −3.5 (5.9) | −7.2 (6.2) | 1.3 (4.2) |
| In NREM sleep | 28.2 (12.4) | 24.1 (11.7) | 21.4 (11.5) | −5.4 (6.2) | −4.3 (3.3) | −5.6 (2.3) |
| Sleep efficiency, % | 77.5 (10.4) | 78.4 (9.6) | 75.3 (12.9) | 7.4 (1.8) | 2.8 (4.3) | 4.6 (3.2) |
| Sleep stage, min | ||||||
| SWS | 53.1 (38.7) | 71.5 (27.2) | 59.5 (29.5) | 23.3 (11.0) | 15.2 (10.1) | −6.4 (6.3) |
| REM | 71.4 (28.1) | 69.3 (26.3) | 61.3 (30.3) | 13.0 (3.4) | 2.6 (5.8) | 6.4 (6.9) |
| Stage 1&2 | 240.7 (27.2) | 222.3 (47.1) | 231.1 (36.2) | −17.9 (16.0) | −2.2 (17.2) | 20.9 (12.0) |
| ESS score | 11.1 (2.2) | 12.1 (3.5) | 13.1 (2.7) | 1.3 (1.4) | 3.3 (0.7) | 0.3 (1.1) |
Data are presented as mean (SD).
Data are presented as mean (SEM).
AHI refers to apnea-hypopnea index; NREM, non-rapid eye movement sleep; ESS, Epworth Sleepiness Scale.
Baseline values in those who completed the protocol. Figures in the improvement columns are positive numbers when that index improved and negative when that index worsened. Positive figures for slow wave sleep (SWS), rapid eye movement sleep (REM), and Stage 1&2 represent an increase in the number of minutes scored as those types of sleep.
Treatment Groups and Compliance with Treatment
Study 1
There were 6 treatment-sequence groups that received 3 of the 5 possible doses in the order listed in Table 4. As such 8 patients received placebo, 13 received 7.5 mg, 12 received 15 mg, 11 received 30 mg, and 10 received 45 mg. Compliance with treatment was assessed verbally and then verified by examining the number of tablets returned at the end of the 2-week treatment period. The median number of doses (range) returned for each dose was as follows: placebo: 3.0 (range 0–7), 7.5 mg: 1.0 (0–5), 15 mg: 2.0 (0–6), 30 mg: 2.5 (0–13), 45 mg: 2.5 (0–10).
Study 2
Twenty-six patients were randomly assigned to mirtazapine: 25 to the combination of mirtazapine and CD0012 and 13 to placebo. The median (range) number of doses returned for placebo was 2 (0–6); for mirtazapine 15 mg, it was 6 (0–16); and for mirtazapine + CD0012, it was 3.5 (0–12). The returned medication figures do not include medication that patients may have lost or discarded.
Effects of Mirtazapine
Study 1
The effects of mirtazapine on sleep apnea, sleep architecture, and daytime sleepiness are listed in Table 1 and, in addition, the primary outcome (AHI) is graphically illustrated in Figure 1. Effect-size calculations22 indicated that the 2 statistically significant increases in AHI caused by the 15-mg (effect size = 1.5) and 30-mg (effect size = 1.8) doses were large in magnitude. These large effect sizes should be interpreted with extreme caution because the AHI range was restricted at baseline, which will have caused underestimation of the true size of the standard deviation, causing inflation of the effect size. Potential side effects are listed in Table 2, with weight gain additionally graphically illustrated in Figure 2. We found no evidence that mirtazapine at any dose worsened periodic limb movements during sleep. We also found no evidence that the changes in the amount of time spent sleeping in the supine position or the changes in AHI in the supine position were significantly different between treatment groups (data not shown but both P > 0.1).
Figure 1.
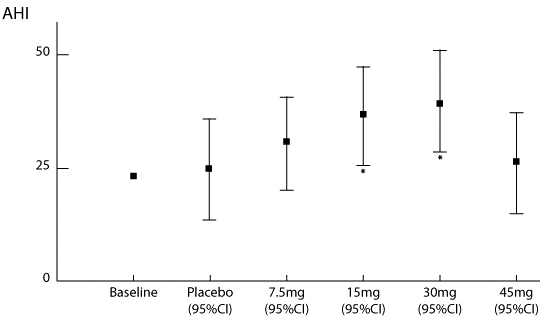
Effects of the various doses of mirtazapine on the apnea hypopnea index (AHI), compared with placebo. Estimates of the end of treatment AHI are derived from mixed-model estimates. Squares indicate the mean (bars = 95% confidence intervals) effects for each dose. Stars indicate significant worsening of the AHI, compared with placebo, adjusted for each patient's baseline value (P < 0.05) with doses of 15 mg and 30 mg. The point estimate of the AHI from the diagnostic sleep studies is included for comparison to show that placebo was not associated with a worsening of sleep apnea.
Figure 2.

Two-week weight gain associated with each dose of mirtazapine compared with placebo. Squares indicate the mean (bars = 95% confidence intervals) effects for each dose. Stars indicate significantly greater weight gain compared with placebo (P < 0.05) was observed with all doses.
Study 2
Treatment effects are listed in Table 3. Potential side effects are listed in Table 5. We found no evidence that mirtazapine worsened periodic limb movements during sleep. We also did not find that there were any changes in the amount of time spent sleeping in the supine position or that the changes in AHI in the supine position were significantly different between treatment groups (data not shown but both P > 0.5).
Table 5.
Effects of 4 Weeks of Mirtazapine, 15 mg, on Safety Variables, Compared with Placebo: Study 2
| Outcome Variable | Baselinea |
Change while on treatmentb |
||||
|---|---|---|---|---|---|---|
| Placebo | Mirtazapine | Mirtazapine + CD0012 | Placebo | Mirtazapine | Mirtazapine + CD0012 | |
| Weight, kg | 92.0 (16.5) | 88.8 (13.4) | 84.1 (13.1) | −0.1 (0.3) | 1.4 (0.4)c | 0.2 (0.3) |
| Blood pressure, mm Hg | ||||||
| Sitting systolic | 127.4 (10.1) | 126.8 (9.9) | 124.4 (9.6) | −3.4 (4.2) | −4.3 (3.0) | 1.1 (2.9) |
| Sitting diastolic | 77.7 (7.6) | 79.7 (7.6) | 79.5 (5.7) | 2.9 (3.1) | −2.6 (2.9) | 2.1 (1.9) |
| Standing systolic | 130.7 (10.1) | 125.6 (9.8) | 122.4 (12.7) | −2.9 (6.1) | 0.3 (3.1) | 2.4 (3.6) |
| Standing diastolic | 81.6 (6.4) | 81.0 (7.9) | 79.9 (8.1) | 3.9 (4.6) | 2.6 (3.1) | 1.4 (2.5) |
| Pulse, bpm | 74.0 (6.7) | 71.9 (9.9) | 71.6 (8.4) | −7.6 (4.2) | 2.4 (2.3) | 2.8 (2.5) |
Data are presented as mean (SD). Weight is shown as total weight at baseline.
Data are presented as mean (SEM). Data in the change columns indicate the arithmetic change from baseline in that index (i.e., a negative number for a blood pressure, pulse, or weight indicates a reduction in that measurement).
P < 0.01 compared with change while taking placebo.
DISCUSSION
Despite various reports supporting a potential therapeutic role for mirtazapine in improving the severity of sleep apnea,16,23,24 the present studies have not demonstrated that mirtazapine improves OSA severity. No index of sleep or sleep apnea was improved by any dose of mirtazapine tested (see Tables 1 and 3). Moreover, in this trial, the drug was associated with weight gain (see Tables 2 and 5), a previously recognized common side effect.25 Weight gain is a recognized risk factor for OSA,26 and, thus, mirtazapine might increase OSA severity over the longer term. Taking these factors together, at this point in time, there is no rationale for using mirtazapine for treating OSA. Clinicians treating depression in patients with comorbid OSA may also want to consider the potential for worsened OSA.
There are a number of studies that provided the rationale for investigating the efficacy of mirtazapine in improving OSA. Mirtazapine is a tetracyclic piperazinoazepine that enhances both central noradrenergic and serotonergic activity by blocking α2 receptors and selectively antagonizing serotonin 5HT2 and 5HT3 receptors.27–29 The rationale for investigating the efficacy of mirtazapine as a pharmacologic treatment of sleep apnea is predicated upon the interaction of mirtazapine with serotonin. Data from animal studies have suggested that serotonin provides a tonic excitatory input to hypoglossal motor neurons innervating the genioglossus and other upper airway-dilating muscles.30–35 Withdrawal of serotonergic input during sleep predisposes to airway obstruction.36 Studies in rodent models of sleep apnea have shown that mirtazapine reduced the apnea index during NREM sleep by more than 50% and during REM sleep by 60% for at least 6 hours.24 Normalized inspiratory minute ventilation was also increased during wake and sleep states. The duration of NREM sleep was unaffected by any dose of mirtazapine, but NREM electroencephalographic delta power was increased by more than 30% at all doses, indicating improved NREM sleep consolidation after mirtazapine injection.24 Mirtazapine also increased genioglossus activity in rats.37 In humans, mirtazapine has beneficial effects upon sleep, increasing both sleep continuity and slow-wave sleep duration.38
These data and encouraging preliminary human trials, first reported in abstract form in 200339 and subsequently published,16 suggested that mirtazapine held promise as potentially effective pharmacologic therapy for OSA. We are unable to easily explain the differences between our results and those of the previous positive trial that was stated to represent “the largest and most consistent drug-treatment effect” in OSA.16 However, our studies had some important strengths in that they employed a wider range and larger number of doses in Study 1, patients were treated for longer in our studies (1 week vs 2 weeks and 4 weeks), and our sample size was larger (12 vs 18 and 39). The disparate findings might be due to mirtazapine having a markedly different effect at 1 week than we have observed at 2 and 4 weeks. Although the patients selected in the 3 trials seem superficially similar, they differ in 2 particular respects. The first is that the previous study included a greater proportion of women than did the 2 trials we have described. These women were also markedly more obese than the men included in the previous trial16 and markedly more obese than all of the participants in the 2 trials we describe here. The second difference is that the previous study may have more strictly excluded potential participants because of adverse medical history. These differences may or may not have caused our patient groups to phenotypically differ in some aspect that is important to the drug's effect on sleep apnea.
Another feature of our data that was puzzling was that the observed worsening of OSA in response to mirtazapine in Study 1 was in a classic dose-response manner, but only up to 30 mg. At the 45-mg dose, the response was not different from that of placebo. This pattern might have been explained by patients showing much lower compliance levels with the 45-mg treatment than with lower doses, though our compliance data do not support this hypothesis. Alternatively, there may be a dose-specific effect of the effect of mirtazapine on OSA. Although a mechanism that would worsen OSA is unclear, dose-specific effects have been observed in which lower doses, compared with higher doses, are associated with greater sedation.40 However, the worsening of OSA associated with the 15-mg mirtazapine dose in Study 1 was not replicated in either arm of Study 2, in which 15-mg doses of mirtazapine were used. The combination of mirtazapine with a dopaminergic and serotinergic agent, CD0012, also resulted in no improvement in OSA. There are no data available on CD0012 as a single agent at this stage.
Mirtazapine is also a potent antagonist of histamine (H1) receptors, resulting in sedative effects. We did not find evidence of increased drowsiness, as measured by the ESS, caused by mirtazapine, compared with placebo in either trial. However, because the ESS is primarily designed to measure propensity to fall asleep rather than sedation, it might not be a sensitive measure for this effect. There were, however, 2 patients who were withdrawn from the Study 1 and 7 from Study 2 due to unacceptable sleepiness that resulted in concern that the patient may be potentially dangerous driving a motor vehicle. In patients who completed the trial, sleep architecture and sleep efficiency were unaffected by mirtazapine. No blood pressure changes were observed in association with mirtazapine use. However, pulse increased by about 10 beats per minute during administration of the 2 higher doses in Study 1 (30 mg and 45 mg, see Table 2) and by approximately the same amount with 15 mg of mirtazapine in Study 2. Weight gain is also associated with mirtazapine use (www.fda.gov). Consistent with these warnings, we observed significant weight gain with all doses of mirtazapine, compared with placebo, in these studies. In Study 1, patients gained about 0.25 kg per week of therapy and lost approximately 0.4 kg when on placebo (see Table 2 and Figure 2). In Study 2, patients gained approximately 1.4 kg in weight in 4 weeks on mirtazapine and remained at a stable weight if they had been randomly assigned to placebo (see Table 5). If such weight gain were to persist over time, patients would gain a considerable amount weight while using this drug. This weight gain would reasonably be expected to further worsen their OSA.26,41
The lack of an effective pharmacologic agent is a major limitation in the therapeutic options for sleep apnea. As such the treatment of sleep apnea is limited to mechanical devices of varying efficacy and effectiveness.42 A range of medications have been tried, typically “repurposing” agents that have been used previously for other conditions. These have included acetazolamide, buspirone, fluoxetine, L-tryptophan, and protriptyline. However, none of these have been truly effective and many have had significant side-effect profiles that have precluded their ongoing usage.12,13 In the light of our data, mirtazapine may have to be added to this list of promising treatments that have not proven to be effective. There is however some hope for pharmacotherapy with weight-loss medication,15 and cholinesterase inhibitory agents.43 However, large, randomized, controlled trials are required to rigorously test these candidates.
These 2 randomized, double-blind, placebo-controlled trials of mirtazapine for the treatment of OSA found no evidence that mirtazapine improves sleep apnea at any dose tested. This, combined with the weight gain that is associated with mirtazapine use that could reasonably be expected to worsen sleep apnea over the longer term,26 leads us to conclude that mirtazapine should not be used to treat sleep apnea.
ACKNOWLEDGMENTS
Financial Support: This study was funded by Cypress Bioscience. Prof. Grunstein is supported by National Health and Medical Research Council of Australia Practitioner Fellowships. Dr. Wong was supported by the NH&MRC Centre of Clinical Research Excellence for Respiratory and Sleep Medicine.
Footnotes
Disclosure Statement
This study was funded by Cypress Bioscience. Dr. Grunstein has participated in studies supported by Takeda, GlaxoSmithKline, Sanofi-Aventis, Cypress Biosciences, and Cephalon and is supported by National Health and Medical Research Council of Australia Practitioner Fellowships. Dr. Wong was supported by the NH&MRC Centre of Clinical Research Excellence for Respiratory and Sleep Medicine. Dr. Kranzler is Chief Executive Officer and Chairman of the Board of Cypress Biosciences. Ms. Zack is Senior Program Manager, Strategy and Clinical Development for Cypress Biosciences. Drs. Rao and Gendreau are employed by Cypress Biosciences. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Peppard P, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. New Eng J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 4.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients—a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 5.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J. 2002;20:1511–8. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- 6.Marin J, Carrizo S, Vincente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Giles T, Lasserson T, Smith B, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD001106.pub2. 19 July 2006. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, White D, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 9.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim J, Lasserson T, Fleetham J, et al. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;1:CD004435. doi: 10.1002/14651858.CD004435.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundaram S, Bridgman S, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004. doi: 10.1002/14651858.CD001004.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Smith I, Lasserson T, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;2:CD003002. doi: 10.1002/14651858.CD003002.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Strollo P, Jr, Atwood C, Jr, Sanders M. Medical therapy for obstructive sleep apnea-hypopnea syndrome. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier/Saunders; 2005. pp. 1053–65. [Google Scholar]
- 14.Whyte K, Gould G, Airlie M, et al. Role of protriptyline and acetazolomide in the sleep apnea/hypopnea syndrome. Sleep. 1988;11:463–72. doi: 10.1093/sleep/11.5.463. [DOI] [PubMed] [Google Scholar]
- 15.Yee B, Phillips C, Banerjee D, et al. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes (Lond) 2007;31:161–8. doi: 10.1038/sj.ijo.0803363. [DOI] [PubMed] [Google Scholar]
- 16.Carley D, Olopade C, Ruigt G, et al. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30:35–41. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. Sleepiness in different situations measured by the Epworth sleepiness scale. Sleep. 1994;17:703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/ Brain Research Institute University of California; 1968. [Google Scholar]
- 19.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 20.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Taskforce. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 21.Brown H, Prescott R. Chichester: John Wiley and Sons; 1999. Applied mixed models in medicine; p. 408. [Google Scholar]
- 22.Kazis L, Anderson J, Meenan R. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–S89. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 23.Castillo J, Menendez P, Segovia L, et al. Effectiveness of mirtazapine in the treatment of sleep apnea/hypopnea syndrome (SAHS) Sleep Med. 2004;5:507–8. doi: 10.1016/j.sleep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160:1824–9. doi: 10.1164/ajrccm.160.6.9902090. [DOI] [PubMed] [Google Scholar]
- 25.Biswas P, Wilton L, Shakir S. The pharmacovigilance of mirtazapine: results of a prescription event monitoring study on 13 555 patients in England. J Psychopharmacology. 2003;17:121–6. doi: 10.1177/0269881103017001716. [DOI] [PubMed] [Google Scholar]
- 26.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 27.de Boer T, Maura G, Raiteri M, et al. Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, org 3770 and its enantiomers. Neuropharmacology. 1988;27:399–408. doi: 10.1016/0028-3908(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 28.Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17:2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 29.Ruigt G, Kemp B, Groenhout C, et al. Effect of the antidepressant org 3770 on human sleep. Eur J Clin Pharmacol. 1990;38:551–4. doi: 10.1007/BF00278580. [DOI] [PubMed] [Google Scholar]
- 30.Bayliss D, Viana F, Talley E, et al. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol. 1997;110:139–50. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 31.Berger A, Bayliss D, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–8. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 32.Douse M, White D. Serotonergic effects on hypoglossal neural activity and reflex responses. Brain Res. 1996;726:213–22. [PubMed] [Google Scholar]
- 33.Kubin L, Tojima H, Davies R, et al. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–8. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro-do-Valle L, Metzler C, Jacobs B. Facilitation of masseter EMG and masseteric (jaw-closure) reflex by serotonin in behaving cats. Brain Res. 1991;550:197–204. doi: 10.1016/0006-8993(91)91318-u. [DOI] [PubMed] [Google Scholar]
- 35.Veasey S, Fornal C, Metzler C, et al. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–59. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veasey S, Panckeri K, Hoffman E, et al. The effects of serotonin antagonists in an animal model of sleep disordered breathing. Am J Respir Crit Care Med. 1996;153:776–86. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- 37.Berry R, Koch G, Hayward L. Low-dose mirtazapine increases genioglossus activity in the anesthetized rat. Sleep. 2005;28:78–84. doi: 10.1093/sleep/28.1.78. [DOI] [PubMed] [Google Scholar]
- 38.Thase M. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry. 1999;60:28–31. [PubMed] [Google Scholar]
- 39.Carley D, Olopade C, Seink S, et al. Serotonin antagonist improves obstructive sleep apnea. Sleep Med. 2003;4:S6. [Google Scholar]
- 40.Kasper S, Praschak-Rieder N, Tauscher J, et al. A risk-benefit assessment of mirtazapine in the treatment of depression. Drug Saf. 1997;17:251–64. doi: 10.2165/00002018-199717040-00005. [DOI] [PubMed] [Google Scholar]
- 41.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 42.Cistulli P, Grunstein R. Medical devices for the diagnosis and treatment of obstructive sleep apnea. Expert Rev Med Devices. 2005;2:749–63. doi: 10.1586/17434440.2.6.749. [DOI] [PubMed] [Google Scholar]
- 43.Hedner J, Kraiczi H, Peker Y, et al. Reduction of sleep-disordered breathing after physostigmine. Am J Respir Crit Care Med. 2003;168:1246–51. doi: 10.1164/rccm.200211-1344OC. [DOI] [PubMed] [Google Scholar]


