Abstract
Objective:
To determine the validity of the phasic electromyographic metric (PEM) to differentiate patients with a history suggestive of rapid eye movement behavior disorder (REMBD) on laboratory nights without overt dream-enactment behavior.
Methods:
PEM was quantified as the % of 2.5-sec intervals with phasic muscle activity of 100-msec duration with an amplitude of at least 4 times background activity in 11 patients and 31 elderly controls. Data were derived from both REM and NREM sleep from 5 muscle groups (mentalis, left/right anterior tibialis, left/right brachioradialis).
Results:
Relative to controls, REMBD patients had significantly higher levels of PEM activity in all recordings. The largest differences occurred during REM sleep for the mentalis and brachioradialis channels. Similar results were obtained by limiting quantification of PEM to the final REM period of the night and could be accomplished by individuals with no previous familiarity with polysomnography.
Discussion:
PEM may be a useful metric to characterize the REM related phasic muscle activity on patients with a history of REMBD, even when no overt dream-enactment behaviors are detected on a laboratory night.
Citation:
Bliwise DL; Rye DB. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. SLEEP 2008;31(6):853–857.
Keywords: Rapid eye movement sleep behavior disorder; electromyography, phasic, tonic
PATIENTS WITH HISTORIES OF DREAM ENACTMENT BEHAVIORS OFTEN UNDERGO POLYSOMNOGRAPHY FOR THE PURPOSE OF CONFIRMING THE DIAGNOSIS of REM behavior disorder (REMBD). A frequent dilemma arises when, despite a compelling clinical history, no overt dream enactment is captured by video-polysomnography. In lieu of frank behavioral episodes, researchers have developed a variety of quantification systems to determine the extent to which muscle activity (primarily recorded during REM) may otherwise signify the absence of normal REM atonia, such as is characteristic of REMBD. The systems rely upon manual scoring of the frequency of occurrence of phasic and/or tonic muscle activity during REM,1–3 though automated scoring algorithms for digitally acquired data are also under development.4 Recently we have applied a modification of the visually derived system originally described by Lapierre and Montplaisir1 to a relatively large number of normal subjects of varying ages and to a patient group with prototypical elevated muscle activity in REM, Parkinson disease (PD).5 We describe here application of this system when applied to patients with REMBD who, with one exception, did not exhibit behavioral abnormalities on their laboratory night.
METHODS
Patients
All patients (9 men, 2 women; mean [SD] age = 68.6 [10.6] demonstrated histories compatible with REMBD of durations varying between 1 and 18 years (see Table 1). Some evidence of subtle neurologic signs (mild unilateral cogwheeling or slightly decreased unilateral arm swing) was noted on neurologic exams in 5 patients (#'s 1, 6, 8, 9, 11) but did not meet criteria for PD. Three (#'s 1, 8, 11) had magnetic resonance imaging (MRI) studies suggestive of old infarcts or small vessel white matter disease. Medication use is shown in Table 1 and indicates that three (#'s 8, 9, 10) received psychotropic medications, such as selective serotonin reuptake inhibitors.
Table 1.
Characteristics of REMBD Patients
| Subject | Age | Gender | Race | Duration (yr) | Medications |
|---|---|---|---|---|---|
| 1 | 75 | M | C | 15 | glyburide, gemfibrozil, oxybutynin |
| 2 | 59 | M | C | 1 | fluticasone spray |
| 3 | 57 | M | C | 9 | loratadine, cetirizine, simvastatin, tamsulosin |
| 4 | 84 | M | C | 2 | furosemide, enalaprilat |
| 5 | 78 | W | C | 18 | furosemide, atenolol, nifedipine |
| 6 | 67 | W | B | 3 | meclizine, tamoxifen, cisapride, nitroglycerin |
| 7 | 51 | M | C | 3 | carbamazepine, lamotrigine |
| 8 | 76 | M | C | 5 | sertraline, diltiazem, lansoprazole |
| 9 | 78 | M | C | 2 | olanzapine, Synthroid |
| 10 | 69 | M | H | 8 | sertraline, rivastigmine, clonazepam, zaleplon, terazosin, meloxicam |
| 11 | 60 | M | B | 10 | terazosin, benazepril, felodipine, insulin |
M, Man; W, Woman; C, Caucasian; B, Black (African American); H, Hispanic
Procedures
Overnight polysomnography with continuous video monitoring was performed using standard methods. In additional to electroencephalography, electrooculography, and mentalis electromyography (EMG), we recorded 4 limb EMG channels as well, from placements over the right and left anterior tibialis and right and left brachioradialis. For mentalis and each limb channel, we used bipolar derivations to minimize pulse artifact and made attempts to maintain electrode pair impedances below 10,000 ohms (when possible, under 5,000 ohms) at the start of night. EMG filter settings were 10 to 100 Hz. We did not alter filter or amplifier settings after initial biocalibrations, but loose electrodes for EMG channels were typically re-referenced or replaced during the night to maintain high quality signals. Polysomnography was recorded on paper with Grass Model 78 polysomnographs at a paper speed of 10 mm/sec, which yielded 30-sec epochs for scoring.
Sleep stages were scored following standard Rechtschaffen and Kales6 criteria using additional criteria as necessary to define REM sleep in the absence of atonia.7 EMG activity was quantified using the system originally described by Lapierre and Montplaisir,1 which yielded a phasic electromyographic metric (PEM). PEM activity was defined separately in the mentalis, anterior tibialis and brachioradialis channels as discrete bursts of EMG activity ≥100 msec duration with an amplitude of at least 4 times the background activity in that particular channel as detected during the pre-sleep baseline. If EMG activity meeting the aforementioned criteria was present in a 2.5-sec interval, then that interval was scored as positive for the presence of PEM. A given 2.5-sec interval might have contained several identifiable discrete bursts of EMG activity within that interval; in such cases that interval was considered positive for PEM. However, any given 2.5-sec interval could receive only a single PEM “score.” The percentage of 2.5-sec intervals containing PEM activity was computed separately for REM and NREM sleep from each of the 5 sites listed above. PEM activity was scored by a single scorer for whom we have previously reported an interrater reliability coefficient of 0.77.5
Comparison of rates of phasic muscle activity in the REMBD patients to normative polysomnographic data relied upon our previously published data from elderly control subjects (n = 31) without periodic leg movements in sleep (PLMS).5 These subjects (9 men, 22 women) were derived from a community-based elderly cohort and had a mean age of 70.3 (SD = 9.3) years. We relied upon 2-group t-tests to compare PEM rates across groups using 2-tailed probabilities. Inequalities of variances were adjusted with the Satterthwaite correction. Effect sizes were computed with the d statistic, as described by Cohen.8
Because visual quantification of PEM using the aforementioned approach can be time consuming, we also evaluated PEM rates in our patients using an abbreviated approach in which data from only the final REM period of the night were evaluated. Finally, in order to determine how well relatively untrained scorers could evaluate PEM, we also examined interrater agreement between 2 scorers (4th year medical students) otherwise naive to polysomnography who independently evaluated such phasic muscle activity.
RESULTS
With the exception of one patient who demonstrated episodes of dream-enactment behavior during 2 REM periods, no behavioral abnormalities were observed on the overnight sleep laboratory recording. Comparisons between the REMBD patients and the 31 controls on PEM measures from all 5 sites in REM and NREM sleep are presented in Table 2. Although all site/stage definitions significantly differentiated the 2 groups, effect size estimates clearly suggested that REM-related mentalis and brachioradialis rates provided the largest differences between groups. Similar results were obtained with elimination of the REMBD patients using psychotropics (cases 8, 9, 10), those with mild neurologic signs (cases 1, 6, 8, 9, 11), those with MRI findings (cases 1, 8, 11), and when the single case with overt dream-enactment behavior (case 5) on the lab night was excluded. PLMS, scored with customary criteria,9 were also significantly higher in REMBD patients relative to controls (for PLMS Index [events per hour of sleep]: 15.8 [20.8] vs 0.2 [0.68], t = 2.48, P = 0.032; for PLMS with arousal index [events per hour of sleep]: 2.7[2.4] vs 0.2[0.5], t = 3.44, P < 0.0001).
Table 2.
Comparison of Mean (SD) PEM Values for REMBD Patients and Controls
| PEM Variable | REMBD (n = 11) | Controls (n = 31) | t | P | d |
|---|---|---|---|---|---|
| Mentalis, REM | 0.201 (0.118) | 0.035 (0.042) | 4.13 | 0.0028 | 2.50 |
| Mentalis, NREM | 0.053 (0.047) | 0.017 (0.015) | 2.50 | 0.0300 | 1.37 |
| Left Brachioradialis, REM | 0.256 (0.091) | 0.004 (0.005) | 6.15 | 0.0035 | 3.90 |
| Left Brachioradialis, NREM | 0.065 (0.029) | 0.005 (0.007) | 4.92 | 0.0031 | 2.78 |
| Right Brachioradialis, REM | 0.254 (0.076) | 0.005 (0.002) | 7.29 | 0.0019 | 4.61 |
| Right Brachioradialis, NREM | 0.082 (0.044) | 0.003 (0.003) | 4.35 | 0.0072 | 2.41 |
| Left Anterior Tibialis, REM | 0.195 (0.189) | 0.033 (0.063) | 2.53 | 0.0337 | 1.55 |
| Left Anterior Tibialis, NREM | 0.134 (0.164) | 0.011 (0.009) | 2.47 | 0.0331 | 1.49 |
| Right Anterior Tibialis, REM | 0.207 (0.208) | 0.019 (0.020) | 2.70 | 0.0268 | 2.00 |
| Right Anterior Tibialis, NREM | 0.162 (0.180) | 0.012 (0.013) | 2.77 | 0.0199 | 1.66 |
REM, rapid eye movement sleep; NREM, non-rapid eye movement sleep
Values shown represent mean and standard deviations of the percentage of 2.5-sec intervals containing phasic muscle activity. A particular 2.5-sec interval could contain several detectable phasic events; however, that interval would receive only a single score indicating the presence of PEM within than interval.
N's vary for some variables because channels were missing
t-test values adjusted for unequal variances using Satterthwaite correction
Effect sizes (d) computed as described by Cohen8
Because whole night quantification of PEM using visual analyses is time consuming, we examined the potential utility of confining analyses to a more limited portion of the overnight polysomnograms. More specifically, because effect sizes in Table 1 suggested that PEM measures derived from REM sleep differentiating REMBD patients from controls were associated with larger effect sizes than those derived from NREM sleep, and because these effects were most notable in both chin and arm leads, we analyzed the final REM period of each recording to determine whether this abbreviated evaluation of PEM activity might also differentiate patients and controls. Final REM periods for the REMBD patients varied widely in length and ranged from 2.5 to 40.5 min in duration (mean [SD] = 20.8 [28.5] min). When computed as the percentage of 2.5-sec intervals containing PEM activity, REMBD patients continued to show significantly higher values than controls (for mentalis REM, t = 3.17, P = 0.012 for left brachioradialis, t = 5.72, P = 0.0045; for right brachioradialis, t = 7.44, P = 0.0017).
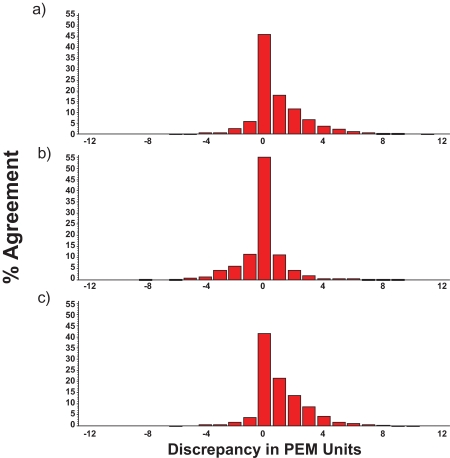
Figure 1 (a-c) shows the agreement on PEM identification achieved among the original trained scorer (a) and the 2 medical students (b and c), unfamiliar with polysomnographic scoring who were asked to tally PEM for the final (previously identified) REM period for the night. Data are presented as frequency distribution histograms of each scorer pair discrepancy for 30-sec epochs based on 2.5-sec intervals of REM sleep for all EMG channels for the final REM period of the night. The range of discrepancy could be as low as −12 (if scorer A determined PEM to be present in all 12 2.5-sec intervals of a 30-sec epoch and scorer B determined PEM to be present in 0 2.5-sec intervals) to as high as +12 (if scorer A determined PEM to be present in 0 of the 12 2.5-sec intervals of the 30-sec epoch whereas scorer B identified PEM as occurring in all 12 2.5-sec intervals of a 30-sec epoch). These data clearly indicate that although occasional discrepancies occurred between scorer pairs, these were, for the most part, seldom more than ±3 PEM units, and were more commonly within ±1 unit.
Figure 1.
Discrepancy in visually scored PEM activity between scorers A and B (a), A and C (b) and B and C (c). X-axis represents the number of discrepant units on identical, independently scored 30-sec epochs containing a maximum of 12 2.5-sec intervals per epoch. Y-axis shows percentage agreement. All data were derived from EMG signals recorded during the final REM period of the night. Scorer A was experienced with polysomnography; scorers B and C were 4th year medical students and had no previous familiarity with polysomnographic recordings. REM epochs were identified for scorers B and C prior to PEM measurements. Distributions show high levels of agreement on visually identified PEM between all scorer pairs.
DISCUSSION
These results suggest that PEM validly differentiates the EMG signals of patients with histories of dream enactment behaviors from elderly controls even when the former do not demonstrate overt behavioral abnormalities on a night of polysomnography. Scoring of PEM, although time consuming when conducted over an entire recording night, can be accomplished reliably by individuals with little or no experience with polysomnographic scoring (assuming that stage scoring has already been provided) and can be limited to the final REM period of the night. Because comparisons of mentalis and upper limbs provide the largest effect sizes, further confining PEM analyses to only those channels provides adequate differentiation. Future studies with such limited scoring would be required to more firmly establish this procedure as a viable approach, and such visual analyses should be performed on video monitor to ensure comparability of analyses across media. Standardized automated approaches relying upon digitization of EMG signals4 might also provide a pragmatic alternative to visual analyses that would be practically and economically feasible.
The neurophysiological basis for the abundant phasic muscle activity during REM sleep remains yet to be fully elucidated in synucleinopathic patients. Whereas sustained tonic muscle activity during REM sleep may represent inhibition of centers within the ventrolateral medulla controlling normal REM-related atonia,10 the basis for phasic activity that overrides such atonia is less certain. One possibility is that the high neuronal firing rates within the substantia nigra reticulata in REM sleep11 may lead to such high rates of phasic activity within skeletal muscle groups. Because both direct and monosynaptic pathways between midbrain regions and more caudal structures such as the pedunculopontine nucleus have been described,12 such pathways represent a viable substrate for such phasic descending influences on motor activity in REM.
Some question may arise as to how tonic EMG activity is handled within the context of the scoring rules for deriving PEM activity that we have employed. The system originally described by Lapierre and Montplaisir1 differentiated tonic activity and phasic activity. We did not attempt to delineate tonic EMG activity on both theoretical and practical grounds. First, “tonic” muscle activity, when recorded from surface EMG electrodes, is considered an amalgam of the firing of various motor units at different frequencies within a muscle.13 This is differentiated from the most fundamental component of any muscle recording, which is the activity of the motor unit. When a sufficient number of such units fire, surface recordings result in what is typically called tonic activity,14 though the visual identification of such tonic activity probably depends on the recruitment of neighboring motor units and the relative tension and stretch of the muscle fibers. Discernment of tonic activity depends on the resting level of firing of the muscle, which may differentially characterize various skeletal muscles in NREM sleep.15 When tonic EMG levels are sufficiently high, it may be impossible to discern firing of individual motor units, i.e., phasic events, with surface recording. In our adaptation of the Lapierre and Montplaisir system, tonic activity precludes the scoring of PEM activity because the noise-to-signal ratio is sufficiently high so as to make identification unreliable.5 For these reasons, we believe that identification of phasic muscle activity on the basis of surface EMG recordings mirrors the physiologic signals closest to individual motor unit potentials that would be otherwise recorded with needle EMG electrodes. Such activity, particularly when recorded in REM, may best represent the firing of neuronal pools within the nigrostriatal system that appear elevated in REM sleep and that appear to attain pathologically high rates in synucleinopathic-like conditions.
Table 3.
Correlations between PEM Values
| Mentalis NREM | Left Brach REM | Left Brach NREM | Right Brach REM | Right Brach NREM | Left Ant Tib REM | Left Ant Tib NREM | Right Ant Tib REM | Right Ant Tib NREM | |
|---|---|---|---|---|---|---|---|---|---|
| Mentalis REM | 0.73*** | 0.72* | 0.92*** | 0.72* | 0.93*** | 0.20 | 0.17 | 0.19 | 0.25 |
| Mentalis NREM | _ _ _ | 0.60 | 0.90*** | 0.52 | 0.86*** | 0.27 | 0.41** | 0.32* | 0.40** |
| Left Brach REM | _ _ _ | 0.76* | 0.82** | 0.71* | 0.92*** | 0.82** | 0.90*** | 0.79** | |
| Left Brach NREM | _ _ _ | 0.75* | 0.95*** | 0.71* | 0.81** | 0.78** | 0.91*** | ||
| Right Brach REM | _ _ _ | 0.75* | 0.94*** | 0.79** | 0.84** | 0.78** | |||
| Right Brach NREM | _ _ _ | 0.70* | 0.64* | 0.72* | 0.83** | ||||
| Left Ant Tib REM | _ _ _ | 0.65*** | 0.58*** | 0.40* | |||||
| Left Ant Tib NREM | _ _ _ | 0.80*** | 0.66*** | ||||||
| Right Ant Tib REM | _ _ _ | 0.89*** |
*P < 0.05; **P < 0.01; ***P < 0.001
N's vary in some cases because of missing data
Values are rho (Spearman rank order correlations)
Abbreviations: Brach = Brachioradialis; Ant Tib = Anterior Tibialis
Table 4.
Mean (SD) PEM Values for Final REM Period in REMBD Patients
| Variable | Value |
|---|---|
| Mentalis | 0.160 (0.116) |
| Left Brachioradialis | 0.215 (0.082) |
| Right Brachioradialis | 0.216 (0.063) |
| Left Anterior Tibialis | 0.204 (0.221) |
| Right Anterior Tibialis | 0.215 (0.215) |
PEM data derived from primary scorer Values represent proportion of 2.5-sec intervals of last REM period containing phasic muscle activity
ACKNOWLEDGMENTS
Support: NS-050595, AG-020269
We gratefully acknowledge the assistance of Farzaneh Pour Ansari, MA, Liqiong He, MD, Monica Habib, MD, and Jacqueline Marcus, MD, in conducting this work.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Rye has participated in speaking engagements for GlaxoSmithKline and has consulted for GlaxoSmithKline, Boehringer Ingelheim, Ortho-McNeill, and Sepracor. Dr. Bliwise has received research support from Takeda, has consulted for Gerson Lehrman Group, Neurocrine, Cephalon, Pfizer, Takeda, and Sepracor and has participated in speaking engagements for Takeda, Boehringer Ingelheim, School of Sleep Medicine, and Sleep Medicine Educational Institute.
REFERENCES
- 1.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–74. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 2.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease and controls. Sleep. 2003;26:507–12. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 3.Consens FB, Chervin RD, Koeppe RA, et al. Validation of a polysomnographic score for REM sleep behavior disorder (RBD) Sleep. 2005;28:993–97. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 4.Burns JW, Consens FB, Little RJ, Angell KJ, Gilman S, Chervin RD. EMG variance during polysomnography as an assessment for REM sleep behavior disorder. Sleep. 2007;30:1771–78. doi: 10.1093/sleep/30.12.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliwise DL, He L, Ansari FP, Rye, DB Quantification of electromyographic activity during sleep: a phasic electromyographic metric. J Clin Neurophysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- 6.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute UCLA; 1968. [Google Scholar]
- 7.Bliwise DL, Williams ML, Irbe D, Ansari FP, Rye DB. Inter-rater reliability for identification of REM sleep in Parkinson's disease. Sleep. 2000;23:671–76. [PubMed] [Google Scholar]
- 8.Cohen J. Statistical power analyses for the behavioral sciences. Orlando: Academic; 1977. [Google Scholar]
- 9.Atlas Task Force of the American Sleep Disorders Association. Recording and scoring of leg movements. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 10.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:855–90. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 11.Datta S, Curro Dossi R, Pare D, Oakson G, Steriade M. Substantia nigra reticulata neurons during sleep-waking states: relation with ponto-geniculo-occipital waves. Brain Res. 1991;566:344–47. doi: 10.1016/0006-8993(91)91723-e. [DOI] [PubMed] [Google Scholar]
- 12.Rye DB, Bliwise DL. Movement disorders specific to sleep and the nocturnal manifestations of waking movement disorders. In: Watts RL, Koller WC, editors. Movement disorders: neurologic principles and practice. 2nd ed. New York: McGraw Hill; 2004. pp. 855–90. [Google Scholar]
- 13.Basmajian JV. Muscles alive: their functions revealed by electromyography. Baltimore: Williams and Wilkins; 1967. [Google Scholar]
- 14.Bliwise D, Coleman R, Bergmann B, Wincor MZ, Pivik RT, Rechtschaffen A. Facial muscle tonus during REM and NREM sleep. Psychophysiology. 1974;11:497–508. doi: 10.1111/j.1469-8986.1974.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson A, Kales A, Lehman D, Hoedemaker FS. Muscle tonus in human subjects during sleep and dreaming. Exp Neurol. 1964;10:418–24. doi: 10.1016/0014-4886(64)90033-0. [DOI] [PubMed] [Google Scholar]