Abstract
During obstructive sleep apnea (OSA) in adults upper airway reopening coincides with a sudden burst in activity of pharyngeal dilating muscles. This has been attributed to arousal from sleep as indicated by increased EEG activity. Recovery from OSA in infants often occurs in the absence of cortical arousal. To investigate mechanisms involved in recovery, we performed experimental airway occlusions in sleeping infants. Based on past work, our hypothesis was that a sleep startle combined with an augmented breath and heart rate acceleration would occur during the occlusion, and that such brainstem mediated reflexes might provide an explanation for recovery from OSA in the absence of cortical arousal. However, this is contrary to expectations, since lung inflation is believed to be necessary for occurrence of an augmented breath.
We studied 16 healthy infants during sleep. We recorded EEG, EOG, ECG, oxygen saturation, diaphragmatic, nuchal and limb electromyograms, face mask pressure, and airflow. A startle, accompanied by neck extension, limb and nuchal EMG activation, as well as heart rate acceleration occurred during all airway occlusions. The startle occurred simultaneously with a large biphasic inspiratory effort, having characteristics of an augmented breath (sigh). In more than a third of cases, this occurred without any evidence of cortical arousal activity. The magnitude of startles as well as the increase in heart rate correlated positively with peak airway negative pressure, indicating that arousal processes are graded in intensity. We conclude that the neck extension and pharyngeal dilating muscle activity associated with the startle and augmented breath may account for recovery of airway patency in infants as they do adults. Lung inflation is not a prerequisite for the reflex to occur.
Citation:
Wulbrand H; McNamara F; Thach BT. The role of arousal related brainstem reflexes in causing recovery from upper airway occlusion in infants. SLEEP 2008;31(6):833–840.
Keywords: Airway obstruction, sleep apnea, annotated breath sigh, startle, arousal
THE RECOVERY FROM PHARYNGEAL AIRWAY OCCLUSION DURING AN OBSTRUCTIVE SLEEP APNEA (OSA) EPISODE HAS BEEN DESCRIBED AS OCCURRING abruptly during a single respiratory effort coinciding with a sudden burst of submental muscle or genioglossus EMG.3,16, It has been suggested that that sudden activation of the cortical EEG occurring at this time produces activation of genioglossus and other upper airway dilating muscles causing airway reopening.17 In contrast, recent work suggests that cortical EEG activation is sometimes absent when adults recover from OSA episodes.16 Moreover, such evidence of cortical arousal is usually absent when infants and children recover from OSA.12,27
Of potential relevance to recovery from OSA episodes in the absence of cortical arousal are the observations of Lijowska et al., who found that an augmented breath (sigh) frequently coupled with a startle, occurs at the onset of arousals in infants.9 These events frequently are not followed by cortical activation in the EEG.12,28 Augmented breaths in infants as well as in animal models are known to be associated with large increases in upper airway dilating muscle activity.4,20,24 Therefore, an augmented breath might explain the “burst” of genioglossus activity described by Remmers et al. in adults and by Wulbrand et al. and Roberts et al. in infants.17,20,27,29 Our primary hypothesis was that lung inflation is not essential for occurrence of augmented breaths. However, early investigations of elimination of pulmonary vagal innervation have shown that augmented breaths are initiated by an inspiration-augmenting reflex mediated via rapidly adapting pulmonary stretch receptors.2,19 Thus, generation of augmented breaths has been believed to be exclusively dependent on lung stretch receptor stimulation.
As suggested by animal models of arousal, in the present study, we hypothesized that during airway occlusion the onset of arousal activity in the brainstem would be sufficient to produce an augmented breath accompanied by a startle, even in the absence of lung inflation.13 We further hypothesized that sighs, startles, and heart rate increase are synchronized and proportionate in terms of magnitude during airway occlusion and that they frequently occur without evidence of cortical arousal.
METHODS
Subjects
We studied 16 normal healthy term infants (7 female, 9 male) between 34 and 134 days of age (median 82.5 days) during daytime naps. Polygraphic recordings started after a feeding when the infant fell asleep. Three of the parents smoked, but all declared that none of the infants had been exposed to smoke in the home. One mother said she smoked during early pregnancy. The study was approved by the Washington University Committee for Human Studies. Informed consent was obtained from the parents, one of whom was present during the recordings.
Polygraphic Recordings
Infants slept in their usual sleeping position (5 prone, 11 supine) during the study, which lasted between 2 and 4 hours. Polygraphic recordings included EEG (C3-P3), EOG (outer canthi - A1), and ECG (Beckman R 611, 8-channel polygraph). Infants who normally slept prone were placed in a prone position, but their heads were turned to the side to facilitate mask placement. In our study, we used 2 scalp electrodes for determining cortical arousals. This was done because placement of multiple leads interfered with the mask occlusion protocols.
To evaluate the accuracy of using 2 scalp electrodes, we analyzed EEG data obtained during the course of a prior study. This analysis has not been previously published. We studied 13 preterm infants during 17 polygraphic recordings. The infants were born at 27 and 35 weeks of gestation. They were 8 to 24 weeks old when studied. The infants had no evidence of cerebral injuries or history of seizures. In the prior study, the infants were studied on 17 occasions. We used the 10/20 monopolar system, with A1 and A2 noncephalic references for electrode positions. Arousal was defined in accordance with the ASDA rules as well as the EEG arousal definition of the International Paediatric Working Group on Arousals, consisting of an abrupt change in EEG background frequency (of at least 1 Hz) ≥3 sec.22 Arousal included heart rate acceleration and body movement; arousal followed by full awakening was excluded from analysis. Sleep state was defined in the standard manner (presence or absence of REMS and chin EMG).
In this study, when we considered C3-P3 alone, we found spontaneous arousal activation by frequency increase meeting the ASDA rules ≥3 sec as well as the rules of the International Paediatric Working Group on Arousals in 85.21% (C3) and 81.7% (P3) of all cortical arousals detected by the 10/20 configuration of electrodes. These cortical arousals were associated with submental EMG activation, heart rate increase, and limb or body movements. Thus using one bipolar EEG channel (C3-P3) indicated arousal activation with 85% confidence.
In the present study an EEG bandpass of 0.5 to 100 Hz was used. Surface EMG of the nuchal muscles right arm (m. biceps brachii and m. triceps brachii), of the diaphragm (electrode position on the right chest wall, subcostal and near the medial clavicular line) was obtained using a 3-channel EMG amplifier (Coulbourn S75-04, Lehigh Valley, PA) and fiberoptic preamplifiers (Coulbourn S75-04B) with a bandpass filter setting of 50 to 3000 Hz (Coulbourn S75-36) (Fig. 1). Abdominal and thoracic breathing movements were recorded using the inductive plethysmography technique with bands placed around the chest and abdomen (Respitrace System, Ambulatory Monitoring, Inc., Ardsley, NY). Oxygen saturation was measured at the right toe (pulse oximeter, Nellcor, Inc., Hayward, CA). All infants were constantly observed for activity, which was also videotaped with an infrared camera (JVC Professional Products, Elmwood Park, NJ). The polygraph signal and video image were displayed on a split video monitor (Videonix, Inc. Campbell, CA).
Figure 1a.
Placement of EEG, EOG, EKG, and EMG leads during the study
Respiratory measurements were performed by using a Puritan Bennet face mask (Nellcor Puritan Bennett, Pleasanton, CA) attached to a Fleisch pneumotachograph and a Statham PM15E differential pressure transducer (Statham Gould, Hato Rey, PR). The flow signal was integrated to give tidal volume. The air pressure within the mask was measured with a Statham Gould PM6 pressure transducer. End-expiratory occlusions were performed by placing a fingertip over the opening of the face mask at end of expiration. The dead space of the mask was 10 mL, but this would have been less when applied to the infant's face. Startles were identified by their characteristic features: a sudden movement resulting in extension in the arm and neck (reflected by an abrupt increase of limb and EMG activity), which was usually followed by a slower adduction (having a pattern similar to the Moro reflex).10,25 All startles occurring when the airway was occluded were analyzed. The respiratory effort that was most closely associated in time with the startle was analyzed for inspiratory contour of the pressure signal, timing, and pressure magnitude characteristics.
Experimental Protocol
We used brief occlusion of the airway of the infant's face mask at end expiration as a respiratory stimulus to evoke startles (Fig. 1b). Detection of end-expiration phase was performed by a flowmeter attached to the outlet of the face mask. The mean interval between the occlusions was 1 min. Occlusions were maintained for a maximum of 11 sec or until a startle or other evidence of arousal or distress occurred. Oxygen saturation was monitored to ensure that saturation remained above 94%.
Figure 1b.
Placement of facemask on the infant and arrangement of the flow meter and mask pressure tubing.
General and Statistical Analysis
EEG recordings were digitized with a sample rate of 1000 Hz using a CED 1401 AD converter (Cambridge Electronic Design, Cambridge, UK) and were stored on an external hard disk. The EMG analysis technique and the ECG artifact elimination procedure of the limb and diaphragmatic EMG are described elsewhere.27 Sleep stages were differentiated according to conventional scoring methods.1
We quantified the intensity of startles by grading them from 1 to 3. Grade 1 startles were characterized by a sudden but small movement observed in one limb, reflecting a weak or incomplete response. Grade 2 designated startles with a sudden extension, abduction, and elevation of 2 limbs, usually both arms.10,25 Grade 3 was used for exceptional strong responses with movements in at least 3 limbs.
For the respiratory efforts most closely associated with startles, the maximum magnitude of face mask pressure was determined. For efforts with a biphasic contour, the pressure of the first and second phase was determined.20 The increase in pressure was expressed over the preceding respiratory efforts. We calculated the mean values of the mask pressure increase for each infant. These values were used to calculate a group mean. To correlate startle intensity with heart rate increase and face mask pressure we used Spearman rank correlation. Linear correlation was used to define confidence limits.
RESULTS
A total of 103 airway end-expiratory occlusion maneuvers (NREM: n = 53, REM: n = 50). The mean number of occlusions maneuvers performed in the infants was 7.5 (± 3.5 SD). The oxygen saturation during the occlusion always remained above 94%. The duration of airway occlusions ranged during NREM sleep from 3.2 sec to 10.7 sec (median 6.7 sec) and during REM sleep from 1.8 sec to 11.7 sec (median 6.9 sec). In no instance did an infant fail to startle during an airway occlusion maneuver. The median number of obstructed breathing efforts during NREM related occlusions was 3 (range = 1 to 7) and during REM sleep related occlusions was 5 (range = 1 to 9). Six startles were preceded only by one occluded breathing effort.
Startles did not cause sleep disruption with 3 exceptions, in which full arousal and crying immediately followed the startle. During 18.4% of the occlusions, there was brief eye opening for ≤2 sec, after which sleep resumed rapidly.
Coinciding or shortly following 45% of the startles, we observed altered EEG activity characterized by an upward shift in frequency. In 96% of these, this activity lasted <3 sec. It was unclear to what extent the movement or EMG artifact associated with the startle altered the cortical EEG, but doubtless this had some effect. The remaining startles occurred without evidence of EEG change.
Characteristics of the Startle That Occurred During Airway Occlusion Maneuvers
The first sign of nonrespiratory behavioral arousal during occlusion was a startle, with 10 exceptions in which the infants showed only slight slow movements of the limbs and legs. The timing of startles following airway occlusion was variable (range = 1.3 to 11.2 sec). In addition to having features of the Moro response, startles were characterized by a sudden rise in limb EMG activity which coincided with simultaneous nuchal EMG (Fig. 2). The nuchal muscle activation was accompanied by a distinct neck extension, which in almost all cases was strong enough to break the seal of the face mask (Fig. 1 and Fig. 2), thus terminating the external airway occlusion.
Figure 2.
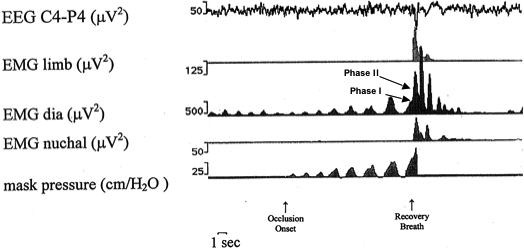
Polygraphic tracings showing effects of airway occlusion in an infant. On spontaneous recovery the mask seal was broken by infant's startle as reflected in the limb and nuchal EMG. Also note biphasic contour of the terminal inspiratory effort (recovery breath) diaphragm EMG. Startle EMG change begins immediately after phase I of the augmented inspiratory effort.
Of 53 occlusions during NREM sleep, 17 accompanying startles were grade 1; 25 were grade 2; and 9 were grade 3. During REM sleep, 16 of the 50 occlusions were grade 1; 30 were grade 2; and 4 were grade 3.
Characteristics of the Respiratory Effort Temporally Associated with a Startle
Startles coincided with a respiratory effort having several distinctive characteristics compared with the preceding occluded breathing efforts. These startle-associated efforts consisted of 2 parts. Phase 1 was followed by phase 2, which had a higher amplitude and shorter inspiratory duration (Fig. 2). In only one instance did we fail to identify these 2 distinct components in the occluded breath associated with a startle.
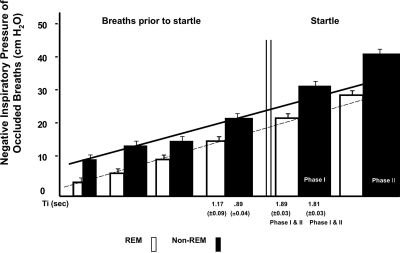
Following onset of face mask occlusion, each occluded breath generated a decrease in peak inspiration pressure over the preceding breath; however, the decrease in pressure of the breath associated with the startle was far greater than the preceding breath. The breath that preceded the startle-associated breath had a mean decrease in of mask pressure of −22.3% (±3.1 SEM) during REM sleep and −25.4% (±4.7) during NREM sleep compared to the breath that came before it. In contrast, the breath coinciding with a startle had a much larger drop in pressure (phase 1: REM: −70.51% (±8.2), NREM: −105.5% (±17.9), phase 2: REM: −109.9% (±8.2), NREM:-132.4% (±15.0) when compared to the breath that preceded it. Face mask pressure decreased during REM sleep occluded breaths from a mean of 18.5 cm/H2O (±1.7) during the occluded breath immediately prior to startle to 34.6 cm/H2O (±3.1) during the startle-related breath; during NREM sleep, it decreased from 20.3 cm/H2O (±1.2) to 42.8 cm/H2O (±2.0) in the startle related breath (P < 0.01 for a difference between REM and NREM startle related breaths). Similarly, the inspiratory duration of pre-startle efforts was 0.89 sec ± 0.04 compared to 1.81 sec ±0.03 for startle associated inspiration efforts (NREM sleep, P < 0.05). For REM the corresponding durations were 1.17 sec ± 0.09 and 1.89 sec ± 0.03 (P < 0.001) (Fig. 3).
Figure 3.
Summed data of all airway occlusions in NREM and REM sleep. Note the large decrease in airway pressure and inspiratory time occurring with sigh-startle complex. The negative pressure of this breath produced was outside of the P < 0.05 confidence interval determined from the preceding breaths. This was true for REM and NREM breaths. Also, note that pressures during REM are significantly less than those during NREM. These differences in pressures of pre-startle breath and augmented breath for REM and NREM were different (P < 0.01).
Timing of Startle Onset during Augmented Breathing Efforts
Startles were closely coupled to the transition of phase 1 to phase 2 of the augmented respiratory effort (Fig. 2). The onset of phase 1 of these efforts, as reflected in the diaphragmatic EMG activation during NREM sleep, occurred 4.8 sec (± 0.24), and during REM sleep 5.5 sec (± 0.33: P < 0.05) after onset of occlusions. The onset of the phase 2 coincided with startle-related activation of limb and nuchal EMG activities. This occurred during NREM sleep after an average of 6.1 sec (± 0.26) and during REM sleep after 6.6 sec (± 0.36: NS) of occlusion onset.4
Correlation of Startle Intensity with Sigh Related Peak Negative Face Mask Pressure
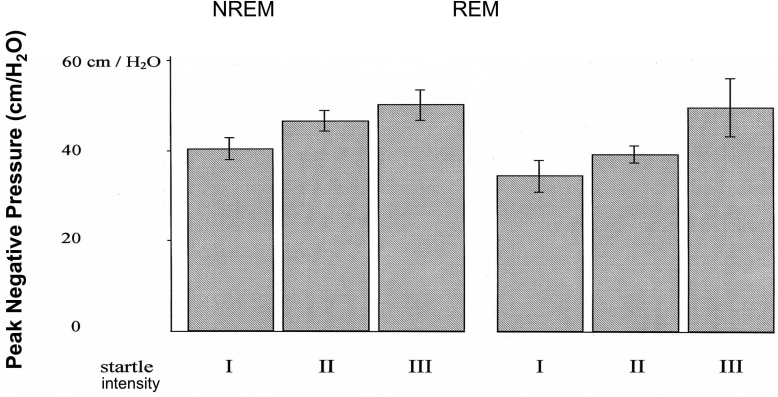
The intensity of a startle correlated positively with the maximum magnitude of the negative face mask pressure (the higher the startle grade, the more negative the face mask pressure during the startle) (Spearman rank correlation: NREM, P < 0.02; REM, P < 0.05; Fig. 5).
Figure 5.
Correlation of peak negative pressure of augmented breaths vs. startle intensity (NREM and REM).
Heart Rate Changes Following Startles
Startles were followed by a heart rate increase (NREM from 126.3 bpm ± 0.9 to 142.9 bpm ± 0.9; REM from 131.2 bpm ± 1.5; SE to 149.9 bpm ± 1.7; SE then followed by a decrease to 110.8 bpm ± 1.4 in NREM and to 115.9 bpm ± 1.8 in REM sleep.
Following startles, heart rate reached its maximum after 3.5 sec ± 0.9 and its minimum after 9.4 sec ± 0.8 during NREM sleep; during REM sleep, heart rate reached its maximum after 3.3 sec ± 1.4 and its minimum after 8.2 sec ± 0.9. Moreover, startle intensity correlated with the percentage heart rate increase (Spearman rank correlation P < 0.001) during NREM sleep (Fig. 6). Cortical arousals were found only during startle grades 2 and 3 during both sleep phases.
Figure 6.
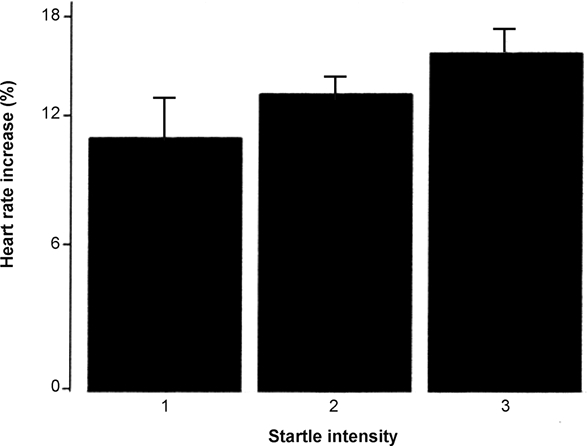
Relation of startle intensity to increase in heart rate at termination of the occlusion.
Effect of Maturation on Latency of Sigh and Startle and Startle Intensity
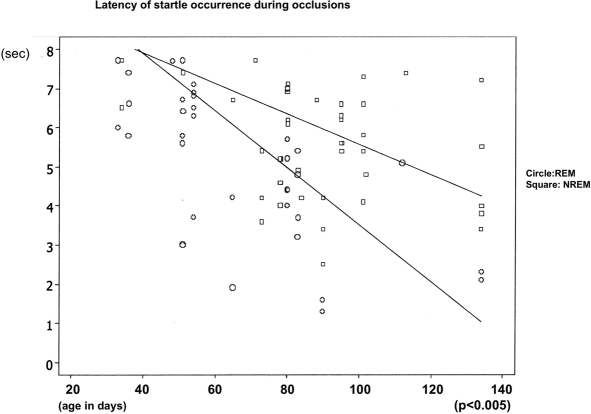
With increasing age, the latency of the sigh and startle occurrence during airway occlusion decreased from a maximum of 7.6 sec to a minimum of 2.5 sec during NREM sleep and from maximum of 7.7 sec to a minimum of 1.4 sec during REM sleep (REM P < 0.005, NREM P < 0.005). The intensity of a startle increased with increased age during NREM sleep (P < 0.02) (Fig. 4 and Fig. 7). We found no significant differences of startle intensity, heart rate changes, or face mask pressure due to body position during the study. No detectable habituation of the startle response or the magnitude of the sigh was found.
Figure 4.
Latency of startle occurrence from occlusion onset related to infants age. Data for both REM and NREM.
Figure 7.
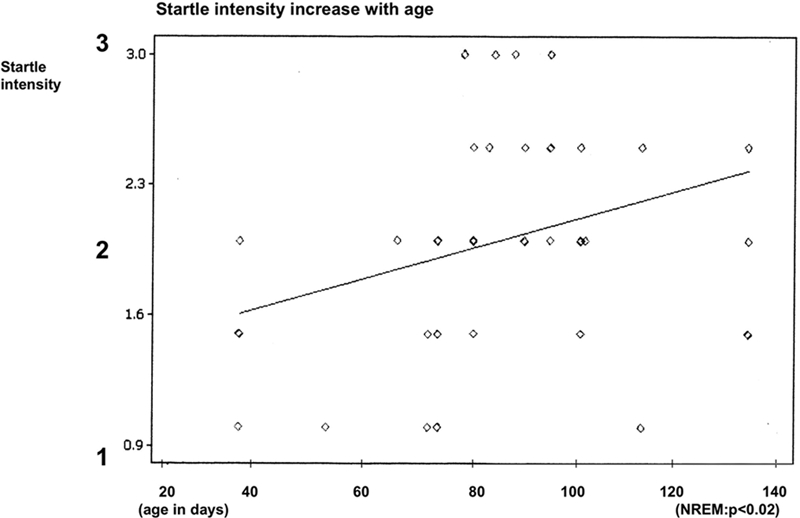
Effect of infant age on startle intensity.
DISCUSSION
The present findings indicate that a sleep startle coordinated with an augmented breath marks the beginning of arousal during airway occlusion in sleeping infants. We also found that all infants startled during the course of airway occlusion. This response was evoked during all occlusion manoeuvres. Stretch receptor activity due to lung inflation was not a precondition for the sigh to occur since the airway was occluded.7,24 Additionally, a cortical arousal reflected by EEG changes according to the ASDA rules and the rules of the international working group on arousals also was not a prerequisite for this response to occur. We have previously reported that sighs and startles terminate the occurrence of EEG sleep spindle activity thus indicating an arousal activation arising from the brainstem to thalamic reticular cells.29 Thus the reflex-like sigh and startle response reflects subcortical arousal activation, which is also supported by the concomitant heart rate increase.
Heart rate accelerations following sighs have been demonstrated in full-term infants at 41 weeks conceptional age and preterm infants (31–37 weeks conceptional age). This study suggested that heart rate acceleration might be caused by a reduced vagotonus initiated by augmented lung volume and body movements. In the present study, the intensity of startles and the magnitude of the sighs reflected by the negative mask pressure correlated with heart rate increase. Surprisingly and in contrast to other species, the response was graded.5 The intensity of startles and sighs and the magnitude of heart rate increase might be crucial for survival during life-threatening events. Conceivably, a weak startle and sigh might be insufficient to reopen a closed airway.
Based on past work, we had predicted that an inspiratory effort having the characteristics of an augmented breath would occur simultaneously with the startle.9,27 This proved to be the case, since large inspiratory efforts always occurred in phase with startles. These efforts resembled spontaneous augmented breaths with respect to longer inspiratory duration, biphasic inspiratory contour, and large amplitude. Importantly, they differed in these respects from the preceding obstructed breaths. During occlusions, a breath-by-breath decrease in inspiratory pressure occurs. So a further decrease in pressure of the startle-associated breath was expected. However, inspiratory pressure of the startle-associated breath (augmented breath) was greatly decreased compared to that predicted on the basis of the gradual decrease in pressure of preceding breaths. Also, inspiratory duration of this breath was longer then predicted. These findings indicate that factors other than the respiratory responses to blood gases had a role in causing the augmented breath.
The close similarities between augmented respiratory efforts during airway occlusion and spontaneous augmented breaths in infants suggest that similar neural mechanisms cause both.9,12 However, this is contrary to what might have been predicted from past studies of the neural regulation of augmented breaths, in which lung inflation stimulating pulmonary mechanoreceptor activity has been viewed as essential for their occurrence in animals with intact pulmonary vagal innervation.7,18 Possibly relevant to our findings is that airway occlusion at FRC suppresses rapidly adapting mechanoreceptor activity but does not eliminate it entirely.7,18 In any event, presence or absence of small volume changes due to decompression of gas in the lungs during respiratory attempts during airway occlusion is not relevant to the mechanisms of reopening the pharyngeal airway.
An important functional role for sleep startles has been suggested in infants sleeping face down, in which case sudden neck extension and repositioning of the head can terminate asphyxia.9,14 Additionally, startle is likely important for the recovery from nasal obstruction that commonly occurs in infants during breastfeeding. The present observations show that the neck extension component of a startle is highly effective in rapidly terminating nasal obstruction, as indicated by the infant's remarkable ability to escape from the face mask. Postmortem studies in infants of the mechanical effects of neck flexion on pharyngeal airway reopening also supports the present findings.15,26
It is of interest that the magnitude of startles correlated with that of sighs. To our knowledge there have been no reports of factors influencing the magnitude of sighs. The present findings suggest that not only the occurrence but also the magnitude of subcortical arousals may determine whether recovery from airway obstruction takes place. Also, we found that the latency from occlusion to recovery and the intensity of startles decreases with age. We have no ready explanation for these findings. Possibly an increase in metabolic rate as infants mature might explain the decreasing latency as arterial CO2 might be expected to accumulate more rapidly, thus stimulating arousal. Another potential explanation is that the threshold for chemoreceptor stimulated arousals might decrease with maturation. Similarly the increasing magnitude of startles is previously unreported. As discussed above, the increased magnitude of the startle could be of benefit to older infants during airway occlusion.
Mechanisms involved in recovery from of obstructive apnea have been the topic of much speculation in the past.3 It has frequently been observed that a burst of GG muscle activity and other upper airway maintaining muscles reflected in submental EMG activity appears to coincide with EEG arousals and airway reopening during apnea episodes.3,17 In contrast, others have pointed out that ≥2 sec of cortical EEG activation as evidence of arousal is not necessary for recovery to occur in either infants or adults, suggesting that activity in subcortical arousal centers may be the primary event critical for recovery of patency rather than the often associated, and perhaps incidental, cortical arousal.11,16 Recently, it has also been argued that recovery from an OSA episode in adults is due to brainstem reflexes that are independent of cortical arousal. This view is consistent with our findings.23
In theory, the simultaneous occurrence of a sigh and a startle should be effective in terminating obstruction of the airway in the pharynx. Neck extension during a startle favors pharyngeal airway reopening.15,26 Also, as mentioned, a sudden increase in genioglossus muscle activity has been shown to occur during the second phase of biphasic augmented breaths in infant and animal studies.2,4,20,24 Our own studies of infants during sleep found that a sudden increase in submental or GG muscle activity coincided with the second phase of an augmented breath and recovery of airway patency.20,27 The infants in these studies had major medical problems, and startles were not recorded. However, the GG, EMG, and submental EMG during the terminal augmented breath were found to exceed the increase in diaphragm EMG activity. These observations are important in that “preferential activation” of the GG muscle is believed to be critical for opening the pharyngeal airway.17 All in all, these past studies suggest that when an augmented breath occurs simultaneously with a startle, the combined effect would be to reopen the collapsed pharyngeal airway.
We found altered EEG activity following startles in terms of a short frequency shift (<3 sec) did not meet the arousal criteria of the ASDA in 45% of all occlusion maneuvers. In our preliminary study, we found that the C3 and P3 electrode placement registered 85% of cortical arousals meeting ASDA criteria. Thus, the use of only these 2 electrodes was sufficient to identify cortical arousal activation in the great majority of instances. Therefore, we conclude that many occlusion-related subcortical arousals we observed in the present study were not associated with cortical arousals.
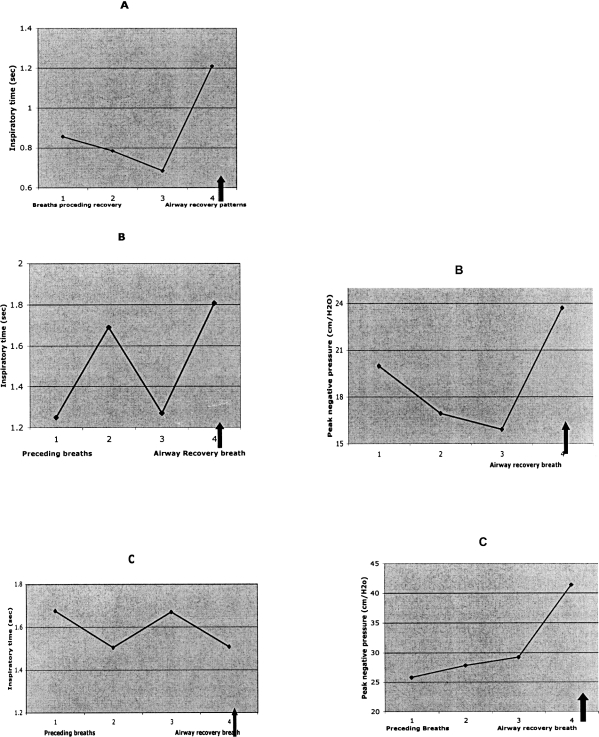
These findings in infants may be highly relevant to airway recovery mechanisms in adults with OSA. In polygraphic recordings of OSA episodes in a patient studied by Remmers et al., the respiratory effort that occurs precisely when airway recovery takes place is biphasic in contour, and both GG and diaphragm EMG activity are much larger than the respiratory efforts that immediately preceded it (Fig 8a).17 Once airway reopening and cortical arousal occur the GG EMG activity of subsequent breaths is immediately reduced in magnitude. This suggests that cortical activity alone is not responsible for the burst in GG activity at reopening. Similar findings are evident in OSA recovery tracings in adult subjects published by others (Fig. 8 b & c). 8 Importantly our findings in infants as well as these in adults suggest that cortical arousal may be an epiphenomenon as it relates to recovery from pharyngeal airway obstruction. This reasoning is based on the observation that recovery takes place during the first second following cortical EEG activation, long before the 2- to 3-sec accepted criteria for cortical arousal. However we feel that cortical arousal may have been important, to some degree, for arousal in a fraction of the infants in the present study. However, in our prior studies, startles clearly preceded cortical arousal.12,28,29
Figure 8.
Data taken from tracings of adult OSA episodes and recovery from obstruction indicating similarities to findings in infants.
A. Note inspiratory time for the recovery breath is much longer than preceding respiratory efforts. The recovery breath was distinctly biphasic, as in infants. Inspiratory peak pressures are not given because the pressure scale went off in the recovery breath. (Data extracted from ref # 17, Fig. 2).
B. Note the increased duration as well as peak inspiratory pressure of the recovery breath. (Data extracted from ref # 5, Fig. 4b).
C. In this example inspiratory time of recovery breath was not prolonged, but inspiratory pressure was distinctly elevated over that of preceding breaths. (Data extracted from ref # 5, Fig. 1a).
In summary, we conclude that sighs and startles provide an efficient defense mechanism for both nasal and pharyngeal airway obstruction in young infants. Not only the occurrence, but also the intensity, of a startle and/or associated augmented breath may be important for recovery from life-threatening events during sleep. Furthermore a large increment in pulmonary rapidly adapting receptor activity is not necessary for occurrence of augmented inspiratory efforts.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Anders T, Emde R, Parmalee A. UCLA Brain Information Service. Los Angeles: BRI Publications Office; 1971. A manual of standardized terminology, technology, and criteria for scoring of states of sleep and wakefulness in newborn infants. [Google Scholar]
- 2.Bartlett D. Origin and regulation of spontaneous deep breaths. Respir Physiol. 1971;12:230–8. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- 3.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–75. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 4.Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. J Appl Physiol. 1980;49:801–8. doi: 10.1152/jappl.1980.49.5.801. [DOI] [PubMed] [Google Scholar]
- 5.Davis, M . The mammalian startle response. In: Eaton RC, editor. Neural mechanisms of startle behavior. New York: Plenum Press; 1984. [Google Scholar]
- 6.Eiselt M, Curzi-Dascalova L, Leffler C, Christova E. Sigh-related heart rate changes during sleep in premature and full-term newborns Neuropediatrics. 1993;23:286–91. doi: 10.1055/s-2008-1071360. [DOI] [PubMed] [Google Scholar]
- 7.Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol. 1972;16:179–96. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- 8.Kimoff RJ, Cheong TH, Olha AE, et al. Mechanisms of apnea termination in obstructive sleep apnea. Am J Respir Crit Care Med. 1994;149:707–14. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- 9.Lijowska A, Reed NW, Chiodini M, Thach BT. Sequential arousal behaviour in infants in asphyxial sleep environments J Appl Physiol. 1997;83:219–28. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- 10.McGraw M. The Moro reflex. Am J Dis Child. 1937;54:240–51. [Google Scholar]
- 11.McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J Appl Physiol. 1996;81:2651–7. doi: 10.1152/jappl.1996.81.6.2651. [DOI] [PubMed] [Google Scholar]
- 12.McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85:2314–21. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- 13.Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol. 1993;74:761–9. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- 14.Paluszynska DA, Harris KA, Thach BT. Influence of sleep position experience on ability of prone-sleeping infants to escape from asphyxiating microenvironments by changing head position. Pediatrics. 2004;114:1634–9. doi: 10.1542/peds.2004-0754. [DOI] [PubMed] [Google Scholar]
- 15.Reed WR, Roberts JL, Thach BT. Factors influencing regional patency and configuration of the human infant upper airway. J Appl Physiol. 1985;58:635–44. doi: 10.1152/jappl.1985.58.2.635. [DOI] [PubMed] [Google Scholar]
- 16.Rees K, Spence PS, Earis JE, Calverly MA. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med. 1995;152:1016–21. doi: 10.1164/ajrccm.152.3.7663777. [DOI] [PubMed] [Google Scholar]
- 17.Remmers JE, De Groot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 18.Richardson P, Sant'Ambrogio G, Mortola J, Bianconi R. The activity of lung afferent nerves during tracheal occlusion. Respir Physiol. 1973;18:273–83. doi: 10.1016/0034-5687(73)90056-x. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds LB. Characteristics of an inspiration-augmenting reflex in anesthetized cats. J Appl Physiol. 1962;17:683–8. doi: 10.1152/jappl.1962.17.4.683. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JL, Reed WR, Matthew OP, Thach BT. Control of respiratory activity of the genioglossus muscle in micrognathic infants. J Appl Physiol. 1986;61:1523–33. doi: 10.1152/jappl.1986.61.4.1523. [DOI] [PubMed] [Google Scholar]
- 21.Thach BT, Taeuch HW. Sighing in newborn human infants: role of inflation-augmenting reflex. J. Appl. Physiol. 1976;41:502–507. doi: 10.1152/jappl.1976.41.4.502. [DOI] [PubMed] [Google Scholar]
- 22.International Paediatric Working Group on Arousals. The scoring of arousals in healthy term infants (between the ages 1 and 6 months) J Sleep Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 23.Trinder J, Ivens C, Kleiman J, Kleverlaan D, White DP. The cardiorespiratory activation response at an arousal from sleep is independent of the level of CO2. J Sleep Res. 2006;15:174–82. doi: 10.1111/j.1365-2869.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 24.Van Lunteren E, Van de Graaff WB, Parker DM, et al. Activity of upper airway muscles during augmented breaths which has been reported by our own group in rabbits Physiology. 1983;53:87–8. doi: 10.1016/0034-5687(83)90018-x. [DOI] [PubMed] [Google Scholar]
- 25.Volpe J. 3rd ed. Philadelphia: WB Saunders; 1995. Neurology of the newborn; p. 106. [Google Scholar]
- 26.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Upper airway patency in the human infant: influence of airway pressure and posture. J Appl Physiol. 1980;48:500–4. doi: 10.1152/jappl.1980.48.3.500. [DOI] [PubMed] [Google Scholar]
- 27.Wulbrand H, Zezschwitz HG, Bentele KHP. Submental and diaphragmatic muscle activity during and at resolution of mixed and obstructive apneas and cardiorespiratory arousal in preterm infants. Pediatr Res. 1995;38:298–305. doi: 10.1203/00006450-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wulbrand H, McNamara F, Thach BT. Suppression of sigma spindle EEG activity as a measure of transient arousal following spontaneous and occlusion evoked sighs and startles. Pediatr Res. 1998;44:767–73. doi: 10.1203/00006450-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Wulbrand H, McNamara F, Thach BT. The mechanism of sleep arousal in infants: relevance of sleep startles. Pediatr Res. 1997;41:308A. [Google Scholar]