Abstract
Atypical imaging features of multiple sclerosis lesions include size >2 cm, mass effect, oedema and/or ring enhancement. This constellation is often referred to as ‘tumefactive multiple sclerosis’. Previous series emphasize their unifocal and clinically isolated nature, however, evolution of these lesions is not well defined. Biopsy may be required for diagnosis. We describe clinical and radiographic features in 168 patients with biopsy confirmed CNS inflammatory demyelinating disease (IDD). Lesions were analysed on pre- and post-biopsy magnetic resonance imaging (MRI) for location, size, mass effect/oedema, enhancement, multifocality and fulfilment of Barkhof criteria. Clinical data were correlated to MRI. Female to male ratio was 1.2 : 1, median age at onset, 37 years, duration between symptom onset and biopsy, 7.1 weeks and total disease duration, 3.9 years. Clinical course prior to biopsy was a first neurological event in 61%, relapsing–remitting in 29% and progressive in 4%. Presentations were typically polysymptomatic, with motor, cognitive and sensory symptoms predominating. Aphasia, agnosia, seizures and visual field defects were observed. At follow-up, 70% developed definite multiple sclerosis, and 14% had an isolated demyelinating syndrome. Median time to second attack was 4.8 years, and median EDSS at follow-up was 3.0. Multiple lesions were present in 70% on pre-biopsy MRI, and in 83% by last MRI, with Barkhof criteria fulfilled in 46% prior to biopsy and 55% by follow-up. Only 17% of cases remained unifocal. Median largest lesion size on T2-weighted images was 4 cm (range 0.5–12), with a discernible size of 2.1 cm (range 0.5–7.5). Biopsied lesions demonstrated mass effect in 45% and oedema in 77%. A strong association was found between lesion size, and presence of mass effect and/or oedema (P < 0.001). Ring enhancement was frequent. Most tumefactive features did not correlate with gender, course or diagnosis. Although lesion size >5 cm was associated with a slightly higher EDSS at last follow-up, long-term prognosis in patients with disease duration >10 years was better (EDSS 1.5) compared with a population-based multiple sclerosis cohort matched for disease duration (EDSS 3.5; P < 0.001). Given the retrospective nature of the study, the precise reason for biopsy could not always be determined. This study underscores the diagnostically challenging nature of CNS IDDs that present with atypical clinical or radiographic features. Most have multifocal disease at onset, and develop RRMS by follow-up. Although increased awareness of this broad spectrum may obviate need for biopsy in many circumstances, an important role for diagnostic brain biopsy may be required in some cases.
Keywords: tumefactive multiple sclerosis, demyelinating disease, biopsy, pathology, MRI
Introduction
Multiple sclerosis plaques on magnetic resonance imaging (MRI) generally appear as multiple, well demarcated, homogenous, small ovoid lesions, lacking mass effect and often oriented perpendicular to the long axis of the lateral ventricles (Paty et al., 1988; Barkhof et al., 2003). However, atypical imaging features have been described, which may confound the diagnostic process. These include a solitary large lesion, size >2 cm, associated mass effect, perilesional oedema and/or the presence of ring enhancement (Rieth et al., 1981; Sagar et al., 1982; Morimoto et al., 1985; Hunter et al., 1987; Gutling and Landis, 1989; Paley et al., 1989; Johnson et al., 1990; Nesbit et al., 1991; Youl et al., 1991; Giang et al., 1992; Kepes, 1993; Guadagnino et al., 1994; Maranhao-Fiho et al., 1995; Rusin et al., 1995; Dagher and Smirniotopoulos, 1996; Kurihara et al., 1996; Masdeu et al., 1996, 2000; Kumar et al., 1998; Al-Bunyan, 2000; Friedman, 2000; Censori et al., 2001; Khoshyomn et al., 2002; McAdam et al., 2002; Di Patre et al., 2003; Iwamoto et al., 2004; Wurm et al., 2004; Schwartz et al., 2006). Clinical presentations are dependent on lesion location and size, and as a result are variable and include headache, cognitive abnormalities, mental confusion, aphasia, apraxia and/or seizures. These atypical clinical and imaging presentations may mimic brain tumour, cerebral abscess or other inflammatory disorders, and may necessitate a brain biopsy for diagnosis. Histologically, the biopsy may be misinterpreted as a neoplasm given the hypercellular nature of these lesions and the frequent presence of atypical reactive astrocytes (i.e. Creutzfeldt-Peters cells) and mitotic figures (Zagzag et al., 1993; Annesley-Williams et al., 2000). A variety of terms including Marburg's disease, fulminant multiple sclerosis, acute disseminated encephalomyelitis (ADEM), Balo's concentric sclerosis, transitional sclerosis, diffuse myelinoclastic sclerosis, pseudotumoral multiple sclerosis and tumefactive multiple sclerosis have been used in the literature when referring to these cases of CNS inflammatory demyelinating disease (IDD) (Niebler et al., 1992; Poser et al., 1992; Bolay et al., 1996; Chen et al., 1996; Dagher and Smirniotopoulos, 1996; Kim et al., 1997; Ernst et al., 1998; Iniguez et al., 2000; Capello et al., 2001; Karaarslan et al., 2001; Kotil et al., 2002). As a result, the literature lacks uniform nomenclature or a clear, comprehensive description of the clinical and radiographic spectrum of CNS IDD with tumefactive features. This is due to the limited number of cases available for study, frequent lack of pathological confirmation, and/or limited clinical or radiographic follow-up (Nesbit et al., 1991; Kepes, 1993; Dagher and Smirniotopoulos, 1996; McAdam et al., 2002; Hayashi et al., 2003). Published series emphasize the unifocal and clinically isolated nature of such tumefactive cases (Kepes, 1993). The purpose of this study is to describe the clinical and radiographic presentations and outcomes in 168 patients with brain biopsy-confirmed CNS IDD. A better appreciation of the broad spectrum of clinical and radiographic features associated with CNS IDD may facilitate diagnosis, reduce unnecessary surgical or medical interventions and ensure proper long-term management and treatment.
Methods and Materials
This study, approved by the Mayo Clinic Institutional Review Board (IRB # 2067–99), is a retrospective review of clinical, pathological and radiographic material amassed from patients with biopsy proven CNS IDD identified from an original cohort of 780 CNS IDD biopsy cases belonging to the multiple sclerosis Lesion Project (MSLP). The MSLP database comprises a unique collection of biopsy-proven CNS IDD cases with detailed pathological, as well as both retrospective and prospective clinical and radiographic data and forms the basis of an international collaborative effort to study the pathologic, clinical and radiologic correlates of the multiple sclerosis lesion (NMSS RG3184-B-3-02). Inclusion criteria for the current study were: (i) brain biopsy performed as part of diagnostic evaluation; (ii) pathological evidence of confluent inflammatory demyelination consistent with multiple sclerosis, confirmed by a certified neuropathologist (B.W.S., W.B., H.L., J.E.P., C.G., F.K.) and (iii) a minimum of 1 MRI available for review. Specifically excluded were patients with ADEM pathologically defined as demyelination limited to perivenular areas (n = 14) (Hart and Earle, 1975), as well as Devic's neuromyelitis optica defined based on published criteria (n = 4) (Wingerchuk et al., 1999, 2005). Also excluded were cases of neoplasm, infection, vascular or other non-demyelinating inflammatory CNS disease, as well as prior history of brain irradiation. A total of 168 patients identified from two medical centres; Mayo Clinic Rochester, USA (n = 127) and University of Göttingen, Germany (n = 41), met inclusion criteria.
Clinical material
Clinical information was obtained by certified neurologists (C.F.L.; I.M.), via medical record review (100%), personal interview and examination (71%), patient letter or telephone contact (22%) and family or physician contact (6%). In 140 patients, there was sufficient clinical documentation obtained via either face-to-face encounter and neurological examination (n = 119 patients; 90 Mayo; 29 Germany), or telephone interview (n = 21 patients) in order to establish birth date, gender, date of symptom onset, date of index attack prompting biopsy, index and other attack symptoms, types and dates of multiple sclerosis treatments administered, estimated EDSS at index attack, date of last follow-up and EDSS at last follow-up. Clinical course at time of biopsy and at last follow-up was categorized as first demyelinating event, monophasic, relapsing–remitting, secondary progressive, primary progressive or uncertain, when it was unclear if an episode represented a discrete attack (e.g. seizure). Patients were classified as having definite or probable multiple sclerosis at last follow-up by either McDonald or Poser criteria (Poser et al., 1983; McDonald et al., 2001). Patients with a single neurological episode at last follow-up were classified as an isolated demyelinating syndrome. Clinical course of multiple sclerosis was based on well accepted criteria for relapsing–remitting, secondary progressive, or primary progressive multiple sclerosis (Lublin and Reingold, 1996). When available, pre-biopsy cerebrospinal fluid (CSF) results were recorded, including white blood cell count/mm3, total protein (mg/dl), intrathecal synthesis rate (normal or elevated) and presence or absence of CSF oligoclonal bands (OB). In addition, visual, brainstem auditory and somatosensory evoked responses performed prior to biopsy were recorded as normal or abnormal.
Median EDSS at last follow-up in the biopsied cohort was compared with a population-based prevalence cohort consisting of patients with definite (n = 201) and/or probable (n = 14) multiple sclerosis (using Poser's criteria), who were residents of Olmsted County (OC) on December 1, 2000 (none underwent brain biopsy) (Mayr et al., 2003; Pittock et al., 2004).
Pathological material
Histopathology was available in all 168 cases. A brain biopsy had been performed at an outside institution in 87% of the cases, and sent for extramural consultation to a study centre. All biopsies were performed between September 11, 1987 and August 13, 2005, with over 1/2 performed since May 6, 2000, likely reflecting the expanding Mayo and University of Göttingen extramural consultative practices. In all instances, the specimen was reviewed by at least one board certified neuropathologist. In 20 patients, a second biopsy was obtained, and in a single patient, a third biopsy performed. Since the majority of biopsies were performed at other institutions, tissue material was obtained retrospectively, typically months to years after the biopsy date, and clinical follow-up was done by clinicians not originally involved in the care of the patient at the time of biopsy, it was not possible to determine the precise indication for brain biopsy in most circumstances.
Radiographic material
A total of 842 brain and 24 spine conventional pre-biopsy and follow-up MRIs obtained on 168 patients were reviewed. Since this was a retrospective study, a variety of imaging techniques and scanners were utilized. However, in the 119 patients who underwent a face-to-face neurological examination at follow-up, a standardized contrast enhanced MRI was also performed on a GE (General Electric Medical Systems, Milwaukee, WI) Signa 1.5T MR scanner using birdcage head coils, a slice thickness of 3 mm and no interslice gap. In all 168 patients, at least one MRI was reviewed, with at least one pre-biopsy MRI available in 151 patients (90%) and at least one post-biopsy MRI in 148 (88%) patients. Among those with a pre-biopsy MRI, 95% had a T1-weighted (T1W), 97% T2W, 69% FLAIR and 95% had a T1W + gadolinium (Gd) sequence. Among those with a post-biopsy MRI, 98% had a T1W, 100% T2W, 93% FLAIR and 99% had a T1W + Gd sequence.
Prior to blinded data collection, the radiographic features were initially defined by consensus among the study investigators (B.E., C.F.L., W.B., R.G. and I.M.), with all training sessions occurring at a single site (Mayo Clinic Rochester). Subsequently, blinded to the clinical data, a single investigator at each centre evaluated the MRIs at their respective institutions (R.G., Mayo; I.M., Göttingen). Results for all MRI variables identified on 10 randomly selected patients were crossed to assess for agreement, which was confirmed on all measures of interest. Questions regarding radiographic interpretations at either study centre were adjudicated by a certified neuroradiologist (B.E.).
Lesions identified on each brain MRI study were defined as either the initially biopsied lesion (‘index lesion’), or as other lesions, enhancing and non-enhancing. These were analysed for a variety of radiographic features: location, number, size range (0.3–2 cm; 2.1–5 cm; >5 cm) of both the T2W margin to margin signal abnormality and the discernible lesion size when it was possible to differentiate discrete lesion borders from the surrounding oedema; actual size (cm) of the largest lesion on the scan, presence and grade of mass effect [mild sulcal effacement, moderate (minimal subfalcine or uncal herniation; <1 cm) or marked (>1 cm subfalcine or uncal herniation)], presence and degree of oedema [mild (<1 mm from the lesion), moderate (1–3 cm from the lesion) or marked (>3 cm from the lesion)], presence of T2W hypointense rim (defined as a discernible smooth complete thin border of T2W hypointensity relative to the hyperintensity of both the lesion centre and surrounding oedema), biopsy defect size (not detected or tract only; <5 mm; 5 mm–2 cm; >2 cm) and T1W intensity relative to normal appearing white matter (isointense, mild/moderate hypointense, severely hypointense, hyperintense). Enhancement patterns were defined as homogenous (uniform and solid enhancement throughout the lesion); ring-like (open-ring when opened toward the gray matter; closed ring when having a complete circular border and incomplete ring or arc when only part of the lesion is bordered), or heterogeneous (variable and complex pattern and distribution of enhancement). In some patients with a heterogeneous enhancement pattern, more specific enhancement patterns could be identified and included the following: diffuse and patchy; ‘fluffy/cotton ball’; nodular with distinct areas of enhancement each >2 mm amongst non-enhancing areas and punctate enhancement characterized by distinct areas of enhancement each < 2 mm. Complex lesions, or multiple MRIs performed during a given imaging interval could result in more than one enhancement pattern.
MRI studies were also evaluated for whether and when multifocal non-enhancing and enhancing lesions developed, or whether Barkhof criteria were fulfilled (Barkhof et al., 1997). Spine MRIs, although included for this analysis, were not analysed for the various radiographic features detailed above. Visual comparison without registration of the first and last MRI study for a given patient was also evaluated for the following radiographic features: overall change in T2W and T1W lesion volume (unchanged, reduced; minimally to moderately increased, markedly increased); extent of confluent periventricular white matter (PVWM) disease (none, mild, moderate, severe); presence of atrophy (none, focal, minimal global, moderate global, severe global) and overall change in PVWM disease and atrophy (unchanged, improved or minimally, moderately or markedly increased). In order to determine if patients with at least one lesion >2 cm on pre-biopsy MRI had a tendency to relapse with additional large uni- or multi-focal lesions, we performed a subgroup analysis of the tumefactive radiographic course in this cohort.
Statistical analysis
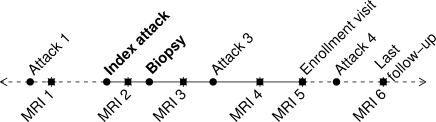
Clinical data were recorded on case report forms and double-data entered to create SAS version 9 data sets (SAS Institute, Cary, NC, USA). Radiographic data were entered into a Microsoft Excel spreadsheet shared between both study sites. All data were subjected to extensive internal consistency checks. Statistical analyses describing clinical data and comparing clinical outcomes to radiographic features were restricted to the 140 patients with detailed clinical data, whereas statistical analyses describing radiographic features included all 168 patients in the cohort in order to increase statistical power and ensure a complete representation of the radiographic spectrum. Given the variability in the number of scans among patients, the unit of analysis was based on individual patients rather than scans, thereby maintaining statistical independence among observations and giving equal weight to each patient. Imaging data from multiple studies per patient were collapsed into a single observation per patient as follows: (i) a patient's studies were first classified into time periods (pre-biopsy, concomitant with biopsy and post-biopsy interval); (ii) for patients with >1 biopsy, the biopsy of interest was considered to be the last biopsy; (iii) if a patient had ≤1 study in a given period, no data reduction was necessary and (iv) if a person had multiple studies in the period, the patient's values on a particular radiographic feature were aggregated by recording the maximal value for ordered variables, such as maximum lesion size observed in a period, greatest number of enhancing lesions on a single study within a period or whether a feature was ever present during the period. Figure 1 shows a schematic of a prototypic patient timeline and radiographic intervals of interest. Because not all subjects had an appropriate study available during each interval of interest, the sample size or denominator depended upon the variables being analysed.
Fig. 1.
Prototypical timeline for study participant. The pre-biopsy MRI interval represents the time between MRI 1 and brain biopsy, whereas the post-biopsy MRI interval represents the time between biopsy and last MRI (MRI 6). Although for most patients the symptoms leading to brain biopsy (index attack) represented the first neurological event, in some cases, the neurological history antedated the index attack (attack 1). At the time of the enrolment visit, 140 patients had a face-to-face neurological history and examination, and underwent a standardized MRI study (MRI 5). Total disease duration was from the time of symptom onset (attack 1) to last follow-up.
Radiographic features were described and analysed separately for biopsied versus non-biopsied lesions. Continuous variables were analysed using medians, interquartile ranges (IQRs) and ranges. Associations between dichotomous variables were analysed using a chi-square test or if counts were small, by Fisher's exact test. Two-sample comparisons of continuous measures were performed using Wilcoxon rank-sum tests. To evaluate associations between two ordinal variables, Spearman rank-order correlation, denoted by rs, was used. When one variable was binary and the other ordinal, we used Armitage's trend test. Kaplan-Meier survival curves were used to determine time to second relapse, and Cox proportional hazards models were used to determine whether specific factors were associated with risk of developing a second multiple sclerosis event. Median EDSS at last follow-up was compared between the biopsy and the OC multiple sclerosis prevalence cohorts using a two sample Wilcoxon rank sum test performed separately among those with a disease duration < 5, 5–10 and > 10 years. Logistic and linear regression models were used to assess the association and/or confounding effect of clinical and radiographic variables such as biopsy defect size, gender and steroids.
All tests were two sided and a P-value of 0.05 was used to indicate significant associations between variables. P-values were not adjusted for multiple comparisons (O’Brien, 1983; Perneger, 1998). However, we do report P-values to several digits so the reader may perform Bonferroni-type corrections if desired.
Results
Clinical characteristics of the cohort
Table 1 summarizes the cohort in terms of demographics, clinical course and diagnoses both prior to biopsy and at last follow-up. Median age at symptom onset was 37 years. The index attack leading to brain biopsy was the first neurological event in the majority of patients (61%), and eight (5%) carried an established diagnosis of multiple sclerosis prior to biopsy.
Table 1.
Clinical features of cohort
| Gender F : M | 1.2 : 1 |
| Age at symptom onset (years) | 37 (8–69) |
| Disease Duration | |
| Symptom onset to biopsy (weeks) | 7.1 (3.7, 28.6) |
| Symptom onset to last F/U (years) | 3.9 (2.0, 9.0) |
| Clinical course prior to biopsy (%) | |
| First neurological event | 61 |
| Relapsing–remitting | 29 |
| Secondary progressive | 1 |
| Primary progressive | 2 |
| Progressive-relapsing | 1 |
| Uncertain | 6 |
| Clinical course at last F/U (%) | |
| Monophasic | 24 |
| Relapsing–remitting | 51 |
| Secondary progressive | 11 |
| Primary progressive | 1 |
| Progressive-relapsing | 3 |
| Uncertain | 9 |
| Diagnosis at last F/U (%) | |
| Multiple sclerosis | 70 |
| Probable multiple sclerosis | 9 |
| Isolated demyelinating syndrome | 14 |
| Unknown | 7 |
| EDSS | |
| At time of index attack | 3.5 (3.0, 4.5) |
| At last follow-up | 3.0 (1.5, 4.0) |
Note: values in parentheses are either minimum – maximum or lower quartile, upper quartile.
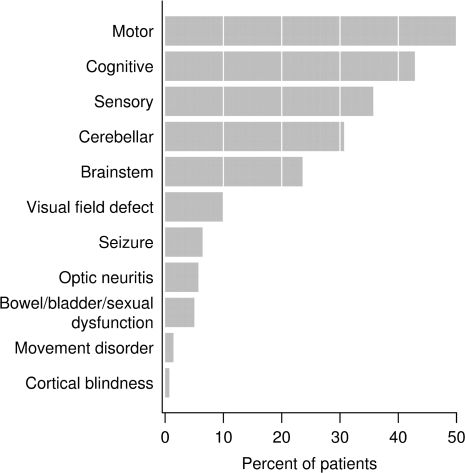
Index attack symptoms were typically polysymptomatic and summarized in Fig. 2, with motor (50%), cognitive (43%), sensory (36%), cerebellar (31%) and brainstem (24%) symptoms being most common. A spectrum of cognitive symptoms was observed, including encephalopathy or confusional state (19%), memory dysfunction (17%), aphasia (17%), apraxia (4%), Gerstmann syndrome (4%) and stupor/coma (2%). Visual field defects were seen in 10%, and seizures in 6%. There were no significant differences in index attack symptoms, including presence of stupor/coma, encephalopathy or cognitive dysfunction, between patients developing multiple sclerosis and those having an isolated demyelinating syndrome at last follow-up.
Fig. 2.
Neurologic symptoms at presentation. Cognitive abnormalities were frequent, and included memory dysfunction, mental confusion and disorders of attention as well as disorders of higher cognitive function including aphasia (17%), apraxia (4%) and agnosia (4%).
Pre-biopsy CSF results were available on a subset of patients. Median protein in 58 patients was 40.5 mg/dl (IQR 30.5, 60.0; range 11–383 mg/dl), with a median white blood cell count/mm3 among 61 patients of 3.0 (IQR 2, 7; range 0–117). In 22 of 62 patients (35%) IgG synthesis rate was elevated, and 20 of 60 (33%) had CSF OB. Pre-biopsy visual evoked responses were prolonged in at least one eye in 13 of 38 (34%) patients; brainstem auditory evoked responses delayed in 3 of 13 (23%) and 15 of 25 patients (60%) had abnormal somatosensory evoked potentials.
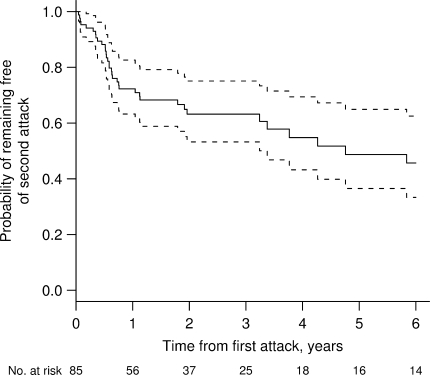
Most patients developed definite (70%) or probable multiple sclerosis (9%) by Poser or McDonald criteria after a median follow-up of 3.9 years (IQR 2.0, 9.0), with only 14% having an isolated demyelinating syndrome at last follow-up. The latter patients were significantly older at symptom onset than were those who developed multiple sclerosis (47 versus 36 years; P < 0.001). Although some patients had a monophasic course (24%), a diagnosis of multiple sclerosis based on McDonald criteria could still be established. The estimated median EDSS at time of index attack was 3.5 (IQR 3.0–4.5, range 0–9.5), and EDSS at last follow-up was 3.0 (IQR 1.5–4.0, range 0.0–9.5). Based on Kaplan-Meier estimates, the median time to the second attack was 4.8 years (Fig. 3). At last clinical follow-up, 25% of patients had been treated with at least one immunosuppressive agent, and 63.5% had received one or more disease modifying drugs.
Fig. 3.
Kaplan-Meier estimates of the probability of remaining free of a second attack among those whose biopsy was performed after the first clinical episode (n = 85). Solid line represents estimated probability, and dotted lines represent 95% CI. The median time to second clinical episode was 4.8 years. There were no specific risk factors (clinical or radiographic) identified which were associated with a greater risk of developing a second multiple sclerosis attack.
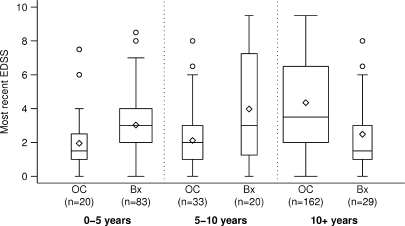
Since patients in the biopsy cohort had a shorter mean disease duration than the population-based multiple sclerosis cohort (3.9 years versus 19 years), we analysed EDSS at last follow-up stratified by disease duration (Fig. 4). The biopsy cohort had a significantly lower median EDSS compared with the OC multiple sclerosis cohort among patients with disease duration >10 years (EDSS 1.5 versus 3.5; P < 0.001), despite a slightly higher EDSS in patients with 0–5 years (EDSS 3.0 versus 1.5; P = 0.01) and 5–10 years (EDSS 3.0 versus 2.0; P = 0.04).
Fig. 4.
Comparison of disability (most recent EDSS) between biopsied cohort and OC multiple sclerosis prevalence cohort stratified by disease duration. The OC multiple sclerosis prevalence cohort is denoted by ‘OC’ and the current biopsy cohort is denoted by ‘Bx’. Boxes extend to cover the middle 50% of the data. Median EDSS scores are indicated by the horizontal lines within each box, mean scores by the diamonds. Whiskers extend to furthest observation within 1.5 IQRs, with outlying points indicated by individual circles. Although median EDSS at last follow-up was slightly higher in the biopsy cohort compared with the OC multiple sclerosis prevalence cohort in patients with disease duration between 0 and 5 years (3.0 versus 1.5; P = 0.01), and 5–10 years (3.0 versus 2.0; P = 0.04), the EDSS was significantly lower in the biopsy cohort for 10+ years (1.5 versus 3.5; P < 0.001). Note that EDSS at last follow-up was unknown in eight patients, therefore, the data reflects 132 rather than 140 biopsied patients in whom detailed clinical information was available for analysis.
Although our study lacked detailed cognitive assessment at the time of last follow-up, based on EDSS evaluation we observed that 22% of the cohort had a Cerebral Functional System's score of ≥3 at last follow-up, reflecting moderate to marked decrease in memory.
Pathological characteristics of the cohort
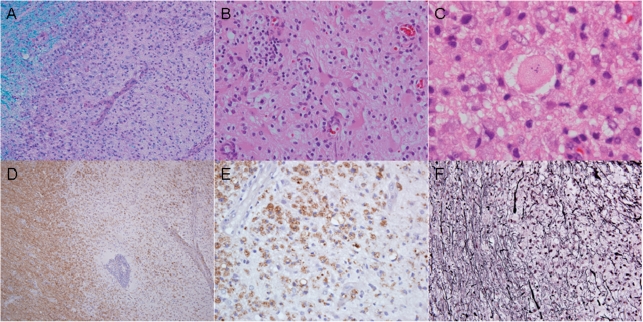
Biopsies in all 168 cases demonstrated characteristic features of active inflammatory demyelinating disease, including hypercellularity with myelin loss (Fig. 5A), reactive gliosis (Fig. 5B), Creutzfeldt cells (Fig. 5C), myelin protein-laden macrophages (Fig. 5D and E), variable lymphocytic inflammation and relative axonal preservation (Fig. 5F). Neoplasia was excluded in every case. The pathological diagnosis was initially interpreted as a non-demyelinating aetiology in 31% of cases by the referring pathologist. Among misdiagnoses (Table 2), a low grade astrocytoma was the most frequent (39%). Table 2 summarizes the pathological features in the cohort.
Fig. 5.
Pathology of an Active Biopsied multiple sclerosis Lesion. Biopsies from all 168 cases demonstrated the characteristic features of active inflammatory demyelinating disease consisting of hypercellular lesions with myelin loss (A; Luxol-fast blue and periodic acid Schiff), reactive gliosis (B; haematoxylin–eosin), Creutzfeldt cells (C; haematoxylin–eosin), lipid-laden macrophages (D and E; immunocytochemistry for proteolipid protein) and relative axonal preservation (F; Bielschowsky silver impregnation).
Table 2.
Pathological features of cohort
| Age at biopsy (years) | 38 (4–74) |
| Disease duration | |
| Index attack to biopsy (weeks) | 5.0 (2.3, 8.5) |
| Initially misread biopsies (%) | 31 |
| Specific diagnoses (%) | |
| Low grade astrocytoma | 39 |
| High grade astrocytoma | 15 |
| Oligodendroglioma | 6 |
| Infarction | 9 |
| Infection | 9 |
| Lymphoma | 3 |
| Non-diagnostic | 18 |
Note: values in parentheses are either minimum – maximum or lower quartile, upper quartile.
Radiographic characteristics of the cohort
A median number of five MRI studies were reviewed per patient (IQR 3–6; range 1–18). The median durations between imaging intervals are summarized in Table 3.
Table 3.
Radiographic features on pre-biopsy MRI of biopsied and non-biopsied lesions
| Feature | Biopsied (%) | Non-biopsied |
|---|---|---|
| Margins | ||
| Well-defined | 73 | NA |
| Diffuse | 38 | NA |
| Size: T2–T2 Margins | ||
| 0.3–2 cm | 20 | NA |
| 2.1–5 cm | 50 | NA |
| >5 cm | 30 | NA |
| Largest size (cm) | 4.0 (IQR 2.5, 6; range 0.5 to 12.0) | |
| Size: discernible margins | ||
| No discernible margins | 26 | NA |
| 0.3–2 cm | 26 | NA |
| 2.1–5 cm | 44 | NA |
| >5 cm | 4 | NA |
| Largest size (cm) | 2.1 (IQR 1, 3.5; range 0.5 to 7.5) | |
| Mass effect present | 45 | 22% |
| Mild | 64 | 100% |
| Moderate/marked | 36 | 0% |
| Edema present | 77 | 69.5% |
| Mild | 59 | 85% |
| Moderate/marked | 41 | 15% |
| Duration between MRI intervals | ||
| Pre-Biopsy MRI Interval | ||
| First MRI to biopsy (weeks) | 1.9 (0.9, 6.8) | |
| Closest MRI to biopsy (days) | 5 (1, 10) | |
| Post biopsy MRI interval (years) | 2.1 (0.9, 4.3) | |
| First to last MRI interval (years) | 2.2 (0.9, 4.4) | |
| Number of scans/patient | 5 (3, 6) | |
Note: unless otherwise indicated, values shown in parentheses are lower quartile, upper quartile.
Lesion number
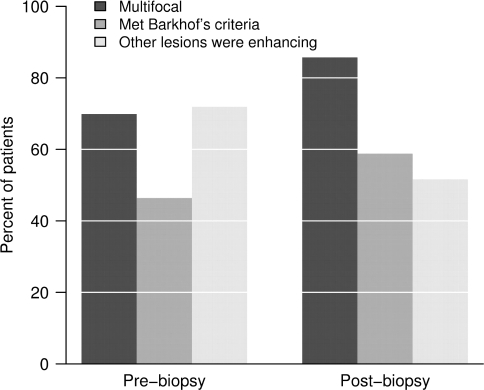
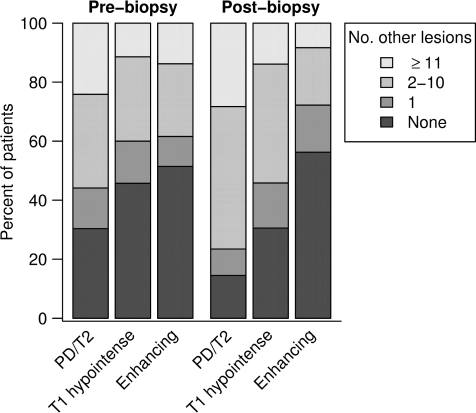
Multiple lesions were present in 70% of cases on pre-biopsy, and in 83% of cases on post-biopsy MRIs. Pre-biopsy MRI spines were available on 24 patients, and 38% had spinal cord lesions. Barkhof criteria were fulfilled in 46% of the cases during the pre-biopsy imaging interval, and 55% at last MRI follow-up (Fig. 6). Figure 7 summarizes the number of additional non-biopsied PD/T2, T1W hypointense and enhancing lesions observed during the pre- and post-biopsy imaging intervals.
Fig. 6.
Percent of patients with multifocality, who fulfilled Barkhof's criteria for multiple sclerosis, and who had other enhancing lesions (among those who were multifocal), pre/concomitant to biopsy and post-biopsy.
Fig. 7.
Number of non-biopsied PD/T2, T1W hypointense and enhancing lesions on pre- and post-biopsy MRI.
Lesion location
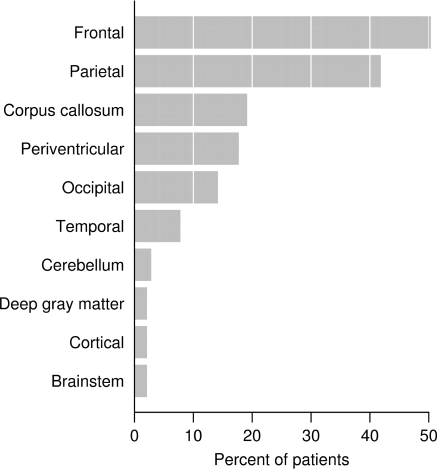
The biopsied lesion location may have involved multiple anatomic areas (Fig. 8). Frontal and parietal subcortical regions were most often affected (50 and 42% of patients, respectively), and a butterfly configuration involving the corpus callosum was observed in 12% of cases. In cases with multifocal lesions, non-biopsied sites included predominantly the periventricular (79%), juxtacortical (61%) or subcortical (54.5%) regions on both pre- and post-biopsy MRI.
Fig. 8.
Biopsy lesion location.
Size, mass effect and oedema
Table 3 summarizes the pre-biopsy MRI characteristics of biopsied and non-biopsied lesions with respect to size, and presence and grade of mass effect and oedema. The median largest lesion size (T2W margin to margin) on pre-biopsy MRI was 4 cm (IQR 2.5–6.0; range 0.5–12.0 cm). When possible to exclude perilesional oedema, the largest median discernible actual lesion size was 2.1 cm (IQR 1.0–3.5; range 0.5–7.5 cm). Typically, biopsied lesions ranged in size from 2.1 to 5 cm for both T2W margin-to-margin (50%) and discernible lesion size (44%). In 45% of cases, a pre-biopsy MRI showed the biopsied lesion was associated with mass effect, which in 36% was moderate to severe, whereas oedema was present in 77% of cases, and was moderate to marked in 41%. Excluding the biopsied lesion, the presence of one or more lesions larger than 2 cm was found in 35% of the cases on pre-biopsy, and in 36% of the cases on post-biopsy MRI. Mass effect and oedema of non-biopsied lesions was usually mild in degree (Table 3).
Enhancement
Representative examples of the different enhancement patterns are illustrated in Fig. 9. In order to avoid a potential confounding effect of the brain biopsy on subsequent enhancement patterns, analysis of biopsied lesion enhancement patterns was restricted to pre-biopsy MRIs, whereas non-biopsied lesions were characterized on both pre- and post-biopsy studies. The biopsied lesion enhanced in 123 (95%) of the cases in whom pre-biopsy T1W Gd scans were available. There was no correlation between lack of enhancement on pre-biopsy MRI, and the use of steroids or other immunosuppressive agents within 4 weeks of the study.
Fig. 9.
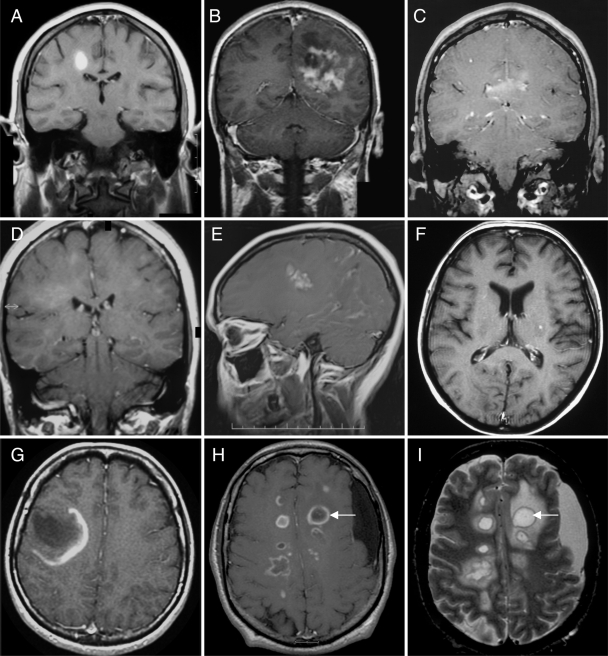
Representative examples of different enhancement patterns. (A) homogenous; (B) heterogenous; (C) patchy and diffuse; (D) cotton-ball; (E) nodular; (F) punctate; (G) open ring; (H) multiple closed rings; (I) multiple T2 hypointense rims co-localize with ring enhancement (arrows; T2W MRI). (A–H) T1W MRI + Gd.
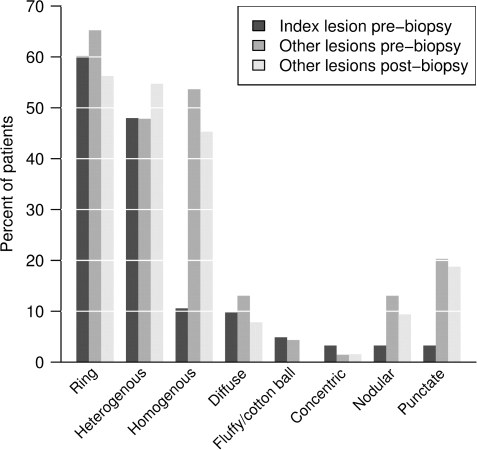
Figure 10 summarizes the spectrum and frequency of enhancement patterns observed. Ring enhancement, either closed, open to gray matter or arc-like, was the most frequent pattern in both biopsied (60%) and non-biopsied lesions (65%) during the pre-biopsy imaging interval. Closed rings (Fig. 9H) were more common than open rings (Fig. 9G) or arcs in both biopsied (45.5 versus 22%) and non-biopsied (52 versus 39%) lesions. On both pre- (54%) and post-biopsy (45%) MRI, non-biopsied lesions more often showed a homogenous enhancement pattern.
Fig. 10.
Frequency of enhancement patterns of biopsied and non-biopsied lesions. The index (biopsied) lesion enhanced in 123 (95%) of the cases in whom pre-biopsy T1W Gd scans were available for review. There was no correlation between lack of enhancement on pre-biopsy MRI, and use of steroids or other immunosuppressive agents within 4 weeks of the scan of interest. Non-enhancing biopsied lesions all demonstrated pathological evidence of active demyelination.
T2W hypointense rim(s)
Pre-biopsy T2W MRI studies showed a T2W hypointense complete rim around the biopsied lesion in 45% of cases. Figure 9I illustrates a classic T2W hypointense rim. T2W hypointense rims were also associated with non-biopsied lesions occurring in 30.5% of cases on pre-biopsy and 18.5% on post-biopsy MRI studies. T2W hypointense rims co-localized with ring enhancement as illustrated in Fig. 8H and I.
T1W hypointensity
The degree of T1W hypointensity of the biopsied lesion on pre-biopsy MRI was isointense in 2%, mild to moderately hypointense in 33% and markedly hypointense in 65% of cases. Post-biopsy MRI studies revealed biopsy defect sizes ranging from none/tract only in 6%, <5 mm in 21%, 5 mm–2 cm in 51% and >2 cm in 22%.
Progression of radiographic disease
Overall T2 lesion volume from first to last MRI was reduced in 15%, unchanged in 27% and increased in 58%, whereas overall T1 lesion volume was reduced in 31%, unchanged in 31% and increased in 38%. Overall progression in periventricular confluent white matter disease between first and last MRI was unchanged in 55% and worsened in 45%. On the first MRI study, there was no apparent global atrophy in 75%, whereas 14% had minimal and 11% moderate or severe global atrophy. In contrast, on last MRI, only half had no apparent global atrophy (50%) while 23% had minimal and 27% had moderate or severe atrophy. The extent of global atrophy between first and last MRI was unchanged in 63%, minimally or moderately worse in 29% and markedly worse in 8%.
Correlations between radiographic features
Across all MRI study periods, a strong association was observed between increasing lesion size and the presence of mass effect (P < 0.001), and oedema (P < 0.001). Only 17% of cases remained unifocal over the imaging follow-up period. The unifocal subgroup was more likely to have mass effect associated with the biopsied lesion on pre-biopsy scan (61.5 versus 41%; P = 0.028), compared with those who developed multifocal lesions. A similar relationship was seen with oedema. Cases that remained unifocal over the imaging interval tended to have oedema associated with the biopsied lesion on a pre-biopsy MRI study (93 versus 73%, P = 0.061). No statistically significant association was observed between lesion size, mass effect or oedema and the likelihood of fulfilling Barkhof criteria by last follow-up.
Relationship between ‘Tumefactive’ features and clinical outcomes
We analysed whether there were any correlations between radiographic features typically associated with neoplasms (size, mass effect, oedema, ring-enhancement) and specific demographic or clinical features. There was a statistically significant, albeit weak correlation, between increasing biopsied lesion size on pre-biopsy MRI and EDSS at follow-up (rs = 0.25, P = 0.009). Patients with biopsied lesions exceeding 5 cm on pre-biopsy MRI had a higher median EDSS at follow-up (median EDSS 4.0), compared with patients with either a 2–5 cm (median EDSS 2.5), or <2 cm (median EDSS 2.0) biopsied lesion size. However, the weak correlation coefficient suggests little of the variability in EDSS can be explained by lesion size. A larger biopsied lesion could prompt a more extensive resection, however, we found no correlation between the post-biopsy defect size and EDSS at last follow-up (r = 0.07; P = 0.46). Lesion size also did not correlate with gender, age at onset or biopsy, or clinical course and diagnosis prior to biopsy and at last follow-up. There was no correlation between biopsied lesion mass effect or oedema on pre-biopsy MRI, and the subsequent clinical course, diagnosis or EDSS at last follow-up. Lastly, the enhancement pattern did not correlate with age, gender, clinical course, diagnosis or EDSS at biopsy or last-follow-up.
Among the 85 patients whose index attack represented their first demyelinating event, no specific clinical or radiographic risk factors were associated with a greater risk of developing a second multiple sclerosis attack. Patients with an isolated demyelinating syndrome at last follow-up were as likely to have an MRI demonstrating multiple lesions (50% of cases) as a solitary lesion (50%). An association between the presence of a greater number of PD/T2 or T1 hypointense lesions on pre-biopsy MRI (P = 0.005 and P = 0.005, respectively), and the subsequent development of multiple sclerosis, however, was noted. Furthermore, disease progression in terms of overall T2W volume change between the first and last MRI was greater in the multiple sclerosis group than in the isolated demyelinating group (P = 0.002).
Twenty-eight patients had relapses associated with radiographic evidence of developing at least one or more lesion(s) >2.0 cm in size. Although these patients had a slightly higher median EDSS at last follow-up [3.0 (IQR 2.0–4.5)], compared with the remainder of the cohort [EDSS 2.5 (IQR 1.0–3.5); P = 0.055], no other significant associations were observed with gender, clinical course or diagnosis.
Discussion
Clinical presentations
Multiple sclerosis is usually diagnosed by demonstrating clinical and/or radiographic evidence of dissemination of disease in time and space (Poser et al., 1983; McDonald et al., 2001). Although the diagnosis of classic multiple sclerosis generally does not require surgical intervention, some cases pose considerable diagnostic difficulty and may require brain biopsy. The occurrence of large demyelinating lesions resembling brain tumours are well recognized, and predominantly described in case reports involving few cases. The largest previously published clinicopathologic published series of CNS IDD consisted of 31 biopsy proven cases, but clinical and imaging follow-up in this series was limited and it was not clear how many patients actually had an MRI versus a computed tomographic (CT) scan (Kepes, 1993). The current study represents the largest clinico-radiographic series of biopsy-confirmed CNS IDD to date, and includes both longitudinal clinical and imaging follow-up.
The occurrence of tumour-like demyelination is reportedly rare, being estimated at 1–2/1000 cases of multiple sclerosis (Poser et al., 1992). A prevalence of three cases per million inhabitants per year has been suggested (Paty et al., 1988). Among 1220 brain biopsies, Hunter et al. observed only four cases of a demyelinating disease initially suggestive of a brain tumour (Hunter et al., 1987). In another series, Annesley-Williams et al., reported 14 cases among 15 394 neuropathological specimens (0.09%) (Annesley-Williams et al., 2000). Although the true incidence is unknown, these figures are likely an underestimation, given the large number of CNS IDD biopsies identified via the multiple sclerosis Lesion Project (NMSS RG3185).
Prior reviews have suggested a female prevalence similar to that seen in classic forms of multiple sclerosis (Comi, 2004), however, no gender predilection was observed in the current series. Atypical CNS IDDs can present at any age, but a median age at onset of 37 years, as reported in the current study, is consistent with previous reports suggesting they occur more frequently in the second and third decades of life (Comi, 2004).
Demyelinating lesions mimicking brain tumours are thought to be exceedingly rare in the paediatric population. Of the 19 previously reported cases of tumefactive demyelinating disease in children, only 10 had pathological confirmation (Hunter et al., 1987; Gutling and Landis, 1989; Giang et al., 1992; Kepes, 1993; Rusin et al., 1995; Dagher and Smirniotopoulos, 1996; Kumar et al., 1998; McAdam et al., 2002; Yapici and Eraksoy, 2002). Several reports suggested that these patients had a comparatively benign, often monophasic course in comparison to other forms of demyelinating disease (Kumar et al., 1998; Yapici and Eraksoy, 2002). In contrast, a more recent series of four paediatric cases reported that three of four patients developed RRMS, and one had a monophasic course (McAdam et al., 2002). Among the seven patients under age 18 years in our current series, the median age at biopsy was 11 years (range 4–17 years), and diagnosis at last follow-up was definite multiple sclerosis (n = 4), probable multiple sclerosis (n = 1), isolated demyelinating syndrome (n = 1) and ‘unknown’ (n = 1). To avoid potentially unnecessary diagnostic interventions, paediatricians, radiologists and neurosurgeons should be aware of the occurrence of tumefactive demyelinating lesions in the paediatric population.
Included in our series were seven patients with pathologically confirmed CNS IDD older than 65 years, (median 69 years, range 66–74) at the time of brain biopsy. Late onset of multiple sclerosis is very rare (Ikeda et al., 1992), with a reported frequency of between 0.6% and 0.75% of multiple sclerosis cases diagnosed past age 60 years (Noseworthy et al., 1983; Iwsamoto et al., 2004). An older age at onset makes distinguishing IDD from tumours such as malignant glioma, metastasis or lymphoma, even more challenging. Since primary CNS lymphoma, especially post-steroid therapy, may present with sentinel ‘demyelinating lesions’ (Alderson et al., 1996), it is important to undertake post-biopsy surveillance imaging, particularly in the elderly.
Although typical symptoms and signs of multiple sclerosis include sensory syndromes, haemiplegia, paraplegia and optic neuritis, the clinical spectrum of CNS IDDs may be associated with systemic symptoms such as fever, or may present with focal, multifocal or non-localizing neurological deficits that mimic tumour or other infectious and/or inflammatory disorders including abscess, vasculitis or granulomatous disease. Despite a predominance of motor symptoms (mono-, hemi-, paraplegia) in the current series, a number of less common clinical presentations were observed. Cognitive dysfunction was a frequent presenting symptom, and included memory dysfunction, mental confusion and disorders of attention. These symptoms may reflect early gray matter cortical involvement. Seizures reportedly occur in only 1–3% of multiple sclerosis cases (Nyquist et al., 2002), but were present in nine cases (6%) in our series. Disorders of higher cognitive function were also observed. Cortical symptoms including apraxia, agnosia and aphasia are rare, but have been described (Kanaha et al., 1971; Olmos-Lau et al., 1977; Roeltgen et al., 1982; Sagar et al., 1982; Morioka et al., 1996; Comi, 2004). Although aphasia is reported in only 1% of multiple sclerosis patients during the course of their disease (Kanaha et al., 1971), 17% of patients experienced language disturbances in our series. The posterior visual pathways are often affected both radiologically and pathologically in multiple sclerosis, but homonymous visual field defects are unusual (Hawkins and Behrens, 1975). In one multiple sclerosis autopsy series (Savitsky and Rangell, 1950), asymptomatic plaques were found in the optic radiations in 23 of 50 cases, but no patient had a visual field cut. In our series, 10% of patients experienced a symptomatic visual field defect. This likely reflects the selection bias for larger lesion sizes in our cohort, the result being a greater impact upon the optic radiations. The presence of symptoms and signs not typically associated with multiple sclerosis may cause the clinician to overlook multiple sclerosis in the differential diagnosis, and increase the suspicion of neoplasm or infection. An accurate diagnosis requires knowledge of the broad spectrum of presentations observed within the family of CNS IDDs. Since misdiagnosis can result in unwarranted procedures and treatments, it is critical for the neurologist to be aware of this diagnostic pitfall.
Although the symptoms leading to brain biopsy in our series represented the first neurological event in most patients, 8 (5%) already carried an established diagnosis of multiple sclerosis prior to biopsy. However, a pre-existing diagnosis of multiple sclerosis does not exclude the possibility of a coexisting tumour, or additional pathology (e.g. infection). In 29% of patients, there was a history of neurological episodes prior to biopsy. In some cases, this previous history was obtained at the time of last follow-up (median 3.9 years after biopsy), by a study investigator, rather than at the time of brain biopsy. These historical details may have been overlooked by the original physician, or not reported by the patient at the time of initial presentation. It is possible that this additional information may have obviated the need for a brain biopsy in some cases, particularly if the symptoms were typical of demyelinating disease (e.g. episode of painful loss of vision). This underscores the importance of obtaining a detailed neurological history in all such patients, with particular attention to prior episodes of transient neurological dysfunction for which the patient may not have sought medical attention.
The frequency of CSF OB noted prior to brain biopsy is relatively low (i.e. 33%) in our study cohort. Although prior studies in acute monosymptomatic demyelinating syndromes have reported CSF OB positivity ranging from 46% to 75% (Martinelli et al., 1991; Frederiksen et al., 1992; Rolack et al., 1996; Tumani et al., 1998), the lower frequency in our study may reflect a selection bias skewed towards OB negative patients who were more likely to have undergone brain biopsy in order to secure a diagnosis. Furthermore, as this was a retrospective study, the quality of CSF analysis was not uniform and performed in different laboratories, possibly leading to a higher false negative rate, particularly if isoelectric focusing was not routinely performed. Interestingly, several studies have suggested that the absence of OB at clinical presentation may reflect a favourable prognostic factor associated with delayed time to a second event, a benign course, as well as delaying disability progression during interferon-B treatment (Stendahl-Brodin and Link, 1980; Sharief and Thompsen, 1991; Paolino et al., 1996; Zeman et al., 1996; Avasarala et al., 2001; Annunziata et al., 2006). Further studies are needed in order to determine whether the longer median interval to second attack and less severe clinical disability observed in our study is related to CSF OB status.
Among the 36 (29%) patients with a history of prior neurological episodes, 28 had multifocal lesions on pre-biopsy MRI, as well as CSF OB. Although in retrospect these 28 patients would have likely met Poser or McDonald criteria based on clinical course, MRI and CSF findings prior to brain biopsy, it must be emphasized that these diagnostic criteria are not absolute, since a number of non-multiple sclerosis diagnoses may also meet Poser and/or McDonald criteria (e.g. primary CNS lymphoma, sarcoidosis, CNS vasculitis, multicentric glioma) (Weinshenker and Lucchinetti, 1998). Therefore, meeting these multiple sclerosis diagnostic criteria does not necessarily eliminate the need for brain biopsy in all circumstances.
Pathological diagnosis
Pathological findings common to all our 168 cases included hypercellular lesions with confluent demyelination, abundant foamy macrophages containing myelin debris, reactive astrogliosis, ‘relative’ axonal preservation and variable perivascular and parenchymal lymphocytic inflammation. Macrophages and astrocytes were commonly closely intermingled. No specific histological features distinguished specimens derived from patients developing classic multiple sclerosis from those who had an isolated demyelinating syndrome.
CNS IDDs, including classic multiple sclerosis, may pose histopathological challenges. Errors of interpretations expose patients to inappropriate treatments (Peterson et al., 1993). In our series, ∼30% of biopsies were originally misdiagnosed at the referring institution. Astrocytoma, more often low-grade than high-grade, was the most frequent misdiagnosis. Since lesions are typically biopsied when symptomatic, an active enhancing lesion is often targeted. Histologic features may mimic tumour including hypercellularity, astrocytic pleomorphism, variable nuclear atypia, a rare mitotic figure and occasional necrosis or cystic changes (Zagzag et al., 1993; Annesley-Williams et al., 2000; Sugita et al., 2001). These features, particularly at frozen section or on small biopsies, pose a potential trap for the pathologist and are a common cause of litigation. Several histologic features, however, do point toward a non-neoplastic demyelinating process. These include (i) abundance of foamy macrophages in the absence of coagulative necrosis, (ii) rather evenly distributed plump, reactive astrocytes, some with multiple micronuclei (Creutzfeldt cells), often closely intermingled with macrophages, (iii) absence of microvascular proliferation, (iv) perivascular inflammation and (v) relative axonal preservation (Zagzag et al., 1993; Annesley-Williams et al., 2000; Sugita et al., 2001).
Distinguishing active demyelination from neoplasm is critical, since a misdiagnosis can lead to inadvertent brain irradiation, which apart from the potential risk of radiation necrosis and post-irradiation neoplasia, is known to exacerbate underlying inflammatory demyelinating disease (Peterson et al., 1993; Miller et al., 2006). Suboptimal tissue sampling also represents a major impediment to accurate histological diagnosis. A good sample should include perilesional brain tissue and not be entirely subject to frozen section; smear preparations are preferred. Diagnostic bias may also be introduced by atypical clinical or imaging presentations. Imaging suggestive of infection or neoplasm tends to bias the pathologists, restricting the differential diagnosis and promoting diagnostic error.
Biopsy of suspicious lesions are often approached stereotactically, thus minimizing tissue injury. Nonetheless, temporary postoperative worsening of neurologic deficits and even death has been reported. For example, in one series, a child died after attempted drainage of a presumed abscess (Rusin et al., 1995). Brain biopsy may also be associated with persistent neurological deficits related to surgical trauma. Although we found no association between the biopsy defect size and EDSS at last follow-up, a number of patients noted persistence of new postoperative neurological symptoms. Apart from defect size, other biopsy related factors such as bleeding or trauma in the region of eloquent anatomy, may contribute to permanent disability in our cohort.
Since perivenular demyelination was an exclusion criteria, no cases of ADEM were included in this study. In-progress studies will compare the clinical and radiographic features of this study cohort with pathologically confirmed cases of ADEM. In addition, a parallel study investigates potential associations between specific pathological features on brain biopsy, including the classification of multiple sclerosis lesions into four subtypes (Lucchinetti et al., 2000) and specific radiographic features (manuscript in preparation) (Gavrilova et al., 2007).
Radiographic presentations
Modern MR imaging is the most sensitive method of detecting the white matter lesions of multiple sclerosis. T2W MRI displays multiple ovoid lesions, which, within PVWM and the corpus callosum, are often oriented perpendicular to the long axis of the ventricular system (Barkhof et al., 1997; Fazekas et al., 1988, 1999; Miller et al., 2004). Although MRI has increased our ability to highlight these lesions, it often fails to provide an unambiguous diagnosis (Butteriss et al., 2003). This is particularly true when the lesions present as large, space occupying lesions misinterpreted as tumour, abscess or infarct. Generally, multiple sclerosis plaques range from 3 to 16 mm in size. In the current series, the majority of cases (80%) had T2W margin-to-margin biopsied lesion sizes >2.0 cm, with a discernible lesion size >2.0 cm in 48%. The largest T2W margin-to-margin lesion size was 12.0 cm. Of those with discernible margins, the largest was 7.5 cm. The largest previously reported multiple sclerosis plaque measured 7.2 cm, a size determined by CT scan (Hershey et al., 1979).
The definition of ‘tumefactive lesions’ is not consistent in the literature, and may refer to various combinations of the following: large size (>2 cm), presence of mass effect or oedema and/or atypical enhancement patterns (ring, heterogenous, etc). Furthermore, some malignancies, such as metastases and primary CNS lymphoma, may be characterized by multiple small lesions with variable or persistent enhancement patterns (nodular, punctate, etc). Although the minimum of the largest lesion size distribution identified on pre-biopsy MRI in our study was 0.5 cm, the lower quartile for the entire cohort was 2.5 cm, while the upper quartile was 6.0 cm. Among the 168 cases, 13 demonstrated lesion size on pre-biopsy MRI <2.0 cm. Although not ‘tumefactive’ based strictly on size, 10 of the cases with lesions <2.0 cm had multiple lesions on pre-biopsy MRI and a spectrum of enhancement patterns were observed among 11 with a Gd study, including ring (3); heterogenous (3); diffuse (3); punctate (1) and concentric (1) patterns. Multiple enhancing lesions were noted in five, and five cases had associated mild oedema, with mild mass effect observed in one. Since the majority of our cases were biopsied elsewhere (87%), and the material was retrospectively obtained well after the brain biopsy was performed, it was often not possible to determine the precise indication for biopsy, particularly among this subgroup with lesions <2.0 cm. Nonetheless, since all but 13 cases had at least one lesion >2.0 cm, it is probable that a large lesion size was the primary, but not exclusive factor contributing to the decision to proceed with brain biopsy.
In comparison with tumours and abscesses, mass effect and oedema in IDD are said to be proportionally minor relative to plaque size (Sagar et al., 1982; Gutling and Landis, 1989; Paley et al., 1989; Nesbit et al., 1991; Giang et al., 1992; Charil et al., 2006; Omuro et al., 2006). The largest previously published radiologic series of pathologically confirmed CNS IDD cases (n = 40), included only a single example with associated mass effect and oedema. The authors suggested that lack of mass effect differentiates multiple sclerosis plaques from other space-occupying lesions (Nesbit et al., 1991). In contrast, our much larger study found both mass effect and oedema frequently associated with the biopsied lesion on pre-biopsy MRI. This also was true of non-biopsied lesions. We also observed a strong statistical association between increasing biopsy lesion size and presence of mass effect and oedema across all MRI study periods. This contradicts prior reports suggesting that sizable IDD lesions lack mass effect and oedema. A recent review by the Magnetic Resonance Network in Multiple Sclerosis (MAGNIMS) defined a series of ‘MRI red flags’ suggestive of alternative diagnoses to multiple sclerosis (Charil et al., 2006). It is important to know that multiple sclerosis and IDDs in general may present with a broad spectrum of radiological findings, ones easily mistaken for tumour, abscess or vascular disease.
Some series suggest tumefactive demyelinating lesions more commonly affect subcortical than PVWM (Kepes, 1993). In our series, the biopsied lesion was most often supratentorial and subcortical in location, involving the frontal, parietal, occipital and temporal lobes in decreasing frequency. In contrast, in the setting of multifocal disease, non-biopsied lesions were most often situated in the PVWM. Among multifocal lesions, the one selected for biopsy is more likely a reflection of surgical bias, rather than a true indicator of pathologic site predilection. This may also account for the relative infrequency of posterior fossa (brainstem/cerebellum) and deep gray matter biopsies in our series. On occasion, IDD spreads across the corpus callosum in a ‘butterfly’ configuration, simulating an infiltrative astrocytoma or lymphoma (Rieth et al., 1981; Hunter et al., 1987; Kalyan-Raman et al., 1987). This pattern was observed in 10 patients (7%) in our series. In such instances, there were no differences in patient age, clinical course prior to biopsy or EDSS at last follow-up between patients with or without a butterfly lesion. Among nine with clinical follow-up, eight developed definite multiple sclerosis, and one an isolated demyelinating syndrome by last follow-up.
A variety of enhancement patterns were observed in this series. Ring enhancement was the most frequent, both among biopsied and non-biopsied lesions during the pre- and post-biopsy imaging intervals. Although a homogenous enhancement pattern was uncommon among biopsied lesions, it was often observed in non-biopsied lesions, both during the pre- and post-imaging interval. In a retrospective study of 25 patients with definite multiple sclerosis, 68% showed homogenous enhancement, 23% a rim pattern and 9% arc-like enhancement (He et al., 2001). A variety of intracranial pathologies can present as a ring enhancing lesion on MRI, including glioma, metastasis, lymphoma, radiation necrosis, infarct, abscess and IDD. In a recent series, the most prevalent pathologies associated with ring enhancement were gliomas (40%), metastasis (30%), abscesses (8%) and multiple sclerosis lesions (6%) (Schwartz et al., 2006). Although less common in prototypic multiple sclerosis, ring enhancing lesions are more likely to be biopsied in order to exclude these other pathologies and may be over-represented in our study.
In the present series, lesions demonstrating ring enhancement, usually had ‘closed rings’. Previous studies have suggested that the pattern of ring enhancement associated with demyelination is more often ‘open’, the incomplete portion abutting cortical gray matter or the basal ganglia (Masdeu et al., 1996, 2000). Although the ring associated with abscesses and neoplasms is more often complete (Haimes et al., 1989), our study underscores the fact that closed rings may be observed with IDD. Indeed, they predominated in our series. Heterogenous enhancement patterns, including punctate and nodular, were common in both biopsied and non-biopsied lesions in our series. Although some studies have suggested that tumefactive demyelinating lesions always enhance (Comi, 2004), we found that 5% of cases with T1W, Gd enhanced studies did not, regardless of concomitant steroid use or other immunosuppressant therapy. Enhancement depends on many factors, including the time from injection to imaging, the dosage of the contrast agent, the magnitude of blood brain barrier abnormalities, the volume of accumulation and the MR pulse sequence (He et al., 2001). It is important to recognize that the lack of enhancement does not necessarily exclude a demyelinating pathology.
Various ring enhancing lesions may have a complete rim of hypointensity on T2W sequences. Such rims were frequent in our cohort, and co-localized with ring enhancement. T2W hypointense rims are most commonly associated with abscesses, wherein they are thought to result from the production of paramagnetic free radicals by macrophages (Haimes et al., 1989). Hypointense T2W rims, more often partial than complete, may also occur in intracranial haematoma, vascular malformations and tumoural haemorrhage, and correlate with the presence of haemosiderin-laden macrophages at the lesion's edge (Gomori et al., 1985; Atlas et al., 1987). Some have proposed that in granulomatous disease, T2W rims correspond to a fibrous rim of granulation and compressed glial tissues (Gupta et al., 1988). A study by Schwartz et al. examined the prevalence of T2W hypointense borders in ring enhancing lesions of various aetiologies (Schwartz et al., 2006). Abscesses had the highest percentage of complete hypointense rims, whereas metastases and gliomas more often featured hypointense arcs and multiple sclerosis lesions equally showed rims and arcs. Interestingly, nearly all multiple sclerosis lesions (92%) were centrally homogenous on T2W MRI compared with abscesses in which 56% were centrally homogenous and 44% heterogenous. Although the presence of a ring enhancing lesion co-localizing with a complete T2W hypointense rim, and having a T2W homogenous centre is most likely due to IDD, given the degree of overlap with other pathologic processes, these trends do not obviate the need for brain biopsy in all circumstances.
Prior series of acute IDD subject to biopsy or autopsy have emphasized their unifocal nature (Nesbit et al., 1991; Kepes, 1993). In contrast, we observed that the majority of cases were radiographically multifocal at presentation. Since these additional lesions were often enhancing, and classic in their locations, they likely represented true demyelinating lesions, rather than non-specific white matter abnormalities. Furthermore, a substantial proportion of patients already met Barkhof criteria of multiple sclerosis at the time of presentation. In a series of 31 patients with large focal cerebral demyelinating lesions (Kepes, 1993), 24 were reportedly unifocal and seven multifocal on either CT or MRI. Technical factors may have limited the demonstration of additional lesions, since many cases were scanned only by CT, and the assessment of all cases antedated 1991. Although relatively uncommon in our series, it is still important to include IDD in the differential diagnosis of a solitary lesion.
Clinical and radiographic disease progression
The long-term evolution of biopsied CNS IDD is not well defined due to the lack of longitudinal studies with adequate clinical or radiographic follow-up. In contrast to prior studies suggesting that acute episodes of tumefactive IDD are usually isolated and rarely progress to more typical multiple sclerosis (Kepes, 1993), we found that the majority (70%) ultimately developed clinically definite multiple sclerosis, only a very small subset remaining isolated at last follow-up. Nonetheless, Kaplan-Meier survival estimates revealed that the median time to the second ‘multiple sclerosis-defining’ clinical episode was quite long (4.8 years). This is longer than expected given the conversion rates observed in untreated non-biopsied patients presenting with a clinically isolated demyelinating syndrome. In the CHAMPS study, 50% of placebo patients with a clinically isolated demyelinating syndrome converted to clinically definite multiple sclerosis by 3 years (Kepes, 1993; Champs Study Group, 2001), whereas in the ETOMS study, 45% converted by 2 years (European, 1998). In a large study of the natural history of multiple sclerosis including 1215 patients, the median time to a second clinical episode was 1.9 years (Confavreux C, 2002). The reason for the longer interval to second episode in biopsied patients with IDD in our cohort is unknown.
Some studies suggest patients with tumefactive multiple sclerosis have a comparatively benign course compared with other forms of multiple sclerosis (Kepes, 1993; Hayashi et al., 2003). We previously reported biopsied multiple sclerosis patients have a similar clinical course and prognosis compared with a population-based multiple sclerosis cohort (Pittock et al., 2005). Of the 168 cases in the current study, 62 (37%) had been reported in our prior series. Since these are overlapping but not identical populations, we compared disability (EDSS) at last follow-up in our study cohort with established disability data from the OC multiple sclerosis prevalence cohort, stratified for disease duration. Although we observed that EDSS at last follow-up was slightly higher in the biopsy cohort compared with the multiple sclerosis prevalence cohort when matched for disease durations between 0–5 and 5–10 years, it is well recognized that EDSS scores in the low range are less reproducible and a 1 point increase in EDSS when <4 is of lesser clinical importance than a 1 point rise in EDSS ≥6. Interestingly, EDSS in biopsied multiple sclerosis cases with a disease duration >10 years was significantly less compared with the prevalence multiple sclerosis cohort matched for disease duration >10 years, (1.5 versus 3.5 P < 0.001). These findings suggest that prognosis is not greatly affected by the appearance of tumefactive lesions, and the presence of such lesions could potentially protect against long-term disease progression.
The current study also demonstrates that the radiographic course of biopsied IDD cases resembles what would be expected in typical multiple sclerosis, with accumulating T2, T1 and PVWM disease burden as well as progressive brain atrophy. Prior reports have also emphasized that large, tumefactive demyelinating lesions tend to remain radiographically unifocal. In the largest previous clinicopathologic series of 31 patients, only three patients developed additional lesions over the follow-up period (Kepes, 1993). In contrast, we found that only 17% of patients remained unifocal at last imaging follow-up, and that they were more likely to be associated with mass effect compared with those of the multifocal group. We conclude that the lack of MRI follow-up in prior studies underestimated the true extent of multifocal disease.
In this study, we assessed the impact of tumefactive radiographic features on course and prognosis. Although lesion size exceeding 5 cm was associated with a higher median EDSS at last follow-up, we found no association between EDSS and mass effect, oedema or enhancement pattern. Furthermore, size, mass effect and oedema were not associated with clinical course or diagnosis at last follow-up. We did identify a small subset of patients who demonstrated a tendency to develop relapsing demyelinating episodes associated with radiographic evidence of recurrent uni- or multi-focal lesions exceeding 2 cm. These patients had a slightly higher median EDSS at last follow-up (3.0 versus 2.5), but no other demographic, clinical or radiographic features that distinguished them from the remainder of the cohort. The reasons why some patients develop recurrent, large multiple sclerosis plaques is unknown.
We did not systematically analyse the prognosis for relapse recovery in our cohort, but prior studies suggest most patients experience symptomatic improvement and reduction or disappearance of radiographic abnormalities after steroid therapy. Plasma exchange should be considered in patients who fail to respond to steroids (Weinshenker, 1999; Keegan et al., 2002).
Indication for brain biopsy
Since the majority (87%) of biopsies were performed elsewhere, and clinical follow-up was obtained a median of 3.9 years after biopsy by neurologists not originally involved in the case, the precise reason(s) for biopsy was difficult to determine retrospectively. Based on the clinical and radiographic features described in our study cohort, reasons for biopsy most likely included atypical clinical presentations (e.g. encephalopathy, seizures, aphasia), older age, the presence of at least one large lesion (>2.0 cm), associated mass effect/oedema and/or atypical enhancement patterns. This study underscores the diagnostically challenging nature of CNS IDD cases that present with atypical clinical or radiographic features, and highlights the need for clinicians, radiologists, pathologists and surgeons to recognize the broad and heterogeneous spectrum of these disorders. Although a better appreciation of this spectrum-supplemented by a consideration of neurological history, CSF and evoked potential findings may obviate the need for brain biopsy in many circumstances, and MR diffusion and perfusion sequences as well as MRS may help elucidate the demyelinating nature of the lesions (Ernst et al., 1998; Metafratzi et al., 2002; Butteriss et al., 2003; Enzinger et al., 2005), an important role for diagnostic brain biopsy remains in some cases.
Conclusions
Despite an atypical clinical or radiographic presentation, the development of multiple sclerosis is the most common clinical outcome among patients presenting with tumefactive features. Although it is still not possible to determine whether a patient presenting with a large tumefactive cerebral lesion will develop typical multiple sclerosis or not, our findings suggest the risk is greater than previously recognized. The majority of patients have a clinical and radiographic course as well as prognosis similar to prototypic multiple sclerosis. Nonetheless, continued clinical surveillance is essential given the relatively long duration between initial clinical presentation and a second event. Although the pathogenesis of large, focal cerebral demyelinating lesions is still controversial, our study, the largest clinicopathologic–radiographic series reported to date, does not support the hypothesis that such lesions occupy an intermediate position between multiple sclerosis and ADEM, a hypothesis forwarded by Kepes (1993), represent recurrent disseminated encephalomyelitis, a theory advocated by Brinar (2004) or represent a distinct variant of multiple sclerosis as proposed by Poser et al. (1992). Rather, these biopsied cases are simply a part of the heterogeneous clinical and radiographic spectrum of multiple sclerosis.
Acknowledgements
This study was supported by the United States National Multiple Sclerosis Society (RG3185; C.F.L.), the National Institute of Health (NS049577; C.F.L.), by UL 1 RR24150-01, from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and the NIH Roadmap for Medical Research, and the Gemeinnützige Hertie-Stiftung (WB).
Glossary
Abbreviations:
- ADEM
acute disseminated encephalomyelitis
- CSF
cerebrospinal fluid
- CT
computed tomographic
- EDSS
expanded disability status scale
- F/U
follow-up
- Gd
gadolinium
- IDD
inflammatory demyelinating disease
- IQRs
interquartile ranges
- MRI
magnetic resonance imaging
- OB
oligoclonal bands
- OC
Olmsted County
- PVWM
periventricular white matter
- T1W
T1-weighted
References
- Al-Bunyan MA. Tumor-like presentation of multiple sclerosis. Saudi Med J. 2000;21:393–5. [PubMed] [Google Scholar]
- Alderson L, Fetell MR, Sisti M, Hochburg F, Cohen M, Louis DN. Sentinel lesions of primary CNS lymphoma. J Neurol Neurosurg Psychiatry. 1996;60:102–5. doi: 10.1136/jnnp.60.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annesley-Williams D, Farrell MA, Staunton H, Brett FM. Acute demyelination, neuropathological diagnosis, and clinical evolution. J Neuropathol Exp Neurol. 2000;59:477–89. doi: 10.1093/jnen/59.6.477. [DOI] [PubMed] [Google Scholar]
- Annunziata P, Giorgio A, De Santi L, Zipoli V, Portaccio E, Amato MP, et al. Absence of cerebrospinal fluid oligoclonal bands is associated with delayed disability progression in relapsing-remitting MS patients treated with interferon-B. J Neurol Sci. 2006;244:97–102. doi: 10.1016/j.jns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Atlas SW, Grossman RI, Gomori JM, Hackney DB, Goldberg HI, Zimmerman RA, et al. Hemorrhagic intracranial malignant neoplasms: spin-echo MR imaging. Radiology. 1987;164:71–7. doi: 10.1148/radiology.164.1.3588929. [DOI] [PubMed] [Google Scholar]
- Avasarala JR, Cross AH, Trotter JL. Oligoclonal band number as a marker for prognosis in multiple sclerosis. Arch Neurol. 2001;58:2044–5. doi: 10.1001/archneur.58.12.2044. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120:2059–69. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Rocca M, Francis G, van Waesberghe JH, Uitdehaag BM, Hommes OR, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta-1a. Ann Neurol. 2003;53:718–24. doi: 10.1002/ana.10551. [DOI] [PubMed] [Google Scholar]
- Bolay H, Karabudak R, Tacal T, Onol B, Selekler K, Saribas O. Balo's concentric sclerosis: report of two patients with magnetic resonance imaging follow-up. J Neuroimaging. 1996;6:98–103. doi: 10.1111/jon19966298. [DOI] [PubMed] [Google Scholar]
- Brinar VV. Non-MS recurrent demyelinating diseases. Clin Neurol Neurosurg. 2004;106:197–210. doi: 10.1016/j.clineuro.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Butteriss DJ, Ismail A, Elison DW, Birchall D. Use of serial proton magnetic resonance spectroscopy to differentiate low grade glioma from tumefactive plaque in patient with multiple sclerosis. Br J Radiol. 2003;76:662–5. doi: 10.1259/bjr/85069069. [DOI] [PubMed] [Google Scholar]
- Capello E, Roccataglioata L, Pagano F, Macardi GL. Tumor-like multiple sclerosis (MS) lesions: neuropathological clues. J Neurosci. 2001;22:S113–6. doi: 10.1007/s100720100047. [DOI] [PubMed] [Google Scholar]
- Censori B, Agostinis C, Partziguian T, Gazzaniga G, Biroli F, Mamoli A. Large demyelinating brain lesion mimicking a herniating tumor. J Neurosci. 2001;22:325–9. doi: 10.1007/s10072-001-8176-5. [DOI] [PubMed] [Google Scholar]
- Champs Study Group. Interferon beta-1a for optic neuritis patients at high risk for multiple sclerosis. Am J Ophthalmol. 2001;132:463–71. doi: 10.1016/s0002-9394(01)01209-0. [DOI] [PubMed] [Google Scholar]
- Charil A, Yousry T, Rovaris M, Barkhof F, De Stefano N, Fazekas F, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of ‘no better explanation’. Lancet Neurol. 2006;5:841–52. doi: 10.1016/S1474-4422(06)70572-5. [DOI] [PubMed] [Google Scholar]
- Chen C, Ro L, Wang L, Wong Y. Balo's concentric sclerosis: MRI. Neuroradiology. 1996;38:322–4. doi: 10.1007/BF00596578. [DOI] [PubMed] [Google Scholar]
- Comi G. Mutiple sclerosis: pseudotumoral forms. Neurol Sci. 2004;25:S374–9. doi: 10.1007/s10072-004-0345-x. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: implications for counselling and therapy. Curr Opin Neurol. 2002;15:257–66. doi: 10.1097/00019052-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Dagher AP, Smirniotopoulos J. Tumefactive demyelinating lesions. Neuroradiology. 1996;38:560–5. doi: 10.1007/BF00626098. [DOI] [PubMed] [Google Scholar]
- Di Patre P, Castillo V, Delavelle J, Vuillemoz S, Picard F, Landis T. ‘Tumor-mimicking’ multiple sclerosis. Clin Neuropathol. 2003;22:235–9. [PubMed] [Google Scholar]
- Enzinger C, Strasser-Fuchs S, Ropele S, Kapeller P, Kleinert R, fazekas F. Tumefactive demyelinating lesions: conventional and advanced magnetic resonance imaging. Mult Scler. 2005;11:135–9. doi: 10.1191/1352458505ms1145oa. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Walot I, Huff K. Physiologic MRI of a tumefactive multiple sclerosis lesion. Neurology. 1998;51:1486–8. doi: 10.1212/wnl.51.5.1486. [DOI] [PubMed] [Google Scholar]
- European S. Group, on Intereron b-1b in Secondary Progressive MS. Placebo-controlled multicentre randomized trial of interferon b-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–7. [PubMed] [Google Scholar]
- Fazekas F, Barkhof F, Filippi M, Grossman RI, Li DK, McDonald WI, et al. The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology. 1999;53:448–56. doi: 10.1212/wnl.53.3.448. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Offenbacher H, Fuchs S, Schmidt R, Niederkorn K, Horner S, et al. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology. 1988;38:1822–5. doi: 10.1212/wnl.38.12.1822. [DOI] [PubMed] [Google Scholar]
- Frederiksen JL, Larsson HB, Olesen J. Correlation of magnetic resonance imaging and CSF findings in patients with acute monosymptomatic optic neuritis. Acta Neurol Scand. 1992;86:317–22. doi: 10.1111/j.1600-0404.1992.tb05093.x. [DOI] [PubMed] [Google Scholar]
- Friedman DI. Multiple sclerosis simulating a mass lesion. J Neuroophthalmol. 2000;20:147–53. doi: 10.1097/00041327-200020030-00001. [DOI] [PubMed] [Google Scholar]
- Gavrilova R, Metz I, Bruck W, Weigand S, Thomsen K, Mandrekar K, et al. MRI correlates of immunopathological patterns. Mult Scler. 2007;13:S76. [Google Scholar]
- Giang DW, Poduri KR, Eskin TA, Ketonen LM, Friedman PA, Wang DD, et al. Multiple sclerosis masquerading as a mass lesion. Neuroradiology. 1992;34:150–4. doi: 10.1007/BF00588163. [DOI] [PubMed] [Google Scholar]
- Gomori JM, Grossman RI, Goldberg HI, Zimmerman RA, Bilaniuk LT. Intracranial hematomas: imaging by high field MR. Radiology. 1985;157:87–93. doi: 10.1148/radiology.157.1.4034983. [DOI] [PubMed] [Google Scholar]
- Guadagnino M, Palma V, Tessitore A. Correlation between neuroradiological and electrophysiological investigations in multiple sclerosis with features of a cerebral tumor. Acta Neuopathol (Berl) 1994;16:19–28. [PubMed] [Google Scholar]
- Gupta RK, Jena A, Sharma A, Guha DK, Khushu S, Gupta AK. MR imaging of intracranial tuberculomas. J Comput Assist Tomogr. 1988;12:28–285. doi: 10.1097/00004728-198803000-00017. [DOI] [PubMed] [Google Scholar]
- Gutling E, Landis T. CT ring sign imitating tumour, disclosed as multiple sclerosis by MRI: a case report. J Neurol Neurosurg Psychiatry. 1989;52:903–6. doi: 10.1136/jnnp.52.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimes AB, Zimmerman RD, Morgello S, Weigarten K, Becker RD, Jennis R, et al. MR imaging of brain abscesses. Am J Roentgenol. 1989;152:1073–85. doi: 10.2214/ajr.152.5.1073. [DOI] [PubMed] [Google Scholar]
- Hart M, Earle K. Haemorrhagic and perivenous encephalitis: a clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry. 1975;38:585–91. doi: 10.1136/jnnp.38.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins K, Behrens MM. Homonymous hemianopia in multiple sclerosis with report of bilateral case. Br J Ophthalmol. 1975;59:334–7. doi: 10.1136/bjo.59.6.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kumabe T, Jokura H, Fujihara K, Shiga Y, Watanabe M, et al. Inflammatory demyelinating disease mimicking malignant glioma. J Nucl Med. 2003;44:565–9. [PubMed] [Google Scholar]
- He J, Rgey G, Manon L. Enhancing patterns in multiple sclerosis: evolution and persistence. AJNR Am J Neuroradiol. 2001;22:64–9. [PMC free article] [PubMed] [Google Scholar]
- Hershey LA, Gado MH, Trotter JL. Computerized tomography in the diagnostic evaluation of multiple sclerosis. Ann Neurol. 1979;5:32–9. doi: 10.1002/ana.410050106. [DOI] [PubMed] [Google Scholar]
- Hunter S, Ballinger W, Rubin J. Multiple sclerosis mimicking primary brain tumor. Arch Pathol Lab Med. 1987;111:464–8. [PubMed] [Google Scholar]
- Ikeda K, Kinosita M, Kurihara T. An autopsy case of multiple sclerosis of late onset: with a review of the literature. Neurol Med. 1992;36:589–95. [Google Scholar]
- Iniguez C, Pascuall L, Ramon y Cajal S, Fayed N, Morales-Asin F. Transitional multiple sclerosis (Schilder's disease): a case report. J Neurol. 2000;247:974–6. doi: 10.1007/s004150070059. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Oka H, Utsuki S, Ozawa T, Fujii K. Late-onset multiple sclerosis mimicking brain tumor: a case report. Brain Tumor pathol. 2004;21:83–6. doi: 10.1007/BF02484515. [DOI] [PubMed] [Google Scholar]
- Noseworthy J, Paty D, Wonnacott T, Feasby T, Ebers G, et al. Multiple sclerosis after age 50. Neurology. 1983;33:1537–44. doi: 10.1212/wnl.33.12.1537. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Lavin P, Whetsell WO Fulminant monophasic multiple sclerosis, Marburg's type. J Neurol Neurosurg Psychiatry. 1990;53:918–21. doi: 10.1136/jnnp.53.10.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyan-Raman U, Garwacki D, Elwood P. Demyelinating disease of the corpus callosum presenting as glioma on magnetic resonance scan: a case documented with pathologic findings. Neurosurgery. 1987;21:247–50. doi: 10.1227/00006123-198708000-00024. [DOI] [PubMed] [Google Scholar]
- Kanaha E, Leibowitz U, Alter M. Cerebral multiple sclerosis. Neurology. 1971;21:1179–85. doi: 10.1212/wnl.21.12.1179. [DOI] [PubMed] [Google Scholar]
- Karaarslan E, Altintas A, Senol U, Yeni N, Dincer A, Bayindir C, et al. Balo's concentric sclerosis: clinical and radiologic features of five cases. Am J Neuroradiol. 2001;22:1362–7. [PMC free article] [PubMed] [Google Scholar]
- Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–6. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- Kepes J. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis: a study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- Khoshyomn O, Braff S, Penar P. Tumefactive multiple sclerosis plaque. J Neurol Neurosurg Psychiatry. 2002;73:85. doi: 10.1136/jnnp.73.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Lee SH, Choi C, Huh J, Lee MC. Balo's concentric sclerosis: a clinical case study of brain MRI, biopsy, and proton magnetic resonance spectroscopic findings. J Neurol Neurosurg Psychiatry. 1997;62:655–8. doi: 10.1136/jnnp.62.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotil K, Kalaychi M, Koseoglu T, Tugral A. Myelinoclastic diffuse sclerosis (Schilder's disease): report of a case and review of the literature. Br J Neurosurg. 2002;16:516–9. doi: 10.1080/026886902320909187. [DOI] [PubMed] [Google Scholar]
- Kumar K, Toth C, Jay V. Focal plaque of demyelination mimicking cerebral tumor in a pediatric patient. Pediatr Neurol. 1998;29:60–3. doi: 10.1159/000028690. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Takahashi S, Furuta A, Higano S, Matsumoto K, Tobita M, et al. MR imaging of multiple sclerosis simulating brain tumor. Clin Imaging. 1996;20:171–7. doi: 10.1016/0899-7071(95)00012-7. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Maranhao-Fiho P, Moraes-Filho LC, Salema C. Fulminant form of multiple sclerosis simulating brain tumor: a case with parkinsonian features and pathologic study. Arq Neuropsiquiatr. 1995;53:503–8. doi: 10.1590/s0004-282x1995000300024. [DOI] [PubMed] [Google Scholar]
- Martinelli V, Comi G, Filippi M, Rovaris M, Ghezzi A. Paraclinical tests in acute optic neuritis: basal data and results of short term follow-up. Acta Neurol Scand. 1991;84:231–6. doi: 10.1111/j.1600-0404.1991.tb04944.x. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Moreira J, Trasi S, Visintainer P, Cavaliere R, Grundman M. The open ring. A new imaging sign in demyelinating disease. J Neuroimaging. 1996;6:104–7. doi: 10.1111/jon199662104. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Quinto C, Olivera C, Tenner M, Leslie D, Visintainer P. Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology. 2000;54:1427–33. doi: 10.1212/wnl.54.7.1427. [DOI] [PubMed] [Google Scholar]
- Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology. 2003;61:1373–7. doi: 10.1212/01.wnl.0000094316.90240.eb. [DOI] [PubMed] [Google Scholar]
- McAdam LC, Blaser SI, Banwell B. Pediatric tumefactive demyelination: case series and review of the literature. Pediatr Neurol. 2002;26:18–25. doi: 10.1016/s0887-8994(01)00322-8. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Metafratzi Z, Argyropoulou MI, Tzoufi M, Papdopoulou Z, Efremidis SC. Conventional MRI and magnetization transfer imaging of tumor-like multiple sclerosis in a child. Neuroradiology. 2002;44:97–99. doi: 10.1007/s002340100704. [DOI] [PubMed] [Google Scholar]
- Miller DH, Filippi M, Fazekas F, Frederiksen JL, Matthews PM, Montalban X, et al. Role of magnetic resonance imaging within diagnostic criteria for multiple sclerosis. Ann Neurol. 2004;56:273–8. doi: 10.1002/ana.20156. [DOI] [PubMed] [Google Scholar]
- Miller RC, Lachance DH, Lucchinetti CF, Brown PD, Keegan BM, Bhatia S, et al. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: the Mayo clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:1178–86. doi: 10.1016/j.ijrobp.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Nagao H, Sano N, Habara S, Takahashi M, Matsuda H, et al. A case of multiple sclerosis with multi-ring-like and butterfly-like enhancement on computerized tomography. Brain Dev. 1985;7:43–5. doi: 10.1016/s0387-7604(85)80057-7. [DOI] [PubMed] [Google Scholar]
- Morioka C, Nameta K, Komatsu Y, Tsujio TA, Kondo H. Higher cerebral dysfunction in a case of atypical multiple sclerosis with concentric features. Psychiatry Clin Neurosci. 1996;50:41–4. doi: 10.1111/j.1440-1819.1996.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Nesbit GM, Forbes GS, Scheithauer BW, Okazaki H, Rodriguez M. Multiple sclerosis: histopathologic and MR and/or CT correlation in 37 cases at biopsy and three cases at autopsy. Radiology. 1991;180:467–74. doi: 10.1148/radiology.180.2.2068314. [DOI] [PubMed] [Google Scholar]
- Niebler G, Harris T, Davis T, Roos K. Fulminant multiple sclerosis. AJNR Am J Neuroradiol. 1992;13:1547–51. [PMC free article] [PubMed] [Google Scholar]
- Nyquist PA, Cascino GD, McClelland RL, Annegers J, Rodriguez MI. Incidence of seizures in patients with multiple sclerosis: a population-based study. Mayo Clin Proc. 2002;77:910–2. doi: 10.4065/77.9.910. [DOI] [PubMed] [Google Scholar]
- O’Brien PC. The appropriateness of analysis of variance and multiple comparison procedures. Biometrics. 1983;39:787–94. [PubMed] [Google Scholar]
- Olmos-Lau N, Ginsberg MD, Geller JB. Aphasia in multiple sclerosis. Neurology. 1977;27:623–6. doi: 10.1212/wnl.27.7.623. [DOI] [PubMed] [Google Scholar]
- Omuro A, Leite C, Mokhtari K, Delattre J. Pitfalls in the diagnosis of brain tumors. Lancet Neurol. 2006;5:937–48. doi: 10.1016/S1474-4422(06)70597-X. [DOI] [PubMed] [Google Scholar]
- Paley RJ, Persing JA, Doctor A, Westwater JJ, Roberson JP, Edlich RF. Multiple sclerosis and brain tumor: a diagnostic challenge. J Emerg Med. 1989;7:241–4. doi: 10.1016/0736-4679(89)90353-3. [DOI] [PubMed] [Google Scholar]
- Paolino E, Fainardi E, Ruppi P, Tola MR, Govoni V, Casetta I, et al. A prospective study on the predictive value of CSF oligoclonal bands and MRI in acute isolated neurological syndromes for subsequent progression to multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;60:572–5. doi: 10.1136/jnnp.60.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paty DW, Oger JJ, Kastrukoff LF, Hashimoto SA, Hooge JP, Eisen AA, et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology. 1988;38:180–5. doi: 10.1212/wnl.38.2.180. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. Br Med J. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K, Rosenblum M, Powers J, Alvord E, Walker R, Posner J. Effect of brain irradiation on demyelinating lesions. Neurology. 1993;43:2105–12. doi: 10.1212/wnl.43.10.2105. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, McClelland RL, Achenbach SJ, Konig F, Bitsch A, Bruck W, et al. Clinical course, pathologic correlations and outcome of biopsy proven inflammatory demyelinating disease. J Neurol Neurosurg and Psychiatry. 2005;767:1693–7. doi: 10.1136/jnnp.2004.060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittock SJ, Mayr WT, McClelland RL, Jorgensen NW, Weigand SD, Noseworthy JH, et al. Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology. 2004;62:51–9. doi: 10.1212/01.wnl.0000101724.93433.00. [DOI] [PubMed] [Google Scholar]
- Poser S, Luer W, Bruhn H, Frahm J, Bruck Y, Felgenhauer K. Acute demyelinating disease. Classification and non-invasive diagnosis. Acta Neurol Scand. 1992;86:579–85. doi: 10.1111/j.1600-0404.1992.tb05490.x. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Rieth K, Di Chiro G, Cromwell L. Primary demyelinating disease stimulating glioma of the corpus callosum. Report of three cases. Neurosurgery. 1981;55:620–4. doi: 10.3171/jns.1981.55.4.0620. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP, Heilman KM, Brennan RW. Aphasia as the presenting symptom of multiple sclerosis. Neurology. 1982;32:A:124. [Google Scholar]
- Rolack LA, Beck RW, Paty DW, Tourtellotte NW, Whitaker JN, Rudick RA. Cerebrospinal fluid in acute optic neuritis: experience of the optic neuritis treatment trial. Neurology. 1996;46:368–72. doi: 10.1212/wnl.46.2.368. [DOI] [PubMed] [Google Scholar]
- Rusin JA, Vezina LG, Chadduck WM, Chandra RS. Tumoral multiple sclerosis of the cerebellum in a child. J Neuroradiol. 1995;16:1164–6. [PMC free article] [PubMed] [Google Scholar]
- Sagar H, Warlow C, Sheldon P, Esitiri M. Multiple sclerosis with clinical and radiological features of cerebral tumor. J Neurol Neurosurg Psychiatry. 1982;45:802–8. doi: 10.1136/jnnp.45.9.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky N, Rangell L. On homonymous hemianopia in multiple sclerosis. J Nerve Ment Dis. 1950;111:225–31. [Google Scholar]
- Schwartz K, Erickson BJ, Lucchinetti CF. Pattern of T2 hypointensity associated with ring enhancing brain lesions can help differentiate pathology. Neuroradiology. 2006;48:143–9. doi: 10.1007/s00234-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Thompsen EJ. The predictive value of intrathecal immunoglobulin synthesis and magnetic resonance imaging in acute isolated syndromes for subsequent development of multiple sclerosis. Ann Neurol. 1991;29:147–51. doi: 10.1002/ana.410290206. [DOI] [PubMed] [Google Scholar]
- Stendahl-Brodin L, Link H. Relation between benign course or multiple sclerosis and low-grade humoral immune response in cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1980;43:102–5. doi: 10.1136/jnnp.43.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y, Terasaki M, Shigemori M, Sakata K, Morimatsu M. Acute focal demyelinating disease simulating brain tumors: histopathologic diagnosis for an accurate diagnosis. Neuropathology. 2001;21:25–31. doi: 10.1046/j.1440-1789.2001.00365.x. [DOI] [PubMed] [Google Scholar]
- Tumani H, Tourtellotte WW, Peter JB, Felenhauer K. Acute optic neuritis: combined immunological markers and magnetic resonance imaging predict subsequent development of multiple sclerosis. The Optic Neuritis Study Group. J Neurol Sci. 1998;155:44–9. doi: 10.1016/s0022-510x(97)00272-4. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG. Therapeutic plasma exchange for acute inflammatory demyelinating syndromes of the central nervous system. J Clin Apheresis. 1999;14:144–8. doi: 10.1002/(sici)1098-1101(1999)14:3<144::aid-jca7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Lucchinetti CF. Acute leukoencephalopathies: differential diagnosis and investigation. Neurologist. 1998;4:148–66. [Google Scholar]
- Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- Wingerchuk D, Pittock S, Lennon V, Lucchinetti C, Weinshenker B. Neuromyelitis optica diagnostic criteria revisited: validation and incorporation of the NMO-IgG serum autoantibody. Neurology. 2005;64:A38. [Google Scholar]
- Wurm G, Parsaei B, Silye R, Fellner F. Distinct supratentorial lesions mimicking cerebral gliomas. Acta Neurochir (Wien) 2004;146:19–26. doi: 10.1007/s00701-003-0151-x. [DOI] [PubMed] [Google Scholar]
- Yapici Z, Eraksoy M. Bilateral demyelinating tumefactive lesions in three children with hemiparesis. J Child Neurol. 2002;17:655–60. doi: 10.1177/088307380201700901. [DOI] [PubMed] [Google Scholar]
- Youl BD, Kermode AG, Thompson AJ, Revesz T, Scaravilli F, Barnard RO, et al. Destructive lesions in demyelinating disease. J Neurol Neurosurg Psychiatry. 1991;54:288–92. doi: 10.1136/jnnp.54.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Miller DC, Kelinman GM, Abatic A, Donnenfeld H, Budzilovich GN. Demyelinating disease versus tumor in surgical neuropathology. Clues to a correct pathological diagnosis. Am J Surg Pathol. 1993;17:537–45. doi: 10.1097/00000478-199306000-00001. [DOI] [PubMed] [Google Scholar]
- Zeman AZJ, Kidd D, McLean BN, Kelly MA, Miller DH, Kendall BE, et al. A study of oligoclonal band negative multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;60:27–30. doi: 10.1136/jnnp.60.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]