Abstract
The dysregulation of the insulin-glucose axis represents the crucial event in insulin resistance syndrome. Insulin resistance increases atherogenesis and atherosclerotic plaque instability by inducing proinflammatory activities on vascular and immune cells. This condition characterizes several diseases, such as type 2 diabetes, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), obesity, hypertension, dyslipidemia, and other endocrinopathies, but also cancer. Recent studies suggest that the pathophysiology of insulin resistance is closely related to interferences with insulin-mediated intracellular signaling on skeletal muscle cells, hepatocytes, and adipocytes. Strong evidence supports the role of free fatty acids (FFAs) in promoting insulin resistance. The FFA-induced activation of protein kinase C (PKC) delta, inhibitor kappaB kinase (IKK), or c-Jun N-terminal kinase (JNK) modulates insulin-triggered intracellular pathway (classically known as PI3-K-dependent). Therefore, reduction of FFA levels represents a selective target for modulating insulin resistance.
1. EPIDEMIOLOGY OF INSULIN RESISTANCE
Historically, the sweetness of urine and other body fluids in diabetic patients suggested that glucose had an important role in the physiopathology of this common disease. Thus, glucose metabolism and the insulin-glucose axis were the leading fields for scientific investigations. To emphasize this concept, diabetes was called “mellitus.” Not only hyperglycaemia is crucial for the diagnosis of diabetes and the development of clinical complications [1], but also increasing evidence demonstrated the involvement of insulin in the physiopathology of this disease. In fact, diabetes is a metabolic disease characterized by hyperglycaemia resulting from either defects in insulin secretion or insulin properties, or both. In the present review, we focus on defects of insulin properties, with particular regard to insulin resistance, which can be defined as a state of reduced responsiveness to normal circulating levels of insulin. This condition is a feature of various disorders, such as type 2 diabetes, which may range from predominantly insulin resistance with relative insulin deficiency to a predominatly secretory defect of insulin [1]. Insulin resistance is also implicated in impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), both considered as “prediabetes” by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [2–4], as well as in obesity, hypertension, dyslipidaemia (all disorders clustering in the so-called metabolic syndrome) [5], other endocrinopathies [6], but also in different diseases, such as cancer, infections or rheumatic, and autoimmune diseases [7–11]. Therefore, given the association between insulin resistance and different diseases, no epidemiological data are available, specifically focused on insulin resistance syndrome prevalence or incidence. Furthermore, only recently (in 1997) the WHO have accepted obesity as an epidemic public health burden in adults, without evaluating a well-defined method to monitoring the problem in children [12–15]. Finally, still few clinical studies on Asian and African cohorts focused on insulin resistance and related diseases have been published [16–19]. For all these reasons, to better define insulin resistance epidemiology syndrome requires more investigations. Mechanisms of insulin resistance remain also unknown. Insulin resistance was initially recognized as an “allergy” to insulin, with the production of antibodies anti-insulin [20, 21]. Further investigations showed that metabolism of both nonesterified fatty acids (NEFA) and free fatty acids (FFAs) was a crucial step in the development of insulin resistance [22, 23]. On the basis of these new evidences, Shafrir and Raz suggested in 2003 that diabetes should be now called “lipidus” instead of “mellitus” [24]. Given the importance of physiological effects of insulin during atherogenesis [25–27], there is a need to better clarify the complexity of mechanisms underlying insulin resistance.
2. MECHANISMS OF INSULIN RESISTANCE
Insulin is an anabolic essential hormone for the maintenance of glucose omeostasis, tissue growth, and development [28]. It is well known that insulin is secreted by the pancreatic β cells mainly in response to increased blood levels of glucose and aminoacids after the meals (extrinsic rhythm) [29]. In addition, the concentration of insulin in the blood displays regular variations independently from the food intake [30]. In fact, two rhythms with periods of 5–10 minutes and 60–120 minutes have been documented (intrinsic rhythm) [31–33]. The extrinsic rhythm was found altered in a lot of diseases including gestational diabetes [34], maturity onset diabetes of the young (MODY) 1 [35, 36], MODY 3 [37], and Chagas’ disease [38]. Furthermore, an altered plasma insulin secretory response has been also observed as an effect of aging processes [39]. On the contrary, the intrinsic rhythm has been found altered in various diseases, such as type 2 diabetes (i.e., MODY 2 as well as maternally inherited diabetes and deafness (MIDD)) [40, 41], obesity [42], and hypertension [43]. Several genetic and molecular studies have been performed to investigate the causes of the dysregulated plasma insulin pattern. Although genetic mutations account for a minor role in the large part of insulin resistance, an alteration of insulin signal transduction, which may be due to genetic mutations, could contribute to the impairment of insulin secretory profile and insulin resistance. Thus, mutations of glucokinase phosphorylated glucose, hepatic nuclear factor-4 alpha, hepatic nuclear factor-1 alpha, mitochondrial tRNALeu(UUR), and also insulin receptor genes have been found [35, 37, 44–46]. For instance, a mutation in the insulin receptor gene of the pancreatic β cells has been correlated with a defective insulin-mediated intracellular signal transduction [46]. On the other hand, obesity and increased FFA levels mediate insulin resistance by inducing a decreased IRS-1-associated phosphatidylinositol 3-kinase (PI3-K) activity [47]. In line with these findings, it has been shown that insulin resistance was reversed when obese persons lose weight [48]. However, this weight loss did not restore normal insulin pulsatiliy in Type 2 diabetes patients [49]. These data suggest that the defective insulin-mediated intracellular signal transduction is not the only cause responsible for insulin resistance, and that the molecular mechanisms of insulin resistance are not completely understood. We will discuss in the following the potential mechanisms contributing to insulin resistance which are currently under investigation.
2.1. Defects on insulin signaling
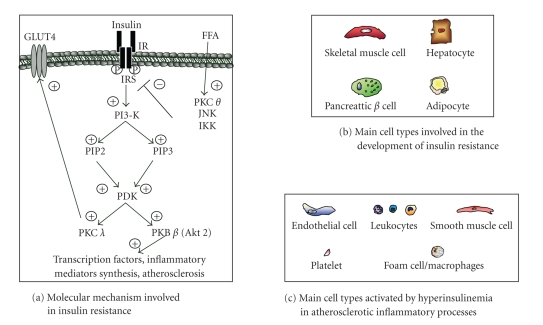
The insulin receptor is considered to play a critical role in insulin resistance. It is a member of the receptor tyrosine kinase family [50], composed of two α-subunits and two β-subunits linked together by disulphide bonds. Two isoforms of insulin receptors are known, exhibiting different affinity for insulin and distribution within tissues. Although it is tempting to suggest that differences in binding activity could contribute to insulin resistance, experimental evidence for the involvement of receptor isoforms or receptor hybrids remains controversial [29]. Apart from the insulin binding step to its receptor, the receptor intracellular tyrosine kinase domains (capable of intrinsic kinase activity) have been investigated in view of their possible implication in insulin resistance. A variety of scaffolding proteins, including insulin receptor substrate (IRS) proteins, casitas B lineage lymphoma (Cbl), or Cbl associated protein (CAP), bind to intracellular receptor sites and become phosphorylated [51–53]. IRS-1 and -2 are considered the most important proteins in regulation of glucose metabolism [54]. As shown in knockout mouse models, IRS-1 or IRS-2 inactivation causes insulin resistance [55, 56]. In addition, in vitro experiments showed an increased serine phosphorylation of IRS by tumor necrosis factor-alpha (TNF-α) or FFA stimulation, thereby causing impaired insulin signal transduction [54, 57]. Finally, a prolonged exposure to insulin (a typical condition in hyperinsulinemic patients) may result in a degradation of IRS protein [58]. All together, these data support IRS-1 and IRS-2 as crucial players in the development of insulin resistance. Furthermore, numerous studies aimed to identify downstream elements of IRS proteins in the insulin-mediated signal transduction pathway. As mentioned above, PI3-K is considered the central mediator [59]. Three different isoforms of this kinase have been identified: Ia, PI3-K/Akt, capable of generating phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3); Ib, G protein regulated kinase; II, incapable of generating PIP2 and PIP3. As shown in Figure 1(a), activated PI3-K is responsible for the beginning of a complex phosphorylation cascade, involving the phospholipids PIP2, PIP3, the phosphoinositide-dependent kinase 1 (PDK1), the protein kinase B (PKB, also called Akt), as well as the protein kinase C (PKC). Akt mediates the effects of insulin on glucose transport (GLUT) [60], glycogen synthesis, protein synthesis, lipogenesis, and suppression of hepatic gluconeogenesis [59]. Once activated, Akt detaches from the plasma membrane and translocates into the nucleus through a still unknown mechanism [61], or activates different substrates, such as glycogen synthase kinase-3 (GSK-3) and transcription factors of the Foxo-family [59]. All these proteins and phospholipids are likely to be implicated in insulin resistance. For instance, a reduced PI3-K activity has been reported in skeletal muscle and adipocytes of patients with insulin resistance [62–64]. In addition, Akt activation has been found reduced in several diseases associated with insulin resistance [65, 66]. However, Akt involvement in insulin resistance is controversial, since other studies did not show any alteration of Akt activation in insulin resistance associated syndromes [67, 68]. On the other hand, insulin-induced PKC activation has been found altered in type 2 diabetic [69] or obese [70] patients. Therefore, although other studies are needed, all these observations suggest that a reduction of insulin-mediated intracellular signaling is crucial for the establishment of insulin resistance.
Figure 1.
Lipid signaling interference generates insulin resistance. (a) Signaling through phosphatidylinositol 3-kinase (PI3-K) is crucial for insulin-mediated glucose transport in hepatocytes and skeletal muscle cells and for inflammatory protein and hormone secretion in adipocytes and pancreatic β cells. Free fatty acids (FFAs) induce a defective insulin-mediated signaling mainly through the activation of protein kinase C (PKC θ), inhibitor κB kinase (IKK) and c-Jun N-terminal kinase (JNK). (b) Main cell types involved in the development of insulin resistance. (c) Main inflammatory cell populations involved in hyperinsulinemia-induced inflammatory states.
Another possible mechanism leading to insulin resistance might be an upregulation of protein-tyrosine phosphatases (PTPases), capable of functioning as negative regulators of the insulin-triggered pathway. Among various proteins, PTP 1B has been shown as a key regulator of insulin signaling [71–73]. Other phosphatases, such as ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1, also known as PC-1), SH-2-containing inositol 5′-phosphatase 2 (SHIP2), and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) have been shown to interfere with insulin sensitivity [74–76]. Although further investigations are warranted to very verify these hypotheses, these proteins may represent future potential targets for the treatment of insulin resistance.
2.2. Glucose metabolism
Glucose uptake into muscle and fat tissue depends on glucose transporter 4 (GLUT4) expression on the cell membrane. Insulin reduces glycaemia, mainly by inducing the secretion of this molecule by muscle and fat cells [77]. However, GLUT4 polymorphisms or mutations inactivating GLUT4 gene were not associated with insulin resistance [78]. In addition, GLUT4 concentrations in skeletal muscle of insulin resistant patients were not reduced [79]. This suggests that alterations in GLUT4 expression are not a primary cause for the development of insulin resistance. In this context, the correct functioning of insulin intracellular signalization appears essential. In fact, GLUT4 upregulation represents the final event of insulin signaling cascade [80–82]. Among various kinases (Figure 1(a)), Aktβ has been shown to play an essential role. Recently, in vitro and in vivo studies suggested that PKBβ alterations [83] or disruption [84] are responsible for the reduction of insulin-induced glucose uptake. Consistent with these data, recent studies in humans detected a missense mutation in the kinase domain of PKBβ (Akt 2) associated with severe insulin resistance [85]. These data suggest that insulin signaling also plays crucial role in the regulation glucose homeostasis.
2.3. Inflammatory molecules
Recent evidence suggests that inflammation might be crucial for the development of insulin resistance [86]. Proinflammatory cytokines and acute-phase reactants are positively correlated with insulin resistance in metabolic syndrome patients [87]. Among these soluble mediators, interleukin (IL)-1 and IL-1 receptor antagonist (RA) have been implicated in the development of insulin resistance in humans [88, 89] and in rodents [90, 91]. IL-1RA is anaturally occurring cytokine and a member of the IL-1 family whose only function is to prevent a biologic response to IL-1 [92]. In humans, the blockade of IL-1 with IL-1 RA improves glycaemia and beta-cell secretory function and reduces markers of systemic inflammation [93]. Accordingly, IL-1 has been shown to induce insulin resistance mainly by inhibiting insulin-mediated signaling [94, 95]. Thus, IL-1 has to be considered as an important factor involved in insulin resistance. TNF-α and IL-6 are also of particular interest, because of their increased expression in adipose tissue and their capacity to induce insulin resistance [96]. Further evidence for the link between TNF-α and insulin resistance was provided by a study using blocking anti-TNF-α antibodies in obese rodents or TNF-α knockout obese mice [97]. In these animals, a reduced insulin resistance was obtained by the suppression of TNF-α. The possible molecular mechanism of TNF-α-induced insulin resistance may involve IRS-1 [98]. Surprisingly, the infusion of anti-TNF-α antibody in humans did not affect insulin resistance. Further investigations are needed to better understand these opposite results obtained in human and mice. On the other hand, the role of IL-6 in insulin resistance is also controversial. IL-6 interferes with the metabolism of both adipose and skeletal muscle tissues [99, 100], but has also a positive effect on skeletal muscle cell insulin sensitivity [101]. In addition, IL-15 has been shown to play a possible role in myocyte-adipocyte crosstalk, but only few studies are published at present to better clarify its role in insulin resistance [102]. Moreover, it is now established that hormones from adipose tissue hormones contribute to insulin resistance. For instance, leptin has been shown to reverse insulin resistance in mice with congenital lipodystrophy [103]. Administration of leptin to patients with lipodystrophy can increase the body fat content and reverse insulin resistance [104]. Resistin, a new adipocyte hormone [105], may be another important link between increased fat mass and insulin resistance [106]. Resistin decreases insulin-dependent glucose transport in vitro and increases fasting blood glucose concentrations and hepatic glucose production in vivo [106–109]. Similarly, the reduction of adiponectin could contribute to insulin resistance. Very recently, adiponectin has been showed as an anti-inflammatory and immunomodulatory molecule [110, 111]. In humans, adiponectin levels correlate with insulin sensitivity. Mice deficient in adiponectin are insulin resistant [112] and the administration of adiponectine to obese and insulin resistant mice has been shown to improve insulin sensitivity [113–115]. In addition, inflammatory mediators such as the proinflammatory chemokine monocyte chemotactic protein-1 (MCP-1) are believed to play a role in the pathogenesis of insulin resistance. Recent in vitro evidence suggests that MCP-1 induces insulin resistance in both adipocytes and skeletal muscle cells [116]. Finally, retinol-binding protein (RBP)-4 and tissue inhibitors of metalloproteinases (TIMP)-1 were recently described to contribute to insulin resistance in vivo, but the underlying mechanism remains unclear [117–119]. In conclusion, at the present state of knowledge, insulin resistance has to be defined as a complex syndrome involving not only glucose and lipids, but also several proinflammatory molecules.
2.4. Lipids and insulin resistance
Lipid abnormalities, such as increased circulating free fatty acids, are frequently associated with insulin resistance [120]. Lipid metabolism induces insulin resistance through a well-known cascade of events. The excessive fat intake causes an increased influx of triglycerides into the blood and an excess of plasma levels of FFAs, which induce insulin resistance, with consequent hyperglycaemia. The increased levels of glucose stimulate pancreatic β cells to secrete more and more insulin, generating hyperinsulinemia, which further triggers the elevation of triglycerides and closes the vicious circle [121]. When insulin secretion is not sufficient and elevated glucose levels prevail, diabetes becomes overt. Defective insulin secretion is a result of chronic exposure to elevated levels of fatty acids, which inhibits insulin gene expression by functioning as true toxic agents for pancreatic β cells [122]. “Lipotoxicity” depends on the interference with insulin-mediated intracellular signaling in various cell types (Figure 1). In particular, FFAs have been shown to activate PKC θ (Figure 1(a)), which not only interferes with insulin signaling (by inducing insulin resistance), but also is implicated in promoting proatherogenic mechanisms, such as endothelial dysfunction, growth, migration, and apoptosis of vascular smooth muscle cells, induction of adhesion molecules and oxidized low-density lipoprotein uptake of oxidized low-density lipoprotein by monocyte-derived macrophages [123]. Furthermore, it was recently shown that FFAs induce insulin resistance in muscle through the activation of inhibitor κB kinase (IKK) and c-Jun N-terminal kinase (JNK) (Figure 1(a)) [124]. Therefore, FFAs induce insulin resistance in hepatocytes and skeletal muscle cells through the activation of different kinases (Figures 1(a) and 1(b)). Both FFAs from plasma and those released from stored triglycerides activate second messangers, which alter insulin signaling [125]. FFAs are also involved in modulating insulin production by pancreatic β-cells and cytokine secretion by hepatocytes, adipocyte, muscle cells, and inflammatory cells (Figure 1(c)). This strongly supports FFA as important proatherosclerotic agents.
3. ROLE OF INSULIN RESISTANCE IN ATHEROSCLEROTIC PLAQUE INSTABILITY
The development of atherosclerotic plaques is dependent on the interaction of multistep biochemical processes that lead to the plaque formation, maturation, and complication [126]. Plaque instability, rupture, and thrombosis are crucial events in the acute artery occlusion, which causes dramatic ischemic consequences in the heart, brain, and also peripheral tissues. Insulin resistance is considered to be a pivotal event in the increased risk of plaque instability through different pathways [127, 128]. High concentrations of insulin directly increase proinflammatory activities of leukocytes, which are involved in atherosclerotic plaque instability. In particular, insulin directly increases neutrophil and monocyte in vitro migration in response to chemokines secreted in atherosclerotic plaques [129, 130]. Insulin could also favor atherosclerotic plaque necrosis by accelerating macrophage death [131]. Furthermore, insulin induces in vivo production of matrix metalloproteinase-9 (MMP-9), which is responsible for plaque instability and rupture [132–134]. The pharmacologic or behavioral treatments to reduce insulin resistance have been shown to inhibit MMP-9 secretion [126, 135, 136]. On the other hand, insulin could also induce a serious atherothrombotic state, by increasing platelet resistance to antiaggregating agents [137] and production of procoagulatory factors, such as plasminogen activator inhibitor-1 (PAI-1), factor VII, factor XII, fibrinogen, and tissue plasminogen activator [126]. These evidences strongly support an emerging role of insulin in plaque instability and rupture. Further investigations are needed to better clarify the specific roles and the interactions of insulin and lipids on inflammatory cells.
4. CONCLUSION REMARKS
In the last years, the standard definition of insulin resistance has been shifted from a traditional “glucocentric” to a new “lipocentric” view [138]. Several soluble mediators are involved in the development of insulin resistance, through generating insulin signaling dysfunction. Among these, FFAs have to be considered as proatherosclerotic agents, capable of interfering with insulin signaling and provoking insulin resistance. New and selective therapies contrasting FFA effects may be promising targets for the treatment of insulin resistance. A possible promising strategy capable of reducing the consequences of excessive lipolysis and reorient FFA flux toward adipose tissue might be represented by peroxisome proliferator-activated receptor (PPAR)-α and -γ agonists [139]. PPAR-γ agonists have been recently shown to regulate trygliceride lipase in adipocytes in vitro and in vivo [140]. Furthermore, PPAR-γ has been shown as crucial in the control of differentiation of human monocytes in M2 macrophages, the subset of macrophages resident in atherosclerotic plaques with anti-inflammatory activity [141]. In vivo studies have also demonstrated that PPAR-γ agonists treatment in patients with type 2 diabetes mellitus is associated with a reduction in plasma NEFA levels [142–145]. However, two recent important clinical studies have shown an increase of acute cardiovascular outcomes induced by treatment with thiazolidinediones (PPAR-γ agonists) [146, 147]. On the other hand, although PPAR-α has been shown to have vascular and metabolic beneficial effects, the activity of PPAR-α agonists on lipid metabolism is still controversial [148, 149]. Therefore, further trials are needed to recommend the use of these pharmacological agents for reducing lipid-mediated insulin resistance.
ACKNOWLEDGMENTS
This work was supported by grants from the Swiss National Science Foundation to Dr F. Mach (Grant no. 320080-105836) and Dr S. Steffens (Grant no. 310000-116324), and Foundation for Medical Research (Geneva). The authors belong to the European Vascular Genomics Network (http://www.evgn.org/) a Network of Excellence supported by the European Community.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(supplement 1):S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 2.Gavin JR, III, Alberti KGMM, Davidson MB, et al. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 3.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 4.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. The American Journal of Medicine. 2007;120(3, supplement 1):S12–S18. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 6.Caprio S, Boulware D, Tamborlane V. Growth hormone and insulin interactions. Hormone Research. 1992;38(supplement 2):47–49. doi: 10.1159/000182594. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American Journal of Clinical Nutrition. 2007;86(3):836S–842S. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin PJ, Ennis M, Bahl M, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. doi: 10.1007/s10549-008-0019-0. Breast Cancer Research and Treatment. In press. [DOI] [PubMed] [Google Scholar]
- 9.Bell DS. Inflammation, insulin resistance, infection, diabetes, and atherosclerosis. Endocrine Practice. 2000;6(3):272–276. doi: 10.4158/EP.6.3.272. [DOI] [PubMed] [Google Scholar]
- 10.Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-α treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. The Journal of Rheumatology. 2008;35(2):355–357. [PubMed] [Google Scholar]
- 11.Oguz A, Dogan EG, Uzunlulu M, Oguz FM. Insulin resistance and adiponectin levels in Behçet's syndrome. Clinical and Experimental Rheumatology. 2007;25(4, supplement 45):S118–S119. [PubMed] [Google Scholar]
- 12.Sarafidis PA, Bakris GL. Insulin resistance, hyperinsulinemia, and hypertension: an epidemiologic approach. Journal of the CardioMetabolic Syndrome. 2006;1(5):334–344. doi: 10.1111/j.1559-4564.2006.05795.x. [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Medica. 2005;47(4):201–210. [PubMed] [Google Scholar]
- 14.James WPT. The epidemiology of obesity: the size of the problem. Journal of Internal Medicine. 2008;263(4):336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 15.Sabin MA, Shield JP. Childhood obesity. Frontiers of Hormone Research. 2008;36:85–96. doi: 10.1159/000115356. [DOI] [PubMed] [Google Scholar]
- 16.Enas EA, Mohammedan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. Journal of the CardioMetabolic Syndrome. 2007;2(4):267–275. doi: 10.1111/j.1559-4564.2007.07392.x. [DOI] [PubMed] [Google Scholar]
- 17.Schuster DP, Gaillard T, Osei K. The cardiometabolic syndrome in persons of the African diaspora: challenges and opportunities. Journal of the CardioMetabolic Syndrome. 2007;2(4):260–266. doi: 10.1111/j.1559-4564.2007.07484.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoang KC, Le TV, Wong ND. The metabolic syndrome in East Asians. Journal of the CardioMetabolic Syndrome. 2007;2(4):276–282. doi: 10.1111/j.1559-4564.2007.07491.x. [DOI] [PubMed] [Google Scholar]
- 19.Reimann M, Schutte AE, Schwarz PEH. Insulin resistance—the role of ethnicity: evidence from Caucasian and African cohorts. Hormone and Metabolic Research. 2007;39(12):853–857. doi: 10.1055/s-2007-993152. [DOI] [PubMed] [Google Scholar]
- 20.Lowell FC. Immunologic studies in insulin resistance I. Report of a case exhibiting variations in resistance and allergy to insulin. The Journal for Clinical Investigation. 1944;23(2):225–231. doi: 10.1172/JCI101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman WB. A case of coexisting insulin allergy and insulin resistance. Journal of Allergy. 1950;21(1):49–54. doi: 10.1016/0021-8707(50)90033-5. [DOI] [PubMed] [Google Scholar]
- 22.Krebs M, Krssak M, Nowotny P, et al. Free fatty acids inhibit the glucose-stimulated increase of intramuscular glucose-6-phosphate concentration in humans. The Journal of Clinical Endocrinology & Metabolism. 2001;86(5):2153–2160. doi: 10.1210/jcem.86.5.7488. [DOI] [PubMed] [Google Scholar]
- 23.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty acids cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. The Lancet. 1963;281(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 24.Shafrir E, Raz I. Diabetes: mellitus or lipidus? Diabetologia. 2003;46(3):433–440. doi: 10.1007/s00125-003-1052-5. [DOI] [PubMed] [Google Scholar]
- 25.Cameron JD, Cruickshank JK. Glucose, insulin, diabetes and mechanisms of arterial dysfunction. Clinical and Experimental Pharmacology and Physiology. 2007;34(7):677–682. doi: 10.1111/j.1440-1681.2007.04659.x. [DOI] [PubMed] [Google Scholar]
- 26.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Current Opinion in Lipidology. 2007;18(3):263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 27.Kashyap SR, DeFronzo RA. The insulin resistance syndrome: physiological considerations. Diabetes & Vascular Disease Research. 2007;4(1):13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- 28.Kones RJ, Phillips JH. Insulin: fundamental mechanism of action and the heart. Cardiology. 1975;60(5):280–303. doi: 10.1159/000169727. [DOI] [PubMed] [Google Scholar]
- 29.Sesti G. Pathophysiology of insulin resistance. Best Practice & Research in Clinical Endocrinology & Metabolism. 2006;20(4):665–679. doi: 10.1016/j.beem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Bergsten P. Pathophysiology of impaired pulsatile insulin release. Diabetes/Metabolism Research and Reviews. 2000;16(3):179–191. doi: 10.1002/1520-7560(200005/06)16:3<179::aid-dmrr115>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Goodner CJ, Walike BC, Koerker DJ, et al. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science. 1977;195(4274):177–179. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- 32.Simon C, Brandenberger G, Follenius M. Ultradian oscillations of plasma glucose, insulin, and C-peptide in man during continuous enteral nutrition. The Journal of Clinical Endocrinology & Metabolism. 1987;64(4):669–674. doi: 10.1210/jcem-64-4-669. [DOI] [PubMed] [Google Scholar]
- 33.Polonsky KS, Sturis J, Van Cauter E. Temporal profiles and clinical significance of pulsatile insulin secretion. Hormone Research. 1998;49(3-4):178–184. doi: 10.1159/000023168. [DOI] [PubMed] [Google Scholar]
- 34.Ryan EA, Imes S, Liu D, et al. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes. 1995;44(5):506–512. doi: 10.2337/diab.44.5.506. [DOI] [PubMed] [Google Scholar]
- 35.Herman WH, Fajans SS, Smith MJ, Polonsky KS, Bell GI, Halter JB. Diminished insulin and glucagon secretory responses to arginine in nondiabetic subjects with a mutation in the hepatocyte nuclear factor- 4α/MODY1 gene. Diabetes. 1997;46(11):1749–1754. doi: 10.2337/diab.46.11.1749. [DOI] [PubMed] [Google Scholar]
- 36.Hani EH, Suaud L, Boutin P, et al. A missense mutation in hepatocyte nuclear factor-4α, resulting in a reduced transactivation activity, in human late-onset non-insulin-dependent diabetes mellitus. The Journal of Clinical Investigation. 1998;101(3):521–526. doi: 10.1172/JCI1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surmely J-F, Guenat E, Philippe J, et al. Glucose utilization and production in patients with maturity-onset diabetes of the young caused by a mutation of the hepatocyte nuclear factor-1α gene. Diabetes. 1998;47(9):1459–1463. doi: 10.2337/diabetes.47.9.1459. [DOI] [PubMed] [Google Scholar]
- 38.Long RG, Albuquerque RH, Prata A, et al. Response of plasma pancreatic and gastrointestinal hormones and growth hormone to oral and intravenous glucose and insulin hypoglycaemia in Chagas's disease. Gut. 1980;21(9):772–777. doi: 10.1136/gut.21.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samos LF, Roos BA. Diabetes mellitus in older persons. Medical Clinics of North America. 1998;82(4):791–803. doi: 10.1016/s0025-7125(05)70024-9. [DOI] [PubMed] [Google Scholar]
- 40.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30(5):435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 41.O'Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. The New England Journal of Medicine. 1988;318(19):1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 42.Van Cauter E, Polonsky KS, Blackman JD, et al. Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity. The Journal of Clinical Endocrinology & Metabolism. 1994;79(6):1797–1805. doi: 10.1210/jcem.79.6.7989487. [DOI] [PubMed] [Google Scholar]
- 43.Andersen UB, Dige-Petersen H, Frandsen EK, Ibsen H, Vølund A. Basal insulin-level oscillations in normotensive individuals with genetic predisposition to essential hypertension exhibit an irregular pattern. Journal of Hypertension. 1997;15(10):1167–1173. doi: 10.1097/00004872-199715100-00015. [DOI] [PubMed] [Google Scholar]
- 44.van den Ouweland JMW, Maechler P, Wollheim CB, Attardi G, Maassen JA. Functional and morphological abnormalities of mitochondria harbouring the tRNA(Leu(UUR)) mutation in mitochondrial DNA derived from patients with maternally inherited diabetes and deafness (MIDD) and progressive kidney disease. Diabetologia. 1999;42(4):485–492. doi: 10.1007/s001250051183. [DOI] [PubMed] [Google Scholar]
- 45.Sakura H, Ashcroft SJH, Terauchi Y, Kadowaki T, Ashcroft FM. Glucose modulation of ATP-sensitive K-currents in wild-type, homozygous and heterozygous glucokinase knock-out mice. Diabetologia. 1998;41(6):654–659. doi: 10.1007/s001250050964. [DOI] [PubMed] [Google Scholar]
- 46.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 47.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. The Journal of Clinical Investigation. 1999;103(2):253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews DR. Oscillatory insulin secretion: a variable phenotypic marker. Diabetic Medicine. 1996;13(9, supplement 6):S53–S58. [PubMed] [Google Scholar]
- 49.Gumbiner B, Van Cauter E, Beltz WF, et al. Abnormalities of insulin pulsatility and glucose oscillations during meals in obese noninsulin-dependent diabetic patients: effects of weight reduction. The Journal of Clinical Endocrinology & Metabolism. 1996;81(6):2061–2068. doi: 10.1210/jcem.81.6.8964829. [DOI] [PubMed] [Google Scholar]
- 50.Ullrich A, Bell JR, Chen EY, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 51.Baumann CA, Ribon V, Kanzaki M, et al. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407(6801):202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 52.Noguchi T, Matozaki T, Inagaki K, et al. Tyrosine phosphorylation of p62Dok induced by cell adhesion and insulin: possible role in cell migration. The EMBO Journal. 1999;18(7):1748–1760. doi: 10.1093/emboj/18.7.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278(5346):2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 54.White MF. IRS proteins and the common path to diabetes. American Journal of Physiology. 2002;283(3):E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 55.Whitehead JP, Humphreys P, Krook A, et al. Molecular scanning of the insulin receptor substrate 1 gene in subjects with severe insulin resistance: detection and functional analysis of a naturally occurring mutation in a YMXM motif. Diabetes. 1998;47(5):837–839. doi: 10.2337/diabetes.47.5.837. [DOI] [PubMed] [Google Scholar]
- 56.Previs SF, Withers DJ, Ren J-M, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. Journal of Biological Chemistry. 2000;275(50):38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 57.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of glucose uptake by hyperosmotic stress. Diabetes & Metabolism. 2003;29(6):566–575. doi: 10.1016/s1262-3636(07)70071-x. [DOI] [PubMed] [Google Scholar]
- 58.Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. Journal of Biological Chemistry. 2001;276(43):40362–40367. doi: 10.1074/jbc.M105332200. [DOI] [PubMed] [Google Scholar]
- 59.Schinner S, Scherbaum WA, Bornstein SR, Barthel A. Molecular mechanisms of insulin resistance. Diabetic Medicine. 2005;22(6):674–682. doi: 10.1111/j.1464-5491.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 60.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Current Opinion in Genetics & Development. 1998;8(1):55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 61.Meier R, Alessi DR, Cron P, Andjelković M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ . Journal of Biological Chemistry. 1997;272(48):30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 62.Brozinick JT, Jr., Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 2003;52(4):935–941. doi: 10.2337/diabetes.52.4.935. [DOI] [PubMed] [Google Scholar]
- 63.Björnholm M, Kawano Y, Lehtihet M, Zierath JR. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes. 1997;46(3):524–527. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- 64.Smith U, Axelsen M, Carvalho E, Eliasson B, Jansson P-A, Wesslau C. Insulin signaling and action in fat cells: associations with insulin resistance and type 2 diabetes. Annals of the New York Academy of Sciences. 1999;892(1):119–126. doi: 10.1111/j.1749-6632.1999.tb07790.x. [DOI] [PubMed] [Google Scholar]
- 65.Kim Y-B, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. The Journal of Clinical Investigation. 1999;104(6):733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47(8):1281–1286. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- 67.Krook A, Björnholm M, Galuska D, et al. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49(2):284–292. doi: 10.2337/diabetes.49.2.284. [DOI] [PubMed] [Google Scholar]
- 68.Beeson M, Sajan MP, Dizon M, et al. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52(8):1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y-B, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase Cλ/ζ activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52(8):1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- 70.Sajan MP, Standaert ML, Miura A, et al. Impaired activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 in cultured preadipocyte-derived adipocytes and myotubes of obese subjects. The Journal of Clinical Endocrinology & Metabolism. 2004;89(8):3994–3998. doi: 10.1210/jc.2004-0106. [DOI] [PubMed] [Google Scholar]
- 71.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 72.Zinker BA, Rondinone CM, Trevillyan JM, et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11357–11362. doi: 10.1073/pnas.142298199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmad F, Considine RV, Bauer TL, Ohannesian JP, Marco CC, Goldstein BJ. Improved sensitivity to insulin in obese subjects following weight loss is accompanied by reduced protein-tyrosine phosphatases in adipose tissue. Metabolism. 1997;46(10):1140–1145. doi: 10.1016/s0026-0495(97)90206-7. [DOI] [PubMed] [Google Scholar]
- 74.Youngren JF, Maddux BA, Sasson S, et al. Skeletal muscle content of membrane glycoprotein PC-1 in obesity. Relationship to muscle glucose transport. Diabetes. 1996;45(10):1324–1328. doi: 10.2337/diab.45.10.1324. [DOI] [PubMed] [Google Scholar]
- 75.Clément S, Krause U, Desmedt F, et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 2001;409(6816):92–97. doi: 10.1038/35051094. erratum in Nature, vol. 431, no. 7010, p. 878, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Butler M, McKay RA, Popoff IJ, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51(4):1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 77.Zierath JR, He L, Gumà A, Odegaard Wahlström E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39(10):1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]
- 78.Buse JB, Yasuda K, Lay TP, et al. Human GLUT4/muscle-fat glucose-transporter gene: characterization and genetic variation. Diabetes. 1992;41(11):1436–1445. doi: 10.2337/diab.41.11.1436. [DOI] [PubMed] [Google Scholar]
- 79.Shepherd PR, Kahn BB. Glucose transporters and insulin action: implications for insulin resistance and diabetes mellitus. The New England Journal of Medicine. 1999;341(4):248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 80.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Molecular and Cellular Biology. 1994;14(7):4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Q, Somwar R, Bilan PJ, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Molecular and Cellular Biology. 1999;19(6):4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bandyopadhyay G, Standaert ML, Sajan MP, et al. Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase C-ζ . Molecular Endocrinology. 1999;13(10):1766–1772. doi: 10.1210/mend.13.10.0364. [DOI] [PubMed] [Google Scholar]
- 83.Brozinick JT, Jr., Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 2003;52(4):935–941. doi: 10.2337/diabetes.52.4.935. [DOI] [PubMed] [Google Scholar]
- 84.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 85.George S, Rochford JJ, Wolfrum C, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2 . Science. 2004;304(5675):1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 87.Hotamisligil GS. Inflammatory pathways and insulin action. International Journal of Obesity. 2003;27(supplement 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 88.Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Letters. 2006;580(27):6289–6294. doi: 10.1016/j.febslet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 89.Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti J-F. Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148(1):241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Somm E, Cettour-Rose P, Asensio C, et al. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia. 2006;49(2):387–393. doi: 10.1007/s00125-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 91.Lagathu C, Yvan-Charvet L, Bastard J-P, et al. Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49(9):2162–2173. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- 92.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. The New England Journal of Medicine. 2000;343(10):732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 93.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England Journal of Medicine. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 94.He J, Usui I, Ishizuka K, et al. Interleukin-1α inhibits insulin signaling with phosphorylating insulin receptor substrate-1 on serine residues in 3T3-L1 adipocytes. Molecular Endocrinology. 2006;20(1):114–124. doi: 10.1210/me.2005-0107. [DOI] [PubMed] [Google Scholar]
- 95.Kim J-A, Yeh DC, Ver M, et al. Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by Mouse Pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. Journal of Biological Chemistry. 2005;280(24):23173–23183. doi: 10.1074/jbc.M501439200. [DOI] [PubMed] [Google Scholar]
- 96.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 97.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 98.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor α-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. Journal of Biological Chemistry. 1995;270(40):23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 99.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. American Journal of Physiology. 2002;283(6):E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 100.van Hall G, Steensberg A, Sacchetti M, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. The Journal of Clinical Endocrinology & Metabolism. 2003;88(7):3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- 101.Weigert C, Hennige AM, Brodbeck K, Häring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. American Journal of Physiology. 2005;289(2):E251–E257. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- 102.Quinn LS, Strait-Bodey L, Anderson BG, Argilés JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biology International. 2005;29(6):449–457. doi: 10.1016/j.cellbi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 104.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. The New England Journal of Medicine. 2002;346(8):570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 105.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 106.Shuldiner AR, Yang R, Gong D-W. Resistin, obesity, and insulin resistance—the emerging role of the adipocyte as an endocrine organ. The New England Journal of Medicine. 2001;345(18):1345–1346. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 107.Moon B, Kwan JJ-M, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. American Journal of Physiology. 2003;285(1):E106–E115. doi: 10.1152/ajpendo.00457.2002. [DOI] [PubMed] [Google Scholar]
- 108.Pravenec M, Kazdová L, Landa V, et al. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. Journal of Biological Chemistry. 2003;278(46):45209–45215. doi: 10.1074/jbc.M304869200. [DOI] [PubMed] [Google Scholar]
- 109.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-β selectively impair insulin action on glucose production. The Journal of Clinical Investigation. 2003;111(2):225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okamoto Y, Folco EJ, Minami M, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circulation Research. 2008;102(2):218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 111.Steffens S, Mach F. Adiponectin and adaptive immunity: linking the bridge from obesity to atherogenesis. Circulation Research. 2008;102(2):140–142. doi: 10.1161/CIRCRESAHA.107.170274. [DOI] [PubMed] [Google Scholar]
- 112.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. Journal of Biological Chemistry. 2002;277(29):25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 113.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature Medicine. 2002;8(7):731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 114.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein ACRP30 enhances hepatic insulin action. Nature Medicine. 2001;7(8):947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 115.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 116.Sell H, Eckel J. Monocyte chemotactic protein-1 and its role in insulin resistance. Current Opinion in Lipidology. 2007;18(3):258–262. doi: 10.1097/MOL.0b013e3281338546. [DOI] [PubMed] [Google Scholar]
- 117.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 118.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. The New England Journal of Medicine. 2006;354(24):2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 119.Kralisch S, Klein J, Lossner U, et al. Proinflammatory adipocytokines induce TIMP-1 expression in 3T3-L1 adipocytes. FEBS Letters. 2005;579(28):6417–6422. doi: 10.1016/j.febslet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 120.Holland WL, Knotts TA, Chavez JA, Wang L-P, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutrition Reviews. 2007;65(supplement 1):39–46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 121.Kraegen EW, Cooney GJ, Ye J, Thompson AL. Triglycerides, fatty acids and insulin resistance—hyperinsulinemia. Experimental and Clinical Endocrinology and Diabetes. 2001;109(4):516–526. doi: 10.1055/s-2001-15114. [DOI] [PubMed] [Google Scholar]
- 122.Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. Journal of Nutrition. 2006;136(4):873–876. doi: 10.1093/jn/136.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(3):487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 124.Gao Z, Zhang X, Zuberi A, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Molecular Endocrinology. 2004;18(8):2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 125.Kovacs P, Stumvoll M. Fatty acids and insulin resistance in muscle and liver. Best Practice & Research in Clinical Endocrinology & Metabolism. 2005;19(4):625–635. doi: 10.1016/j.beem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 126.Grant PJ. The genetics of atherothrombotic disorders: a clinician's view. Journal of Thrombosis and Haemostasis. 2003;1(7):1381–1390. doi: 10.1046/j.1538-7836.2003.00276.x. [DOI] [PubMed] [Google Scholar]
- 127.Amar J, Perez L, Burcelin R, Chamontin B. Arteries, inflammation and insulin resistance. Journal of Hypertension. 2006;24(supplement 5):S18–S20. doi: 10.1097/01.hjh.0000240042.50838.61. [DOI] [PubMed] [Google Scholar]
- 128.Theuma P, Fonseca VA. Inflammation, insulin resistance, and atherosclerosis. Metabolic Syndrome and Related Disorders. 2004;2(2):105–113. doi: 10.1089/met.2004.2.105. [DOI] [PubMed] [Google Scholar]
- 129.Montecucco F, Bianchi G, Bertolotto M, Viviani G, Dallegri F, Ottonello L. Insulin primes human neutrophils for CCL3-induced migration: crucial role for JNK 1/2. Annals of the New York Academy of Sciences. 2006;1090:399–407. doi: 10.1196/annals.1378.043. [DOI] [PubMed] [Google Scholar]
- 130.Kappert K, Meyborg H, Clemenz M, et al. Insulin facilitates monocyte migration: a possible link to tissue inflammation in insulin-resistance. Biochemical and Biophysical Research Communications. 2008;365(3):503–508. doi: 10.1016/j.bbrc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 131.Tabas I, Seimon T, Arellano J, et al. The impact of insulin resistance on macrophage death pathways in advanced atherosclerosis. Novartis Foundation Symposium. 2007;286:99–109. doi: 10.1002/9780470985571.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roberts CK, Won D, Pruthi S, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. Journal of Applied Physiology. 2006;100(5):1657–1665. doi: 10.1152/japplphysiol.01292.2005. [DOI] [PubMed] [Google Scholar]
- 133.Głowińska-Olszewska B, Urban M. Elevated matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in obese children and adolescents. Metabolism. 2007;56(6):799–805. doi: 10.1016/j.metabol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 134.Boden G, Song W, Pashko L, Kresge K. In vivo effects of insulin and free fatty acids on matrix metalloproteinases in rat aorta. Diabetes. 2008;57(2):476–483. doi: 10.2337/db07-1261. [DOI] [PubMed] [Google Scholar]
- 135.Ceriello A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes/Metabolism Research and Reviews. 2008;24(1):14–26. doi: 10.1002/dmrr.790. [DOI] [PubMed] [Google Scholar]
- 136.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106(6):679–684. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 137.Anfossi G, Russo I, Trovati M. Platelet resistance to the anti-aggregating agents in the insulin resistant states. Current Diabetes Reviews. 2006;2(4):409–430. doi: 10.2174/1573399810602040409. [DOI] [PubMed] [Google Scholar]
- 138.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 139.Leroyer SN, Tordjman J, Chauvet G, et al. Rosiglitazone controls fatty acid cycling in human adipose tissue by means of glyceroneogenesis and glycerol phosphorylation. Journal of Biological Chemistry. 2006;281(19):13141–13149. doi: 10.1074/jbc.M512943200. [DOI] [PubMed] [Google Scholar]
- 140.Kershaw EE, Schupp M, Guan H-P, Gardner NP, Lazar MA, Flier JS. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. American Journal of Physiology. 2007;293(6):E1736–E1745. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Charo IF. Macrophage polarization and insulin resistance: PPARγ in control. Cell Metabolism. 2007;6(2):96–98. doi: 10.1016/j.cmet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 142.Bajaj M, Suraamornkul S, Pratipanawatr T, et al. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52(6):1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 143.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. The Journal of Clinical Endocrinology & Metabolism. 2002;87(6):2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 144.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51(3):797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tiikkainen M, Häkkinen A-M, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53(8):2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 146.Lipscombe LL, Gomes T, Lévesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. Journal of the American Medical Association. 2007;298(22):2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 147.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England Journal of Medicine. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 148.Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia. 2007;50(8):1723–1731. doi: 10.1007/s00125-007-0698-9. [DOI] [PubMed] [Google Scholar]
- 149.Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor-α activators. Hypertension. 2005;46(5):1086–1092. doi: 10.1161/01.HYP.0000187900.36455.4c. [DOI] [PubMed] [Google Scholar]