Abstract
Addictive drugs can profoundly affect social behaviour both acutely and in the long-term. Effects range from the artificial sociability imbued by various intoxicating agents to the depressed and socially withdrawn state frequently observed in chronic drug users. Understanding such effects is of great potential significance in addiction neurobiology. In this review we focus on the ‘social neuropeptide' oxytocin and its possible role in acute and long-term effects of commonly used drugs. Oxytocin regulates social affiliation and social recognition in many species and modulates anxiety, mood and aggression. Recent evidence suggests that popular party drugs such as MDMA and gamma-hydroxybutyrate (GHB) may preferentially activate brain oxytocin systems to produce their characteristic prosocial and prosexual effects. Oxytocin interacts with the mesolimbic dopamine system to facilitate sexual and social behaviour, and this oxytocin-dopamine interaction may also influence the acquisition and expression of drug-seeking behaviour. An increasing body of evidence from animal models suggests that even brief exposure to drugs such as MDMA, cannabinoids, methamphetamine and phencyclidine can cause long lasting deficits in social behaviour. We discuss preliminary evidence that these adverse effects may reflect long-term neuroadaptations in brain oxytocin systems. Laboratory studies and preliminary clinical studies also indicate that raising brain oxytocin levels may ameliorate acute drug withdrawal symptoms. It is concluded that oxytocin may play an important, yet largely unexplored, role in drug addiction. Greater understanding of this role may ultimately lead to novel therapeutics for addiction that can improve mood and facilitate the recovery of persons with drug use disorders.
Keywords: addiction, antisocial, anxiety, cannabinoid, GHB, MDMA, methamphetamine, oxytocin, social, vasopressin
Introduction
The social causes and social consequences of drug use, and their underlying neural correlates, are important but as yet understudied areas in the study of addiction. Even the most cursory reflection on human drug use suggests that the motivation to consume drugs is inextricably linked to the social context. Obvious examples are the widespread use of alcohol for ‘social lubrication', the popular use of ‘party drugs' such as 3,4-methylenedioxymethamphetamine (MDMA) (Ecstasy) and gamma-hydroxybutyrate (GHB) for their prosocial and prosexual effects, and drug initiation in adolescence as a result of ‘peer pressure'. Drug use may even define social groups, with the psychedelic movement of the 1960s and rave phenomenon of the late 1980s and 1990s seeing entire subcultures identified by their drug choice and drug-induced behaviours.
The adverse consequences of drug use are often expressed in terms of costs to society; encapsulating the idea that repeated drug use entails profound social costs. Familiar examples include the random violence inflicted by intoxicated pub patrons, the aggressive psychosis and paranoia seen in heavy crystal methamphetamine users, the poor parenting of drug addicted parents and the disintegrating social life and resulting isolation of compulsive drug users. Some 80% of prisoners have a history of drug abuse and most are incarcerated for antisocial acts related to or committed under the influence of licit or illicit drugs (see for example, Dolan et al., 2007). Most people detained by police test positive for one or more drugs of abuse in the 24 h following arrest (Mouzos et al., 2006). Moreover, psychiatric diagnoses of social dysfunction such as borderline personality disorder and antisocial personality disorder are over-represented in drug abusing populations (Brady et al., 2007; van den Bosch and Verheul, 2007).
Untangling the genetic, environmental, epigenetic, pharmacological and neural determinants of social breakdown consequent to drug use is a major undertaking in addiction neurobiology and a task that has barely started. Part of the problem is that the neural and genetic determinants of social behaviour remain obscure, with the fledgling area of ‘social neuroscience' having only recently emerged (for example, Caldu and Dreher, 2007; Sanfey, 2007). Another problem has been that animal models of drug addiction, which are the cornerstone of basic research in addiction neurobiology, rarely take social factors into account. Experiments typically involve rats or mice self-administering a drug under solitary conditions and frequently such animals are housed individually to prevent damage to chronically indwelling catheters or to allow clear assessment of individual drug or alcohol intake. Nonetheless, occasional preclinical studies of social factors in drug abuse make clear the social implications of acute intoxication (for example, Miczek et al., 2004; Pedraza et al., 2007; Thompson et al., 2007), and the adverse social consequences of repeated drug exposure (for example, Morgan et al., 2002; Clemens et al., 2007).
In the present review, we review the general ideas that (1) commonly used drugs of abuse exert major acute effects on sociability, (2) repetitive drug use may cause lasting adverse neuroadaptations in key neural substrates subserving social behaviour and (3) both of the above processes may involve the ‘social' neuropeptide oxytocin (OT).
Oxytocin: the social neuropeptide
Oxytocin is the most abundant neuropeptide in the hypothalamus and is best known in mammalian species for the characteristic peripheral effects that it promotes, including uterine contractions during parturition and milk ejection during lactation (for review see Gimpl and Fahrenholz, 2001). The neurosecretory cells of the supraoptic nucleus (SON) and paraventricular nucleus of the hypothalamus (PVN) are the main sites in the brain in which OT is synthesized, and these cells release OT and the closely related neuropeptide arginine vasopressin (AVP), via the posterior pituitary into the bloodstream to produce peripheral actions (see Figure 1). Independently of this, the SON and PVN also release OT and AVP from their dendrites and cell bodies and this OT diffuses locally within the hypothalamus, and presumably over much larger distances to influence a wide network of central OT receptors (Landgraf and Neumann, 2004; Ludwig and Leng, 2006; Neumann, 2007). Limbic and brainstem regions also receive direct innervation of OT and AVP positive fibres from the parvocellular PVN. Local release of OT has been documented in sites such as the amygdala, septum and bed nucleus, particularly in relation to stress and defensive behaviours (see for example, Ebner et al., 2005) and such OT release may depend upon such direct projections from the PVN or perhaps as yet undocumented extrahypothalamic OT-containing cell bodies in these regions (Neumann, 2007). OT receptors are to be found in many sites that are relevant to drug seeking behaviour, including the nucleus accumbens, ventral tegmental area, bed nucleus of the stria terminalis, central amygdala, medial amygdala, hippocampus and ventral pallidum (Vaccari et al., 1998).
Figure 1.
Schematic showing the possible interaction between central oxytocin release and addiction-relevant brain regions. (a) depicts dendritic release of oxytocin from the SON and its interaction with distal oxytocin receptor containing brain regions (volume transmission). (b) indicates both dendritic release of oxytocin from magnocellular PVN neurons and/or classical neurotransmission from PVN parvocellular oxytocinergic projections to forebrain, midbrain and spinal regions. Prosocial drugs (that is MDMA) release 5-HT via raphefugal fibres within PVN and SON and cause oxytocin release while oxytocinergic projections from the PVN to the VTA and NAS may modulate mesolimbic dopamine activity. PVN, paraventricular hypothalamic nucleus; SON, supraoptic hypothalamic nucleus; NAS, nucleus accumbens; LS, lateral septum; BNST, bed nucleus of the stria terminalis; MPO, medial preoptic area; VMH, ventromedial hypothalamus; CeA, central amygdaloid nucleus; MeA, medial amygdaloid nucleus; VTA, ventral tegmental area; SN, substantia nigra pars compacta; brainstem nuclei; spinal cord. AC, anterior commissure; OT, olfactory tract, SC, superior colliculus, IC, inferior colliculus; Hippo, hippocampus; DA, dopamine; CSF, cerebrospinal fluid.
Correlating central OT release with behavioural changes via techniques such as microdialysis involves considerable technical challenges (Neumann, 2007). Measuring changes in peripheral OT in plasma is accomplished much more readily, but only gives an imperfect index of the complexities of central oxytocin release. An increasing number of experimental conditions have been documented in which blood OT levels are uncorrelated with central OT levels and our understanding of the dynamics and determinants of central OT release are rudimentary at best (Neumann, 2007).
Despite this, there is compelling evidence that OT acts in the brain to promote a variety of vital adaptive responses, including maternal behaviour, the formation of monogamous pair-bonds, sexual arousal and orgasm, peer to peer social interaction, social memory and anxiety reduction (Keverne and Curley, 2004; Storm and Tecott, 2005; Lim and Young, 2006; Neumann, 2007). These functions are highlighted not only from microdialysis studies but also from those in which behaviour has been observed following administration of OT receptor antagonists. Other important evidence comes from OT (OTKO) or OT receptor (OTR-KO) knockout mice, with these mutants showing various deficits in social behaviour, maternal behaviour, social recognition and an anxious and aggressive phenotype (Winslow et al., 2000; Winslow and Insel, 2002; Ragnauth et al., 2005; Takayanagi et al., 2005; Pedersen et al., 2006; but see also Crawley et al., 2007).
Recent studies also highlight remarkable anxiolytic and prosocial effects of intranasally administered OT in humans, including increased ‘trust', decreased amygdala activation towards fear-inducing stimuli, improved recognition of social cues and increased gaze directed towards the eye regions of others (Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2006; Guastella et al., 2008). This has generated immense current interest in the role of OT in human psychopathologies such as autism, schizophrenia and social phobia (Carter, 2007; Heinrichs and Gaab, 2007; Hollander et al., 2007; Schulkin, 2007) and a growing realization that OT and AVP may play a key role in social and other processes relating to drug intoxication and drug addiction (Zhou et al., 2008).
AVP inhabits many of the same neural locations as OT. However in contrast to OT, AVP tends to promote aggression, defensiveness and fear (Ebner et al., 2005; Huber et al., 2005). Blockade of central AVP type V1b, receptors (nomenclature in the present article is according to Alexander et al., 2007) elicits anxiolytic, antidepressant and prosocial effects (Griebel et al., 2002; Stemmelin et al., 2005; Shimazaki et al., 2006). Indeed, OT and AVP circuits appear to have reciprocally inhibitory connections in regions such as the amygdala such that boosting OT ‘shuts down' AVP circuitry and vice versa (Huber et al., 2005). However, the situation is rather complex given that vasopressin V1a receptors may have a prosocial role, with V1a knockout mice showing social interaction deficits (Egashira et al., 2007) and overexpression of V1a receptors in regions such as the ventral pallidum linked to enhanced social bonding and mating-induced neural activation (Lim and Young, 2004). Arginine vasopressin is of great potential relevance to a range of behaviours relevant to addiction: for example a very recent report suggests that V1b antagonists may prevent stress and prime-induced heroin seeking in rats via an action on the amygdala (Zhou et al., 2008).
Oxytocin involvement in acute drug effects: MDMA as an example
The involvement of OT in the effects of abused drugs is a relatively under-researched area. Early findings, reviewed a decade ago (Kovacs et al., 1998), indicated that OT administration can influence opiate self-administration, cocaine-induced sensitization and the development of tolerance to opiates, cocaine and alcohol. Summarizing these early experiments, Kovacs et al. (1998) speculated that OT exerts a potent effect in inhibiting key neuroadaptations in mesolimbic, basal forebrain and hippocampal sites that may underlie addiction and tolerance to a wide range of drugs.
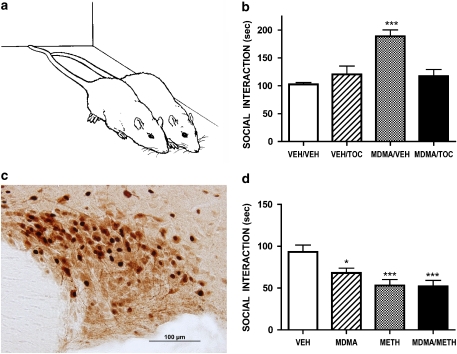
However, OT may also mediate some aspects of acute drug reward and intoxication. Systemically delivered OT is rewarding in rats, as shown in the place preference paradigm (Liberzon et al., 1997) and our own research has recently highlighted a role for OT in the acute effects of the popular dance party drug MDMA. At low doses, MDMA has a powerful ‘prosocial' effect in rats, increasing social interaction in pairs of rats meeting for the first time (Morley and McGregor, 2000; Thompson et al., 2007). This effect is primarily due to an increase in a behaviour termed ‘adjacent lying' (Figures 2a and b). MDMA causes hyperactivity in individually tested rats, so adjacent lying is not due to motoric impairment. Neither is it a ‘huddling' response to perceived cold, as high ambient temperatures augment MDMA-induced adjacent lying and associated neural effects (Cornish et al., 2003; Hargreaves et al., 2007). Rather the effect may well model the powerful prosocial action of MDMA in humans (Dumont and Verkes, 2006; Sumnall et al., 2006).
Figure 2.
(a) Sketch of typical OT-mediated ‘adjacent lying' behaviour seen in pairs of rats given low doses of MDMA. (b) The facilitation of social interaction produced by acute MDMA (5 mg kg−1, i.p.) is reversed by intracerebroventricular administration of the OT receptor antagonist tocinoic acid (TOC, 20 μg). When administered alone tocinoic acid did not affect social interaction. Redrawn from Thompson et al., 2007. (c) Picture of the supraoptic nucleus of the hypothalamus showing staining for OT (brown) and Fos (black). MDMA (10 mg kg−1 significantly increases the number of double-labelled (oxytocin+Fos) neurons (see Thompson et al., 2007) (d) Administration of MDMA (8 mg kg−1, i.p.), methamphetamine (METH, 8 mg kg−1, i.p.) or the combination of MDMA (4 mg kg−1, i.p.)+METH (4 mg kg−1, i.p.) once per week for 16 weeks to rats causes a long-term decline in baseline social interaction measured 7 weeks after the last drug dose was administered. Redrawn from data presented by Clemens et al., 2007. *P<0.05, ***P<0.001.
These effects appear to involve the hypothalamic release of OT. Low, human-like, doses of MDMA activate OT-containing neurons in the SON and PVN of the hypothalamus (Figure 2c) and increase plasma OT levels in rats (Thompson et al., 2007). This interaction reflects the close proximity and confluence of 5-HT containing terminals and OT-containing cell bodies within the PVN and SON (Emiliano et al., 2006). In agreement with the findings from rats, raised peripheral OT is also seen in human MDMA users tested at a dance party (Wolff et al., 2006). Importantly, central administration of the OT receptor antagonist tocinoic acid (TOC, 20μg ICV), attenuates the prosocial effects of MDMA in rats, while not affecting baseline social behaviour (Figure 2b). High ambient temperatures increase brain OT release (Uvnas-Moberg et al., 1993) and this may explain our findings that high ambient temperatures potentiate the social, neural and rewarding effects of MDMA (Cornish et al., 2003; Hargreaves et al., 2007) and the tendency for human users to take MDMA under hot and sweaty conditions (Parrott, 2004).
The prosocial effects of MDMA are mimicked by the 5-HT1A receptor agonist 8-OH-DPAT, which is also a potent releaser of OT. Moreover the 5-HT1A antagonist WAY 100 635 blocks both MDMA-induced OT release and prosocial effects (Thompson et al., 2007; Thompson et al., 2008). These results give a preliminary indication that the functional social effects of MDMA, which are a key reason for the widespread use of the drug, depend upon 5-HT1A-mediated OT release. Tolerance to this effect with repeated MDMA exposure may underlie the loss of sensitivity to the positive effects of MDMA in heavy users, and may result in the dose escalation commonly seen in MDMA users over time (Thompson et al., 2008).
While MDMA provides a strong prima facie case for OT involvement in positive drug effects, evidence for other OT involvement in the effects of other commonly used drugs is rather scarce. The other popular dance party drug GHB is similarly prosocial to MDMA, has anti-aggressive and anxiolytic properties (Schmidt-Mutter et al., 1998; Pedraza et al., 2007; Sumnall et al., 2008), and causes powerful activation of SON oxytocinergic neurons (Van Nieuwenhuijzen et al., unpublished data). Indeed, GHB was once used to promote uterine contractions in childbirth, suggestive of a powerful OT-stimulating effect (Geldenhuys et al., 1968). Low doses of nicotine also powerfully activate SON neurons via cholinergic innervation of this structure (Matta et al., 1993) and SON and PVN neurons show profound alterations in firing rate during the development of opiate tolerance and withdrawal (Brown and Russell, 2004). Acute cocaine produces a dose-dependent increase in hypothalamic and hippocampal OT release (Kovacs et al., 1998). Further circumstantial evidence suggestive of a role for OT in the incentive motivational properties of drugs comes from considering the interaction between OT and brain dopamine pathways.
Oxytocin interaction with the mesolimbic dopamine system
The mesolimbic dopamine system, connecting the midbrain ventral tegmental area (VTA) with the nucleus accumbens (NAS) and other forebrain structures, plays a long-celebrated and much discussed role in the incentive motivational processes underlying the response to natural rewarding stimuli, addictive drugs and drug-related cues. A critical interaction between OT and this brain module is evident from several elegant studies of rodent sexual and social behaviour.
OT-containing neurons in the PVN promote penile erection and copulatory behaviour via their central and spinal projections (Melis and Argiolas, 2003). Oxytocinergic projections from the PVN to the VTA innervate the dopaminergic neurons that project to the nucleus accumbens and this circuit has a prosexual effect: OT infused into the caudal VTA or PVN causing penile erections, increased sexual motivation and increased mesolimbic dopamine activity (Melis et al., 2007). OT is also released during ejaculation, an event that is associated with VTA activation in human subjects (Holstege et al., 2003) and a recent case report suggests the utility of intranasal OT for male anorgasmia (Ishak et al., 2008). OT also appears responsible for the reduced anxiety that is typical of the post-orgasmic state (Waldherr and Neumann, 2007). To the extent that drugs of abuse ‘piggyback' upon or ‘hijack' natural reward circuits (Kauer and Malenka, 2007), we have an interesting liaison between the substrates of sexual reward and drug reward that may involve OT. The prosexual effects of drugs such as GHB, cocaine and methamphetamine might conceivably have an OT component.
Sexual intercourse in monogamous species gives rise to lasting pair bonds with compelling evidence that OT acting within the dopaminergic circuitry of the nucleus accumbens facilitates this process (Lim and Young, 2006). The enduring partner preference that is produced by sexual intercourse in monogamous prairie voles is blocked by both dopamine antagonists or OT antagonists injected into the NAS (Aragona et al., 2006; Lim and Young, 2006). Thus OT, acting in concert with dopamine D2 receptors in the NAS, serves to imbue a particular conspecific with special significance. This has lead to the speculations that social attachment may be some form of addictive disorder (Insel, 2003) and that the same OT circuitry that mediates addiction to love in monogamous species may well mediate addiction to drugs (Edwards and Self, 2006). At present, these remain fascinating ideas in search of confirmatory data.
The mesolimbic and ventral pallidal processes involving OT and AVP that allow social bonding between two mammals may also serve to generate social exclusion, so that the opportunity to bond with other prospective partners is subsequently rejected once a monoaganous bond is formed (Aragona et al., 2006; Lim and Young, 2006). This process appears to involve a proliferation of D1 receptors in the rostral shell of the NAS. One is reminded here of the exclusivity with which addicts bond to their drugs, with the pathological salience of drug-related cues relegating family, friends and natural rewards to a peripheral role in the addict's life (Robinson and Berridge, 1993). It is notable here that exclusivity of bonding is also manifest in maternal behaviour, with OT appearing to subserve an important role not only in maternal ‘calmness' but in the protective aggression seen in new mothers (Slattery and Neumann, 2008).
Clearly much work remains to be done to confirm exactly how, and to what extent, OT impinges upon mesolimbic dopamine activity to influence the acute reinforcing actions of drugs and the neuroplasticity in this and related regions (VTA, dorsal striatum, prefrontal cortex and amygdala) that underlies compulsive drug seeking. The ability of OT to attenuate the repetitive, stereotyped behaviours that are characteristic of autism (Hollander et al., 2003), stimulant-induced hyperactivity (Qi et al., 2008) and drug-induced sensitization (Kovacs et al., 1998) suggests that although OT may contribute towards the acute reinforcing properties of drugs it may also serve to put a ‘brake' on their more excessive long-term, habit-promoting effects. Interestingly, a ‘brake-like' effect of OT is also evident from studies of palatable fluid consumption where OT knockout mice are found to consume greater amounts of saccharin, sucrose and nonsweet carbohydrate (cornstarch, polycose) solutions than wild types (Amico et al., 2005; Billings et al., 2006; Sclafani et al., 2007). While this may simply reflect a general role of hypothalamic OT in the regulation of appetite and fluid balance (Verty et al., 2004) it may speaks of a more specific role of the neuropeptide in promoting satiety, a role that could be relevant to the termination of drug and as well as food intake.
Oxytocin amelioration of drug withdrawal effects
Some intriguing evidence speaks about the ability of OT to ameliorate physical and behavioural effects associated with drug withdrawal. To the extent that drug withdrawal represents an aversive, anxiogenic and agitated state it is perhaps not surprising that this neuropeptide with anxiolytic, serenic and anti-stress properties should be potentially therapeutic in this situation (see Table 1).
Table 1.
Schema of general psychological effects of brain oxytocin and those associated with acute drug intoxication, addiction and withdrawal
| Effect | Oxytocin | Intoxicateda | Addicted | Withdrawal |
|---|---|---|---|---|
| Sociability | ↑ | ↑ | ↓ | ↓ |
| Trust | ↑ | ↑ | ↓ | ↓ |
| Mood | ↑ | ↑ | ↓ | ↓ |
| Coping with stress | ↑ | ↑ | ↓ | ↓ |
| Aggression/irritation | ↓ | ↓↑ | ↑ | ↑ |
| Stereotyped behaviours | ↓ | ? | ↑ | ? |
| Drug craving | ? | ↓ ↑ | ↑ | ↑ |
| Anxiety | ↓ | ↓ | ↑ | ↑ |
See text for relevant references.
↑ Increase, ↓ Decrease, ? Effects unclear.
It is acknowledged that these acute effects may vary from one drug to another.
In a key study, withdrawal symptoms arising from sudden precipitated cannabinoid abstinence in rats and were reversed by administration of lithium, via an OT-dependent mechanism (Cui et al., 2001). Lithium produces pronounced activation of OT-positive hypothalamic nuclei and the ability of lithium to prevent abstinence effects was reversed by co-administration of the OT antagonist L-368,899. Moreover, L-368,899 given in the absence of lithium, exacerbated the cannabinoid withdrawal syndrome. In an analogous fashion, symptoms arising from naloxone-precipitated withdrawal from morphine in mice were attenuated by co-administration of lithium via an OT-dependent mechanism (You et al., 2001). These findings have prompted preliminary trials of lithium in treating cannabis withdrawal with encouraging results (Bowen et al., 2005; Winstock et al., in press) and such trials could conceivably be extended to future examination of intranasal OT or other OT-releasing compounds as therapeutics.
The neural mechanics underlying such effects are worthy of some speculation. Withdrawal from many drugs of abuse, including ethanol, cannabinoids, nicotine and opioids is associated with increased release of corticotropin releasing factor (CRF) in the amygdala. Accordingly, CRF antagonists have a potent and well-documented effect in blocking withdrawal-induced anxiety and drug-seeking behaviour in rodent models (Rodriguez de Fonseca et al., 1997; George et al., 2007; Heilig and Koob, 2007). It is therefore interesting to note that OT has marked inhibitory effects on activation of the hypothalamic pituitary adrenal axis (Uvnas-Moberg, 1998; Legros, 2001; Windle et al., 2004) and centrally administered OT, but not AVP, exerts a potent action in suppressing stress-induced Fos expression in sites such as the hypothalamus and hippocampus (Windle et al., 2004). It would therefore appear timely to explore the action of OT in animal models that explore the influence of stress on drug seeking behaviour, such as the reinstatement paradigm. The capacity of OT to antagonize AVP effects in the amygdala (Huber et al., 2005), and the demonstrated role of amygdala AVP in drug-seeking (Zhou et al., 2008), invites the suggestion that OT might inoculate against stress-induced relapse to drug seeking.
Incidentally, a recent theoretical analysis of autism (Schulkin, 2007) sees the interplay between CRF and OT in the central and medial amygdala as a fundamental determinant of social approach and avoidance. Dominance of CRF over OT is hypothesized to cause profound social withdrawal and an anxious, stress-sensitive and novelty-averse state typical of autism and perhaps drug withdrawal. Recent clinical studies of OT as a therapeutic for autism have lead to some encouraging results (Hollander et al., 2003, 2007).
Residual social deficits caused by drug exposure
While MDMA exerts an acute prosocial effect on rats (Figure 2a), we and others have repeatedly noted that rats given even brief exposure to small doses of MDMA show subsequent deficits in social interaction, effects that can be detected many weeks and months after drug exposure (Figure 2d, Table 2). Thus the short-term ‘ultrasocial' state produced by MDMA appears to be followed by a much longer lasting ‘antisocial' state. The behavioural changes occurring as a result of MDMA pre-exposure are not only social in nature: pre-exposed rats show prolonged increases in anxiety on the elevated plus maze and emergence tests, have poorer memory than controls and are impaired in their coping responses to acute stress in the forced swim test (Morley et al., 2001; McGregor et al., 2003b; Thompson et al., 2004). These effects are mirrored in some recent studies of human MDMA users, where anxiety and depressive disorders may be over-represented and impairment in processing of social cues is evident (Parrott and Marsden, 2006; Reay et al., 2006).
Table 2.
Lasting social interaction deficits in rodents following drug exposure
| Drug | Example references |
|---|---|
| MDMA (Ecstasy) | Bull et al., 2003; Clemens et al., 2004, 2007; Fone et al., 2002; Morley et al., 2001; Morley et al., 2004; Thompson et al., 2004 |
| Methamphetamine | Clemens et al., 2004, 2007 |
| Cannabinoids | O'Shea et al., 2004, 2006; Quinn et al., 2008 |
| Phencyclidine | Boulay et al. 2004; Sams-Dodd, 1996; Snigdha and Neill, 2008 |
| Ketamine | Becker and Grecksch, 2004; Koros et al., 2007 |
| Opiates | Blatchford et al. (2005) |
| GHB | Van Nieuwenhuijzen et al. (2007) |
MDMA is not the only drug that leads to lasting social interaction deficits in animal models. Our group, and others, have reported similar lasting social deficits in rats given brief exposure to methamphetamine, cannabinoids and NMDA antagonists such as ketamine and phencyclidine (Table 2). Indeed, the social withdrawal caused by subchronic exposure to NMDA antagonists is an increasingly popular animal model of negative symptomatology in schizophrenia. Importantly, the social withdrawal caused by repeated exposure to PCP is reversed by intra-amygdala administration of OT (Lee et al., 2005) suggesting that long-term adaptations in brain OT systems plays a critical role in the effect. Vasopressin V1a agonists can also have a beneficial effect in restoring social behaviour after exposure to NMDA antagonists (Matsuoka et al., 2005).
If drug abuse were causing lasting neuroadaptations in brain OT systems then major deficits in maternal behaviour would also be predicted as a result of drug exposure (Jin et al., 2007). In humans, child neglect appears to be both a cause and consequence of drug abuse and there is evidence that such effects may involve OT (Carter, 2005). Consonant with this, various studies have documented OT-related deficits in mothering in rodents following exposure to methamphetamine or cocaine, including reduced pup-directed behaviours, impaired nest building and overly aggressive maternal defence (Johns et al., 2005; Slamberova et al., 2005; Jarrett et al., 2006). Moreover, in utero exposure to drugs of abuse such as cocaine cause lasting changes in the brain OT system of offspring (Johns et al., 2005; Jarrett et al., 2006), and this could conceivably lead to lasting adverse effects on their social behaviour into childhood and beyond (Tronick et al., 2005; Barr et al., 2006). It is tempting to speculate that the high prevalence of early trauma and abuse reported in adults with drug use disorders (Reed et al., 2007) may reflect a vulnerability that arises from abnormal development of brain OT systems.
Oxytocin-related neuroadaptations: lasting adverse effect of drug use?
Lasting social dysfunction as a result of drug exposure is thus evident in studies with laboratory animals (Table 2), although the neuroadaptations that mediate such effects are not well defined. While MDMA has well-documented effects in depleting brain 5-HT, this does not appear to be the key factor causing lasting social changes, since these occur with low or intermittent doses of MDMA that do not affect 5-HT (McGregor et al., 2003a; Clemens et al., 2007) and are seen with exposure of other drugs of abuse that do not greatly influence 5-HT systems (Table 2). Rather, it appears that some other major neuroadaptation is occurring, and it is therefore interesting to turn our attention to brain OT.
The OT systems of the brain display profound neuroplasticity in response to a variety of environmental and pharmacological stimuli and in relation to developmental milestones such as puberty, pregnancy and parturition (Gimpl and Fahrenholz, 2001; Kramer et al., 2006; Neumann, 2007; Slattery and Neumann, 2008). With respect to drugs of abuse, repeated administration of low-dose THC caused downregulation of OT receptor expression and diminished OT innervation in the nucleus accumbens of rats (Butovsky et al., 2006). It is notable here that cannabinoids are known to cause lasting social deficits in rats (O'Shea et al., 2004, 2006; Quinn et al., 2008). In other relevant studies, chronic morphine exposure was found to decrease brain OT synthesis (You et al., 2000), chronic ethanol exposure was associated with degeneration of OT containing magnocellular neurons in the hypothalamus of both humans and rats (Silva et al., 2002; Sivukhina et al., 2006) and repeated cocaine administration caused a decline in hippocampal and hypothalamic OT levels (Kovacs et al., 1998). More recent, albeit preliminary, findings suggest that both MDMA and GHB can cause lasting alterations in OT and OT receptor gene expression in rats that are associated with the social interaction deficits described above (Van Nieuwenhuijzen et al., 2007). Brain-wide assessment of altered OT and OT receptor levels and associated gene expression after repeated drug exposure would clearly be a worthy pursuit for future studies.
Conclusions and future directions
The evidence reviewed here suggests that OT may act as a key component of the rewarding prosocial effects of certain drugs, may have a strong capacity to influence addiction-relevant neuroadaptations, may ameliorate anxiety and irritability during drug withdrawal and that drug-induced disturbances in brain OT may underlie the adverse social consequences arising from repeated drug exposure. On the other hand, it has also long been known that psychosocial support can be a vital component in allowing recovery for addictions and one wonders whether OT might play a role as a natural substrate for such therapeutic effects (see for example, Grewen et al., 2005). In addition, to the extent that addicts are caught in something of a behavioural loop, it is interesting to note the ability of OT to attenuate highly repetitive and compulsive stereotyped behaviours (Hollander et al., 2003). Clearly, an improved characterization of OT and related neuropeptide involvement in a range of acute drug actions and addiction-related neuroadaptations is a timely endeavour that may yield major dividends.
Most interesting perhaps is the possibility that drugs targeting central OT receptors may provide a novel pharmacotherapy for treatment of drug withdrawal and drug craving. A major problem here is that systemically delivered OT has very poor penetration of the CNS, with nasal delivery being the only successful current CNS delivery system in human studies. Even then, there is still uncertainty as to how well intranasal OT penetrates the brain and the longevity of its action (Born et al., 2002). Development of novel OT agonists is at an early stage and faces considerable technical hurdles (Chini and Manning, 2007). Nonetheless, even with the current limited range of tools available, there is considerable scope to further examine the role of OT in the acute effects of popular recreational drugs and the capacity of this ‘social neuropeptide' to reverse the anxiety, depression and social disconnection experienced by many who battle drug addiction.
Acknowledgments
Supported by the National Health and Medical Research Council of Australia. We acknowledge the input of many students and colleagues who have studied animal models of the social effects of drug exposure, including Vince Cakic, Rose Chesworth, Kelly Clemens, Jennifer Cornish, Kirsten Morley, Clint Gurtman, Paul Mallet, Heidi Quinn, Melanie O'Shea, Murray Thompson and Petra Van Nieuwenhuijzen. We also thank John Creighton for the illustration shown in Figure 2a.
Abbreviations
- AVP
arginine vasopressin
- GHB
gamma-hydroxybutyrate
- L-368,899
(2S)-2-Amino-N-[(1S,2S,4R)-7,7-dimethyl-1-[[[4-(2-methy lphenyl)-1-piperazinyl]sulphonyl]methyl]bicyclo[2.2.1]he pt-2-yl]-4-(methylsulfonyl)butanamide
- MDMA
3,4,-methylenedioxymethamphetamine
- NAS
nucleus accumbens
- OT
oxytocin
- PVN
paraventricular nucleus of the hypothalamus
- SON
supraoptic nucleus
- THC
delta-9-tetrahydrocannabinol
- TOC
tocinoic acid
- WAY 100,635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2 pyridinylcyclohexanecarboxamide maleate salt
- VTA
ventral tegmental area
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)-tetraline
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 2nd edition (2007 revision) Br J Pharmacol. 2007;150 Suppl 1:S1–S168. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O'Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the structured clinical interview for DSM-IV in young adult offspring. Am J Psychiatry. 2006;163:1061–1065. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1267–1277. doi: 10.1016/j.pnpbp.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Billings LB, Spero JA, Vollmer RR, Amico JA. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res. 2006;171:134–141. doi: 10.1016/j.bbr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Blatchford KE, Diamond K, Westbrook RF, McNally GP. Increased vulnerability to stress following opiate exposures: behavioral and autonomic correlates. Behav Neurosci. 2005;119:1034–1041. doi: 10.1037/0735-7044.119.4.1034. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Louis C, Perrault G, Griebel G, Soubrie P. SSR181507, a putative atypical antipsychotic with dopamine D2 antagonist and 5-HT1A agonist activities: improvement of social interaction deficits induced by phencyclidine in rats. Neuropharmacology. 2004;46:1121–1129. doi: 10.1016/j.neuropharm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Bowen R, McIlwrick J, Baetz M, Zhang X. Lithium and marijuana withdrawal. Can J Psychiatry. 2005;50:240–241. doi: 10.1177/070674370505000410. [DOI] [PubMed] [Google Scholar]
- Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9:374–380. doi: 10.1007/s11920-007-0048-0. [DOI] [PubMed] [Google Scholar]
- Brown CH, Russell JA. Cellular mechanisms underlying neuronal excitability during morphine withdrawal in physical dependence: lessons from the magnocellular oxytocin system. Stress. 2004;7:97–107. doi: 10.1080/10253890410001727776. [DOI] [PubMed] [Google Scholar]
- Bull EJ, Hutson PH, Fone KC. Reduced social interaction following 3,4-methylenedioxymethamphetamine is not associated with enhanced 5-HT 2C receptor responsivity. Neuropharmacology. 2003;44:439–448. doi: 10.1016/s0028-3908(02)00407-0. [DOI] [PubMed] [Google Scholar]
- Butovsky E, Juknat A, Elbaz J, Shabat-Simon M, Eilam R, Zangen A, et al. Chronic exposure to Delta9-tetrahydrocannabinol downregulates oxytocin and oxytocin-associated neurophysin in specific brain areas. Mol Cell Neurosci. 2006;31:795–804. doi: 10.1016/j.mcn.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Caldu X, Dreher JC. Hormonal and genetic influences on processing reward and social information. Ann N Y Acad Sci. 2007;1118:43–73. doi: 10.1196/annals.1412.007. [DOI] [PubMed] [Google Scholar]
- Carter CS. The chemistry of child neglect: do oxytocin and vasopressin mediate the effects of early experience. Proc Natl Acad Sci USA. 2005;102:18247–18248. doi: 10.1073/pnas.0509376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders. Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Chini B, Manning M. Agonist selectivity in the oxytocin/vasopressin receptor family: new insights and challenges. Biochem Soc Trans. 2007;35:737–741. doi: 10.1042/BST0350737. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. Repeated weekly exposure to MDMA, methamphetamine or their combination: long-term behavioural and neurochemical effects in rats. Drug Alcohol Depend. 2007;86:183–190. doi: 10.1016/j.drugalcdep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Van Nieuwenhuyzen PS, Li KM, Cornish JL, Hunt GE, McGregor IS. MDMA (‘ecstasy'), methamphetamine and their combination: long-term changes in social interaction and neurochemistry in the rat. Psychopharmacology (Berl) 2004;173:318–325. doi: 10.1007/s00213-004-1786-x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, et al. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol. 2003;482:339–341. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cui SS, Bowen RC, Gu GB, Hannesson DK, Yu PH, Zhang X. Prevention of cannabinoid withdrawal syndrome by lithium: involvement of oxytocinergic neuronal activation. J Neurosci. 2001;21:9867–9876. doi: 10.1523/JNEUROSCI.21-24-09867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K, Khoei EM, Brentari C, Stevens A.Prisons and drugs: a global review of incarceration, drug use and drug services The Beckley Foundation Drug Policy Program 2007. Report 12, available on-line at
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves ‘mind-reading' in humans. Biol Psychiatry. 2006;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Verkes RJ. A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20:176–187. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Edwards S, Self DW. Monogamy: dopamine ties the knot. Nat Neurosci. 2006;9:7–8. doi: 10.1038/nn0106-7. [DOI] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, et al. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Emiliano AB, Cruz T, Pannoni V, Fudge JL. The interface of oxytocin-labeled cells and serotonin transporter-containing fibers in the primate hypothalamus: a substrate for SSRIs therapeutic effects. Neuropsychopharmacology. 2006;32:977–988. doi: 10.1038/sj.npp.1301206. [DOI] [PubMed] [Google Scholar]
- Fone KC, Beckett SR, Topham IA, Swettenham J, Ball M, Maddocks L. Long-term changes in social interaction and reward following repeated MDMA administration to adolescent rats without accompanying serotonergic neurotoxicity. Psychopharmacology (Berl) 2002;159:437–444. doi: 10.1007/s00213-001-0931-z. [DOI] [PubMed] [Google Scholar]
- Geldenhuys FG, Sonnendecker EWW, De Klerk MCC. Experience with sodium-gamma-4-hydroxybutyric acid (gamma-OH) in obstetrics. J Obstet Gynaec Brit Cwlth. 1968;75:405–413. doi: 10.1111/j.1471-0528.1968.tb00137.x. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, Hunt GE, Cornish JL, McGregor IS. High ambient temperature increases 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy')-induced Fos expression in a region-specific manner. Neuroscience. 2007;145:764–774. doi: 10.1016/j.neuroscience.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatry. 2007;20:158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AA. Brain activation during human male ejaculation. J Neurosci. 2003;23:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder. Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Ishak WW, Berman DS, Peters A. Male anorgasmia treated with oxytocin. J Sex Med. 2008;5:1022–1024. doi: 10.1111/j.1743-6109.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides. 2006;40:161–167. doi: 10.1016/j.npep.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, et al. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav Neurosci. 2005;119:1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koros E, Rosenbrock H, Birk G, Weiss C, Sams-Dodd F. The selective mGlu5 receptor antagonist MTEP, similar to NMDA receptor antagonists, induces social isolation in rats. Neuropsychopharmacology. 2007;32:562–576. doi: 10.1038/sj.npp.1301133. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Szabo G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones. Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Trujillo KA, Akil H, Young EA. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 1997;17:353–359. doi: 10.1016/S0893-133X(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Sumiyoshi T, Tanaka K, Tsunoda M, Uehara T, Itoh H, et al. NC-1900, an arginine-vasopressin analogue, ameliorates social behavior deficits and hyperlocomotion in MK-801-treated rats: therapeutic implications for schizophrenia. Brain Res. 2005;1053:131–136. doi: 10.1016/j.brainres.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology. 1993;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Clemens KJ, Van der Plasse G, Li KM, Hunt GE, Chen F, et al. Increased anxiety 3 months after brief exposure to MDMA (‘Ecstasy') in rats: association with altered 5-HT transporter and receptor density. Neuropsychopharmacology. 2003a;28:1472–1484. doi: 10.1038/sj.npp.1300185. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Gurtman CG, Morley KC, Clemens KJ, Blokland A, Li KM, et al. Increased anxiety and ‘depressive' symptoms months after MDMA (‘ecstasy') in rats: drug-induced hyperthermia does not predict long-term outcomes. Psychopharmacology (Berl) 2003b;168:465–474. doi: 10.1007/s00213-003-1452-8. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Central oxytocinergic neurotransmission: a drug target for the therapy of psychogenic erectile dysfunction. Curr Drug Targets. 2003;4:55–66. doi: 10.2174/1389450033347190. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, DE Almeida RM, Bannai M, Fish EW, Debold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS. Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (‘ecstasy') Eur J Pharmacol. 2001;433:91–99. doi: 10.1016/s0014-2999(01)01512-6. [DOI] [PubMed] [Google Scholar]
- Morley KC, Li KM, Hunt GE, Mallet PE, McGregor IS. Cannabinoids prevent the acute hyperthermia and partially protect against the 5-HT depleting effects of MDMA (‘Ecstasy') in rats. Neuropharmacology. 2004;46:954–965. doi: 10.1016/j.neuropharm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Morley KC, McGregor IS. (+/−)-3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy') increases social interaction in rats. Eur J Pharmacol. 2000;408:41–49. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- Mouzos J, Hind N, Smith L, Adams K. Research and Public Policy Series. Australian Institute of Criminology: Canberra; 2006. Drug use monitoring in Australia: 2006 annual report on drug use among police detainees. [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA (3,4-Methylenedioxymethamphetamine) or ecstasy: the neuropsychobiological implications of taking it at dances and raves. Neuropsychobiology. 2004;50:329–335. doi: 10.1159/000080961. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Marsden CA. MDMA (3,4-methylenedioxymethamphetamine) or ecstasy: the contemporary human and animal research perspective. J Psychopharmacol. 2006;20:143–146. doi: 10.1177/0269881106063263. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Pedraza C, Davila G, Martin-Lopez M, Navarro JF. Anti-aggressive effects of GHB in OF.1 strain mice: Involvement of dopamine D(2) receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:337–342. doi: 10.1016/j.pnpbp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, et al. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Reay JL, Hamilton C, Kennedy DO, Scholey AB. MDMA polydrug users show process-specific central executive impairments coupled with impaired social and emotional judgement processes. J Psychopharmacol. 2006;20:385–388. doi: 10.1177/0269881106063269. [DOI] [PubMed] [Google Scholar]
- Reed PL, Anthony JC, Breslau N. Incidence of drug problems in young adults exposed to trauma and posttraumatic stress disorder: do early life experiences and predispositions matter. Arch Gen Psychiatry. 2007;64:1435–1442. doi: 10.1001/archpsyc.64.12.1435. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving—an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- Sanfey AG. Social decision-making: insights from game theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- Schmidt-Mutter C, Pain L, Sandner G, Gobaille S, Maitre M. The anxiolytic effect of gamma-hydroxybutyrate in the elevated plus maze is reversed by the benzodiazepine receptor antagonist, flumazenil. European Journal of Pharmacology. 1998;342:21–27. doi: 10.1016/s0014-2999(97)01503-3. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Autism and the amygdala: an endocrine hypothesis. Brain Cogn. 2007;65:87–99. doi: 10.1016/j.bandc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–R1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Iijima M, Chaki S. The pituitary mediates the anxiolytic-like effects of the vasopressin V1B receptor antagonist, SSR149415, in a social interaction test in rats. Eur J Pharmacol. 2006;543:63–67. doi: 10.1016/j.ejphar.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, Paula-Barbosa MM. Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Res. 2002;925:76–88. doi: 10.1016/s0006-8993(01)03261-9. [DOI] [PubMed] [Google Scholar]
- Sivukhina EV, Dolzhikov AA, Morozov Iu E, Jirikowski GF, Grinevich V. Effects of chronic alcoholic disease on magnocellular and parvocellular hypothalamic neurons in men. Horm Metab Res. 2006;38:382–390. doi: 10.1055/s-2006-944522. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Charousova P, Pometlova M. Maternal behavior is impaired by methamphetamine administered during pre-mating, gestation and lactation. Reprod Toxicol. 2005;20:103–110. doi: 10.1016/j.reprotox.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Neill JC. Efficacy of antipsychotics to reverse phencyclidine-induced social interaction deficits in female rats-A preliminary investigation. Behav Brain Res. 2008;187:489–494. doi: 10.1016/j.bbr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Lukovic L, Salome N, Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- Storm EE, Tecott LH. Social circuits: peptidergic regulation of mammalian social behavior. Neuron. 2005;47:483–486. doi: 10.1016/j.neuron.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Woolfall K, Edwards S, Cole JC, Beynon CM. Use, function, and subjective experiences of gamma-hydroxybutyrate (GHB) Drug Alcohol Depend. 2008;92:286–290. doi: 10.1016/j.drugalcdep.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, McGregor IS.Reduced sensitivity to MDMA-induced facilitation of social behavior in MDMA pre-exposed rats Prog Neuropsychopharmacol Biol Psychiatry 2008 10.1016/j.pnpbp.2008.01.014[e-pub ahead of print: doi [DOI] [PubMed]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (‘ecstasy') Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Li KM, Clemens KJ, Gurtman CG, Hunt GE, Cornish JL, et al. Chronic fluoxetine treatment partly attenuates the long-term anxiety and depressive symptoms induced by MDMA (‘Ecstasy') in rats. Neuropsychopharmacology. 2004;29:694–704. doi: 10.1038/sj.npp.1300347. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Messinger DS, Weinberg MK, Lester BM, Lagasse L, Seifer R, et al. Cocaine exposure is associated with subtle compromises of infants' and mothers' social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Dev Psychol. 2005;41:711–722. doi: 10.1037/0012-1649.41.5.711. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Bruzelius G, Alster P, Lundeberg T. The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993;149:199–204. doi: 10.1111/j.1748-1716.1993.tb09612.x. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- van den Bosch LM, Verheul R. Patients with addiction and personality disorder: Treatment outcomes and clinical implications. Curr Opin Psychiatry. 2007;20:67–71. doi: 10.1097/YCO.0b013e328011740c. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhuijzen PS, Bayes PM, Hunt GE, McGregor IS. Lasting social deficits in rats following repeated exposure to Gamma-hydroxybutyrate (GHB) and 3,4-Methylenedioxymethamphetamine (MDMA) Society for Neuroscience Abstracts: 2007;810:819. [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE. Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology. 2004;47:593–603. doi: 10.1016/j.neuropharm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Lea T, Copeland J. Lithium carbonate in the management of cannabis withdrawal. J Psychopharmacology. in press. [DOI] [PubMed]
- Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, et al. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol. 2006;20:400–410. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Lu CL, He C. Oxytocin mediates the inhibitory action of acute lithium on the morphine dependence in rats. Neurosci Res. 2001;41:143–150. doi: 10.1016/s0168-0102(01)00272-3. [DOI] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Wang CH, Lu CL. Chronic morphine treatment inhibits oxytocin synthesis in rats. Neuroreport. 2000;11:3113–3116. doi: 10.1097/00001756-200009280-00015. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]