Abstract
Background and purpose:
Neuropeptide S (NPS) was recently identified as the endogenous ligand of an orphan receptor, now referred to as the NPS receptor. In vivo, NPS produces a unique behavioural profile by increasing wakefulness and exerting anxiolytic-like effects. In the present study, we further evaluated the effects of in vivo supraspinal NPS in mice.
Experimental approach:
Effects of NPS, injected intracerebroventricularly (i.c.v.), on locomotor activity (LA), righting reflex (RR) recovery and on anxiety states (measured with the elevated plus maze (EPM) and stress-induced hyperthermia (SIH) tests) were assessed in Swiss mice.
Key results:
NPS (0.01–1 nmol per mouse) caused a significant increase in LA in naive mice, in mice habituated to the test cages and in animals sedated with diazepam (5 mg kg−1). In the RR assay, NPS dose dependently reduced the proportion of animals losing the RR in response to diazepam (15 mg kg−1) and their sleeping time. In the EPM and SIH test, NPS dose dependently evoked anxiolytic-like effects by increasing the time spent by animals in the open arms and reducing the SIH response, respectively.
Conclusions and implications:
We provide further evidence that NPS acts as a novel modulator of arousal and anxiety-related behaviours by promoting a unique pattern of effects: stimulation associated with anxiolysis. Therefore, NPS receptor ligands may represent innovative drugs for the treatment of sleep and anxiety disorders.
Keywords: Neuropeptide S, locomotor activity, righting reflex recovery, elevated plus maze test, stress induced hyperthermia, mice
Introduction
Neuropeptide S (NPS) is a recently discovered peptide (Xu et al., 2004) with the following primary structure SFRNGVGTGMKKTSFQRAKS for the human peptide. The primary sequence of NPS is highly conserved among species (Reinscheid et al., 2005). NPS selectively binds and activates an orphan G-protein coupled receptor, previously known as GPR154, which shows low homology to other members of the GPCR family (Xu et al., 2004). Following formal pairing with NPS, the GPR154 receptor was named the NPS receptor (Xu et al., 2004). In cells expressing the NPS receptor, NPS concentration dependently increases intracellular calcium concentration and cAMP levels, suggesting that this receptor may be coupled with both Gq and Gs proteins (Xu et al., 2004; Reinscheid et al., 2005). Results from structure–activity studies performed in different laboratories (Reinscheid et al., 2005; Bernier et al., 2006; Roth et al., 2006) consistently indicate the N-terminal sequence of the peptide, in particular Phe2-Arg3-Asn4, is crucial for receptor binding. NPS and its receptor are expressed in various tissues with the highest levels found in the brain, thyroid, salivary and mammary glands (Xu et al., 2004). A detailed study regarding the distribution of NPS receptor and the neurochemical characteristics of neurons expressing NPS in the rat brain was also performed by the same group (Xu et al., 2007). Interestingly, leukocytes also express both the peptide and its receptor (Pulkkinen et al., 2006).
With respect to biological activity, genetic studies indicate a possible peripheral involvement of the NPS/NPS receptor system in chronic inflammatory conditions of barrier organs, such as asthma (Laitinen et al., 2004) and ulcerative colitis/Crohn's disease (D'Amato et al., 2007). It has, therefore, been proposed that this system may contribute to innate immunity and antimicrobial defence (D'Amato et al., 2007). However, NPS-receptor knockout studies failed to demonstrate major phenotypic differences in terms of airway functions between wild-type and mutant mice (Allen et al., 2006). Similar human genetic studies suggest a possible role of NPS receptor in centrally mediated responses, such as sleep (Gottlieb et al., 2007), and panic disorders (Okamura et al., 2007). No published data are available regarding the phenotype of NPS-receptor knockout mice, in terms of sleep and anxiety. In contrast, several animal studies have implicated the NPS/NPS-receptor system in the control of different centrally mediated behaviours, including locomotor activity (LA) (Xu et al., 2004; Roth et al., 2006; Smith et al., 2006), wakefulness (Xu et al., 2004), anxiety (Xu et al., 2004; Leonard et al., 2007), food intake (Beck et al., 2005; Ciccocioppo et al., 2006; Smith et al., 2006; Cline et al., 2007), but also see Niimi (2006) and more recently, drug abuse (Ciccocioppo et al., 2007; Paneda et al., 2007).
In the present study, we further evaluated the effects of supraspinal NPS in mice, using a range of behavioural assays (i) LA performed with naïve mice, mice habituated to the test cage and mice sedated with 5 mg kg−1 diazepam, (ii) recovery of righting reflex (RR) following treatment with a hypnotic dose of diazepam 15 mg kg−1, (iii) elevated plus maze (EPM) and (iv) stress-induced hyperthermia (SIH). The effects elicited by NPS in these behavioural assays were compared to those evoked under the same experimental conditions by caffeine (as a reference stimulant drug) and diazepam (as a reference anxiolytic).
Methods
Animals
All experimental procedures for in vivo studies complied with the standards of the European Communities Council directives (86/609/EEC) and National regulations (DL 116/92). Male Swiss mice (2–3 months old, 30–35 g) were used. They were housed in 425 × 266 × 155 mm cages (Tecniplast, MN, Italy), eight animals per cage, under standard conditions (22 °C, 55% humidity, 12-h light–dark cycle, lights on 0700 hours) with food (MIL, standard diet Morini RE, Italy) and water ad libitum for at least 10 days before experiments began. Each animal was used only once. Diazepam and caffeine were administered to the animals intraperitoneally (i.p.) whereas NPS was given to the cerebral ventricle (intracerebroventricularly; i.c.v.). The i.c.v. injections (2 μl per mouse) were given under light (just sufficient to produce a loss of the righting reflex) ether anaesthesia into the left ventricle according to the procedure described by Laursen and Belknap (1986) and routinely adopted in our laboratory (Rizzi et al., 2002; Gavioli et al., 2003). All procedures were randomised across test groups. In all the assays, food was not available during testing.
Locomotor activity
Experiments were performed during the light cycle (0900–1300 hours). Naïve mice (that is, animals not habituated to the test cage before the experiment) were injected with NPS i.c.v. 5 min before the beginning of the test. Habituated mice were acclimatised to the test cage for 60 min before the i.c.v. injection of NPS and then returned to the test cage. Diazepam-treated mice received an i.p. injection (100 μl) of diazepam (5 mg kg−1) in Tween 80 (0.5%) 15 min before the i.c.v. injection of NPS or the i.p. injection of caffeine. In all the three groups of animals (naïve, habituated and diazepam-treated mice), caffeine and NPS were assessed at 20 mg kg−1and over the 0.01–1 nmol dose range, respectively. LA was assessed using basile activity cages, which consist of a four-channel resistance detector circuit, which converts the bridges ‘broken' by the animals paws into pulses that are summed by an electronic counter every 5 min (Rizzi et al., 2001).
Recovery of the righting reflex
This assay was performed according to the procedures described by Marley et al., 1986. Mice were given an i.p. injection of diazepam 15 mg kg−1. When the animals lost RR, they were placed in a plastic cage and the time was recorded by an expert observer, unaware of the drug treatments. Animals were judged to have regained the RR response when they could right themselves three times within 30 s. Sleeping time is defined as the amount of time between the loss and regaining of the RR; it was rounded to the nearest minute. The ability of caffeine (20 mg kg−1, i.p.) and NPS (0.01–1 nmol, i.c.v.) to modify the proportion of animals responding to diazepam 15 mg kg−1 and their sleeping time (minutes) were evaluated. Caffeine and NPS were administered 15 and 5 min before the injection of diazepam, respectively.
Elevated plus maze
The EPM assay was carried out essentially as previously described by Pellow et al. (1985). The EPM apparatus (Hamilton–Kinder, Poway, CA, USA) consists of two open arms (30 × 5 × 0.6 cm), which are facing two opposite wall-enclosed arms (30 × 5 × 20 cm) connected by a central platform (5 × 5 cm) elevated 50 cm from the floor. A red dim light was focused on the central platform (∼100 lux). Animals were placed at the centre of the maze, with the head facing an open arm. The number of entries and the time spent in both closed and open arms and some ethological variables (that is, rearing, head dipping and stretch attend postures) were recorded during a 5 min period by an experienced observer. An entry was scored as such only when the animal placed all four limbs into any given arm. The ratio of ‘time spent in the open arms divided by time spent in all (that is, open and closed) arms' and ‘number of entries into open arms divided by total entries into all arms' was calculated and multiplied by 100, to yield the percentage of time spent in and the frequency of entries into open arms, respectively. The dose-related effects of NPS (0.001–1 nmol, i.c.v.) and a comparison with that produced by caffeine (20 mg kg−1, i.p.) and diazepam (1 mg kg−1, i.p.) were assessed.
Stress induced hyperthermia
The test was performed according to the method previously reported by Olivier et al. (2002). Rectal temperatures were measured to the nearest 0.1 °C using an ELLAB instruments thermometer (Copenhagen, Denmark) using a lubricated thermistor probe designed for mice (2-mm diameter) inserted 20 mm into the rectum, while the mouse was handheld near the base of the tail. The probe was left in place until steady readings were obtained (approximately 10 s). Rectal temperatures were measured twice in each mouse, at t=0 min (T1) and t=10 min (T2). The first rectal body temperature measurement (T1) induces a mild stress that causes an increase in the second value (T2). The difference in temperature (T2−T1) was considered to reflect SIH. A comparison between T1 in saline-treated mice and in animals treated with a given dose of a test compound was used to determine whether a test compound affected body temperatures, per se. NPS (0.01–1 nmol, i.c.v.) and diazepam (1, 3 and 5 mg kg−1, i.p.) were administered 60 min before the test to evaluate their ability to modify the SIH response. This relatively long period of pretreatment is needed because the injection procedure (handling plus injection) evokes a similar increase in core body temperature as the rectal temperature measurement procedure. This stress effect had waned after 60 min and the basal body temperature returned to the undisturbed baseline (Olivier et al., 2003).
Data analysis
The pharmacological terminology adopted in this paper is consistent with IUPHAR recommendations (Neubig et al., 2003). Data are expressed as mean±s.e.m. of n animals. Data were analysed using Student's t-test or one-way analysis of variance (ANOVA) followed by Dunnett's test, as specified in figure or table legends. Differences were considered statistically significant when P<0.05.
Drugs
Neuropeptide S was synthesised according to published methods (Roth et al., 2006) using Fmoc/tBu chemistry with a SYRO XP multiple peptide synthesiser (MultiSyntech, Witten Germany). Crude peptides were purified by preparative reversed-phase HPLC and the purity checked by analytical HPLC and mass spectrometry, using a matrix-assisted laser desorption ionisation time of flight (Bruker BioScience, Billerica, MA, USA) and an ESI Micromass ZMD-2000 mass spectrometer (Waters corporation, MS, USA). All other reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA). The vehicle used for injecting NPS and caffeine was saline, whereas that for diazepam was Tween 80 (0.5%).
Results
Locomotor activity
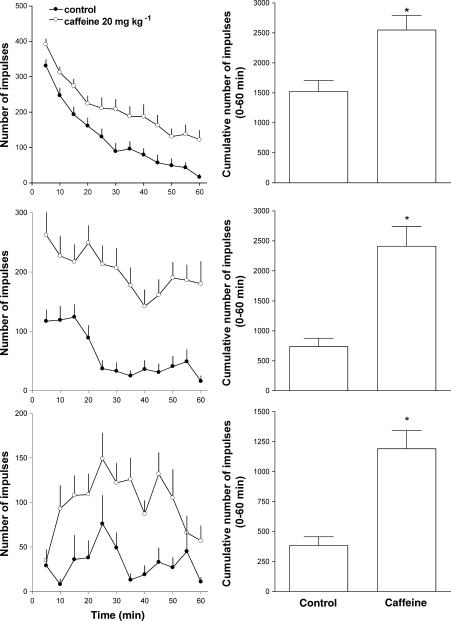
Naïve mice injected with saline (2 μl per mouse, i. c.v.) displayed a progressive reduction in spontaneous LA over the time course of the experiment. Their cumulative LA in 60 min was >1000 impulses. Mice habituated to the test cage for 60 min before the experiment showed an important reduction in LA with cumulative impulses <500. An even greater inhibitory effect was produced by i.p. administration of a sedative dose of diazepam (5 mg kg−1), such that mice treated with the benzodiazepine displayed a cumulative LA of approx 250 impulses in 60 min.
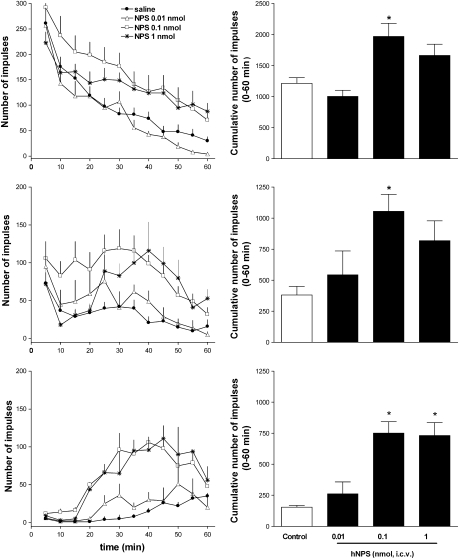
Caffeine administration promoted a robust and consistent increase in locomotion in naïve, habituated and diazepam-treated mice (Figure 1). The stimulatory effect of caffeine was more pronounced in habituated (327% of controls) and diazepam-treated (310% of controls) animals than that in naïve mice (167% of controls). Similar results were obtained following i.c.v. injection of NPS in naïve, habituated and diazepam-treated mice (Figure 2). NPS in the range of 0.01–1 nmol per mouse dose dependently stimulated LA. At 0.01 nmol, the peptide was inactive whereas at 0.1 and 1 nmol, the peptide produced a robust stimulatory effect. However, in naïve and habituated animals, the effect produced by 0.1 nmol was higher than that produced by the 1 nmol dose. This was due to the fact that the onset of action of the latter dose of NPS was delayed (Figure 2, top and middle panels). The maximal stimulatory effect of NPS was 162, 276 and 488% of controls in naïve, habituated and diazepam-treated mice, respectively.
Figure 1.
Locomotor activity assay. Effect of caffeine in naive (top panels), habituated (middle panels) and in diazepam-treated (bottom panels) mice. Locomotor activity of mice is displayed over the time course of the experiment in the left panels and as cumulative impulses over the 60 min observation period in the right panels. Data are mean±s.e.m. of 16 mice per group. *P<0.05 vs control, Student's t-test for unpaired data.
Figure 2.
Locomotor activity assay. Dose–response curve to i.c.v. injected neuropeptide S (NPS; 0.01–1 nmol) in naive (top panels), habituated (middle panels) and in diazepam-treated (bottom panels) mice. Locomotor activity of mice is displayed over the time course of the experiment in the left panels and as cumulative impulses over the 60 min observation period in the right panels. Data are mean±s.e.m. of 20 mice per group. *P<0.05 vs control, analysis of variance (ANOVA) followed by the Dunnett's test for multiple comparison.
Recovery of righting reflex
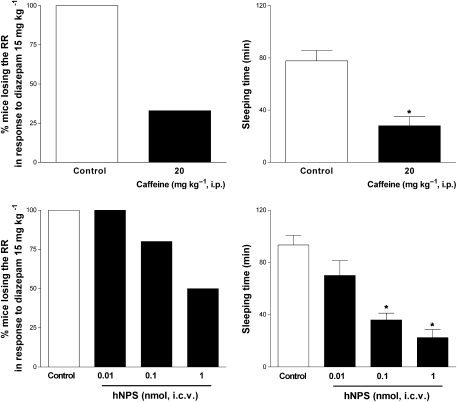
As shown in Figure 3, i.p. injection of diazepam at the hypnotic dose of 15 mg kg−1 produced loss of RR in 100% of the mice and approx 80 min were needed to regain this reflex. Pretreatment with caffeine (20 mg kg−1 i.p.) decreased both the percentage of mice losing RR in response to the benzodiazepine and their sleeping time. Only 33% of caffeine-treated mice lost RR and the sleeping time of those animals responding to diazepam was reduced to 28 min (Figure 3, top panels). This arousal promoting effect of caffeine was mimicked by i.c.v. injection of NPS. Over the 0.01–1 nmol range, the peptide dose dependently reduced the proportion of animals responding to diazepam and their sleeping time. The maximal arousal-promoting effect of NPS was obtained with the dose of 1 nmol, which reduced the percentage of animals showing loss of RR to 50% and their sleeping time to 22 min (Figure 3, bottom panels).
Figure 3.
Recovery of righting reflex in mice. Effect of caffeine 20 mg kg−1, i.p. (top panels) and of i.c.v. injected neuropeptide S (NPS; 0.01–1 nmol, bottom panels) on the per cent of animals losing of the righting reflex in response to diazepam 15 mg kg−1 i.p. (left panels) and on their sleeping time (right panels). Sleeping time is defined as the amount of time between the loss and regaining of the righting reflex. Data are mean±s.e.m. of 14 mice per group. *P<0.05 vs control, analysis of variance (ANOVA) followed by the Dunnett's test for multiple comparison.
Elevated plus-maze test
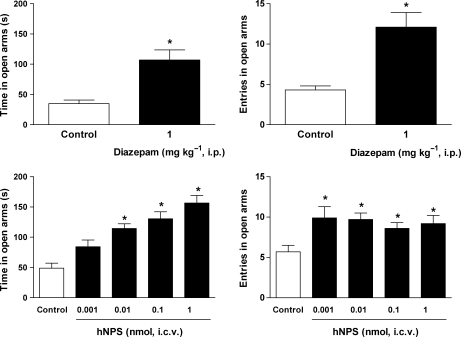
In the EPM test, control mice spent about 35 s in the open arms (corresponding to 19% of the total time spent in open and closed arms) and their entries into open arms were approximately four (corresponding to 27% of the total entries into open and closed arms). Diazepam (1 mg kg−1; i.p.) significantly increased both the percentage of time spent in and the number of entries into open arms (Figure 4, top panels). Similar anxiolytic-like effects were promoted by i.c.v. injection of NPS (Figure 4, bottom panels). In the range 0.001–1 nmol, NPS dose dependently increased the time spent by animals in the open arms with a maximal effect, evoked at 1 nmol, equal to 157 s (corresponding to 68% of the total time spent in open and closed arms). This effect was associated with a statistically significant increase in entries into the open arms, which was, however, dose independent. Under the same experimental conditions, caffeine produced the opposite result reducing to 15 s, the time spent by animals in the open arms (corresponding to 11% of the total time spent in open and closed arms) and the number of entries into open arms to three (corresponding to 21% of the total entries into open and closed arms) (Table 1). All the behavioural parameters measured in the EPM in saline as well as drug-treated animals are summarised in Table 1.
Figure 4.
Effects of diazepam (1 mg kg−1 i.p., top panels) and of i.c.v. injected neuropeptide S (NPS; 0.001–1 nmol, bottom panels) on the time spent in (left panel) and in the number of entries into the open arms (right panel) in the mouse elevated plus maze test. Data are mean±s.e.m. of 16 mice per group. *P<0.05 vs control, analysis of variance (ANOVA) followed by the Dunnett's test for multiple comparison.
Table 1.
Effects of diazepam, caffeine and neuropeptide S on various behavioural parameters displayed by mice subjected to the elevated plus maze test
| Treatment | Time in open arms (s) | Entries in open arms | Time in closed arms (s) | Entries in closed arms | Stretch attend posture | Head-dipping | Rearing |
|---|---|---|---|---|---|---|---|
| Control | 34.9±6.0 | 4.3±0.5 | 161.6±8.4 | 11.1±1.3 | 8.1±0.7 | 3.8±0.7 | 11.1±1.4 |
| Diazepam 1 mg kg−1 | 107±16.6* | 12.1±1.8* | 111.9±17.4* | 13.3±1.9 | 4.9±1.2* | 8.9±1.0* | 12.5±1.4 |
| Control | 39.8±6.9 | 4.8±0.9 | 129.7±6.5 | 8.8±0.8 | 4.1±0.6 | 5.7±0.9 | 11.8±1.6 |
| Caffeine 20 mg kg−1 | 14.8±3.5* | 2.7±0.4* | 125.4±10.2 | 10.1±0.7 | 7.5±0.8* | 4.5±0.7 | 12.3±1.6 |
| Control | 48.9±8.2 | 5.7±0.8 | 133.6±10.1 | 10.3±0.7 | 5.3±0.9 | 8.2±1.2 | 10.3±1.6 |
| NPS 0.001 nmol | 84.2±11.2 | 9.9±1.4 | 111.8±6.6 | 10.8±0.7 | 3.2±1.2 | 11.1±1.7 | 12.0±1.8 |
| NPS 0.01 nmol | 114.4±7.9* | 9.7±0.8* | 107.2±7.1 | 8.8±0.8 | 1.8±0.4* | 14.9±2.3 | 11.6±1.9 |
| NPS 0.1 nmol | 130.4±11.6* | 8.6±0.7* | 89.2±10.6* | 7.1±0.5* | 1.3±0.3* | 16.3±2.8 | 8.6±1.7 |
| NPS 1 nmol | 156.6±12.4* | 9.2±1.0* | 68.2±4.9* | 6.1±0.7* | 0.5±0.1* | 18.8±4.4* | 10.2±1.5 |
All values are expressed as mean±s.e.m. of 12–16 mice per group.
*P<0.05 vs control according to Student's t-test for unpaired data or analysis of variance followed by Dunnett's test for multiple comparison.
Stress-induced hyperthermia test
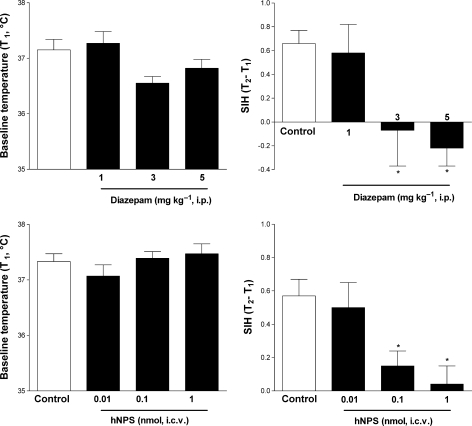
In control mice, stress induced by the measurement of body temperature evoked a significant increase in T2 compared with T1 corresponding to a ΔT of about 0.6 °C. Diazepam (1–5 mg kg−1, i.p.) produced a dose-dependent reduction in baseline temperature, although this effect did not reach statistical significance (Figure 5, top left panel). In the same dose range, the benzodiazepine reduced ΔT to negative values (Figure 5, top right panel). These effects of diazepam were statistically significant at the doses of 3 and 5 mg kg−1. NPS (i.c.v.) did not modify baseline temperature but produced a dose-related reduction in ΔT, which reached statistical significance at doses of 0.1 and 1 nmol (Figure 5, bottom panels).
Figure 5.
Effects of diazepam (1, 3 and 5 mg kg−1, i.p.) (top panels) and neuropeptide S (NPS; 0.01–1 nmol. i.c.v.) (bottom panels) on baseline temperature (T1) (left panels) and stress-induced hyperthermic response (SIH) (T2–T1) (right panels) in the mouse SIH test. Data are mean±s.e.m. of 14 mice per group. *P<0.05 vs control, analysis of variance (ANOVA) followed by the Dunnett's test for multiple comparison.
Discussion and conclusions
Collectively, the present findings demonstrate that the human form of NPS was able to evoke a robust and consistent arousal response in mice. This stimulant effect was associated with a clear anxiolytic-like action. Thus, the present findings corroborate the proposal of NPS as a unique neuropeptidergic signal: an activating anxiolytic (Koob and Greenwell, 2004; Xu et al., 2004).
In LA assays, NPS mimicked the stimulatory effect of caffeine and this effect is highly consistent across different experimental conditions, that is, naïve and habituated mice, and animals sedated with diazepam. Similar results in terms of degree of stimulation, NPS potency, onset and duration of action were previously reported both in mice (Xu et al., 2004) and in rats (Smith et al., 2006). This suggests that the locomotor stimulant effect of NPS is highly consistent among experimental conditions and animal species. The activating properties of NPS were further confirmed in the RR assay, where NPS mimicked the arousal-promoting action of caffeine in mice by reducing the number of animals losing RR in response to a hypnotic dose of diazepam and markedly decreasing the sleeping time in those animals responding to the benzodiazepine. These findings parallel the results obtained by Xu et al. (2004) in rats in electroencephalographic studies, where NPS given i.c.v. in the same dose range (that is, 0.1 and 1 nmol) increases the amount of wakefulness and decreases SWS1, SWS2 and REM sleep. These latter experiments and relative findings were independently replicated in a different laboratory (Ahnaou et al., 2006). Collectively, these data demonstrated that the arousal-promoting action of NPS is a robust effect, which can be easily replicated in different laboratories and animal species. There is no experimental evidence to conclusively ascribe these in vivo biological actions of NPS to the activation of the NPS receptor. However, it is worthy of mention that [Ala3]NPS, a peptide displaying partial agonist features at recombinant NPS receptor in vitro (Roth et al., 2006), partially stimulated LA in mice when given alone and counteracted the stimulatory effect of NPS when coinjected (Calo et al., 2006). This initial indication should be confirmed in future studies, by testing the effects of NPS in mice with the gene for the NPS receptor deleted and by challenging NPS actions with selective and pure NPS receptor antagonists.
Collectively, our findings are in line with those already published, indicating that NPS behaves as an important signal in the brain to stimulate LA and wakefulness. In this respect, the effects of NPS are similar to those elicited by caffeine (and other psychostimulant drugs) and opposite to those of diazepam. The parallel between caffeine and NPS can be extended to the regulation of food intake as several independent studies demonstrated that NPS is able to promote an important anorectic effect in rats (Beck et al., 2005; Ciccocioppo et al., 2006; Smith et al., 2006) and chicks (Cline et al., 2007). Interestingly, the inhibitory effect of NPS on palatable food intake was found to be sensitive to the antagonist action of [Ala3]NPS (Ciccocioppo et al., 2006), suggesting that this effect of the peptide is also due to selective NPS receptor activation. A possible role of the NPS–NPS receptor system in mediating some of the effects of caffeine has been recently proposed based on PCR studies that demonstrated acute and chronic caffeine treatments to modulate the expression of NPS and its receptor in the rat hypothalamus and brainstem (Lage et al., 2006). Although we must acknowledge that the use of the i.c.v. route of administration does not allow investigation of the brain site responsible for a given effect, the brain areas possibly relevant to the stimulant action of NPS are worthy of mention. A recent detailed study demonstrated that NPS receptors are expressed in several regions known to play a major role in the regulation of arousal, including the thalamus, hypothalamus, ventral tuberomammilary nucleus, substantia nigra and ventral tegmental area, and the pontine reticular nucleus (Xu et al., 2007). Further studies using microinjection and neurochemical techniques are now needed to identify which of these areas play a crucial role in the stimulant action of NPS.
In EPM experiments, 1 mg kg−1 diazepam produced clear anxiolytic-like effects, increasing the time spent by mice in the open arms. In contrast, caffeine promoted anxiogenic-like effects. These results agree with those from earlier work, for instance by El Yacoubi et al. (2000) and Gavioli et al. (2002), and validate our EPM experimental conditions. In this assay, the supraspinal administration of NPS mimicked the effects of diazepam promoting dose dependent anxiolytic-like effects. These results are similar to those obtained by Xu et al. (2004) not only in the same assay but also in the light–dark and open field tests. Moreover, an anxiolytic-like action of NPS was also reported in mice subjected to the four-plate test and elevated zero maze (Leonard et al., 2007). Thus, similar to the stimulant effects of NPS, its anxiolytic-like action also seems to be a robust effect easily reproduced in different laboratories and with different assays. However, the stimulatory action of NPS can bias the interpretation of results obtained in the above-mentioned assays, in which anxiety levels are measured as inhibited behaviours.
This prompted us to investigate NPS effects in the SIH assay, a model of anxiety, which is not sensitive to LA (Bouwknecht et al., 2007). As expected, in this assay, diazepam produced anxiolytic-like effects, that is, it counteracted the SIH response in a dose-dependent manner (Bouwknecht et al., 2007). However, higher benzodiazepine doses were required in this assay compared to EPM to elicit statistically significant effects. In addition, the effect of diazepam on body temperature (T1), although not statistically significant, may represent a bias for the analysis of its effect on SIH. However, this is unlikely because it has been clearly demonstrated using different mouse strains and several benzodiazepines (diazepam, oxazepam, alprazolam and chlodiazepoxide) that the decrease in ΔT is indeed independent of the effect of the drug on temperature T1 (Olivier et al., 2002). The action of caffeine was not evaluated in this assay because of the reported hyperthermic/hypothermic response of this drug (Yang et al., 2007) and because this assay failed to detect anxiogenic-like effects of several drugs, such as pentylenetetrazol, the beta-carboline FG7142, meta-chlorophenylpiperazine (Olivier et al., 2002). Thus, anxiogenic-like actions are extremely difficult to assess using this test, probably due to a ceiling effect of ΔT (Bouwknecht et al., 2007).
Under the same experimental conditions, NPS did not produce any modification of T1 but dose dependently prevented SIH. This same result has been independently replicated in a different laboratory (Leonard et al., 2007). Thus the ability of NPS to counteract SIH, a physiological parameter insensitive to locomotion, indicates that the action of NPS should be considered as a genuine (that is, not merely dependent on experimental bias) anxiolytic-like effect. This proposal is corroborated by the anxiolytic-like effects measured in mice in response to NPS in the marble-burying assay (Xu et al., 2004), a model where anxiety levels are measured as an active (marble burying) behaviour. Collectively, the effects promoted by NPS in both the EPM and SIH assays are similar to those elicited by diazepam and opposite to those of caffeine. As far as brain areas relevant to the action of NPS on stress and anxiety are concerned, the study by Xu et al. (2007) demonstrated that NPS receptor messenger is expressed in various stress-related regions, including the amygdala, bed nucleus of the stria terminalis, hypothalamus, raphe nucleus and ventral tegmental area. However, as already mentioned for the stimulant action of NPS, further studies are needed to identify the brain region(s) and the neurotransmitter(s) mediating the anxiolytic-like effects of NPS.
Collectively, the present study confirmed and extended previous findings (Xu et al., 2004), demonstrating that NPS produced a unique behavioural profile: stimulation associated with anxiolysis. The only known drug displaying a similar pattern of effect is nicotine (Koob and Greenwell, 2004). Interestingly the NPS–NPS receptor system, to some extent, seems to be upregulated in response to nicotine treatment in rats (Lage et al., 2007), thus suggesting a role, in part, for this peptidergic system in mediating the behavioural actions of nicotine. It is expected that the pharmacological analysis of the actions elicited by selective NPS receptor agonists and antagonists together with the phenotypic characterisation of mice lacking the NPS receptor will substantially extend our knowledge of the neurobiological mechanisms that regulate the sleep/wakefulness cycle, the response to stress and anxiety. Hopefully, this information may be useful in the near future for the design of innovative drugs for the treatment of sleep and anxiety disorders and possibly other pathological conditions, such as drug dependence and food intake disorders.
Acknowledgments
This work was supported by funds from the University of Ferrara (FAR grant to GC) and the Italian Ministry of Universities (PRIN 2006 grant to GC and SS).
Abbreviations
- EPM
elevated plus maze
- LA
locomotor activity
- NPS
neuropeptide S
- RR
righting reflex recovery
- SIH
stress-induced hyperthermia
Conflict of interest
The authors state no conflict of interest.
References
- Ahnaou A, Drinkenburg W, Huysmans H, Heylen A, Steckler T, Dautzenberg F.Differential roles of hypothalamic neuropeptides in sleep–wake modulation in rats Society for Neuroscience 2006 2006Atlanta, GA, USA; pp. 458.1/CC11 [Google Scholar]
- Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN, et al. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1005–L1017. doi: 10.1152/ajplung.00174.2006. [DOI] [PubMed] [Google Scholar]
- Beck B, Fernette B, Stricker-Krongrad A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem Biophys Res Commun. 2005;332:859–865. doi: 10.1016/j.bbrc.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Bernier V, Stocco R, Bogusky MJ, Joyce JG, Parachoniak C, Grenier K, et al. Structure-function relationships in the neuropeptide S receptor: molecular consequences of the asthma-associated mutation N107I. J Biol Chem. 2006;281:24704–24712. doi: 10.1074/jbc.M603691200. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Calo G, Roth A, Marzola E, Rizzi A, Arduin M, Trapella C, et al. Structure activity studies on Neuropeptide S: identification of the amino acid residues crucial for receptor activation Society for Neuroscience 2006 2006Atlanta, GA, USA; pp. 726.18/D56 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cannella N, Fedeli A, Cippitelli A, Calo G, et al. Inhibition of palatable food intake by Neuropeptide S is reversed by its analogue (Ala3)hNPS Society for Neuroscience 2006 2006Atlanta, GA, USA; pp. 809.7/O15 [Google Scholar]
- Ciccocioppo R, Kallupi M, Cannella N, Braconi S, Stopponi S, Economidou D.Neuropeptide s system activation facilitates conditioned reinstatement of cocaine-seeking in the rat Society for Neuroscience 2007 2007San Diego; pp. 271.18/Z1 [Google Scholar]
- Cline MA, Godlove DC, Nandar W, Bowden CN, Prall BC. Anorexigenic effects of central neuropeptide S involve the hypothalamus in chicks (Gallus gallus) Comp Biochem Physiol A Mol Integr Physiol. 2007;148:657–663. doi: 10.1016/j.cbpa.2007.08.016. [DOI] [PubMed] [Google Scholar]
- D'Amato M, Bruce S, Bresso F, Zucchelli M, Ezer S, Pulkkinen V, et al. Neuropeptide s receptor 1 gene polymorphism is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2007;133:808–817. doi: 10.1053/j.gastro.2007.06.012. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology (Berl) 2000;148:153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, et al. Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci. 2003;17:1987–1990. doi: 10.1046/j.1460-9568.2003.02603.x. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rae GA, Calo G, Guerrini R, De Lima TC. Central injections of nocistatin or its C-terminal hexapeptide exert anxiogenic-like effect on behaviour of mice in the plus-maze test. Br J Pharmacol. 2002;136:764–772. doi: 10.1038/sj.bjp.0704739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, O'Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Med Genet Suppl. 2007;1:S9. doi: 10.1186/1471-2350-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Greenwell TN. Neuropeptide S: a novel activating anxiolytic. Neuron. 2004;43:441–442. doi: 10.1016/j.neuron.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lage R, Dieguez C, Lopez M. Caffeine treatment regulates neuropeptide S system expression in the rat brain. Neurosci Lett. 2006;410:47–51. doi: 10.1016/j.neulet.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Lage R, Gonzalez CR, Dieguez C, Lopez M. Nicotine treatment regulates neuropeptide S system expression in the rat brain. Neurotoxicology. 2007;28:1129–1135. doi: 10.1016/j.neuro.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, et al. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Logue SF, Dwyer J, Sukoff Rizzo SJ, Schechter LE, Rosenzweig-Lipson S, et al. Anxiolytic-like and locomotor-promoting effects of neuropeptide S are distinguished in the mouse four-plate test: comparison to amphetamine Society for Neuroscience 2007 2007San Diego; pp. 841.15/UU30 [Google Scholar]
- Marley RJ, Miner LL, Wehner JM, Collins AC. Differential effects of central nervous system depressants in long-sleep and short-sleep mice. J Pharmacol Exp Ther. 1986;238:1028–1033. [PubMed] [Google Scholar]
- Neubig RR, Spedding M, Kenakin T, Christopoulos A. International union of pharmacology committee on receptor nomenclature and drug classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- Niimi M. Centrally administered neuropeptide S activates orexin-containing neurons in the hypothalamus and stimulates feeding in rats. Endocrine. 2006;30:75–79. doi: 10.1385/ENDO:30:1:75. [DOI] [PubMed] [Google Scholar]
- Okamura N, Hashimoto K, Iyo M, Shimizu E, Dempfle A, Friedel S, et al. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1444–1448. doi: 10.1016/j.pnpbp.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Olivier B, Bouwknecht JA, Pattij T, Leahy C, van Oorschot R, Zethof TJ. GABAA-benzodiazepine receptor complex ligands and stress-induced hyperthermia in singly housed mice. Pharmacol Biochem Behav. 2002;72:179–188. doi: 10.1016/s0091-3057(01)00759-6. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, et al. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–132. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- Paneda C, de Lecea L, Roberts AJ.Neuropeptide S increases cocaine seeking behaviour Society for Neuroscience 2007 2007San Diego; pp. 611.1/KK13 [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pulkkinen V, Majuri ML, Wang G, Holopainen P, Obase Y, Vendelin J, et al. Neuropeptide S and G protein-coupled receptor 154 modulate macrophage immune responses. Hum Mol Genet. 2006;15:1667–1679. doi: 10.1093/hmg/ddl090. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, et al. Pharmacological characterization of human and murine neuropeptide S receptor variants. J Pharmacol Exp Ther. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Bigoni R, Marzola G, Guerrini R, Salvadori S, Regoli D, et al. Characterization of the locomotor activity-inhibiting effect of nociceptin/orphanin FQ in mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:161–165. doi: 10.1007/s002100000358. [DOI] [PubMed] [Google Scholar]
- Rizzi D, Rizzi A, Bigoni R, Camarda V, Marzola G, Guerrini R, et al. Arg(14), Lys(15)]nociceptin, a highly potent agonist of the nociceptin/orphanin FQ receptor: in vitro and in vivo studies. J Pharmacol Exp Ther. 2002;300:57–63. doi: 10.1124/jpet.300.1.57. [DOI] [PubMed] [Google Scholar]
- Roth AL, Marzola E, Rizzi A, Arduin M, Trapella C, Corti C, et al. Structure-activity studies on neuropeptide S: identification of the amino acid residues crucial for receptor activation. J Biol Chem. 2006;281:20809–20816. doi: 10.1074/jbc.M601846200. [DOI] [PubMed] [Google Scholar]
- Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, et al. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Yang JN, Tiselius C, Dare' E, Jaohansson B, Valen G, Fredhholm BB. Sex diffrences in mouse heart rate and body temperature and their regulation by adenosine A1 receptors. Acta Physiol. 2007;190:63–75. doi: 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]