Abstract
Background and purpose:
Effects of imatinib mesylate, a Kit receptor tyrosine kinase inhibitor, on spontaneous activity of interstitial cells of Cajal (ICC) and smooth muscles in the stomach were investigated.
Experimental approach:
Effects of imatinib on spontaneous electrical and mechanical activity were investigated by measuring changes in the membrane potential and tension recorded from smooth muscles of the guinea-pig stomach. Its effects on spontaneous changes in intracellular concentration of Ca2+ ([Ca2+]i) (Ca2+ transients) were also examined in fura-2-loaded preparations.
Key results:
Imatinib (1–10 μM) suppressed spontaneous contractions and Ca2+ transients. Simultaneous recordings of electrical and mechanical activity demonstrated that imatinib (1 μM) reduced the amplitude of spontaneous contractions without suppressing corresponding slow waves. In the presence of nifedipine (1 μM), imatinib (10 μM) reduced the duration of slow waves and follower potentials in the antrum and accelerated their generation, but had little affect on their amplitude. In contrast, imatinib reduced the amplitude of antral slow potentials and slow waves in the corpus.
Conclusions and implications:
Imatinib may suppress spontaneous contractions of gastric smooth muscles by inhibiting pathways that increase [Ca2+]i in smooth muscles rather than by specifically inhibiting the activity of ICC. A high concentration of imatinib (10 μM) reduced the duration of slow waves or follower potentials in the antrum, which reflect activity of ICC distributed in the myenteric layers (ICC-MY), and suppressed antral slow potentials or corporal slow waves, which reflect activity of ICC within the muscle bundles (ICC-IM), presumably by inhibiting intracellular Ca2+ handling.
Keywords: imatinib mesylate, interstitial cells of Cajal, smooth muscle, stomach
Introduction
Interstitial cells of Cajal (ICC) have a fundamental role in both the generation of electrical rhythmicity and neuromuscular transmission in the gastrointestinal tract (Sanders et al., 2006). Myenteric ICC (ICC-MY) form an extensive network in the myenteric layers between the circular and longitudinal muscle layers, which generate driving potentials that propagate to adjacent smooth muscle layers to initiate slow waves (Dickens et al., 1999). Another population of ICC (intramuscular ICC; ICC-IM), distributed between the smooth muscle cells within the circular layers, mediate signal transmission from both cholinergic excitatory and nitrergic inhibitory neurons to smooth muscle, and thus modulate spontaneous activity (Hirst and Ward, 2003).
ICC-like cells (ICC-LCs) have also been identified in non-gastrointestinal tissues, including the urinary tract and male genital system by their immunoreactivity against Kit receptor tyrosine kinase, a cell surface marker specific for ICC, or vimentin filament, a marker for cells of mesenchymal origin (Hashitani, 2006; Lang et al., 2006). However, the physiological function of ICC-LCs in non-gastrointestinal tissue still remains to be elucidated, with the exception of those in the rabbit urethra, which may act as electrical pacemakers such as ICC in the gastrointestinal tract (Hashitani et al., 1996; Sergeant et al., 2000; Johnston et al., 2005; Hashitani and Suzuki, 2007).
In general, blockers of L-type Ca2+ channels either abolish or largely diminish the electrical discharge of smooth muscle, although having little effect on ICC or ICC-LC activity. Thus, Ca2+ channel blockade has allowed researchers to differentiate ICC or ICC-LC activity from smooth muscle activity in situ (Yamazawa and Iino, 2002; Hashitani et al., 2003; Hennig et al., 2004; Hashitani and Suzuki, 2007). On the other hand, although ICC activity generally depends on Ca2+ handling by intracellular Ca2+ stores and mitochondria (Sanders et al., 2006), blockers of the function of these organelles would also be affecting neighbouring smooth muscle cells. Thus, insights into the role of ICC or ICC-LC in smooth muscle activity are not readily available in situ, except when genetically modified mouse models with loss-of-function mutations in the Kit signalling pathway are studied (Sanders and Ward, 2007). The identification of specific blockers for ICC or ICC-LC function should boost our understanding of the physiological importance of these cells, which are being discovered in many smooth muscle systems.
As both development and maintenance of ICC require Kit (Beckett et al., 2007), an inhibitor of Kit signalling could be used as a specific blocker for the function of ICC or ICC-LCs. Recently, imatinib mesylate, a Kit tyrosin kinase inhibitor has been shown to suppress contractile activity in various phasic smooth muscles, including the guinea-pig bladder (Kubota et al., 2004, 2006), human uterus and small intestine (Popescu et al., 2006) and guinea-pig gall bladder (Lavoie et al., 2007). Imatinib alters the pattern of action potential generation in the bladder (Kubota et al., 2004) and gall bladder (Lavoie et al., 2007), and exhibits comparable effects on spontaneous Ca2+ transients in both smooth muscle and ICC-LCs in the gall bladder (Lavoie et al., 2007). Relatively high concentrations of imatinib (50–100 μM) also suppress L-type Ca2+ channel and K-selective outward currents in single detrusor smooth muscle cells, indicating a nonspecific action (Kubota et al., 2004). However, none of the studies has demonstrated that imatinib, by inhibiting Kit signalling, specifically inhibits ICC or ICC-LCs.
In the present study, we have investigated the effects of imatinib on electrical, Ca2+ and mechanical activity of smooth muscles of the guinea-pig stomach, where the function of two distinct populations of ICC, ICC-MY and ICC-IM, has been well established (Dickens et al., 1999; Hirst and Ward, 2003).
Methods
Male guinea-pigs weighing 250–400 g were anaesthetized with fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether (sevoflurane), and exsanguinated by decapitation. All animals were treated ethically according to the guiding principles for the care and use of animals in the field of physiological sciences approved by The Physiological Society of Japan. The stomach was excised, and opened by cutting along the lesser curvature. The mucosal layer, connective tissues were then removed leaving the underlying smooth muscles intact.
Isometric tension recordings
For contractile studies, full thickness antral smooth muscle preparations, 8–10 mm long and 1–2 mm wide, were prepared along the axis of circular smooth muscle. Preparations were transferred to 2 mL organ baths and were superfused with warmed (36 °C) physiological saline at a constant flow rate (2 mL min–1). Silk threads were tied around both ends of a strip, one of them was fixed at the bottom of the organ bath and the other was connected to an isometric force transducer connected to a bridge amplifier. Isometric tension changes were digitized using a Digidata 1200 interface and stored on a personal computer for later analysis. After being set up, the preparations were allowed to equilibrate for 60–90 min, and a tension of approximately 4 mN was applied to the preparations that were then further left to equilibrate for around 60 min until spontaneous phasic contractions, which were stable in both amplitude and frequency, were generated.
Intracellular recordings
For intracellular recordings, whole wall, circular or longitudinal smooth muscle preparations, around 3 mm long and 2 mm wide, were prepared from the antral region. Circular smooth muscle preparations were also prepared from the corporal region. Preparations were pinned out on a Sylgard plate at the bottom of the recording chamber (volume, approximately 1 mL), which was mounted on the stage of an inverted microscope. The preparations were superfused with warmed (36 °C) physiological salt solution (PSS) at a constant flow rate (2 mL min−1). After being set up, the preparations were allowed to equilibrate for about 120 min. Individual smooth muscle cells in the muscle bundles were impaled with glass capillary microelectrodes, filled with 0.5 M KCl (tip resistance, 120–250 MΩ). Membrane potential changes were recorded using a high input impedance amplifier, and displayed on a cathode-ray oscilloscope (SS-5702). After low-pass filtering (cutoff frequency, 1 kHz), membrane potential changes were digitized using either Digidata 1322 or Digidata 1200 interfaces and stored on a personal computer for later analysis.
In some experiments, isometric tension recordings were made simultaneously with intracellular recordings. Approximately 1–2 mm of one end of our whole wall preparations of antral smooth muscle was pinned out on a Sylgard plate, at the bottom of the recording chamber (volume, approximately 1 mL), which was mounted on a stage of an inverted microscope, while a thread was tied around the other end and attached to the force transducer as above. The preparations were superfused with warmed (36 °C) PSS at a constant flow rate (2 mL min−1). After a 90 min initial equilibration period, a tension of approximately 1 mN was applied to preparations and they were then further left to equilibrate for about 60 min.
Intracellular calcium measurements
For measurements of changes in intracellular concentration of Ca2+ ([Ca2+]i), antral smooth muscle preparations were pinned out on the bottom of a recording chamber, which was similar to that used for electrical recordings. After 60 min incubation with warmed (36 °C) PSS, and the generation of spontaneous contractions, preparations were loaded with fluorescent dye, fura-2, by incubation in low Ca2+ PSS (Ca2+, 1 mM) containing 10 μM fura-2 AM and cremphor EL (0.01%) for 1 h at room temperature. After being loaded, preparations were superfused with dye-free, warmed (36 °C) PSS at a constant flow (about 2 mL min−1) for 30 min.
The recording chamber was mounted on the stage of an upright fluorescence microscope (BX51WI; Olympus) equipped with an electron multiplier CCD camera (C9100; Hamamatsu Photonics) and a high-speed scanning polychromatic light source (C7773; Hamamatsu Photonics). Preparations were viewed under a water immersion objective (LUMPlanFI × 60; Olympus) and illuminated with ultraviolet light, wave lengths of 340 and 380 nm. The fluorescence emission in a rectangular window (250 × 250 pixels) was measured through a barrier filter above 510 nm, and pairs of frames with excitations at 340 and 380 nm were obtained every 200 ms with an exposure time of 8.7–39.1 ms using a microphotoluminescence measurement system (AQUACOSMOS; Hamamatsu Photonics). The ratio of fluorescence (R340/380) was taken as an index of [Ca2+]i.
Solutions
The composition of PSS was (in mM): Na+ 137.5, K+ 4.7, Ca2+ 2.5, Mg2+ 1.2, HCO3− 15.5, H2PO4− 1.2, Cl− 134 and glucose 15. The pH of PSS was 7.2 when bubbled with 95% O2 and 5% CO2 and the measured pH of the recording bath was approximately 7.4.
Materials
Sevoflurane was obtained from Maruishi Pharm. (Osaka, Japan); Sylgard plate (silicone elastomer) from Dow Corning Corporation (Midland, MI, USA); high input impedance amplifier and Digidata 1322, Digidata 1200 interfaces from Axoclamp-2B (Axon Instruments Inc., Foster City, CA, USA); cathode-ray oscilloscope (SS-5702), Iwatsu (Tokyo, Japan); fura-2 AM, Dojindo (special packaging); cremphor EL (Sigma, St Louis, MO, USA).
Drugs used: imatinib mesylate was kindly provided by Novartis Pharma (Basel, Switzerland), nifedipine and nicardipine (from Sigma). Imatinib mesylate was dissolved in distilled water. Nifedipine was dissolved in absolute ethanol and nicardipine was dissolved in dimethylsulphoxide. The final concentration of these solvents in the PSS did not exceed 1:1000.
Calculations and statistics
Measured values are expressed as means±s.d. Statistical significance was tested using paired t-test, and it was significant if P<0.05.
For isometric tension changes, the following parameters were measured: peak amplitude, measured as the value from the basal tension level to the peak of phasic contractions; half-width, measured as the time between 50% peak amplitude on the rising and falling phases; frequency which was defined as an average over a 5 min recording period.
For Ca transients, peak amplitude was measured as the value from the basal Ca level to the peak of Ca transients and frequency was averaged over 3 min.
The following parameters of spontaneous electrical activity were measured: peak amplitude, measured as the value from the resting membrane potential to the slow wave peak; half-width, measured as the time between 50% peak amplitude on the rising and falling phases; frequency which was averaged over 5 min. Maximum rate of rise (dV/dtMax) of follower potentials was also measured.
Results
Effects of imatinib mesylate on spontaneous contractions of smooth muscles in the antrum
All smooth muscle preparations taken from the antrum generated about 4–5 spontaneous contractions every minute, which were stable with respect to both their amplitude and frequency.
Imatinib mesylate (1 μM) reduced the amplitude and half-width of the spontaneous contractions to 77.8±12.1% (from 7.2±1.7 to 5.6±1.4 mN, n=12, P<0.05; Figure 1Aa) and 92.6±4.7% (from 6.6±0.53 to 6.1±0.48 s, n=12, P<0.05; Figure 1Ca and b) of control, respectively. However imatinib increased contraction frequency to 117.6±10.6% (from 4.6±0.51 to 5.4±0.30 min−1, n=12, P<0.05; Figure 1Ab and c).
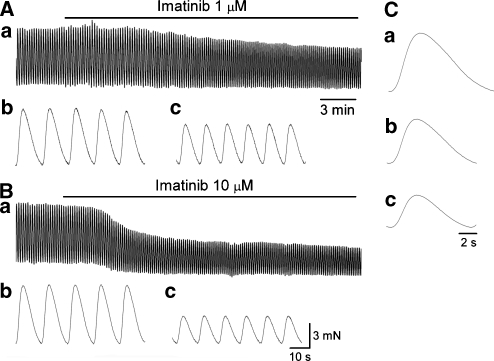
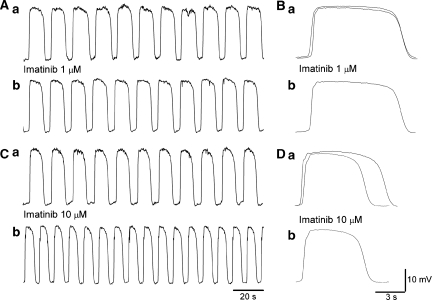
Figure 1.
Effects of imatinib on spontaneous contractions of smooth muscles in the antrum. (Aa) In an antral preparation, imatinib (1 μM) reduced the amplitude of spontaneous contractions. It also increased the frequency of contractions (Ab, c), whereas reducing their half-width (Ca, b). (Ba) In the same preparation, imatinib (10 μM) suppressed spontaneous contractions and reduced basal tension. It also increased the frequency of contractions (Bb, c), whereas reducing their half-width (Ca, c). Traces in (C) are averaged traces of 20 contractions each.
A higher concentration of imatinib (10 μM) further suppressed spontaneous contractions to 68.6±18.6% (from 6.9±1.6 to 4.7±1.6 mN, n=10, P<0.05; Figure 1Ba) and reduced basal tension. It also reduced their half-width to 89±4.6% (from 6.6±0.57 to 5.9±0.64 s, n=10, P<0.05; Figure 1Ca and c) and increased their frequency 121.3±12.8% (from 4.8±0.47 to 5.8±0.50 min−1, n=10, P<0.05; Figure 1Bb and c).
Effects of imatinib mesylate on electrical–mechanical coupling in antral smooth muscle
The effects of imatinib on electrical–mechanical coupling were investigated by monitoring its action on slow waves and their corresponding contractions simultaneously recorded from antral smooth muscle preparations.
Imatinib (1 μM) suppressed spontaneous contractions without affecting either the amplitude of slow wave (28.9±3.7 mV in control, 28.9±3.5 mV in imatinib, n=6; Figure 2Aa and b) or the resting membrane potential (−66.3±2.9 mV in control, −66.6±2.1 mV in imatinib, n=6). However, it reduced the half-width of slow waves to 95.3±4.2% (from 6.0±0.62 to 5.7±0.66 s, n=6, P<0.05), and increased their frequency to 113.6±10.5% (from 4.5±0.87 to 5.1±0.97 min−1, n=6, P<0.05; Figure 2Ba and b).
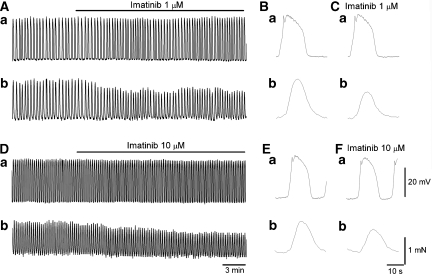
Figure 2.
Effects of imatinib on the correlation between electrical and mechanical activity in antral smooth muscle. In an antral preparation, imatinib (1 μM) suppressed spontaneous contractions (Ab) without affecting the amplitude of slow waves (Aa). It also reduced the half-width of slow waves and suppressed corresponding contractions (B, C). In another antral preparation, imatinib (10 μM) suppressed spontaneous contractions (Db) with small reductions in the amplitude of slow waves (Da). It also reduced the half-width of slow waves and suppressed associated contractions (E, F).
A higher concentration of imatinib (10 μM) suppressed spontaneous contractions with small reductions in the amplitude of slow waves to 90.3±3.8% (29.2±3.4 mV in control, 26.4±4.3 mV in imatinib, n=7, P<0.05; Figure 2Ca and b) without changing the resting membrane potential (−65.3±2.4 mV in control, −65.6±1.9 mV in imatinib, n=7). It also reduced the half-width of slow waves to 92.1±3.6% (from 6.0±0.66 to 5.5±0.66 s, n=7, P<0.05), and increased their frequency to 126.4±9.8% (from 4.3±0.83 to 5.4±0.96 min−1, n=7, P<0.05; Figure 2Da and b).
Effects of imatinib mesylate on spontaneous Ca2+ transients in antral smooth muscles
As imatinib (1 μM) suppressed spontaneous contractions without reducing the amplitude of slow waves, the effects of imatinib on spontaneous Ca2+ transients in antral smooth muscle were examined.
Imatinib (1 μM) reduced the amplitude of Ca2+ transients to 74.4±6.6% (from 0.21±0.039 R340/380 to 0.15±0.029 R340/380, n=6, P<0.05) and increased their frequency to 107.8±2.9% (from 3.5±0.88 to 3.9±0.85 min−1, P<0.05), but did not change their half-width (3.8±0.46 s in control, 3.8±0.46 s in imatinib; Figure 3Aa and b). Imatinib (10 μM) reduced the amplitude of Ca2+ transients to 49.7±9.8% (from 0.24±0.067 R340/380 to 0.12±0.053 R340/380, n=5, P<0.05) and shortened their half-width to 90.3±1.6% (from 3.8±0.59 to 3.4±0.46 s, P<0.05). It also increased their frequency to 111.2±3.9% (from 3.7±1.1 to 4.2±1.1 min−1, P<0.05; Figure 3Ba and b).
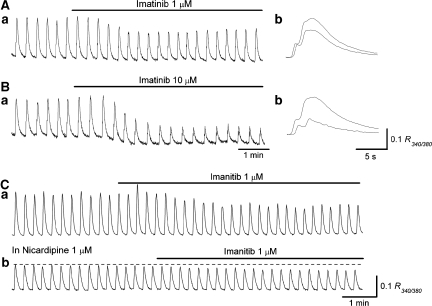
Figure 3.
Effects of imatinib on spontaneous Ca2+ transients in antral smooth muscle. In an antral preparation, imatinib (1 μM) reduced the amplitude of spontaneous Ca2+ transients (Aa, b). In the same preparation, imatinib (10 μM) suppressed Ca2+ transients and reduced basal Ca2+ level (Ba, b). In another antral preparation, imatinib (1 μM) reduced the amplitude of spontaneous Ca2+ transients (Ca). In the same preparation, which had been exposed to nicardipine (1 μM), imatinib (1 μM) was still capable of reducing the amplitude of the residual Ca2+ transients (Cb). In (Ab, Bb), overlaid traces (solid line in control, dotted line in Imatinib) show the effects of imatinib on Ca2+ transients configuration.
In five preparations, nicardipine (1 μM) reduced the amplitude of Ca2+ transients to 49.4±5.3% (from 0.21±0.023 R340/380 to 0.10±0.023 R340/380, P<0.05). In the preparations that had been exposed to nicardipine for 20 min, imatinib (1 μM) further reduced the amplitude of Ca2+ transients to 77.2±3.9% (from 0.096±0.018 R340/380 to 0.074±0.013 R340/380, P<0.05, n=5; Figure 3Ca and b).
Effects of imatinib mesylate on slow waves in the antrum
The effects of imatinib on slow waves were further investigated in the presence of nifedipine (1 μM) to exclude its possible action on L-type Ca2+ channels.
Imatinib (1 μM) had no effect on the amplitude of slow waves (29.1±1.4 mV in control, 28.1±2.2 mV in imatinib, n=5; Figures 4A and B), their half-width (from 6.0±1.9 to 6.0±1.9 s, n=5; Figure 4Ba and b) or frequency (from 4.0±0.89 to 4.1±1.04 min−1, n=5; Figure 4Aa and b). Also, it did not change the resting membrane potential (−66.1±2.4 mV in control, −65.8±1.1 mV in imatinib, n=5).
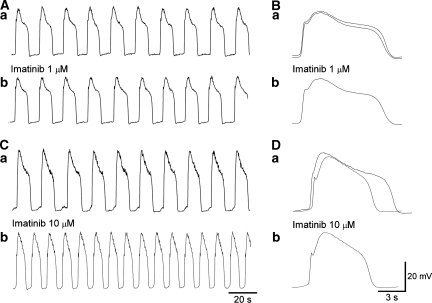
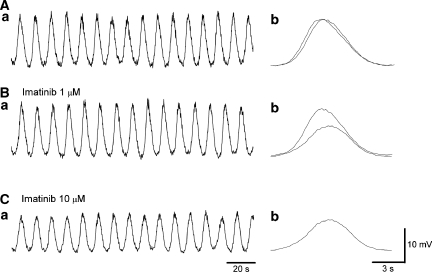
Figure 4.
Effects of imatinib on slow waves in the antrum. In an antral preparation that had been treated with nifedipine (1 μM) for 30 min, imatinib (1 μM) did not reduce the amplitude of slow waves, their frequency (Aa, b) or half-width (Ba, b). In another nifedipine-treated antral preparation, imatinib (10 μM) slightly reduced the amplitude of slow waves but increased their frequency (Ca, b). It also reduced the half-width of slow waves (Da, b). Traces in (B, D) are averaged traces of 25–30 slow waves each. Overlaid traces (solid line in control, dotted line in imatinib) show the effects of imatinib on slow wave configuration.
A higher concentration of imatinib mesylate (10 μM) slightly reduced the amplitude of slow waves to 93.4±3.7% (9.6±9.6 mV in control, 27.5±9.5 mV in imatinib, n=10, P<0.05; Figures 4C and D), reduced the half-width of slow waves to 84.6±4.1% (from 7.0±0.86 to 5.9±0.74 s, n=7, P<0.05; Figure 4Da and b), although increasing their frequency to 127.3±8.7% (from 4.5±0.71 to 5.7±0.97 min−1, n=7, P<0.05; Figure 4Ca and b) but did not change the resting membrane potential (−66.3±2.8 mV in control, −65.2±3.1 mV in imatinib, n=10).
Effects of imatinib mesylate on follower potentials in longitudinal smooth muscle of the antrum
To specify the effects of imatinib on slow waves further, its effects on follower potential in longitudinal smooth muscle, which is thought to represent the passive propagation of the pacemaker potential generated in ICC-MY (Dickens et al., 1999), were investigated in the presence of nifedipine (1 μM).
Imatinib (1 μM) did not reduce the amplitude of follower potentials (24.6±2.2 mV in control, 24.1±2.1 mV in imatinib, n=4; Figures 5A and B), their half-width (from 8.7±2.3 to 8.7±2.2 s, n=4; Figure 5Ba and b), dV/dtMax (from 0.12±0.017 mV ms−1 to 0.12±0.016 s, n=4), frequency (from 4.3±0.42 to 4.3±0.62 min−1, n=4; Figure 5Aa and b) or the resting membrane potential (−65.3±2.9 mV in control, −64.3±2.5 mV in imatinib, n=4).
Figure 5.
Effects of imatinib on follower potentials in longitudinal smooth muscle of the antrum. In a longitudinal smooth muscle preparation that had been treated with nifedipine (1 μM) for 30 min, imatinib (1 μM) did not reduce the amplitude of follower potentials, their frequency (Aa, b) or half-width (Ba, b). In another nifedipine-treated longitudinal muscle preparation, imatinib (10 μM) slightly reduced the amplitude of slow waves but increased their frequency (Ca, b). It also reduced the half-width of slow waves (Da, b). Traces in (B, D) are averaged traces of 25–30 follower potentials each. Overlaid traces (solid line in control, dotted line in imatinib) show the effects of imatinib on follower potential configuration.
A higher concentration of imatinib (10 μM) slightly reduced the amplitude of follower potentials to 89.1±6.9% (25.1±4.9 mV in control, 22.6±5.4 mV in imatinib, n=6, P<0.05; Figures 5C and D) without changing the resting membrane potential (−65.0±2.9 mV in control, −63.9±1.6 mV in imatinib, n=6). It reduced the half-width of follower potentials to 85.2±4.1% (from 7.5±0.92 to 6.4±0.52 s, n=6, P<0.05; Figure 5Da and b), although increasing their frequency to 138.1±13.5% (from 4.2±0.69 to 5.8±0.58 min−1, n=6, P<0.05; Figure 5Ca and b), but did not change their dV/dtMax (from 0.11±0.018 mV ms−1 to 0.11±0.016 s, n=6).
Effects of imatinib mesylate on slow potential in circular smooth muscle of the antrum
Effects of imatinib on slow potential in circular smooth muscle, which reflect the activity of ICC-IM without contribution of ICC-MY (Suzuki and Hirst, 1999), were investigated in the presence of nifedipine (1 μM). As slow potentials exhibited substantial variations in their amplitude or frequency between preparations, analysis was carried out on the recordings from a single cell in three preparations.
Representative effects of imatinib on antral slow potentials are summarized in Figure 6. Imatinib (1–10 μM) reduced the amplitude of slow potentials (P<0.05; Figures 6a–d) and their frequency (P<0.05; Figure 6f), without affecting their half-width (Figure 6e).
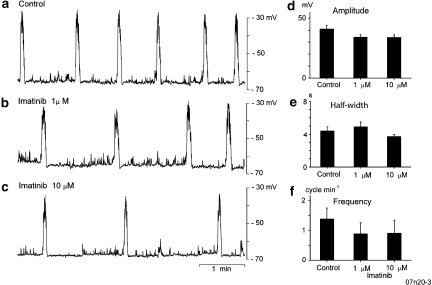
Figure 6.
Effects of imatinib on slow potentials in circular smooth muscle of the antrum. In a circular smooth muscle preparation of the antrum, slow potentials were generated (a). Imatinib (1–10 μM) reduced the amplitude and frequency of slow potentials without altering their half-width (b, c). These results are summarized in (d–f). These values were expressed as mean±s.d of at least 10 slow potentials in each experimental condition.
Effects of imatinib mesylate on slow waves in circular smooth muscle of the corpus
To investigate whether the effects of imatinib have any regional selectivity, its action on slow waves in circular smooth muscles of the corpus, which exclusively reflect the activity of ICC-IM (Hashitani et al., 2005), were investigated.
Imatinib (1 μM) did not alter the amplitude of corporal slow waves (19.3±6.1 mV in control, 18.7±6.1 mV in imatinib, n=5; Figures 7Ab and Bb), their half-width (from 3.9±0.11 to 4.0±0.16 s, n=5; Figures 7Ab and Bb), frequency (from 5.2±0.31 to 5.2±0.22 min−1, n=5; Figures 7Aa and Ba) or the resting membrane potentials (−55.7±2.8 mV in control, −55.1±1.8 mV in imatinib, n=5).
Figure 7.
Effects of imatinib on slow waves in circular smooth muscle of the corpus. In a corporal smooth muscle preparation that had been treated with nifedipine (1 μM), imatinib (1 μM) did not reduce the amplitude of slow waves, their frequency (Aa, Ba) or half-width (Ab, Bb). In the same preparation, imatinib (10 μM) reduced the amplitude of slow waves but did not alter either their frequency (Aa, Ca) or half-width of slow waves (Bb, Cb). Traces in (Ab, Bb, Cb) are averaged traces of 25–30 slow waves each. Overlaid traces (solid line in control, dotted line in imatinib) show the effects of imatinib on corporal slow wave configuration.
A higher concentration of imatinib mesylate (10 μM) reduced the amplitude of slow waves to 65.3±5.7% (22.5±5.7 mV in control, 14.7±4.0 mV in imatinib, n=8, P<0.05; Figures 7Bb and Cb) without changing the resting membrane potential (−57.1±2.3 mV in control, −56.6±2.7 mV in imatinib, n=8). However, it did not alter either the half-width of slow waves (3.8±0.26 s in control, 3.8±0.32 s in imatinib, n=8; Figures 7Bb and Cb) or their frequency (5.3±0.51 min−1 in control, 5.2±0.49 min−1 in imatinib, n=8; Figures 7Ba and Bb).
Discussion
In the present study, imatinib (1–10 μM) suppressed spontaneous phasic contractions in antral smooth muscles of the guinea-pig as it does in other types of smooth muscle. Simultaneous recordings of electrical and mechanical activity from antral muscle strips revealed that the suppression of spontaneous contractions with imatinib (1 μM) was not associated with a reduction in the amplitude of their corresponding slow waves, suggesting that imatinib suppresses spontaneous contractions by inhibiting pathways that increase [Ca2+]i in smooth muscles rather than by specifically acting on Kit signalling in ICC. This was supported by the finding that imatinib (1 μM) reduced the amplitude of spontaneous Ca2+ transients by about 25%. In preparations where L-type Ca2+ channels had been blocked with nicardipine, imatinib (1 μM) was capable of further suppressing the residual Ca2+ transients by about 20%, thus it was not possible to determine if imatinib acts exclusively on either L-type Ca2+ channels or the release of Ca2+ from intracellular stores.
In the stomach and other smooth muscles, ICC determine the rhythmicity of spontaneous contractile and electrical activity (Dickens et al., 1999), whereas contraction amplitude is largely attributed to Ca influx through L-type Ca channels on the smooth muscle cells (Dickens et al., 2000). Thus, inhibition of ICC with imatinib would be expected largely to reduce the frequency of spontaneous activity. However, in various other smooth muscles, imatinib predominantly reduces the amplitude of spontaneous contractions without altering their frequency much (Kubota et al., 2004; Popescu et al., 2006), in a manner similar to the effects of nifedipine (Dickens et al., 2000). We have preliminary data demonstrating that imatinib (10 μM) reduces the amplitude of Ca2+ transients in detrusor smooth muscle of the guinea-pig bladder by about 20% (H Hashitani et al., personal observations). As detrusor smooth muscles generate spontaneous action potentials, which exclusively rely on the opening of L-type Ca2+ channels (Hashitani and Brading, 2003) and associated Ca2+ transients (Hashitani et al., 2003), this suggests that imatinib (10 μM) could inhibit Ca2+ influx through L-type Ca2+ channels, although previous studies showed an apparent lack of inhibition on action potentials with this concentration of imatinib (Kubota et al, 2004, 2006). After all, interpretation of the effects of imatinib in in vitro experiments should be treated with caution, particularly for contractile studies.
Imatinib (1–10 μM) increased the frequency of antral slow waves and corresponding contractions. As the rhythmicity of antral slow waves is determined by ICC-MY, which generate driving potentials to compose the first components of slow waves (Dickens et al., 1999), this suggests that imatinib may act on ICC-MY. This was consistent with its effect on the frequency of follower potentials recorded from longitudinal smooth muscles, which more directly reflect driving potentials generated by ICC-MY (Dickens et al., 1999). The rising phase of driving potentials results from the opening of T-type-like, voltage-dependent Ca2+ channels (Kito et al., 2002) that are sensitive to nickel or mibefradil (Kito and Suzuki, 2003; Kito et al., 2005). Imatinib has been shown to block recombinant T-type Ca2+ channels expressed in human embryonic kidney-293 cells by a protein tyrosine kinase-independent mechanism (Cataldi et al., 2004). However, imatinib did not alter the maximum rise slope of follower potentials, although their duration was reduced upon inhibition of a sustained component. Both depolarization and Ca2+ influx during the initial components evoke following sustained components by opening Ca2+-activated chloride channels (Kito et al., 2002). Thus, the sustained components are selectively diminished by blockers of Ca2+-activated chloride channels or SERCA, or by membrane depolarizations (Kito et al., 2002). Imatinib did not affect either the initial components of driving potentials or electrical coupling between ICC-MY and adjacent circular or longitudinal smooth muscles. Rather, imatinib might inhibit either SERCA or the opening of Ca2+-activated chloride channels to shorten the duration of pacemaker potentials, as does cycliopazonic acid (Kito et al, 2002). Such an inhibition of Ca2+ release is consistent with the suppression of Ca2+ transients by imatinib in the presence of nicardipine. Alternatively, imatinib might cause the release of neurohumoral factors to accelerate the generation of slow waves and therefore follower potentials.
If imatinib has an inhibitory action on the Ca2+ release from intracellular stores and the subsequent activation of Ca-activated chloride channels, it would be expected to suppress ICC-IM activity, which contributes to the secondary, regenerative components of slow waves (Suzuki and Hirst, 1999). Although inhibition of the regenerative components with imatinib was not obvious in whole layer antral preparations, both antral slow potentials and corporal slow waves were clearly suppressed. As these electrical events are exclusively generated by ICC-IM (Suzuki and Hirst, 1999; Hashitani et al., 2005), our results suggest that imatinib inhibits ICC-IM activity selectively. However, imatinib did not alter the frequency of corporal slow waves. Therefore, imatinib may nonspecifically inhibit intracellular Ca2+ handling and associated activation of Ca-activated chloride channels rather than inhibit ICC activity through its action on the Kit signalling pathway. In isolated detrusor smooth muscle cells, imatinib inhibits outward K current in a Ca2+-dependent manner (Kubota et al., 2004), suggesting that it inhibits intracellular Ca2+ handling rather than Ca2+-activated chloride channels.
The effects of imatinib on slow waves are small; therefore, the Kit receptors, although necessary for maintaining the ICC phenotype, are not directly required for the pacemaker mechanism. This result is consistent with data from previous studies where neither imatinib (Beckett et al., 2007) nor ACK-2, an anti-Kit antibody (Rich et al., 2002), had an effect on slow wave activity. Although this insensitivity of ICC to imatinib (1–10 μM) seems to argue against the usefulness of imatinib as a specific inhibitor of Kit-dependent ICC activity, this effect may be beneficial for its clinical applications. A lower concentration of imatinib (0.1 μM), which does not affect the function of differentiated ICC or ICC-LCs distributed in many types of smooth muscle, effectively suppressed proliferation of the GIST cell line in culture (Mukaisho et al., 2001; Tuveson et al., 2001). This suggests that imatinib used at these low concentrations is unlikely to have unwanted effects on smooth muscle activity. In addition, imatinib could well be used for the treatment of overactivity in smooth muscles, a condition that has been correlated with pathological differentiations of ICC or ICC-LCs. In the guinea-pig bladder, outlet obstruction increases the population of ICC-LCs and their Kit immunoreactivity (Kubota et al., 2007), suggesting that the altered distribution of ICC-LCs may contribute to the pathophysiology of bladder overactivity. This may well explain why imatinib was more effective at inhibiting spontaneous and evoked contractions in overactive bladders than in normal bladders (Biers et al., 2006). In conclusion, acute application of imatinib in vitro is unlikely to suppress ICC activity specifically through its inhibition of Kit signalling, instead it probably induces this effect by inhibiting intracellular Ca2+ handling. As imatinib is able to inhibit pathways that increase [Ca2+]i in smooth muscle at concentrations that do not affect ICC activity, it seems inappropriate to infer the effects of imatinib on ICC or ICC-LC activity after monitoring only smooth muscle function. Rather the effects of imatanib on identified ICC or ICC-LC activity need to be investigated directly.
Acknowledgments
We thank Dr RJ Lang for his critical reading of the paper. This project was supported by Grant-in-Aid for Scientific Research (B) from JSPS to HH (no. 19390418).
Abbreviations
- [Ca2+]i
intracellular concentration of Ca2+
- ICC
interstitial cells of Cajal
- ICC-IM
intramuscular ICC
- ICC-MY
myenteric ICC
Conflict of interest
The authors state no conflict of interest.
References
- Beckett EA, Ro S, Bayguinov Y, Sanders KM, Ward SM. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–616. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- Cataldi M, Gaudino A, Lariccia V, Russo M, Amoroso S, di Renzo G, et al. Imatinib-mesylate blocks recombinant T-type calcium channels expressed in human embryonic kidney-293 cells by a protein tyrosine kinase-independent mechanism. J Pharmacol Exp Ther. 2004;309:208–215. doi: 10.1124/jpet.103.061184. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GD. Vagal inhibition in the antral region of guinea pig stomach. Am J Physiol Gastrointest Liver Physiol. 2000;279:G388–G399. doi: 10.1152/ajpgi.2000.279.2.G388. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576:707–714. doi: 10.1113/jphysiol.2006.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Garcia-Londono AP, Hirst GD, Edwards FR. Atypical slow waves generated in gastric corpus provide dominant pacemaker activity in guinea pig stomach. J Physiol. 2005;569:459–465. doi: 10.1113/jphysiol.2005.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Properties of spontaneous Ca transients recorded from interstitial cells of Cajal-like cells (ICC-LCs) of the rabbit urethra in situ. J Physiol. 2007;583:505–519. doi: 10.1113/jphysiol.2007.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2003;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, et al. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556:585–599. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005;288:C710–C720. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Biers SM, Kohri K, Brading AF. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006;25:205–210. doi: 10.1002/nau.20085. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, et al. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction Neurourol Urodyn 2007(in press) [DOI] [PubMed]
- Kubota Y, Kajioka S, Biers SM, Yokota E, Kohri K, Brading AF. Investigation of the effect of the c-kit inhibitor Glivec on isolated guinea-pig detrusor preparations. Auton Neurosci. 2004;115:64–73. doi: 10.1016/j.autneu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WF, Wendt I, Parkington HC. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695–705. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487–501. doi: 10.1113/jphysiol.2006.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaisho K, Miwa K, Totsuka Y, Shimomura A, Sugihara H, Wakabayashi K, et al. Induction of gastric GIST in rat and establishment of GIST cell line. Cancer Lett. 2001;231:295–303. doi: 10.1016/j.canlet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Popescu LM, Vidulescu C, Curici A, Caravia L, Simionescu AA, Ciontea SM, et al. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur J Pharmacol. 2006;546:177–181. doi: 10.1016/j.ejphar.2006.06.068. [DOI] [PubMed] [Google Scholar]
- Rich A, Hanani M, Ermilov LG, Malysz J, Belzer V, Szurszewski JH, et al. Physiological study of interstitial cells of Cajal identified by vital staining. Neurogastroenterol Motil. 2002;14:189–196. doi: 10.1046/j.1365-2982.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hirst GD. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]