Abstract
Background and purpose: The endogenous cannabinoid anandamide (AEA) acts at cannabinoid (CB1) and vanilloid (TRPV1) receptors. AEA also shows antinociceptive properties; although the underlying mechanism for this is not fully understood, both CB1 and TRPV1 may be involved. Voltage-activated Ca2+ channels in rat-cultured dorsal root ganglion (DRG) neurons are modulated by AEA. However, AEA in different populations of neurons enhanced or attenuated KCl-evoked Ca2+ influx; these effects were linked with soma size. The aim of this study was to determine how AEA or its metabolites might produce these variable responses.
Experimental approach: The whole cell patch-clamp technique and fura-2 Ca2+ imaging were used to characterize the actions of AEA on action potential firing and voltage-activated K+ currents and to determine whether AEA metabolism plays any role in its effects on cultured DRG neurons.
Key results: AEA attenuated multiple action potential firing evoked by 300 ms depolarizing current commands in a subpopulation of DRG neurons. Application of 1 μM AEA attenuated voltage-activated K+ currents and the recovery of KCl-evoked Ca2+ transients. The insensitivity of these responses to the CB1 receptor antagonist rimonabant (100 nM) and preincubation of DRG neurons with pertussis toxin suggested that these actions are not CB1 receptor-mediated. Preincubating DRG neurons with the fatty acid amide hydrolase (FAAH) inhibitor phenylmethylsulphonyl fluoride (PMSF) attenuated the inhibitory actions of AEA on K+ currents and Ca2+ influx.
Conclusion and implications: These data suggest that the products of AEA metabolism by FAAH contribute to the attenuation of K+ conductances and altered excitability of cultured sensory neurons.
Keywords: cannabinoids, sensory neurons, potassium channels, anandamide, fatty acid amide hydrolase, phenylmethylsulphonylfluoride, arachidonic acid, pain
Introduction
Anandamide (N-arachidonoyl-ethanolamide, AEA) was the first endogenous ligand of the G-protein-coupled cannabinoid receptors CB1 and CB2 to be discovered (Devane et al., 1992). AEA is rapidly hydrolysed within cells to arachidonic acid (AA) and ethanolamine by the microsomal enzyme fatty acid amide hydrolase (FAAH). It is also subject to metabolism by cyclooxygenase-2 (COX-2) (Yu et al., 1997). AEA acts as a partial agonist at both types of cannabinoid receptor; it has a slightly higher affinity for and greater efficacy at CB1 receptors. CB1 receptors are synthesized in the cell bodies of dorsal root ganglion (DRG) neurons before being transported along axons and inserted into central and peripheral terminals (Hohmann and Herkenham, 1999a). The profile of CB1 receptor expression on subtypes of DRG neurons is controversial. Ahluwalia et al. (2000, 2002) found that most CB1 receptors are expressed on the nociceptive fibres that arise from small- to medium-sized neurons but in contrast, others (Hohmann and Herkenham, 1999b; Bridges et al., 2003) found most CB1 receptors are expressed on medium- to large-sized neurons. CB2 receptors are also present in cultures of DRG neurons (Ross et al., 2001); they may be expressed on neurons or non-neuronal cells.
Cannabinoids have long been associated with the modulation of pain. Constituents of the cannabis plant, as well as synthetic and endogenous cannabinoids, are analgesic in both inflammatory and neuropathic pain (for review see Iversen and Chapman, 2002; Pertwee, 2004). Consistent with this, CB1 receptor antagonists, for example, rimonabant (SR141716A), can produce hyperalgesia in mice (Richardson et al., 1997), indicating that low levels of endogenous cannabinoid may be having a tonic effect that controls thresholds of nociception. However, others have found that rimonabant is both anti-inflammatory and antinociceptive (Croci and Zarini, 2007). Increases in levels of AEA are associated with pain, possibly reflecting endogenous pain control (Rice et al., 2002; Walker and Huang, 2002). Furthermore, FAAH inhibitors are antinociceptive in models of acute and inflammatory pain, an effect that is mediated by CB1 receptors. In line with this, mice lacking FAAH display a hypoalgesic phenotype (Cravatt and Lichtman, 2004). In the mouse tetrad model in vivo, AEA and its primary metabolite AA display characteristic hypomobility, hypothermia, catalepsy and antinociception, but these effects are not antagonized by the CB1 receptor antagonist rimonabant (Wiley et al., 2006). However, in the presence of inhibitors of AEA metabolism, the antinociceptive effect of AEA is attenuated but not completely reversed by rimonabant (Wiley et al., 2006). Furthermore, although the in vivo effects of AA were blocked by COX inhibitors, those of AEA were not. Taken together, these results suggest that CB1 receptors are not the only receptors involved in the antinociceptive properties of cannabinoids and that a component of AEA-mediated antinociceptive effects occur via mechanisms that differ from classical CB1 receptors.
CB1 receptor activation is linked to the inhibition of voltage-activated Ca2+ currents (VACCs) (Caulfield and Brown, 1992; Mackie and Hille, 1992; Vásquez and Lewis, 1999; Ross et al., 2001). AEA can cause CB1-mediated inhibition of N- and P/Q-type VACCs and can also act via CB1 receptors to activate inwardly rectifying K+ channels (Mackie et al., 1995). These mechanisms probably contribute to the analgesic actions of cannabinoids through attenuation of primary afferent neurotransmission and inhibition of excitability. As well as activating G-protein-coupled inwardly rectifying K+ channels via CB1 receptors, AEA can also attenuate A-type K+ channels by inhibiting the formation of cAMP. AEA may also be capable of directly interacting with K+ channels to inhibit their activity (Maingret et al., 2001; Di Marzo et al., 2002). Mu et al. (1999) also showed cannabinoid modulation of K+ currents in cultured hippocampal neurons, with WIN55212-2 enhancing IA and decreasing ID. Cannabinoids have effects on K+ conductances that are insensitive to pertussis toxin (PTX) and antagonists such as rimonabant (Ross et al., 2004). These effects may be by an unidentified receptor or by direct ion channel modulation. This modulation of K+ conductances has been associated with the antinociceptive properties displayed by cannabinoids and endocannabinoids.
We have previously shown that AEA can have a dual effect on KCl-evoked Ca2+ transients in DRG neurons, with one subpopulation showing an enhanced Ca2+ influx and another subpopulation showing a reduction. The enhancement in response to AEA was insensitive to PTX. In contrast, the reduction in Ca2+ influx in response to AEA was sensitive to PTX. This suggests that in a subpopulation of cultured DRG neurons, AEA is acting via a G-protein-coupled receptor to elicit an inhibitory effect on depolarization-evoked Ca2+ entry, whereas in another subpopulation, AEA has the opposite effect, which is not mediated by a PTX-sensitive mechanism (Evans et al., 2004). Here, we further investigate the modulation of K+ currents in DRG neurons by AEA, with regard to the possible involvement of both CB receptors and products of AEA metabolism.
Methods
DRG neuron culture
Primary cultures of neonatal rat sensory neurons from dorsal root ganglia were used in this study and were prepared as described previously (Ross et al., 2001). Briefly, 2-day-old neonatal Sprague–Dawley rats were decapitated, and cells within the dorsal root ganglia (∼40 per rat) were enzymatically (collagenase 0.125% for 13 min at 37 °C, trypsin 0.25%, 6 min) and mechanically dissociated. The sensory neurons were plated on to laminin–polyornithine-coated coverslips and bathed in F14 culture medium (Imperial Laboratories, Andover, UK) supplemented with 10% horse serum (Gibco, Paisley, Scotland), penicillin (50 U ml−1), streptomycin (5000 ng ml−1), NaHCO3 (14 mM) and nerve growth factor (NGF-2.5S; 20 ng ml−1). The DRG neurons were used between 1 and 7 days in culture. Some cultures were treated for 18–24 h with 500 ng ml−1 PTX to uncouple G-proteins from effectors, before experiments were conducted (Dolphin and Scott, 1987).
Electrophysiology
Electrophysiological experiments were conducted at room temperature (18–20 °C) using the whole-cell variant of the patch-clamp technique. The acute actions of AEA on voltage-activated K+ channel currents and the action potential firing properties were investigated. Patch pipettes with resistances of 3–9 MΩ were made from Pyrex borosilicate glass tubing (1.4–1.6 mm outer diameter, 0.8–1.0 mm bore with 0.15 mm fibre attached to the inside wall; Plowden and Thompson Ltd, Dial Glass Works, Stourbridge, UK) using a two-stage vertical microelectrode puller. An Axoclamp 2A switching amplifier (operated at 20 kHz) or Axopatch 1D amplifier (Axon Instruments Molecular Devices, Sunnyvale, CA, USA) was used and 80% series resistance compensation was applied. For all electrophysiological experiments, the patch pipettes were filled with the following KCl-based solution containing (in mM): 140 KCl, 0.1 CaCl2, 5 EGTA (ethylene glycol bis(β-aminoethylether)-N,N,N′,N′,-tetraacetic acid), 2 MgCl2, 2 ATP and 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). The pH and osmolarity of the patch pipette solution were corrected to 7.2 and 310–320 mosM l−1 with Tris and sucrose, respectively. The extracellular recording solution used contained (in mM) 130 NaCl, 2 CaCl2, 3 KCl, 0.6 MgCl2, 1 NaHCO3, 10 HEPES, 5 glucose and 0.01% dimethyl sulphoxide (DMSO). The pH and osmolarity of this extracellular bathing solution were corrected to 7.4 and 320 mosM l−1 with NaOH and sucrose, respectively. Some experiments were carried out in nominally Ca2+-free extracellular recording medium; this medium was the standard NaCl-based extracellular recording medium but without the addition of 2 mM CaCl2. Further experiments were conducted in the continual presence of the FAAH inhibitor phenylmethylsulphonyl fluoride (PMSF) (Deutsch et al., 2002; Sugiura et al., 2002), and standard NaCl-based extracellular recording medium containing 10 μM PMSF was used.
The low solubility of AEA in water necessitated the use of solvent, so DMSO was used to dissolve AEA, the final DMSO concentration in test solutions being 0.01%. For this reason, all experiments were conducted in the continual presence of DMSO (0.01%). Previously, we found that 0.01% DMSO had no acute or chronic effects on VACCs (Evans et al., 2004). Stock solutions of AEA, methanandamide (methAEA), PMSF, AA, WIN55212-2 and rimonabant were all made up at a concentration of 10 mM in 100% DMSO. Drugs were either continually present in the extracellular media or delivered by low-pressure ejection from a blunt pipette positioned about 50–100 μm away from the cell being recorded.
For recordings made under single-electrode voltage clamp, current–voltage relationships were generated between –130 and +80 mV from a holding voltage (Vh) of –70 mV. Action potential properties were investigated in current clamp mode. Continuous hyperpolarizing current (−30 to −120 pA) was applied to hold the membrane potential at –70 mV. Action potentials were evoked every 30 s by 300–500 ms depolarizing current commands (+100 to +800 pA).
Fura-2 calcium imaging
For intracellular Ca2+ imaging, DRG neurons were incubated for 1 h in NaCl-based extracellular solution containing (in mM) 130 NaCl, 3 KCl, 0.6 MgCl2, 2 CaCl2, 1 NaHCO3, 10 HEPES, 5 glucose and 0.01 fura-2AM (1 mM stock in dimethylformamide). The pH was adjusted with NaOH to 7.4 and the osmolarity to 310–320 mosM l−1 with sucrose (Sutton et al., 2002). After the neurons had been loaded with fura-2, the cultures were washed three times with normal extracellular recording medium and preincubated in NaCl-based extracellular solution containing 0.01% DMSO and either AEA, PMSF, AA or WIN-55212 for 20 min. For DRG neurons treated with both PMSF and AEA, a 20-min preincubation with PMSF preceded the 20-min preincubation with AEA and PMSF. Cultures were protected from light during the incubation periods, before the start of the experiment. Some cultures were preincubated overnight with PTX as above. The neurons were then moved to the imaging set up and constantly perfused (1–2 ml min−1) with NaCl-based extracellular solution containing 0.01% DMSO and the drug treatment of interest. Preincubation protocols were used because the electrophysiological data showed that the inhibitory modulation of K+ currents developed over a 20-min period. Additionally, preincubation with AEA might reduce potential problems produced by the acute activation of CB1 and transient receptor potential vanilloid type 1 (TRPV1) receptors, but we cannot rule out a longer term influence of receptor crosstalk (Evans et al., 2007). The Ca2+ imaging experiments were conducted to evaluate the decay of Ca2+ transients, which, in part, reflect membrane potential repolarization through K+ channel activity. The cultures were stimulated for 15 s with high KCl–Na+ recording medium that contained (in mM) 103 NaCl, 60 KCl, 0.6 MgCl2, 2 CaCl2, 1 NaHCO3, 10 HEPES, 5 glucose and 0.01% DMSO (adjusted with NaOH and sucrose to give pH 7.4 and 310–320 mosM l−1). A concentration of 60 mM KCl was used instead of 30 mM in most of the experiments, as evidence from studies using soft coral preparations that contained carboxy-1-methyl pyridinium (trigonelline) indicated that some neurons do not fire action potentials or show an increase in intracellular [Ca2+] in response to 30 mM KCl unless also exposed to another stimulant (Temraz et al., 2006).
The mean time taken for the Ca2+ transients evoked by 60 mM KCl to recover by 90% of their peak amplitude was assessed under control conditions. This time was then used as a point of reference for neurons in subsequent experiments when drugs were applied, and the percentage recovery occurring in this time frame was determined under each experimental condition.
Neuron size was also determined so that response profiles could be correlated with soma areas. All experiments were conducted at room temperature.
Materials
Collagenase, trypsin, laminin–polyornithine, penicillin, streptomycin, NGF-2.5S, PTX, DMSO, AEA, AA, PMSF and fura-2AM were obtained from Sigma (Poole, UK); F14 culture medium from Imperial Laboratories; 10% horse serum from Gibco; methAEA and WIN55212-2 ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)-pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone) from Tocris Cookson (Avonmouth, UK); rimonabant from Sanofi Recherche (Paris, France). The Pyrex borosilicate glass tubing was purchased from Plowden and Thompson Ltd (Dial Glass Works); vertical microelectrode puller (Model 730) from David Kopf Instruments (Tujunga, CA, USA); and Axopatch 1D amplifier from Axon Instruments.
Data analysis
Electrophysiological data were captured and stored on digital audiotape using a Biologic digital tape recorder (DTR 1200). Analysis of data was performed off-line using Cambridge Electronic Design voltage clamp analysis software (version 6.0). All voltage-activated K+ currents had scaled linear leakage and capacitance currents subtracted to obtain values for the net outward K+ current.
The intracellular Ca2+ imaging data were analysed with OraCal pro, Merlin morphometry temporal mode (Life Sciences Resources, Brock University, Ontario, Canada). Both electrophysiological and intracellular Ca2+ imaging data are given as means±s.e.mean, and statistical significances were determined using Student's paired or independent t-test or ANOVA as appropriate. One-way ANOVA followed by Newman–Keuls post-test was used for multiple comparisons of normalized Ca2+ imaging data. The Fisher test was also used to assess differences between population response patterns.
Results
Inhibition of voltage-activated potassium currents by AEA
The stability of voltage-activated potassium currents in the presence of 0.01% DMSO vehicle was assessed. Neurons were voltage clamped at –70 mV, and potassium currents evoked by 100 ms depolarizing voltage steps to +30 mV every 30 s for 15 min. No significant change in the mean peak K+ current amplitude was observed over this time. The mean initial outward K+ current (recorded immediately on entering whole-cell recording configuration) was 3.25±0.50 nA, after 7 min the current amplitude was 3.34±0.41 nA and at 15 min it was 2.93±0.36 nA (n=6).
Under all experimental conditions described below, AEA predominantly had an inhibitory effect on K+ currents. However, a small proportion of DRG neurons under a variety of experimental conditions showed an enhancement of K+ current (∼40%) in response to AEA. At 10 nM, in 2 out of 6 neurons, AEA enhanced K+ current; at 1 μM AEA, 2 out of 14 neurons showed an increase during the 3–5 min drug application. The inhibitory actions of AEA were analysed separately from the enhancements, and the increases in K+ current evoked by AEA will not be considered in detail in this paper.
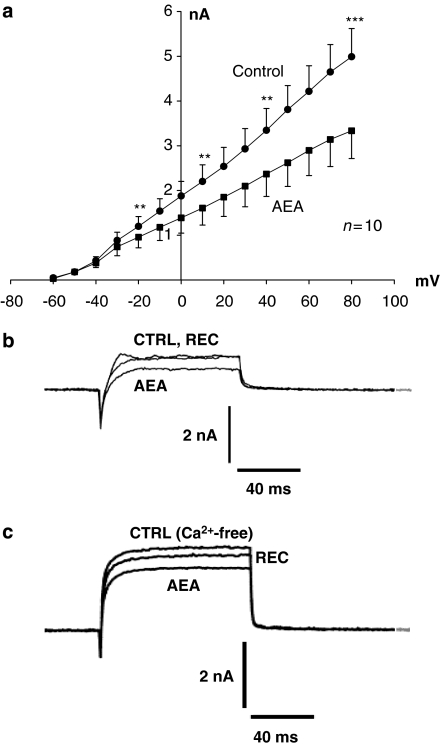
Three to six minutes application of 1 μM AEA reduced the mean peak K+ current by 22±3% (n=12) from a control level of 2.77±0.44 nA at +30 mV to 2.22±0.39 nA (n=12; considering only inhibitory actions of AEA, P<0.001, Student's paired t-test). Complete net current–voltage relationships were constructed for test potentials between –60 and +80 mV for 10 neurons under control conditions and after AEA treatment (Figure 1a). The inhibition of K+ current by AEA was consistent across voltages from –20 to +80 mV (P<0.009 across the stated range, n=10, Student's paired t-test). Recoveries of between 25 and 100% were observed in five neurons after varying time periods up to 40 min (Figure 1b). However, it was noted that in some cells, the inhibitory action of AEA continued to develop for 10–20 min after AEA was no longer being applied. This was not due to rundown of the K+ current because subsequent recovery could still take place. The mean additional percentage inhibition seen 15 min after removing the perfusion pipette containing AEA was 30±5% (n=3). We hypothesized that this additional, delayed effect was due to the persistence of either low concentrations of poorly soluble AEA or to the possible formation of active AEA metabolites within the neurons.
Figure 1.
Anandamide (AEA) inhibits voltage-activated K+ currents recorded from cultured dorsal root ganglion (DRG) neurons from neonatal rats. (a) Mean current–voltage relationships (±s.e.mean, n=10) for K+ currents elicited from a holding potential of –70 mV by voltage step commands to potentials between –60 and +80 mV under control conditions, and a 3–5 min application of 1 μM AEA. Significant inhibition (Student's paired t-test; **P<0.01 and ***P<0.005) of K+ current amplitude by 1 μM AEA was evident at potentials between –20 and +80 mV. Inset trace (b) shows currents recorded from one neuron under control conditions, following a 5-min application of 1 μM AEA, and recovery of K+ current after 5 min. (c) Currents recorded from one neuron under nominally Ca2+-free conditions, following a 5-min application of 1 μM AEA, and recovery of K+ current after 5 min.
Previously, we found that 1 μM AEA inhibited VACCs (Evans et al., 2004). The above results could be explained by an indirect mechanism through inhibition of Ca2+ channels and attenuation of Ca2+-activated K+ channel activity because of reduced Ca2+ entry. To determine the influence of Ca2+-activated potassium conductances, the actions of AEA were assessed in a nominally Ca2+-free recording medium. In Ca2+-free recording medium, the mean peak K+ current amplitudes were 2.46±0.60, 1.93±0.60 and 2.74±0.79 nA for control currents, AEA treated and recoveries, respectively (n=8; P<0.005). This represents a significant reduction of 25±5% in peak K+ current amplitude induced by AEA (Figure 1c). Control K+ current amplitudes in Ca2+-free recording conditions and the level of AEA-evoked inhibition were not significantly different from those recorded from DRG neurons in standard extracellular medium that contained Ca2+. The persistent inhibition of K+ current in Ca2+-free conditions indicates that AEA was having a genuine primary effect on potassium conductances, and not merely acting through a secondary mechanism involving Ca2+ channel inhibition.
The cannabinoid CB1 receptor is coupled to Gi−o-proteins, and the CB1-mediated effects have been shown to be sensitive to PTX treatment in DRG neurons (Ross et al., 2001). To determine whether AEA-induced inhibition of K+ current was via a Gi−o-dependent mechanism, the effects of AEA were investigated in cultured DRG neurons pretreated with PTX. The mean control current amplitude in PTX-treated neurons was 2.29±0.47 nA (n=11), and not significantly different from control currents in untreated neurons (one-way ANOVA). After the application of 1 μM AEA, the mean K+ current amplitude was reduced to 1.74±0.47 nA (30±5%; P<0.0001). This AEA-evoked inhibition was partially reversible; recoveries (6–100%) were recorded in 6 of 11 neurons. The persistence of AEA-induced inhibition of K+ current in PTX-treated neurons (Figure 2a) suggests that this effect of AEA was not mediated through inhibitory G-proteins. This is similar to the failure of PTX treatment to abolish AEA-induced potentiation of Ca2+ flux that we observed previously (Evans et al., 2004).
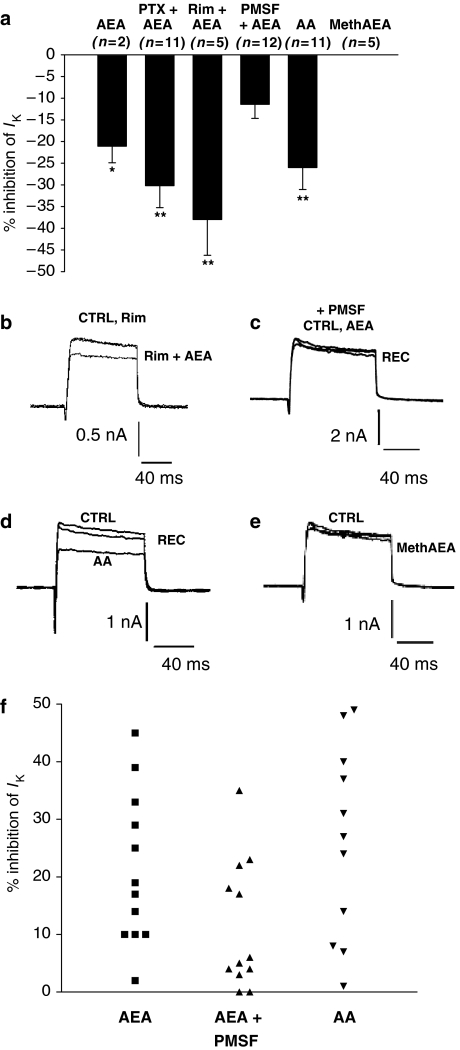
Figure 2.
Anandamide (AEA) metabolites appear to be involved in the inhibition of voltage-activated K+ currents. (a) The mean (±s.e.mean) percentage inhibition of voltage-activated K+ currents recorded at a clamp potential of +30 mV (n-values are given for each condition; * indicates significant inhibition from control currents, *P<0.05; **P<0.01). Data show that AEA responses were not attenuated by PTX treatment or rimonabant, that PMSF treatment does inhibit AEA responses and that AA but not methanandamide (methAEA) mimic the actions of AEA. (b) Current records under control conditions (CTRL) in the presence of rimonabant (Rim) and in the presence of rimonabant and anandamide (Rim+AEA). (c) Current records in the continual presence of phenylmethylsulphonyl fluoride (PMSF), before (CTRL) and during (AEA) AEA treatment, and during recovery from AEA (REC). (d) Current records under control conditions (CTRL) in the presence of arachidonic acid (AA) and during recovery from AA (REC). (e) Current records under control conditions (CTRL) in the presence of methanandamide (methAEA). All currents shown in panels b–e were activated at +30 mV from a resting holding potential of –70 mV. (f) Scatter diagram showing the variability of percentage inhibition of K+ currents (IK) activated at a clamp potential of +30 mV produced by anandamide alone (AEA), AEA+PMSF together and AA alone.
Furthermore, experiments with the CB1 receptor antagonist rimonabant showed that the AEA-evoked inhibition was not sensitive to this drug. A 3–5 min application of rimonabant (100 nM) had no significant effect on K+ current amplitude (n=7). However, subsequent application of rimonabant with 1 μM AEA resulted in significant inhibition (38±8%) of the mean peak K+ current activated at +30 mV from 3.62±0.74 to 2.44±0.66 nA (n=5, P<0.005; Figures 2a and b). The data from experiments with PTX-treated DRG neurons combined with rimonabant indicate that the effects of AEA on K+ current are probably not mediated through G-protein-coupled CB1 receptors.
A role for AEA metabolites in the modulation of potassium conductances in cultured DRG neurons
The development of further inhibition of K+ current after AEA was removed raised the question of whether metabolic products of AEA might be involved in the response. To investigate a role for downstream metabolites of AEA, we firstly determined whether the irreversible hydrolase–protease inhibitor PMSF, which has been shown to inhibit FAAH and prevent AEA metabolism (Deutsch et al., 2002), would influence AEA-evoked inhibition of K+ currents.
PMSF was used at 10 μM, as this was the highest concentration that was soluble in 0.01% DMSO under our recording conditions. The mean amplitude of the K+ current recorded in PMSF-treated neurons was 2.65±0.35 nA (n=18) and not significantly different to the mean control current in untreated neurons (2.95±0.32 nA; n=37; one-way ANOVA).
AEA (1 μM) was applied with PMSF (10 μM) to neurons that had been continually bathed with PMSF containing media for a minimum of 30 min. The mean K+ current amplitude was 2.94±0.69 nA in PMSF alone and after application of 1 μM AEA, the current amplitude was 2.39±0.46 nA (n=7, NS (nonsignificant); Figures 2a and c). Although the mean data show no significant inhibition of the K+ current, some reversible inhibition was observed in individual neurons. Clear recovery was seen, and 3–6 min after removing the AEA-containing perfusion pipette, the mean current amplitude was 2.92±0.62 nA (n=6). Hydrolysis of AEA by FAAH produces ethanolamine and AA, and the next stage in this study was to determine whether either of these metabolites mimicked the effect of AEA on K+ currents. The mean K+ current amplitudes in this experiment were 3.70±0.90 and 2.77±0.73 nA under control conditions and after application of 1 μM AA, respectively (n=11, P<0.005, Student's paired t-test). This represents a mean reduction of current by 26±5% (Figures 2a and d). Recoveries of between 25 and 100% were observed in seven of the neurons after washout of AA (mean recovery of 72±13%). No enhancement in the K+ current was observed in the presence of AA. Similarly, ethanolamine (1 μM) application also induced significant inhibition of 18±4% (n=8; P<0.05) of the mean peak K+ current. Another approach to investigating the role of metabolites of AEA in the inhibition of K+ current was to test the action of a methAEA, a non-hydrolyzable analogue of AEA, and a potent CB1 receptor agonist (Abadji et al., 1994). Our data clearly showed that a 4–5 min application of 1 μM methAEA had no inhibitory effects on K+ current on any of five DRG neurons studied (Figures 2a and e) and interestingly in two cases, methAEA increased current amplitude by 8 and 18%, respectively.
Some caution is required in the interpretation of the AEA and PMSF data because of the heterogeneity of DRG neurons and the distinctive responsiveness to drugs. The scatter diagram (Figure 2f) indicated that there was considerable variation in the inhibition produced by AEA and AA. Additionally, the responses to AEA in the presence of PMSF varied greatly from one DRG neuron to another, which could be indicative of several mechanisms of action.
Effects of AEA on neuronal repolarization
The time courses of intracellular Ca2+ transients may be influenced by many physiological factors, but we expected that the inhibition of voltage-activated K+ currents might slow membrane potential repolarization after a KCl stimulus and prolong Ca2+ influx through voltage-activated Ca2+ channels. Some evidence for the inhibition of K+ currents resulting in longer lasting depolarization-evoked intracellular Ca2+ transients has come from using the K+ channel blocker tetraethylammonium chloride (TEA; McClelland et al., 2004). Even a quite low concentration of 5 mM TEA prolonged KCl-evoked Ca2+ transients by 25–100%. To assess the potential consequences of the AEA-induced inhibition of K+ conductances, fura-2 Ca2+ imaging experiments were performed on DRG neurons transiently depolarized with KCl. Preliminary experiments showed that AEA prolonged Ca2+ transients evoked by 30 mM KCl and altered the responsiveness of neurons to subsequent stimulation with 60 mM KCl (Figure 3). More detailed analysis was conducted, in which the effects of AEA on the recoveries of Ca2+ transients evoked by depolarizing 60 mM KCl stimuli were assessed. Directed by the whole-cell recording experiments that showed long-term development of responses, for Ca2+ imaging experiments, neurons were incubated with the appropriate test compound(s) for 20 min before beginning the experiment, and the drug remained present throughout the recordings.
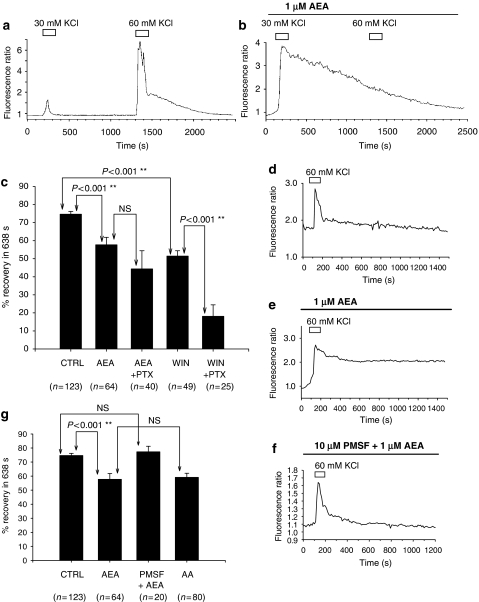
Figure 3.
Anandamide (AEA) attenuated the recovery of Ca2+ transients evoked by depolarization (60 mM KCl). (a) Shows a record from a single representative dorsal root ganglion (DRG) neuron of Ca2+ transients evoked by 30 and 60 mM KCl under control conditions. (b) A record from a different neuron of a prolonged Ca2+ transient evoked by 30 mM KCl applied in the continued presence (20 min) of 1 μM AEA. Under these conditions, 60 mM KCl failed to evoke a second response. (c) Comparisons of mean (±s.e.mean and n-values) percentage recovery in 638 s as a measure of the recovery of Ca2+ transients by 90% recorded under control conditions in the presence of 1 μM AEA alone, in DRG neurons pretreated with PTX and then exposed to AEA (AEA+PTX) in the presence of 1 μM WIN55212-2 alone (WIN) and in neurons pretreated with PTX and then exposed to WIN55212-2 (WIN+PTX). AEA significantly attenuated recovery from depolarization evoked by 60 mM KCl. This effect was not abolished by PTX treatment of neurons and was mimicked by WIN55212-2. In PTX-treated neurons, the effects of WIN55212-2 on repolarization were significantly enhanced. Significance was determined by one-way ANOVA. (d–f) Individual records of intracellular Ca2+ transients from neurons illustrating typical responses seen under control conditions (d), in the presence of 1 μM AEA (e) and in neurons treated with 10 μM phenylmethylsulphonyl fluoride (PMSF) and then 1 μM AEA (f). (g) Comparisons of mean (±s.e.mean and n-values) percentage recovery in 638 s as a measure of the recovery of Ca2+ transients recorded under control conditions in the presence of 1 μM AEA alone, in DRG neurons pretreated with PMSF together with (AEA+PMSF) and in the presence of 1 μM arachidonic acid (AA). AEA slowed recovery of the KCl-evoked Ca2+ transients, an effect attenuated by pretreatment of neurons with PMSF, and mimicked by AA. Significance values presented were determined by one-way ANOVA.
In initial experiments on control neurons, the mean time taken for the Ca2+ transient evoked by 60 mM KCl to recover by 90% of its peak amplitude (T90%) was found to be 638±50 s (n=41). To confirm that the time taken to recover from the stimulus was not merely a product of the amplitude of the response, or of neuronal soma size, correlation analyses were performed on the control population data. No linear relationship was observed between recovery time and peak amplitude of the Ca2+ transient evoked by 60 mM KCl or soma area in the population of control neurons used to determine T90%. These observations support the use of the T90% values in assessing recovery rates independently of these variables. With each experimental run using the drug preincubation protocols, the percentage recovery occurring in 638 s was determined. In this series of experiments, no intraexperimental controls were possible, so for every culture preparation on which drug-treated experiments were performed, an additional control run was also carried out. On completion of this series of experiments, all control data were accumulated, and percentage recovery occurring in 638 s determined to provide a reference point for comparison (Figure 3c). This complex approach to the data analysis was taken because, although the responses to 60 mM KCl stimuli were clearly transient (Figure 3d), in some experimental situations various neurons had very prolonged Ca2+ responses (Figure 3e). Neurons treated with 1 μM AEA for 20 min, before the start of the recording and throughout the experiment had Ca2+ transients that recovered by 57±4% in 638 s (n=64) compared with the mean control value of 74±1% (n=123). This indicated that AEA significantly slowed the recovery of the Ca2+ transient evoked by 60 mM KCl (P<0.001, one-way ANOVA, Figures 3c and e). The data were examined for any evidence of different responses in subpopulations of DRG neurons but none was found. The soma areas of neurons that had Ca2+ transients that decayed by less than 50% in 638 s were compared with neurons that showed decays of more than 50%. The mean area of neurons with slow recoveries under 50% was 275±56 μm2 (n=22), and for neurons with faster recoveries, greater than 50%, mean soma area was 234±73 μm2 (n=42, NS, Student's unpaired t-test).
A potential role for inhibitory G-proteins was investigated by using PTX-treated DRG neurons. PTX treatment alone had no effect on the recovery of Ca2+ transients. For PTX-treated neurons, the mean recovery of the Ca2+ transient in 638 s was 81±5% (n=20), a recovery rate not significantly different from controls (one-way ANOVA). The mean recovery in 638 s for neurons pretreated with PTX and exposed to 1 μM AEA was 44±10% (n=40; Figure 3c). Therefore, the effects of AEA in neurons treated with PTX remained significantly different from control values (P<0.001, one-way ANOVA), but not different from those assessed in the AEA experiment on naive neurons not pretreated with PTX. These data showed that PTX did not abolish the effect of 1 μM AEA on repolarization after KCl stimulation.
The above protocol was then repeated using the synthetic CB1 receptor agonist WIN55212-2 (1 μM) in place of AEA, with the aim of ruling out a role for this receptor in the AEA response (Figure 3c). In neurons treated for 20 min with 1 μM WIN55212-2, the mean recovery in 638 s was 51±3% (n=49). This represents a significant slowing of the decay rate of the KCl response as compared with control values (P<0.001, one-way ANOVA) and is not statistically different from the recovery value obtained from neurons treated with 1 μM AEA. In contrast to expectations, the synthetic cannabinoid receptor agonist mimicked the actions of AEA on the repolarization phase of the transient depolarization-evoked response. However, the effects of WIN55212-2 were also assessed in PTX-treated neurons. In PTX- and WIN55212-2-treated DRG neurons, mean recovery value in 638 s was 18±6% (n=25). This result was significantly different from control recovery values (P<0.001, one-way ANOVA), and PTX treatment of neurons significantly enhanced the effect of WIN55212-2 in slowing the repolarization phase of the transient response (P<0.001, one-way ANOVA). This action of WIN55212-2 was clearly not mediated through a PTX-sensitive G-protein-coupled receptor, and we have no explanation as to why WIN55212-2 should have such a strong effect in PTX-treated neurons.
The role of AEA metabolites in AEA-induced inhibition of repolarization was also investigated by blocking FAAH activity, and by investigating the ability of AA to mimic the actions of AEA. Firstly, the effects of treating neurons with 10 μM PMSF were assessed in the absence of AEA; 42 neurons were tested under this condition, and the mean recovery value in 638 s was 82±2%. This is not statistically different from the recovery value in control neurons (one-way ANOVA), indicating that PMSF was not affecting the decay of KCl-evoked Ca2+ transients in its own right.
Secondly, 1 μM AEA was applied to PMSF-treated DRG neurons. Neurons were first treated with 10 μM PMSF for 20 min and then with a combined 10 μM PMSF and 1 μM AEA solution for a further 20 min before stimulation with 60 mM KCl. Both PMSF and AEA remained in the extracellular recording media throughout the experiments. The mean recovery value in 638 s for these DRG neurons was 77±4% (n=20), which is not significantly different from control recovery values, but is significantly different from the recovery rates observed for neurons treated with 1 μM AEA in the absence of PMSF (57±4%, n=64, P<0.05, one-way ANOVA; Figures 3g and f).
The ability of 1 μM AA to mimic the actions of AEA on Ca2+ transient recovery was next examined. Neurons were treated with 1 μM AA in the way described previously for AEA. The mean recovery value in 638 s for neurons in this experiment was 55±3% (n=80). This represents a prolonged recovery time with respect to control values (P<0.001, one-way ANOVA; Figure 3g), which was not significantly different from that seen in neurons treated with 1 μM AEA.
The data from calcium imaging studies are thus consistent with those obtained in patch-clamp recording experiments and suggest that an AEA-induced inhibition of K+ conductances (as evidenced by a slower recovery from responses to 60 mM KCl-evoked depolarization) is prevented by blocking the activity of FAAH with PMSF and mimicked by the application of AA, an immediate downstream product of AEA metabolism by FAAH.
Ethanolamine also slowed the recovery of Ca2+ transients evoked by 60 mM KCl, although the effect was less pronounced compared to the actions of AEA and AA. The mean recovery value in 638 s for neurons in this experiment was 68±4% (n=18).
In contrast to AEA, 1 μM methAEA did not alter the recovery phase of the KCl-evoked Ca2+ transients. In the continual presence of methAEA, the mean percent recovery in 638 s of the Ca2+ transients was 88±4% (n=9) and not significantly different from the recovery observed under control conditions.
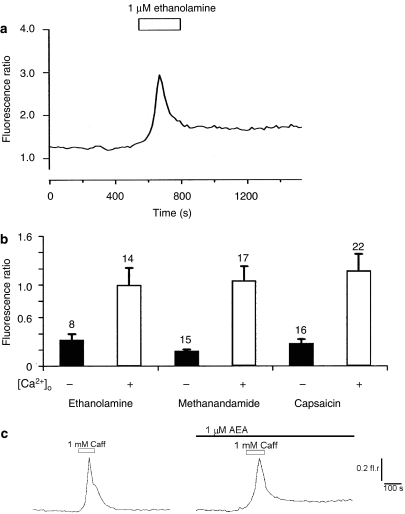
Additionally, ethanolamine alone evoked transient increases in intracellular Ca2+ concentration (Figure 4a). Acute responses to ethanolamine were compared with the actions of methAEA and the TRPV1 agonist capsaicin. Figure 4b shows that both in the presence and absence of extracellular Ca2+, ethanolamine, methAEA and the TRPV1 agonist all produced similar increases in intracellular Ca2+. The mean amplitudes of these responses were similarly reduced in nominally Ca2+-free conditions. However, there was no correlation between responsiveness of DRG neurons to the three drugs. Out of 34 DRG neurons that responded to at least one drug, 15 responded to ethanolamine, 17 responded to methAEA and 22 responded to capsaicin. Only 2 out of 34 neurons responded to all three drugs but three, three and eight neurons responded only to ethanolamine, methAEA or capsaicin, respectively. Additionally, some neurons responded to every combination of only two of the drugs tested.
Figure 4.
Ethanolamine produced complex actions on intracellular Ca2+ in cultured dorsal root ganglion (DRG) neurons. (a) Example record showing an increase in intracellular Ca2+ evoked by 1 μM ethanolamine applied alone. (b) A comparison of increases in intracellular Ca2+ levels evoked by ethanolamine, methanandamide and the TRPV1 agonist capsaicin in the presence and absence of extracellular Ca2+ [Ca2+]o. Although there appears to be similar patterns of activity, different populations of DRG neurons responded to these ligands. The mean±s.e.mean and n-values of responding cells are given. Significantly larger responses (P<0.05) were seen with Ca2+ present in the extracellular environment compared with nominally Ca2+-free conditions, but no significant differences were seen when comparing responses to each of the drugs. (c) Treatment of DRG neurons with 1 μM AEA had no obvious effect on the dynamics of Ca2+ release from caffeine-sensitive stores, although the late phase of recovery was incomplete this may relate to a voltage-dependent component of Ca2+ entry. Records show responses to 1 mM caffeine recorded in the absence and presence of AEA.
The observed effects of AEA on the repolarization phase of the Ca2+ transient were consistent with an attenuation of voltage-activated K+ currents. However, this effect could also be explained, in part, by an action of AEA on Ca2+ homeostasis, particularly any effect on Ca2+ release from stores or reuptake into stores. To assess whether intracellular Ca2+ store activities could be contributing significantly to the observed effect, caffeine responses were examined in naive DRG neurons and in those treated with 1 μM AEA for 20 min. No obvious difference in caffeine-sensitive release mechanisms was evident, with caffeine producing similar-sized Ca2+ transients. However, in some neurons exposed to AEA the late stage of the recovery phase of Ca2+ transients was delayed (Figure 4c). Although this might reflect an influence of AEA on Ca2+ reuptake, the change in response duration is very modest compared to the effects seen with AEA slowing the decay of Ca2+ transients evoked by 60 mM KCl stimuli.
The influence of AEA on neuronal excitability—action potential firing
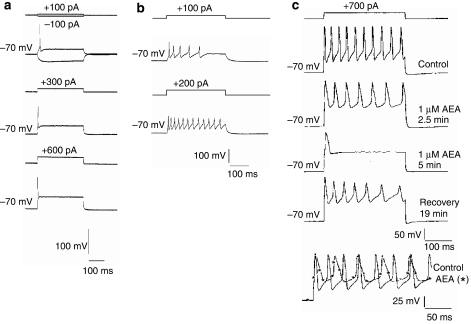
Under our culture and recording conditions, the majority (∼80%) of DRG neurons fired only a single action potential in response to a depolarizing stimulus, regardless of the amplitude of the stimulus (Figure 5a). However, a subpopulation of approximately 20% of DRG neurons (Figure 5b) fired a train of action potentials (these neurons we call ‘multiple firers') in response to a prolonged depolarizing stimulus. A supramaximal stimulus can be applied repeatedly, and provided the frequency is not too high (∼0.033 Hz), the number of action potentials fired by the neurons remained constant over a period of 20–30 min.
Figure 5.
Anandamide attenuates multiple firing in a subpopulation (∼20%) of cultured dorsal root ganglion (DRG) neurons. (a) The majority of cultured DRG neurons fired only one action potential in response to 300 ms depolarizing current commands, regardless of increasing stimulus amplitudes increasing from +100 pA to +300 pA or +600 pA. (b) A minority of DRG neurons fired a train of action potentials in response to a 300 ms depolarizing current step. The number of action potentials fired increased with increasing stimulus amplitude (in this example +100 pA gives five action potentials and +200 pA gives 12), up to a set point determined by frequency and the duration of the stimulus. A stable number of potentials were then fired in response to a supramaximal stimulus. (c) Application of 1 μM AEA reduced the number of action potentials fired in response to a 300 ms depolarizing current step from 10 to 1 over a period of 5 min. After a recovery period of 19 min, the multiple firing behaviour was partially restored. The bottom trace in panel c shows two superimposed records from the same neuron, under control conditions, following a 2-min AEA application (*) overlaid for comparison. AEA slowed the rate of firing, increased action potential duration and lessened the extent of the repolarization phase of the action potentials.
The effects of AEA on multiple firing properties of DRG neurons were assessed to provide some indication of what the overall effect of AEA on neuronal excitability may be. From resting membrane potentials of approximately –60 mV, constant hyperpolarizing current was injected to hold neurons at –70 mV. Action potentials were activated by 300 ms supramaximal current step commands of between +100 and +800 pA applied every 30 s, and stable multiple firing (of between 3 and 12 action potentials during 300 ms) was obtained before AEA, AA or ethanolamine were applied. In neurons investigated in this manner, AEA at both 1 μM and 100 nM consistently reduced the number of action potentials fired in response to a stable stimulus. For example, the number of action potentials evoked in 300 ms was reduced from between 5 and 11 to 1 during the 3–6 min application of AEA (1 μM, n=3 and 100 nM, n=3). Figure 5c shows an example of one such neuron, in which the multiple firing behaviour was restored following a recovery period after AEA application. It was also noted that AEA application apparently slowed the repolarization phase of the action potentials, consistent with inhibition of K+ conductances. AA (1 μM) produced very similar effects to AEA, reducing evoked multiple action potential firing in 7 out of 8 DRG neurons from 4–11 to 1–3 action potentials. Data obtained with ethanolamine were less consistent, but still 3 out of 6 neurons showed a marked decrease in action potentials from 3–11 under control conditions to 1–2 in the presence of ethanolamine. These data show that both metabolites of AEA have the potential to reduce neuronal excitability.
Discussion
Our previous investigations into the effects of AEA on the membrane properties of cultured DRG neurons revealed a number of distinct effects, namely (1) inhibition of VACCs (Evans et al., 2004), (2) both reduction and enhancement of depolarization-induced Ca2+ flux in different subpopulations of neurons (Evans et al., 2004). In this study, we have found (3) a predominantly inhibitory effect on voltage-activated K+ currents, (4) a prolongation of the repolarization phase of transient responses to depolarization, probably mediated by a reduction in K+ conductance and (5) a reduction in multiple action potential firing evoked by depolarizing current step commands. The overall action of AEA on functional neuronal excitability probably varies between subpopulations of DRG neurons and is also likely to depend on the spatial distributions of receptors and ion channels on cell membranes and their kinetics of activation and inactivation.
Results from our DRG neurons indicate that the net response to AEA is neuroinhibitory. Reductions in the number of action potentials fired were consistent with an inhibition of voltage-activated Ca2+ channel activity, whereas the slowed repolarization is consistent with an inhibition of voltage-activated K+ conductances. However, caution must be applied in interpretation of this data. Although the phenomenon of multiple firing behaviour in our cultured DRG neurons has been widely reported, its functional significance is still not well understood. Only a minority of DRG neurons cultured under our conditions display this behaviour, and it is unclear if these neurons map onto a functionally or anatomically distinct subpopulation of DRG neurons in vivo. An additional complexity is that different sensory neuron preparations contain differing proportions of multiple firers. A further consideration is that endogenous intracellular AEA induces Ca2+ influx via the activation of TRPV1 receptors that may counter the effects described in this paper and enhance neurotransmitter release and contribute to synaptic plasticity (van der Stelt et al., 2005). However, we have recently demonstrated that in cultures exposed to low NGF (this study, 20 ng ml−1), AEA produces little activation of TRPV1 receptors, in comparison to cultures exposed to high NGF (200 ng ml−1) (Evans et al., 2007). Furthermore, as can be seen in Figure 3b, AEA-induced prolongation of depolarization evoked Ca2+ transients that may under some circumstances introduce longer refractory periods and thus reduce excitability.
Diverse K+ channel currents have been identified in a number of studies on DRG neurons (Gold et al., 1996; Everill et al., 1998), and their expression and modulation has been implicated in hyperexcitability associated with pain states (Rasband et al., 2001). We found that AEA predominantly produced modest, variable but significant inhibition of voltage-activated K+ currents, which reflects attenuation of both transient and delayed components of outward current. However, in a subpopulation of DRG neurons, the outward K+ current was enhanced by AEA, an effect that reflects the heterogeneity of DRG neurons in culture as well as diverse direct and indirect responses of distinct K+ channels to cannabinoids (Mackie et al., 1995; Hampson et al., 2000; Maingret et al., 2001; Di Marzo et al., 2002; Vásquez et al., 2003; Ross et al., 2004). The inhibitory action of AEA persisted in Ca2+-free conditions, indicating that the modulation of voltage-activated K+ current occurred independently of the inhibition of VACC, as previously demonstrated (Evans et al., 2004).
An AEA-induced inhibition of Shaker-related Kv1.2 channels, which was found to be insensitive to both PTX and CB1 antagonist rimonabant, has been previously identified (Poling et al., 1996). Similarly, the actions of AEA on DRG neuron K+ current were insensitive to PTX treatment and the CB1 receptor antagonist, which raises the possibility that the effect was occurring independently of CB1 receptor-mediated pathways. The fact that the inhibitory action developed slowly further indicated a potential role for active, downstream metabolites of AEA in this effect. This hypothesis was supported by the findings that hydrolysis-resistant methAEA was inactive in our assays and that PMSF attenuated actions of AEA. PMSF irreversibly blocks the actions of FAAH, therefore preventing FAAH from hydrolyzing AEA (Deutsch et al., 2002). Thus PMSF increases levels of AEA and attenuates increases in the intracellular levels of downstream metabolites, such as AA and ethanolamine. Our data indicate that, in the majority of neurons, metabolism of AEA is necessary for K+ channel inhibition. The finding that both AA and ethanolamine mimicked the action of AEA provides further support for this hypothesis. However, inspection of the data showed that in some neurons, PMSF did not attenuate AEA action, suggesting that a further mechanism independent of CB1 receptors and FAAH-mediated metabolism accounts for some AEA activity that was not identified when methAEA was applied to DRG neurons. This could involve COX-2, which has been found to limit retrograde endocannabinoid signalling in the hippocampus (Kim and Alger, 2004). Additionally, COX-2 production of prostaglandins results in a number of effects through cAMP and PKA activation, including inhibition of voltage-activated K+ currents (Vanegas and Schaible, 2001). Alternatively, it may be that AEA is acting on a novel PTX insensitive receptor. Wiley et al. (2006) have observed effects of AEA on nociception in vivo that are neither CB1 receptor-mediated nor mediated via active metabolites. There is a growing body of evidence for non-CB1 and non-CB2 receptors that are activated by the endogenous cannabinoid but have distinct pharmacology (Di Marzo et al., 2002; Pertwee and Ross, 2002; Wiley and Martin, 2002). AEA and WIN55212-2 stimulate [35S]-GTPγS binding in both CB1 knockout and wild-type mice (Breivogel et al., 2001; Wiley and Martin, 2002), and AEA is an agonist at the orphan receptor GPR55 (Baker et al., 2006; Ryberg et al., 2007).
Voltage-activated K+ channel activity, in part, mediates the recovery of the KCl-evoked Ca2+ transients, which are enhanced by K+ channel inhibitors such as TEA (McClelland et al., 2004). We therefore predicted, from the actions of AEA on K+ currents, that Ca2+ transients evoked by high KCl-induced depolarizations would be prolonged by AEA. This was the case and the pharmacological features of this effect were similar to those seen in the electrophysiological studies. Specifically, PTX pretreatment did not attenuate AEA action but PMSF pretreatment did, and the slowing of the decay in the Ca2+ transient was also observed with AA. Furthermore, although the synthetic CB1 agonist WIN-55212-2 also slowed the Ca2+ transient, recovery from this effect was enhanced by PTX treatment. The mechanism for this is unknown, but the results from this experiment implicated distinct mechanisms of modulation produced by WIN-55212-2 compared with that seen with AEA and AA.
Studies with acute application of ethanolamine showed that, similar to methAEA and the TRPV1 receptor agonist capsaicin, this metabolite of AEA evoked Ca2+ transients. These responses were reduced in amplitude but persisted in Ca2+-free extracellular conditions implicating intracellular Ca2+ stores. Although there was no correlation between the responsiveness of DRG neurons to the three drugs, these data suggest that the AEA metabolite ethanolamine may contribute to increases in intracellular Ca2+ signals. We speculate that ethanolamine mobilizes Ca2+ from intracellular stores and may also desensitize TRPV1 receptors by transiently increasing intracellular Ca2+.
The action of AEA on Ca2+ transients appeared disproportionate to the action of AEA on K+ currents, so caffeine was used as a tool to release Ca2+ from stores and evaluate any potential effect of AEA on Ca2+ homeostatic mechanisms. Modest effects were observed that could contribute but not fully account for the actions of AEA on KCl-evoked Ca2+ transients.
Taken together the results from this study suggest that metabolites of AEA, AA and ethanolamine and/or subsequent metabolites modulate K+ conductances and alter the excitability of cultured DRG neurons. AA is usually formed from membrane phospholipids and is metabolized by COX-1 and -2 to form prostaglandins (for example, PGE2, PGF2α), prostacyclin (PGI2) and thromboxanes. AA metabolism by 5-lipoxygenase produces leukotrienes and AA metabolism by 15-lipoxygenase produces lipoxins. Aspirin and NSAIDs (non-steroidal anti-inflammatory drugs), such as ibuprofen, act via inhibition of COX, thus inhibiting the formation of these metabolites of AA. Prostaglandins have been shown to increase the excitability of sensory neurons and, as such, increase sensitivity to noxious stimuli. Jiang et al. (2003) showed that PGE2 inhibited K+ currents to contribute to hyperalgesia. PGE2 suppresses K+ current in embryonic rat sensory neurons (Nicol et al., 1997) via the cAMP transduction pathway (Evans et al., 1999).
A further complexity of the effects of AEA involves actions through TRPV1 receptors. In the dorsal horn, AEA activates TRPV1 receptors to increase the frequency of miniature excitatory post-synaptic currents (Jennings et al., 2003). We previously found that in cultured DRG neurons, the effects of AEA on TRPV1 receptors were best achieved through intracellular delivery of AEA and were not easily produced by the extracellular application of the endocannabinoid (Evans et al., 2004). There is evidence, although conflicting, that TRPV1 and CB1 receptors are preferentially expressed on small-diameter sensory neurons. If the observed effects were mediated via CB1 or TRPV1 receptors then it might be expected that cell size, which reflects functional neuronal type, would show some correlation with the observed effect. This was not the case with the effects of AEA described here, which adds weight to the argument that the effects observed in this study were not mediated by either CB1 or TRPV1 receptors.
Although AEA is primarily considered to control pain, its metabolism leads directly to AA and other pro-nociceptive and pro-inflammatory agents. The overall physiological effect of AEA is difficult to predict by studying sensory neurons alone, being dependent on the localization of AEA, receptor and channel expression of neurons and non-neuronal cells in the area of AEA and metabolic activity of the cells. Our study points to a predominant overall suppression of excitability of cultured DRG neurons by AEA and to a complex pharmacology of this endocannabinoid that may involve both FAAH metabolites and novel CB receptors.
Acknowledgments
RME thanks the College of Life Sciences and Medicine at the University of Aberdeen for a PhD studentship. HAK thanks the Egyptian Government for support.
Abbreviations
- AA
arachidonic acid
- AEA
anandamide (N-arachidonoyl-ethanolamide)
- COX-2
cyclooxygenase-2
- DMSO
dimethyl sulphoxide
- DRG
dorsal root ganglion
- FAAH
fatty acid amide hydrolase
- methAEA
methanandamide
- PMSF
phenylmethylsulphonyl fluoride
- PTX
pertussis toxin
- VACC
voltage-activated Ca2+ current
Conflict of interest
The authors state no conflict of interest.
References
- Abadji V, Lin SY, Taha G, Griffin G, Stevenson LA, Pertwee RG, et al. (R)—methanandamide: a chiral novel anandamide possessing high potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience. 2002;110:747–753. doi: 10.1016/s0306-4522(01)00601-7. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley R. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Greiffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Bridges D, Rice ASC, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992;106:231–232. doi: 10.1111/j.1476-5381.1992.tb14321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol. 2004;61:149–160. doi: 10.1002/neu.20080. [DOI] [PubMed] [Google Scholar]
- Croci T, Zarini E. Effect of the cannabinoid CB1 receptor antagonist rimonabant on nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br J Pharmacol. 2007;150:559–566. doi: 10.1038/sj.bjp.0707138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Ueda N, Yamamoto S. The fatty acid amide hydrolase (FAAH) Prostaglandins Leukot Essent Fatty Acids. 2002;66:201–210. doi: 10.1054/plef.2001.0358. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essent Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Scott RH. Calcium channel currents and their inhibition by baclofen in rat sensory neurons: modulation by guanine nucleotides. J Physiol. 1987;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J Physiol. 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Scott RH, Ross RA. Multiple actions of anandamide on neonatal rat cultured sensory neurones. Br J Pharmacol. 2004;141:1223–1233. doi: 10.1038/sj.bjp.0705723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Scott RH, Ross RA. Chronic exposure of sensory neurones to increased levels of nerve growth factor modulates CB1-TRPV1 receptor crosstalk. Br J Pharmacol. 2007;152:404–413. doi: 10.1038/sj.bjp.0707411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Rizzo MA, Kocsis JD. Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J Neurophysiol. 1998;79:1814–1824. doi: 10.1152/jn.1998.79.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurones. J Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Mu J, Deadwyler SA. Cannabinoid and kappa opioid receptors reduce potassium K current via activation of Gs proteins in cultured hippocampal neurons. J Neurophysiol. 2000;84:2356–2364. doi: 10.1152/jn.2000.84.5.2356. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999a;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999b;90:923–932. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Iversen L, Chapman V. Cannabinoids: a real prospect for pain relief. Curr Opin Pharmacol. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Jennings EA, Vaughan CW, Roberts LA, Christie MJ. The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2003;548:121–129. doi: 10.1113/jphysiol.2002.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang H, Clark JD, Tempel BL, Nicol GD. Prostaglandin E2 inhibits the potassium current in sensory neurons from hyperalgesic Kv1.1 knockout mice. Neuroscience. 2003;119:65–72. doi: 10.1016/s0306-4522(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cycooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma–glioma cells. Proc Natl Acad Sci USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;10:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Zhuang S, Kirby MT, Hampson RE, Deadwyler SA. Cannabinoid receptors differentially modulate potassium A and D currents in hippocampal neurons in culture. J Pharmacol Exp Ther. 1999;291:893–902. [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Novel pharmacological targets for cannabinoids. Curr Neuropharmacol. 2004;1:9–29. [Google Scholar]
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Poling JS, Rogawski MA, Salem N, Vincini S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-activated K+ channels. Neuropharmacology. 1996;35:983–991. doi: 10.1016/0028-3908(96)00130-x. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci USA. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ASC, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Aanonsen L, Hargreaves KM. SR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur J Pharmacol. 1997;319:R3–R4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Ross RA, Evans RM, Scott RH. Cannabinoids and sensory neurones. Curr Neuropharmacol. 2004;1:59–73. [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson N-O, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temraz TA, Houssen WE, Jaspars M, Woolley DR, Wease KN, Davies SN, et al. A pyrdinium derivative from Red Sea soft corals inhibited voltage-activated potassium conductances and increased excitability of cultured sensory neurones. BMC Pharmacol. 2006;6:10. doi: 10.1186/1471-2210-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stelt M, Trevisani M, Vellani V, DePetrocellis L, Moriello AS, Campi B, et al. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas HN, Schaible HG. Prostaglandins and cycloxygenases in the spinal cord. Prog Neurobiol. 2001;64:327–363. doi: 10.1016/s0301-0082(00)00063-0. [DOI] [PubMed] [Google Scholar]
- Vásquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez C, Navarro-Polanco RA, Huerta M, Trujilo X, Andrade F, Trrujilo-Hernandez B, et al. Effects of cannabinoids on endogenous K+ and Ca2+ currents in HEK293 cells. Can J Physiol Pharmacol. 2003;81:436–442. doi: 10.1139/y03-055. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM. Endocannabinoids in pain modulation. Prostgland Leukotr Essent Fatty Acids. 2002;66:235–242. doi: 10.1054/plef.2001.0361. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem Phys Lipids. 2002;121:57–63. doi: 10.1016/s0009-3084(02)00146-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic cascade in anandamide's in vivo effects in mice. Life Sci. 2006;80:24–35. doi: 10.1016/j.lfs.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E-2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–211866. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]