Abstract
Background and purpose: There is increasing evidence that angiotensin II (Ang II) is associated with the occurrence of ventricular arrhythmias. However, little is known about the electrophysiological effects of Ang II on ventricular repolarization. The rapid component of the delayed rectifier K+ current (IKr) plays a critical role in cardiac repolarization. Hence, the aim of this study was to assess the effect of Ang II on IKr in guinea-pig ventricular myocytes.
Experimental approach: The whole-cell patch-clamp technique was used to record IKr in native cardiocytes and in human embryonic kidney (HEK) 293 cells, co-transfected with human ether-a-go-go-related gene (hERG) encoding the α-subunit of IKr and the human Ang II type 1 (AT1) receptor gene.
Key results: Ang II decreased the amplitude of IKr in a concentration-dependent manner with an IC50 of 8.9 nM. Action potential durations at 50% (APD50) and 90% (APD90) repolarization were prolonged 20% and 16%, respectively by Ang II (100 nM). Ang II-induced inhibition of the IKr was abolished by the AT1 receptor blocker, losartan (1 μM). Ang II decreased hERG current in HEK293 cells and significantly delayed channel activation, deactivation and recovery from inactivation. Moreover, PKC inhibitors, stausporine and Bis-1, significantly attenuated Ang II-induced inhibition of IKr.
Conclusions and implications: Ang II produces an inhibitory effect on IKr/hERG currents via AT1 receptors linked to the PKC pathway in ventricular myocytes. This is a potential mechanism by which elevated levels of Ang II are involved in the occurrence of arrhythmias in cardiac hypertrophy and failure.
Keywords: angiotensin II, ion channels, action potentials, ventricle, receptors
Introduction
Electrophysiological remodelling in cardiac hypertrophy and failure predisposes the heart to lethal arrhythmias, which account for half of the deaths of patients with heart failure (Tomaselli and Marban, 1999). The renin–angiotensin system (RAS) plays a pivotal role in maintaining cardiovascular homeostasis but may contribute to cardiac arrhythmias in various cardiovascular diseases, including cardiac hypertrophy and heart failure (Griendling et al., 1996; Delpon et al., 2005). Large-scale clinical trials have provided evidence that inhibition of angiotensin II (Ang II) synthesis by angiotensin-converting enzyme inhibitors or direct blockade of angiotensin receptor type 1 (AT1) with the antagonist losartan results in a significant decrease in sudden cardiac death in patients with heart failure, which may be linked to fewer episodes of complex arrhythmias (Brooksby et al., 1999; Lindholm et al., 2003). In addition, experimental studies have demonstrated the efficacy of RAS inhibitors in the treatment of reperfusion-induced arrhythmias (Harada et al., 1998; Yahiro et al., 2003). Different mechanisms, including the attenuation of electrophysiological remodelling caused by Ang II, have been postulated to explain the beneficial influence of RAS inhibition (Alberte and Zipes, 2003; Rubart and Zipes, 2005).
The delayed rectifier K+ currents (IK) are the major repolarizing outward currents of ventricular action potentials in mammalian species, including humans, and consist of rapidly and slowly activating components (IKr and IKs, respectively). Human ether-a-go-go-related gene (hERG or KCNH2) (Warmke and Ganetzky, 1994) encodes the pore-forming subunit of the channel underlying IKr (Sanguinetti et al., 1995), which is crucial for the repolarization of cardiac action potentials. A reduction in hERG currents due to either genetic defects, or adverse drug effects can lead to hereditary or acquired long QT syndrome in humans characterized by action potential prolongation, lengthening of the QT interval on the surface ECG, and an increased risk for ‘torsade de pointes' ventricular arrhythmias and sudden cardiac death (Marban, 2002). IKr also represents a target for modulation by autonomic neurotransmitters and hormones. Recent studies have revealed that hERG channels are modulated by G protein-coupled receptors including α- and β-adrenoceptors through the intracellular second messengers, such as cAMP, protein kinase A (PKA; Cui et al., 2000) and protein kinase C (PKC; Thomas et al., 2003; Cockerill et al., 2007). However, the information available is limited regarding the effect of Ang II on repolarizing K+ currents and resultant changes in action potential duration (APD) in cardiac ventricular myocytes. The present study was designed to examine the possible regulation of IKr/hERG currents by Ang II in guinea-pig isolated ventricular myocytes and heterologous expression system using the whole-cell patch-clamp technique. Our results provide direct evidence that Ang II produces an acute inhibitory effect on IKr/hERG currents via the AT1 receptor linked to the PKC pathway in ventricular myocytes.
Methods
Isolation of single ventricular myocytes
All experimental procedures were carried out in accordance with protocols approved by the Hebei Medical University Institutional Animal Care and Use committee. Single ventricular myocytes were enzymatically dissociated from the heart of adult guinea-pigs as described previously (Ahmmed et al., 2000) with slight modification. In brief, hearts were retrogradely perfused with Ca2+-free modified Tyrode solution composed of (in mM) NaCl, 140; KCl, 5.4; MgCl2, 1; HEPES, 10 and glucose, 10 (pH 7.4 with NaOH). After 5 min of perfusion, the solution was switched to one containing Type II collagenase (0.4 mg mL−1) and hearts were removed after 10–15 min of perfusion from the perfusion apparatus. Left ventricular free wall was cut into small pieces in high K+ solution, which contained (in mM) KOH, 80; KCl, 40; KH2PO4, 25; MgSO4, 3; glutamic acid, 50; taurine, 20; EGTA, 0.5; HEPES, 10 and glucose, 10 (pH 7.3 with KOH). Cells were then harvested and were used for electrophysiological recording within 6–8 h after isolation.
Transient transfection of hERG and AT1 receptor
Human embryonic kidney (HEK) 293 cells were maintained in Dulbecco's modified Eagles medium supplemented with 10% fetal bovine serum, 1 mM L-glutamine and 1% penicillin–streptomycin solution. Cell cultures were kept at 37 °C in a 5% CO2 incubator. Cells were transiently transfected with plasmids containing hERG and green fluorescent protein (GFP) and AT1 receptor cDNA using Lipofectamine 2000. GFP-positive cells were identified using the epifluorescence system and studied within 24–48 h of transfection.
Electrophysiological recordings
The rapid component of IK (IKr)/hERG currents were recorded using the conventional whole-cell patch-clamp technique (Hamill et al., 1981). Borosilicate glass electrodes had tip resistances of 1–3 MΩ when filled with the pipette solution. Uncompensated capacitance currents in response to small hyperpolarizing voltage steps were recorded for offline integration, as a means of measuring cell capacitance. Action potentials were recorded in ventricular myocytes using the perforated patch technique (Korn et al., 1991). Action potentials were evoked at a rate of 1 Hz with suprathreshold current pulse of 4−6 ms duration applied via patch electrodes in the current-clamp mode. The APD was measured at 50 and 90% repolarization (APD50 and APD90). All experiments were performed at room temperature (22–23 °C) using an Axopatch 200B amplifier (Axon Instrument, Foster City, CA, USA). The electrical signals were sampled at 2.5–10 kHz and filtered at 1 kHz using a low-pass filter, and digitized with an A/D converter (Digidata 1322; Axon Instruments). A pClamp software (Version 8.1; Axon Instrument) was used to generate voltage-pulse protocols, acquire and analyse data.
Solutions and chemicals
For IKr recordings in ventricular myocytes, the external solution contained (in mM) NaCl, 132; KCl, 4; CaCl2, 1.8; MgCl2, 1.2; glucose, 5 and HEPES, 10 (pH 7.4 with NaOH). The pipette solution contained (in mM) KCl, 140; Mg-ATP, 4; MgCl2, 1; EGTA, 5 and HEPES 10 (pH 7.2 with KOH). Nimodipine (Nim, 1 μM) was added to the external solution to block the L-type Ca2+ current. Na+ and T-type Ca2+ currents were inactivated by holding potential of −40 mV. IKs was blocked by the addition of 20 μM chromanol 293B. For action potential recordings, the patch pipettes were backfilled with amphotericin (600 μg). Pipette solution contained (in mM) potassium glutamate, 120; KCl, 25; MgCl2, 1; CaCl2, 1 and HEPES 10 (pH 7.4 with KOH). The external solution contained (in mM) NaCl, 138; KCl, 4; MgCl2, 1; CaCl2, 2; NaH2PO4, 0.33; glucose, 10 and HEPES, 10 (pH 7.4 with NaOH).
For hERG recording in HEK cells, the external solution contained (in mM) NaCl, 140; KCl, 5.4; MgCl2, 1; CaCl2, 2; glucose, 10 and HEPES, 10 (pH 7.4 with NaOH). The pipette solution contained (in mM) KCl, 140; Mg-ATP, 4; MgCl2, 1; EGTA, 5; and HEPES 10 (pH 7.2 with KOH).
Angiotensin II (Sigma, St Louis, MO, USA) was prepared as a 1-mM stock solution in water and stored at −20 °C. Chromanol 293B (Sigma) was prepared as a 10-mM stock solution in DMSO and stored at −20 °C. E-4031 (Sigma) was prepared as a 1-mM stock in water and stored at −20 °C. Nim (Sigma) was prepared as a 100-mM stock solution in DMSO and stored in the dark. The highest final concentration of DMSO in external solution was ⩽0.1%, a concentration that had no effect on IKr.
Type II collagenase was obtained from Worthington Biochemical Corporation (Lakewood, NJ, USA). hERG cDNA was kindly provided by Dr Chiamvimonvat (University of California, Davis, CA, USA). The AT1 receptor cDNA was kindly provided by Dr Vauquelin (Vrije Universiteit Brussel, Brussel, Belgium) and Lipofectamine 2000 was from Invitrogen (Grand Island, NY, USA).
Statistical analysis
All averaged values presented are means±s.e.mean. Statistical comparisons were made using Student's unpaired or paired t-tests. A value of P<0.05 was considered statistically significant.
Results
Effects on IKr in guinea-pig ventricular myocytes
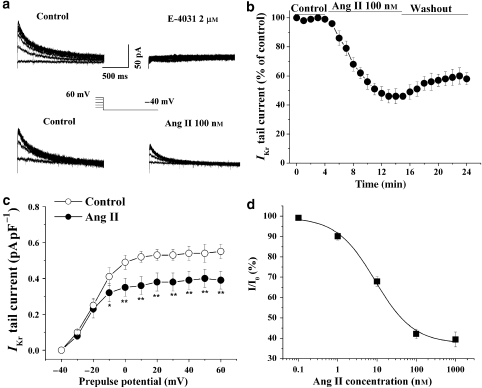
Ventricular myocytes were depolarized from a holding potential of −40 mV to various prepulse potentials of −30 to 60 mV for 2 s and repolarized to −40 mV to evoke outward tail currents in the presence of IKs blocker, chromanol 293B (20 μM). The tail currents were abolished by a specific IKr blocker, E-4031 (2 μM) (Figure 1a). IKr was recorded in the control conditions and after application of Ang II in the bath. The results showed that Ang II markedly reduced the tail currents during repolarizations (Figure 1a). The time course of the inhibitory action of Ang II on IKr was determined by measuring the amplitude of tail currents elicited on repolarization to a test potential of −40 mV after a 2-s prepulse potential of 40 mV every minute (Figure 1b). The reduction of IKr tail current occurred within 2–3 min and reached saturation about 10 min after addition of Ang II (100 nM) into the bath. The inhibition of IKr by Ang II was not reversible. The voltage dependence of the activation was calculated from tail current density (Figure 1c). Ang II was significantly more potent at inhibiting the tail current at more positive potentials. The tail current density of IKr was decreased from 0.54±0.04 to 0.39±0.05 pA pF−1 at a prepulse potential of 40 mV (n=6, P<0.01). The percentage of inhibition of the amplitude of IKr tail current was calculated and plotted against concentrations of Ang II (Figure 1d). The mean data are well described by a Hill equation with an IC50 value of 8.9 nM.
Figure 1.
Effects of angiotensin II (Ang II) on the rapid component of IK (IKr) in guinea-pig isolated ventricular cardiomyocytes. (a) Representative tail traces of IKr before (left), and after application of 2 μM E-4031 in one myocyte and 100 nM Ang II (right) to another myocyte. The ventricular myocyte was pulsed as shown. (b) The time course of the IKr current reduction by Ang II (100 nM). The effect of Ang II on IKr was quantitatively evaluated by measuring the amplitude of tail currents elicited on return to the potential of −40 mV after 2 s depolarizing pulse to 40 mV every minute. The percentage of decrease in the tail current was calculated in six cells. (c) Voltage-dependent activation of IKr was calculated from IKr tail current density before and 10 min after an application of 100 nM Ang II (n=6, *P<0.05, **P<0.01, versus before Ang II). (d) The concentration–response curve for the effect of Ang II on the IKr, IC50=8.9 nM (n=4–6 cells at each concentration). IK, the delayed rectifier K+ currents.
Effects on action potentials
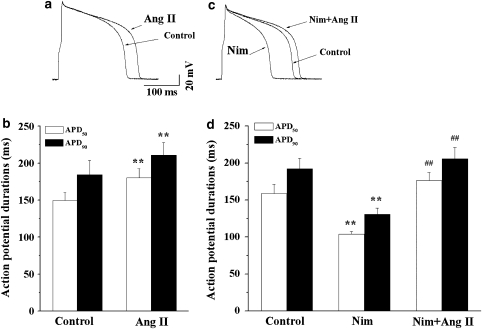
We next investigated the effects of Ang II on action potentials in guinea-pig ventricular myocytes. The perforated patch technique in current-clamp mode was used to avoid dialysis of the intracellular milieu. Figure 2a represents superimposed traces of action potentials recorded before and during exposure to Ang II (100 nM). Ang II markedly prolonged APD, but did not influence the amplitude of the action potential and the resting potentials. In 10 myocytes, APD50 and APD90 were increased from the control values of 149.7±10.4 and 184.2±15.3 ms to 180.3±12.3 (P<0.01) and 210.8±17.2 ms (P<0.01), respectively (Figure 2b).
Figure 2.
Effects of angiotensin II (Ang II) on action potentials. (a) Superimposed action potentials recorded before and 10 min after exposure to 100 nM Ang II. (b) A summary of the data for changes in APD at 50% of repolarization (APD50) and APD at 90% of repolarization (APD90; n=6, **P<0.01, Ang II versus control). (c) Superimposed action potentials recorded during exposure to Ang II (100 nM) in the presence of nimodipine (Nim, 1 μM). (d) A summary of the data for changes in APD50 and APD90 during exposure to Ang II in the presence of Nim (n=6, **P<0.01, Nim versus control, ##P<0.01, Nim plus Ang II versus Nim).
Action potential duration is dependent on the balance between depolarizing inward and repolarizing outward currents. Some evidence has been presented showing that Ang II increases the L-type Ca2+ current in ventricular myocytes (Aiello and Cingolani, 2001; De Mello and Monterrubio, 2004). This effect of Ang II may result from its potentiation of the inward Ca2+ current or inhibition of the IKr. If the prolongation of APD is predominantly due to the former, we would expect an L-type Ca2+ channel antagonist to counteract the prolongation of APD by Ang II. Hence, we determined the effect of Ang II on APD in the presence of an L-type Ca2+ channel blocker, Nim (1 μM). Figure 2c shows the superimposed traces of action potentials recorded first in the control conditions, then, during exposure to Nim and, lastly, in the presence of Nim plus Ang II. Application of Nim caused a significant shortening of APD, and after that Ang II produced a marked prolongation of APD in the presence of Nim (Figure 2c). A summary of corresponding data is shown in Figure 2d. In seven myocytes, APD50 and APD90 were shortened by Nim from the control values of 158.6±12.6 and 192.4±14.1 ms to 104.0±3.7 (P<0.01) and 130.6±8.6 ms (P<0.01), respectively. After that, Ang II prolonged APD50 and APD90 to 176.5±10.9 and 205.6±15.5 ms, respectively (Figure 2d). This result indicates that Nim does not antagonize the prolongation of AP by Ang II and suggests that this L-type Ca2+ currents are not involved in this effect of Ang II.
Effects on hERG channels
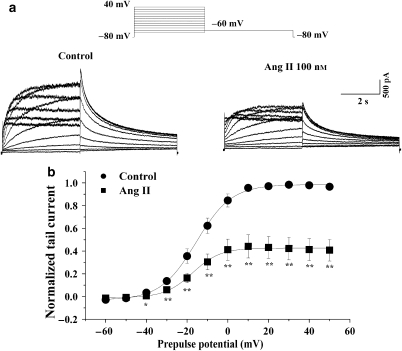
We investigated the effects of Ang II on hERG channels in human embryonic kidney 293 (HEK293) cells transiently co-expressed with hERG and the human AT1 receptor cDNA. The hERG current was elicited from a holding potential of −80 mV to prepulses from −60 to 50 mV for a 4-s duration and was followed by a test pulse to −60 mV to evoke large, slowly decaying outward tail currents. Figure 3a shows an example of a voltage-clamp recording from an HEK293 cell, with representative current traces obtained under control conditions and after exposure to Ang II (100 nM). Ang II reduced both the hERG current during the prepulse potentials and the tail current. Tail current amplitude, normalized to the maximum tail current amplitude, was used to construct the activation curve shown in Figure 3b. The activation curve showed that Ang II reduced the tail current by 50.0±1.1% at prepulse of 0 mV (Figure 3b). When fitted to a Boltzmann function, the half-maximum activation voltage (V1/2) and slope factor under control conditions were −15.5±3.8 mV and 8.5±1.3, respectively, values which are not significantly different from those in the presence of Ang II (V1/2: –17.5±3.3 mV; slope factor: 7.8±1.8) (n=5, P>0.05).
Figure 3.
Effects of angiotensin II (Ang II) on ether-a-go-go-related gene (hERG) current. (a) Representative traces of hERG current recorded, using the pulse protocol shown, before (left) and after application of 100 nM Ang II (right) in a human embryonic kidney 293 (HEK293) cell. (b) Normalized I–V relationships for tail currents in control conditions and in the presence of 100 nM Ang II (n=5, *P<0.05, **P<0.01, Ang II versus control). The solid lines represent fits to a Boltzmann function.
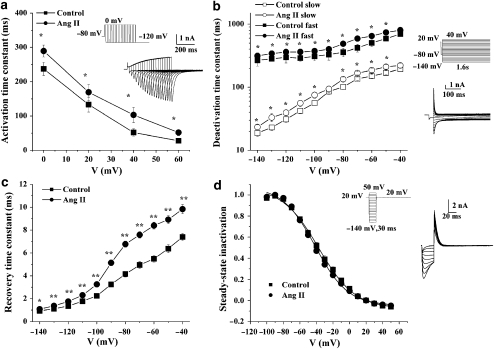
We further determined the effects of Ang II on hERG channel kinetics including activation, deactivation, inactivation and recovery from inactivation. The activation time course was estimated using tail current measurements. From the holding potential of −80 mV, cells were clamped to 0, 20, 40 and 60 mV for varying durations before it was repolarized to −120 mV (Figure 4a). The time constant obtained by fitting a single exponential function to the envelope of tail currents was used for evaluation of the hERG current activation. Time constants were significantly slowed at different voltages in the presence of Ang II (n=5, Figure 4a). Deactivation tail current was elicited by 10 mV step pulses from −140 to 40 mV after a prepulse to −80 mV from a holding potential of 20 mV for 0.03 s (Figure 4b). The deactivation time constants were obtained by fitting the decay phase of the tail current with double exponential functions. Both the fast and slow components of the deactivation were significantly inhibited by Ang II (Figure 4b). Recovery from inactivation and steady-state inactivation were measured using a two-pulse protocol (Sharma et al., 2004). Cells were first stepped between −140 and 50 mV in 10-mV increments for 30 ms from the holding potential of 20 mV to elicit tail currents. Then, a test pulse of 20 mV was applied for 0.17 s. The rising phase ‘hook' of the tail current represents the rapid recovery of hERG from inactivated to open states (Figure 4d, inset). The time constants of recovery from inactivation were determined as a monoexponential fit to the rising phase of the tail current. Ang II markedly increased the time constant of recovery from inactivation at all potentials (Figure 4c). The steady-state inactivation curve is shown in Figure 4d. When fit as a Boltzmann function, the V1/2 (−37.3±0.4 mV) and slope factor (21.9±0.4) under control conditions were not significantly different from those in the presence of Ang II (V1/2: −41.6±0.3 mV; slope factor: 19.7±0.2).
Figure 4.
Effects of angiotensin II (Ang II; 100 nM) on ether-a-go-go-related gene (hERG) channel kinetics. (a) Activation time constants at test potential of 0, 20, 40 and 60 mV (n=5, *P<0.05, control versus Ang II). Inset: representative tracings for hERG current activation and pulse protocol. Activation time constants were obtained by fit with a single exponential function. (b) Time constants for fast and slow deactivation were plotted against the membrane potentials (n=6, *P<0.05, Ang II versus control). Inset: representative traces for hERG current deactivation and pulse protocol. Deactivation time constants were obtained by fit with double exponential function to the decay phase of the tail current. (c) The recovery time constants were plotted against the membrane potentials (n=5, *P<0.05, **P<0.01, control versus Ang II). Recovery from inactivation was determined by fitting a single exponential function to the initial ‘hook' preceding slower deactivation of tail currents shown in the inset of (d). (d) Normalized steady-state inactivation curves for control and after application of Ang II (n=5). Solid lines represent fits with Boltzmann function. Inset: representative current traces for steady-state inactivation and pulse protocol.
Modulation of IKr/hERG currents via AT1 receptor
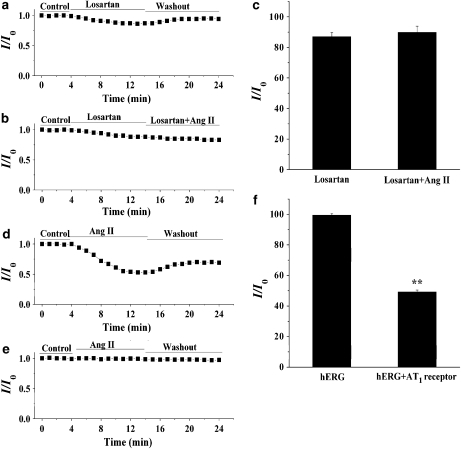
It is known that most of the cardiovascular effects of Ang II are through the AT1 receptor. To investigate whether the IKr response to Ang II is mediated through the AT1 receptor, we determined the effect of Ang II on the amplitude of the IKr tail current in the presence of the selective AT1 receptor antagonist losartan. As illustrated in Figure 5a, losartan (1 μM) alone decreased the amplitude of the IKr tail current, and this effect was completely reversed after washing. This result is identical to that obtained previously where losartan was shown to have a direct inhibitory action on the hERG channel (Caballero et al., 2000). However, pretreatment of ventricular myocytes with losartan almost totally prevented the inhibitory effect of Ang II (100 nM) on IKr (Figure 5b). A summary of the percentage of decrease in the amplitude of the IKr tail current under various conditions is shown in Figure 5c. Similar results were obtained in HEK293 cells (data not shown). These results support the view that losartan prevents the inhibitory action of Ang II by blocking the binding of Ang II to the AT1 receptor. We further compared the effects of Ang II on hERG current in HEK293 cells expressing hERG channel in the absence and presence of the AT1 receptor. As shown in Figure 5d, the hERG tail current was decreased by Ang II (100 nM) in HEK293 cells co-expressing the AT1 receptor with the hERG channel. However, in HEK293 cells expressing the hERG channel without the AT1 receptor this current was not altered by Ang II (Figure 5e). A summary of these data is shown in Figure 5f. These results further confirm that the inhibitory action of Ang II on hERG is mediated by AT1 receptors.
Figure 5.
The angiotensin II (Ang II) type 1 (AT1) receptor mediates the inhibition of the rapid component of IK (IKr)/ether-a-go-go-related gene (hERG) current by Ang II. For the measurement of the amplitude of IKr tail currents, myocytes were stepped to 0 mV for 2 s from a holding potential of −40 mV and then repolarized to a test potential of −40 mV. In human embryonic kidney 293 (HEK293) cells, hERG tail currents were elicited on repolarization to a test potential of −60 mV after a 0-mV, 4-s long step from the holding potential of −80 mV. (a) The effect of losartan (1 μM) on IKr. (b) The effect of Ang II (100 nM) on IKr in the presence of losartan. (c) A summary of the percentage of decrease in the amplitude of IKr tail current (n=5). (d) Effect of Ang II (100 nM) on the hERG current recorded from HEK293 cells co-expressing hERG and AT1 receptors. (e) Effect of Ang II (100 nM) on the hERG current recorded from cells expressing the hERG channel without the AT1 receptor. (f) A summary of the results obtained from (e) (n=5, **P<0.01, hERG versus hERG plus AT1 receptor). IK, the delayed rectifier K+ currents.
Intracellular signal pathways involved in AT1 receptor-mediated inhibition of IKr/hERG
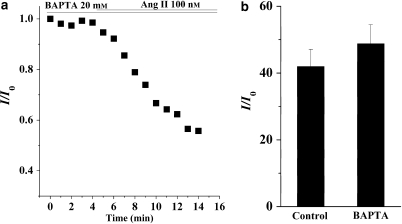
It is known that the AT1 receptor is coupled to G proteins and activation of the AT1 receptor stimulates phospholipase C, resulting in the hydrolysis of phosphatidylinositol 4,5-bisphosphate into IP3 (inositol 1,4,5-trisphosphate) and DAG (1,2-diacylglycerol). IP3 elicits Ca2+ release from the endoplasmic reticulum and elevates the concentration of intracellular free Ca2+, whereas DAG activates conventional and novel isotypes of PKC. To investigate whether the actions of Ang II are due to an increase of intracellular free Ca2+, ventricular myocytes were dialysed via the patch pipette with an intracellular solution containing 20 mM BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) for 10 min after the whole-cell configuration had been achieved. BAPTA is a faster and more efficient Ca2+ chelator than EGTA. Nevertheless, Ang II still decreased the amplitude of IKr even in the myocyte dialysed with BAPTA (Figure 6a). A summary of the data showed that the extent of the inhibition of the IKr when the pipette solution containing 20 mM BAPTA was similar to that with the control solution containing 5 mM EGTA (Figure 6b), indicating that the release of intracellular Ca2+ was not required for the inhibitory effect of Ang II on IKr.
Figure 6.
Effects of buffering intracellular Ca2+ on the action of angiotensin II (Ang II; 100 nM). (a) The effects of an internal application of 20 mM BAPTA on the response of the rapid component of IK (IKr) to Ang II. (b) A summary of the data for the normalized IKr tail current in the presence of Ang II in myocytes dialysed with control pipette solution containing 5 mM EGTA and pipette solution containing 20 mM BAPTA (n=6). The amplitude of the IKr tail currents was measured using the same pulse protocol as that described in Figure 5. IK, the delayed rectifier K+ currents.
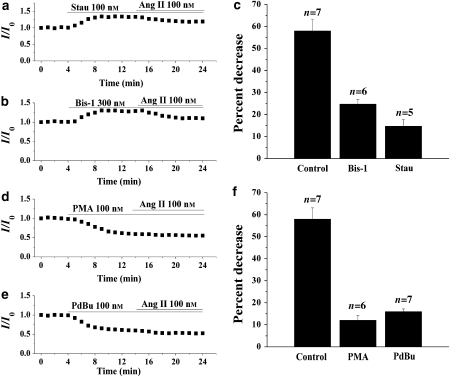
To assess whether PKC mediates the inhibition of AT1 receptor stimulation, we investigated the effects of PKC inhibitors and activators on the inhibitory action of Ang II. The nonspecific PKC inhibitor stausporine (Stau) (100 nM) or the specific PKC inhibitor Bis-1 (300 nM) was applied in the bath for 10 min and then co-applied with Ang II (100 nM). As illustrated in Figures 7a and b, the inhibitory effects of Ang II on IKr were substantially attenuated by the pretreatment of ventricular myocytes with Stau or Bis-1. In the presence of Stau or Bis-1, the percentage of decrease in the amplitude of the IKr tail current was reduced from the control level of 58.0 to 24.8 or 14.8%, respectively (Figure 7c). The results suggest a role for PKC in IKr modulation by Ang II. In addition, we investigated whether Ang II could further decrease IKr after attenuation by PKC activation. The nonspecific PKC activators phorbol-12-myristate-13-acetate (PMA) (100 nM) or phorbol-12,13-dibutyrate (PdBu) (100 nM) were applied in the bath for 10 min and then co-applied with Ang II (100 nM). As illustrated in Figures 7d and e, the PKC activators decreased the amplitude of IKr. Additional application of Ang II produced a much smaller decrease in IKr. Indeed, the percentage of decrease in the amplitude of IKr tail current was reduced from the control level of 58.0 to 12.1 or 16.0% in the presence of PMA or PdBu, respectively (Figure 7f).
Figure 7.
Effects of protein kinase C (PKC) inhibitors and activators on the response of the rapid component of IK (IKr) to angiotensin II (Ang II; 100 nM). (a and b) Effects of (a) the nonspecific PKC inhibitor, staurosporin (Stau; 100 nM) and (b) the specific PKC inhibitor, Bis-1 (300 nM), on the inhibition of IKr tail current by Ang II. (c) A summary of the data expressed as a percentage of decrease of IKr tail current by Ang II in the presence of Stau or Bis-1. (d and e) Effects of the PKC activators (d) PMA (100 nM) and (e) PdBu (100 nM), on the inhibition of IKr tail current by Ang II. (f) A summary of the data expressed as a percentage of decrease of IKr tail current by Ang II in the presence of PMA or PdBu. The amplitude of the IKr tail currents was measured using the same pulse protocol described in Figure 5. IK, the delayed rectifier K+ currents.
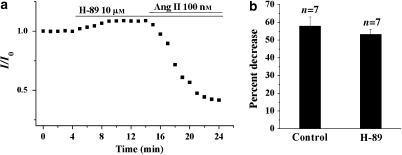
To rule out the possible involvement of PKA in the AT1-evoked attenuation of IKr, the effect of Ang II was examined in the presence of the specific PKA inhibitor H-89 (10 μM). As demonstrated in Figures 8a and b, there were no significant differences in the degree of IKr attenuation by Ang II in the absence and presence of H-89, supporting the notion that PKA activation is not involved in the AT1-mediated attenuation of IKr. Taken together, our results suggest that the attenuation of IKr induced by stimulation of the AT1 receptor is mediated primarily through the activation of PKC.
Figure 8.
The effect of the protein kinase A (PKA) inhibitor, H-89, on the response of the rapid component of IK (IKr) to angiotensin II (Ang II). (a) Effects of H-89 (10 μM) on the inhibition of IKr by Ang II. (b) A summary of the data expressed as a percentage of decrease of IKr tail current by Ang II in the presence of H-89. The amplitude of the IKr tail currents was measured using the same pulse protocol described in Figure 5. IK, the delayed rectifier K+ currents.
Discussion and conclusion
Inhibitory action of Ang II on IKr/hERG current
Previous evidence has suggested that Ang II can modulate cardiac ionic channels. Experiments with isolated cardiomyocytes have shown that stimulation of the AT1 receptor results in the inhibition of the transient outward potassium channel, KV4.3, in myocytes from rat or canine ventricles (Yu et al., 2000; Shimoni and Liu, 2003). Ang II increases cardiac L-type Ca2+ current by intracellular Ca2+- and PKC-dependent mechanisms in cat isolated myocytes (Aiello and Cingolani, 2001). Recently, it has been demonstrated that Ang II potentiates IKs via the AT1 receptor in guinea-pig atrial myocytes (Zankov et al., 2006). Our results demonstrate, for the first time, that Ang II inhibits IKr channel in guinea-pig ventricular myocytes. This inhibitory effect occurs within several minutes and is concentration dependent, suggesting an acute effect of Ang II on the channels. A more detailed analysis of the effects was performed on the hERG channel, which was co-expressed with human AT1 receptor cDNA in HEK293 cells. A similar reduction of the hERG current to that obtained with the cardiomyocytes was observed after exposure to Ang II. The inhibitory effect on the current was associated with significant changes in channel kinetics, that is, a significant delay in the activation, deactivation and recovery from inactivation. The specific AT1 receptor blocker, losartan, antagonized the inhibitory action of Ang II on the IKr/hERG current. The inhibitory effect of Ang II on the hERG current in HEK293 cells was dependent on the co-expression of AT1 receptors. Taken together, our results suggest that the inhibitory action of Ang II on the IKr/hERG current is mediated via the AT1 receptor.
It has been demonstrated previously (Daleau and Turgeon, 1994) that Ang II decreases IKs but increases IKr in guinea-pig ventricular myocytes, which is in contrast to our present findings. One possible explanation for this discrepancy could be related to the different techniques utilized to dissect the two components of IK into IKs and IKr. In the previous study, IKs and IKr were differentiated on the basis of their characteristic channel kinetics. We isolated IKr by using a specific IKs inhibitor in the native cardiomyocytes. Moreover, to confirm the observed inhibitory effect of Ang II on IKr, we used a heterologous expression system and obtained similar attenuation of the current by Ang II.
Our results showed that Ang II effectively inhibited IKr at a concentration of 1 nM and an IC50 value of 8.9 nM (Figure 1d), which appears to be much higher than the plasma level of Ang II in humans at baseline conditions (approximately 5 pM) (Bragat et al., 1997) and in patients with essential hypertension (about 70 pM) (Jalil et al., 2003). However, it is known that Ang II formation in the heart is also mediated by a local RAS, in addition to the circulating RAS. By using a microdialysis technique, it has been found that the baseline concentration of Ang II in the interstitial fluid of canine heart is about 6 nM, which is 100-fold higher than the plasma level (Dell'Italia et al., 1997). Increased gene transcript levels of the components of the RAS have been identified in animal models of cardiac hypertrophy and heart failure (De Mello and Danser, 2000). Thus, it is likely that the concentration of Ang II in the interstitial fluid of the heart is much higher than 6 nM in pathophysiological conditions and could be comparable to the concentration we used to inhibit IKr.
PKC involvement in the inhibitory effect of Ang II on IKr
It has been documented that the hERG K+ channel can be directly modulated by cAMP, PKA (Cui et al., 2000) and PKC (Thomas et al., 2003; Cockerill et al., 2007). Stimulation of different G protein-coupled receptors, including α1A- and β-adrenoceptors, regulates IKr through intracellular messengers and provides a link between autonomic stimulation and cardiac repolarization (Thomas et al., 2006). AT1 receptors belong to the G protein-coupled receptor family and are mainly coupled to heterotrimeric G protein αq. Stimulation of AT1 receptors results in the activation of PKC and an increase in intracellular Ca2+ (Touyz and Schiffrin, 2000). Strong intracellular Ca2+ buffering (20 mM BAPTA) did not affect the inhibitory action of Ang II on IKr. In contrast, a nonspecific PKC inhibitor, Stau and a specific PKC inhibitor, Bis-1, significantly attenuated the Ang II-induced decrease in the amplitude of IKr. In the presence of the nonspecific PKC activators, PMA and PdBu, Ang II produced little further effect on IKr. The specific PKA inhibitor, H-89, had no effect on the action of Ang II. Therefore, the results from the present study support the view that the inhibitory action of Ang II on IKr is, predominantly, mediated by a PKC-dependent pathway, induced by activation of AT1 receptors. Moreover, our study has demonstrated that PKC inhibitors decrease, whereas PKC activators increase IKr in guinea-pig ventricular myocytes, suggesting a tonic inhibitory regulation of PKC on IKr channel in the cardiomyocytes. This result is consistent with previous observations that PKC activation leads to an inhibitory effect on hERG current either through direct PKC-dependent phosphorylation of the channel or an indirect mechanism (Thomas et al., 2003; Cockerill et al., 2007). Indeed, the precise mechanism by which PKC regulates IKr in native cardiomyocytes remains to be elucidated. For example, which isoform of PKC mediates the attenuation of IKr via AT1 receptors in ventricular myocytes is not known. Buffering of the intracellular Ca2+ with BAPTA did not attenuate the response of IKr current to Ang II stimulation, suggesting that the Ca2+-independent novel isoform, rather than Ca2+-dependent conventional PKC isoforms, is preferentially involved in the IKr response under the present experimental conditions.
APD prolongation by Ang II
One of the most characteristic electrophysiological remodelling in hypertrophied or failing heart is APD prolongation, which is believed to mainly result from the downregulation of repolarizing outward potassium currents, including Ito (transient outward K+ current), IKs and IKr in heart failure (Tomaselli and Marban, 1999; Tsuji et al., 2000). The mechanism underlying ionic channel remodelling in heart failure is not fully understood. The RAS has been well studied in the heart (Lindpaintner et al., 1990). In experimental animal models and in the clinical setting, cardiac hypertrophy and heart failure are associated with an increase in the stimulation of cardiac angiotensinogen as well as an increased local level of Ang II (De Mello and Danser, 2000). It has been shown that local application of Ang II to the ventricular wall of cardiomyopathic hamsters results in an increase in APD, an effect blocked by losartan (De Mello, 2001). Transgenic animal models with high Ang II levels in the heart show a prolongation of the cardiac repolarization and sudden arrhythmic death (Domenighetti et al., 2007; Fischer et al., 2007). The present study provides evidence that Ang II-mediated inhibition of IKr may result in the delay in cardiac repolarization via stimulation of AT1 receptors in ventricular cardiomyocytes. Indeed, one of the pro-arrhythmic actions of Ang II may be related to the delay in ventricular repolarization. Furthermore, our finding that an AT1 receptor antagonist can abolish the Ang II-mediated inhibitory action on IKr provides a potential mechanism for some of the clinical benefits of angiotensin-converting enzyme inhibitors and AT1 blockers on cardiac arrhythmias associated with heart failure. One limitation of the present study is that all the experiments were performed at room temperature. Although we would not expect to obtain qualitatively different results at various temperatures, they could be somewhat different in quantitative terms. Therefore, the effect of Ang II on IKr and the cardiac repolarization should be studied at body temperature in the future.
In summary, the present study demonstrated that Ang II produces an inhibitory effect on IKr/hERG currents via stimulation of AT1 receptors, and this effect is induced by activation of the PKC pathway in ventricular myocytes. These results suggest a potential mechanism by which elevated levels of Ang II may be involved in the occurrence of arrhythmias in cardiac pathophysiology. They also provide a possible explanation for some of the clinical benefits of angiotensin-converting enzyme inhibitors and AT1 blockers on cardiac arrhythmias associated with heart failure.
Acknowledgments
This work was supported by NCET Program in China 04–0253, National Natural Science Foundation of China 30370571 and Natural Science Foundation of Hebei Province 200400628 and partly supported by National Basic Research Program of China 2007CB512100.
Abbreviations
- Ang II
angiotensin II
- APD
action potential duration
- APD50
APD at 50% of repolarization
- APD90
APD at 90% of repolarization
- AT1
Ang II type 1 receptor
- HEK293
human embryonic kidney 293 cells
- hERG
human ether-a-go-go-related gene
- IK
the delayed rectifier K+ currents
- IKr
the rapid component of IK
- IKs
the slow component of IK
- Nim
nimodipine
- RAS
renin–angiotensin system
Conflict of interest
The authors state no conflict of interest.
References
- Ahmmed GU, Dong PH, Song G, Ball NA, Xu Y, Walsh RA, et al. Changes in Ca(2+) cycling proteins underlie cardiac action potential prolongation in a pressure-overloaded guinea pig model with cardiac hypertrophy and failure. Circ Res. 2000;86:558–570. doi: 10.1161/01.res.86.5.558. [DOI] [PubMed] [Google Scholar]
- Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca(2+) current by a Ca(2+)- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol. 2001;280:H1528–H1536. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- Alberte C, Zipes DP. Use of nonantiarrhythmic drugs for prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2003;14:S87–S95. doi: 10.1046/j.1540-8167.14.s9.23.x. [DOI] [PubMed] [Google Scholar]
- Bragat AC, Blumenfeld J, Sealey JE. Effect of high-performance liquid chromatography on plasma angiotensin II measurements in treated and untreated normotensive and hypertensive patients. J Hypertens. 1997;15:459–465. [PubMed] [Google Scholar]
- Brooksby P, Robinson PJ, Segal R, Klinger G, Pitt B, Cowley AJ. Effects of losartan and captopril on QT dispersion in elderly patients with heart failure. ELITE Study Group. Lancet. 1999;354:395–396. doi: 10.1016/s0140-6736(99)01354-9. [DOI] [PubMed] [Google Scholar]
- Caballero R, Delpon E, Valenzuela C, Longobardo M, Tamargo J. Losartan and its metabolite E3174 modify cardiac delayed rectifier K(+) currents. Circulation. 2000;101:1199–1205. doi: 10.1161/01.cir.101.10.1199. [DOI] [PubMed] [Google Scholar]
- Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol. 2007;581:479–493. doi: 10.1113/jphysiol.2006.123414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Melman Y, Palma E, Fishman GI, McDonald TV. Cyclic AMP regulates the HERG K(+) channel by dual pathways. Curr Biol. 2000;10:671–674. doi: 10.1016/s0960-9822(00)00516-9. [DOI] [PubMed] [Google Scholar]
- Daleau P, Turgeon J. Angiotensin II modulates the delayed rectifier potassium current of guinea pig ventricular myocytes. Pflugers Arch. 1994;427:553–555. doi: 10.1007/BF00374275. [DOI] [PubMed] [Google Scholar]
- De Mello WC. Cardiac arrhythmias: the possible role of the renin–angiotensin system. J Mol Med. 2001;79:103–108. doi: 10.1007/s001090100195. [DOI] [PubMed] [Google Scholar]
- De Mello WC, Danser AH. Angiotensin II and the heart: on the intracrine renin–angiotensin system. Hypertension. 2000;35:1183–1188. doi: 10.1161/01.hyp.35.6.1183. [DOI] [PubMed] [Google Scholar]
- De Mello WC, Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension. 2004;44:360–364. doi: 10.1161/01.HYP.0000139914.52686.74. [DOI] [PubMed] [Google Scholar]
- Dell'Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpon E, Caballero R, Gomez R, Nunez L, Tamargo J. Angiotensin II, angiotensin II antagonists and spironolactone and their modulation of cardiac repolarization. Trends Pharmacol Sci. 2005;26:155–161. doi: 10.1016/j.tips.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Boixel C, Cefai D, Abriel H, Pedrazzini T. Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1 potassium current downregulation. J Mol Cell Cardiol. 2007;42:63–70. doi: 10.1016/j.yjmcc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, et al. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1242–H1253. doi: 10.1152/ajpheart.01400.2006. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Lassegue B, Alexander RW. Angiotensin receptors and their therapeutic implications. Annu Rev Pharmacol Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harada K, Komuro I, Hayashi D, Sugaya T, Murakami K, Yazaki Y. Angiotensin II type 1a receptor is involved in the occurrence of reperfusion arrhythmias. Circulation. 1998;97:315–317. doi: 10.1161/01.cir.97.4.315. [DOI] [PubMed] [Google Scholar]
- Jalil JE, Palomera C, Ocaranza MP, Godoy I, Roman M, Chiong M, et al. Levels of plasma angiotensin-(1–7) in patients with hypertension who have the angiotensin-I-converting enzyme deletion/deletion genotype. Am J Cardiol. 2003;92:749–751. doi: 10.1016/s0002-9149(03)00847-6. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Bolden A, Horn R. Control of action potentials and Ca2+ influx by the Ca(2+)-dependent chloride current in mouse pituitary cells. J Physiol. 1991;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm LH, Dahlof B, Edelman JM, Ibsen H, Borch-Johnsen K, Olsen MH, et al. Effect of losartan on sudden cardiac death in people with diabetes: data from the LIFE Study. Lancet. 2003;362:619–620. doi: 10.1016/S0140-6736(03)14183-9. [DOI] [PubMed] [Google Scholar]
- Lindpaintner K, Jin MW, Niedermaier N, Wilhelm MJ, Ganten D. Cardiac angiotensinogen and its local activation in the isolated perfused beating heart. Circ Res. 1990;67:564–573. doi: 10.1161/01.res.67.3.564. [DOI] [PubMed] [Google Scholar]
- Marban E. Cardiac channelopathies. Nature. 2002;415:213–218. doi: 10.1038/415213a. [DOI] [PubMed] [Google Scholar]
- Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sharma D, Glatter KA, Timofeyev V, Tuteja D, Zhang Z, Rodriguez J, et al. Characterization of a KCNQ1/KVLQT1 polymorphism in Asian families with LQT2: implications for genetic testing. J Mol Cell Cardiol. 2004;37:79–89. doi: 10.1016/j.yjmcc.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Role of PKC in autocrine regulation of rat ventricular K+ currents by angiotensin and endothelin. Am J Physiol Heart Circ Physiol. 2003;284:H1168–H1181. doi: 10.1152/ajpheart.00748.2002. [DOI] [PubMed] [Google Scholar]
- Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des. 2006;12:2271–2283. doi: 10.2174/138161206777585102. [DOI] [PubMed] [Google Scholar]
- Thomas D, Zhang W, Wu K, Wimmer AB, Gut B, Wendt-Nordahl G, et al. Regulation of HERG potassium channel activation by protein kinase C independent of direct phosphorylation of the channel protein. Cardiovasc Res. 2003;59:14–26. doi: 10.1016/s0008-6363(03)00386-9. [DOI] [PubMed] [Google Scholar]
- Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- Tsuji Y, Opthof T, Kamiya K, Yasui K, Liu W, Lu Z, et al. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res. 2000;48:300–309. doi: 10.1016/s0008-6363(00)00180-2. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiro E, Ideishi M, Wang LX, Urata H, Kumagai K, Arakawa K, et al. Reperfusion-induced arrhythmias are suppressed by inhibition of the angiotensin II type 1 receptor. Cardiology. 2003;99:61–67. doi: 10.1159/000069722. [DOI] [PubMed] [Google Scholar]
- Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, et al. Effects of the renin–angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- Zankov DP, Omatsu-Kanbe M, Isono T, Toyoda F, Ding WG, Matsuura H, et al. Angiotensin II potentiates the slow component of delayed rectifier K+ current via the AT1 receptor in guinea pig atrial myocytes. Circulation. 2006;113:1278–1286. doi: 10.1161/CIRCULATIONAHA.104.530592. [DOI] [PubMed] [Google Scholar]