Abstract
Background and purpose:
Neutrophil migration into tissues is involved in the genesis of inflammatory pain. Here, we addressed the hypothesis that the effect of CXC chemokines on CXCR1/2 is important to induce neutrophil migration and inflammatory hypernociception.
Experimental approach:
Mice were treated with a non-competitive allosteric inhibitor of CXCR1/2, DF 2162, and neutrophil influx and inflammatory hypernociception were assessed by myeloperoxidase assay and electronic pressure meter test, respectively, in various models of inflammation.
Key results:
DF 2162 inhibited neutrophil chemotaxis induced by CXCR1/2 ligands but had no effect on CXCL8 binding to neutrophils. A single mutation of the allosteric site at CXCR1 abrogated the inhibitory effect of DF 2162 on CXCL-8-induced chemotaxis. Treatment with DF 2162 prevented influx of neutrophils and inflammatory hypernociception induced by CXCL1 in a dose-dependent manner. The compound inhibited neutrophil influx and inflammatory hypernociception induced by carrageenan, lipopolysaccharide and zymosan, but not hypernociception induced by dopamine and PGE2. DF 2162 had a synergistic effect with indomethacin or the absence of TNFR1 to abrogate carrageenan-induced hypernociception. Treatment with DF 2162 diminished neutrophil influx, oedema formation, disease score and hypernociception in collagen-induced arthritis.
Conclusions and implications:
CXCR1/2 mediates neutrophil migration and is involved in the cascade of events leading to inflammatory hypernociception. In addition to modifying fundamental pathological processes, non-competitive allosteric inhibitors of CXCR1/2 may have the additional benefit of providing partial relief for pain and, hence, may be a valid therapeutic target for further studies aimed at the development of new drugs for the treatment of rheumatoid arthritis.
Keywords: chemokines, CXCR1/2, arthritis, neutrophil, TNF-α, hyperalgesia
Introduction
Chemokines are small molecular weight proteins (8–10 kDa) thought to play an important role in the recruitment of leukocytes from the blood into tissues during inflammatory processes. Among the four classes of chemokines, CXC chemokines acting on their CXCR1 and CXCR2 receptors have been shown to coordinate neutrophil migration and activation in several models of inflammation (Bizzarri et al., 2006; Busch-Petersen, 2006; Reutershan, 2006). For example, pharmacological blockade of CXCR2 or administration of anti-CXC chemokines prevented neutrophil influx and tissue injury following intestinal, hepatic and cerebral ischaemia and reperfusion in rats (Souza et al., 2004; Cavalieri et al., 2005; Garau et al., 2005). More recently, we also showed that blockade of CXCR1/2 receptors prevents neutrophil influx and joint damage following adjuvant-arthritis in rats (Barsante et al., 2007).
In addition to mediating inflammatory tissue injury, neutrophil migration into tissue appears to be involved in the genesis of inflammatory pain (Levine et al., 1984). The sensitization of primary afferent nociceptors is a common denominator of all kinds of inflammatory pain that leads to a state of hyperalgesia and/or allodynia, better described as hypernociception in animal models (Millan, 1999). Hypernociception is induced by the direct action of the final mediators prostaglandins and sympathetic amines (for example, dopamine, adrenaline) on peripheral nociceptors (Ferreira et al., 1978; Nakamura and Ferreira, 1987; Khasar et al., 1999). These direct-acting hyperalgesic mediators are ultimately released in the inflamed tissue in response to a range of inflammatory stimuli that trigger the release of a cascade of cytokines (tumour necrosis factor (TNF)-α, interleukin-1β and CXC chemokines) by resident and incoming cells (Cunha et al., 2005; Verri et al., 2006). In mice, the chemokine CXCL1 (also known as KC, keratinocyte-derived chemokine) is released in response to several inflammatory stimuli, including carrageenan and lipopolysaccharide (LPS), and induces neutrophil influx and hypernociception when given intraplantarly (i.pl.). CXCL1-induced hypernociception is partially dependent on the production of sympathetic amines (Cunha et al., 2005). CXCL1 and TNF-α may also trigger the interleukin-1β/prostaglandin pathway, which has a synergistic effect with sympathetic amines to induce inflammatory hypernociception (Cunha et al., 2005).
In the present paper, we addressed the hypothesis that the effect of CXC chemokines on CXCR1/2 is important to induce neutrophil migration and inflammatory hypernociception induced by several inflammatory stimuli in mice. To address this hypothesis, mice were treated with DF 2162 (4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate), a compound belonging to the class of CXCR1/2 allosteric modulators (Bertini et al., 2004; Bizzarri et al., 2006; Barsante et al., 2007). Initial experiments confirmed the selectivity and mode of action of DF 2162. The compound was then tested in models in which neutrophil influx and inflammatory hypernociception were induced by i.pl. injection of the following stimuli: the chemokine CXCL1, carrageenan and LPS. We also determined whether DF 2162 could have a synergistic effect with blockade of prostaglandin synthesis (indomethacin) or with TNF-α blockade (using TNF receptor type-1 (TNFR1)-deficient mice) to prevent inflammatory hypernociception in mice. In addition, the effects of oral treatment with DF 2162 were investigated in a model of zymosan-induced arthritis and in a model of collagen-induced arthritis (CIA) in DBA/1J mice.
Methods
Cell isolation and tissue culture
Human mononuclear cells and polymorphonuclear leukocytes (PMNs) were obtained from buffy coats of heparin-treated blood from normal volunteers through the courtesy of Centro Trasfusionale, Ospedale S Salvatore, L'Aquila, Italy. Ethical clearance was obtained to perform these experiments. Mononuclear cells were obtained by centrifugation on Ficoll/Hipaque. Monocytes were separated by Percoll gradient centrifugation (Colotta et al., 1984). Human PMNs (95% purity) were prepared by dextran sedimentation followed by hypotonic lysis of contaminating red blood cells (McPhail and Snyderman, 1983).
L1.2 cells were maintained as described previously in suspension at 37 °C with 5% CO2 at a density of not more than 1 × 106 cells per ml (Wise et al., 2007). Cells were transiently transfected by electroporation as previously described (Imai et al., 1998). In brief, 1 × 106 cells per 3 μg of DNA were electroporated and incubated overnight with medium supplemented with 5 mM sodium butyrate. Cells were harvested and assayed the following day. Cellular viability was >95% in all the experiments, as measured by trypan blue dye exclusion.
Generation of mutants
wtCXCR1 cDNA coding sequence (GenBank accession no. M73969) was cloned using pcDNA3.1 expression vector as Not I–Xho I fragment. K99A CXCR1 mutant (Bertini et al., 2004) was made using a Quick Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) starting from wtCXCR1 pcDNA3.1 construct as template and following the instructions provided by the manufacturer.
Migration assay
Cell migration of human PMNs and monocytes was evaluated using a 48-well micro-chemotaxis chamber, as described previously (Bizzarri et al., 2001). Briefly, 29 μl of control medium (phosphate-buffered saline for monocytes and Hank's buffered salt solution for PMNs) or chemoattractant solution was seeded in the lower compartment of the chemotaxis chamber; 50 μl of cell suspension (1.5 × 106 per ml human PMNs or monocytes) preincubated at 37 °C for 15 min in the presence or absence of different concentrations of DF 2162 was seeded in the upper compartment. The two compartments of the chemotactic chamber were separated by 5-mm polycarbonate filter (PVP-free for PMN chemotaxis). The chamber was incubated at 37 °C in air with 5% CO2 for 45 min (human PMNs) or 2 h (monocytes). At the end of incubation, filters were removed, fixed, stained with Diff-Quik and five oil immersion fields at high magnification (× 100) were counted after sample coding. Cell migration of L1.2 was evaluated in 5-μm pore size Transwell filters (Bowman et al., 1998). The Transwell filter was incubated at 37 °C in air with 5% CO2 for 4 h and the cells were counted in a Burker chamber.
Radioligand-binding assay
Isolated PMNs (107 per ml) were resuspended in RPMI-1640 and incubated at 37 °C for 15 min in the presence of DF 2162 (1 μM) or vehicle. After incubation, cells were resuspended (2 × 107 per ml) in binding medium (RPMI-1640 containing 10 mg ml−1 bovine serum albumin, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid and 0.02% NaN3) in the presence of DF 2162 or vehicle. Aliquots of 0.2 nM of [125I]-CXCL8 and serial dilution of unlabelled CXCL8 were added to 106 cells in 100 μl of binding medium and incubated at room temperature for 1 h under gentle agitation. Unbound radioactivity was separated from cell-bound radioactivity by centrifugation through an oil gradient (80% silicon and 20% paraffin) on a microcentrifuge. Nonspecific binding was determined by adding a 100-fold molar excess of unlabelled CXCL8. Scatchard analysis and all calculations were performed with the LIGAND program (Munson and Rodbard, 1980).
Peritoneal murine macrophage preparation and LPS-induced PGE2 production
Peritoneal exudate cells were collected from peritoneal washings of mice, 5 days after i.p. inoculum of 3% thioglycollate in saline (1.5 ml per mouse), as previously described (Mascagni et al., 2000). Cells were placed at 1 × 106 per ml in 96-well plates and non-adherent cells removed by gentle washing 2 h later. Drugs were then added to adherent macrophages 20 min before adding LPS (1 μg ml−1). Control cells received vehicle at the appropriate dilution. Total prostaglandin E2 (PGE2) production was determined in the supernatant 24 h after LPS stimulation. PGE2 levels were measured by EIA kit (sensitivity 2.5 pg per well).
Animals
Experiments were performed in C57BL/6, wild-type (WT) and TNFR1-deficient (TNFR1−/−) mice (male, 20–30 g weight), except for the studies in which CIA was evaluated. In the latter, DBA/1J mice were used (male, 25–30 g weight). Mice were housed in the animal care facility of the Faculty of Medicine of Ribeirão Preto. They were taken to the testing room at least 1 h before experiments and were used once. The animal care and handling procedures were in accordance with the International Association for the Study of Pain Guidelines on the use of animals in pain research, and they were approved by the Animal Ethics Committee of the School of Medicine of Ribeirão Preto (University of São Paulo).
Experimental protocol
DF 2162 (5–45 mg kg−1) suspended in caboximetilcelulose 0.05% was administered 50 min before the inflammatory stimuli by oral gavage. Effects of treatment with DF 2162 given 2 or 3 h after injection of carrageenan or zymosan, respectively, were also evaluated. The following stimuli were used: carrageenan (100 μg in 25 μl saline), CXCL1/KC (10 ng in 25 μl saline), LPS (100 ng in 25 μl saline), zymosan (30 μg in 5 μl saline), PGE2 (100 ng in 25 μl saline) and dopamine (10 μg in 25 μl saline). The relevant vehicle (saline) was used as control in all experiments. Hypernociception and neutrophil influx were determined 3 h after the i.pl. injection of stimuli, with the exception of zymosan-induced arthritis in which hypernociception was determined at 3, 5 and 7 h and neutrophil influx was determined 3 h after the intra-articular injection of zymosan (30 μg in 5 μl saline). Previous studies determined that these parameters are optimal for evaluation of inflammatory hypernociception (Cunha et al., 2005; Guerrero et al., 2006). Indomethacin (2.5 mg kg−1, s.c.; 30 min before stimulus injection) and DF 2162 (15 mg kg−1) were used in combination at the given doses after injection of carrageenan.
Measurement of mechanical hypernociception
Paw test
The term hypernociception was used to define the decrease of nociceptive withdrawal threshold (Cunha et al., 2007). Mechanical hypernociception was tested in mice, as previously described (Cunha et al., 2004). The test consisted of evoking hindpaw flexion reflex with a hand-held force transducer (electronic anaesthesiometer; IITC Life Science, Woodland Hills, CA, USA) adapted with a 0.5-mm2 polypropylene tip. The investigator was trained to apply the tip perpendicularly to the central area of the hind paw with a gradual increase in pressure. The end point was characterized by removal of the paw followed by clear flinching movements. After paw withdrawal, the intensity of pressure was automatically recorded. The value for the response was obtained by averaging three measurements. The results are expressed by delta (Δ) withdrawal threshold (in g) calculated by subtracting zero-time mean measurements from time-interval mean measurements.
Articular nociception
Articular hypernociception was evaluated as previously described (Guerrero et al., 2006). For this model, a polypropylene tip probe with 4.2 mm2 area size adapted for the hand-held force transducer, instead of the standard tip probe (0.5 mm2), was applied on the plantar surface of hind paw to produce tibio-tarsal flexion movement. An increasing perpendicular force was applied to the central area of the plantar surface of the hind paw to induce the dorsal flexion of the tibio-tarsal joint, followed by paw withdrawal. A tilted mirror below the grid provided a clear view of the animal's hind paw. The electronic pressure-meter apparatus automatically recorded the intensity of the force applied when the paw was withdrawn. The test was repeated until three sequential, consistent measurements (that is, the variation between these measurements was less than 2 g). The flexion-elicited withdrawal threshold is expressed in grams.
Neutrophil migration into the paw and joint
Neutrophil migration into the subcutaneous plantar and tibio-tarsal joint tissues of mice hind paw was evaluated by using a myeloperoxidase (MPO) kinetic colorimetric assay, as previously described (Bradley et al., 1982; Guerrero et al., 2006; Valerio et al., 2007). Samples of subcutaneous plantar and joint tissues were collected in 50 mM K2HPO4 buffer (pH 6.0) containing 0.5% hexadecyl trimethylammonium bromide and kept at −80 °C until use. Samples were homogenized using a Polytron (PT3100), centrifuged at 16 100 g for 4 min and the resulting supernatant assayed spectrophotometrically for MPO activity determination at 450 nm (Spectra max), with three readings in 1 min. The MPO activity of samples was compared to a standard curve of neutrophils. Briefly, 10 μl of sample was mixed with 200 μl of 50 mM phosphate buffer pH 6.0, containing 0.167 mg ml−1 O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The results were presented as the MPO activity (number of neutrophils per mg of tissue).
Induction and assessment of collagen-induced arthritis
Male DBA/1 mice (12–14 weeks old) received 200 μg of bovine type-II collagen in complete Freund's adjuvant by i.d. injection (day 0). Collagen (200 μg in phosphate-buffered saline) was given again on day 21 by i.p. injection (Leung et al., 2003). Mice were monitored daily for signs of arthritis for which severity scores were derived as follows: 0=normal, 1=erythema, 2=erythema plus swelling, 3=extension/loss function and total score=sum of four limbs. Disease onset characterized by erythema and/or paw swelling was seen between days 25 and 35. Paw thickness was measured with a plesthismometer (Ugo Basile, Comerio, VA, Italy) before the CIA induction (Vo) and every day after the beginning of the disease (VT), as described previously (Winter et al., 1962). The amount of paw swelling was determined for each mice and the difference between VT and Vo was taken as the oedema value (Δ oedema in mm3). For the therapeutic approach, DBA/1 mice were treated with DF 2162 (15 mg kg−1) p.o. or vehicle twice a day for a total of 7 days. The treatment began 1 day after CIA became clinically detectable. At day 8, the hind paws were used for the determination of tissue MPO contents.
Drugs and reagents
The following materials were obtained from the indicated sources: chemokines were purchased from PeproTech (London, UK). Bovine type-II collagen, zymosan, PGE2 and dopamine were purchased from Sigma Chemical Co. (St Louis, MO, USA). DF 2162 was produced by the chemical department of Dompe S.p.A, L'Aquila, Italy. Bacterial endotoxin from Escherichia coli referred to here as LPS (LPS-Difco Laboratories Ltd, West Molsey, Surrey, UK). Carrageenan was obtained from FMC (Philadelphia, PA, USA), and indomethacin was obtained from Prodome Química e Farmacêutica (São Paulo, Brazil). Diff-Quik was from Dade Behring (Düdingen, Switzerland). Polycarbonate filters and micro-Boyden chambers were from Neuroprobe Inc. (Pleasanton, CA, USA). Transwell filters were from Costar (Cambridge, MA, USA). pcDNA3 was from Invitrogen (Carlsbad, CA, USA). Cell culture reagents were from Life Technologies (Grand Island, NY, USA). [125I]-CXCL8 (0.2–0.02 nM, specific activity 2200 Ci mmol−1 (1 Ci=37 GBq)) was from Amersham Pharmacia (Buckinghamshire, England, UK).
Statistical analysis
For in vivo experiments, the results are presented as means±s.e.mean for groups of five animals and they are representative of two independent experiments. For in vitro chemotaxis assays, data are expressed as percentage of inhibition related to the control group (media±s.d. of two separate experiments) whereas PGE2 levels (pg per well) are presented as means±s.d. of three independent experiments. Differences between experimental groups were compared by ANOVA and individual comparisons were subsequently made with Tukey's post hoc test. The level of significance was set at P<0.05.
Results
Effect of DF 2162 on human leukocyte chemotaxis
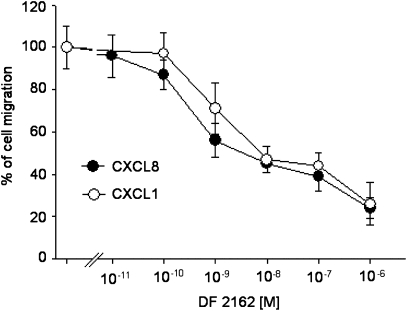
The effect of DF 2162 on human PMN chemotaxis induced by CXCL8 and CXCL1 was assessed over a wide range of concentrations (10 pM–1 μM). As shown in Figure 1, pretreatment of human PMNs with DF 2162 inhibited PMN migration induced by an optimal concentration of CXCL8 or CXCL1 (1 or 10 nM, respectively). The inhibitory effect was concentration-dependent and the IC50 values were 8.4 and 26 nM for CXCL8- and CXCL1-induced chemotaxis, respectively. In the absence of chemokine stimulation, DF 2162 was unable to modify spontaneous migration of PMNs (data not shown).
Figure 1.
The effects of DF 2162 on CXCL8 (1 nM)- or CXCL1 (10 nM)-induced migration of human PMNs. PMNs were preincubated at 37 °C for 15 min with vehicle (control) or increasing concentrations of DF 2162. Data are expressed as percentage of cell migration (mean±s.d. of three separate experiments). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; PMNs, polymorphonuclear leukocytes.
Next, we examined the effect of DF 2162 on fMLP-, C5a- or CCL2-induced chemotaxis. As shown in Table 1, DF 2162 (1 μM) did not affect migration of human leukocytes induced by C5a, fMLP or CCL2. Similarly, DF 2162 (10 μM) did not affect LPS-induced PGE2 production on murine peritoneal macrophages (Table 2). It is noteworthy that DF 2162 did not alter the basal production of PGE2 by murine macrophages (Table 2).
Table 1.
Selectivity of the effect of DF 2162
| Cells | Chemotatic factor | DF 2162 concentration (M) | % inhibition of migration |
|---|---|---|---|
| Human PMN | fMLP | 10−6 | 2±6 |
| Human PMN | C5a | 10−6 | −5±11 |
| Human monocyte | CCL2 | 10−6 | 0±4 |
Abbreviations: LPS, lipopolysaccharide; PMN, polymorphonuclear leukocyte.
Human PMNs and monocytes were preincubated at 37 °C for 15 min with DF 2162. PMNs were then tested for their ability to migrate in response to fMLP (10 nM) or C5a (1 nM). Monocytes were tested for their ability to migrate in response to CCL2 (2.5 nM). Data (subtracted from spontaneous migration) are presented as percentage of inhibition of migration (mean±s.d. of two independent experiments).
Table 2.
Effect of DF 2162 on LPS-induced PGE2 production
| Treatment | PGE2 (pg per well) |
|---|---|
| Vehicle | 0.18±0.11 |
| DF 2162 (10 μM) | 0.10±0.12 |
| LPS (1 μg ml−1) | 3.64±1.18 |
| LPS+DF 2162 | 3.95±1.09 |
Abbreviations: LPS, lipopolysaccharide; PGE2, prostaglandin E2.
Mouse peritoneal macrophages were exposed to DF 2162 (10 μM) for 20 min before addition of LPS (1 μg ml−1). Total PGE2 production was measured 24 h after LPS stimulation. Data are presented as mean±s.d. of three independent experiments.
Effect of DF 2162 on CXCL8 binding to human PMNs
DF 2162 (1 μM) did not affect the binding (9.9±1.1 × 104 and 10.3±1.3 × 104 sites per cell in vehicle- and DF 2162-pretreated groups, respectively) or affinity (Kd=1.5±0.30 nM and 1.7±0.4 nM in vehicle- and DF 2162-pretreated groups, respectively) of CXCL8 to human PMNs.
Effect of DF 2162 on L1.2 cell line CXCR1 transfectants
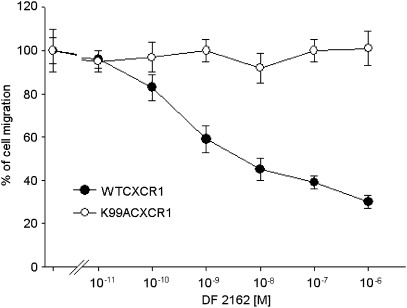
A series of experiments were then conducted to confirm that the inhibitory effects of DF 2162 were indeed mediated by binding to a previously described allosteric site on CXCR1 (Bertini et al., 2004). As shown in Figure 2, CXCL8 (10 nM)-induced migration of L1.2 transfectants expressing CXCR1wt was inhibited by DF 2162, with an efficacy and potency in the same order as that observed on human PMN migration. These cells did not express CXCR2 (data not shown). Using the same CXCR1wt/L1.2 transfectants, DF 2162 did not affect CXCL12-induced migration (data not shown). In experiments conducted with L1.2 transfectants expressing CXCR1K99A, previously found to be totally resistant to the action of reparixin (Bertini et al., 2004), DF 2162 (10 pM–1 μM) did not affect CXCL8-induced chemotaxis (Figure 2).
Figure 2.
The effects of DF 2162 on CXCR1wt/L1.2 or K99A CXCR1K99A/L1.2 transfectant migration in response to 10 nM CXCL8. Transfectants were preincubated at 37 °C for 30 min with vehicle or increasing concentrations of DF 2162. Data are expressed as percentage of inhibition of CXCL8-mediated cell migration (mean±s.d. of two separate experiments). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate.
DF 2162 inhibits neutrophil recruitment and inflammatory hypernociception induced by CXCL1, carrageenan and LPS
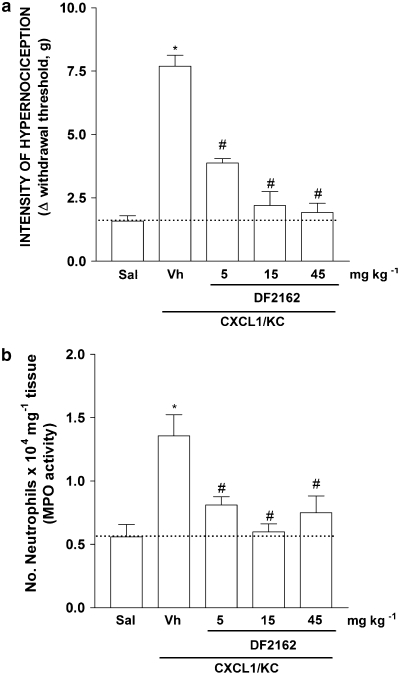
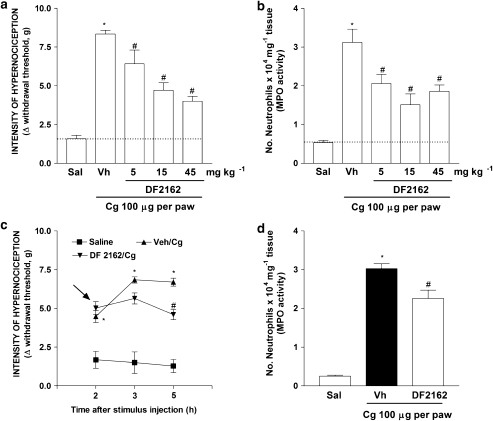
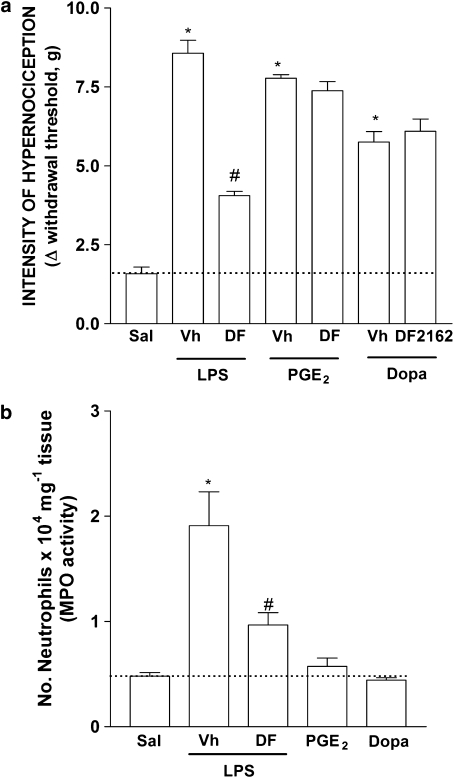
Treatment of mice with DF 2162 reduced in a dose-dependent (5–45 mg kg−1) manner both neutrophil influx and inflammatory hypernociception induced by the chemokine CXCL1 (10 ng per paw, Figures 3a and b). There was greater than 95% inhibition of CXCL1-induced responses at 15 mg kg−1of DF 2162. The compound also reduced carrageenan (100 μg per paw)-induced neutrophil influx and mechanical hypernociception in a dose-dependent manner (Figures 4a and b). There was 60% inhibition of neutrophil influx and 56% inhibition of hypernociception when DF 2162 was used at 15 mg kg−1. There was no further inhibition with higher doses of DF 2162 (Figures 4a and b). As DF 2162 at 15 mg kg−1 was fully effective at inhibiting CXCL1-induced responses, this dose was used in all further experiments. Treatment of mice with DF 2162 (15 mg kg−1) 2 h after injection of carrageenan was also accompanied by a similar inhibition of mechanical hypernociception and neutrophil migration (Figures 4c and d). It is of note that administration of DF 2162 was less effective when given after than before injection of carrageenan (Figure 4). The compound also reduced LPS (100 ng per paw)-induced neutrophil recruitment and hypernociception by 63 and 65%, respectively (Figures 5a and b).
Figure 3.
Treatment with DF 2162 dose-dependently inhibited CXCL1-induced (a) mechanical hypernociception and (b) neutrophil migration. Mice were treated with DF 2162 (5, 15 and 45 mg kg−1, p.o.) or vehicle (Vh) 50 min before the intraplantar injection of CXCL1 (100 ng per paw) or saline (Sal). Hypernociceptive responses were evaluated 3 h after CXCL1, and hind paws were then collected for MPO analysis (a and b, respectively). *P<0.05 when compared to saline-injected paws and #P<0.05 when compared to vehicle-treated group (one-way ANOVA followed by Tukey's post hoc test; n=5 in each group). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; MPO, myeloperoxidase.
Figure 4.
Treatment with DF 2162 dose-dependently reduced carrageenan-induced mechanical hypernociception and neutrophil migration in the mice paw. Mice were treated with DF 2162 (5, 15 and 45 mg kg−1, p.o.) or vehicle (Vh) 50 min before the intraplantar injection of carrageenin (Cg, 100 μg per paw) or saline (Sal). Hypernociceptive responses were evaluated 3 h after carrageenan and hind paws were then collected for MPO analysis (a and b, respectively). Mice were also treated with DF 2162 (indicated by arrows; 15 mg kg−1, p.o.) or vehicle (Vh) 2 h after Cg injection. Hypernociceptive responses were evaluated 3 and 5 h later and hind paws were then collected for MPO analysis (c and d, respectively). *P<0.05 when compared to saline-injected paws and #P<0.05 when compared to vehicle-treated group (one-way ANOVA followed by Tukey's post hoc test; n=5 in each group). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; MPO, myeloperoxidase.
Figure 5.
Treatment with DF 2162 reduced LPS-induced (a) mechanical hypernociception and (b) neutrophil migration but had no effect on PGE2- and dopamine-induced hypernociception. Mice were treated with DF 2162 (15 mg kg−1, p.o.) or vehicle (Vh) 50 min before the intraplantar injection of LPS (100 ng per paw), PGE2 (100 ng per paw), dopamine (Dopa, 10 μg per paw) or saline (Sal). Hypernociceptive responses were evaluated 3 h after stimuli and hind paws were then collected for MPO analysis (a and b, respectively). *P<0.05 when compared to saline-injected paws and #P<0.05 when compared to vehicle-treated group (one-way ANOVA followed by Tukey's post hoc test; n=5 in each group). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; LPS, lipopolysaccharide; MPO, myeloperoxidase; PGE2, prostaglandin E2.
DF 2162 does not modify the inflammatory hypernociception induced by PGE2 and dopamine
Treatment of mice with DF 2162 had no significant effect on hypernociception induced by i.pl. injection of two directly acting hypernociceptive mediators, PGE2 (100 ng per paw) or dopamine (10 μg per paw) (Figure 5a). These substances cause hypernociception because they act directly on receptors present in the membrane of primary nociceptive neurons leading to their sensitization (Ferreira et al., 1978; Nakamura and Ferreira, 1987; Khasar et al., 1999). Therefore, the hypernociception induced by PGE2 and dopamine is independent of neutrophil migration (Cunha et al., 2008). Supporting this suggestion, i.pl. injection of PGE2 or dopamine, at doses that produced intense hypernociception, failed to induce significant recruitment of neutrophils above levels observed in saline-injected paws (Figure 5b).
Additive effect of DF 2162 plus indomethacin on inflammatory hypernociception
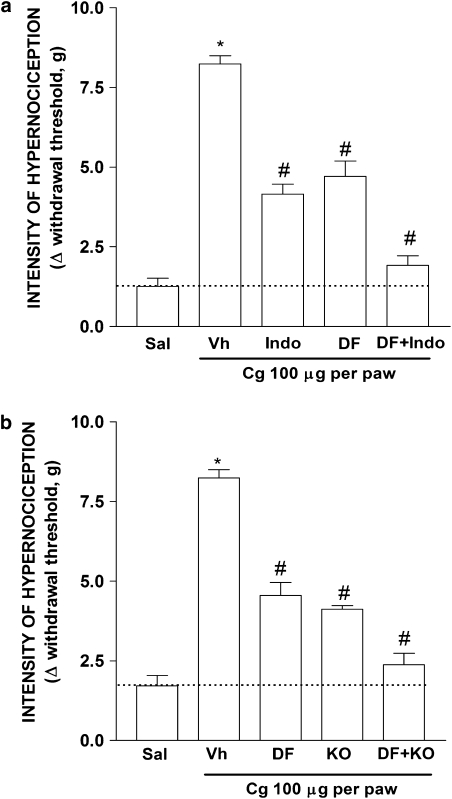
Cyclooxygenase inhibitors, such as indomethacin, are the most frequently used medication for the relief of inflammatory pain (Kean and Buchanan, 2005; Hinz and Brune, 2007). Previous studies from our laboratory have shown that carrageenan-induced inflammatory hypernociception in mice relies on the co-operation between sympathetic mediators, triggered by CXCL1, and prostaglandins triggered mostly by TNF-α (Cunha et al., 2005). Figure 6a shows that treatment of carrageenan-injected mice with DF 2162 diminished inflammatory hypernociception by 51%. In the same experiment, indomethacin diminished carrageenan-induced responses by 59%. Combination treatment with DF 2162 and indomethacin abrogated (greater than 90% inhibition) carrageenan-induced hypernociception. Moreover, hypernociception induced by carrageenan was decreased by 40% in TNFR1−/− mice when compared to their WT counterparts. Treatment of TNFR1−/− mice with indomethacin produced no significant further reduction of carrageenin-induced mechanical hypernociception when compared with vehicle-treated TNFR1−/− mice (data not shown). Consistent with the synergy between the CXCL1/adrenergic and TNF-α/prostaglandin pathways, treatment of TNFR1−/− mice with DF 2162 (15 mg kg−1) abolished (greater than 90% inhibition) carrageenan-induced hypernociception (Figure 6b).
Figure 6.
Combined treatment with DF 2162 and indomethacin or administration of DF 2162 to TNFR1−/− mice abrogated carrageenan-induced mechanical hypernociception. (a) Mice treated with DF 2162 (15 mg kg−1, p.o.), indomethacin (Indo, 2.5 mg kg−1, s.c.), DF 2162 plus Indo or vehicle (Vh). (b) The effect of treatment of WT and TNFR1−/− mice with DF 2162 (15 mg kg−1, p.o.). Mice were then injected with carrageenan (Cg, 100 μg per paw) or Saline (Sal) and hypernociceptive responses evaluated after 3 h. *P<0.05 when compared to saline-injected paws and #P<0.05 when compared to vehicle-treated group (one-way ANOVA followed by Tukey's post hoc test; n=5 in each group). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; TNFR1, TNF receptor type-1.
DF 2162 inhibits neutrophil recruitment and inflammatory hypernociception in zymosan-induced arthritis
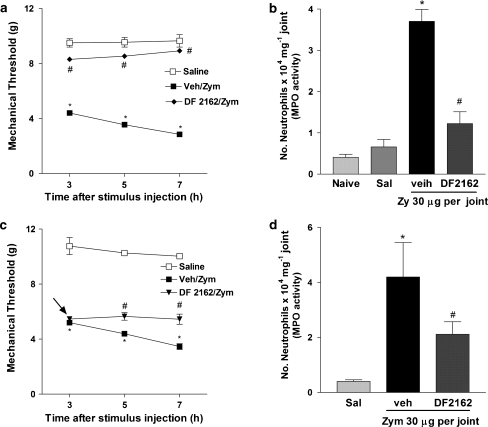
We have demonstrated that administration of zymosan into the tibio-tarsal joint of mice induces mechanical hypernociception, which was closely associated with the recruitment of neutrophil into the joint (Guerrero et al., 2006). Treatment of mice with DF 2162 (15 mg kg−1) inhibited both neutrophil migration and mechanical hypernociception induced by intra-articular injection of zymosan (Figures 7a and b). From a therapeutic perspective, treatment of mice with DF 2162 (15 mg kg−1) 3 h after injection of zymosan partially inhibited articular hypernociception and neutrophil migration (Figures 7c and d). Again, the effects of the post-treatment were of lesser intensity than when the compound was given before injection of zymosan.
Figure 7.
Treatment with DF 2162 inhibited mechanical hypernociception and neutrophil migration induced by intra-articular injection of zymosan. Mice were treated with DF 2162 (15 mg kg−1, p.o.) or vehicle (Vh) 50 min before the intra-articular injection of zymosan (zy, 30 μg per joint) or saline. Hypernociceptive responses were evaluated 3, 5 and 7 h after stimuli and joints were then collected for MPO analysis (a and b, respectively). Mice were also treated with DF 2162 (indicated by an arrow; 15 mg kg−1, p.o.) or vehicle (Vh) 3 h after zymosan injection. Hypernociceptive responses were evaluated 5 and 7 h later and joints were then collected for MPO analysis (c and d, respectively). *P<0.05 when compared to saline-injected paws and #P<0.05 when compared to vehicle-treated group (one-way ANOVA followed by Tukey's post hoc test; n=5 in each group). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; MPO, myeloperoxidase.
DF 2162 ameliorates collagen-induced arthritis in mice
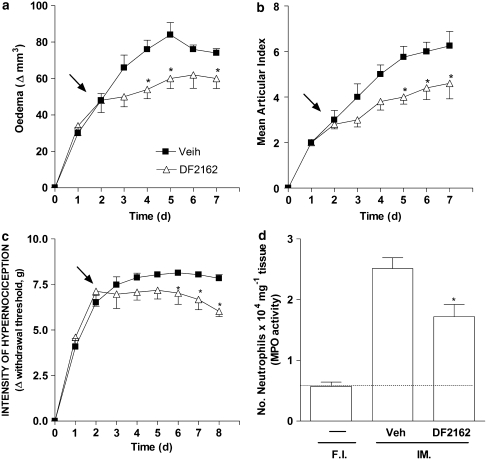
Next, we examined whether DF 2162 would have a similar inhibitory effect on neutrophil influx and inflammatory hypernociception in a murine model of CIA. DF 2162 (15 mg kg−1, p.o., twice a day) was given for 7 days starting from the day after onset of disease. Treatment with DF 2162 significantly ameliorated signs of CIA, as assessed by a decrease in paw oedema and mean articular index (Figures 8a and b). Mechanical hypernociception was partially inhibited (maximal inhibition was 25%) and only after the fifth day of DF 2162 treatment (Figure 8c). DF 2162 reduced the number of neutrophils infiltrating into the affected paws by 40% (Figure 8d).
Figure 8.
Treatment with DF 2162 ameliorated collagen-induced arthritis. DBA/1 mice were immunized with collagen and the appearance of arthritis was monitored daily. Treatment with DF 2162 (15 mg kg−1, twice a day) or saline was started 1 day after disease became clinically significant (demonstrated by arrows in the figure). Paw oedema (a), mean articular index (b) and hypernociception (c) were evaluated daily. Hind paws were collected and the number of neutrophils (d) determined by measuring MPO activity at day 8 after start of treatment. *P<0.05 when compared to the vehicle-treated group (one-way ANOVA followed by Tukey's post hoc test; n=8 in each group). DF 2162, 4-[(1R)-2-amino-1-methyl-2-oxoethyl]phenyl trifluoromethane sulphonate; MPO, myeloperoxidase.
Discussion
DF 2162 is an orally active compound that belongs to the class of 2-arylpropionamide drugs, a novel class of allosteric modulators of CXCR1 and CXCR2 (Bertini et al., 2004; Barsante et al., 2007). In the present study, we confirmed that DF 2162 blocks the action of CXCR1- and CXCR2-active chemokines. Moreover, evidence is provided to show that, akin to reparixin (Bertini et al., 2004), DF 2162 does not displace the ligand but appears to act via binding to an allosteric site at the chemokine receptor. In our in vivo experiments, treatment with DF 2162 greatly prevented the influx of neutrophils induced by CXCL1, a chemokine active on murine CXCR1/2 receptors, carrageenan, LPS and zymosan. More importantly, the compound diminished by a similar extent inflammatory hypernociception induced by the same stimuli, suggesting that CXCR1/2-mediated neutrophil influx is an important element in the cascade of events leading to inflammatory hypernociception. Further experiments in a model of CIA confirmed the ability of the compound to diminish neutrophil influx, oedema, tissue damage (arthritic index) and hypernociception.
Initial experiments were carried out to confirm the ability of DF 2162 to block the activity of the relevant chemokine in mice. Our results clearly show that DF 2162 dose-dependently inhibited the ability of the CXCR2-active chemokine CXCL1 to induce neutrophil recruitment after i.pl. injection. DF 2162 also blocked the neutrophil recruitment induced by carrageenan and LPS. These results are consistent with the known neutrophil-recruiting activity of CXCR2 in mice and the importance of this receptor for neutrophil influx in several models of inflammation (Bozic et al., 1994; Lee et al., 1995; McColl and Clark-Lewis, 1999; Bertini et al., 2004; Souza et al., 2004; Bizzarri et al., 2006; Reutershan, 2006; Barsante et al., 2007). More recently, Fan et al. (2007) described murine CXCR1, a functional receptor for CXCL6 and human IL-8. DF 2162 blocked both CXCR1 and CXCR2 in human cells (Barsante et al., 2007), but the tools are not yet widely available to test whether DF 2162 will block murine CXCR1 with a similar efficacy and potency as it blocks the function of murine CXCR2 and human CXCR1 and CXCR2.
Previous studies from our and other groups (Levine et al., 1984; Bezerra et al., 2007; Cunha et al., 2008), especially in rat models of inflammation, have demonstrated that neutrophils may play a role in mediating inflammatory hypernociception induced by several stimuli, including carrageenan and LPS. The relevance of CXCR2 in the neutrophil-mediated effects was, to the best of our knowledge, not known. In the present study, we showed that blockade of neutrophil influx with DF 2162 was associated with inhibition of inflammatory hypernociception induced by CXCL1, carrageenin and LPS. Indeed, there was a good association between the extent of inhibition of neutrophil influx and the extent of inhibition of inflammatory hypernociception. The inhibition of neutrophil migration and consequently of hypernociception by DF 2162 in a model of zymosan-induced arthritis also support a neutrophil-dependent hypernociceptive mechanism. In the latter model, inhibition of neutrophil migration, by fucoidin or neutralizing antibody against neutrophils, blocked mechanical hypernociception (Guerrero et al., 2008). The ability of CXCL1 to induce neutrophil recruitment by a mechanism that can be inhibited by DF 2162 is consistent with the role of neutrophils in mediating inflammatory hypernociception. Thus, our results clearly indicate that neutrophils recruited in response to CXCR1/2 stimulation are essential in the cascade of events leading to inflammatory hypernociception induced by several inflammatory stimuli.
Blockade of prostaglandins and sympathetic amines is known to diminish hypernociception induced by known mediators of inflammation (Cunha et al., 2005; Verri et al., 2006). Indeed, prostaglandins and sympathetic amines are considered to be direct-acting hypernociceptive mediators (Ferreira et al., 1978; Khasar et al., 1999). They do not induce neutrophil recruitment but act directly on their receptors, present in the nociceptor, to induce hypernociception. DF 2162 greatly reduced the hypernociception induced by carrageenan and LPS but had no effect on the hypernociception induced by PGE2 and dopamine. Two important conclusions may be derived from these latter results. First, the effects of DF 2162 on hypernociception appear to be specific, as the drug does not block hypernociception induced by all stimuli. Second, the action of DF 2162 and, by inference, the role of CXCR1/2, appear to be upstream of the release of prostaglandins and sympathetic amines and, therefore, this compound does not directly affect nociceptor sensitization. Thus, our studies clearly favour the hypothesis that CXCR1/2 is essential in the cascade of non-neuronal events leading to the release of the direct-acting mediators of inflammatory pain, which ultimately cause sensitization of nociceptors and consequently mechanical hypernociception. This is consistent with our findings in mice (Cunha et al., 2005) and studies showing that inhibition of neutrophil influx prevents the local release of PGE2 in rats (Cunha et al., 2008). Thus, the antihypernociceptive actions of DF 2162 appear to be secondary to its ability to prevent neutrophil influx or activation and consequent neutrophil-induced release of other mediators. However, we cannot discard the possibility that DF 2162 also prevents the action of CXCL1 directly on sensory nerves. Indeed, CXCR2 is present on sensory nerves and the activation of this receptor may trigger nociceptor sensitization directly (Qin et al., 2005).
The experiments showing a synergism between DF 2162 and indomethacin or with TNF-α blockade favour our previous hypothesis that two main hypernociceptive pathways mediate carrageenan-induced mechanical inflammatory hypernociception in mice. One pathway, initiated by TNF-α, triggers interleukin-1β and prostaglandin release, and the other pathway, initiated by CXCL1, triggers the release of sympathetic amines and may also stimulate the interleukin-1β/prostaglandin pathway (Cunha et al., 2005).
Although there are a reasonable number of effective drugs used for symptomatic relief of inflammatory disease (for example, non-steroidal anti-inflammatory drugs), there are few safe drugs that modify the pathological processes responsible for chronic inflammation (Olsen and Stein, 2004). Cytokine-based therapies, especially strategies that prevent the effects of TNF-α, have been used for the treatment of rheumatoid arthritis and found to be useful in preventing progression of this disease in several groups of patients. However, the latter therapies are based on the use of exogenous proteins (such as antibodies) that are costly, are given by injection and have the inherent possibility of eliciting an immune response against the administered protein (Olsen and Stein, 2004). As DF 2162 blocked two important components of inflammation, neutrophil influx and hypernociception, thought to be central in human arthritis, we tested the effects of the compound in a model of CIA. We chose to give the compound in a therapeutic regime, that is, 1 day after the onset of the disease. Our experiments showed that DF 2162 decreased paw oedema, arthritic index and neutrophil influx into affected paws. This was accompanied by a small but significant decrease of inflammatory hypernociception, which appeared to be greater towards the end of the treatment. These results expand our recent findings showing that DF 2162 decreased adjuvant-induced arthritis in rats. However, the findings are novel as they indicate that, in addition to decreasing tissue inflammation and damage, CXCR2 blockade may also have the additional benefit of providing relief to the unpleasant symptoms of pain.
In conclusion, this study shows that treatment with DF 2162 greatly prevented neutrophil influx induced by a range of stimuli injected i.pl. or in the knee joint in mice. More importantly, this study is the first to show that treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR2, greatly diminished inflammatory hypernociception. Taken together with the protective effects of the drug in the model of CIA and with our previous studies (Barsante et al., 2007), we suggest that blockade of CXCR2 may be a useful strategy for the treatment of chronic arthritis in humans. In addition to modifying fundamental pathological processes, these compounds may have the additional benefit of providing partial relief for pain. These possibilities need to be tested in the clinical situation.
Acknowledgments
This work received financial support from Fundação de Amparo a Pesquisas do Estado de Minas Gerais (FAPEMIG, Brazil), Fundação de Amparo a Pesquisas do Estado de São Paulo (FAPESP, Brazil), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Brazil) and the European Union FP6 (INNOCHEM, grant number LSHB-CT-2005-518167).
Abbreviations
- IL-1
interleukin-1
- KC/CXCL1
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- PGE2
prostaglandin E2
- TNF
tumour necrosis factor
- TNFR1−/−
TNF receptor type-1 knockout
- WT
wild-type
Conflict of interest
Marcello Allegretti, Riccardo Bertini and Claudia Di Giacinto are employees of Dompé pha.r.ma s.p.a., Italy. The company has interests in the development of CXCR2 allosteric modulators for the treatment of inflammatory diseases.
References
- Barsante MM, Cunha TM, Allegretti M, Cattani F, Policani F, Bizzarri C, et al. Blockade of the chemokine receptor CXCR2 ameliorates adjuvant-induced arthritis in rats. Br J Pharmacol. 2007;153:992–1002. doi: 10.1038/sj.bjp.0707462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra MM, Brain SD, Girao VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112:139–149. doi: 10.1016/j.pharmthera.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Bizzarri C, Pagliei S, Brandolini L, Mascagni P, Caselli G, Transidico P, et al. Selective inhibition of interleukin-8-induced neutrophil chemotaxis by ketoprofen isomers. Biochem Pharmacol. 2001;61:1429–1437. doi: 10.1016/s0006-2952(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Bowman EP, Campbell JJ, Druey KM, Scheschonka A, Kehrl JH, Butcher EC. Regulation of chemotactic and proadhesive responses to chemoattractant receptors by RGS family members. J Biol Chem. 1998;273:28040–28048. doi: 10.1074/jbc.273.43.28040. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski LF, Jr, Conklyn MJ, Breslow R, et al. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Busch-Petersen J. Small molecule antagonists of the CXCR2 and CXCR1 chemokine receptors as therapeutic agents for the treatment of inflammatory diseases. Curr Top Med Chem. 2006;6:1345–1352. doi: 10.2174/15680266106061345. [DOI] [PubMed] [Google Scholar]
- Cavalieri B, Mosca M, Ramadori P, Perrelli MG, De Simone L, Colotta F, et al. Neutrophil recruitment in the reperfused-injured rat liver was effectively attenuated by repertaxin, a novel allosteric noncompetitive inhibitor of CXCL8 receptors: a therapeutic approach for the treatment of post-ischemic hepatic syndromes. Int J Immunopathol Pharmacol. 2005;18:475–486. doi: 10.1177/039463200501800307. [DOI] [PubMed] [Google Scholar]
- Colotta F, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984;132:936–944. [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Poole S, Parada CA, Cunha FQ, Ferreira SH.Pain facilitation by proinflammatory cytokine actions at peripheral nerve terminals Immune and Glial Regulation of Pain 2007IASP Press: Seattle; In: DeLeo J, Sorkin L, Watkins L (eds)pp. 67–83 [Google Scholar]
- Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha F.Crucial role of neutrophils in the development of mechanical inflammatory hypernociception J Leukoc Biol 200883in press [DOI] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Nakamura M, De Abreu Castro MS. The hyperalgesic effects of prostacyclin and prostaglandin E2. Prostaglandins. 1978;16:31–37. doi: 10.1016/0090-6980(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, et al. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Guerrero AT, Verri WA, Jr, Cunha TM, Silva TA, Schivo IR, Dal-Secco D, et al. Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol. 2008;83:122–130. doi: 10.1189/jlb.0207123. [DOI] [PubMed] [Google Scholar]
- Guerrero AT, Verri WA, Jr, Cunha TM, Silva TA, Rocha FA, Ferreira SH, et al. Hypernociception elicited by tibio-tarsal joint flexion in mice: a novel experimental arthritis model for pharmacological screening. Pharmacol Biochem Behav. 2006;84:244–251. doi: 10.1016/j.pbb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Hinz B, Brune K. Antipyretic analgesics: nonsteroidal antiinflammatory drugs, selective COX-2 inhibitors, paracetamol and pyrazolinones. Handb Exp Pharmacol. 2007;177:65–93. doi: 10.1007/978-3-540-33823-9_3. [DOI] [PubMed] [Google Scholar]
- Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- Kean WF, Buchanan WW. The use of NSAIDs in rheumatic disorders 2005: a global perspective. Inflammopharmacology. 2005;13:343–370. doi: 10.1163/156856005774415565. [DOI] [PubMed] [Google Scholar]
- Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–1530. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- Levine JD, Lau W, Kwiat G, Goetzl EJ. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science. 1984;225:743–745. doi: 10.1126/science.6087456. [DOI] [PubMed] [Google Scholar]
- Mascagni P, Sabbatini V, Biordi L, Martinetti S, Allegretti M, Marullo A, et al. R- and S-isomers of nonsteroidal anti-inflammatory drugs differentially regulate cytokine production. Eur Cytokine Netw. 2000;11:185–192. [PubMed] [Google Scholar]
- McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- McPhail LC, Snyderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocytes by chemoattractants and other soluble stimuli. Evidence that the same oxidase is activated by different transductional mechanisms. J Clin Invest. 1983;72:192–200. doi: 10.1172/JCI110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ferreira SH. A peripheral sympathetic component in inflammatory hyperalgesia. Eur J Pharmacol. 1987;135:145–153. doi: 10.1016/0014-2999(87)90606-6. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Reutershan J. CXCR2—the receptor to hit. Drug News Perspect. 2006;19:615–623. doi: 10.1358/dnp.2006.19.10.1068009. [DOI] [PubMed] [Google Scholar]
- Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio DA, Cunha TM, Arakawa NS, Lemos HP, Da Costa FB, Parada CA, et al. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: inhibition of cytokine production-dependent mechanism. Eur J Pharmacol. 2007;562:155–163. doi: 10.1016/j.ejphar.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development. Pharmacol Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Wise EL, Duchesnes C, Da Fonseca PC, Allen RA, Williams TJ, Pease JE. Small molecule receptor agonists and antagonists of CCR3 provide insight into mechanisms of chemokine receptor activation. J Biol Chem. 2007;282:27935–27943. doi: 10.1074/jbc.M703255200. [DOI] [PMC free article] [PubMed] [Google Scholar]