Abstract
In this paper I present an updated account that attempts to explain, in cognitive processing and neural terms, the nonverbal intellectual impairments experienced by most children with deletions of chromosome 22q11.2. Specifically, I propose that this genetic syndrome leads to early developmental changes in the structure and function of clearly delineated neural circuits for basic spatiotemporal cognition. This dysfunction then cascades into impairments in basic magnitude and then numerical processes, because of the central role that representations of space and time play in their construction. I propose that this takes the form of “spatiotemporal hypergranularity”; the increase in grain size and thus reduced resolution of mental representations of spatial and temporal information. The result is that spatiotemporal processes develop atypically and thereby produce the characteristic impairments in nonverbal cognitive domains that are a hallmark feature of chromosome 22q11.2 deletion syndrome. If this hypothesis driven account is supported by future research, the results will create a neurocognitive explanation of spatiotemporal and numerical impairments in the syndrome that is specific enough to be directly translated into the development of targeted therapeutic interventions.
Keywords: Space, Time, Number, Brain, Cognition, Attention
The physical, behavioral and cognitive phenotype resulting from microdeletions of chromosome 22q11.2 is quite stable and aspects of it has been given the labels of DiGeorge (DiGeorge 1965), Velocardiofacial (Shprintzen et al. 1978) and Conotruncal Anomaly Face (Burn et al. 1993) syndromes, and some cases of Cayler Cardiofacial syndrome (Giannotti et al. 1994) and Opitz G/BBB syndrome (McDonald-McGinn et al. 1995). A more complete review can be found in the paper by Shprintzen elsewhere in this issue. Therefore I will use the more inclusive label of chromosome 22q11.2 deletion sydrome (hereafter DS22q11.2) to describe the broad scope of the disorder.
Within the last ten years a fairly broad consensus characterization of the neuropsychological phenotype of children with DS22q11.2 has emerged (Bearden et al. 2001; Moss et al. 1999; Swillen et al. 1997; Swillen et al. 1999; Wang et al. 2000; Woodin et al. 2001). It will not be reviewed in detail here because that is the scope of another article in this edition (ref Antshel et al). Instead, this article will focus on presenting a possible explanation for the consistent finding that many children with DS22q11.2 perform at a significantly lower level than their age-matched peers in the nonverbal domain. Often, nonverbal function is poorer than the child's own functioning in the verbal domain, though PIQ/VIQ differences are not consistently found. It seems clear, however, that impairments are extremely common in the following domains: attention, particularly visuospatial attention; spatial cognition, including spatial memory; quantitative cognition, involving at least enumeration and numerical and temporal magnitude estimation and, later, arithmetical and procedures and concepts.
Here I present the hypothesis that dysfunctions in neural circuits that are well established as the basis for typical processing of spatial and temporal information (e.g. Coull et al. 2004; Meck 2005; Rao et al. 2001) impair the development of spatiotemporal cognitive competence in children with DS22q11.2. I contend also that this dysfunction creates a suboptimal foundation for the subsequent development of numerical and mathematical competence, thereby “cascading” impairments into those more academic domains. This view is essentially a refinement of my earlier claim that visuospatial dysfunction is the basis for most of the nonverbal cognitive impairments manifested by children with DS22q11.2 (Simon et al. 2005a; Simon et al. 2005b). Recent findings lead me to suggest that many of the structures involved in these circuits, especially the subcortical ones, suffer atypical early development, possibly driven to some extent by the genetics of the disorder. As a result, the representations and processes necessary for typical function in the spatiotemporal domain likely cannot develop optimally during childhood, when formation and sculpting of cortical circuits that will come to support these functions takes place (Giedd et al. 1999; Sowell et al. 2002). A growing literature identifies circuitry for processing timing information in the milliseconds to seconds and minutes range in typical animals and humans that consists mainly of the cerebellum, basal ganglia, thalamus, subthalamic nucleus and substantia nigra in conjunction with the hippocampus, frontal cortex and, in many human tasks, the posterior parietal cortex and insular/operculum regions (e.g. Mangels et al. 1998; Meck 2005; Rao et al. 2001). The large literature on spatial attention points to the involvement of a dorsal, or upper brain network in typical individuals that connects parietal and frontal cortical regions, among others (e.g. Corbetta and Shulman 2002; Culham et al. 2001). However, it is also clear that subcortical structures, including the cerebellum, the basal ganglia, and especially the pulvinar in the posterior thalamus, are critical parts of this network (Allen et al. 1997; Karnath et al. 2002; Petersen et al. 1987; Shipp 2004). Studies of spatial memory clearly implicate the hippocampal formation as a critical structure (Burgess et al. 2002).
One of the main effects of these dysfunctions, I propose, is a reduced resolution, or clarity, of mental representations processed by spatial attentional and broader spatiotemporal cognitive functions that I will refer to as “spatiotemporal hypergranularity”. Spatiotemporal hypergranularity refers to the increased grain size and reduced number of elements in mental representations of spatial and temporal information. By analogy to an increase in the size of individual pixels and a reduction in their overall number that make up a digital image, mental representations processed by spatiotemporal cognitive functions in children with DS22q11.2 have a coarser, “grainier” resolution than in a typical system. This makes identification of specific spatial locations or time points less accurate and requires larger differences between values before they are perceived as distinct.
What determines the grain size of this resolution? It is well known that an object presented in the visual periphery and surrounded by other similar objects is difficult to recognize or scrutinize (e.g. Bouma 1970; Intriligator and Cavanagh 2001). Figure 1 demonstrates the viewer's inability to count the peripherally viewed bars when fixating on the plus sign. The source of this limitation likely arises at multiple stages, from early visual processing (e.g. Levi et al. 1985; Pelli et al. 2004) to capacity-limited higher level processes of spatial attention (Cavanagh 2004; He et al. 1997; He et al. 1996; Intriligator and Cavanagh 2001). While we can move our attentional focus, or “spotlight”, its size is sufficiently large that we can perceive many lines in Figure 1 without being able to count sequentially through them or scrutinize the individuals. I hypothesize that the relatively low resolution of spatial attention, not vision, is exaggerated, or hypergranular, in children with DS22q11.2. Temporal perception also depends on low-level temporal visual acuity (e.g. Kelly 1979) as well as temporal attention, which also has limited resolution (Battelli et al. 2007). For example, humans can typically detect individual events at almost 60Hz, just slower than flicker of fluorescent lighting, but cannot do so at temporal frequencies above about 7Hz (e.g. Battelli et al. 2003). This limited temporal resolution of attention is not a failure of low-level vision, but a constraint on our ability to modulate attention over time (Holcombe and Cavanagh 2001; Verstraten et al. 2000). Thus, I also hypothesize that DS22q11.2 creates a differential, hypergranular temporal resolution of attention as well.
Figure 1.

When fixating the plus sign, the vertical bars can be seen. They are thin, parallel and all of equal length. But it is very hard to count them, indicating that at least the middle bars cannot be easily individuated (Adapted from Cavanagh, 2004).
My position is similar to Cavanagh's (2004) account, stating that the resolution of attention is determined by “attention routines” that function as an intermediate processing stage between automatic low-level visual, and conscious, multi-step cognitive processes. They are specialized for processing information about salience or importance, spatial and temporal relations and for tracking objects. The routines have limits on Capacity, or the amount of information that can be passed on to higher processing and Acuity, or resolution, which is a “restriction on the density of items that still permits access to individual items” (p.13). So, if the bars in Figure 1 were spread out, the individual items could be attended. Cavanagh assumes that multiple ‘spotlights” exist, enabling multiple objects to be tracked at once (Pylyshyn and Storm 1988) and that the resolution of attention resembles visual acuity in that it degrades with distance from “focal point” (Intriligator and Cavanagh 2001). Since the resolution of attention is “unexpectedly coarse in both space and time” (Cavanagh 2004), spatial and temporal information together influence how much can be attended and processed by higher-level cognitive routines. In the case of children with DS22q11.2, I hypothesize that early development of the basic spatiotemporal processing circuitry in the brains of such children is suboptimal and that this reduces the resolution of spatial attentional selection. This then impairs the typical development of spatial, temporal and numerical cognitive processes that are constructed upon the foundation of attentional function.
In what follows I will present data from a selection of studies that indicate two stable characteristics of cognition in children with DS22q11.2. The first is that basic functions that typically depend on basic spatial and temporal attention processes are impaired in children with the deletion. The second is that, in children with the deletion, processes in these domains appear to operate as if the mental representations they are processing are of coarser granularity than is true for typically developing age-matched controls.
First, I will review impairments in spatial attention. By adapting a classic spatial attention task we assessed children's ability to select target objects in the visual environment in the presence of helpful or confusing cues about their location (Simon et al. 2005a). Children with DS22q11.2 and TD controls saw a central stimulus (a solid black diamond) on either side of which was a square box. Targets were black and white diamond checkerboards appearing inside the boxes and their appearance was cued by a white triangle appearing within the black diamond, pointing either left or right. A valid cue pointed in the same direction as the subsequent appearance of the target while an invalid cue pointed in the opposite direction. Neutral cues were white diamonds pointing both ways and providing no location information about the upcoming target. The child's task was to press the left button on a button box when a target was detected on the left and the right button for a target on the right. Most of the cues correctly predicted the target's location but some were invalid and required disengagement from the (incorrectly) cued location and reorienting to the correct location of the target. We found that children with DS22q11.2 were not only slower overall than TD controls (F(1, 34)=5.367; p=.027) they were also more impaired at responding to invalid vs. valid cues, F(1,34)=5.273, p=.028, than were TD controls.
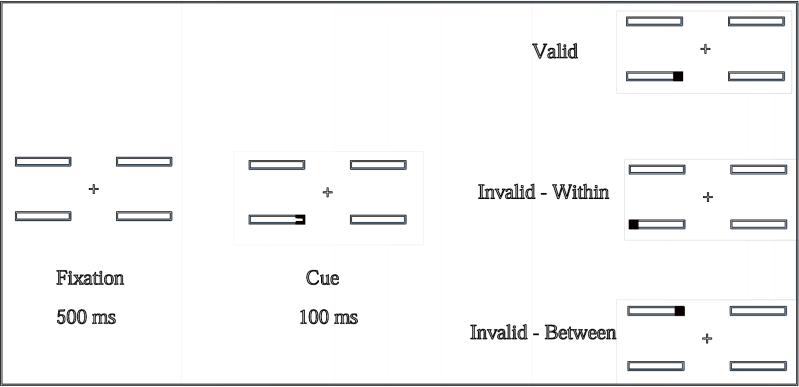
In a related experiment (Bish et al. 2007) we presented children with DS22q11.2 and TD controls with a display containing four horizontally oriented oblong boxes arranged one above the other on either side of a centrally presented fixation cross. Spatial cues were created by darkening the lines on one end of one of the boxes, followed by a small black square target filling one end of a box, either at the same location as the (valid) cue or in different locations, either in the same box or a different box to the (invalid) cue (See Figure 2). The key detail is that ALL targets appeared at the same distance from the cue so differences in attention shifting could only be influenced by the presence of a containing object. The results clearly differentiated the groups. While both invalid cue types increased the cost of shifting attention, the “invalid-between” cost was significantly greater for children with DS22q11.2. In contrast, although the difference was not statistically significant, children with DS22q11.2 actually incurred a lower “invalid-within” cost, in that they performed slightly better than TD controls, when they were required to move their attention from one (invalidly cued) end of a box to the target in the other end of the same box. In other words, when attention had to be shifted within the bounds of a clearly defined object, children with DS22q11.2 showed a distinct advantage compared to situations where they had to move their attention the same distance but over unbounded space (i.e. in the space between two objects). This suggests that the object boundaries acted as guides within coarser representations of spaces and so reduced error in the spatial attentional search.
Figure 2.
Design of experiment to test attention to spatial locations versus objects. Children saw the display on the left, followed by the display in the center and then one of the three displays on the right. Their task was to press a button when the black square appeared (From Bish et al., 2007).
We also examined the interaction of spatial attention and numerical processing by using an enumeration task (Simon et al. 2005a) where children were asked to report the number of dots in a random pattern as quickly as possible. Regardless of the number of target items presented for enumeration, each must be detected and represented uniquely, designated as a target for enumeration, and subjected to further processing. Children and adults can individuate small sets (i.e. fewer than 4) almost effortlessly, without using spatial attention (e.g. Chi and Klahr 1975; Trick and Pylyshyn 1993, 1994) in a process known as subitizing. However, larger sets are individuated mostly one item at a time with the aid of spatial attention (Piazza et al. 2003; Sathian et al. 1999). In our task, children were required to respond by speaking into a microphone, as quickly as possible, the number of objects (1−8) that were presented on a computer display. Targets were small bright green squares presented on a red background square within 2 degrees of visual angle so that only attention and not eye movements could be used to navigate the display. Both the TD and DS22q11.2 groups subitized 2 items. Regression equations revealed significant slopes for subitizing 74.89ms/item, t(18)=4.18, p<.01 and counting 530.05ms/item, t(18)=12.23, p<.001 in the TD group and 74.59ms/item, t(33) =4.51, p<.001 (subitizing) and 739.62ms/item, t(33)=13.41, p<.001 (counting) for the DS22q11.2 group. As predicted, we found near identical subitizing performance across groups, consistent with no SRT differences when spatial attention is not required. In contrast, counting produced significantly steeper slopes in children with DS22q11.2. We assume that this was largely due to spatial attention impairments. The slope increases are consistent with our hypergranularity hypothesis and underscored by a significant group difference in error patterns. Almost all error responses were one more or one less than the target value. For TD controls the distribution of +1/−1 errors was 49.5%/50.5%. The DS22q11.2 group's +1/−1 error pattern was 26.75%/73.25%, showing that they consistently undercounted. We hypothesize that hypergranular representations caused dots in the stimulus array to merge with one another so fewer targets were perceived. Alternatively, poor control of spatial attentional search could cause some objects to be missed and so not counted. Our evidence appears to support the former account but even the latter does not rule out revisiting dots and recounting them, thereby overestimating the total number.
Next, I will review the effect of hypergranular representations on spatiotemporal processing. In the “Landmark” task, adapted from a neurological bedside test of spatial neglect (Bish and Simon in preparation), Children saw a 40 mm long horizontal line with a picture of the Muppet character Miss Piggy at one end and Fozzie Bear at the other. Above a short vertical line that bisected the horizontal was a picture of Kermit the Frog. The location of Kermit's line was presented in 2 mm increments from the center to the ends of the line. Children indicated, with a button press, which Muppet character Kermit was closest to. We found that children with DS22q11.2 made significantly more errors when Kermit was 6 to 10mm to the right of center and 4 to 10mm to the left of center than did TD controls. The central area in which these errors occurred, the “zone of indifference”, was more than a third larger (14.93mm versus 11.07mm) for the DS22q11.2 than the TD group, F(1,27)=14.177 p=.001. This suggests that, in unconstrained space without object boundaries or guides, a difference of at least 33% greater than that required by TD controls is needed before children with DS22q11.2 can accurately judge relative location. This is perhaps the only study to date that puts an actual quantitative value on the degree of impairment in spatial processing experienced by children with DS22q11.2.
However, precisely the same assessment has been made for temporal processing (Debbané et al. 2005). In this study, forty-two individuals with DS22q11.2 aged 6−32 years were compared to 35 TD controls on a task where two sounds were presented consecutively and the participant was required to determine which duration was longer. Again, those with DS22q11.2 required a much greater difference between the two durations than did controls in order to tell them apart. In this case the difference required was 20% to 50% longer depending on whether the durations were presented visually or auditorily. The authors link the greater auditory threshold to cerebellar dysfunction. In another task, each participant was asked to tap first one and then both index fingers in time with a string of tones. When the tones were stopped the participants’ task was to continue tapping at the same rate. Spacing between tones was varied on different trials. The results showed that individuals with DS22q11.2 consistently tapped more quickly, i.e. they underestimated the duration between tones to be much shorter than they really were. This was not done by the controls. The fact that Debbané et al. found significant correlations between performance on both of the tasks led them to conclude that “an underlying temporal perception mechanism [was] common to both tasks (p. 1758)”.
Related brain-based evidence of spatiotemporal processing impairments does, however, come from an electrophysiological study of adolescents and young adults (Baker et al. 2005). A task measured “mismatch negativity” (MMN), a neural signal seen as a negative deflection in the ongoing EEG wave 100 milliseconds or more after the presentation of an “oddball”, or unusual stimulus, within a stream of similar stimuli. The study reported a significantly impaired MMN in the DS22q11.2 group for duration, but not pitch, of a stimulus tone. This indicates that overall detection was not impaired but a specific inability to detect a change in duration from 50 to 100 milliseconds existed in children with the deletion.
Further evidence for spatiotemporal hypergranularity also comes from our use of magnitude comparison tasks. These test the hypothesis that numerical quantities are represented as if on a mental “number line” where the difficulty of a comparison increases as the numerical “distance” between the two values decreases. Having demonstrated (Simon et al. 2005a) that children with DS22q11.2 could not perform as well as TD controls children, we used a new task requiring children to simply choose the larger of a pair of objects, either bars varying in length differences of 1 to 7cm or Arabic numbers varying in numerical differences of 1 to 7. . Our results (Simon et al. 2007) show both a significant increase in response time for all distances and significantly greater response times with increasing number in the DS22q11.2 group. Slopes for distances 1−5 indicated were significantly larger for the DS22q11.2 than the TD group (p=.001); DS22q11.2 97.86ms/distance vs. TD 45.43ms/distance for blocks and 100.29ms/distance vs. 30.88ms/distance for numbers. That children with DS22q11.2 required larger differences than did TD controls before they could respond similarly, suggests that their number line representations are hypergranular.
What might be the neural basis for these kinds of findings? The literature on brain imaging results in DS22q11.2, has been reviewed recently (e.g. Eliez and Van Amelsvoort 2005) and, in general, there are consistent reports of reduced volume of around 10% for the entire brain of children and adolescents with DS22q11.2, with similar reductions in gray and white matter. Most of the reductions have been reported in the posterior brain (parietal, occipital and temporal lobes) (Campbell et al. 2006; Eliez et al. 2000; Kates et al. 2001; Simon et al. 2005c) with the frontal lobes showing little to no reduction in volumes, except in terms of the deep white matter (Kates et al. 2004). More specific measures, acquired from manual tracings of specific regions (ROIs) on MRI images have been reported also and some of these involve the subcortical structures implicated in the spatiotemporal circuits outlined earlier (e.g. Bish et al. 2004; Eliez et al. 2002). Some of the published results differ from one another because of the ages of the variations in methodology, sample size, age of people scanned and characteristics of the comparison group recruited. However, some general consensus appears to be evident.
More specifically, changes to neural structures involved in spatiotemporal processing have also been reported in children with DS22q11.2. For the basal ganglia, Sugama's (2000) study of 16 individuals with DS22q11.2 from 8 months to 21 years old and 15 age-matched controls reported that the absolute volume of the head of the caudate was larger in the left hemisphere in TD controls. Conversely, the whole brain adjusted right head of the caudate was larger in the DS22q11.2 group. Eliez (2002) studied 30 children and adolescents with DS22q11.2 and 30 age-matched TD controls. Total grey matter adjusted volumes were larger in those with DS22q11.2 for left and right whole caudate. The same was true for the head of the caudate head but not for the body. A study by Kates (2004) of 10 children and adolescents with DS22q11.2 and 10 age-matched TD controls reported larger right caudate volumes adjusted for whole brain volume in the DS22q11.2 group. Finally, Campbell's study of 39 children with DS22q11.2 (mean age 11 years) and 26 TD sibling controls also reported that the right, but not left, caudate was larger in the DS22q11.2 group, with a trend towards a large total volume of the caudate in that group also.
There have been at least two reports of changes to the nearby insular cortex. Simon (Simon et al. 2005c) reported increased gray matter volumes in the right insula of 18 children aged 7−14 compared to similarly aged TD controls, while Campbell (Campbell et al. 2006) reported a bilateral increase in affected children. The posterior thalamus, especially the pulvinar nucleus, is critically involved in spatial attention and cognition (Petersen et al. 1987; Ward et al. 2002). There is apparently only one study (Bish et al. 2004) of this structure in children with DS22q11.2 and it shows that, while the entire thalamic area measured was reduced by the same percentage as the overall brain in children with DS22q11.2 (i.e. 9.2% & 9.9% respectively), the posterior thalamic region that contains the pulvinar was reduced by 22.7% compared to controls. The effect was significant even after taking total brain volume, age and gender into account.
The hippocampus, which is another critical structure in this circuitry, is located in the inferior lateral temporal lobes. Two studies have reported reductions in this structure in those affected with DS22q11.2. Campbell (2006) did not find hippocampal differences in hand traced volumes when they were calculated as a percentage of intracranial volume, as is appropriate when one group of brains is characterized by smaller volumes than the other. However, whole brain analyses did show bilateral clusters in which the hippocampal region was significantly decreased in volume in children with DS22q11.2. Debbané's (2006) study included 43 people with the deletion ranging in age from 6 to 37 years, though only 37 apparently had a confirmed chromosome 22q11.2 deletions. Controls were 40 similarly aged typicals. Hippocampal volumes adjusted for total gray matter revealed significant bilateral reductions in hippocampal body volumes in those with DS22q11.2 and a trend towards reductions in the tail. Covarying for age did not change the results but covarying for IQ did remove the trend for differences in the tail. So, with so much atypical development in structures that have been identified as key to the processing of spatial and temporal information, it appears likely that these changes might be the neural basis for the development of hypergranular representations and impaired function in these processing domains.
While no direct relationship has been established, at least in the case of children with DS22q11.2, it is possible that the size of the lateral ventricles could affect the development of many of the structures reviewed above because of their proximity to the ventricular zone. There have now been several reports of dilation of the lateral ventricles in affected children. While cases of spina bifida and spina bifida oculta are observed (Shprintzen 2005), there is no evidence linking such dilation to normal pressure hydrocephalus. Simon's (Simon et al. 2005c) whole brain study showed this quite clearly when CSF volumes where measured. Two studies have taken the approach of measuring the lateral ventricles directly. Campbell's (2006) study used a manual tracing technique and reported significantly larger ventricular volumes in children with the deletion than in the controls. Machado (2007) reported that the ventricles from the same children Simon's (2005c) whole brain study were segmented using a semi-automated method and, again, significantly larger volumes were found in children with the deletion.
It is possible that a similar relationship exists between cerebellar volumes and fourth ventricle dilation and, if so, may even be due to same mechanism that causes dilation of the lateral ventricles. However, this is currently little more than speculation. At least three studies have reported reductions in the cerebellum in children with DS22q11.2. Eliez (2001) measured the area of cerebellar vermis regions in 24 children and adults with DS22q11.2 and the same number of age-matched TD controls. Adjusting for total brain volume, the area of vermal lobules VI –VII (including the neocerebellum). Bish (2006) used a similar method to that of Eliez to measure vermal area in 54 children aged 7−14 years, 31 of whom had DS22q11.2 with the remainder being TD controls. After covarying for age, gender and total brain volume, area of the entire cerebellum, the anterior lobe, the neocerebellum and the tonsils was found to be significantly smaller in the DS22q11.2 than the control group. Campbell's (2006) study reported reduced gray matter volume in the left cerebellar area in children with DS22q11.2 from the whole brain analyses and reduced volume of the total cerebellum of this group when hand drawn measurements were adjusted by total intracranial volume.
Changes to patterns of connectivity within the brains of children with DS22q11.2 have also been reported. These have been detected using Diffusion Tensor MRI methods that indirectly generate measures of white matter organization inferred from the motion of water molecules. The primary measure is called fractional anisotropy (FA), which indicates how much of the overall motion is highly oriented because it is constrained by myelin wrapped around fiber tracts, versus multidirectional because it is unconstrained, such as in the CSF in ventricular spaces. The first report (Barnea-Goraly et al. 2003) focused on reporting reduced FA, and thus connectivity, between the frontal and temporal lobes in children and young adults with DS22q11.2. They also mentioned increased FA in the posterior corpus callosum nearby optic tract. This latter finding was replicated and extended in our studies (Simon et al. 2005c), where showed that the entire organization and location of the corpus callosum is altered in children with DS22q11.2. We suggested that this might a key factor in cognitive dysfunction because the corpus callosum is the main connective pathway between the two cortical hemispheres in the brain.
Some evidence in favor of that position now exists. We recently reported (Machado et al. 2007) that at least one change in the corpus callosum (its area) relates quite differently to performance on the attentionally demanding counting part of the enumeration task described earlier in children with DS22q11.2 than in TD controls. This suggests that affected children may be using a different, and atypical, cortical network to complete this task and this may partially explain their difficulties. Barnea-Goraly (2005) showed that performance on a standardized arithmetic measure correlates with FA in children and young adults in the left parietal inferior parietal region often associated with numerical processing. To date, no studies of subcortical connectivity appear to have been published.
Strong new evidence (Simon et al. submitted) for altered connectivity in children with DS22q11.2 and its relationship to cognitive impairments comes from further analyses of the diffusion imaging data we reported previously (Simon et al. 2005c). Here we focused on measures that can be extracted from the diffusion data that describe the main directions of diffusion (and thus can be interpreted as indicators of white matter tract characteristics). We detected a result that indicated opposite patterns of connectivity in common clusters within the parietal and frontal lobes of children with DS22q11.2 and TD controls in the superior longitudinal and fronto-occipital fasciculi respectively (see Figure. 3). Though the data resolution does not support direct visualization, the results can be interpreted as indicating that TD controls had more connections branching out of these clusters to adjoining parietal and frontal cortex than did those with DS22q11.2. This is because radial diffusion is, by definition, that which is perpendicular to the primary axis of diffusion, in this case within the longitudinal fasciculi. By contrast, most of the connectivity these clusters in children with DS22q11.2 appeared to be along the major white matter tracts with little branching out to adjoining cortex.
Figure 3.
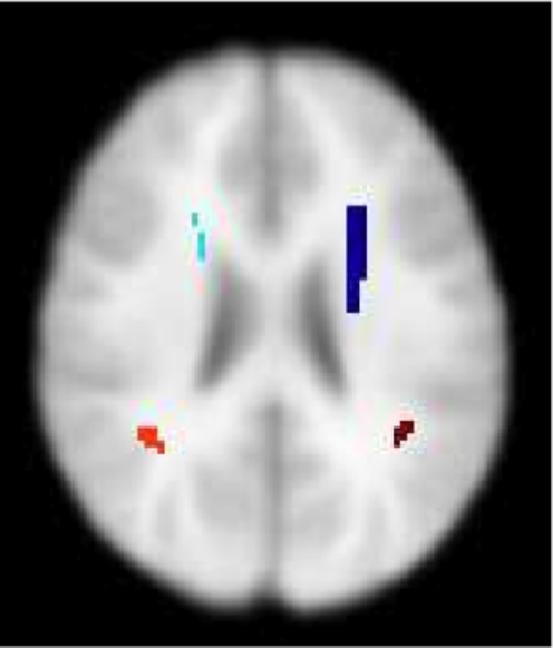
Axial slice from average brain template showing the locations of clusters with opposite patterns of connectivity in children with DS22q11.2 and typically developing controls.
It is possible, therefore, that differences in spatial and temporal attentional functions are related to patterns of connectivity in crucial cortical regions. This interpretation is strengthened by the correlations we found between the relevant diffusion values in those clusters and scores representing the cost of processing an invalid spatial cue in the Cueing task described above. This task is presumed to depend most heavily upon the parietal parts of a network connecting regions of parietal and frontal cortices, among other regions. In the DS22q112 group we found a positive correlation in the right parietal cluster (r=.78, p<.05) indicating that the cost increased (i.e. performance got worse) as FA values increased. In the TD group, a negative correlation in the right frontal cluster (r=−.58, p<.01) indicating that the cost decreased (i.e. performance got better) as FA values increased. This suggests that excessive FA in these clusters indicates atypical connectivity that was related to worse performance. The lower FA values, complemented by a much higher value in another measure that we took to indicate cortical branching, in the TD group suggests a more appropriate connective pattern that is related to better performance.
DISCUSSION
In this paper I have presented an updated account of the possible neurocognitive basis of nonverbal intellectual impairments observed in most children with DS22q11.2. It is based on two main hypotheses. One is that neural circuits for the basic processing of spatial and temporal information develop atypically and that this impaired circuitry forms a suboptimal foundation for the development of typical spatiotemporal and numerical cognitive competence. It is likely that the subcortical components of the spatiotemporal processing networks are the earliest source of dysfunction that affects the development of higher cortical circuitry supporting acquired cognitive functioning. Changes in the shape, size and patterns of connections between these structures appear to be one index of the impairments within the system. The other hypothesis is that the changes described above lead to the development of mental representations of space and time that have a reduced, or grainier, resolution. This means that they can less accurately represent the spatial and temporal information that perceptual systems take in. This leads to a characteristic set of cognitive impairments when children with DS22q11.2 need to process information concerning space, time and, later, numbers.
The last five years of research into DS22q11.2 has made the generation of such hypothesis driven accounts possible as it has moved beyond characterization of the cognitive phenotype using descriptive standardized measures to the examination of specific representation and processing hypotheses using experimental cognitive processing tests. The results of these experiments have been integrated with data from structural, connective and, to a lesser extent, functional neuroimaging studies to form the foundation of mechanistic, and therefore more explanatory, accounts. It is now critical that the hypothesis upon which such accounts are built, and the predictions they make undergo rigorous testing with new, and better, explanations emerging as a result. Another critical step will be to undertake developmental studies in order to further explain the neurocognitive progression from early childhood to the middle and later childhood profiles that have been characterized so far. If this is done, we should be able to explain how characteristic cognitive impairments, as well as the variation in those difficulties between individuals with DS22q11.2 come about. At that point, we will be well positioned to develop specific cognitive and possibly pharmacological, interventions designed specifically to impact early atypical neurocognitive systems and reduce the cognitive and academic challenges currently faced by most young people with a chromosome 22q11.2 deletion.
Acknowledgements
I would like to thank David Whitney for his insightful and expert contributions to sections of this paper on spatial and temporal acuity of vision and resolution of attention. Thanks also to two very thorough reviewers whose comments significantly increased the quality of this manuscript.
Funded by NIH RO1HD42974
Footnotes
Call Outs:
○ “I present the hypothesis that dysfunctions in neural circuits that are well established as the basis for typical processing of spatial and temporal information impair the development of spatiotemporal cognitive competence in children with DS22q11.2”.
○ “One of the main effects of these dysfunctions, I propose, is a reduced resolution, or clarity, of mental representations processed by attentional and broader spatiotemporal cognitive functions that I will refer to as “spatiotemporal hypergranularity”.
○ “neural circuits for the basic processing of spatial and temporal information develop atypically and that this impaired circuitry forms a suboptimal foundation for the development of typical spatiotemporal and numerical cognitive competence”.
References
- Allen G, Buxton RB, Wong EC, et al. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Baker K, Baldeweg T, Sivagnanasundaram S, et al. COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry. 2005;58(1):23–31. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Menon V, et al. Arithmetic ability and parietal alterations: A diffusion tensor imaging study in Velocardiofacial syndrome. Brain Res Cogn Brain Res. 2005 doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Krasnow B, et al. Investigation of white matter structure in velocardiofacial syndrome: A diffusion tensor imaging study. American Journal of Psychiatry. 2003;160(10):1863–1869. doi: 10.1176/appi.ajp.160.10.1863. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Martini P, et al. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;126(Pt 10):2164–2174. doi: 10.1093/brain/awg221. [DOI] [PubMed] [Google Scholar]
- Battelli L, Pascual-Leone A, Cavanagh P. The ‘when’ pathway of the right parietal lobe. Trends Cogn Sci. 2007;11(5):204–210. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, et al. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. Journal of Clinical and Experimental Neuropsychology. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bish JP, Chiodo R, Mattei V, et al. Domain specific attentional impairments in children with chromosome 22q11.2 deletion syndrome. Brain & Cognition. 2007;64:265–273. doi: 10.1016/j.bandc.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish JP, Nguyen V, Ding L, et al. Thalamic reductions in children with chromosome 22q11.2 deletion syndrome. Neuroreport. 2004;15:1413–1415. doi: 10.1097/01.wnr.0000129855.50780.85. [DOI] [PubMed] [Google Scholar]
- Bish JP, Pendyal A, Ding L, et al. Specific cerebellar reductions in children with chromosome 22q11.2 deletion syndrome. Neurosci Lett. 2006;399(3):245–248. doi: 10.1016/j.neulet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bish JP, Simon TJ. Spatial processing impairments in children with chromosome 22q11.2 deletion syndrome. in preparation. [DOI] [PMC free article] [PubMed]
- Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226(5241):177–178. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The Human Hippocampus and Spatial and Episodic Memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burn J, Takao A, Wilson D, et al. Conotruncal anomaly face syndrome is associated with a deletion within chromosome 22. Journal of Medical Genetics. 1993;30:822–824. doi: 10.1136/jmg.30.10.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006 doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Attention routines and the architecture of selection. In: Posner MI, editor. Cognitive Neurosciecne of Attention. Guilford Press; NY: 2004. pp. 13–28. [Google Scholar]
- Chi MTH, Klahr D. Span and rate of apprehension in children and adults. Journal of Experimental Child Psychology. 1975;19:434–439. doi: 10.1016/0022-0965(75)90072-7. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, et al. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303(5663):1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanaugh P, Kanwisher NG. Attention response functions: Characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Debbané M, Glaser B, Gex-Fabry M, et al. Temporal perception in velo-cardio-facial syndrome. Neuropsychologia. 2005;43:1754–1762. doi: 10.1016/j.neuropsychologia.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Debbané M, Schaer M, Farhoumand R, et al. Hippocampal volume reduction in 22q11.2 deletion syndrome. Neuropsychologia. 2006;44(12):2360–2365. doi: 10.1016/j.neuropsychologia.2006.05.006. [DOI] [PubMed] [Google Scholar]
- DiGeorge A. A new concept of the cellular basis of immunity. Journal of Pediatrics. 1965;67:907. [Google Scholar]
- Eliez S, Barnea-Goraly N, Schmitt EJ, et al. Increased basal ganglia volumes in velocardio-facial syndrome (Deletion 22q11.2). Biol Psychiatry. 2002;52:68–70. doi: 10.1016/s0006-3223(02)01361-6. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, et al. Children and adolescents with Velocardiofacial Syndrome: A Volumetric Study. American Journal of Psychiatry. 2000;157(3):409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, et al. A quantitative MRI study of posterior fossa development in velocardiofacial syndrome. Biological Psychiatry. 2001;49:540–546. doi: 10.1016/s0006-3223(00)01005-2. [DOI] [PubMed] [Google Scholar]
- Eliez S, Van Amelsvoort T. Neuroimaging in the velo-cardio-facial syndrome. In: Murphy KC, Scambler PJ, editors. Velo-Cardio-Facial Syndrome: A Model for Understanding Microdeletion Disorders. Cambridge University Press; Cambridge, UK: 2005. pp. 165–180. [Google Scholar]
- Giannotti A, Digilio MC, Marino B, et al. Cayler cardiofacial syndrome and del 22q11: part of the CATCH22 phenotype. American Journal of Medical Genetics. 1994;53(3):303–304. doi: 10.1002/ajmg.1320530320. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intiligator J. Attentional resolution. Trends in Cognitive Sciences. 1997;1(3):115–121. doi: 10.1016/S1364-6613(97)89058-4. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383(6598):334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Holcombe AO, Cavanagh P. Early binding of feature pairs for visual perception. Nat Neurosci. 2001;4(2):127–128. doi: 10.1038/83945. [DOI] [PubMed] [Google Scholar]
- Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cognit Psychol. 2001;43(3):171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125(2):350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Bessette BA, et al. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2). J Child Neurol. 2004;19(5):337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, et al. Regional cortical white matter reductions in velocardiofacial syndrome: A volumetric MRI analysis. Biological Psychiatry. 2001;49(677−684) doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Motion and vision. II. Stabilized spatio-temporal threshold surface. J Opt Soc Am. 1979;69(10):1340–1349. doi: 10.1364/josa.69.001340. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Res. 1985;25(7):963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Machado AM, Simon TJ, Nguyen V, et al. Corpus callosum morphology and ventricular size in chromosome 22q11.2 deletion syndrome. Brain Res. 2007;1131(1):197–210. doi: 10.1016/j.brainres.2006.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7(1):15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Driscoll DA, Bason L, et al. Autosomal dominant “Opitz” GBBB syndrome due to a 22q11.2 deletion. American Journal of Medical Genetics. 1995;59(1):103–113. doi: 10.1002/ajmg.1320590122. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropsychology of timing and time perception. Brain Cogn. 2005;58(1):1–8. doi: 10.1016/j.bandc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, et al. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. The Journal of Pediatrics. 1999;134(2):193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J Vis. 2004;4(12):1136–1169. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25(1A):97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Piazza M, Giacomini E, Le Bihan D, et al. Single-trial classification of parallel pre-attentive and serial attentive processes using functional magnetic resonance imaging. Proc Biol Sci. 2003;270(1521):1237–1245. doi: 10.1098/rspb.2003.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: Evidence for a parallel tracking mechanism. Spatial Vision. 1988;3(3):179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nature Neuroscience. 2001;4(3):317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Sathian K, Simon TJ, Peterson S, et al. Neural evidence linking visual object enumeration and attention. Journal of Cognitive Neuroscience. 1999;11(1):36–51. doi: 10.1162/089892999563238. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8(5):223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-Cardio-Facial Syndrome. In: Cassidy SB, Allanson JE, editors. Management of Genetic Syndromes. Second ed Wiley-Liss; 2005. pp. 615–631. [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, et al. A new syndrome involving cleft palate, cardiac anomalies, typical faces, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate Journal. 1978;15:56–62. [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, McDonald-McGinn DM, et al. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005a;41(2):145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bish JP, Bearden CE, et al. A Multiple Levels Analysis of Cognitive Dysfunction and Psychopathology Associated With Chromosome 22q11.2 Deletion Syndrome in Children. Development and Psychopathology. 2005b;17:753–784. doi: 10.1017/S0954579405050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, et al. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 2005c;25(1):169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Takarae Y, DeBoer TL, et al. Overlapping Numerical Cognition Impairments In Chromosome 22q11.2 Deletion And Turner Syndromes. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.08.016. doi:10.1016/j.neuropsychologia.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Wu Z, Avants B, et al. Atypical Cortical Connectivity and Visuospatial Cognitive Impairments are Related in Children with Chromosome 22q11.2 Deletion Syndrome. Journal of Neuroscience. doi: 10.1186/1744-9081-4-25. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, et al. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44(01):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sugama S. Morphometry of the head of the caudate nucleus in patients with velocardiofacial syndrome (del 22q11. 2). Acta Paediatrica. 2000;89(5):546–549. doi: 10.1080/080352500750027826. [DOI] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, et al. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. The Journal of Medical Genetics. 1997;34(6):453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Vandeputte L, Cracco J, et al. Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychology. 1999;5(4):230–241. doi: 10.1076/0929-7049(199912)05:04;1-R;FT230. [DOI] [PubMed] [Google Scholar]
- Trick LM, Pylyshyn ZW. What enumeration studies can tell us about spatial attention. Evidence for limited capacity preattentive processing. Journal of Experimental Psychology: Human Perception and Performance. 1993;19:331–351. doi: 10.1037//0096-1523.19.2.331. [DOI] [PubMed] [Google Scholar]
- Trick LM, Pylyshyn ZW. Why are small and large numbers enumerated differently? A limited capacity preattentive stage in vision. Psychological Review. 1994;101:80–102. doi: 10.1037/0033-295x.101.1.80. [DOI] [PubMed] [Google Scholar]
- Verstraten FA, Cavanagh P, Labianca AT. Limits of attentive tracking reveal temporal properties of attention. Vision Res. 2000;40(26):3651–3664. doi: 10.1016/s0042-6989(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Wang PP, Woodin MF, Kreps-Falk R, et al. Research on behavioral phenotypes: velocardiofacial syndrome (deletion 22q11.2). Developmental Medicine & Child Neurology. 2000;42:422–427. doi: 10.1017/s0012162200000785. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, Owen V, et al. Deficits in spatial coding and feature binding following damage to the spatiotopic maps in the human pulvinar. Nature Neuroscience. 2002;5(2):99–100. doi: 10.1038/nn794. [DOI] [PubMed] [Google Scholar]
- Woodin MF, Wang PP, Aleman D, et al. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genetics in Medicine. 2001;3(1):34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]