Abstract
This study investigated associations between CpG island methylator phenotype (CIMP) colon cancer and genetic polymorphisms relevant to one-carbon metabolism and thus, potentially the provision of methyl groups and risk of colon cancer. Data from a large, population-based case–control study (916 incident colon cancer cases and 1972 matched controls) were used. Candidate polymorphisms in methylenetetrahydrofolate reductase (MTHFR), thymidylate synthase (TS), transcobalamin II (TCNII), methionine synthase (MTR), reduced folate carrier (RFC), methylene-tetrahydrofolate dehydrogenase 1 (MTHFD1), dihydrofolate reductase (DHFR) and alcohol dehydrogenase 3 (ADH3) were evaluated. CIMP− or CIMP+ phenotype was based on five CpG island markers: MINT1, MINT2, MINT31, p16 and MLH1. The influence of specific dietary factors (folate, methionine, vitamin B12 and alcohol) on these associations was also analyzed. We hypothesized that polymorphisms involved in the provision of methyl groups would be associated with CIMP+ tumors (two or more of five markers methylated), potentially modified by diet. Few associations specific to CIMP+ tumors were observed overall, which does not support the hypothesis that the provision of methyl groups is important in defining a methylator phenotype. However, our data suggest that genetic polymorphisms in MTHFR 1298A > C, interacting with diet, may be involved in the development of highly CpG-methylated colon cancers. AC and CC genotypes in conjunction with a high-risk dietary pattern (low folate and methionine intake and high alcohol use) were associated with CIMP+ (OR = 2.1, 95% CI = 1.3–3.4 versus AA/high risk; P-interaction = 0.03). These results provide only limited support for a role of polymorphisms in one-carbon metabolism in the etiology of CIMP colon cancer.
Introduction
Colorectal cancer appears to arise via at least four distinct molecular pathways (1,2). A specific molecular pathway for colon carcinogenesis has recently emerged, which is characterized by a large number of hypermethylated CpG islands with subsequent transcriptional silencing (3–5). It is currently believed that perhaps up to 30% of colon cancers are characterized by this CpG island methylator phenotype (CIMP), in which numerous CpG islands are methylated and tumor suppressor genes such as the cell-cycle regulator, p16, are inactivated (3,6,7). Weisenberger et al. (4) concluded from screening of 195 CpG island methylation markers in 295 colorectal cancers that convincing evidence exists that CIMP+ tumors represent a distinct subset of tumors, as originally proposed by Toyota et al. (8).
In colorectal carcinogenesis, both CpG island promoter hypermethylation and global DNA hypomethylation (largely at repeat or satellite regions) occur concurrently (9). S-adenosylmethionine (SAM), the universal donor of methyl groups in humans, and S-adenosylhomocysteine, the product and an inhibitor of DNA methyltransferases, provide strong links between folate-mediated one-carbon metabolism and DNA methylation (10). Global DNA hypomethylation in both lymphocytes and colon tissue has been linked to low intakes of folate in animal models and several human studies (11–17). Similarly, folic acid supplementation for 10 weeks among patients with colorectal adenoma resulted in increases in global DNA methylation of both leukocytes and colonic mucosa (18). However, the role of folate status in the etiology of promoter-specific DNA methylation and in CIMP has been investigated to a limited extent (19–21).
Polymorphisms in folate-metabolizing enzymes and genes involved in DNA methylation have been reported to be associated with colon cancer (22–25). It has been hypothesized that genetic polymorphisms in folate-metabolizing enzymes affect global DNA methylation and changes in the availability of nucleotides for DNA synthesis and repair (26). Animal experiments have shown that disruption of the methylenetetrahydrofolate reductase (MTHFR) gene results in decreased methylation capacity (27,28). Similarly, studies of MTHFR polymorphisms in humans provide evidence for an association between genotype and global DNA methylation, particularly in the presence of a low-folate diet (13,15,28). However, it is unclear whether the provision of methyl groups and genetic variants in one-carbon metabolism also play a role in defining a CIMP phenotype. The evaluation of CIMP in colon tumors and other cancers for possible relationships with one-carbon metabolism polymorphisms has been limited to few and small studies (29–32). We recently reported in Slattery et al. (19) findings that did not support an association between dietary intakes of folate, vitamins B6 and B12 and methionine and a CIMP phenotype. In the current investigation, however, we sought to establish whether one-carbon metabolism genotypes and dietary factors together may better define CIMP+ tumors, as has been suggested by van Engeland et al. (20).
The purpose of this study was to build on our previous work by evaluating associations between genetic polymorphisms relevant to folate-mediated one-carbon metabolism and colon cancer risk and to furthermore investigate the impact of dietary factors on these associations. We examined polymorphisms in MTHFR, thymidylate synthase (TS), transcobalamin II (TCNII), methionine synthase (MTR), reduced folate carrier (RFC), methylenetetrahydrofolate dehydrogenase (MTHFD1), dihydrofolate reductase (DHFR) and alcohol dehydrogenase (ADH3) genes based on their involvement in the production of the methyl donor SAM (MTR reaction), the provision of the B12 cofactor for the MTR reaction (TCNII), cellular folate availability (RFC), folate absorption (ADH3) or other central roles in folate-mediated one-carbon metabolism (MTHFR, TS, MTHFD1 and DHFR), some of which have been clearly linked to altered genomic DNA methylation (MTHFR) (33) (Figure 1). We hypothesized that polymorphisms in MTHFR, MTR and RFC would have the strongest impact on DNA methylation. We evaluated genetic variants with folate and relevant B-vitamins, methionine and alcohol to more comprehensively evaluate the possibility of dietary interactions with polymorphisms and CIMP, as little information is currently available in this regard. We used data from individuals enrolled in a multi-center case–control study of colon cancer with available genetic, diet and lifestyle data. Cases were classified by their CIMP status in tumors to better define disease pathways.
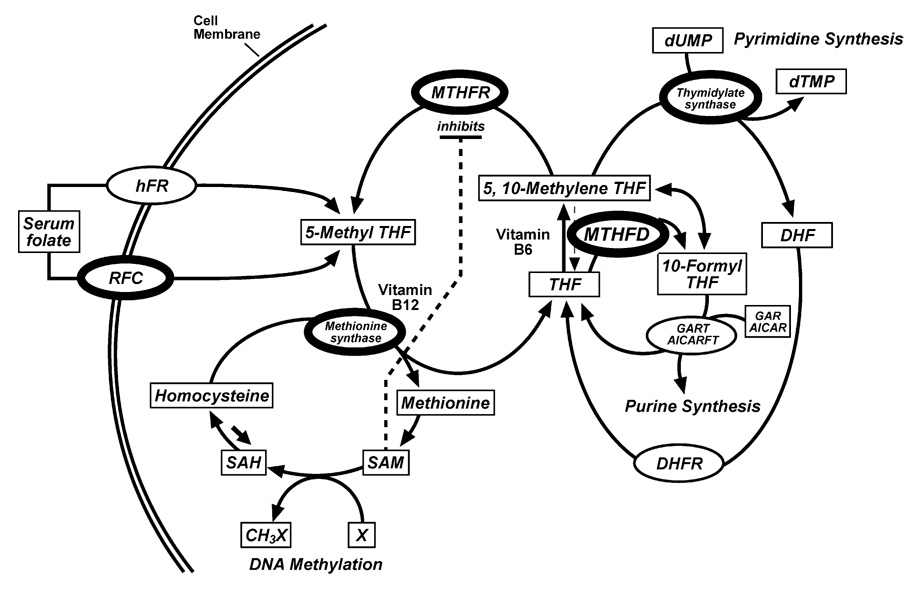
Fig. 1.
Simplified version of folate-mediated one-carbon metabolism, highlighting proteins with polymorphisms investigated in this study (figure modified from ref. 51). Key enzymes are denoted as ovals and substrates as rectangles. THF, tetrahydrofolate; DHF, dihydrofolate; DHFR, dihydrofolate reductase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; dUMP, deoxyuridine monophosphate; dTMP, deoxythymidine monophosphate; X, a variety of substrates for methylation; RFC, reduced folate carrier; hFR, human folate receptor; MTHFR, 5,10-methylenetetrahydrofolate reductase; MTHFD, methylenetetra-hydrofolate dehydrogenase; GART, phosphoribosylglycinamide formyltransferase; AICARFT, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase; AICAR, 5-aminoimidazole-4-carboxamine ribotide; GAR, glycinamide ribonucleotide and X, a variety of substrates for methylation.
Materials and methods
Study data
The Institutional Review Board from all centers approved all aspects of the study. Study participants were predominantly non-Hispanic white (90.2% of cases and 93.2% of controls), Hispanic (4.4% of cases and 4.0% of controls) or African-American (5.4% of cases and 2.8% of controls). Participants were from either the Kaiser Permanente Medical Care Program of Northern California, an eight county area in Utah (Davis, Salt Lake, Utah, Weber, Wasatch, Tooele, Morgan and Summit counties), or the Twin Cities Metropolitan area in Minnesota. Eligibility criteria for cases included diagnosis with first-primary incident colon cancer (International Classification of Diseases for Oncology, 2nd edition codes 18.0 and 18.2–18.9) between 1 October 1991 and 30 September 1994, between 30 and 79 years of age at time of diagnosis, and mentally competent to complete the interview. Cases with adenocarcinoma or carcinoma of the rectosigmoid junction or rectum (defined as the first 15 cm from the anal opening), with known familial adenomatous polyposis, ulcerative colitis or Crohn’s disease were not eligible. In addition, seven individuals were excluded from the analyses as hereditary non-polyposis colorectal cancer cases based on sequencing of mismatch repair genes (34). Of all cases asked to participate, 75.6% cooperated. Age- and sex-matched controls, in addition to eligibility criteria for cases, could not have had a previous colorectal tumor and were selected from eligibility lists for Kaiser Permanente Medical Care Program, driver’s license lists, random digit dialing or Health Care Finance Administration lists. Of controls selected, 63.7% participated. These methods have been described in detail (35).
All data were collected in person by trained and certified interviewers using a laptop-administered computerized questionnaire. The referent period for the study was the calendar year ~2 years prior to date of diagnosis for cases and date of selection for controls. Information was collected on demographic factors such as age, sex, center, diet, physical activity, height and weight 2 years prior to diagnosis, regular use of aspirin and/or non-steroidal anti-inflammatory drugs, cigarette smoking history and medical history (35). Participants were asked to self-report their race/ethnicity. Dietary intake data were obtained from an extensive diet history questionnaire that was adapted for the case–control study and validated as described previously (36,37). Participants were asked to recall activity levels by level of intensity and consumption of alcoholic beverages (beer, wine and liquor) for the referent year and 10 and 20 years ago. Body mass index of weight (kg)/height (m2) was used as an indicator of body size. Participants were asked the usual number of cigarettes smoked in a day along with when they started and stopped smoking.
CIMP
Of 362 study cases in Utah and 984 cases in Kaiser Permanente Medical Care Program, we obtained tissue and tumor DNA from 97 and 85%, respectively. Of 647 cases in Minnesota, tumor DNA was available on patients who were subsequently contacted and consented for tissue release, ~35%. Of cases with tumor DNA, 82% (1154) had an amount that was adequate to perform an evaluation of a CIMP panel. Although the tissue acquisition rate was considerably lower in Minnesota, the distribution of subject characteristics, genotypes and dietary intakes were similar in cases without CIMP status compared with cases included in this study, and we adjusted for study center in our models. Colon cancer tissue was microdissected and DNA extracted from formalin-fixed paraffin-embedded tissue blocks as described previously (38). Sodium bisulfite modification was performed on tumor DNA and methylation-specific polymerase chain reaction was then performed in accordance with methods described in Derks et al. (39) for the following CpG islands: MINT1, MINT2, MINT31, p16 and MLH1. In our analysis, each primer set used in methylation-specific polymerase chain reaction assessed between 4 and 7 methylated bases per assay at each CpG island. CIMP+ (positive or high) was methylation of two or more of these CpG islands. CIMP− (negative or low) was defined as zero or one of five markers methylated (3). The use of established assays, selection of genetic loci and criterion for CIMP+ or CIMP− was based on the pioneering work of other groups that previously defined the CIMP phenotype (40,41).
Genotyping methods
Genotyping of MTHFR 677C > T and 1298A > C polymorphisms was described previously (23,42). Of 2410 controls and 1993 cases with diet and lifestyle data, 85% of controls and 83% of cases who consented to have blood collected subsequently had DNA extracted; genotype information for MTHFR and other one-carbon polymorphisms was available for 1972 controls and 1608 cases (23). Of 1154 cases successfully evaluated for CIMP, 916 had genotype information available. Genotyping of TS variants (TSER and 3′-untranslated region 1494delTTAAAG), MTR (D919G) and RFC (80G > A) was performed as detailed previously (24). Genotyping of MTHFD1 R134K and R653Q variants was performed as described (43,44).
The 19 bp deletion polymorphism in intron 1 of the DHFR gene was genotyped as described by Johnson et al. (45) with the exception of using a 6-FAM-labeled reverse primer and an ABI3100 genetic analyzer. Genotyping for the ADH I349V (1045A > G) polymorphism (ADH3 *1*2, also known as ADH1C) and the TCNII R259P (776C > G) polymorphism was performed by allelic discrimination using the 5′ nuclease assay on a 7900HT sequence detection system (Applied Biosystems, Foster City, CA). The 5′ nuclease genotyping assays were validated by genotyping 92 individuals by both 5′ nuclease assay and restriction fragment length polymorphism. There were no discrepancies between the assays. The ADH3 I349V genotyping reactions contained 1× Taqman Core Reagents (Applied Biosystems), 5 mM MgCl2, 0.5 U AmpliTaq DNA polymerase, 0.2 U AmpErase uracil-N-glycosylase, 200 nM each primer (5′-CAATGATATTTTCTTCTTTTCAGGCTTT-3′ and 5′-GCGAAGCAGGTCAAATCCTT-3′), 150 nM ADH3 1045A probe (5′-VIC-CATTAATAACAAATaTTTTACC-3′-non-fluorescent quencher), 100 nM ADH3 1045G probe (5′-6-FAM-CATTAATAACAAATgTTTTACCT-3′-non-fluorescent quencher) and 4 ng genomic DNA. Cycling was 50°C for 2 min, 95°C for 10 min and 50 cycles of 95°C for 15 s and 60°C for 1 min. The TCNII R259P reactions contained 1× Taqman Core Reagents, 4 mM MgCl2, 0.5 U AmpliTaq DNA polymerase, 0.2 U AmpErase UNG, 200 nM each primer (5′-CACTCTATCACCAGTTCCTCATGACTT-3′ and 5′-CTTGAGACATGCTGTTCCCAGTT-3′), 150 nM each probe (G-allele 5′-6-FAM-GCCCCACGCATG-3′ and C-allele 5′-VIC-CTGCCCCAGGCAT-3′) and 4 ng genomic DNA. Cycling was 50°C for 2 min, 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. Positive controls for all genotypes as well as four negative controls were included on each 384-well plate. For quality control purposes, genotyping for 94 randomly selected samples was repeated. There were no discrepancies.
Statistical methods
To test for differences in the number of methylated markers in cases, ordinal logistic proportional odds models were used to calculate a Wald Chi-square statistic for the parameter estimate of markers methylated, ordered from zero through five. Data were analyzed using polytomous logistic regression models comparing cases with and without CIMP+ to controls. We compared CIMP+ to CIMP− cases to better define unique associations with CIMP+. We also evaluated associations for men and women separately, as we have previously reported evidence of sex-specific colon cancer risks and genetic variation in MTHFR and TS (23,24). Adjustment variables included in the logistic regression models were age at diagnosis (cases) or selection (controls), sex (for models with men and women combined), race and study center. Additionally, interaction models of alcohol, polymorphisms and CIMP were adjusted for smoking (usual number of cigarettes/day smoked regularly) as smoking is associated with alcohol use and CIMP status (46). Other potential confounders (energy, body mass index, physical activity, calcium, dietary fiber, folate and use of aspirin or non-steroidal anti-inflammatory drugs were not used as adjustment covariates as they were not related to one-carbon variants, and their effect on the estimates was negligible. It has been suggested that because both MTHFR polymorphisms are in high-linkage disequilibrium, analyses of combined 677C > T and 1298A > C genotypes are needed to understand genetic variability in the MTHFR gene and draw appropriate conclusions (22,47). Associations with CIMP phenotype in single-polymorphism models of MTHFR were adjusted for the other polymorphism as a covariate, and results were consistent with combined MTHFR genotype models.
We evaluated long-term use of alcoholic beverages (average of 10 and 20 years ago) and smoking behavior (never smoked regularly and ≤20 cigarettes/day or >20 cigarettes/day for current or former smokers). Diet exposures were categorized by sex-specific tertiles calculated from the distribution in the control population. High- or low-risk dietary pattern was based on tertile intake of folate and methionine and long-term alcohol consumption, as defined in Slattery et al. (42). High-risk diet was defined as the lowest two tertiles of folate and methionine along with high long-term alcohol intake. Low-risk diet was defined as the highest tertile folate and methionine and no or moderate long-term alcohol intake. Those not defined as high- or low-risk diet were designated as intermediate risk. High- and low-risk groups in the current study each represented ~20% of the control population. A multiplicative model was used to test for interaction between polymorphisms and diet and lifestyle exposures in CIMP+ or CIMP− tumors. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
Results
In our predominantly white study population, allele frequencies did not differ significantly between non-Hispanic white and Hispanic controls with the exception of the most common ADH3 *1 allele, which was more frequent (69 versus 58%) in 79 Hispanic subjects. In 56 African-American subjects (<3% of controls), allele frequencies generally differed from non-Hispanic whites; however, the most common variant was generally the same in both groups (exceptions: TS 3′-untranslated region, RFC and DHFR).
In Table I, the population is described in terms of one-carbon metabolism polymorphisms and number of CpG island methylated tumor markers, as previously defined: a CIMP− phenotype (zero or one marker methylated) and CIMP+ phenotype (two or more of five markers methylated), in addition to overall case and control status (3,19). A CIMP+ phenotype characterized nearly 30% of all cancers. Individuals whose genotype contained one or two variant MTHFR 1298 C alleles had a higher proportion of CIMP markers methylated than individuals homozygous for the A allele. Results from adjusted ordinal logistic models, for cases only, showed a statistically significant difference (P = 0.01) when using number of methylated markers as an ordered dependent variable for the MTHFR 1298A > C polymorphism. Genotypes for other one-carbon metabolism polymorphisms were not associated with the number of markers that were methylated.
Table 1.
Study population characteristics
| Controls |
Cases |
CIMP− (zero or one marker) |
CIMP+ (two or more of five markers) |
Chi-square |
Ordinal |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | P-valuea | P-valueb | |
| All subjects | 1972 | 916 | 655 | 71.5 | 261 | 28.5 | ||||
| Men | 1042 | 52.8 | 518 | 56.6 | 383 | 58.5 | 135 | 51.7 | ||
| Women | 930 | 47.2 | 398 | 43.5 | 272 | 41.5 | 126 | 48.3 | 0.06 | <0.01 |
| Age at diagnosis or selectionc | 65.0 | (10.2) | 65.3 | (9.5) | 64.5 | (9.6) | 67.2 | (9.1) | 0.01 | <0.001 |
| MTHFR 677C > T | ||||||||||
| CC | 887 | 45.0 | 432 | 47.2 | 310 | 47.3 | 122 | 46.7 | ||
| CT | 858 | 43.5 | 402 | 43.8 | 289 | 44.1 | 113 | 43.3 | ||
| TT | 227 | 11.5 | 82 | 9.0 | 56 | 8.6 | 26 | 10.0 | 0.67 | 0.15 |
| MTHFR 1298A > C | ||||||||||
| AA | 929 | 47.1 | 435 | 47.5 | 326 | 49.8 | 109 | 41.8 | ||
| AC | 827 | 41.9 | 394 | 43.0 | 270 | 41.2 | 124 | 47.5 | ||
| CC | 216 | 11.0 | 87 | 9.5 | 59 | 9.1 | 28 | 10.7 | 0.04 | 0.01 |
| TSER 28 bp repeat | ||||||||||
| 3R/3R | 542 | 27.6 | 287 | 31.6 | 206 | 31.6 | 81 | 31.6 | ||
| 3R/2R | 983 | 50.1 | 425 | 46.8 | 306 | 46.9 | 119 | 46.5 | ||
| 2R/2R | 437 | 22.3 | 195 | 21.5 | 139 | 21.3 | 56 | 21.9 | 0.90 | 0.83 |
| TS 3′-UTR 1494delTTAAAG | ||||||||||
| ins/ins | 881 | 44.9 | 417 | 45.9 | 300 | 46.0 | 117 | 45.7 | ||
| ins/del | 866 | 44.1 | 387 | 42.6 | 273 | 41.9 | 114 | 44.5 | ||
| del/del | 215 | 11.0 | 104 | 11.5 | 79 | 12.1 | 25 | 9.8 | 0.53 | 0.66 |
| TCNII P259R (776C > G) | ||||||||||
| CC | 616 | 31.3 | 319 | 34.9 | 215 | 32.9 | 104 | 39.9 | ||
| CG | 972 | 49.4 | 441 | 48.3 | 322 | 49.3 | 119 | 45.6 | ||
| GG | 379 | 19.2 | 154 | 16.9 | 116 | 17.8 | 38 | 14.6 | 0.05 | 0.39 |
| MTR D919G (2756A > G) | ||||||||||
| AA | 1272 | 64.4 | 572 | 62.5 | 413 | 63.1 | 159 | 60.9 | ||
| AG | 610 | 30.9 | 309 | 33.7 | 215 | 32.8 | 94 | 36.0 | ||
| GG | 92 | 4.7 | 35 | 3.8 | 27 | 4.1 | 8 | 3.1 | 0.79 | 0.32 |
| RFC 80G > A | ||||||||||
| GG | 588 | 29.8 | 294 | 32.1 | 202 | 30.8 | 92 | 35.3 | ||
| GA | 982 | 49.8 | 433 | 47.3 | 319 | 48.7 | 114 | 43.7 | ||
| AA | 402 | 20.4 | 189 | 20.6 | 134 | 20.5 | 55 | 21.1 | 0.47 | 0.28 |
| MTHFD1 R134K (401G > A) | ||||||||||
| GG | 1319 | 66.9 | 624 | 68.1 | 438 | 66.9 | 186 | 71.3 | ||
| GA | 585 | 29.7 | 265 | 28.9 | 199 | 30.4 | 66 | 25.3 | ||
| AA | 68 | 3.5 | 27 | 3.0 | 18 | 2.8 | 9 | 3.5 | 0.35 | 0.43 |
| MTHFD1 R653Q (1958G > A) | ||||||||||
| GG | 627 | 31.8 | 314 | 34.3 | 222 | 33.9 | 92 | 35.3 | ||
| GA | 949 | 48.1 | 436 | 47.6 | 314 | 47.9 | 122 | 46.7 | ||
| AA | 396 | 20.1 | 166 | 18.1 | 119 | 18.2 | 47 | 18.0 | 0.92 | 0.89 |
| DHFR 19 bp del (intron 1) | ||||||||||
| wt/wt | 620 | 31.5 | 277 | 30.2 | 196 | 29.9 | 81 | 31.0 | ||
| wt/del | 945 | 48.0 | 437 | 47.7 | 316 | 48.2 | 121 | 46.4 | ||
| del/del | 406 | 20.6 | 202 | 22.1 | 143 | 21.8 | 59 | 22.6 | 0.95 | 0.57 |
| ADH3 I349V (1045A > G) | ||||||||||
| *1*1 | 708 | 36.0 | 326 | 35.6 | 234 | 35.7 | 92 | 35.4 | ||
| *1*2 | 928 | 47.1 | 434 | 47.4 | 305 | 46.6 | 129 | 49.6 | ||
| *2*2 | 333 | 16.9 | 155 | 16.9 | 116 | 17.7 | 39 | 15.0 | 0.64 | 0.46 |
Mantel-Haenszel Chi-square test, CIMP− and CIMP+; for age at diagnosis/selection, 30–64 and 65–79.
Wald Chi-Square test, ordinal logistic regression model with number of markers methylated (zero to five), cases only.
Mean (SD).
Estimated ORs for various genetic polymorphisms and colon tumors characterized as CIMP− (zero or one marker methylated) or CIMP+ (two or more of five markers methylated), each compared with controls, showed few associations unique to either type of tumor (Table II). Certain genetic polymorphisms did exhibit associations with CIMP in colon tumors. A decreased risk of MTHFR 677 TT and MTHFR 1298 CC genotypes and colon cancer in comparison with MTHFR 677 CC and MTHFR 1298 AA genotypes reported previously (23) was seen only in CIMP− tumors. Estimates for combined MTHFR genotypes were consistent with individual MTHFR polymorphism results, as were estimates for MTHFR haplotype (data not shown). In men, specifically, having one or two variant MTHFR 1298 C alleles resulted in an ~2-fold increased risk of a CIMP+ tumor (OR= 1.9, 95% CI = 1.2–2.9). Results from a case–case comparison showed this association was statistically significantly different between CIMP+ compared with CIMP− tumors (OR = 2.5, 95% CI = 1.6–4.0). A modest inverse association with TS variant alleles that we have previously reported (24) was seen in both CIMP+ and CIMP− colon cancers, although it was not statistically significant due to the smaller sample size of cases with completed CIMP assays. Individuals with one or two variant TCNII 776 G alleles in their genotype had a modestly reduced risk of a CIMP+ tumor, in either a case–control or case–case comparison. Other genotypes showed no differences between CIMP+ and CIMP−. Estimates for men and women were similar in sex-stratified analyses, with the exception of MTHFR 1298A > C (data not shown).
Table 2.
Associations between polymorphisms in one-carbon metabolism genes and CIMP status in colon tumors, case–control comparisona,b
| Polymorphism | Genotype | Controls |
CIMP− (zero or one marker) |
CIMP+ (two or more of five markers) |
||||
|---|---|---|---|---|---|---|---|---|
| N | N | OR | 95% CI | N | OR | 95% CI | ||
| MTHFR 677C > T | CC | 885 | 310 | 1.0 | — | 121 | 1.0 | — |
| CT | 858 | 288 | 0.9 | (0.7, 1.1) | 113 | 1.0 | (0.8, 1.4) | |
| TT | 227 | 56 | 0.6 | (0.4, 0.9) | 26 | 1.0 | (0.6, 1.6) | |
| MTHFR 1298A > C | AA | 929 | 326 | 1.0 | — | 108 | 1.0 | — |
| AC | 826 | 269 | 0.9 | (0.7, 1.1) | 124 | 1.3 | (0.97, 1.8) | |
| CC | 215 | 59 | 0.7 | (0.5, 1.01) | 28 | 1.1 | (0.7, 1.9) | |
| MTHFR 677C > T and 1298A > Cc | CC/AA | 247 | 109 | 1.0 | — | 31 | 1.0 | — |
| CT/AA | 455 | 161 | 0.9 | (0.6, 1.2) | 51 | 0.9 | (0.6, 1.5) | |
| TT/AA | 227 | 56 | 0.6 | (0.4, 0.9) | 26 | 0.9 | (0.5, 1.6) | |
| CC/AC | 423 | 142 | 0.8 | (0.6, 1.1) | 62 | 1.2 | (0.7, 1.9) | |
| CT/AC | 403 | 127 | 0.8 | (0.6, 1.1) | 62 | 1.3 | (0.8, 2.1) | |
| CC/CC | 215 | 59 | 0.7 | (0.5, 1.01) | 28 | 1.0 | (0.6, 1.9) | |
| TSER 28 bp repeat | 3R/3R | 540 | 205 | 1.0 | — | 811 | 1.0 | — |
| 3R/2R | 982 | 306 | 0.8 | (0.7, 1.02) | 118 | 0.8 | (0.6, 1.1) | |
| 2R/2R | 437 | 139 | 0.8 | (0.6, 1.1) | 56 | 0.8 | (0.6, 1.2) | |
| TS 3′-UTR 1494delTTAAAG | ins/ins | 877 | 298 | 1.0 | — | 116 | 1.0 | — |
| ins/del | 863 | 271 | 0.9 | (0.7, 1.1) | 114 | 0.9 | (0.7, 1.3) | |
| del/del | 214 | 78 | 0.9 | (0.7, 1.2) | 24 | 0.8 | (0.5, 1.2) | |
| TSER and TS 3′-UTRd | wt/wt | 148 | 64 | 1.0 | — | 28 | 1.0 | — |
| wt/h or wt/v | 1141 | 369 | 0.8 | (0.5, 1.03) | 146 | 0.7 | (0.4, 1.1) | |
| h/h, h/v or v/v | 670 | 218 | 0.8 | (0.5, 1.1) | 81 | 0.6 | (0.4, 1.03) | |
| TCNII P259R (776C > G) | CC | 616 | 215 | 1.0 | — | 104 | 1.0 | — |
| CG | 971 | 322 | 1.0 | (0.8, 1.2) | 118 | 0.7 | (0.6, 0.98) | |
| GG | 378 | 115 | 0.9 | (0.7, 1.2) | 38 | 0.6 | (0.4, 0.9) | |
| MTR D919G (2756A > G) | AA | 1270 | 412 | 1.0 | — | 159 | 1.0 | — |
| AG | 609 | 215 | 1.1 | (0.9, 1.3) | 93 | 1.2 | (0.9, 1.6) | |
| GG | 91 | 27 | 0.9 | (0.6, 1.4) | 8 | 0.7 | (0.3, 1.4) | |
| RFC 80G > A | GG | 587 | 202 | 1.0 | — | 92 | 1.0 | — |
| GA | 980 | 318 | 0.9 | (0.8, 1.1) | 114 | 0.7 | (0.6, 0.99) | |
| AA | 402 | 134 | 0.9 | (0.7, 1.2) | 54 | 0.8 | (0.6, 1.2) | |
| MTHFD1 R134K (401G > A) | GG | 1317 | 437 | 1.0 | — | 185 | 1.0 | — |
| GA | 585 | 199 | 1.0 | (0.8, 1.2) | 66 | 0.8 | (0.6, 1.1) | |
| AA | 67 | 18 | 0.8 | (0.5, 1.4) | 9 | 0.9 | (0.5, 1.9) | |
| MTHFD1 R653Q (1958G > A) | GG | 626 | 221 | 1.0 | — | 91 | 1.0 | — |
| GA | 948 | 314 | 1.0 | (0.8, 1.2) | 122 | 0.9 | (0.6, 1.2) | |
| AA | 395 | 119 | 0.9 | (0.7, 1.2) | 47 | 0.8 | (0.5, 1.2) | |
| DHFR 19 bp del (intron 1) | wt/wt | 619 | 195 | 1.0 | — | 81 | 1.0 | — |
| wt/del | 945 | 316 | 1.1 | (0.9, 1.3) | 120 | 1.0 | (0.7, 1.3) | |
| del/del | 404 | 143 | 1.1 | (0.8, 1.4) | 59 | 1.1 | (0.8, 1.6) | |
| ADH3 I349V | *1*1 | 706 | 234 | 1.0 | — | 92 | 1.0 | — |
| *1*2 | 927 | 304 | 1.0 | (0.8, 1.3) | 129 | 1.1 | (0.8, 1.5) | |
| *2*2 | 333 | 116 | 1.1 | (0.8, 1.4) | 38 | 0.9 | (0.6, 1.3) | |
ORs and 95% CIs adjusted for age, sex, race, center and other MTHFR, TS, or MTHFD1 polymorphism where appropriate.
Number of subjects varies slightly from Table I due to excluding observations with one or more missing values for adjustment variables.
MTHFR combined 677 and 1298 TT/AC, TT/CC and CT/CC genotypes were not observed.
TS combined wt/wt = 3R/3R and ins/ins; wt/h = 3R/3R and ins/del or 3R/2R and ins/ins; wt/v = 3R/3R and del/del or 2R/2R and ins/ins; h/h = 3R/2R and ins/del; h/v = 3R/2R and del/del or 2R/2R and ins/del and v/v = 2R/2R and del/del.
We evaluated interactions between folate, methionine, vitamin B12, long-term alcohol intake and smoking with one-carbon metabolism polymorphisms in association with CIMP status; in general, interactions were not observed. An exception was with the MTHFR 1298A > C polymorphism, where a statistically significant interaction with alcohol in determining CIMP status was observed (Table III). Individuals homozygous for the 1298 A allele who were long-term consumers of alcohol were at reduced risk of a highly methylated tumor (P-interaction < 0.01). Individuals with low intakes of folate who had one or two variant (slow catabolizing *2) ADH3 alleles were at increased risk of a CIMP+ tumor. There was no interaction between alcohol use, ADH3, and CIMP. Results of a case–case comparison and interactions with polymorphisms and diet with CIMP were consistent with these findings, and associations were similar in men and women (data not shown).
Table 3.
Association between one-carbon metabolism polymorphisms and diet and CIMP in colon tumors, case-control comparisona,b
| Polymorphismc and exposure | Tertiled | Controls |
CIMP− (zero or one marker methylated) |
CIMP+ (two or more of five markers methylated) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt |
Het/Var |
wt |
Het/Var |
Pe | wt |
Het/Var |
Pe | ||||||||||
| N | N | N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | ||||
| MTHFR 677C > T | |||||||||||||||||
| Folate | Low | 281 | 362 | 115 | 1.0 | — | 136 | 0.9 | (0.6, 1.2) | 53 | 1.0 | — | 52 | 0.8 | (0.5, 1.2) | ||
| Middle | 324 | 339 | 106 | 0.8 | (0.6, 1.2) | 119 | 0.8 | (0.6, 1.1) | 35 | 0.5 | (0.3, 0.9) | 44 | 0.7 | (0.5, 1.1) | |||
| High | 280 | 384 | 89 | 0.7 | (0.5, 1.1) | 89 | 0.5 | (0.4, 0.7) | ns | 33 | 0.5 | (0.4, 0.9) | 43 | 0.6 | (0.4, 0.9) | ns | |
| Alcoholf (long term) | None | 350 | 404 | 114 | 1.0 | — | 126 | 0.9 | (0.6, 1.2) | 42 | 1.0 | — | 64 | 1.4 | (0.9, 2.2) | ||
| Moderate | 339 | 453 | 120 | 1.0 | (0.7, 1.4) | 135 | 0.8 | (0.6, 1.1) | 48 | 1.2 | (0.8, 1.9) | 43 | 0.9 | (0.6, 1.5) | |||
| High | 194 | 227 | 76 | 1.0 | (0.7, 1.5) | 83 | 0.9 | (0.6, 1.3) | ns | 31 | 1.2 | (0.7, 1.9) | 32 | 1.2 | (0.7, 1.9) | ns | |
| MTHFR 1298A > C | |||||||||||||||||
| Folate | Low | 304 | 339 | 126 | 1.0 | — | 125 | 0.9 | (0.6, 1.1) | 42 | 1.0 | — | 63 | 1.4 | (0.9, 2.2) | ||
| Middle | 317 | 346 | 110 | 0.8 | (0.6, 1.1) | 115 | 0.8 | (0.6, 1.04) | 32 | 0.7 | (0.4, 1.1) | 47 | 1.0 | (0.6, 1.5) | |||
| High | 308 | 356 | 90 | 0.7 | (0.5, 1.0) | 88 | 0.5 | (0.4, 0.8) | ns | 34 | 0.7 | (0.5, 1.2) | 42 | 0.8 | (0.5, 1.2) | ns | |
| Alcoholf (long term) | None | 337 | 417 | 117 | 1.0 | — | 123 | 0.8 | (0.6, 1.1) | 56 | 1.0 | — | 50 | 0.7 | (0.5, 1.2) | ||
| Moderate | 387 | 405 | 124 | 0.9 | (0.6, 1.2) | 131 | 0.8 | (0.6, 1.1) | 32 | 0.5 | (0.3, 0.9) | 59 | 1.0 | (0.6, 1.5) | |||
| High | 203 | 218 | 85 | 1.0 | (0.7, 1.4) | 74 | 0.8 | (0.5, 1.1) | ns | 20 | 0.5 | (0.3, 0.97) | 43 | 1.1 | (0.7, 1.8) | <0.01 | |
| ADH3 I349V *1/*2 | |||||||||||||||||
| Folate | Low | 243 | 399 | 90 | 1.0 | — | 161 | 1.1 | (0.8, 1.5) | 29 | 1.0 | — | 76 | 1.6 | (1.03, 2.6) | ||
| Middle | 200 | 460 | 73 | 1.0 | (0.7, 1.4) | 152 | 0.9 | (0.7, 1.3) | 24 | 1.0 | (0.6, 1.6) | 54 | 1.0 | (0.6, 1.6) | |||
| High | 263 | 401 | 71 | 0.7 | (0.5, 1.01) | 107 | 0.7 | (0.5, 1.00) | ns | 39 | 1.1 | (0.7, 1.9) | 37 | 0.7 | (0.4, 1.2) | 0.02 | |
| Alcoholf (long term) | None | 276 | 476 | 87 | 1.0 | — | 153 | 1.1 | (0.8, 1.4) | 42 | 1.0 | — | 64 | 0.9 | (0.6, 1.3) | ||
| Moderate | 288 | 502 | 95 | 1.0 | (0.7, 1.4) | 160 | 1.0 | (0.7, 1.4) | 29 | 0.7 | (0.4, 1.1) | 61 | 0.8 | (0.5, 1.3) | |||
| High | 141 | 280 | 52 | 1.0 | (0.7, 1.5) | 107 | 1.0 | (0.7, 1.5) | ns | 21 | 0.9 | (0.5, 1.5) | 42 | 0.9 | (0.5, 1.5) | ns | |
ORs and 95% CIs adjusted for age, sex, race, center, smoking amount and other MTHFR polymorphism where appropriate.
Number of subjects varies slightly from Table I due to excluding observations with one or more missing values for adjustment variables.
Genotypes are homozygous for the common allele (wt), heterozygous (Het) and homozygous for the minor allele (Var); Het and Var genotypes are combined.
Cutpoints for middle and high based on controls, nutrient density/1000/kcal/day of folate (µg): 135/180 men and 152/201 women.
P-value for multiplicative interaction term; values >.0.05 not shown (ns).
High consumption of long-term alcohol (average of 10 and 20 years ago), g/day: ≥ 20 men, ≥ 10 women.
An evaluation of the combined effects of folate, methionine and alcohol and one-carbon metabolism polymorphisms and CIMP indicated MTHFR 1298 AC or CC genotypes and a high-risk dietary pattern (low in folate or methionine intake, high in alcohol consumption) was associated with a CIMP+ phenotype, but not a CIMP− phenotype (Table IV). This was not seen for the MTHFR 677C > T polymorphism or the ADH3 polymorphism (data not shown).
Table 4.
Association between one-carbon metabolism polymorphisms and high- and low-risk diets and CIMP in colon tumors, case–control comparisona,b
| Polymorphismc | Dietary patternd | Controls |
CIMP− (zero or one marker methylated) |
CIMP+ (two or more of five markers methylated) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt |
Het/Var |
wt |
Het/Var |
Pe | wt |
Het/Var |
Pe | ||||||||||
| N | N | N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | ||||
| MTHFR 677C > T | High risk | 257 | 304 | 95 | 1.0 | — | 116 | 0.9 | (0.7, 1.3) | 45 | 1.0 | — | 39 | 0.7 | (0.5, 1.2) | ||
| Intermediate | 486 | 622 | 175 | 1.1 | (0.8, 1.5) | 186 | 0.9 | (0.6, 1.2) | 66 | 0.9 | (0.6, 1.4) | 76 | 0.9 | (0.6, 1.4) | |||
| Low risk | 141 | 151 | 37 | 0.9 | (0.6, 1.4) | 41 | 0.9 | (0.6, 1.4) | ns | 11 | 0.6 | (0.3, 1.2) | 22 | 1.2 | (0.6, 2.2) | ns | |
| MTHFR 1298A > C | High risk | 270 | 293 | 104 | 1.0 | — | 106 | 1.0 | (0.6, 1.2) | 27 | 1.0 | — | 57 | 2.1 | (1.3, 3.4) | ||
| Intermediate | 531 | 575 | 175 | 0.9 | (0.7, 1.2) | 188 | 0.9 | (0.7, 1.3) | 62 | 1.2 | (0.8, 2.0) | 80 | 1.5 | (0.9, 2.4) | |||
| Low risk | 124 | 169 | 45 | 1.0 | (0.7, 1.5) | 33 | 0.6 | (0.4, 0.9) | ns | 18 | 1.6 | (0.8, 2.7) | 15 | 1.0 | (0.5, 1.8) | 0.03 | |
ORs and 95% CIs adjusted for age, sex, race, center, smoking amount and other MTHFR polymorphism where appropriate.
Number of subjects varies slightly from Table I due to excluding observations with one or more missing values for adjustment variables.
Genotypes are homozygous for the common allele (wt), heterozygous (Het) and homozygous for the minor allele (Var); Het and Var genotypes are combined.
High-risk dietary pattern is lowest two tertiles of folate and methionine (per 1000/kcal/day) and high long-term alcohol (≥20 g/day men and ≥10 g/day women); low-risk pattern is highest tertile of folate and methionine and no or moderate long-term alcohol use. All others are intermediate risk.
P-value for multiplicative interaction term; values >0.05 not shown (ns).
We reported previously an association with heavy smoking (>20 cigarettes/day) and an increased risk of both CIMP+ colon cancer and BRAF V600E tumor mutations (46). In our current assessment, cigarette smoking and CIMP status did not interact with one-carbon genotypes (data not shown).
Discussion
We investigated the associations between CIMP and polymorphisms relevant to one-carbon metabolism and thus, to some extent, the provision of methyl groups in relation to colon cancer risk in data from a large, population-based case–control study. We observed few associations specific to CIMP+ colon tumors, suggesting that the provision of methyl groups may not be critical in the development of CIMP. Our data, however, suggest that a genetic polymorphism at MTHFR 1298A > C (but not 677C > T) interacts with diet to increase the risk of highly CpG-methylated colon tumors. The 1298A > C variant occurs in the MTHFR regulatory region, where SAM binds as an allosteric inhibitor. This provides some basis for observing stronger associations between MTHFR 1298A > C and CIMP rather than the 677C > T polymorphism, which, in contrast, affects the enzyme’s stability. Nevertheless, the 677 TT variant has been associated with reduced genomic DNA methylation, whereas the 1298 CC variant has not. Both the MTHFR 677C > T and 1298A > C polymorphisms are in high linkage disequilibrium and thus should not be considered in isolation; however, the single-polymorphism models had increased power to detect associations, and results were consistent with combined polymorphism analyses.
Recent findings, as well as the current study support the notion of MTHFR 1298A > C as a predictor of colon cancer risk (23,48). Consistent with earlier reports, we found no evidence to support that MTHFR 677C > T polymorphisms were associated with CIMP in tumors (29,32). There was no interaction between either MTHFR polymorphism and folate intake in association with CIMP-defined colon tumors. However, when the joint effect of folate and alcohol intake was considered as part of a ‘dietary pattern’, we observed an interaction between a high- or low-risk diet and MTHFR 1298A > C in regard to CIMP status. In the Netherlands Cohort Study, van Engeland et al. (20) found that the prevalence of promoter hypermethylation was higher in colorectal cancers in 61 subjects with low intake of folate and high intake of alcohol; however, the difference was not statistically significant as power was limited. The authors suggested that the observed effect of folate deficiency on promoter methylation may be stronger after stratification for functionally important polymorphisms in folate metabolism genes (20).
We observed an inverse association of CIMP+ colon tumors with an increasing number of variant alleles for TCNII. TCNII encodes holotranscobalamin, the carrier protein for vitamin B12. Vitamin B12 is an essential cofactor for the methionine synthase reaction, which is essential for the provision of SAM. The TCNII variant has been associated with several biomarkers of reduced vitamin B12 status (49,50), adding biologic plausibility to this association. However, the associations we observed were modest and certainly require confirmation.
In this investigation, an interaction between alcohol intake and MTHFR 1298A > C, in association with CIMP status, was observed whereby the AA genotype was associated with half the risk of CIMP+ in drinkers compared with the AA genotype in non-drinkers. No risk difference between drinkers and non-drinkers was observed in variant genotypes. We previously reported in Slattery et al. (19) that high long-term alcohol use was associated with increased likelihood of a CIMP− tumor among individuals with microsatellite unstable (MSI+) cancers; when CIMP status was assessed for associations with alcohol use without regard to genotype, we did not observe an association. This suggests that one-carbon metabolism enzyme activity may modify the risk associated with alcohol in determining the CIMP status of colon cancers.
Prior to this study, we reported that heavy cigarette smoking was associated with increased risk of CIMP-high colon cancer (46). Although smoking-induced DNA damage may require nucleotides provided via one-carbon metabolism, we did not hypothesize that smoking would interact with one-carbon polymorphisms to alter colon cancer risk in our current investigation. Our assessment of one-carbon variants and CIMP status showed no significant interactions with cigarette smoking, suggesting that smoking is not directly involved in further defining risk in the one-carbon metabolism and colon cancer pathway. We also recently reported that higher dietary fiber intake decreased the likelihood of having a CIMP+ tumor, unconfounded by folate (19). In this investigation, some suggestion of a gene–fiber interaction was observed. At present, we have no potential mechanism to explain these observations, which may be spurious; however, if confirmed, these findings may explain some of the heterogeneity in results previously reported for fiber and colon cancer risk.
Few published studies have evaluated CIMP in colon or other cancers for possible relationships with one-carbon metabolism polymorphisms. However, several studies have evaluated one-carbon polymorphisms in relation to promoter methylation at specific loci, including some used as CIMP markers in our study. In a study of 233 patients with colorectal, breast or lung tumors, Paz et al. (29) reported that five homozygous carriers of the variant MTR G allele showed a lower rate of CpG island hypermethylation in tumor suppressor genes, including p16 and MLH1. Kang et al. (30) reported that the MTHFR 677 CT genotype was associated with decreased promoter hypermethylation of O(6)-methylguanine DNA methyltransferase in 82 uterine cervical cancers. In our much larger study, this association was in the same direction, but not statistically significant. In a study of 194 Japanese colorectal cases, proximal tumors classified as CIMP+ were more frequent in subjects with alleles conferring low MTHFR enzymatic activity (31), consistent with our comparison of MTHFR 1298 variants in CIMP+ (the majority being proximal tumors) and CIMP− in Table I. In a study of several methyl metabolism variants and frequency of CpG island hypermethylation in 227 breast cancers, Li et al. (32) reported that cases homozygous for MTHFD1 R653Q exhibited more frequent CpG hypermethylation in the promoter regions of seven genes, including p16. We observed no association between MTHFD1 and CIMP in our set of markers. Overall, these studies are not conclusive and suggest that further research is needed to define the role of genetic polymorphisms in relation to CIMP status and the promoter hypermethylation of specific genes.
We recognize that the few associations we observed in our investigation of specific study hypotheses may reflect chance, because a number of comparisons were made; thus, replication in other studies is important to confirm or disprove our results. We believe our diet history questionnaire was an accurate assessment of intake during the referent year based on a validation study (37). However, if the relevant time of intake was prior to the referent year, we may not have captured nutrient intake for the period of interest. For alcohol consumption, we assessed long-term use in addition to that reported in the referent period to give a broader perspective on intake.
A limitation of studying DNA methylation in colon tumors is that the classification of CIMP is not defined universally. Although we used an established panel (40,41), CIMP may have been potentially misclassified in our analyses. Generally, at least two markers methylated out of five markers (MINT1, MINT2, MINT31, p16 and MLH1) have been used to define a CIMP+ phenotype (40,41). We have previously reported on different classifications of methylation and dietary associations with colon cancer risk (19). Low methylation (one marker) was consistent with CIMP− (no markers methylated), whereas results were similar when examining two through five markers methylated relative to CIMP+. In a report of aberrant DNA methylation of CpG islands using a systematic, stepwise screen of 195 methylation markers in 295 colorectal cancers published subsequent to this study, Weisenberger et al. (4) used clustering routines to propose a new panel of markers that would optimally distinguish between CIMP− and CIMP+ in tumors. Although the Weisenberger panel probably supports a more precise CIMP classification than the standard panel that was used here, if associations existed, we would have identified medium to strong associations with CIMP because of the large sample size in our study and the similarity in CIMP classification panels.
Another limitation is that only few of the polymorphisms studied have to date been directly associated with methyl-group availability or global DNA methylation, partly because of the complex cycles of folate-mediated one-carbon metabolism (Figure 1). Thus, null findings for some of these variants do not necessarily abrogate a role of methyl-group availability in DNA methylation or CIMP status. Although our analysis of variants in one-carbon metabolism was fairly comprehensive, genotyping was completed prior to assessment of CIMP in our study cases; funding was unavailable to investigate polymorphisms in DNA methyltransferases and other methionine cycle variants. Future studies should more directly evaluate polymorphisms in the methionine cycle, including variants in MAT2A and in DNA methyltransferases, as well as other enzymes that are critical in determining the SAM to S-adenosylhomocysteine ratio.
Conclusion
Although a substantial body of literature supports the relevance of one-carbon metabolism polymorphisms and dietary factors for global DNA methylation, our data provide only limited support for a role of these factors in the promoter-specific methylation characterizing the CIMP subset of colon cancer. Possible explanations for a lack of association are as follows: (i) there is truly no association, perhaps because the availability of methyl groups is not relevant to the development of the CIMP; (ii) not all polymorphisms we investigated have been directly linked to the availability of methyl groups and (iii) CIMP status may have been moderately misclassified, thus attenuating associations.
Acknowledgements
We would like to acknowledge the contributions of Dr Bette Caan and Dr Kristen Anderson, Sandra Edwards, Leslie Palmer and Judy Morse to the data collection and management efforts of this study; Michael Hoffman for genotyping and the core facility at the University of Utah Health Sciences Center for sequencing. This study was funded by National Institutes of Health Grants R01 CA48998, R01 CA59045 and R01 CA61757. This research was supported by the Utah Cancer Registry, which is funded by contract no. N01-PC-67000 from the National Cancer Institute, with additional support from the State of Utah Department of Health and the University of Utah, the Northern California Cancer Registry and the Sacramento Tumor Registry. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute.
Abbreviations
- ADH3
alcohol dehydrogenase 3
- CIMP
CpG island methylator phenotype
- DHFR
dihydrofolate reductase
- MTHFD1
methylenetetrahydrofolate dehydrogenase 1
- MTHFR
methylenetetrahydrofolate reductase
- MTR
methionine synthase
- RFC
reduced folate carrier
- SAM
S-adenosylmethionine
- TCNII
transcobalamin II
- TS
thymidylate synthase
Footnotes
Conflict of Interest Statement: None declared.
Contributor Information
Karen Curtin, Department of Internal Medicine, University of Utah Health Sciences Center, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108, USA.
Martha L. Slattery, Department of Internal Medicine, University of Utah Health Sciences Center, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108, USA
Cornelia M. Ulrich, Cancer Prevention Research Program, Fred Hutchinson Cancer Research Center, Seattle,WA, 98109, USA
Jeannette Bigler, Cancer Prevention Research Program, Fred Hutchinson Cancer Research Center, Seattle,WA, 98109, USA.
Theodore R. Levin, Kaiser Permanente Medical Center, Walnut Creek, CA 94596, USA
Roger K. Wolff, Department of Internal Medicine, University of Utah Health Sciences Center, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108, USA
Hans Albertsen, Department of Internal Medicine, University of Utah Health Sciences Center, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108, USA.
John D. Potter, Cancer Prevention Research Program, Fred Hutchinson Cancer Research Center, Seattle,WA, 98109, USA
Wade S. Samowitz, Department of Pathology, University of Utah Health Sciences Center, Salt Lake City, UT 84132, USA
References
- 1.Potter JD. Colorectal cancer: molecules and populations. J. Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR, et al. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862–876. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 3.Samowitz WS, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Weisenberger DJ, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 5.Schuebel K, et al. CIMPle origin for promoter hypermethylation in colorectal cancer? Nat. Genet. 2006;38:738–740. doi: 10.1038/ng0706-738. [DOI] [PubMed] [Google Scholar]
- 6.van Rijnsoever M, et al. Characterisation of colorectal cancers showing hypermethylation at multiple CpG islands. Gut. 2002;51:797–802. doi: 10.1136/gut.51.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins N, et al. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 8.Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc. Natl Acad. Sci. USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J. Nutr. 2005;135:2703–2709. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- 10.James SJ, et al. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J. Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- 11.Kim YI, et al. Exon-specific DNA hypomethylation of the p53 gene of rat colon induced by dimethylhydrazine. Modulation by dietary folate. Am. J. Pathol. 1996;149:1129–1137. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YI, et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am. J. Clin. Nutr. 1997;65:46–52. doi: 10.1093/ajcn/65.1.46. [DOI] [PubMed] [Google Scholar]
- 13.Rampersaud GC, et al. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am. J. Clin. Nutr. 2000;72:998–1003. doi: 10.1093/ajcn/72.4.998. [DOI] [PubMed] [Google Scholar]
- 14.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ. Mol. Mutagen. 2004;44:10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 15.Shelnutt KP, et al. Methylenetetrahydrofolate reductase 677C–>T polymorphism affects DNA methylation in response to controlled folate intake in young women. J. Nutr. Biochem. 2004;15:554–560. doi: 10.1016/j.jnutbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Pufulete M, et al. Influence of folate status on genomic DNA methylation in colonic mucosa of subjects without colorectal adenoma or cancer. Br. J. Cancer. 2005;92:838–842. doi: 10.1038/sj.bjc.6602439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis SR, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C-> T polymorphism and by dietary folate restriction in young women. J. Nutr. 2005;135:1045–1050. doi: 10.1093/jn/135.5.1045. [DOI] [PubMed] [Google Scholar]
- 18.Pufulete M, et al. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut. 2005;54:648–653. doi: 10.1136/gut.2004.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slattery ML, et al. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int. J. Cancer. 2007;120:656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 20.van Engeland M, et al. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res. 2003;63:3133–3137. [PubMed] [Google Scholar]
- 21.Zhu K, et al. Methyl-group dietary intake and risk of breast cancer among African-American women: a case-control study by methylation status of the estrogen receptor alpha genes. Cancer Causes Control. 2003;14:827–836. doi: 10.1023/b:caco.0000003823.97506.be. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich CM. Nutrigenetics in cancer research—folate metabolism and colorectal cancer. J. Nutr. 2005;135:2698–2702. doi: 10.1093/jn/135.11.2698. [DOI] [PubMed] [Google Scholar]
- 23.Curtin K, et al. MTHFR C677T and A1298C polymorphisms: diet, estrogen, and risk of colon cancer. Cancer Epidemiol. Biomarkers Prev. 2004;13:285–292. doi: 10.1158/1055-9965.epi-03-0083. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich CM, et al. Polymorphisms in the reduced folate carrier, thymidylate synthase, or methionine synthase and risk of colon cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14:2509–2516. doi: 10.1158/1055-9965.EPI-05-0261. [DOI] [PubMed] [Google Scholar]
- 25.Little J, et al. Colon cancer and genetic variation in folate metabolism: the clinical bottom line. J. Nutr. 2003;133:3758S–3766S. doi: 10.1093/jn/133.11.3758S. [DOI] [PubMed] [Google Scholar]
- 26.Stempak JM, et al. Cell and stage of transformation-specific effects of folate deficiency on methionine cycle intermediates and DNA methylation in an in vitro model. Carcinogenesis. 2005;26:981–990. doi: 10.1093/carcin/bgi037. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 28.Stern LL, et al. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol. Biomarkers Prev. 2000;9:849–853. [PubMed] [Google Scholar]
- 29.Paz MF, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res. 2002;62:4519–4524. [PubMed] [Google Scholar]
- 30.Kang S, et al. Polymorphism in folate- and methionine-metabolizing enzyme and aberrant CpG island hypermethylation in uterine cervical cancer. Gynecol. Oncol. 2005;96:173–180. doi: 10.1016/j.ygyno.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Oyama K, et al. The association between methylenetetrahydrofolate reductase polymorphism and promoter methylation in proximal colon cancer. Anticancer Res. 2004;24:649–654. [PubMed] [Google Scholar]
- 32.Li SY, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in human breast cancer. Oncol. Rep. 2006;15:221–225. [PubMed] [Google Scholar]
- 33.Ulrich C. Genetic variability in folate-mediated one-carbon metabolism and cancer risk. In: Choi SW, Friso S, editors. Nutrient-Gene Interactions in Cancer. Boca Raton, FL: Taylor & Francis Group; 2006. pp. 75–91. [Google Scholar]
- 34.Samowitz WS, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–838. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- 35.Slattery ML, et al. Energy balance and colon cancer—beyond physical activity. Cancer Res. 1997;57:75–80. [PubMed] [Google Scholar]
- 36.Slattery ML, et al. A comparison of two methods to ascertain dietary intake: the CARDIA Study. J. Clin. Epidemiol. 1994;47:701–711. doi: 10.1016/0895-4356(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, et al. A study of the reliability and comparative validity of the cardia dietary history. Ethn. Dis. 1994;4:15–27. [PubMed] [Google Scholar]
- 38.Spirio LN, et al. Alleles of APC modulate the frequency and classes of mutations that lead to colon polyps. Nat. Genet. 1998;20:385–388. doi: 10.1038/3865. [DOI] [PubMed] [Google Scholar]
- 39.Derks S, et al. Methylation-specific PCR unraveled. Cell. Oncol. 2004;26:291–299. doi: 10.1155/2004/370301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frazier ML, et al. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003;63:4805–4808. [PubMed] [Google Scholar]
- 41.Park SJ, et al. Frequent CpG island methylation in serrated adenomas of the colorectum. Am. J. Pathol. 2003;162:815–822. doi: 10.1016/S0002-9440(10)63878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slattery ML, et al. Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol. Biomarkers Prev. 1999;8:513–518. [PubMed] [Google Scholar]
- 43.Brody LC, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am. J. Hum. Genet. 2002;71:1207–1215. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hol FA, et al. Molecular genetic analysis of the gene encoding the trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase) in patients with neural tube defects. Clin. Genet. 1998;53:119–125. doi: 10.1111/j.1399-0004.1998.tb02658.x. [DOI] [PubMed] [Google Scholar]
- 45.Johnson WG, et al. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am. J. Med. Genet. A. 2004;124:339–345. doi: 10.1002/ajmg.a.20505. [DOI] [PubMed] [Google Scholar]
- 46.Samowitz WS, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J. Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 47.Robien K, et al. Methylenetetrahydrofolate reductase and thymidylate synthase genotypes modify oral mucositis severity following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:799–800. doi: 10.1038/sj.bmt.1705330. [DOI] [PubMed] [Google Scholar]
- 48.Keku T, et al. 5,10-Methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol. Biomarkers Prev. 2002;11:1611–1621. [PubMed] [Google Scholar]
- 49.Pangilinan F, et al. Snowmass Village, CO: FASEB; 2004. Transcobalamin II Polymorphisms Influence Plasma Levels in B12 but are not Major Risk Factors for Neural Tube Defects. [Google Scholar]
- 50.von Castel-Dunwoody KM, et al. Transcobalamin 776C->G polymorphism negatively affects vitamin B-12 metabolism. Am. J. Clin. Nutr. 2005;81:1436–1441. doi: 10.1093/ajcn/81.6.1436. [DOI] [PubMed] [Google Scholar]
- 51.Ulrich CM, et al. Pharmacogenetics and folate metabolism—a promising direction. Pharmacogenomics. 2002;3:299–313. doi: 10.1517/14622416.3.3.299. [DOI] [PubMed] [Google Scholar]