Abstract
The significance of cell wall invertase (cwINV) for plant defense was investigated by comparing wild-type tobacco (Nicotiana tabacum) Samsun NN (SNN) with plants with RNA interference (RNAi)-mediated repression of cwINV (SNN∷cwINV). In source leaves of SNN∷cwINV, the activity of cwINV was repressed by about 90%. Sucrose export and apoplastic carbohydrate levels were significantly reduced, while photosynthesis and dark respiration exhibited little or no change. Activities of sucrose synthase and phosphofructokinase were depressed moderately, while ADP-glucose pyrophosphorylase was diminished greatly. Yet, the content of cytosolic/vacuolar carbohydrates was not significantly lower, which correlated with the absence of phenotypic effects in SNN∷cwINV under normal growing conditions. By contrast, defense-related processes in primary metabolism and hypersensitive cell death were impaired and delayed in correlation with repression of cwINV. The increase in cwINV observed in source leaves of the resistant wild type following infection with Phytophthora nicotianae was absent in SNN∷cwINV. Also, defense-related callose deposition at cell-to-cell interfaces, the related decline in sugar export, and accumulation of apoplastic carbohydrates were reduced and delayed. Expression of pathogenesis-related proteins and increase in phenylalanine ammonia-lyase and glucose-6-phosphate dehydrogenase activities were alleviated. Formation of hydrogen peroxide and development of hypersensitive lesions were weak and heterogeneous, and the pathogen was able to sporulate. We conclude that in photosynthetically active leaves of the apoplastic phloem loader, tobacco cwINV plays an essential role for acquisition of carbohydrates during plant-pathogen interactions and that the availability of these carbohydrates supports the onset of the hypersensitive reaction and ensures successful defense.
When plant cells are attacked by pathogens, a network of cellular reactions is initiated. These include generation of reactive oxygen species (ROS), synthesis of pathogenesis-related (PR) proteins and phytoalexins, reorganization of the cytoskeleton, cell wall fortification, the hypersensitive reaction (HR), and cell death (Garcia-Brugger et al., 2006). Most of these reactions occur within the first few hours postinfection (hpi). These concerted cellular reactions are expensive in terms of energy, reducing power and carbon skeletons. For example, reorganization of the cytoskeleton (for review, see Takemoto and Hardham, 2004) requires ATP, which has to be generated, e.g. by respiration. ROS generation by the plasmalemma oxidase is driven by NADPH, mainly generated by the cytosolic Glc-6-P dehydrogenase (G6PDH; Pugin et al., 1997). Defense-related callose deposition (for review, see Maor and Shirasu, 2005) consumes large amounts of Glc units and is presumably one of the strongest sink reactions in plant cells. Carbohydrates can fuel all the above-mentioned pathways, satisfying the extra requirement for ATP, NADPH, and metabolites. In addition to their function as metabolic fuel, soluble carbohydrates are known to control the expression of various metabolic and defense-related genes (for review, see Rolland et al., 2006). Thus, carbohydrates may be essential for successful plant defense.
There is increasing evidence that the availability of carbohydrates indeed affects the plant resistance against pathogens and that plants have evolved mechanisms to modulate their carbohydrate fluxes in response to infections. Increased levels of soluble carbohydrates support plant resistance against diseases (high-sugar resistance; Horsfall and Dimond, 1957), while reduction in carbohydrates can increase its susceptibility (sink-induced loss of resistance; Horsfall, 1975). Several lines of evidence suggest that plants establish high hexose levels in response to invading pathogens, which in turn support defense responses of the host (e.g. Herbers et al., 1996, 2000; Roitsch et al., 2003; Biemelt and Sonnewald, 2006; Swarbrick et al., 2006; Berger et al., 2007; Seo et al., 2007). On the other hand, those hexoses could be a nutrient source for pathogens. Rapid mobilization of carbohydrates, in particular the reprogramming of the carbon flow from Suc to hexose, seems to be an important factor determining the outcome of plant-pathogen interactions. Yet, a consistent concept of the interrelationship between primary metabolism and successful plant defense does not exist (Conrath et al., 2003).
Acquisition of carbohydrates for defense may be a particular problem in fully developed photoautotrophic source leaves: Due to rapid sugar export, the level of soluble carbohydrates is usually low, and carbohydrate-consuming pathways important for defense, such as the glycolysis, respiration, oxidative pentose phosphate (OPPP), and shikimic acid pathways, are reduced. In this metabolic state, mesophyll cells may be not well suited for defense. Nonetheless, photosynthetically active leaves of resistant plants exhibit strong defense reactions, including HR.
Cell wall invertase (cwINV) is a sink-specific enzyme, normally found in various kinds of carbohydrate-consuming tissues, and its activity is usually low in source leaves (e.g. Sturm, 1999). It catalyzes the irreversible cleavage of Suc into hexoses and is the key enzyme of the apoplastic phloem unloading pathway. CwINV plays a crucial role in carbohydrate acquisition and various aspects of plant growth and development by linking intra- and extracellular stimuli to the regulation of source/sink relations (for review, see Roitsch, 1999; Roitsch and González, 2004).
It was shown previously that a rapid induction of cwINV is one of the early defense-related reactions in resistant tobacco (Nicotiana tabacum) Samsun NN (SNN) source leaves after infection with Phytophthora nicotianae (Scharte et al., 2005). Similar observations were made in barley (Hordeum vulgare) leaves during an interaction with powdery mildew (Blumeria graminis; Swarbrick et al., 2006) and in various other plant-pathogen interactions (for review, see Roitsch et al., 2003). A specific role of cwINV during plant defense has been suggested (Ehness et al., 1997), based on observations that cwINV activity is often induced during various plant-pathogen interactions (for review, see Roitsch et al., 2003) and the finding that overexpression of a yeast (Saccharomyces cerevisiae) invertase in the apoplast increases plant resistance against viruses (Herbers et al., 1996, 2000). It is also reported that pathogens can stimulate host cwINV activity (Swarbrick et al., 2006). Because plant pathogens possess their own invertase activity (e.g. Voegele et al., 2006), it is often difficult to distinguish whether induced cwINV activity during plant-pathogen interactions is of host or parasite origin. Despite evidence for a role for cwINV in plant-pathogen interactions, details of the causal relationship between an increase in invertase activity and the outcome of the interaction remain unclear (Berger et al., 2007).
In the case of defense-related increase in cwINV activity in source leaves (e.g. Scharte et al., 2005; Swarbrick et al., 2006), it was hypothesized that the cwINV plays an essential role in generating hexoses and reprogramming the mesophyll from source to sink metabolism. Due to the induction of cwINV activity, hexoses will be produced in the apoplast from photosynthetically generated, exported Suc, and then reimported into the infected mesophyll cells to support defense processes. A transgenic approach with tobacco plants expressing a yeast invertase in the apoplast (von Schaewen et al., 1990) further supports this concept. In source leaves, constitutive expression of cwINV leads to the accumulation of carbohydrates and a metabolic source-to-sink shift. These plants also show increased gene expression of PR proteins and enhanced resistance against viral infection (Herbers et al., 1996, 2000).
Yet, it still remains unclear whether cwINV activity and the related reprogramming of the carbon metabolism are indispensable elements of defense in leaves. To address this question, we generated transgenic tobacco plants (SNN∷cwINV) in which the cwINV activity in source leaves is constitutively suppressed by an RNA interference (RNAi) construct. We investigate changes in the carbohydrate status, callose deposition, and HR/cell death in source leaves during an interaction with the oomycete P. nicotianae van Breda de Haan and compare them with the resistant wild-type tobacco SNN.
The oomycete P. nicotianae is a soil-borne, hemibiotrophic plant pathogen with a broad host range of over 70 tropical and temperate crops, predominately Solanaceae. In susceptible tobacco plants, it causes the Black Shank disease, which includes root rot, leaf wilting, stem blackening, and eventual death (for review, see Erwin and Ribeiro, 1996). In this study, the resistant wild type exhibited hypersensitive lesions at about 24 hpi. Although the transgenic tobacco SNN∷cwINV plants developed normally under standard growth conditions, they exhibited weaker HR symptoms and were less tolerant of the pathogen. We discuss the significance of cwINV for the acquisition of carbohydrates in source leaves of the apoplastic loader tobacco and their impact on plant resistance.
RESULTS
Differential CwINV Deficiency in Tobacco Leaves Caused by RNAi-Mediated Silencing
There is only limited sequence information available concerning number and tissue-specificity of tobacco cwINV isoforms. To generate transgenic tobacco plants with reduced cwINV activity, we made use of an intron-spliced hairpin RNAi construct targeted to silence cwINV isoenzymes (LINs) LIN8 and LIN6 in tomato (Solanum lycopersicum; N. Kocal and S. Sonnewald, unpublished data). LIN8 and LIN6 share about 90% identity with the published cwINV isoforms from tobacco (Nt-CWI [Greiner et al., 1995] and NtINV [EMBL database accession no. AB055500]). RNAi is homology dependent, and earlier studies revealed that 90% sequence identity between two nucleotide sequences is sufficient to trigger effective RNA degradation (Schweizer et al., 2000; Le et al., 2006; Chen et al., 2008).
Thirty-five transgenic plants (SNN∷cwINV) were obtained and screened for reduced cwINV activity in source leaves. One line showing the strongest inhibition was selected for further analyses, and homozygous plants of the T3 generation (selection of plants as described in “Materials and Methods”) and their wild-type siblings were used in the disease assays and biochemical measurements of this study.
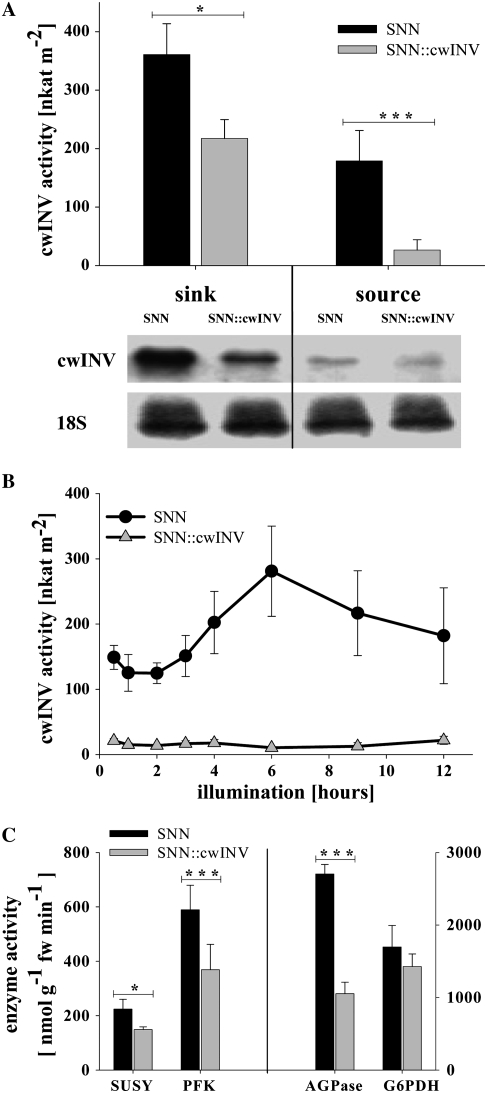
In SNN∷cwINV, the activity of cwINV was significantly reduced: 50% in sink and about 90% in source leaves compared to the wild type (Fig. 1A; Table I). As shown by representative northern blots probed with Nt-CWI cDNA (Greiner et al., 1995), transcripts of cwINV were hardly measurable in source leaves of SNN as well as of SNN∷cwINV. In sink leaves, transcripts of cwINV were detectable in both lines but much more in the wild type (Fig. 1A). Also, a characteristic circadian cycle in the cwINV activity found in source leaves of the wild type was absent in the transgenic plants (Fig. 1B). In the mean over the whole day, Suc efflux and the absolute content of apoplastic carbohydrates were reduced in SNN∷cwINV plants compared to the wild type (Table I).
Figure 1.
RNAi-mediated silencing of cwINV and its impact on primary metabolism enzymes. A, CwINV activity and representative northern blot of cwINV transcripts from sink and source leaves of tobacco. Probing with an 18S cDNA served as loading control. Similar results were obtained with at least three individual plants; samples were taken in the middle of the light phase. B, Time course of cwINV activity in source leaves. C, Enzyme activities of SUSY, ATP-dependent PFK, AGPase, and G6PDH in source leaves (mean values over whole day). Samples were taken from control leaves. Data are means ± se of at least three individual plants at each time point. Black, SNN; gray, SNN∷cwINV. An asterisk indicates a significant difference (t test, * P <0.05, ** P <0.01, *** P <0.001).
Table I.
CwINV activity and content of apoplastic carbohydrates in source leaves of wild type (SNN) and transgenic line (SNN∷cwINV)
Results represent mean and se during the light phase of at least three individual plants at each time point. An asterisk indicates a significant difference (t test, * P <0.05, ** P <0.01, *** P <0.001) between SNN and SNN∷cwINV plants.
| cwINV | Apoplastic Carbohydrates
|
Suc Efflux | ||
|---|---|---|---|---|
| Hexoses | Suc | |||
| nkat m−2 | μmol m−2 | μmol m−2 h−1 | ||
| *** | *** | ** | * | |
| SNN | 179 ± 53 | 128 ± 14 | 85 ± 14 | 149 ± 18 |
| SNN∷cwINV | 16 ± 4 | 86 ± 22 | 61 ± 15 | 105 ± 17 |
CwINV Repression Affects Primary Metabolism
Photosynthesis was not affected, whereas the dark respiration was slightly increased in source leaves of the SNN∷cwINV plants (Table II). The activities of cytosolic and vacuolar invertase were not altered due to the repression of the cwINV (Table II), whereas Suc synthase (SUSY), the reversible Suc cleaving enzyme in the cytosol, and ATP-dependent phosphofructokinase (PFK), a key enzyme of the glycolysis, were significantly reduced (Fig. 1C). The G6PDH, important for the OPPP, was only slightly and not significantly depressed, whereas ADP-Glc pyrophosphorylase (AGPase), one key enzyme of governing starch synthesis, was significantly diminished by about 60% compared to the wild type (Fig. 1C).
Table II.
Activities of vacuolar/cytosolic invertases, contents of vacuolar/cytosolic carbohydrates, respiration, and photosynthesis in source leaves (mean values over the whole day) of wild type (SNN) and transgenic line (SNN∷cwINV)
Results represent mean and se during the light phase of at least three individual plants at each time point. An asterisk indicates a significant difference (t test, * P <0.05, ** P <0.01, *** P <0.001) between SNN and SNN∷cwINV plants.
| Invertase
|
Cytosolic/Vacuolar Carbohydrates
|
Respiration | Photosynthesis | ||||
|---|---|---|---|---|---|---|---|
| Cytosolic | Vacuolar | Hexoses | Suc | Starch | |||
| nkat m−2 | μmol m−2 | μmol m−2 s−1 | μmol e m−2 s−1 | ||||
| * | |||||||
| SNN | 189 ± 48.2 | 1,924 ± 367 | 1,030 ± 252 | 473 ± 61 | 2,203 ± 1,322 | 1.01 ± 0.04 | 122 ± 3.1 |
| SNN∷cwINV | 183 ± 14.9 | 1,908 ± 346 | 832 ± 213 | 359 ± 58 | 1,828 ± 1,167 | 1.15 ± 0.30 | 123 ± 3.5 |
Although activities of some enzymes of the primary metabolism were significantly reduced, the content of cytosolic/vacuolar carbohydrates was not significantly lower in source leaves of the transgenic plants (Table II). This correlated with the absence of phenotypic effects (e.g. size and quantity of roots, leaves, and seeds) in SNN∷cwINV plants under normal growing conditions (data not shown).
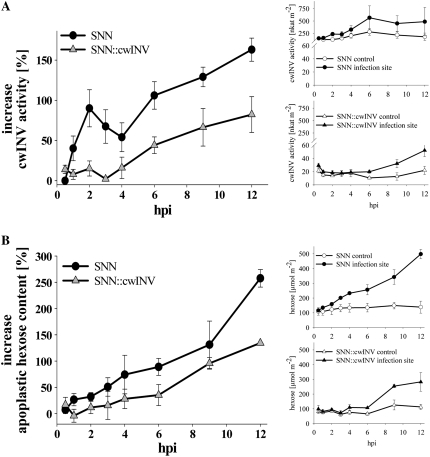
Defense-Induced Increase in CwINV Activity and Apoplastic Hexose Content Are Impaired in SNN∷cwINV
In wild-type source leaves, the activity of cwINV increased in a biphasic manner immediately after infection with P. nicotianae (Fig. 2A); a first maximum at 2 hpi was followed by a secondary increase (from 6 hpi on) of more than 150% compared to the noninfected control (Fig. 2A). This early increase in cwINV activity was completely absent in SNN∷cwINV plants. A second increase occurred but was much weaker, not even reaching the absolute cwINV activity level of noninfected wild-type tissue (Fig. 2A, inset).
Figure 2.
Defense-induced increase in cwINV activity and apoplastic carbohydrate content was impaired in SNN∷cwINV source leaves. A, CwINV activity at the infection site. B, Content of apoplastic hexoses (Glc, Fru) at the infection site. All data points taken from noninfected parts of the plants in each individual experiment and each point along the time scale of an experiment are set as 0%. At least three independent infections are averaged and their means are presented as percentage changes ± se (circles, SNN; triangles, SNN∷cwINV). Insets show the means of the absolute amount of activities and contents (white symbols, control; black symbols, infection site).
In the wild type, the content of apoplastic hexoses slightly increased during the first cwINV activity peak. At later stages of infection, it raised parallel to the secondary cwINV activity increase (Fig. 2B). In source leaves of SNN∷cwINV, the apoplastic hexose content remained almost constant during the first hpi. It just rose at 9 hpi but never reached the absolute amount found in infected wild-type leaves (Fig. 2B, inset).
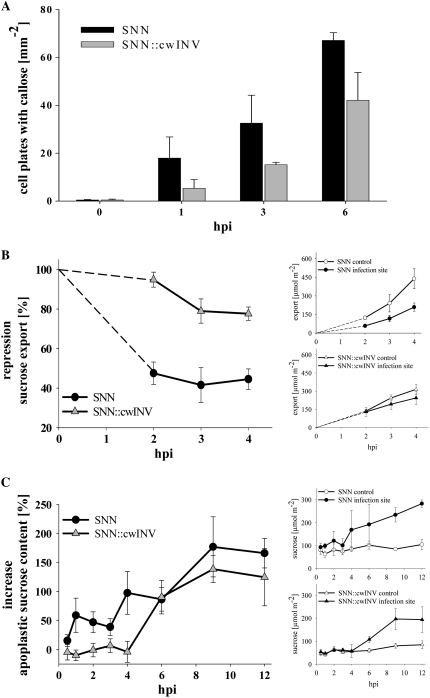
Defense-Induced Callose Deposition, Reduction of Suc Export, and Increase in Apoplastic Suc Content Are Decelerated in SNN∷cwINV
The formation of callose could be observed at the infection site of the resistant wild type as well as in plants with cwINV deficiency. But the frequency of callose depositions was clearly reduced in the transgenic line (Fig. 3A). Initially, callose distinctively appeared at plasmodesmata of cell-to-cell contacts of spongy mesophyll cells. During the following hours callose spread over the whole cell wall of infected cells (Scharte et al., 2005). The callose formation proceeded over the course of infection in both lines (Fig. 3A) but never occurred in the particular controls (data not shown).
Figure 3.
Defense-induced callose deposition, repression of Suc export, and increase in Suc content were impaired in SNN∷cwINV source leaves. A, Defense-induced callose deposition at cell plates in infected mesophyll of at least three independent infections ± se (black bars, SNN; gray bars, SNN∷cwINV). B, Suc export via the petiole. The Suc efflux from 0 to 2 hpi was extrapolated (dotted line). C, Content of apoplastic Suc at the infection site. All data points taken from noninfected parts of the plants in each individual experiment and each point along the time scale of an experiment are set as 100% for Suc efflux and 0% for Suc content. At least three independent infections are averaged and their means are presented as percentage changes ± se (circles, SNN; triangles, SNN∷cwINV). Insets show the means of the absolute amount of activities and contents (white symbols, control; black symbols, infection site).
From the first hpi on, the export of Suc through the petiole in infected source leaves decreased in the wild type of more than 50% compared to noninfected control leaves (Fig. 3B). Simultaneously, the content of apoplastic Suc increased at the infection site (Fig. 3C).
In SNN∷cwINV plants, the Suc export rate remained relatively stable during the first 2 hpi followed by a slow decline (Fig. 3B). Accordingly, the content of apoplastic Suc remained constant. At about 4 hpi, it increased, but the absolute content was reduced compared to the infected wild type (Fig. 3C, inset).
Impaired Defense Reactions, Hypersensitive Cell Death, and Disease Symptoms Occur in SNN∷cwINV
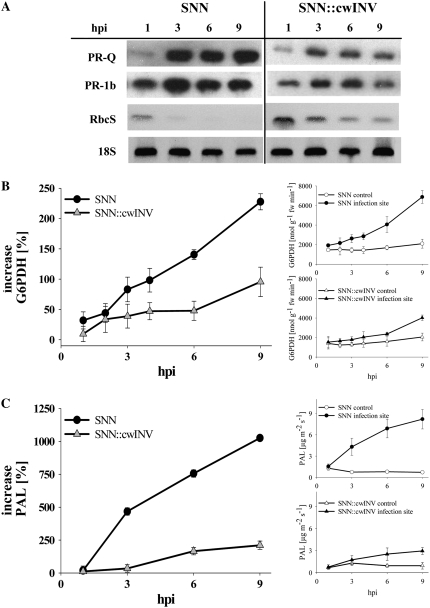
Transcripts of the acidic, salicylate-inducible protein PR-1b (Herbers et al., 1996, 2000) accumulated at the infection site in the wild type from an early stage of infection on, whereas the expression of PR-1b was weaker and only slightly induced in cwINV-deficient plants (Fig. 4A). A similar expression profile (Fig. 4A) was observed for the sugar-inducible PR-Q (Herbers et al., 2000). In the noninfected controls of both plants, neither PR-1b nor PR-Q transcripts could be detected (data not shown). Transcripts of the small nuclear-encoded subunit of Rubisco (RbcS), whose gene expression is known to be sugar mediated (e.g. Rolland et al., 2006; Seo et al., 2007), completely disappeared from 3 hpi on in the resistant wild type but not in the SNN∷cwINV plants (Fig. 4A). Here, only a slight decrease in transcript abundance was observed during the course of infection. Noninfected controls of both plants showed diurnal variations in the RbcS expression (data not shown) as was previously shown for source leaves of tobacco by Scharte et al. (2005).
Figure 4.
Defense-induced increase in the expression of PR proteins and G6PDH/PAL activity were delayed and alleviated in SNN∷cwINV source leaves. A, Representative northern blots of RbcS, PR-1b, and PR-Q from infection sites. Probing with an 18S cDNA served as loading control, and similar results were obtained with at least three individual plants at each time point. B and C, G6PDH (B) and PAL (C) activities at the infection site. All data points taken from noninfected parts of the plants in each individual experiment and each point along the time scale of an experiment are set as 0%. At least three independent infections are averaged and their means are presented as percentage changes ± se (circles, SNN; triangles, SNN∷cwINV). Insets show the means of the absolute amount of activities and contents (white symbols, control; black symbols, infection site).
Activities of G6PDH and Phe ammonia-lyase (PAL), key enzymes of the OPPP and shikimic pathways, exhibited a steep rise in the wild type and only moderately increased in SNN∷cwINV plants (Fig. 4, B and C).
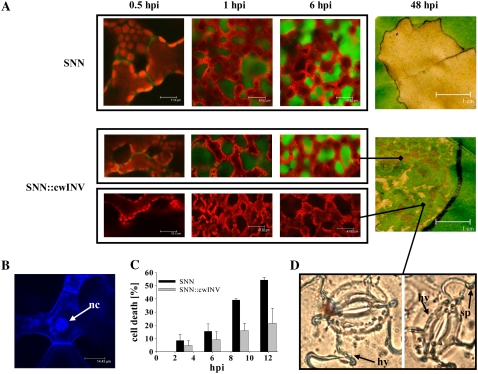
Hydrogen peroxide (H2O2) release was examined by confocal laser scanning microscopy (cLSM) probing the ROS-dependent oxidation of the nonfluorescent 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), to the highly fluorescent 2′,7′-dichlorofluorescein (DCF). The fluorescent signal has been demonstrated to be proportional to the production of ROS (Rat et al., 1997). In wild-type plants, H2O2 accumulation already appeared in the apoplast during the first hpi and than further increased (Fig. 5A). Due to the infiltration of the dye into the leaf tissue, H2O2 is washed out and the DCF-fluorescence appeared also in the intercellular space. In SNN∷cwINV plants, the accumulation of ROS was heterogeneously distributed throughout the inoculation area; some parts of the infected tissue reacted like the resistant wild type, while other mesophyll areas did not produce any ROS, even at late infection stages (Fig. 5A). Similarly, macroscopic visible hypersensitive lesions developed heterogeneously and in a patchy manner (Fig. 5A).
Figure 5.
Impaired formation of H2O2 and hypersensitive lesions/cell death and disease symptoms in SNN∷cwINV source leaves. A, Representative images of H2O2 release in mesophyll cells at the infection site (cLSM pictures: DCF fluorescence, green; chlorophyll fluorescence, red) and macroscopic visible hypersensitive lesions. Similar results were obtained in at least three individual plants at each time point. B, Intense staining of nucleus (nc) by propidium iodide (blue fluorescence) and extensive cell shrinkage in a representative mesophyll cell undergoing hypersensitive cell death. C, Cell death at the infection site. Data are means ± se of at least six individual plants at each time point. Black bars, SNN; gray bars, SNN∷cwINV. D, Representative lactophenol trypan blue stainings of hyphae (hy) with growing sporangiophores (sp) emerging from the stomata in the epidermis of a source leaf of SNN∷cwINV 6 d after infection.
Cell death was observed by cLSM using propidium iodide. Intense staining of nuclei by propidium iodide and extensive cell shrinkage (Fig. 5B) followed the release of H2O2. Between 9 and 12 hpi, nearly 50% of all spongy mesophyll cells at the infection site of the wild type were dead compared to 20% to 25% averaged over the infection site in SNN∷cwINV plants (Fig. 5C). From 24 hpi on, hypersensitive lesions covered the whole infection area in wild-type leaves, indicating that all cells had undergone hypersensitive cell death. In contrast, the infection site of SNN∷cwINV leaves exhibited only small spots of hypersensitive lesions (Fig. 5A).
The rapidly induced hypersensitive cell death in wild-type leaves after infection with P. nicotianae limited the rate and extent of pathogen infection indicated by the absence of hyphal growth (not shown). By contrast, in SNN∷cwINV plants, hyphal growth and growing sporangiophores could be detected using lactophenol trypan blue staining (Fig. 5D) in those parts of the infected leave tissue where hypersensitive cell death had not occurred.
DISCUSSION
This study focuses on the impact of cwINV on a successful establishment of plant defense in leaves. Therefore, it is most important that the cwINV activity of the transgenic tobacco plants (SNN∷cwINV) is drastically reduced (about 90% in source and 50% in sink leaves; Fig. 1A; Table I) irrespective of which particular cwINV isoforms are repressed. It is also relevant that the activities of other intracellular invertases, vacuolar and cytosolic, are not affected (Table II).
CwINVs are encoded by a multigene family with a highly differential tissue-specific expression, mainly in the phloem conductive tissues (Sturm, 1999). Fully sequenced genomes indicate nine putative cwINVs from rice (Oryza sativa; Ji et al., 2005) and six from Arabidopsis (Arabidopsis thaliana; Sherson et al., 2003), though recent results suggest that Arabidopsis AtcwINV3 and AtcwINV6 are fructan exohydrolases rather than invertases (De Coninck et al., 2005). Similar cwINV families are also reported for poplar (Populus spp.), maize (Zea mays), and potato (Solanum tuberosum; for review, see Huang et al., 2007). In tomato, cwINVs are encoded by a gene family compromising four tissue-specific members (LIN5, LIN6, LIN7, and LIN8; Godt and Roitsch, 1997; Fridman and Zamir, 2003).
The RNAi construct used in this study was originally designed to silence LIN8 and LIN6 in tomato (N. Kocal and S. Sonnewald, unpublished data). Both were shown to be expressed in leaves and other tissues upon stress-related stimuli and under conditions that require a high carbohydrate supply (Godt and Roitsch, 1997; Fridman and Zamir, 2003). Assuming that the construct selectively represses homologous isoforms, we cannot exclude that the residual cwINV activity is due to the expression of different isoforms. For tobacco, about seven cwINV isoforms (T. Roitsch, personal communication) with tissue- and developmental-specific expression patterns are postulated, but no sufficient sequence information was available until now. We cannot exclude the possibility that a strong expression of cwINV transcripts in tobacco, homolog to LIN8, could overcome the repression by the RNAi construct. For example, particularly strong expression signals are expected in sink leaves (e.g. Sturm, 1999; Fig. 1A).
Despite the reduced cwINV activity, plant growth and development of the transgenic plants seem to be normal under standard growing conditions. The relatively high residual cwINV activity in the sink leaves may enable the normal development of the transgenic plants. However, cwINV repression leads to significant alterations in the carbohydrate metabolism of source leaves. The activities of SUSY, PFK, and G6PDH were moderately reduced (Fig. 1C). SUSY is the key enzyme of symplastic Suc unloading (for review, see Koch, 2004) and its activity, in concert with invertases, can determine the sink capacity of plant tissues (Nguyen-Quoc and Foyer, 2001). The products of the SUSY reaction (Fru, UDP-Glc) feed respiration and the synthesis of cell wall polymers such as cellulose and callose (Koch, 2004).
The most significant change in source leaves of SNN∷cwINV was a 60% decline in the activity of AGPase. Despite this drastic repression in enzyme activity, the steady-state level of starch was only slightly diminished (Table II). Similar observations were made in Solanaceae-antisense mRNA transformants, where the starch level is little affected over a wide range and decreases only when the AGPase activity is repressed by more than 50% of the wild-type level. The levels of soluble sugars are also largely unaffected by the AGPase repression (Müller-Röber et al., 1992; Leidreiter et al., 1995; Geigenberger et al., 1999). Possibly the contribution of AGPase to the control of starch synthesis is low, and other enzymes or pathways, e.g. starch phosphorylase and phosphoglucomutase, also influence the rate of starch accumulation (for review, see Zeeman et al., 2007). Posttranslational redox activation of AGPase in response to cytosolic sugars is part of a signaling mechanism linking the rate of starch synthesis to the availability of carbon in diverse plant tissues (Geigenberger et al., 2005). In this sense, the AGPase activity might be a sensitive indicator for reduced carbon availability in the mesophyll of SNN∷cwINV plants. On the other hand, our data do not show a significant decline in the steady-state levels of leaf carbohydrates (Table II). Moderate restriction in the carbon availability by repressed cwINV activity does not necessarily have to lead to a drastic decline in the steady-state sugar levels. Significant differences in carbohydrate levels between wild-type and SNN∷cwINV leaves, however, were difficult to demonstrate, as even in wild-type plants sugar levels were highly fluctuant between individual plants (not shown). Moderate restriction of carbon availability by cwINV repression may not harm the regular leaf functions in nonstressed plants, but could diminish their metabolic flexibility and capability to cope with unusual metabolic demands that occur, e.g., during plant-pathogen interactions.
The activity of cwINV in source leaves of resistant wild-type tobacco rapidly increased in a biphasic manner during an incompatible interaction with P. nicotianae. Elicitor studies showed that the increased invertase activity in the apoplast is of plant origin (Scharte et al., 2005). We assign the initial transient activity peak (1–4 hpi) to the induction of preexisting but mostly inactivated invertase in the cell wall. The observed defense-induced blockage of sugar export in tobacco source leaves actually leads to Suc accumulation in the apoplast (Fig. 3). The cwINV activation state could be controlled in a Suc-dependent manner by an inhibitor protein (Weil et al., 1994). Also, a transient cell wall acidification occurs in SNN source leaves postinfection with P. nicotianae (data not shown). It is well known that the activity of cwINV depends on pH-value (Goetz and Roitsch, 1999). It is reasonable to assume that preexisting cwINV in source leaves of tobacco is activated and/or enhanced by the combined action of Suc and cell wall acidification during defense. The secondary increase in cwINV activity is accompanied by the appearance of cwINV transcripts and seems to be related to de-novo synthesis of cwINV (Scharte et al., 2005). Increased cwINV activity will lead to reallocation of photosynthetically generated sugars to infected mesophyll cells by the uptake of hexoses after the cleavage of apoplastic Suc. Here, the retained carbohydrates can fuel defense-related processes and generate sugar-sensing signals.
Major carbohydrate-consuming reactions are the generation of callose and ROS, immediately elicited after infection (Figs. 3A and 5A). The defense-induced callose deposition at cell-to-cell interfaces closes Suc export routes in tobacco leaves (Scharte et al., 2005). Tobacco possesses relatively few plasmodesmatal connections and is expected to use an apoplastic route of phloem loading (Kingston-Smith et al., 1998). The defense-induced callose-dependent export blockage (Fig. 3, A and B), in conjunction with the enhanced cwINV activity (Fig. 2A), causes the sharp decline in apoplastic and symplastic sugar export (see also Scharte et al., 2005). This supports the reallocation and retention of carbohydrates in the infected cells and is a prerequisite for ROS generation and the formation of hypersensitive lesions.
The release of ROS (Fig. 5A) is an additional carbohydrate-consuming reaction during plant defense. It is assumed that ROS generation by a plasmalemmal NADPH-oxidase is important for the initiation of HR. Depletion of NADPH by the oxidase reaction in turn activates the cytosolic G6PDH reaction (and subsequently the OPPP), which generates NADPH (Pugin et al., 1997) and thereby consumes carbohydrates.
In addition to its function as metabolic fuel, reflux of sugars affects gene expression via sugar sensing. Hexokinases are known to sense soluble hexoses and initiate sugar-sensitive signaling pathways (Rolland et al., 2006). Here, sugar-sensitive gene expression is actually indicated by the induction of PR-Q and PR-1b and the decline in the RbcS after infection (Fig. 4A). Recent results show that hexokinase also regulates the execution of programmed cell death in plant cells (Kim et al., 2006), suggesting a direct link between carbohydrate metabolism and cell death mediated via sugar sensing.
In infected SNN∷cwINV leaves, both HR (ROS generation, lesion formation) and defense-related alterations in the carbohydrate status were diminished or decelerated. Apoplastic sugar levels remained low, and callose deposition and the inhibition of Suc export were delayed (Figs. 2 and 3). It is reasonable to assume that low sugar supply and reduced SUSY activity restrict callose synthesis and are responsible for the delay of defense-induced callose deposition. Other enzymes, which also could provide carbohydrates, do not compensate the reduced carbohydrate availability due to cwINV repression. For example, there was no enhanced activity of vacuolar and cytosolic invertases detectable in SNN∷cwINV plants (Table II). The insufficient carbohydrate supply could also restrict ROS generation (by depletion of the cytosolic G6PDH reaction; see above) and, thus, the execution of programmed cell death in plants (Fig. 5). Additionally, weak changes in PR-Q, PR-1b, and RbcS expression in the transgenic line compared to wild-type leaves (Fig. 4A) actually indicate absent or insufficient sugar-sensing signals most likely due to the weaker induction of cwINV activity. As a result of all these diminished or decelerated defense-induced reactions, source leaves of SNN∷cwINV are less tolerant to P. nicotianae, indicated by hyphal growth and growing sporangiophores in those cells where hypersensitive cell death did not occur (Fig. 5D). In compatible interactions, successful colonization of the host typically culminates in sporulation with the development of asexual sporangia on the plant surface or sexual oospores within the host tissues (Hardham, 2007). The majority of spores produced by most Phytophthora species are asexual and develop at the termini of specialized hyphae called sporangiophores bearing several multinucleate spores, which are called sporangia (or zoosporangia), as they can release zoospores (Judelson and Blanco, 2005).
Summarizing, we propose that in leaves of SNN∷cwINV plants, the carbohydrate supply required for a successful plant defense is reduced to low, sometimes critical levels. In this sense, the patchy appearance or complete absence of HR/cell death could be explained by an inhomogeneous distribution of carbohydrate allocation within the leaf. Sugar gradients occur, for example, between mesophyll cells adjacent to veins and more distant cells (for review, see Tomos and Sharrock, 2001). In some leaf areas of SNN∷cwINV, the carbohydrate supply drops below a critical limit where the execution of HR fails. In wild-type tobacco, the defense-related increase in cwINV activity and the homogenous establishment of a high-sugar state at the infection site would compensate for any unequal sugar allocation within the leaf.
In plants, mechanisms have evolved to modulate carbohydrate levels in response to infections and to use carbohydrates as signals to initiate as well as to fuel defense reactions. Our results strongly support the hypothesis that insufficient carbohydrate supply can indeed delay, hinder, or even completely suppress hypersensitive cell death. Furthermore, the cwINV plays a key role in the acquisition of carbohydrates during plant defense. We show that in the case of the photosynthetically active apoplastic loader, tobacco cwINV acts as enzymatic resistance gene, as was described for genes encoding photorespiratory enzymes (Taler et al., 2004). Transgenic plants with differential suppression of cwINV activity could be helpful to analyze the threshold of cwINV activity required for the acquisition of carbohydrates during defense.
It is possible that the significance of cwINV activity for defense is a special case of photosynthetically active, apoplastic loaders only. During the night when the sugar export rate is low (e.g. Gibon et al., 2004), carbohydrates could be provided by the mobilization of internal starch storages, while the blockage of the apoplastic pathway is less important for the acquisition of carbohydrates during defense. It will be of interest in future studies to investigate whether cwINV is of any significance for successful plant defense in cases where the apoplastic system is not involved in the allocation of carbohydrates.
MATERIALS AND METHODS
RNAi-Plasmid Construction and Tobacco Transformation
The Gateway technology (Invitrogen) was used to generate an RNAi construct. Because we aimed at silencing closely related isoforms LIN8 (accession no. AF506007) and LIN6 (accession no. AF506005), primers were designed from the highly homologous sequence region of both sequences (bp 755–1,162 according to LIN8 sequence). In this region, both sequences share 81.9% sequence identity, which is thought to be sufficient to silence both isoforms by a single construct (Le et al., 2006; Chen et al., 2008). A 400-bp cDNA fragment of LIN8 was amplified by PCR using the primers LIN8-5′ 5′-caccggtactggaaattggg-3′ and LIN8-3′ 5′-acaggccattgaaccaattg-3′ (nt 755–1,162 of LIN8), and tomato (Solanum lycopersicum) leaf cDNA as a template. The resulting PCR product was subcloned into pENTR/D (Invitrogen) to create an entry clone containing the attL-recombination sites. Subsequently, the lambda reconstruction reaction was performed to introduce the fragment into the destination vector pK7GWIWG2 (Karimi et al., 2002) containing the attR-attachment sites. This procedure was performed according to the manufacturer's instructions, yielding the final construct designated as LIN8-RNAi. The construct was transformed into Agrobacterium tumefaciens strain CV58C1 carrying the pGV2260 virulence plasmid (Deblaere et al., 1985). Tobacco (Nicotiana tabacum) SNN plants were transformed by Agrobacterium-mediated gene transfer as described by Rosahl et al. (1987).
Selection of Tobacco Transformants
Homozygous lines of SNN∷cwINV were selected by kanamycin resistance and invertase activity levels in the progeny of self-pollinated primary transformants. Thirty-five kanamycin-resistant transformants were screened for reduction in cwINV level by activity assays, compared to the wild type (data not shown), and then vegetatively multiplied in tissue culture. Seeds (T2 generation) resulting from self-pollination of the T1-transformants with the lowest cwINV activities were scored for kanamycin resistance on Murashige and Skoog medium containing 100 μg mL−1 kanamycin. Some of these chosen lines were not even fertile or the seeds did not germinate. T2 seed populations expressing the lowest levels of cwINV activity and exhibited 3:1 (resistant:susceptible) segregation for the kanamycin marker were chosen for further analysis. Vital lines homozygous for the transgene were then identified by allowing 10 kanamycin-resistant T2 progeny to self-pollinate and set seed and by screening for plants whose seeds (T3 generation) were 100% kanamycin resistant.
Plant Growth and Characterization
Seeds were germinated on Murashige and Skoog agar plates supplemented with 100 μg mL−1 kanamycin in the case of transformants. After 3 weeks, seedlings were transferred to soil. Soil-cultured plants (SNN and SNN∷cwINV) were grown in a growth chamber at 24°C/22°C day/night temperature and a 14-h photoperiod (400 μmol quanta m−2 s−1). Eight- to 10-week-old homozygous transformants of the T3 generation and their wild-type siblings were exposed to the pathogen as described below.
Oomycete Growth, Zoospores Production, and Inoculation
Phytophthora nicotianae van Breda de Haan isolate 1828 (DSMZ) was cultivated at 24°C on clarified tomato agar as described by von Broembsen and Deacon (1996). Zoospores were produced under aseptic conditions according to von Broembsen and Deacon (1996). Finally, the zoospores were stored in sterile tap water. Source leaves from 8- to 10-week-old tobacco plants were infiltrated with a suspension containing 500 to 1,000 zoospores μL−1 according to Colas et al. (2001). We have chosen this zoospore-leaf infiltration assay to achieve a rapid, synchronized start of infection of all parenchymatic cells in the infiltrated area. For mock-inoculation, we infiltrated sterile tap water, further named as control.
To consider individual plant or developmental variations, the samples for control and infection site were taken from adjacent intercostal areas of the same source leaf, except for the measurements of the Suc efflux. Inoculation of the plants was always performed at the beginning of the photoperiod.
Total RNA Extraction and Northern-Blot Hybridization
Total RNA was extracted according to Logemann et al. (1987). RNA (7 μg) was subjected to electrophoresis in a 1% agarose-formaldehyde gel, capillary transferred, and linked onto Hybond N+ membrane (Amersham Biosciences Europe). Probes were labeled with biotin by PCR according to the method described by Löw and Rausch (1996). Hybridization was performed in a reaction mixture containing 50% (v/v) formamide, 1% (w/v) SDS, 1 m NaCl, 6% (w/v) polyethylene glycol 6000, and 250 μg mL−1 salmon sperm DNA at 42°C. Membranes were washed with 0.2× SSC/0.5% (w/v) SDS at 65°C, developed as described by Löw and Rausch (1996) and exposed to Hyperfilm ECL (Amersham Biosciences Europe).
Microscopic Detection of H2O2, Callose, Cell Death, and Disease Symptoms
To detect H2O2, the tissue was incubated for 10 min in 10 μm H2DCF-DA (Invitrogen). H2DCF-DA is a nonfluorescent cell-permanent dye that after oxidation with H2O2 becomes the fluorescent compound DCF. The fluorescent signal of DCF (excitation, 488 nm; emission, 535 nm) is proportional to ROS production (Rat et al., 1997). The fluorescence of DCF and chlorophyll were detected upon excitation with 488 nm at 500 to 535 nm and 670 to 710 nm, respectively, using a cLSM (TCS SP2 with inverse DMIRB-microscope, Leica).
Callose was detected with aniline blue as described by Gómez-Gómez et al. (1999). Stained sections were viewed using a fluorescence microscope (DMRBE, Leica) equipped with a filter set (340- to 380-nm excitation filter, 400-nm dichroic mirror, 425-nm barrier filter) and cell plates with callose deposition were counted.
Cell death studies were performed with 0.5 mg mL−1 propidium iodide. Cells with disrupted membranes allow propidium iodide to enter the cell and fluoresce, indicating cell death. The fluorescence is detected at 590 to 650 nm after excitation at 488 nm using a cLSM (TCS SP2 with inverse DMIRB-microscope, Leica).
To monitor hyphal growth, plant leaves and epidermal stripes were stained with lactophenol trypan blue as described previously (Takemoto et al., 2003). Leaves were cleared in Farmer's fluid (3:1 ethanol:acetic acid) for more than 24 h and boiled for 3 min in lactophenol trypan blue stain (10 mL of water, 10 mL of lactic acid, 10 mL of glycerol, 10 g of phenol, 10 mg of trypan blue). After cooling to room temperature, the stain was replaced and decolorized with 1 g mL−1 chloral hydrate. Stained probes were viewed using a DMRBE microscope (Leica).
Sample Preparation for Carbohydrate and Invertase Measurements
For measurements of cwINV activity and apoplastic carbohydrate content, preparation of the apoplastic fluid was carried out as described by Lohaus et al. (2001) with the following modifications: plant tissues were infiltrated with 1 m NaCl, the surface was dried, and the leaf discs were centrifuged at 1,000g for 10 min at 4°C in a 25-mL syringe barrel placed in a centrifuge tube.
To assay vacuolar and cytosolic invertase activity, plant material was homogenized in liquid nitrogen and resuspended in homogenization buffer (50 mm Tris, pH 6.9, 5 mm MgCl2, 15% [v/v] glycerol, 1 mm EDTA, 1 mm EGTA, 5 mm dithiothreitol, and 0.1 mm phenylmethylsulfonyl fluoride). The homogenate was centrifuged for 10 min at 10,000g and 4°C. The supernatant was removed and used for invertase activity measurements.
For measurements of cytosolic/vacuolar carbohydrates, leaf pieces were ground in liquid nitrogen and homogenized with 3 m HClO4 after the extraction of the apoplastic fluid. The homogenate was centrifuged for 5 min at 10,000g at 4°C. The supernatant was neutralized with 3 m KHCO3 to pH 7.0. For starch determination, the pellet was washed with distilled water, resuspended in 5 m KOH, and incubated at 55°C overnight, followed by hydrolysis via amyloglucosidase in 7% (v/v) acetic acid, pH 4.6, for 3 h at 55°C.
Determination of Enzyme Activities, Carbohydrate Content, and Suc Efflux
Invertase activity was measured in a medium containing the respective extract, 100 mm Suc and either 50 mm sodium acetate at pH 4.9 or pH 4.6 for cwINV and vacuolar invertase or 50 mm HEPES-NaOH, pH 7.5, for alkaline invertase. The amount of liberated hexoses was measured according to the method of Kunst et al. (1984) after incubation for 60 min at 37°C and inactivation after alkalization with 0.1 m Tris-HCl pH 8.0 and heat inactivation for 5 min at 95°C.
Carbohydrates in the apoplastic fluid or the perchloric extract were measured according to the method of Kunst et al. (1984).
The measurements of Suc efflux from petioles were performed according to Murillo et al. (2003) with the following modifications: to determine the Suc efflux, two source leaves (control and infection) from the same plant were cut from the base of their petiole 1 h after inoculation of the whole leaf with zoospores or water. Then the leaves were immersed immediately in 10 mm sodium phosphate buffer, pH 7.0, containing 20 mm EDTA to prevent callose formation. The effluxing sugars were collected in a growth chamber with almost 100% humidity to prevent transpiration. Every hour, an aliquot was removed and the soluble sugars were determined enzymatically as described above. The first aliquot was taken after 1 h (=2 hpi) and the Suc efflux from 0 to 2 hpi was extrapolated.
Phe ammonia-lyase was determined according to Rongrong et al. (2006). Extraction of SUSY, ATP-dependent PFK, G6PDH, and AGPase were performed according to Gibon et al. (2004). The assays were prepared in 96-well microplates using a robot-based platform to measure multiple enzymes using a set of cycling assays at the Max-Planck Institute of Molecular Plant Physiology, Golm, Germany.
Determination of Respiration and Photosynthesis
Photosynthetic electron transport and respiration measured as CO2 production in the dark were derived from a chlorophyll-a fluorescence-imaging system connected with a gas exchange system as described in Scharte et al. (2005). Attached leaves were placed in a sandwich-type gas exchange cuvette, and the gas exchange was measured with a two-channel gas flow system. Gas flow rate through the chamber was 2,000 mL min−1, the leaf temperature was maintained at 22°C to 24°C, and the relative humidity at 55% to 57%. CO2 and O2 concentrations were adjusted using gas flow controllers (Tylan General).
Analysis and Statistics
Due to the high variability of the carbohydrate contents from plant to plant and over the day (well known from other studies e.g. Herbers et al., 2000; Swarbrick et al., 2006), we presented both absolute and percentage values of defense-induced changes. Percentage values were not calculated from the mean of the absolute values (insets). All data points taken from noninfected parts of the plants, in each individual experiment and each point along the time scale of an experiment, was set as 0% and 100% for Suc efflux, respectively. At least three independent infections were averaged, and their means were presented as percentage changes including ses.
Differences described as significant were analyzed using the t test algorithm incorporated into Microsoft Excel (v9.0; Microsoft) that yielded a value below 5% (P < 0.05).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF506005 and AF506007.
Acknowledgments
We thank Yves Gibon and Phillip Bones for helpful assistance with some enzyme measurements, Karin Topp for technical assistance, Antje von Schaewen and Silvia Haferkamp for critical discussion, and Uwe Sonnewald and Thomas Rausch for providing RbcS, PR-Q, PR-1b, and invertase cDNAs, respectively.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Judith Scharte (jschart@uni-muenster.de).
Open Access articles can be viewed online without a subscription.
References
- Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant pathogen interactions. J Exp Bot 58 4019–4026 [DOI] [PubMed] [Google Scholar]
- Biemelt S, Sonnewald U (2006) Plant-microbe interactions to probe regulation of plant carbon metabolism. J Plant Physiol 163 307–318 [DOI] [PubMed] [Google Scholar]
- Chen S, Hajirezaei MR, Zanor MI, Hornyik C, Debast S, Lacomme C, Fernie AR, Sonnewald U, Börnke F (2008) RNA interference-mediated repression of Suc-phosphatase in transgenic potato tubers (Solanum tuberosum) strongly affects the hexose-to-Suc ratio upon cold storage with only minor effects on total soluble carbohydrate accumulation. Plant Cell Environ 31 165–176 [DOI] [PubMed] [Google Scholar]
- Colas V, Conrod S, Venard P, Keller H, Ricci P, Panabieres F (2001) Elicitin genes expressed in vitro by certain tobacco isolates of Phytophthora parasitica are down regulated during compatible interactions. Mol Plant Microbe Interact 14 326–335 [DOI] [PubMed] [Google Scholar]
- Conrath U, Linke C, Jeblick W, Geigenberger P, Quick WP, Neuhaus HE (2003) Enhanced resistance to Phytophthora infestans and Alternaria solani in leaves and tubers, respectively, of potato plants with decreased activity of the plastidic ATP/ADP transporter. Planta 217 75–83 [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, DeGreve H, Beboeck F, Schell J, Van Montagu M, Leemanns J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium mediated gene transfer to plants. Nucleic Acids Res 13 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coninck B, Le Roy K, Francis I, Clerens S, Vergauwen R, Halliday AM, Smith SM, Van Laere A, Van den Ende W (2005) Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant Cell Environ 28 432–443 [Google Scholar]
- Ehness R, Ecker M, Godt D, Roitsch T (1997) Glucose and stress independently regulate source/sink relations and defence mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin DC, Ribeiro OK (1996) Phytophthora Diseases Worldwide. APS Press, St. Paul
- Fridman D, Zamir D (2003) Functional divergence of a syntenic invertase gene family in tomato, potato, and Arabidopsis. Plant Physiol 131 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant Microbe Interact 19 711–724 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Kolbe A, Tiessen A (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56 1469–1479 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Müller-Röber B, Stitt M (1999) Contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta 209 338–345 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39 847–862 [DOI] [PubMed] [Google Scholar]
- Godt DE, Roitsch T (1997) Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 115 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Roitsch T (1999) The different pH-optima and substrate specificities of extracellular and vacuolar invertases are determined by a single amino acid substitution. Plant J 20 707–711 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18 277–284 [DOI] [PubMed] [Google Scholar]
- Greiner S, Weil M, Krausgrill S, Rausch T (1995) A tobacco cDNA coding for cell-wall invertase. Plant Physiol 108 825–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham AR (2007) Cell biology of plant-oomycete interactions. Cell Microbiol 9 1–39 [DOI] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer W, Metraux J, Sonnewald U (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Takahata Y, Melzer M, Mock HP, Hajirezaei M, Sonnewald U (2000) Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol Plant Pathol 1 51–59 [DOI] [PubMed] [Google Scholar]
- Horsfall J (1975) The story of a nonconformist. Annu Rev Phytopathol 13 1–13 [DOI] [PubMed] [Google Scholar]
- Horsfall J, Dimond A (1957) Interactions of tissue sugar, growth substances and disease susceptibility. Z Pflanzenkr Pflanzenschutz 64 415–421 [Google Scholar]
- Huang LF, Bocock PN, Davis JM, Koch KE (2007) Regulation of invertase: a ‘suite’ of transcriptional and post-transcriptional mechanisms. Funct Plant Biol 34 499–507 [DOI] [PubMed] [Google Scholar]
- Ji X, Van den Ende W, Van Laere A, Cheng S, Benett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60 615–634 [DOI] [PubMed] [Google Scholar]
- Judelson HS, Blanco FA (2005) The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol 3 47–58 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Kim M, Lim JH, Ahn CS, Park K, Kim GT, Kim WT, Pai HS (2006) Mitochondria-associated hexokinases play a role in the control of programmed cell death in Nicotiana benthamiana. Plant Cell 18 2341–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston-Smith AH, Galtier N, Pollock CJ, Foyer CH (1998) Soluble acid invertase activity in leaves is independent of species differences in leaf carbohydrates, diurnal sugar profiles and paths of phloem loading. New Phytol 139 283–292 [Google Scholar]
- Koch K (2004) Suc metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235–246 [DOI] [PubMed] [Google Scholar]
- Kunst A, Draeger B, Ziegenhorn J (1984) D-Glc. In HU Bergmeyer, J Bergmeyer, M Graßl, eds, Methods of Enzymatic Analysis, Ed 3, Vol 4. Verlag Chemie, Weinheim, Germany, pp 1469–1473
- Le QL, Lorenz Y, Scheurer S, Fötisch K, Enrique E, Bartra J, Biemelt S, Vieths S, Sonnewald U (2006) Design of tomato fruits with reduced allergenicity by dsRNAi-mediated inhibition of ns-LTP (Lyc e 3) expression. Plant Biotechnol J 4 231–242 [DOI] [PubMed] [Google Scholar]
- Leidreiter K, Heineke D, Heldt HW, Müller-Röber B, Sonnewald U, Willmitzer L (1995) Leaf-specific antisense inhibition of starch biosynthesis in transgenic potato plants leads to an increase in photoassimilate export from source leaves during the light period. Plant Cell Physiol 36 615–624 [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163 16–20 [DOI] [PubMed] [Google Scholar]
- Lohaus G, Pennewiss K, Sattelmacher B, Hussmann M, Muehling K (2001) Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol Plant 111 457–465 [DOI] [PubMed] [Google Scholar]
- Löw R, Rausch T (1996) Detection of nucleic acids with biotinylated PCR-amplified probes. In T Meier, F Fahrenholz, eds, A Laboratory Guide to Biotinin-Labelling in Biomolecule Analysis. Birkhäuser Verlag, Basel, 201–213
- Maor R, Shirasu K (2005) The arms race continues: battle strategies between plants and fungal pathogens. Curr Opin Microbiol 8 399–404 [DOI] [PubMed] [Google Scholar]
- Müller-Röber BT, Sonnewald U, Willmitzer L (1992) Inhibition of ADP-Glc pyrophosphorylase leads to sugar storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11 1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo I, Roca R, Bortolotti C, Segundo BS (2003) Engineering photoassimilate partitioning in tobacco plants improves growth and productivity and provides pathogen resistance. Plant J 36 330–341 [DOI] [PubMed] [Google Scholar]
- Nguyen-Quoc B, Foyer CH (2001) A role for ‘futile cycles’ involving invertase and Suc synthase in Suc metabolism of tomato fruit. J Exp Bot 52 881–889 [DOI] [PubMed] [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J (1997) Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell 9 2077–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat P, Osseni R, Christen MO, Thevenin M, Warnet J-M, Adolphe M (1997) Microtitration fluorimetric assays on living cells (MiFALC tests): new tools for screening in cell pharmacotoxicology. In LFM Van Zutphen, M Balls, eds, Animal Alternatives, Welfare and Ethics. Elsevier, Paris, pp 813–825
- Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2 198–206 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54 513–524 [DOI] [PubMed] [Google Scholar]
- Roitsch T, González MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9 606–613 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 675–709 [DOI] [PubMed] [Google Scholar]
- Rongrong L, Shaohua X, Jialin L, Yuanlei H, Zhongping L (2006) Expression profile of a PAL gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Rep 25 705–710 [DOI] [PubMed] [Google Scholar]
- Rosahl S, Schmidt R, Schell J, Willmitzer L (1987) Expression of a tuber specific storage protein in transgenic tobacco plants: demonstration of an esterase activity. EMBO J 6 1155–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharte J, Schön H, Weis E (2005) Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant Cell Environ 28 1421–1435 [Google Scholar]
- Schweizer P, Pokorny J, Schulze-Lefert P, Dudler R (2000) Technical advance. Double-stranded RNA interferes with gene function at the single-cell level in cereals. Plant J 24 895–903 [DOI] [PubMed] [Google Scholar]
- Seo YS, Cho JI, Lee SK, Ryu HS, Han M, Hahn TR, Sonnewald U, Jeon JS (2007) Current insights into the primary carbon metabolic flux that occurs in plants undergoing a defense response. Plant Stress 1 42–49 [Google Scholar]
- Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54 525–531 [DOI] [PubMed] [Google Scholar]
- Sturm A (1999) Invertases. Primary structures, functions, and roles in plant development and Suc partitioning. Plant Physiol 121 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick PJ, Schulze-Lefert P, Scholes JD (2006) Metabolic consequences of susceptibility and resistance (race specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ 29 1061–1076 [DOI] [PubMed] [Google Scholar]
- Takemoto D, Hardham AR (2004) The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol 136 3864–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33 775–792 [DOI] [PubMed] [Google Scholar]
- Taler D, Galperin M, Benjamin I, Cohen Y, Kenigsbuch D (2004) Plant eR genes encoding for photorespiratory enzymes confer resistance against disease. Plant Cell 16 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomos AD, Sharrock RA (2001) Cell sampling and analysis (SiCSA): metabolites measured at single cell resolution. J Exp Bot 52 623–630 [PubMed] [Google Scholar]
- Voegele RT, Wirsel SGR, Möll U, Lechner M, Mendgen K (2006) Cloning and characterization of a novel invertase from the obligate biotroph “Uromyces fabae” and analysis of expression patterns of host and pathogen invertases in the course of infection. Mol Plant Microbe Interact 19 625–634 [DOI] [PubMed] [Google Scholar]
- von Broembsen SL, Deacon JW (1996) Germination and further zoospore release from zoospore cysts of Phytophthora parasitica. Mycol Res 100 1498–1504 [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Willmitzer L (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate, inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M, Krausgrill S, Schuster A, Rausch T (1994) A 17-kDa Nicotiana tabacum cell-wall peptide acts as an in vitro inhibitor of the cell-wall isoform of acid invertase. Planta 193 438–445 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM (2007) The diurnal metabolism of leaf starch. Biochem J 401 13–28 [DOI] [PubMed] [Google Scholar]