The fundamental discoveries of Darwin and Mendel established the scientific basis for plant breeding and genetics at the turn of the 20th century. Similarly, the recent integration of advances in biotechnology, genomic research, and molecular marker applications with conventional plant breeding practices has created the foundation for molecular plant breeding, an interdisciplinary science that is revolutionizing 21st century crop improvement. Though the methods of molecular plant breeding continue to evolve and are a topic of intense interest among plant breeders and crop scientists (for review, see Cooper et al., 2004; Nelson et al., 2004; Lörz and Wenzel, 2005; Varshney et al., 2006; Eathington et al., 2007; Mumm, 2007), they have received relatively little attention from the majority of plant biologists engaged in basic scientific research. The objective of this article for an Editor's Choice series on future advances in crop biotechnology is to briefly review important historical developments in molecular plant breeding, key principles influencing the current practice of molecular plant breeding, and factors that influence the adoption of molecular plant breeding in crop improvement programs. Furthermore, we emphasize how the application of molecular plant breeding is now contributing to discoveries of genes and their functions that open new avenues for basic plant biology research.
HISTORICAL DEVELOPMENT OF MOLECULAR PLANT BREEDING
Plant breeding describes methods for the creation, selection, and fixation of superior plant phenotypes in the development of improved cultivars suited to needs of farmers and consumers. Primary goals of plant breeding with agricultural and horticultural crops have typically aimed at improved yields, nutritional qualities, and other traits of commercial value. The plant breeding paradigm has been enormously successful on a global scale, with such examples as the development of hybrid maize (Zea mays; Duvick, 2001), the introduction of wheat (Triticum aestivum) and rice (Oryza sativa) varieties that spawned the Green Revolution (Everson and Golin, 2003), and the recent commercialization of transgenic crops (James, 2007). These and many other products of plant breeding have contributed to the numerous benefits global society has received from greater sustainable supplies of carbon that may be harvested as food, feed, forests, fiber, and fuel.
Plant breeding has a long history of integrating the latest innovations in biology and genetics to enhance crop improvement. Prehistoric selection for visible phenotypes that facilitated harvest and increased productivity led to the domestication of the first crop varieties (Harlan, 1992) and can be considered the earliest examples of biotechnology. Darwin outlined the scientific principles of hybridization and selection, and Mendel defined the fundamental association between genotype and phenotype, discoveries that enabled a scientific approach to plant breeding at the beginning of the 20th century (e.g. Shull, 1909). Despite the immediate recognition among some plant breeders of the importance of Mendelian genetics, full integration was delayed for nearly 20 years until quantitative genetics reconciled Mendelian principles with the continuous variation observed for most traits considered important by most plant breeders (Paul and Kimmelman, 1988). Subsequent advances in our understanding of plant biology, the analysis and induction of genetic variation, cytogenetics, quantitative genetics, molecular biology, biotechnology, and, most recently, genomics have been successively applied to further increase the scientific base and its application to the plant breeding process (e.g. Baenziger et al., 2006; Jauhar, 2006; Varshney et al., 2006).
The plant biotechnology era began in the early 1980s with the landmark reports of producing transgenic plants using Agrobacterium (Bevan et al., 1983; Fraley et al., 1983; Herrera-Estrella et al., 1983). Molecular marker systems for crop plants were developed soon thereafter to create high-resolution genetic maps and exploit genetic linkage between markers and important crop traits (Edwards et al., 1987; Paterson et al., 1988). By 1996, the commercialization of transgenic crops demonstrated the successful integration of biotechnology into plant breeding and crop improvement programs (Koziel et al., 1993; Delannay et al., 1995). As depicted in Figure 1, introgression of one or a few genes into a current elite cultivar via backcrossing is a common plant breeding practice. Methods for marker-assisted backcrossing were developed rapidly for the introgression of transgenic traits and reduction of linkage drag, where molecular markers were used in genome scans to select those individuals that contained both the transgene and the greatest proportion of favorable alleles from the recurrent parent genome (e.g. Ragot et al., 1995; Johnson and Mumm, 1996). During the past 25 years, the continued development and application of plant biotechnology, molecular markers, and genomics has established new tools for the creation, analysis, and manipulation of genetic variation and the development of improved cultivars (for review, see Sharma et al., 2002; Varshney et al., 2006; Collard and Mackill, 2008). Molecular breeding is currently standard practice in many crops, with the following sections briefly reviewing how molecular information and genetic engineering positively impacts the plant breeding paradigm.
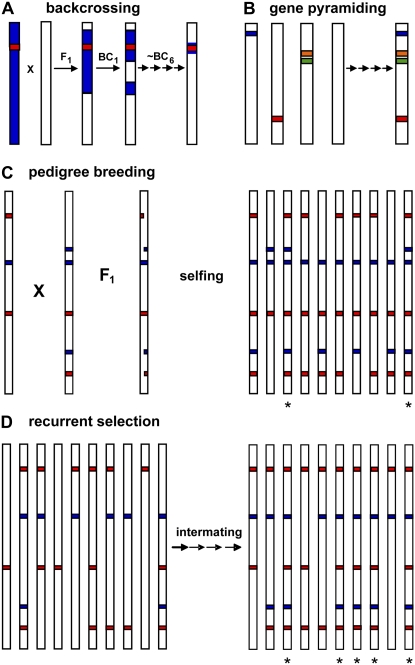
Figure 1.
Common breeding and selection schemes. Each vertical bar is a graphical representation of the genome for an individual within a breeding population, with colored segments indicating genes and/or QTLs that influence traits under selection. Genes associated with different traits are shown in different colors (e.g. red, blue). “X” indicates a cross between parents, and arrows depict successive crosses of the same type. Asterisk below an individual signifies a desirable genotype. A, Backcrossing. A donor line (blue bar) featuring a specific gene of interest (red) is crossed to an elite line targeted for improvement (white bar), with progeny repeatedly backcrossed to the elite line. Each backcross cycle involves selection for the gene of interest and recovery of increased proportion of elite line genome. B, Gene pyramiding. Genes/QTLs associated with different beneficial traits (blue, red, orange, green) are combined into the same genotype via crossing and selection. C, Pedigree breeding. Two individuals with desirable and complementary phenotypes are crossed; F1 progeny are self-pollinated to fix new, improved genotype combinations. D, Recurrent selection. A population of individuals (10 in this example) segregate for two traits (red, blue), each of which is influenced by two major favorable QTLs. Intermating among individuals and selection for desirable phenotypes/genotypes increases the frequencies of favorable alleles at each locus. For this example, no individual in the initial population had all of the favorable alleles, but after recurrent selection half of the population possesses the desired genotype. For hybridized crops, recurrent selection can be performed in parallel within two complementary populations to derive lines that are then crossed to form hybrids; this method is called reciprocal recurrent selection.
PRINCIPLES AND PRACTICES OF MOLECULAR PLANT BREEDING
Breeding Schemes and the Genetic Gain Concept
Conceptually, plant breeding is simple: cross the best parents, and identify and recover progeny that outperform the parents. In practice, plant breeding is a three step process, wherein populations or germplasm collections with useful genetic variation are created or assembled, individuals with superior phenotypes are identified, and improved cultivars are developed from selected individuals. A wide diversity of approaches, tailored to the crop species and breeding objectives, have been developed for improving cultivars (Fehr, 1987; Stoskopf et al., 1993). These breeding methods feature different types of populations, selection procedures, and outcomes.
Figure 1 summarizes the three breeding methods that are commonly employed in crop improvement programs. As mentioned previously, when the goal is to upgrade an established elite genotype with trait(s) controlled by one or a few loci, backcrossing is used either to introgress a single gene (Fig. 1A) or to pyramid a few genes (Fig. 1B). For genetically complex traits, germplasm improvement instead requires reshuffling of the genome to produce new favorable gene combinations in the progeny. The pedigree breeding method produces such novelty via crossing and recombination among superior, yet complementary, parents and selection among segregating progeny for improved performance (Fig. 1C). Recurrent selection aims to simultaneously increase the frequencies of favorable alleles at multiple loci in breeding populations through intermating of selected individuals (Fig. 1D). For hybridized crops such as maize, recurrent selection may be extended to improve the performance of distinct complementary populations (e.g. heterotic groups) that are used as parents to form superior hybrid combinations. This practice is referred to as reciprocal recurrent selection.
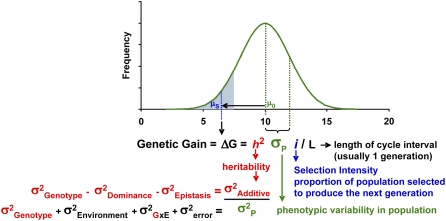
Quantitative genetic principles have been particularly powerful as the theoretical basis for both population improvement and methods of selecting and stabilizing desirable genotypes (Hallauer, 2007). An important concept in quantitative genetics and plant breeding is genetic gain (ΔG), which is the predicted change in the mean value of a trait within a population that occurs with selection. Regardless of species, the trait of interest, or the breeding methods employed, ΔG serves as a simple universal expression for expected genetic improvement (Fehr, 1987; Falconer and Mackay, 1996). Figure 2 shows the genetic gain equation and an expansion of its terms to fundamental parameters of quantitative genetics. Though clearly an oversimplification of the advanced quantitative genetic principles employed in plant breeding, the genetic gain equation effectively relates the four core factors that influence breeding progress: the degree of phenotypic variation present in the population (represented by its sd, σP), the probability that a trait phenotype will be transmitted from parent to offspring (heritability, h2), the proportion of the population selected as parents for the next generation (selection intensity, i, expressed in units of sd from the mean), and the length of time necessary to complete a cycle of selection (L). L is not only a function of how many generations are required to complete a selection cycle, but also how quickly the generations can be completed and how many generations can be completed per year.
Figure 2.
The genetic gain equation and its component variables. The top portion illustrates an idealized distribution showing the frequency of individuals within a breeding population (y axis) that exhibit various classes of phenotypic values (x axis). Mean phenotypic value (μ0) of the original population (shown as entire area under the normal curve) and mean (μS) for the group of selected individuals (shaded in blue) are indicated. In this generalized example, trait improvement is achieved by selecting for a lower phenotypic value, e.g. grain moisture at harvest in maize. Components of variation (σ2) that contribute to the sd of the phenotypic distribution (σP) are indicated below the histogram.
It is clear that ΔG can be enhanced by increasing σP, h2, or i, and by decreasing L. Thus, the genetic gain equation provides a framework for comparing the predicted effectiveness of particular breeding strategies and is often used as a guide to the judicious allocation of resources for achieving breeding objectives. When considered in the context of the genetic gain concept, molecular plant breeding offers powerful new approaches to overcome previous limitations in maximizing ΔG. The following sections cite examples where molecular plant breeding positively impacts ΔG and each of its component variables. For brevity, we focus on examples from maize where molecular breeding is most advanced, and has now become the primary means to develop improved commercial hybrids.
Molecular Plant Breeding Expands Useful Genetic Diversity for Crop Improvement
The maximum potential for genetic gain is proportional to the phenotypic variation (σP) present in the original source population and maintained in subsequent cycles of selection. Phenotypic variation is positively associated with genetic diversity, yet also depends on environmental factors and the interactions between genotype and environment. Genetic diversity may be derived from breeding populations (either naturally occurring or synthetic), segregating progeny from a cross of selected parental lines, exotic materials that are not adapted to the target environment, wide interspecific crosses, naturally occurring or induced mutations, the introduction of transgenic events, or combinations of these sources.
However, not all phenotypic variation is equal. For example, the use of exotic germplasm has been extremely successful for improving many crop species, but difficulties may be encountered through the introduction of undesirable alleles associated with lack of adaptation. The need for genetic diversity must be balanced by elite performance, because choosing the best parents is key to maximizing the probability for successful improvement. In contrast, the expected increase in linkage disequilibrium among elite populations derived from intense prior selection may also limit the creation of new genetic combinations for future gain. Intermating source populations for genetic recombination may overcome this problem, but delays cultivar development.
Molecular markers and more recently, high-throughput genome sequencing efforts, have dramatically increased knowledge of and ability to characterize genetic diversity in the germplasm pool for essentially any crop species. Using maize as one example, surveys of molecular marker alleles and nucleotide sequence variation have provided basic information about genetic diversity before and after domestication from its wild ancestor teosinte, among geographically distributed landraces, and within historically elite germplasm (for review, see Cooper et al., 2004; Niebur et al., 2004; Buckler et al., 2006). This information enriches investigations of plant evolution and comparative genomics, contributes to our understanding of population structure, provides empirical measures of genetic responses to selection, and also serves to identify and maintain reservoirs of genetic variability for future mining of beneficial alleles (McCouch, 2004; Slade et al., 2005). In addition, knowledge of genetic relationships among germplasm sources may guide choice of parents for production of hybrids or improved populations (e.g. Dudley et al., 1992; Collard and Mackill, 2008).
While molecular markers and other genomic applications have been highly successful in characterizing existing genetic variation within species, plant biotechnology generates new genetic diversity that often extends beyond species boundaries (Gepts, 2002; Johnson and McCuddin, 2008). Biotechnology enables access to genes heretofore not available through crossing and creates an essentially infinite pool of novel genetic variation. Genes may be acquired from existing genomes spanning all kingdoms of life, or designed and assembled de novo in the laboratory. Both subtle and extreme examples of the power of transgenes to introduce novel phenotypic variation can be found in the three different transgenes developed for resistance to glyphosate herbicides in maize and other crops. The first glyphosate-tolerant maize hybrids used a modified version of the endogenous maize gene encoding 5-enol-pyruvylshikimate-3-P synthase (Spencer et al., 2000), which was followed later by events produced with a 5-enol-pyruvylshikimate-3-P synthase gene isolated from Agrobacterium (Behr et al., 2004). More recently, a synthetic gene with enhanced glyphosate acetyltransferase activity was created via gene shuffling and selection in a microbial system (Castle et al., 2004). Each of these glyphosate-tolerant maize events also illustrates another benefit of biotechnology, where new combinations of regulatory sequences (e.g. the cauliflower mosaic virus 35S and rice actin1 promoters) may be used to achieve optimal trait expression with respect to overall activity and tissue distribution relative to what might be possible with endogenous genes (Heck et al., 2005).
Molecular Plant Breeding Increases Favorable Gene Action
Quantitative genetics uses the theoretical concept of heritability to quantify the proportion of phenotypic variation that is controlled by genotype. In practice, heritability is greatly influenced by the genetic architecture of the trait of interest, which is described by the number of genes, the magnitude of their effects, and the type of gene action associated with phenotypes. Better knowledge of genetic architecture and favorable gene action (that which is more amenable to selection) often has the greatest impact on improving genetic gain. For the genetic gain formula, heritability (h2) is used in its narrow sense, representing the proportion of phenotypic variation due to additive genetic effects (those that reflect changes in allele dosage or allelic substitutions). Additive genetic effects are also referred to as the breeding value because they are predictably transmitted to progeny. Deviations from additive effects are significant for many traits, and are partitioned into either dominance effects that reflect the interactions between different alleles at the same locus or epistatic effects resulting from interactions among different loci. Gene action and breeding values are characterized by progeny testing, where the phenotypes of individuals in a population are compared to their parents and siblings produced from either self-pollination or outcrossing.
Previous efforts to develop large numbers of molecular markers, high density genetic maps, and appropriately structured mapping populations have now made routine for many crop species the ability to simultaneously define gene action and breeding value at hundreds and often thousands of loci distributed relatively uniformly across entire genomes. The results from such mapping studies provide greatly improved estimates for the number of loci, allelic effects, and gene action controlling traits of interest. More importantly, genomic segments can be readily identified that show statistically significant associations with quantitative traits (quantitative trait loci [QTLs]). In addition to genetic mapping in families derived from biparental crosses, new advances in association genetics with candidate genes and approaches that combine linkage disequilibrium analysis in families and populations (Holland, 2007; Yu et al., 2008) further enhance power for QTL discovery.
Information about QTLs can be used in a number of ways to increase heritability and favorable gene action. For traits exhibiting low to moderate heritability, such as grain yield, QTLs, and their associated molecular markers often account for a greater proportion of the additive genetic effects than the phenotype alone. Furthermore, knowledge of genetic architecture can be exploited to add or delete specific alleles that contribute to breeding value. When either genetic linkage or epistasis among loci with antagonistic effects on a trait limits genetic gain, QTL information can be used to break these undesirable allelic relationships.
Success in using information about QTLs to increase genetic gain depends greatly on the magnitude of QTL effects, precise estimation of QTL positions, stability of QTL effects across multiple environments, and whether QTLs are robust across relevant breeding germplasm. Prediction of QTL positions is enhanced by further fine mapping, which facilitates testing QTL effects and breeding values in additional populations. When the density of observed recombinations approaches the resolution of single genes, the causal genetic change for a QTL can be determined (for review, see Salvi and Tuberosa, 2005; Yu and Buckler, 2006; Beló et al., 2008; Harjes et al., 2008). Molecular isolation of QTLs permits the development of perfect or functional molecular markers at the potential resolution of the fundamental unit of inheritance, the nucleotide, and dramatically increases the specificity and precision by which genetic effects are estimated and manipulated in breeding programs.
The use of transgenes can further simplify the genetic architecture for desirable traits, in ways that may be superior to or not possible even when perfect markers are available for robust QTLs of large effect. Transgenes typically condition strong genetic effects at operationally single loci, which also exhibit dominant gene action where only one copy of the event is needed for maximal trait expression in a hybrid cultivar. These features of transgenes can reduce complex quantitative improvement to a straightforward, often dramatic, solution. Excellent examples are provided by the expression in transgenic corn hybrids of insecticidal toxin proteins from Bacillus thuringiensis (Bt) to reduce feeding damage by larvae of the European corn borer (Ostrinia nubilalis) or the corn rootworm beetle (Diabrotica spp.). Partial resistance in maize germplasm to these insect pests had been previously characterized as quantitatively inherited traits with low heritability (Papst et al., 2004; Tollefson, 2007), but the Bt transgenic events offer a simply inherited alternative that is efficiently manipulated in breeding programs.
By simplifying genetic architecture, transgenes may also permit disruption of allelic interactions between factors controlling the trait of interest and other important performance characteristics. For example, employing a transgenic source of insect resistance (e.g. a single locus Bt transgene) may facilitate selection for favorable alleles for yield improvement that are tightly linked in repulsion with endogenous genes for resistance to the same class of insect pests. In addition, transgenic events may be engineered to uncouple negative pleiotropic effects from beneficial phenotypes conditioned by recessive mutations. This application is illustrated by the use of RNA interference to specifically down-regulate zein seed storage protein gene expression (Segal et al., 2003; Huang et al., 2004). This strategy mimics the effects of the opaque2 mutation on improving the amino acid profile of maize grain for animal feed, while circumventing the softer endosperm texture and susceptibility to fungal pathogens typically associated with opaque2.
Transgenic events can also be designed to intervene at key regulatory steps for entire metabolic or developmental pathways, such that gene action for the corresponding traits are largely inherited as single dominant factors that are less sensitive to environmental effects. Examples include the expression of a transcription factor that increases drought tolerance (Nelson et al., 2007), and altering the balance between levels of the GLOSSY15 transcription factor relative to its repressor, microRNA172, to delay flowering time in maize hybrids (Lauter et al., 2005).
Biotechnology also facilitates the molecular stacking of transgenes that control a trait or suite of traits into a single locus haplotype defined by a transgenic event. Examples of such an approach include the initial Golden Rice (Ye et al., 2000), recently released YieldGuard VT Triple transgenic maize hybrids where herbicide tolerance and multiple insect resistance traits are integrated as one genomic locus (http://www.yieldgardvt.com/VTScience/Default.aspx), or the combination of transgenes that simultaneously increase synthesis and decrease catabolism of Lys in maize seeds (Frizzi et al., 2008). Recent reports of improvements in gene targeting technology (Ow, 2007) and the construction of meiotically transmissible plant minichromosomes (Carlson et al., 2007; Yu et al., 2007) pave the way for introducing more traits with increasing complexity. With such advances, biotechnology is now poised to assemble useful genetic diversity from essentially any source into constructs that concentrate favorable gene action and maximize heritability for a greatly expanded set of traits.
In closing this section about how molecular plant breeding increases favorable gene action, it is important to emphasize that QTL studies, when conducted with appropriate scale and precision to identify causal genes, represent a powerful functional genomics approach. The molecular cloning of QTLs has yielded novel insights about the biology of quantitative traits that were not likely to be discovered from the analysis of gene knockouts or overexpression strategies, in particular the impacts of regulatory variation on phenotypic variation and evolution (e.g. Cong et al., 2002; Clark et al., 2006; Yan et al., 2004; Salvi et al., 2007). Furthermore, molecular markers, genomics, and biotechnology are now applied in an iterative network to exploit genetic diversity for crop improvement. Genomic information enables the discovery of beneficial alleles via QTL mapping and cloning, followed by the use of information learned from the molecular characterization of QTLs to design optimal transgenic strategies for crop improvement.
Molecular Plant Breeding Increases the Efficiency of Selection
Conventional plant breeding that relies only on phenotypic selection has been historically effective. However, for some traits, phenotypic selection has made little progress due to challenges in measuring phenotypes or identifying individuals with the highest breeding value. The effects of environment, genotype by environment interaction, and measurement errors also contribute to observed differences. Evaluation of genotypes in multiple environments with replicated designs allows better estimation of breeding values, but requires additional time and expense. For some traits, it may be necessary to sacrifice the individual to measure phenotypes, or trait expression may depend on variable environmental conditions (e.g. disease pressure) and the stage of development (e.g. grain quality can only be assessed after flowering). Furthermore, plant breeders typically must simultaneously improve a suite of commercially valuable traits, which may limit gains from selection. Just as molecular plant breeding helps to expand genetic diversity, characterize genetic architecture, and modify gene action, its methods can also be applied to increasing the efficiency of selection.
An extensive body of literature has considered the utility of molecular marker-assisted selection and its fit with different breeding methods (Fig. 1), with the reader being referred to a number of recent excellent reviews on this topic (Dekkers and Hospital, 2002; Holland, 2004; Johnson, 2004; Varshney et al., 2006; Collard and Mackill, 2008). Molecular marker genotypes that are either within genes or tightly linked to QTL influencing traits under selection can be employed as a supplement to phenotypic observations in a selection index (Lande and Thompson, 1990). In cases where genetic correlations are high, further efficiencies can be gained by substituting genotypic for phenotypic selection during some selection cycles, which can reduce phenotyping efforts and cycle times by permitting the use of off-season nurseries. Johnson (2004) summarized an early example of combining phenotypic data and molecular marker scores to increase selection gains for maize grain yield and resistance to European corn borer. An effective strategy to simultaneously modify multiple traits is the use of selection indices that consider multiple factors in choosing the final improved genotype. Eathington et al. (2007) recently reported on results obtained from the use of multiple trait indices and marker-assisted selection for nearly 250 unique corn breeding populations. Use of molecular markers increased breeding efficiency approximately 2-fold relative to phenotypic selection alone, with similar gains also observed in soybean (Glycine max) and sunflower (Helianthus annuus) populations.
Marker-assisted selection can also significantly enhance genetic gain for traits where the phenotype is difficult to evaluate because of its expense or its dependence on specific environmental conditions. Molecular markers may be used to increase the probability of identifying truly superior genotypes, by focusing testing resources on genotypes with the greatest potential (i.e. early elimination of inferior genotypes), by decreasing the number of progeny that must be screened to recover a given level of gain, and by enabling simultaneous improvement for traits that are negatively correlated (Knapp, 1998). Successful examples include resistance to soybean cyst nematode (Young, 1999), resistance to cereal diseases (for review, see Varshney et al., 2006), and drought tolerance in maize (Ribaut and Ragot, 2007; Tuberosa et al., 2007).
The efficiency of phenotypic selection for some complex traits can be enhanced by including physiological or biochemical phenotypes as secondary traits, if these exhibit strong genetic correlations with the target trait and possess high heritability. Recent advances in functional genomics permit the population-scale profiling of RNA abundance, protein levels and activities, and metabolites that are associated with important traits. In addition to molecular markers that tag DNA sequence variation, such genetical genomics approaches may provide additional secondary phenotypes as selection targets (Jansen and Nap, 2001; Johnson, 2004), particularly for traits defined by responses to environmental, developmental, or physiological cues.
Marker-assisted selection also accelerates the deployment of transgenes in commercial cultivars. Typically, this has been achieved through marker-assisted backcrossing. However, for future biotechnology improvements such as tolerance to drought or nutrient limitation, forward breeding may be required to cooptimize transgene expression and genetic background because endogenous genes and environmental factors may have the potential to influence the phenotypes resulting from transgenic modifications (Mumm, 2007). Of course, use of molecular markers could aid forward breeding efforts as well. Alternatively, discovery efforts for additional genes or QTLs that are necessary for dependable trait performance may suggest design of new transgene constructs that stack primary transgenes with known genetic modifiers into second-generation transgenic events.
INCREASING ADOPTION OF MOLECULAR PLANT BREEDING
The adoption of molecular plant breeding approaches has occurred at different rates among crop species and institutions engaged in crop improvement, due to the combined influence of scientific, economic, and sociological factors. Important early scientific barriers included the recalcitrance of cereal crop species to Agrobacterium-mediated transformation and lack of knowledge about genetic control of traits already defined as important breeding targets. Continued research and technology development has largely overcome obstacles for plant transformation of nearly all important crop and horticultural species (Wenzel, 2006). Similarly, information gained from genomics research in plant species and other organisms has generated a wealth of information about gene structure and function, as well as large numbers of molecular markers for use in plant breeding. Despite these resources, genetic specificity of robust QTLs remains elusive, unless breeding programs and associated information management systems are restructured to fully integrate knowledge of pedigrees, phenotypes, and marker genotypes that can be leveraged to optimize response to selection (Cooper et al., 2004; Eathington et al., 2007). Even with such integration, modifying regulatory functions remains a scientific challenge for molecular breeding because it is difficult to determine the sequence basis for regulatory changes and to predict their phenotypic effects (Morgante and Salamini, 2003).
Once enabling technologies in biotechnology and genomics become available, economic factors often dictate the degree to which these innovations are integrated into existing plant breeding programs. The expense of gaining governmental regulatory approval for commercial release of transgenic varieties (recently estimated at $7–$10 million by Kalaitzandonakes et al., 2007) is a significant economic barrier. The costs associated with the development, establishment, and operation of molecular plant breeding are greater than conventional plant breeding practices (Koebner and Summers, 2003; Morris et al., 2003), requiring significant investments in new research infrastructure and intellectual capacity. Such resources initially existed only in private agribusiness firms and a handful of larger public institutions, further accelerating an ongoing trend for increased industrialization of plant breeding programs among major crops such as corn, soybeans, cotton (Gossypium hirsutum), and wheat (Johnson, 2007). Where there has been adoption in companies, the balance may favor biotechnology over QTLs for improving complex traits, despite greater product development costs, because transgenics can be designed to produce stronger, even dramatic, phenotypic effects, and can often be more rapidly deployed across a broader range of germplasm, resulting in new solutions sooner.
Though molecular breeding is now considered an essential component of current crop improvement efforts for major crops by large companies, the broad applicability of modern molecular approaches to conventional plant breeding remains a source of debate among some practicing plant breeders in the public sector, particularly for minor crops (e.g. Gepts, 2002; Goodman, 2004). In addition to the valid scientific and economic factors that have delayed or prevented adoption of molecular approaches in meeting some plant breeding objectives, there are at least three additional reasons contributing to this view. First, molecular plant breeding requires training and expertise in both molecular biology and plant breeding. Educational efforts that delivered such interdisciplinary training were initially established in the early 1990s, but still remain limited to a relatively small group of academic institutions with historic strengths in plant breeding (Guner and Wehner, 2003; Gepts and Hancock, 2006; Guimarães and Kueneman, 2006). A second reason for reduced enthusiasm to embrace biotechnology among some plant breeders is the problems with acceptance of transgenic crops among certain governments and groups of consumers, as exemplified by the shelving of wheat varieties with transgenes for resistance to glyphosate herbicides (Sokstad, 2004). Finally, excitement about the potential of molecular plant breeding also stimulated shifts in funding at public institutions to enhance intellectual capacity and infrastructure for molecular genetics and genomics research, which ironically often occurred at the expense of conventional plant breeding (Knight, 2003). This emphasis may have been temporarily necessary to establish the foundations for 21st century plant biology, but there is currently a growing recognition that increased investment in plant breeding capacity and translational research linking molecular methods with breeding objectives is necessary to fully realize the potential of recent advances in biotechnology and genomics (Guimarães and Kueneman, 2006; National Research Council, 2008).
CONCLUSION
The above review emphasizes that despite recent advances and successful examples of molecular plant breeding, one of the current grand challenges in plant biology remains identifying those gene combinations that lead to significant crop improvement. This commentary closes by suggesting that the most effective approach to accelerate such efforts is to better integrate the different research disciplines and activities that form core components of molecular plant breeding. As illustrated here and described by others previously (e.g. Gepts and Hancock, 2006; Bliss, 2007), this integration requires knowledge of the genomic organization and function of genes, a solid foundation in statistical approaches to estimate genetic effects, strong background in plant biology, experience with both the laboratory methods of molecular biology/functional genomics and field-based breeding practices, and the ability to manage large datasets with diverse data types. Though an awareness and appreciation for each of these disciplines is recognized as important by all plant scientists, student education and training efforts, funding streams, and research programs still typically emphasize specific subsets of the molecular breeding paradigm. This is likely justified given the breadth of the disciplines involved, but greater efforts are needed to implement research and education programs that promote active participation in molecular breeding for crop improvement. Such programs will most likely be developed by fostering collaborations groups with complementary expertise. Funding agencies must absolutely expand their portfolio of projects that support translational efforts by requiring proposals to integrate basic research endeavors with plant improvement outcomes. Existing examples that deserve emulation include the HarvestPlus program (http://www.harvestplus.org/), MASwheat (Dubcovsky, 2004), and U.S. Department of Agriculture Coordinated Agricultural Projects. The private sector must also continue investments that stimulate integration and provide the appropriate training environment for future scientists entering the molecular breeding workforce. In addition to direct support for graduate training and sponsored research, companies can often provide in-kind support that helps bridge the expanding technology gap between public and private sector research in molecular plant breeding. With the collective efforts of the broad community of scientists committed to plant biology and crop improvement, molecular plant breeding will further expand its contributions and impacts to meeting global needs for sustainable increases in agricultural productivity.
This work was supported by the National Science Foundation (grant no. NSF–PGRP–0501700) and the U.S. Department of Agriculture (award no. 2007–35100–18335).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Stephen P. Moose (smoose@uiuc.edu).
References
- Baenziger PS, Russell WK, Graef GL, Campbell BT (2006) Improving lives: 50 years of crop breeding, genetics, and cytology (C-1). Crop Sci 46 2230–2244 [Google Scholar]
- Behr CF, Heck GR, Hironaka CH, You J, inventors (2004) Corn plants comprising event PV-ZMGT32 (NK603). U.S. Patent No. 6,825,400
- Beló A, Zhen P, Luck S, Shen B, Meyer DJ, Li B, Tingey S, Rafalski A (2008) Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol Genet Genomics 279 1–10 [DOI] [PubMed] [Google Scholar]
- Bevan MW, Flavell RB, Chilton MD (1983) A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304 184–187 [PubMed] [Google Scholar]
- Bliss FA (2007) Education and preparation of plant breeders for careers in global crop improvement. Crop Sci 47 S250–S261 [Google Scholar]
- Buckler ES, Gaut BS, McMullen MD (2006) Molecular and functional diversity of maize. Curr Opin Plant Biol 9 172–176 [DOI] [PubMed] [Google Scholar]
- Carlson SR, Rudgers GW, Zieler H, Mach JM, Luo S, Grunden E, Krol C, Copenhaver GP, Preuss D (2007) Meiotic transmission of an in vitro-assembled autonomous maize minichromosome. PLoS Genet 3 1965–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle LA, Siehl DL, Gorton R, Patten PA, Chen YH, Bertain S, Cho HJ, Duck N, Wong J, Kiu D, et al (2004) Discovery and directed evolution of a glyphosate tolerance gene. Science 304 1151–1154 [DOI] [PubMed] [Google Scholar]
- Clark RM, Wagner TN, Quijada P, Doebley J (2006) A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescence architecture. Nat Genet 38 594–597 [DOI] [PubMed] [Google Scholar]
- Collard BCY, Mackill DJ (2008) Marker assisted selection: an approach for precision plant breeding in the twenty-first century. Philos Trans R Soc Lond Ser B Biol Sci 363 557–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B, Liu J, Tanksley SD (2002) Natural alleles at a tomato fruit size quantitative trait locus differ by heterchronic regulatory mutations. Proc Natl Acad Sci USA 99 13606–13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Smith OS, Graham G, Arthur L, Feng L, Podlich DW (2004) Genomics, genetics, and plant breeding: a private sector perspective. Crop Sci 44 1907–1913 [Google Scholar]
- Dekkers JCM, Hospital F (2002) The use of molecular genetic in the improvement of agricultural populations. Nat Rev Genet 3 22–32 [DOI] [PubMed] [Google Scholar]
- Delannay X, Baumann TT, Beighley DH, Buettner MJ, Coble HD, Defelice MS, Derting CW, Diedrick TJ, Griffin JL, Hagood ES, et al (1995) Yield evaluation of a glyphostae-tolerant soybean line after treatment with glyphosate. Crop Sci 35 1461–1467 [Google Scholar]
- Dubcovsky J (2004) Marker-assisted selection in public breeding programs: the wheat experience. Crop Sci 44 1895–1898 [Google Scholar]
- Dudley JW, Saghai Maroof MA, Rufener GK (1992) Molecular marker information and selection of parents in corn breeding programs. Crop Sci 32 301–304 [Google Scholar]
- Duvick DN (2001) Biotechnology in the 1930s: the development of hybrid maize. Nat Rev Genet 2 69–74 [DOI] [PubMed] [Google Scholar]
- Eathington SR, Crosbie TM, Edwards MD, Reiter RD, Bull JK (2007) Molecular markers in a commercial breeding program. Crop Sci 47 S154–S163 [Google Scholar]
- Edwards MD, Stuber CW, Wendel JF (1987) Molecular marker-facilitated investigations of quantitative-trait loci in maize. I. Numbers, genomic distribution and types of gene action. Genetics 116 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson RE, Golin D (2003) Assessing the impact of the Green Revolution, 1960 to 2000. Science 300 758–762 [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC (1996) Introduction to Quantitative Genetics, Ed 4. Longman and Company, Essex, UK
- Fehr WR, editor (1987) Principles of Cultivar Development: Theory and Technique, Vol 1. Macmillan, New York
- Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML, Brand LA, Fink CL, Fry JS, et al (1983) Expression of bacterial genes in plant cells. Proc Natl Acad Sci USA 80 4803–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzi A, Huang S, Gilbertson LA, Armstrong TA, Luethy MH, Malvar TM (2008) Modifying lysine biosynthesis and catabolism in corn with a single bifunctional expression/silencing transgene cassette. Plant Biotechnol J 6 13–21 [DOI] [PubMed] [Google Scholar]
- Gepts P (2002) A comparison between crop domestication, classical plant breeding, and genetic engineering. Crop Sci 42 1780–1790 [Google Scholar]
- Gepts P, Hancock J (2006) The future of plant breeding. Crop Sci 46 1630–1634 [Google Scholar]
- Goodman MM (2004) Plant breeding requirements for applied molecular biology. Crop Sci 44 1913–1914 [Google Scholar]
- Guimarães EP, Kueneman E (2006) Assessment of national plant breeding and biotechnology capacity worldwide. HortScience 41 50–52 [Google Scholar]
- Guner N, Wehner TC (2003) Survey of U.S. land-grant universities for training of plant breeding students. Crop Sci 43 1938–1944 [Google Scholar]
- Hallauer AR (2007) History, contribution, and future of quantitative genetics in plant breeding: lessons from maize. Crop Sci 47 S4–S19 [Google Scholar]
- Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni E, Williams M, Wurtzel ET, et al (2008) Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319 330–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JR (1992) Crops and Man. American Society of Agronomy and Crop Science Society of America, Madison, WI
- Heck GR, Armstrong CL, Astwood JD, Behr CF, Bookout JT, Brown SM, Cavato TA, DeBoer DL, Deng MY, George C, et al (2005) Development and characterization of a CP4 EPSPS-based glyphosate-tolerant corn event. Crop Sci 44 329–339 [Google Scholar]
- Herrera-Estrella L, Depicker A, van Montagu M, Schell J (1983) Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303 209–213 [PubMed] [Google Scholar]
- Holland J (2004) Implementation of molecular markers for quantitative traits in breeding programs—challenges and opportunities. In T Fischer, N Turner, J Angus, L McIntyre, M Robertson, A Borrell, D Lloyd, eds, New Directions for a Diverse Planet: Proceedings for the 4th International Crop Science Congress. Regional Institute, Gosford, Australia, www.cropscience.org.au/icsc2004
- Holland J (2007) Genetic architecture of complex traits in plants. Curr Opin Plant Biol 10 156–161 [DOI] [PubMed] [Google Scholar]
- Huang S, Adams WR, Zhou Q, Malloy KP, Voyles DA, Anthony J, Kriz AL, Luethy MH (2004) Improving nutritional quality of maize proteins by expressing sense and antisense zein genes. J Agric Food Chem 52 1958–1964 [DOI] [PubMed] [Google Scholar]
- James C (2007) Global status of commercialized biotech/GM crops: 2007. In ISAAA Briefs No. 37. International Service for the Acquisition of Agri-Biotech Applications, Ithaca, NY
- Jansen RC, Nap JP (2001) Genetical genomics: the added value from segregation. Trends Genet 17 388–391 [DOI] [PubMed] [Google Scholar]
- Jauhar P (2006) Modern biotechnology as an integral supplement to conventional plant breeding: the prospects and challenges. Crop Sci 46 1841–1859 [Google Scholar]
- Johnson GR (2004) Marker assisted selection. In J Janick, ed, Plant Breeding Reviews, Long Term Selection: Maize, Vol 24. John Wiley and Sons, Hoboken, NJ, pp 293–309
- Johnson GR (2007) Corn breeding in the twenty-first century. In MS Kang, PM Priyadarshan, eds, Breeding Major Food Staples. Blackwell Publishing, Ames, IA
- Johnson GR, McCuddin ZP (2008) Maize and the biotech industry. In JL Bennetzen, SC Hake, eds, Handbook of Maize: Its Biology. Springer, Berlin (in press)
- Johnson GR, Mumm RH (1996) Marker assisted maize breeding. In Proceedings of the 51st Corn and Sorghum Conference. American Seed Trade Association, Washington, DC, pp 75–84
- Kalaitzandonakes N, Alston JM, Bradford KJ (2007) Compliance costs for regulatory approval of new biotech crops. Nat Biotechnol 25 509–511 [DOI] [PubMed] [Google Scholar]
- Knapp S (1998) Marker-assisted selection as a strategy for increasing the probability of selecting superior genotypes. Crop Sci 38 1164–1174 [Google Scholar]
- Knight J (2003) Crop improvement: a dying breed. Nature 421 568–570 [DOI] [PubMed] [Google Scholar]
- Koebner RMD, Summers RW (2003) 21st century wheat breeding: plot selection or plate detection? Trends Biotechnol 21 59–63 [DOI] [PubMed] [Google Scholar]
- Koziel TM, Beland GL, Bowman C, Carozzi NB, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S, et al (1993) Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Biotechnology (N Y) 11 194–200 [Google Scholar]
- Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter N, Kampani A, Carlson S, Goebel M, Moose SP (2005) microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci USA 102 9412–9417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörz H, Wenzel G (2005) Molecular Marker Systems in Plant Breeding and Crop Improvement. Springer, New York
- McCouch S (2004) Diversifying selection in plant breeding. PLoS Biol 2 e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante M, Salamini F (2003) From plant genomics to breeding practice. Curr Opin Biotechnol 14 214–219 [DOI] [PubMed] [Google Scholar]
- Morris M, Dreher K, Ribaut JM, Khariallah M (2003) Money matters (II): costs of maize inbred line conversion schemes at CIMMYT using conventional and marker-assisted selection. Mol Breed 11 235–247 [Google Scholar]
- Mumm RH (2007) Backcross versus forward breeding in the development of transgenic maize hybrids: theory and practice. Crop Sci (Suppl 3) 47 S164–S171 [Google Scholar]
- National Research Council (2008) Achievements of the National Plant Genome Initiative and New Horizons in Plant Biology. National Academies Press, Washington, DC
- Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, et al (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA 104 16450–16455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Naylor RL, Jahn MM (2004) The role of genomics research in improvement of “orphan” crops. Crop Sci 44 1901–1904 [Google Scholar]
- Niebur WS, Rafalski JA, Smith OS, Cooper M (2004) Applications of genomics technologies to enhance rate of genetic progress for yield of maize within a commercial breeding program. In T Fischer, N Turner, J Angus, L McIntyre, M Robertson, A Borrell, D Lloyd, eds, New Directions for a Diverse Planet: Proceedings for the 4th International Crop Science Congress. Regional Institute, Gosford, Australia, www.cropscience.org.au/icsc2004
- Ow DW (2007) GM maize from site-specific recombination technology, what next? Curr Opin Biotechnol 18 115–120 [DOI] [PubMed] [Google Scholar]
- Papst C, Bohn M, Utz HF, Melchinger AE, Klein E, Eder J (2004) QTL mapping for European corn borer resistance (Ostrinia nubilalis Hb.), agronomic and forage quality traits of testcross progenies in early-maturing European maize (Zea mays L.) germplasm. Theor Appl Genet 108 1545–1554 [DOI] [PubMed] [Google Scholar]
- Paterson AH, Lander ES, Hewitt JD, Peterson S, Lincoln SE, Tanksley SD (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 335 721–726 [DOI] [PubMed] [Google Scholar]
- Paul DB, Kimmelman BA (1988) Mendel in America: theory and practice. In R Rainger, KR Benson, J Maienschien, eds, The American Development of Biology. University of Pennsylvania Press, Philadelphia, pp 281–310
- Ragot M, Biasiolli M, Delbut MF, Dell'Orco A, Malgarini L, Thevenin P, Vernoy J, Vivant J, Zimmermann R, Gay G (1995) Marker-assisted backcrossing: a practical example. In A Bervillé, M Tersac, eds, Les Colloques, No. 72, Techniques et Utilisations des Marqueurs Moléculaires. INRA, Paris, pp 45–56
- Ribaut JM, Ragot M (2007) Marker-assisted selection to improve drought adaptation in maize: the backcross approach, perspectives, limitations, and alternative. J Exp Bot 58 351–360 [DOI] [PubMed] [Google Scholar]
- Salvi S, Sponza G, Morgante M, Tomes D, Niu X, Fengler KA, Meeley R, Ananiev EV, Svitashev S, Bruggemann E, et al (2007) Conserved noncoding genomic sequences associated with a flowering time quantitative trait locus in maize. Proc Natl Acad Sci USA 104 11376–11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Tuberosa R (2005) To clone or not to clone plant QTLs: present and future challenges. Trends Plant Sci 10 297–304 [DOI] [PubMed] [Google Scholar]
- Segal G, Song R, Messing J (2003) A new opaque variant of maize by a single dominant RNA-interference-inducing transgene. Genetics 165 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HC, Crouch JH, Sharma KK, Seetharama N, Hash CT (2002) Applications of biotechnology for crop improvement: prospects and constraints. Plant Sci 163 381–395 [Google Scholar]
- Shull GH (1909) A pure-line method in corn breeding. Am Breeders Assoc Rep 5 51–59 [Google Scholar]
- Slade AJ, Fuerstenberg SI, Loeffler D, Steine MN, Facciotti D (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol 23 75–81 [DOI] [PubMed] [Google Scholar]
- Sokstad E (2004) Monsanto pulls the plug on genetically modified wheat. Science 304 1088–1089 [DOI] [PubMed] [Google Scholar]
- Spencer M, Mumm R, Gwyn J, inventors (2000) Glyphosate resistant maize lines. U.S. Patent No. 6,040,497
- Stoskopf NC, Tomes DT, Christie BR (1993) Plant Breeding Theory and Practice. Westview Press, Boulder, CO
- Tollefson JJ (2007) Evaluating maize for resistance to Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). Maydica 52 311–318 [Google Scholar]
- Tuberosa R, Salvi S, Giuliani S, Sanguineti MC, Belotti M, Conti S, Landi P (2007) Genome-wide approaches to investigate and improve maize response to drought. Crop Sci 47 S120–S141 [Google Scholar]
- Varshney RV, Hoisington DA, Tyagi AK (2006) Advances in cereal genomics and applications in crop breeding. Trends Biotechnol 24 490–499 [DOI] [PubMed] [Google Scholar]
- Wenzel G (2006) Molecular plant breeding: achievements in green biotechnology and future perspectives. Appl Microbiol Biotechnol 70 642–650 [DOI] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, San Miguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (catrotenoid-free) rice endosperm. Science 287 303–305 [DOI] [PubMed] [Google Scholar]
- Young ND (1999) A cautiously optimistic vision for marker-assisted breeding. Mol Breed 5 505–510 [Google Scholar]
- Yu J, Buckler ES (2006) Genetic association mapping and genome organization of maize. Curr Opin Biotechnol 17 155–160 [DOI] [PubMed] [Google Scholar]
- Yu J, Holland JB, McMullen MD, Buckler ES (2008) Genetic design and statistical power of nested association mapping in maize. Genetics 178 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Han F, Gao Z, Vega JM, Birchler JA (2007) Construction and behavior of engineered minichromosomes in maize. Proc Natl Acad Sci USA 104 8924–8929 [DOI] [PMC free article] [PubMed] [Google Scholar]