Abstract
Lotus japonicus accumulates the hydroxynitrile glucosides lotaustralin, linamarin, and rhodiocyanosides A and D. Upon tissue disruption, the hydroxynitrile glucosides are bioactivated by hydrolysis by specific β-glucosidases. A mixture of two hydroxynitrile glucoside-cleaving β-glucosidases was isolated from L. japonicus leaves and identified by protein sequencing as LjBGD2 and LjBGD4. The isolated hydroxynitrile glucoside-cleaving β-glucosidases preferentially hydrolyzed rhodiocyanoside A and lotaustralin, whereas linamarin was only slowly hydrolyzed, in agreement with measurements of their rate of degradation upon tissue disruption in L. japonicus leaves. Comparative homology modeling predicted that LjBGD2 and LjBGD4 had nearly identical overall topologies and substrate-binding pockets. Heterologous expression of LjBGD2 and LjBGD4 in Arabidopsis (Arabidopsis thaliana) enabled analysis of their individual substrate specificity profiles and confirmed that both LjBGD2 and LjBGD4 preferentially hydrolyze the hydroxynitrile glucosides present in L. japonicus. Phylogenetic analyses revealed a third L. japonicus putative hydroxynitrile glucoside-cleaving β-glucosidase, LjBGD7. Reverse transcription-polymerase chain reaction analysis showed that LjBGD2 and LjBGD4 are expressed in aerial parts of young L. japonicus plants, while LjBGD7 is expressed exclusively in roots. The differential expression pattern of LjBGD2, LjBGD4, and LjBGD7 corresponds to the previously observed expression profile for CYP79D3 and CYP79D4, encoding the two cytochromes P450 that catalyze the first committed step in the biosyntheis of hydroxynitrile glucosides in L. japonicus, with CYP79D3 expression in aerial tissues and CYP79D4 expression in roots.
β-Glycosidases that belong to the family 1 glycoside hydrolases catalyze hydrolysis of the β-glycosidic bond in β-glycosides consisting of two carbohydrate moieties or a carbohydrate moiety linked to an aryl or alkyl aglucone. In plants, β-glycosidases serve a number of diverse and important functions, including bioactivation of defense compounds (Nisius, 1988; Poulton, 1990; Morant et al., 2003; Halkier and Gershenzon, 2006; Suzuki et al., 2006), cell wall degradation in endosperm during germination (Leah et al., 1995), activation of phytohormones (Kristoffersen et al., 2000; Lee et al., 2006), and lignification (Dharmawardhana et al., 1995; Escamilla-Trevino et al., 2006). In addition, β-glycosidases play key roles in aroma formation in tea, wine, and fruit juice (Mizutani et al., 2002; Fia et al., 2005; Maicas and Mateo, 2005). Plants produce myriad secondary metabolites involved in defense against pathogens and herbivores. These defense compounds are often stored as β-glycosides and bioactivated by specific β-glycosidases (Morant et al., 2008). Glycosylation serves to protect the plant against the toxic effects of its own chemical defense system, to increase solubility, and to facilitate storage. Examples of two-component plant defense systems wherein β-glycosidases act as the bioactivator include the α-hydroxynitrile glycosides (cyanogenic glycosides) that are found in numerous different plant species (Poulton, 1990; Hughes, 1993; Bak et al., 2006; Morant et al., 2007; Bjarnholt and Møller, 2008; Bjarnholt et al., 2008), benzoxazinoid glycosides in Zea mays, Triticum aestivum, and Secale cereale (Niemeyer, 1988; Sue et al., 2000a, 2000b), avenacosides in Avena sativa (saponins; Nisius, 1988; Kim et al., 2000), isoflavonoid glycosides in legumes (Cairns et al., 2000; Chuankhayan et al., 2005, 2007a, 2007b; Suzuki et al., 2006), and glucosinolates found mainly in Brassicales (Halkier and Gershenzon, 2006). All of these different types of glycosides contain an O-linked β-glucosidic bond except for the glucosinolates, which are thioglucosides. This difference is reflected in the catalytic machinery of the corresponding β-glycosidases. In all β-glycosidases except the glucosinolate-degrading myrosinases (β-thioglucoside glucohydrolases), two Glu residues act as the catalytic nucleophile and the catalytic proton donor. In myrosinases, the proton donor Glu is replaced by a Gln residue (for review, see Bones and Rossiter, 2006; Morant et al., 2008).
One of the most well characterized plant defense systems is the α-hydroxynitrile glucoside/β-glucosidase system generally referred to as the “cyanide bomb.” This two-component system, in which each of the components when separated is chemically inert, provides cyanogenic plants with an immediate defense against attacking pathogens and herbivores by hydrolyzing α-hydroxynitrile glucosides, resulting in the release of toxic hydrogen cyanide. α-Hydroxynitrile glucosides thus exercise their effect as preformed defense compounds in the first line of chemical defense against pathogens or herbivores. α-Hydroxynitrile glucosides are derived from amino acids and found in more than 2,650 different plant species (Bak et al., 2006), including several important crops (Jones, 1998). α-Hydroxynitrile glucosides and their degrading β-glucosidases are stored separately in different subcellular compartments and/or in different cell types. The α-hydroxynitrile glucosides accumulate in the vacuole (Saunders et al., 1977; Saunders and Conn, 1978; Gruhnert et al., 1994). α-Hydroxynitrile glucoside cleaving β-glucosidases from monocotyledenous plants contain an N-terminal chloroplast transit peptide and are hence deposited in the chloroplast (Thayer and Conn, 1981). Eudicotyledenous α-hydroxynitrile glucoside-cleaving β-glucosidases contain an N-terminal signal peptide directing them through the secretory pathway, which results in protein glycosylation and deposition in the apoplast (Kakes, 1985; Frehner and Conn, 1987; Mkpong et al., 1990) or retention of the glycosylated proteins before secretion, resulting in intracellular deposition in protein bodies (Swain et al., 1992; Poulton and Li, 1994).
In some cyanogenic plant species, including the legumes Trifolium repens (white clover) and Lotus corniculatus (common bird's foot trefoil), cyanogenesis is a polymorphic trait (Hughes, 1991; Gebrehiwot and Beuselinck, 2001; Olsen et al., 2007, 2008). Acyanogenic L. corniculatus and T. repens plants have lost their ability either to synthesize α-hydroxynitrile glucosides, to produce a functional α-hydroxynitrile glucoside hydrolyzing β-glucosidase, or both. The polymorphic trait is supposedly retained in populations of white clover by the action of two opposing selective forces constituted of herbivory (giving an evolutionary advantage of cyanogenesis through reduction of herbivory) and temperature (giving an evolutionary disadvantage of cyanogenesis in cold climates where plant tissue exposed to frost is disrupted, causing cyanogenesis to occur with resulting exposure of the plant to the toxic effects of its own chemical defense system; Till, 1987). In addition, the growth rate has been shown to be higher in low-cyanogen versus high-cyanogen varieties of the same plant species (Gleadow and Woodrow, 2002; Goodger et al., 2004).
The enzymes and corresponding genes encoding the entire α-hydroxynitrile glucoside biosynthetic pathway are known from the monocotyledenous crop Sorghum bicolor (great millet), which produces the α-hydroxynitrile glucoside dhurrin with Tyr as the parent amino acid. Tyr is converted into dhurrin via the concerted action of two endoplasmic reticulum-anchored cytochromes P450, CYP79A1 and CYP71E1, and a soluble UDPG-glucosyltransferase, UGT85B1 (Sibbesen et al., 1995; Kahn et al., 1997; Bak et al., 1998; Jones et al., 1999), which function as a metabolon to secure efficient channeling of the intermediates (Møller and Conn, 1980; Jørgensen et al., 2005b; Nielsen et al., 2008). Lotus japonicus (bird's foot trefoil) contains the Ile- and Val-derived α-hydroxynitrile glucosides lotaustralin and linamarin as well as the Ile-derived γ- and β-hydroxynitrile glucosides rhodiocyanoside A and rhodiocyanoside D (Forslund et al., 2004; Bjarnholt and Møller, 2008; Bjarnholt et al., 2008), collectively referred to as hydroxynitrile glucosides. While α-hydroxynitrile glucosides are known to be implicated in plant defense, the biological function of the γ- and β-hydroxynitrile glucosides remains unknown. In apical L. japonicus leaves, the lotaustralin-linamarin and rhodiocyanoside A-rhodiocyanoside D ratios are high (Forslund et al., 2004; Bjarnholt et al., 2008). In the same plant species, the two isoenzymes CYP79D3 and CYP79D4 catalyze the first and rate-limiting step in hydroxynitrile glucoside biosynthesis, the conversion of Ile and Val into the corresponding oximes (Forslund et al., 2004; Morant et al., 2007; Bjarnholt et al., 2008). The enzymes that catalyze the remaining steps remain elusive. CYP79D3 is expressed in aerial parts of L. japonicus, whereas CYP79D4 is expressed exclusively in the roots, as determined by semiquantitative reverse transcription (RT)-PCR (Forslund et al., 2004). Hydroxynitrile glucosides are generally only detected in the aerial parts of L. japonicus (Forslund et al., 2004). This questions the in vivo function of CYP79D4.
In this work, we have identified and characterized two β-glucosidases, LjBGD2 and LjBGD4, responsible for bioactivation of the hydroxynitrile glucosides in L. japonicus leaves. The two isoenzymes share 85% sequence identity at the amino acid level. LjBGD2 and LjBGD4 are coexpressed with the biosynthetic CYP79D3 in aerial parts of L. japonicus. Staining for β-glucosidase activity demonstrated a preferential localization in the palisade tissue of the leaves. Heterologous expression of LjBGD2 and LjBGD4 in Arabidopsis (Arabidopsis thaliana) confirmed that LjBGD2 and LjBGD4 both hydrolyze hydroxynitrile glucosides and have very similar substrate specificity profiles. A third β-glucosidase, LjBGD7, with approximately 82% amino acid sequence identity to LjBGD2 and LjBGD4, is coexpressed with the biosynthetic CYP79D4 in L. japonicus roots. This implies that two parallel pathways for the biosynthesis and turnover of hydroxynitrile glucosides exist in L. japonicus.
RESULTS
Hydrolysis of Lotaustralin and Rhodiocyanosides upon Cell Disruption
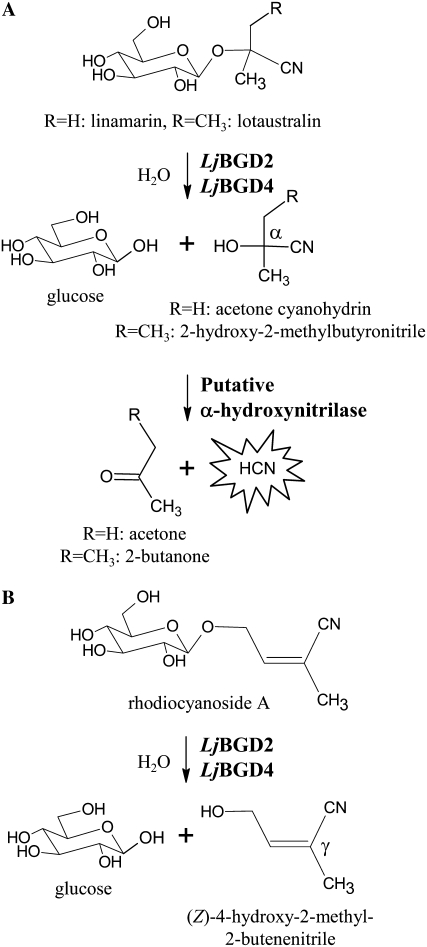
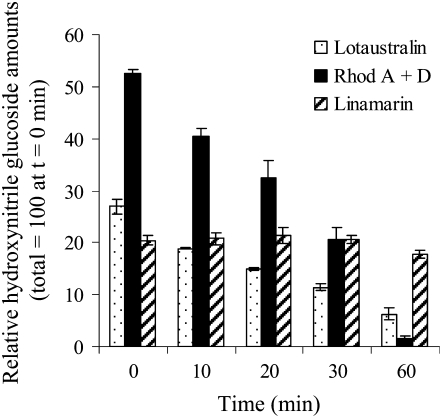
Upon cell disruption, both α-hydroxynitrile glucosides and rhodiocyanosides are hydrolyzed by β-glucosidases in L. japonicus, as measured using crude L. japonicus leaf extracts (Figs. 1 and 2). The aglucone resulting from hydrolysis of α-hydroxynitrile glucosides dissociates either spontaneously or by the action of α-hydroxynitrilases to release toxic HCN and a ketone or an aldehyde (Fig. 1A). In contrast, hydrolysis of rhodiocyanoside A and D affords γ- and β-hydroxynitriles, respectively, which do not result in HCN release (Fig. 1B). To examine the rate of hydrolysis of the individual hydroxynitrile glucosides upon cell disruption, leaves from a L. japonicus 35S∷CYP79D2 line (Forslund et al., 2004) were employed. This transgenic L. japonicus line accumulates approximately 20-fold higher amounts of linamarin compared with the wild type and was obtained by introduction of the CYP79D2 gene of Manihot esculenta (cassava) under the control of the constitutive cauliflower mosaic virus (CaMV) 35S promoter. Compared with L. japonicus CYP79D3 and CYP79D4, M. esculenta CYP79D2 shows enhanced activity toward Val and thus favors the formation of linamarin (Forslund et al., 2004). The use of the L. japonicus 35S∷CYP79D2 line thus allows for a careful examination of changes in lotaustralin, rhodiocyanoside A and D, and linamarin levels in response to tissue disruption. The rhodiocyanosides are most rapidly hydrolyzed, followed by lotaustralin, while linamarin hydrolysis proceeds at a very slow rate (Fig. 2). When the corresponding experiment was conducted with L. japonicus wild-type leaves, the same relative pattern of rhodiocyanoside and lotaustralin degradation was observed (data not shown), while degradation of linamarin could not be detected due to the very low amounts of linamarin initially present. These results show that L. japonicus leaves contain β-glucosidase activity, which upon cell disruption preferentially catalyzes the hydrolysis of rhodiocyanosides and lotaustralin compared with linamarin.
Figure 1.
Bioactivation of lotaustralin, linamarin, and rhodiocyanoside A in L. japonicus leaves. A, Cyanogenic glucosides are β-glucosides of α-hydroxynitriles. Upon cell disruption, β-glucosidases catalyze the hydrolysis of the O-β-glucosidic bond to yield Glc and an α-hydroxynitrile aglucone. The α-hydroxynitrile either spontaneously or enzymatically breaks down to liberate a ketone and toxic HCN. B, Rhodiocyanosides are β-glucosides of β- or γ-hydroxynitriles. In contrast to α-hydroxynitrile glucosides, aglucone formation is not accompanied by the release of HCN.
Figure 2.
Degradation of lotaustralin, linamarin, and rhodiocyanosides in leaves of L. japonicus following cell disruption. The rate of degradation of endogenous hydroxynitrile glucosides in transgenic L. japonicus 35S∷CYP79D2 leaves following tissue disruption was monitored by LC-MS and analysis of the amounts of metabolites remaining at time points between 0 and 60 min following cell disruption. The total amount of hydroxynitrile glucosides (linamarin + lotaustralin + rhodiocyanosides) at time 0 was defined as 100. Rhodiocyanosides are most rapidly degraded, followed by lotaustralin, which is degraded at a moderate rate; only negligible degradation of linamarin is observed.
Identification of Two β-Glucosidases Involved in Hydroxynitrile Glucoside Hydrolysis in L. japonicus Leaves
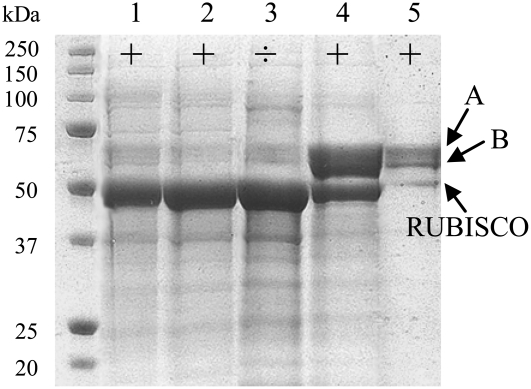
To identify the enzymes responsible for hydrolysis of hydroxynitrile glucosides in L. japonicus, the enzymes showing activity with these substrates were purified from crude soluble protein extracts of young L. japonicus leaves by a simple two-step procedure using cation exchange followed by gel filtration chromatography. Purification using the cation exchange resin was carried out by batch elution to prevent aggregation and immediately followed by gel filtration to remove Rubisco (Fig. 3). The fractions from the gel filtration column that showed hydrolytic activity toward hydroxynitrile glucosides were enriched in proteins with an apparent molecular mass of 107 kD, as monitored by comparison with known molecular mass standards coapplied to the gel filtration chromatography column (data not shown). SDS-PAGE analysis and Coomassie Blue staining revealed that the most active gel filtration fraction contained three distinct protein bands, with apparent molecular masses of 60 kD (band A), 55 kD (band B), and 48 kD (lower band; Fig. 3). In the course of fractionation, no lotaustralin- or linamarin-hydrolyzing activity was found in the discarded fractions (Fig. 3). Hence, no indications of the occurrence of hydroxynitrile glucoside-cleaving β-glucosidases that exhibited a different fractionation pattern were observed. The cation exchange and gel filtration chromatography resulted in 8- and 18-fold purification, respectively, of the hydroxynitrile glucoside-cleaving β-glucosidases, as measured by HCN release with lotaustralin as substrate (data not shown). The relatively low purification fold is a reflection of the high abundance of the enzymes in the crude leaf protein extract.
Figure 3.
Purification of hydroxynitrile glucoside-cleaving β-glucosidases from L. japonicus leaves. Protein fractions obtained during the purification procedure were analyzed by 12% SDS-PAGE, and the protein composition was visualized by Coomassie Blue staining. The lanes show protein composition in the total crude leaf extract (1), soluble crude leaf extract (2), cation exchange supernatant (3), cation exchange eluate (4), and fractions from gel filtration chromatography containing β-glucosidase activity against hydroxynitrile glucosides (5). The presence (+) or absence (÷) of β-glucosidase activity toward lotaustralin and linamarin as measured by HCN release is indicated at the top of each lane. Protein sequencing identified the protein bands indicated by A and B as a mixture of LjBGD2 and LjBGD4, while the faster migrating protein band was identified as Rubisco.
The proteins migrating with apparent molecular masses of 60, 55, and 48 kD (Fig. 3, lane 5) were subjected to in-gel trypsin digestion, and the obtained peptides were sequenced by mass spectrometry. Peptide fingerprints of the 60- and 55-kD protein bands were nearly identical, and subsequent alignment of the 13 peptide sequences obtained from the 60- and 55-kD protein bands with the L. japonicus β-glucosidase sequences provided by the Kazusa DNA Research Institute revealed that both sequenced protein bands contained a mixture of two different L. japonicus β-glucosidases, LjBGD2 and LjBGD4 (Supplemental Fig. S1). Of the 13 peptide sequences obtained, seven matched perfectly to LjBGD2, three matched perfectly to LjBGD4, and three matched perfectly to both sequences. An overall amino acid sequence coverage of 24% and 14% was obtained for LjBGD2 and LjBGD4, respectively. The deduced LjBGD2 and LjBGD4 polypeptide sequences are 514 and 518 amino acids long, respectively, and share 85% amino acid sequence identity and 91% similarity. PSORT and TargetP analysis of the full-length LjBGD2 and LjBGD4 amino acid sequences predicted that the polypeptides both contain an N-terminal signal peptide consisting of 27 amino acids (Supplemental Fig. S1) and are destined for the secretory pathway, in agreement with other known dicotyledenous α-hydroxynitrile glucoside-cleaving β-glucosidases (Kakes, 1985; Frehner and Conn, 1987; Mkpong et al., 1990; Swain et al., 1992; Poulton and Li, 1994). No amino acid sequences corresponding to the N terminus were obtained. Sequencing of peptides obtained from the 48-kD protein band revealed a perfect match to Rubisco (data not shown).
The β-Glucosidases Isolated from L. japonicus Leaves Preferentially Hydrolyze Rhodiocyanosides and Lotaustralin
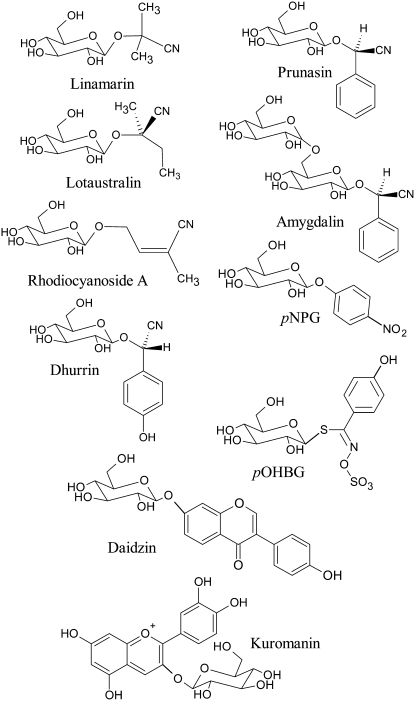
To characterize the biochemical activity of the L. japonicus leaf β-glucosidases, a range of β-glucosides (Fig. 4) were tested as substrates for the isolated mixture of LjBGD2 and LjBGD4. Km and Vmax values were determined for a number of different aliphatic and aromatic hydroxynitrile glucosides. Of those tested, the L. japonicus β-glucosidases exhibited the lowest Km for rhodiocyanoside A, followed by dhurrin, prunasin, and lotaustralin (Table I). In agreement with the data obtained using leaf extracts, linamarin proved to be a poor substrate, with a Km 10-fold higher than that for rhodiocyanoside A (Table I). The Vmax values obtained using different hydroxynitrile glucosides as substrates were similar. Accordingly, the low turnover of linamarin observed (Fig. 2) is primarily due to a high Km value and not a lower Vmax (Table I). The kinetic data obtained with the purified mix of LjBGD2 and LjBGD4 correspond well with the degradation profile observed using crude L. japonicus leaf extracts (Fig. 2) and support the idea that the purified β-glucosidases catalyze the hydrolysis of hydroxynitrile glucosides in planta. As expected, the α-hydroxynitrile diglucoside amygdalin and the thioglucoside p-hydroxybenzylglucosinolate (pOHBG) were not hydrolyzed (data not shown). Hydrolysis of the flavonoid glucoside kuromanin was not detected using concentrations up to 100 μm. Daidzin was cleaved, and the Km value was lower than that observed for rhodiocyanoside A (Table I). The Vmax for daidzin was low compared with the Vmax values obtained for the hydroxynitrile glucosides (Table I).
Figure 4.
Structures of the main β-glucosides used to examine the substrate specificity of the hydroxynitrile glucoside-cleaving β-glucosidases from L. japonicus. Val-derived linamarin and Ile-derived lotaustralin and rhodiocyanoside A are synthesized by L. japonicus. The α-hydroxynitrile glucosides dhurrin and prunasin are derived from Tyr and Phe, respectively. Amygdalin is the diglucoside derived from glucosylation of prunasin. pNPG is an artificial chromogenic β-glucoside degraded by a wide range of β-glycosidases. Daidzin and kuromanin are isoflavonoid and flavonoid glucosides, respectively. All glucosides tested contain an O-β-glucosidic bond except for the glucosinolates, which contain an S-β-glucosidic bond.
Table I.
Catalytic parameters of a mixture of LjBGD2 and LjBGD4 isolated from L. japonicus leaves
| Parameter | Lotaustralin | Linamarin | Rhodiocyanoside A | Prunasin | Dhurrin | pNPG | Daidzin |
|---|---|---|---|---|---|---|---|
| Km (mm) | 0.69 ± 0.06 | 6.6 ± 1.1 | 0.19 ± 0.03 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.65 ± 0.02 | 0.03 ± 0.02 |
| Vmax (nmol min−1) | 2.47 ± 0.06 | 1.6 ± 0.2 | 3.4 ± 0.3 | 1.8 ± 0.1 | 1.1 ± 0.1 | 3.78 ± 0.03 | 0.5 ± 0.1 |
| r2 | 0.99 | 0.99 | 0.96 | 0.97 | 0.97 | 0.99 | 0.77 |
Heterologous Expression of LjBGD2 and LjBGD4 in Arabidopsis and Analysis of Their Individual Substrate Specificities
LjBGD2 and LjBGD4 could not be separated by the protein purification methods applied in this study. This may reflect the co-occurrence of differently glycosylated forms of each protein. To examine the substrate specificity of the two β-glucosidases independently, LjBGD2 and LjBGD4 were heterologously expressed in transgenic Arabidopsis. Arabidopsis was chosen as expression host because of the straightforward transformation protocol, the absence of endogenous hydroxynitrile and isoflavonoid glucosides, and because crude extracts from this plant were shown not to possess any endogenous β-glucosidase activity toward hydroxynitrile and isoflavonoid glucosides (Fig. 5). As a dicotyledenous plant, Arabidopsis would be expected to properly process LjBGD2 and LjBGD4 with respect to signal peptide recognition, targeting of the enzymes through the secretory pathway, and cotranslational signal peptide processing and glycosylation, as required for enzyme stability (Keresztessy et al., 1996; Cicek and Esen, 1999; Zhou et al., 2002).
Figure 5.
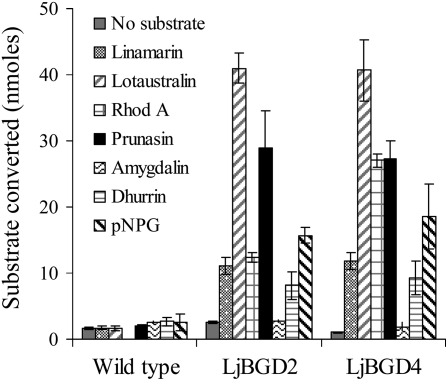
Substrate specificity profiles of LjBGD2 and LjBGD4 expressed in Arabidopsis. Arabidopsis leaf discs producing recombinant LjBGD2 and LjBGD4 were assayed for the ability to hydrolyze a range of β-glycosides. Rhod A, Rhodiocyanoside A. All incubation assays (10 min) were conducted using 1 mm substrate corresponding to a total of 200 nmol. Arabidopsis wild-type leaf discs served as a negative control.
The LjBGD4 cDNA was cloned, including the first intron, to overcome difficulties in PCR amplification of the full-length cDNA. Moreover, the use of Escherichia coli SURE cells as recipients for transformation with the ligation product was critical for successful cloning. These observations suggested that the LjBGD4 nucleotide sequence formed secondary structures that interfered with PCR and standard cloning procedures. Analysis of the LjBGD4 nucleotide sequence using the Arabidopsis intron splice site prediction server NetPlantGene (http://www.cbs.dtu.dk/services/NetPGene/) predicted that Arabidopsis would recognize the L. japonicus-derived intron.
Six independent kanamycin-resistant Arabidopsis 35S∷LjBGD2 transformants and five independent gentamycin-resistant Arabidopsis 35S∷LjBGD4 transformants that showed β-glucosidase activity toward lotaustralin were obtained. The ability of Arabidopsis 35S∷LjBGD2 and 35S∷LjBGD4 to hydrolyze lotaustralin confirmed that both L. japonicus β-glucosidases were successfully expressed in an active form and that the intron included in the LjBGD4 construct was properly recognized and excised in Arabidopsis.
LjBGD2 and LjBGD4 activity in the transgenic Arabidopsis leaves was measured using excised leaf discs and discs from wild-type plants as controls. LjBGD2 and LjBGD4 were found to possess very similar hydrolytic activities toward the different β-glucosides tested (Fig. 5). Both aliphatic and aromatic α-hydroxynitrile monoglucosides were hydrolyzed, whereas the α-hydroxynitrile diglucoside amygdalin was not (Fig. 5). Due to the presence of endogenous myrosinases in Arabidopsis, glucosinolates were not applied as substrates. The background activity observed in Arabidopsis wild-type plants was due to hydrolysis of endogenous indole glucosinolates, which results in the formation of SCN− (for review, see Bones and Rossiter, 2006). SCN− is known to react in the colorimetric assay for CN− determination, as described previously (Bak et al., 1999, 2001; Bak and Feyereisen, 2001). The background activity observed in wild-type Arabidopsis with p-nitrophenyl β-d-glucopyranoside (pNPG) is most likely due to pNPG being a general β-glucosidase substrate that is hydrolyzed by endogenous Arabidopsis β-glucosidases. These results show that despite their co-occurrence (Figs. 3 and 8 [below]), LjBGD2 and LjBGD4 are individually able to hydrolyze the same range of aliphatic and aromatic α-hydroxynitrile monoglucosides and rhodiocyanoside A as observed for the partially purified mixture of the two enzymes. Accordingly, heteromer formation is not required for activity.
Figure 8.
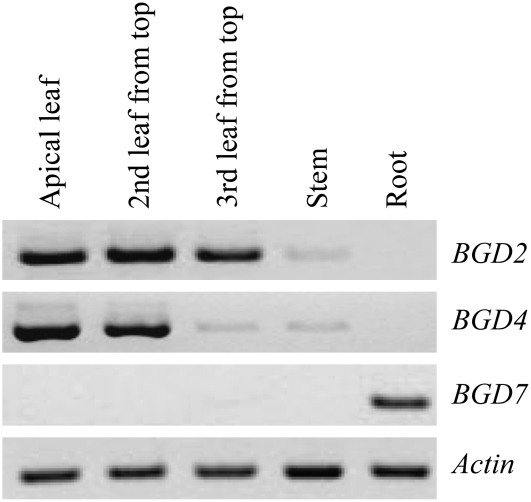
Expression profiles of LjBGD2, LjBGD4, and LjBGD7 in 21-d-old L. japonicus seedlings. cDNA produced from mRNA isolated from different tissues was obtained from Forslund et al. (2004), and PCR was performed with specific primers amplifying LjBGD2, LjBGD4, LjBGD7, and ACTIN. LjBGD2 and LjBGD4 are detected in aerial tissues, while LjBGD7 is detected exclusively in roots.
The assays with leaf discs expressing either LjBGD2 or LjBGD4 showed the ability of each of these β-glucosides to hydrolyze daidzin (data not shown). This cross-reactivity is consistent with previous observations that almond (Prunus dulcis) β-glucosidase hydrolyzes daidzin (Ismail and Hayes, 2005; Chuankhayan et al., 2007a), whereas β-glucosidases hydrolyzing isoflavonoid glucosides have not been found to hydrolyze cyanogenic glucosides (Ketudat Cairns et al., 2000; Chuankhayan et al., 2005). The isoflavonoid glucoside profile in L. japonicus is currently not known, and daidzin has not been reported to be present (Farag et al., 2007; Suzuki et al., 2008). This argues that the in planta function of LjBGD2 and LjBGD4 is to hydrolyze hydroxynitrile glucosides and not isoflavonoid glucosides. The data obtained using leaf discs to independently characterize the substrate specificity of LjBGD2 and LjBGD4 are in accordance with the results obtained with the purified mixture of the two β-glucosidases obtained from L. japonicus leaves (Table I). As in the experiments with the mixture of the two purified BGDs, hydrolysis of kuromanin could not be detected in the leaf disc assay using transgenic Arabidopsis expressing LjBGD2 or LjBGD4 (Table I).
No or limited activities were observed with extracts of transgenic Arabidopsis leaves obtained by maceration or freeze/thaw cycles. This apparent inactivation prevented the isolation of active LjBGD2 and LjBGD4 from the transgenic Arabidopsis lines. An obvious explanation for the observed lack of activity of L. japonicus β-glucosidase in Arabidopsis leaf extracts following homogenization would be the inhibition by endogenous glucosinolates or breakdown products thereof liberated in the course of the maceration process. However, no inhibitory effect of glucosinolates on LjBGD2 and LjBGD4 was observed upon the addition of a 5-fold molar excess of aliphatic or aromatic glucosinolates [4-(methylthio)butyl glucosinolate and pOHBG, respectively] or NaSCN to purified LjBGD2 and LjBGD4 (data not shown).
Exploration of the Active Site Architecture of LjBGD2 and LjBGD4 by Homology Modeling
To gain insight into the active site architecture of LjBGD2 and LjBGD4 and their respective abilities to accommodate the L. japonicus hydroxynitrile glucosides, models of the protein structures were built based on three-dimensional structures of T. repens linamarase (TrCBG), S. bicolor dhurrinase 1 (SbDhr1), and Z. mays DIMBOA-Glc-hydrolase (Zm-Glu-1), which have all been solved at high resolution by x-ray chrystallography (Barrett et al., 1995; Czjzek et al., 2001; Verdoucq et al., 2004). TrCBG, SbDhr1, and Zm-Glu-1 show high amino acid sequence identity to LjBGD2 and LjBGD4 (approximately 55%, 45%, and 46%, respectively) and hence represent excellent templates for modeling of the two L. japonicus β-glucosidases. The models obtained of the three- dimensional structures of the LjBGD2 and LjBGD4 monomers, with closeup views of the active sites into which lotaustralin, linamarin, and rhodiocyanoside A are docked, are presented in Figure 6. The predicted structures of LjBGD2 and LjBGD4 are highly similar to those of TrCBG (Fig. 6A), SbDhr1, and Zm-Glu-1 (data not shown). In Figure 6B, the amino acids within the LjBGD2 and LjBGD4 aglucone-binding sites and the two catalytic glutamates (indicated by magenta and blue arrowheads in Supplemental Fig. S1) are displayed with lotaustralin docked in the active site. Only the amino acids corresponding to those located within the aglucone-binding pocket of SbDhr1 (Verdoucq et al., 2004) plus Gly-211/Val-215 and Trp-210/Trp-214, which in this study were also predicted to lie within the aglucone-binding pocket of LjBGD2 and LjBGD4, are shown. Studies on Zm-Glu-1 and SbDhr1 have demonstrated the importance of additional amino acid residues in determining aglucone specificity. Substitution of single amino acids in Zm-Glu-1 with their SbDhr1 counterparts by site-directed mutagenesis demonstrated that Ala-467, Tyr-473, and Asp-261 (Zm-Glu-1 amino acids and numbering), which are located in close proximity of the aglucone-binding pocket, played key roles in aglucone specificity in Zm-Glu-1 and SbDhr1 (Verdoucq et al., 2003). The corresponding amino acids in LjBGD2 and LjBGD4 (marked by “Z” in Supplemental Fig. S1) are replaced by Ser, Phe, and Val, respectively, and conserved between LjBGD2 and LjBGD4. In Prunus serotina (black cherry), two additional amino acids at positions corresponding to Val-219 and Thr-391 in LjBGD2 (indicated by “P” in Supplemental Fig. S1) were shown to be crucial for the aglucone specificity of amygdalin hydrolase 1 and the prunasin hydrolases (Zhou et al., 2002). These were likewise located in proximity to the aglucone-binding pocket, but interestingly, the corresponding amino acids differ between LjBGD2 and LjBGD4 (Supplemental Fig. S1). The Thr-391 (LjBGD2) and Asp-395 (LjBGD4) immediately preceded a highly conserved Trp residue located in the aglucone-binding pocket (shown in gray in Fig. 6B). According to Czjzek et al. (2001), the amino acid residue at this position could be responsible for determining the particular conformation of the Trp in the active site. Interestingly, this Trp is preceded by a Pro in Zm-Glu-1 (Czjzek et al., 2001) and by a Gly in P. serotina amygdalin hydrolase 1 (Zhou et al., 2002).
Figure 6.
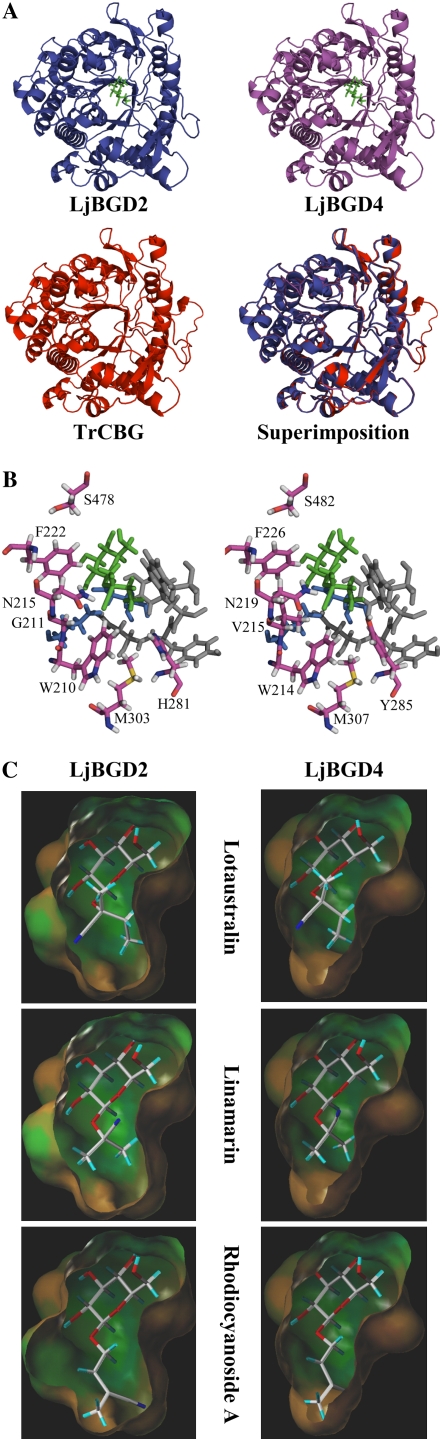
Models of the three-dimensional architecture of the substrate-binding pockets of LjBGD2 and LjBGD4 with docking of lotaustralin, linamarin, and rhodiocyanoside A. A, Comparison of the backbone configurations of the modeled LjBGD2 and LjBGD4 monomers and the known structure of TrCBG. A superimposition of the three structures illustrates the highly conserved structure of α-hydroxynitrile glucoside-cleaving β-glucosidases. B, Stick models of the amino acids lining the aglucone-binding pockets of LjBGD2 and LjBGD4. Lotaustralin (green) is docked with the two catalytic Glu residues (E208 and E420 [LjBGD2 numbering], shown in blue) positioned on both sides of the β-glucosidic bond. Four highly conserved amino acids (N349, Y350, Y351, and W392 [LjBGD2 numbering], shaded in gray) line one side of the aglucone-binding pocket, while the amino acids lining the opposite side (magenta) are highly variable. Only the unconserved amino acids are specified. The LjBGD2 and LjBGD4 aglucone-binding pockets differ at two positions (G211/V215 and H281/Y285). The amino acid numbering corresponds to that applied in Supplemental Figure S1. The amino acids that define the glucone-binding sites are highly conserved in all β-glucosidases belonging to the family 1 glycoside hydrolases and are not shown for reasons of simplicity. C, Lipophilic surface representations of the active sites of LjBGD2 and LjBGD4. Lipophilic areas are shown in brown, hydrophilic areas in blue, and neutral areas in green. The orientation of the active site is the same as in B. The three endogenous L. japonicus substrates lotaustralin, linamarin, and rhodiocyanoside A are docked into the active sites.
The lipophilic surface representations of the active site pockets in Figure 6C are shown using the same orientations as in Figure 6B to allow comparison of the amino acid residues that form the aglucone-accommodating part of the active site pocket. The amino acids involved in glucone binding are highly conserved in all β-glucosidases belonging to the family 1 glycoside hydrolases (Czjzek et al., 2001; Supplemental Fig. S2). This is reflected in the identical topologies of the glucone-binding pockets that form the upper parts of the active sites (Fig. 6C). As a starting model for docking of the L. japonicus substrates in LjBGD2 and LjBGD4, Zm-Glu-1 complexed with the inhibitor p-nitrophenyl β-d-thioglucoside was used (Czjzek et al., 2001). The glucone part of this complex is poorly defined, probably due to the glucone taking multiple conformations during catalysis; accordingly, it was decided to model the hydroxynitrile glucosides with the glucone in a chair conformation. The hydroxynitrile glucoside glucones docked into the predicted active sites of LjBGD2 and LjBGD4 were in hydrogen-bonding distance to the same amino acids as those reported in the Zm-Glu-1-inhibitor template (Czjzek et al., 2001), except His-162/His-166, which was not in hydrogen-bonding distance to the glucone in the conformation chosen. The predicted LjBGD2 and LjBGD4 aglucone-binding pockets have similar overall topologies. Only two amino acid differences between LjBGD2 and LjBGD4 are found within the aglucone-binding pockets (Gly-211/Val-215 and His-281/Tyr-285). The relatively bulkier residues of LjBGD4 are predicted to result in a narrower aglucone-binding pocket in LjBGD4 compared with LjBGD2. The three L. japonicus hydroxynitrile glucosides are equally accommodated into the predicted active sites. Based on the nearly identical substrate binding pockets, it was predicted that LjBGD2 and LjBGD4 would have similar catalytic activities and substrate specificities.
The predicted similar active site topologies of LjBGD2 and LjBGD4 (Fig. 6) agree well with the similar substrate specificities and activities (Fig. 5).
Phylogenetic Analysis Reveals Three Putative Hydroxynitrile Glucoside-Cleaving β-Glucosidases in L. japonicus
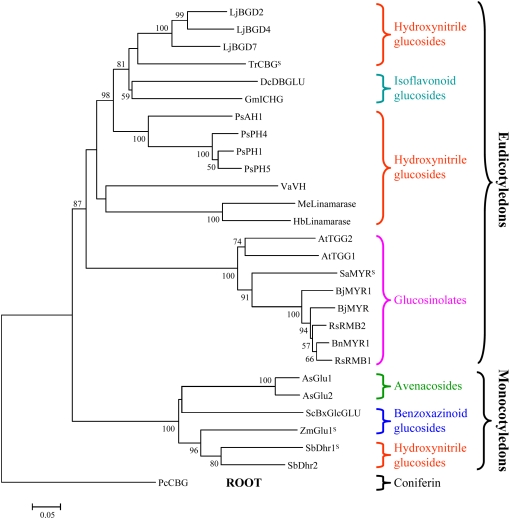
Several genes encoding putative β-glucosidases have been sequenced from the L. japonicus genome as part of the L. japonicus genome sequencing project (Kazusa DNA Research Institute). Figure 7 shows the results of a phylogenetic analysis that focuses on plant β-glucosidases involved in the bioactivation of defense compounds. In the phylogenetic tree, the β-glucosidases known from the literature to hydrolyze α-hydroxynitrile glucosides form separate clades in monocotyledons and eudicotyledons. This argues that the ability to hydrolyze α-hydroxynitrile glucosides has evolved independently in monocotyledons and eudicotyledons. A more elaborate phylogenetic analysis of plant β-glycosidases, including those presented in Figure 7, is available at http://www.p450.kvl.dk/BGD.shtml. The phylogenetic analysis identified three L. japonicus β-glucosidases within the group of eudicotyledenous α-hydroxynitrile glucoside β-glucosidases: LjBGD2 and LjBGD4, which were isolated from L. japonicus leaves, and a third β-glucosidase, LjBGD7, with approximately 82% amino acid sequence identity and approximately 90% similarity to LjBGD2 and LjBGD4. LjBGD2, LjBGD4, and LjBGD7 form a cluster with TrCBG, a β-glucosidase from white clover that hydrolyzes linamarin. In agreement with the phylogenetic analysis provided by Chuankhayan et al. (2007b), the isoflavonoid glucoside-cleaving β-glucosidases from Glycine max and Dalbergia cochinchinensis were found to cluster between hydroxynitrile glucoside-specific β-glucosidases from legumes and P. serotina. Based on the phylogenetic analysis, isoflavonoid glucoside- cleaving β-glucosidases in legumes appear to have evolved from the β-glucosidases involved in the hydrolysis of hydroxynitrile glucosides.
Figure 7.
Phylogenetic analysis of selected plant β-glucosidases involved in the bioactivation of defense compounds. The phylogenetic tree includes hydroxynitrile and isoflavonoid glucoside-cleaving β-glucosidases from eudicotyledons, glucosinolate-degrading myrosinases (Brassicales), and selected β-glucosidases involved in the bioactivation of defense compounds in monocotyledons. The defense compounds degraded are indicated for the different groups of β-glucosidases. “S” indicates enzymes for which the crystal structures have been solved. LjBGD2, LjBGD4, and LjBGD7, L. japonicus β-glucosidases (this study); TrCBG, T. repens cyanogenic β-glucosidase (Barrett et al., 1995); GmICHG, G. max isoflavone conjugate-hydrolyzing β-glucosidase (Suzuki et al., 2006); DcBDGLU, D. cochinchinensis dalcochinase (Cairns et al., 2000); PsAH1, PsPH1, PsPH4, and PsPH5, P. serotina amygdalin hydrolase and prunasin hydrolase isoenzymes (Kuroki and Poulton, 1987; Zheng and Poulton, 1995; Zhou et al., 2002); VaVH, Vicia angustifolia vicianin hydrolase (Ahn et al., 2007); MeLinamarase, M. esculenta linamarase (Hughes et al., 1992; Keresztessy et al., 2001); HbLinamarase, H. brasiliensis linamarase (Selmar et al., 1987); AtTGG1 and AtTGG2, Arabidopsis myrosinases (Barth and Jander, 2006); SaMYR, Sinapis alba (white mustard) myrosinase (Burmeister et al., 1997); BjMYR and BjMYR1, Brassica juncea (mustard greens) myrosinases (Heiss et al., 1999); RsRMB1 and RsRMB2, Raphanus sativus (radish) myrosinases (Hara et al., 2000); BnMYR1, Brassica napus (rape) myrosinase (Chen and Halkier, 1999); As-Glu-1 and As-Glu-2, A. sativa avenacosidases (Gusmayer et al., 1994; Kim et al., 2000); ScBxGlcGLU, S. cereale DIBOA-Glc β-glucosidase (Nikus et al., 2003); Zm-Glu-1, Z. mays glucosidase 1 (Czjzek et al., 2001); SbDhr1 and SbDhr2, S. bicolor dhurrinases (Hösel et al., 1987; Verdoucq et al., 2004); PcCBG, Pinus contorta (lodgepole pine) coniferin β-glucosidase (Dharmawardhana et al., 1995, 1999). The bootstrapped neighbor-joining tree was built in MEGA 4.0 (Tamura et al., 2007). The tree was bootstrapped with 1,000 iterations (node cutoff value, 50%). The underlying amino acid sequences in fastA format and the multiple alignment can be accessed at http://www.p450.kvl.dk/VintherMorant_etal_Figure7.tfa and http://www.p450.kvl.dk/VintherMorant_etal_Figure7_Alignment.pdf, respectively. The phylogenetic tree was rooted using PcCBG as an outgroup. For the bootstrap analysis, 1,000 trials were performed, and the bootstrap values are shown in percentages; bootstrap node values below 50% are not shown. A more elaborate phylogenetic analysis of plant β-glycosidases, including those presented here, is available at http://www.p450.kvl.dk/BGD.shtml.
LjBGD2 and LjBGD4 Are Expressed in Aerial Parts of L. japonicus, while LjBGD7 Is Expressed in Roots
To determine the expression profiles of the three β-glucosidase-encoding genes from L. japonicus identified in the phylogenetic analysis (Fig. 7), semiquantitative RT-PCR was performed using cDNA prepared from leaves, stems, and roots of 21-d-old L. japonicus seedlings (Forslund et al., 2004). Based on the RT-PCR analysis, LjBGD2 and LjBGD4 are expressed in aerial parts of L. japonicus, with an apparent highest expression level in young leaves, and LjBGD7 is expressed exclusively in roots (Fig. 8). If LjBGD7 is a hydroxynitrile glucoside-cleaving β-glucosidase, as suggested, the different expression profiles explain why LjBGD2 and LjBGD4 and not LjBGD7 were obtained from L. japonicus leaves. The expression profile of LjBGD2 and LjBGD4 is in agreement with results from T. repens, a legume closely related to L. japonicus, which show that the α-hydroxynitrile glucoside-cleaving β-glucosidase (linamarase) is present in young leaves and not in roots (Dunn et al., 1988).
Localization of β-Glucosidase Activity in L. japonicus and Transgenic Arabidopsis by in Tissue 6-Bromo-2-Naphtyl β-d-Glucopyranoside Staining
In order to determine the tissue localization of β-glucosidase activity in L. japonicus wild type and transgenic Arabidopsis expressing LjBGD2 and LjBGD4, tissue sections were stained with the chromogenic substrate 6-bromo-2-naphtyl β-d-glucopyranoside (BNG) in the presence of 4-benzoylamino-2,5-diethoxybenzenediazonium chloride hemi[zinc chloride] salt (Fast Blue BB salt). Upon hydrolysis of BNG, the aglucone adheres to proteins (Cohen et al., 1952) and forms an insoluble complex with the Fast Blue BB salt, resulting in red/brown staining at the site of hydrolysis. BNG is an artificial β-glucosidase substrate cleaved by some β-glucosidases and has previously been used to monitor the localization of α-hydroxynitrile glucoside β-glucosidase activity in L. corniculatus (Rissler and Millar, 1977) and almond (Sánchez-Pérez et al., 2008). The reason that BNG may be used to specifically show the localization of hydroxynitrile-cleaving β-glucosidases is that these are abundant.
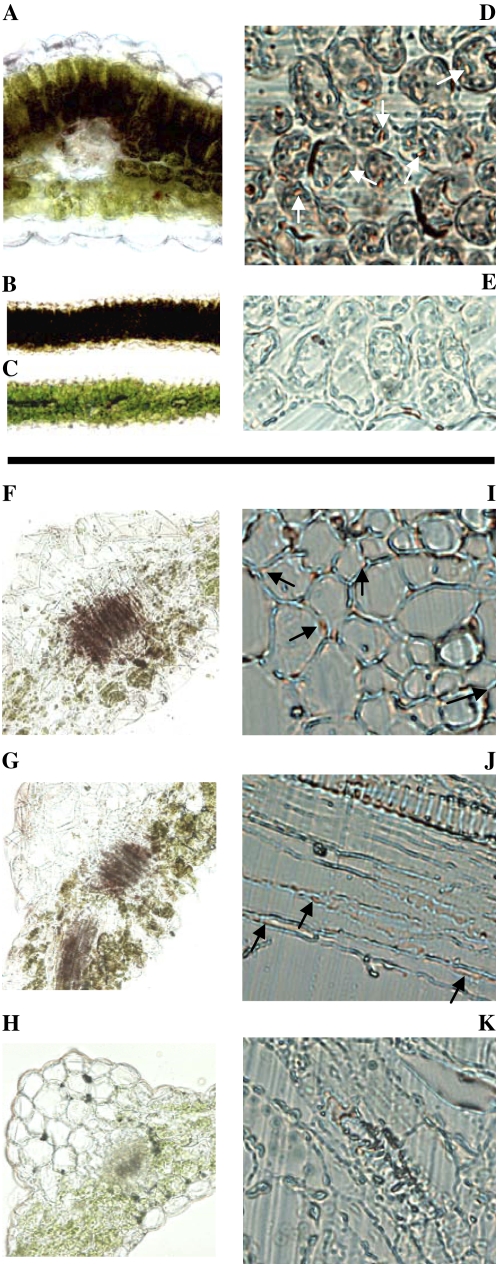
In L. japonicus wild-type leaves, color development representing β-glucosidase activity was observed in nearly all mesophyll cells, while none was detected in epidermal cells (Fig. 9, A and B). The strongest β-glucosidase activity was observed in palisade cells and in the spongy cells adjacent to these (Fig. 9, A and B). At the cellular level, distinct areas within the symplast showed strong staining (Fig. 9D). No or very weak color development was observed upon the addition of Fast Blue BB salt in the absence of BNG, demonstrating that the chromogenic reaction is dependent on the presence of the BNG aglucone formed by β-glucosidase hydrolytic activity (Fig. 9C).
Figure 9.
Localization of β-glucosidase activity in leaves of L. japonicus wild type (A–E) and transgenic Arabidopsis expressing LjBGD2 and LjBGD4 (F–K). β-Glucosidase activity is represented by red/brown staining resulting from the hydrolysis of the artificial substrate BNG and subsequent aglucone complex formation with Fast Blue BB salt. Cross sections (80 μm) of apical leaves (fresh tissue) of L. japonicus wild type show strong color development in leaf palisade tissue in the presence of BNG and Fast Blue BB salt (A and B), while no staining is observed in the absence of BNG (C). A represents a 20× magnification, while B and C show 5× magnifications. Visualization of the subcellular localization of β-glucosidase activity is possible at 40× magnification of 6-μm cross sections of fixed apical leaf tissue. Application of BNG results in strong staining (indicated by white arrows) localized to the symplast (D), whereas only diffuse and weak apoplastic background color development is observed in the absence of BNG (E). BNG staining for β-glucosidase activity in 80-μm cross sections (20× magnification, fresh tissue) of rosette leaves of Arabidopsis expressing LjBGD2 (F), LjBGD4 (G), and the wild type (H) show that LjBGD2 and LjBGD4 both hydrolyze BNG but no BNG hydrolysis is observed in Arabidopsis wild type. I, J, and K are 40× magnifications of 10-μm sections of fixed Arabidopsis rosette leaves. I shows the subcellular localization of LjBGD2 in a cross section (black arrows). J illustrates the subcellular localization of LjBGD4 in a longitudinal section (black arrows). Very weak apoplastic background staining is observed around the xylem in Arabidopsis wild type (K) as well as in transgenic Arabidopsis. Identical subcellular and tissue localizations of LjBGD2 and LjBGD4 activity are observed upon heterologous expression of the enzymes in Arabidopsis, with activity localized in the symplast at the subcellular level (in agreement with L. japonicus) and in and around the vascular tissue at the tissue level (in contrast to L. japonicus).
The transgenic Arabidopsis expressing either LjBGD2 or LjBGD4 showed strong β-glucosidase activity upon the addition of Fast Blue BB salt in the presence of BNG (Fig. 9, F and G). In contrast, no BNG-specific β-glucosidase activity was observed in Arabidopsis wild-type leaves (Fig. 9H). Thus, the observed staining in the transgenic leaves reflects the expression of LjBGD2 or LjBGD4 and demonstrates that these enzymes are active after fixation.
The color development and, hence, BNG hydrolysis proceeded at a slower rate compared with L. japonicus. This is most likely due to a more abundant accumulation of the β-glucosidases in L. japonicus and/or the presence of an inhibitor in Arabidopsis. The presence of an inhibitor is in agreement with the observed lack or low levels of enzyme activity in Arabidopsis leaf homogenates. In agreement with the results observed in L. japonicus leaves, both LjBGD2 and LjBGD4 are localized within distinct areas in the symplast upon heterologous expression in Arabidopsis (Fig. 9, I and J). No or very weak color development was observed in the apoplast, demonstrating that only a weak background reaction takes place in the absence of BNG (Fig. 9, E and K) in L. japonicus wild type and in both wild-type and transgenic Arabidopsis.
In all transgenic Arabidopsis lines, LjBGD2 and LjBGD4 activity was concentrated in the phloem parenchyma, and the activity was very low in other cell types (Fig. 9, F and G). This is unexpected, as expression of LjBGD2 an LjBGD4 is under the control of the generally regarded constitutive CaMV 35S promoter. These results suggest that the activity of the heterologously expressed LjBGD2 and LjBGD4 could be specifically inhibited in Arabidopsis leaf cells except those surrounding the vascular tissue. This is in agreement with the observed ability to measure hydroxynitrile glucoside β-glucosidase activity only when assaying leaf discs and not upon tissue disruption caused by maceration or freezing/thawing. The data obtained with the Arabidopsis transgenic lines complement the symplastic localization of hydroxynitrile glucoside-cleaving β-glucosidase observed in L. japonicus (compare Fig. 9, D and I).
DISCUSSION
LjBGD2 and LjBGD4 Are Hydroxynitrile Glucoside β-Glucosidases Present in L. japonicus Leaves
Purification, identification by protein sequencing, and biochemical characterization of LjBGD2 and LjBGD4 showed that these two enzymes are the hydroxynitrile glucoside-cleaving β-glucosidases in L. japonicus leaves. Heterologous expression in Arabidopsis verified these conclusions and demonstrated that LjBGD2 and LjBGD4 possess very similar activity profiles and that the activity is not dependent on heterodimer formation. The localization of LjBGD2 and LjBGD4 in wild-type leaves of L. japonicus and in transgenic Arabidopsis leaves was determined using the chromogenic β-glucosidase substrate BNG. The data indicate that the hydroxynitrile glucoside-cleaving β-glucosidases are localized intracellularly, possibly in protein bodies, as observed for the P. serotina prunasin and amygdalin hydrolases (Swain et al., 1992).
The predicted molecular masses of the mature LjBGD2 and LjBGD4 proteins are 55.9 and 56.9 kD, respectively. Analysis of the primary sequences revealed the presence of three and five potential N-glycosylation sites in LjBGD2 and LjBGD4, respectively. Other dicotyledenous α-hydroxynitrile glucoside β-glucosidases are known to be glycosylated (Hughes et al., 1992; Li et al., 1992; Ahn et al., 2007), and heterogeneity in the overall glycosylation pattern within LjBGD2 and LjBGD4 may account for the observed presence of both proteins in the 60- and 55-kD protein bands following SDS-PAGE (Fig. 3, lane 5). The apparent molecular mass of 107 kD observed by gel filtration chromatography suggests that the native LjBGD2 and LjBGD4 proteins exist as dimers, as reported previously for a selection of other α-hydroxynitrile glucoside β-glucosidases (Hughes and Dunn, 1982; Kuroki and Poulton, 1987; Verdoucq et al., 2004). It cannot be excluded that a third β-glucosidase specific for rhodiocyanoside A cleavage was lost during purification, because protein fractions that did not show an ability to hydrolyze lotaustralin or linamarin were not separately analyzed for hydrolytic activity toward rhodiocyanoside A due to a shortage of this substrate. However, this is unlikely to have happened, because LjBGD2 and LjBGD4 both showed high activity toward rhodiocyanoside A. The hydroxynitrile glucoside-cleaving β-glucosidases were purified 18-fold from young L. japonicus leaves. The low purification fold reflects the high concentration of these enzymes among the soluble leaf proteins and corresponds to the levels of other abundantly expressed plant β-glucosidases from T. repens, Hevea brasiliensis (rubber tree), Z. mays, Prunus avium (sweet cherry), and S. bicolor (Hughes and Dunn, 1982; Selmar et al., 1987; Pocsi et al., 1989; Esen, 1992; Gerardi et al., 2001; Verdoucq et al., 2004). Purification of linamarase from M. esculenta and prunasin and amygdalin hydrolases from P. serotina seeds provided significantly higher purification folds (Kuroki and Poulton, 1986, 1987; Li et al., 1992; Yeoh and Woo, 1992) because of the minor abundance of these α-hydroxynitrile glucoside-cleaving β-glucosidases.
Linamarin Is a Poor Substrate for L. japonicus Hydroxynitrile Glucoside-Cleaving β-Glucosidases in Contrast to Lotaustralin and Rhodiocyanoside A
Upon tissue disruption of L. japonicus leaves, the rhodiocyanosides and lotaustralin were rapidly hydrolyzed, whereas linamarin was not (Fig. 2). This pattern is reflected in the catalytic parameters observed for the partially purified LjBGD2 and LjBGD4, with the Km values for rhodiocyanoside A, lotaustralin, and linamarin being 0.2, 0.7, and 6.7 mm, respectively (Table I). The Km values for rhodiocyanoside A and lotaustralin are similar to those obtained for other purified α-hydroxynitrile glucoside β-glucosidases (Fan and Conn, 1985; Hösel et al., 1987; Kuroki and Poulton, 1987; Mkpong et al., 1990; Li et al., 1992). Although the Km of the L. japonicus enzymes toward linamarin appeared unphysiologically high, it remains comparable to the reported Km values of 4.3, 5.6, and 7.6 mm toward linamarin for linamarases purified from T. repens, Phaseolus lunatus (butter bean), and H. brasiliensis (Itoh-Nashida et al., 1987; Selmar et al., 1987; Pocsi et al., 1989), respectively. It has been suggested that the high abundance of some α-hydroxynitrile glucoside β-glucosidases compensates for their low turnover (Esen, 1992), the most significant example being H. brasiliensis linamarase, which constitutes approximately 30% of the total soluble leaf protein (Selmar et al., 1987).
The low abundance of linamarin in L. japonicus wild-type leaves (less than 5% of the total hydroxynitrile glucosides; Forslund et al., 2004; Bjarnholt et al., 2008) could suggest that linamarin is not a relevant physiological substrate for LjBGD2 and LjBGD4. In L. japonicus cotyledons, however, linamarin constitutes a higher proportion (20%) of the total α-hydroxynitrile glucoside content (Forslund et al., 2004). It is possible that an α-hydroxynitrile glucoside-cleaving β-glucosidase that more efficiently degrades linamarin is specifically expressed in cotyledons. However, among the β-glucosidase sequences obtained from the L. japonicus genome sequencing project, only LjBGD2, LjBGD4, and LjBGD7 fall within the hydroxynitrile-cleaving β-glucosidase cluster in the phylogenetic analysis (Fig. 7; http://www.p450.kvl.dk/BGD.shtml), which argues against this possibility. Alternatively, rapid degradation of lotaustralin results in an immediate release of HCN to provide a cyanide bomb-type defense to instantly repel chewing insects, whereas slow degradation of linamarin would result in a less pronounced but long-term release of HCN, signaling ongoing cyanogenesis as a warning to potential attackers.
Docking of the hydroxynitrile glucosides in the predicted LjBGD2 and LjBGD4 active sites (Fig. 6C) suggested that the small aglucone of linamarin would enable relatively free movement within the active site. This could prevent orientation of the β-glucosidic bond in a fixed position between the two catalytic Glu residues, as required for hydrolysis, and thus result in a higher Km. The bulkier aglucones of rhodiocyanoside A and lotaustralin are expected to direct a more rigid binding of these hydroxynitrile glucosides in the active site and thus favor a more efficient hydrolysis of the β-glucosidic bond.
The in Planta Function of LjBGD2 and LjBGD4 Is to Hydrolyze Hydroxynitrile Glucosides
The substrate specificity of plant β-glucosidases has been a matter of much debate (Hösel and Conn, 1982; Conn, 1993). The S. bicolor dhurrinases show very narrow substrate specificity and only hydrolyze the natural substrate dhurrin, the Phe-derived sambunigrin, and, in the case of dhurrinase 2, the artificial fluorescent substrate 4-methylumbelliferyl β-glucoside (Hösel et al., 1987). P. serotina prunasin hydrolases likewise show high substrate specificity toward prunasin and no activity against aliphatic α-hydroxynitrile glucosides, but in contrast to S. bicolor dhurrinase 1 and 2, they do hydrolyze pNPG (Kuroki and Poulton, 1987). As opposed to these highly specific α-hydroxynitrile glucoside β-glucosidases, linamarases isolated from Linum ussitassimum (flax) and P. lunatus efficiently hydrolyze aromatic α-hydroxynitrile glucosides and pNPG in addition to their endogenous aliphatic substrates, linamarin and lotaustralin (Fan and Conn, 1985; Itoh-Nashida et al., 1987). The broad substrate specificity of LjBGD2 and LjBGD4 toward hydroxynitrile glucosides resembles those of the L. ussitassimum and P. lunatus linamarases.
The observed ability of LjBGD2 and LjBGD4 (our data) and almond β-glucosidase (Chuankhayan et al., 2007b) to hydrolyze daidzin is in agreement with the hypothesis that the isoflavonoid glucoside-cleaving β-glucosidases have evolved from the hydroxynitrile glucoside-cleaving β-glucosidases, as suggested by the phylogenetic relationship in Figure 7 and by Chuankhayan et al. (2007b). Legumes are characterized by containing cyanogenic glucosides as well as isoflavonoid glucosides. It remains to be investigated to what extent isolated β-glucosidases are able to hydrolyze cyanogenic glucosides as well as isoflavonoid glucosides. The α-hydroxynitrile diglucoside-specific β-glucosidases amygdalin hydrolase and linustatinase, purified from P. serotina and L. ussitassimum, catalyze the hydrolysis of the terminal O-linked Glc residue of endogenously occurring amygdalin and linustatin, respectively, to provide the corresponding monoglucosides prunasin and linamarin (Fan and Conn, 1985; Kuroki and Poulton, 1986). In addition, amygdalin hydrolase shows activity toward the aliphatic counterparts linustatin and neolinustatin and vice versa for linustatinase (Fan and Conn, 1985; Kuroki and Poulton, 1986). The lack of ability of LjBGD2 and LjBGD4 to degrade the diglucoside amygdalin, while the corresponding monoglucoside is readily hydrolyzed, is a common trait for all of the above-mentioned hydroxynitrile glucoside-cleaving β-glucosidases: all are highly specific for either monoglucosides or diglucosides, except for a single report stating that linustatinase is able to hydrolyze the α-hydroxynitrile monoglucoside dhurrin (Fan and Conn, 1985). The α-hydroxynitrile diglucosides have been proposed to be nonhydrolyzable transport forms of the corresponding monoglucosides (Selmar et al., 1988). Absolute specificity of the β-glucosidases toward either monoglucosides or diglucosides would enable tight control of α-hydroxynitrile glucoside transport through compartmentalization and specific developmental expression of the β-glucosidases. Until now, neither linustatin nor neolinustatin, the glucosylated forms of linamarin and lotaustralin, have been detected in L. japonicus (A.V. Morant, C.E. Olsen, B.L. Møller, and S. Bak, unpublished data). This may be due to their occurrence in very low concentrations and/or their exclusive presence at defined developmental stages and in specific cell types. In H. brasiliensis, linustatin is detected only during a 48-h period in seedling development (Selmar et al., 1988).
The Biological Implications of Rhodiocyanoside Hydrolysis Is Unknown
The L. japonicus hydroxynitrile glucoside-cleaving β-glucosidases exhibit a low Km and high Vmax toward rhodiocyanoside A, and this compound is rapidly hydrolyzed upon tissue disruption of apical L. japonicus leaves (Fig. 2). L. corniculatus (Zagrobelny et al., 2004, 2007a), T. repens (data not shown), and M. esculenta (data not shown) all accumulate linamarin and lotaustralin but do not contain rhodiocyanosides. Nevertheless, leaf extracts of L. corniculatus and T. repens hydrolyze rhodiocyanoside A as rapidly as linamarin, whereas M. esculenta extracts hydrolyze rhodiocyanide A at a somewhat lower rate (A.V. Morant, N. Bjarnholt, K. Jørgensen, B.L. Møller, and S. Bak, unpublished data). Hence, the ability to degrade γ-hydroxynitrile glucosides in addition to the α-hydroxynitrile glucosides might be an intrinsic characteristic of linamarases.
The biological function of rhodiocyanosides remains unknown (Bjarnholt and Møller, 2008). Hydrolysis of rhodiocyanosides does not result in the liberation of toxic HCN. The observed parallel hydrolysis of rhodiocyanosides and α-hydroxynitrile glucosides suggests that the corresponding aglucones have an antimicrobial or herbicidal effect and act in concert with the α-hydroxynitrile glucosides to deter pathogens and herbivores. The Ile-derived rhodiocyanosides might have emerged as a side product of lotaustralin biosynthesis in L. japonicus. Because the hydroxynitrile glucoside-cleaving β-glucosidases hydrolyze rhodiocyanosides, the biosynthesis of rhodiocyanosides could represent an evolutionary adaptation of L. japonicus to specialized insects that are attracted rather than repelled by the α-hydroxynitrile glucosides and their hydrolysis products. In free-choice laboratory experiments, larvae of Zygaena filipendulae (burnet moth) prefer feeding on L. japonicus to L. corniculatus, which does not contain rhodiocyanosides. However, the larvae raised on L. japonicus suffer during pupation and the transition into an imago, as evidenced by reduced growth and increased mortality rates. While α-hydroxynitrile glucosides are transferred from the larval instars to the imago, the rhodiocyanosides are not (Zagrobelny et al., 2007a, 2007b, 2008).
Expression Profiles of Genes Encoding Enzymes Involved in the Biosynthesis and Bioactivation of Hydroxynitrile Glucosides Suggest the Evolution of Parallel Pathways in Leaves and Roots of L. japonicus
LjBGD2 and LjBGD4 are expressed in L. japonicus aerial parts, as revealed by purification of the encoded enzymes from young leaves and by RT-PCR analysis. In contrast, LjBGD7 is expressed exclusively in L. japonicus roots. This differential expression pattern corresponds well to that observed for the genes encoding the hydroxynitrile glucoside biosynthetic enzymes (Forslund et al., 2004), with coexpression of CYP79D3 with LjBGD2 and LjBGD4 in aerial tissues and coexpression of CYP79D4 with LjBGD7 in roots. Although the biochemical function of LjBGD7 has not been demonstrated, its position within the eudicotyledenous hydroxynitrile glucoside-specific β-glucosidase cluster in the phylogenetic tree (Fig. 7) and its very high amino acid sequence identity to LjBGD2 and LjBGD4 imply that LjBGD7 is a hydroxynitrile glucoside-cleaving β-glucosidase. Hydroxynitrile glucosides are absent, or present only in minute amounts, in wild-type roots of L. japonicus, but upon ectopic expression of M. esculenta CYP79D2 under the control of the constitutive CaMV 35S promoter, linamarin and lotaustralin but no rhodiocyanosides do accumulate in roots (Forslund et al., 2004). Unless this is a mere consequence of the transport of α-hydroxynitrile glucosides produced in aerial parts to the roots, these results indicate that L. japonicus roots contain the biosynthetic machinery for the production of α-hydroxynitrile glucosides. In accordance, LjBGD7 could represent the corresponding hydroxynitrile glucoside-cleaving β-glucosidase. The physiological conditions required to induce hydroxynitrile glucoside synthesis in wild-type roots of L. japonicus remain unknown, as does the possible biological significance of the presence of hydroxynitrile glucosides in this tissue (Bjarnholt and Møller, 2008). Apart from an obvious function in defense, α-hydroxynitrile glucosides have been suggested to serve as nitrogen storage compounds (Selmar et al., 1988; Jenrich et al., 2007). In roots, the α-hydroxynitrile glucosides or the aglucone or HCN formed after hydrolysis may serve as important signaling molecules secreted into the rhizosphere. Preliminary results from the expression of a GFP-GUS reporter construct under the control of the CYP79D3 and CYP79D4 promoters in L. japonicus indicate that, in addition to being constitutively expressed in roots, CYP79D4 is wound inducible in aerial parts. These results are in agreement with metabolite analyses showing that the concentration of hydroxynitrile glucosides in L. japonicus leaves increases in response to mechanical wounding (A.V. Morant, N. Bjarnholt, M.E. Kragh, C.H. Kjærgaard, K. Jørgensen, S.M. Paquette, M. Piotrowski, A. Imberty, C.E. Olsen, B.L. Møller, and S. Bak, unpublished data). Further experiments are required to determine whether LjBGD7 is able to hydrolyze hydroxynitrile glucosides and, in parallel to CYP79D4, whether it is expressed in aerial parts of L. japonicus in response to wounding. If the hydroxynitrile glucoside/β-glucosidase system in L. japonicus is truly wound inducible, this would extend the classification of α-hydroxynitrile glucosides in L. japonicus to phytoalexins (defense compounds synthesized in response to pathogen or herbivore attack) in addition to phytoanticipins (preformed defense compounds). Insect-induced changes in the profile of secondary metabolites otherwise characterized as phytoanticipins were previously observed for glucosinolates in Arabidopsis (Mewis et al., 2005).
Why Do L. japonicus Leaves Contain Two Apparently Highly Similar Hydroxynitrile Glucoside β-Glucosidases?
The presence of LjBGD2 and LjBGD4 in L. japonicus leaves raises the question of why L. japonicus produces two apparently redundant hydroxynitrile glucoside-cleaving β-glucosidases. LjBGD2 is located on chromosome 3 and LjBGD4 on chromosome 5. This, in combination with the approximately 85% amino acid sequence identity shared between the encoded enzymes, argues against LjBGD2 and LjBGD4 as resulting from a recent gene duplication and as being truly redundant. The chromosomal location rather suggests that both paralogues have been retained through evolution due to different biochemical activities or expression profiles. Furthermore, pairwise alignment of a 1,000-nucleotide region immediately upstream from the ATG start codon revealed that the promoter regions of LjBGD2 and LjBGD4 share only approximately 50% sequence identity, with no increase in sequence identity in the region approaching the start codon (data not shown). Combined, these data indicate that LjBGD2 and LjBDG4 are not redundant enzymes but have acquired separate biological functions in the process of subfunctionalization. The chromosomal location of LjBGD7 is currently not known. Future experiments using in tube in situ PCR and promoter fusion constructs will reveal whether LjBGD2 and LjBGD4 are differentially expressed at the cellular or developmental level and might be involved in different phases of cyanogenesis, depending on the cellular location or nature of pathogen or insect attack.
One potential reason for the coaccumulation of LjBGD2 and LjBGD4 in L. japonicus leaves could be that heterodimer formation could lead to a change in substrate specificity or activity. However, the highly similar predicted active site topologies and the nearly identical substrate specificity profiles obtained when LjBGD2 and LjBGD4 were separately expressed in transgenic Arabidopsis and when the two copurifying β-glucosidases were tested together argue against this scenario.
The presence of two or more isoforms of β-glucosidases has been observed in several other cyanogenic and noncyanogenic plants. P. serotina contains several isoforms of both prunasin and amygdalin hydrolases (Kuroki and Poulton, 1986, 1987; Li et al., 1992), and multiple isoforms of linamarase are found in M. esculenta (Mkpong et al., 1990). Likewise, Z. mays, S. bicolor, and A. sativa each contain two isoenzymes of the β-glucosidases that bioactivate the DIMBOA-Glc, dhurrin, and avenacosides found in these three crops, respectively (Hösel et al., 1987; Kim and Kim, 1998; Cicek and Esen, 1999, Morant et al., 2008). As-Glu-1 and As-Glu-2, the two avenacoside β-glucosidases in A. sativa (Fig. 7), exist as homooligomers of As-Glu-1 as well as heterooligomers of both isoenzymes (Kim and Kim, 1998; Kim et al., 2000). Whether the homooligomers and the heterooligomers display different biological functions in vivo is unknown, except that the encoding genes are differentially expressed (Kim and Kim, 1998), as are the Z. mays and S. bicolor paralogues (Hösel et al., 1987; Cicek and Esen, 1999). In addition, two apparently redundant myrosinases, TGG1 and TGG2 (Fig. 7), with nearly identical expression patterns are found in the aerial parts of Arabidopsis (Barth and Jander, 2006). Hence, it appears that plant β-glucosidases constitute a highly fine-tuned system in which even subtle differences in biochemical activity, localization, or expression are important for the bioactivation of defense compounds in response to diverse biotic stresses at different stages of plant development. A number of interacting factors may control and modulate β-glucosidase activity in accordance with demands (Morant et al., 2008). Recently, the complexity of the pathways responsible for the induction of isoflavonoid synthesis and β-glucosidase-mediated activation was documented by thorough studies in Medicago truncatula (Naoumkina et al., 2007; Farag et al., 2008) and in G. max (Suzuki et al., 2006).
MATERIALS AND METHODS
Chemicals and Plant Growth Conditions
Lotus japonicus GIFU B-129-S9 plants were grown hydroponically at approximately 24°C in a greenhouse fitted with extra light bulbs, ensuring a minimum photosynthetic flux of 100 to 120 μmol photons m−2 s−1, as described by Forslund et al. (2004). Arabidopsis (Arabidopsis thaliana) plants were grown in 9-cm-diameter plastic pots in autoclaved soil supplemented with vermiculite (10%, v/v) in an insect-free growth chamber at 22°C, with 70% humidity, a photosynthetic flux of 100 μmol photons m−2 s−1, and a 16-h-light/8-h-dark regime, as described by Kristensen et al. (2005).
Linamarin was purchased from AG Scientific, lotaustralin from Toronto Research Chemicals, and dhurrin from Carl Roth. pNPG, BNG, Fast Blue BB salt, and amygdalin were from Sigma. Daidzin was purchased from Tauto Biotech, and kuromanin chloride salt was from Polyphenols. Rhodiocyanoside A was purified from L. japonicus leaves (Bjarnholt and Møller, 2008). Prunasin was kindly synthesized by Dr. Mohammed Saddik Motawia. pOHBG and 4-(methylthio)butyl glucosinolate were from C2 Bioengineering.
Analysis of Hydroxynitrile Glucoside Degradation in Crude L. japonicus Extracts
Apical leaf extracts of L. japonicus 35S∷CYP79D2 line number 5 (Forslund et al., 2004) were made by subjecting two leaf discs (diameter, 6 mm) to three freeze/thaw cycles while submersed in 200 μL of MES (20 mm, pH 6.5). The apical leaf extract was diluted 10 times and incubated at 30°C (300 rpm). Aliquots (25 μL) were collected at 0, 10, 20, 30, and 60 min and immediately transferred to an equal volume of 100% methanol to stop further β-glucosidase activity. The relative concentrations of linamarin, lotaustralin, and rhodiocyanosides were quantified by liquid chromatography-mass spectrometry (LC-MS; described below) based on the extracted ion chromatograms and taking advantage of the similar ionization efficiency of all three compounds (Bjarnholt and Møller, 2008).
Isolation of Hydroxynitrile Glucoside-Cleaving β-Glucosidases from L. japonicus Leaves and Protein Sequencing
Soluble proteins from apical leaves of L. japonicus were extracted by grinding the leaves in ice-cold extraction buffer (5 v/w plant material of 100 mm MES [pH 7.0], 250 mm Suc, 50 mm NaCl, 2 mm EDTA, 2 mm DTT, 0.5 mm phenylmethylsulfonyl fluoride, 10% [w/v] polyvinylpolypyrrolidone, and 0.5% [w/v] 6-capronic acid). After removal of the cell debris by centrifugation, the soluble protein extract (pH adjusted to 5.5) was incubated (4°C, 30 min, gentle shaking) with 10% (v/v) cation exchange resin preequilibrated in 100 mm MES (pH 5.5; Source 15S; Amersham Biosciences). The resin was recovered by centrifugation, and bound protein was extracted with 100 mm MES (pH 7.0) fortified with 500 mm NaCl. The protein extract was dialyzed (SpectraPor, 12- to 14-kD molecular mass cutoff [Spectrum Laboratories]) against 100 mm MES (pH 7.0, 4°C) and stored at 4°C. An aliquot of the protein extract (500 μg of protein; Bradford, 1976) was size fractionated by gel filtration chromatography (Superdex G200 HR 10/ 30 column; Amersham Biosciences) using 50 mm Tris-HCl (pH 7.0) and 150 mm NaCl as equilibration and elution buffer. Elution of proteins with β-glucosidase activity was monitored using lotaustralin and linamarin as substrates and colorimetric detection of cyanide release (Halkier and Møller, 1989; Jørgensen et al., 2005a). The polypeptide composition of the main fraction showing β-glucosidase activity was analyzed by 12% SDS-PAGE and showed enrichment in two proteins at 60 and 55 kD. In-gel digestion of the Coomassie Blue-stained protein bands with trypsin and sequencing of the peptides by mass spectrometry were performed as described by Piotrowski and Volmer (2006). Database searches (nrdb95) were done with MS Blast (Shevchenko et al., 2001), available at http://dove.embl-heidelberg.de/Blast2/msblast.html. LjBGD2 and LjBGD4 were identified by manual assignment of the peptide sequences to assembled β-glucosidase sequences from L. japonicus.
Biochemical Characterization of LjBGD2 and LjBGD4
Km and Vmax values were determined using assay mixtures (total volume, 200 μL) containing purified L. japonicus β-glucosidases (200× diluted) and a number of different substrates (10 concentrations between 25 μm and 4 mm) in 20 mm MES (pH 6.5). At the end of the incubation period (10 min, 30°C, 300 rpm), the enzyme reaction was stopped and metabolic conversions were measured. Assay mixtures containing linamarin, lotaustralin, dhurrin, prunasin, amygdalin, and pOHBG hydrolysis were stopped by the addition of NaOH (40 μL, 6 n), and metabolism was measured by colorimetric detection of cyanide release (Halkier and Møller, 1989; Jørgensen et al., 2005a) or SCN− release in the case of pOHBG (Bak et al., 1999). KCN and NaSCN standard curves were included to enable quantification. Assay mixtures containing rhodiocyanoside A were stopped by the addition of an equal volume of 100% methanol, and metabolism was measured by quantification of the remaining amounts of rhodiocyanoside A by LC-MS based on a rhodiocyanoside A standard curve. For pNPG, 100 μL of 0.4 m Na2CO3 was added and pNPG metabolism was measured (optical density at 410 nm) by colorimetric detection of aglucone release. For quantification, a pNPG standard curve was produced by reaction of 0 to 50 μm pNPG with an excess of cation exchange-purified L. japonicus leaf protein. Daidzin hydrolysis was tested at seven concentrations between 25 and 400 μm (added in 5 μL of dimethyl sulfoxide). Kuromanin hydrolysis was evaluated at a concentration of 1 mm, and the reaction was stopped by the addition of 200 μL of methanol containing 4% formic acid for stabilization of the remaining kuromanin. The daidzin and kuromanin results were quantified by LC-MS as described below.
Assays to determine the biochemical activity of recombinant LjBGD2 and LjBGD4 were performed using leaf discs from Arabidopsis T3 transformants homozygous for the respective transgenes. Relative turnovers of glucosides by Arabidopsis expressing LjBGD2 and LjBGD4 were determined using assay mixtures (total volume, 200 μL) containing two 6-mm leaf discs from rosette leaves of 4- to 5-week-old plants and 1 mm substrate in 20 mm MES (pH 6.5) and incubation for 10 min (30°C, 300 rpm). Reactions were stopped and analyzed as described above. Daidzin turnover was determined in the same way except that the substrate concentration was 0.75 mm added in 10 μL of dimethyl sulfoxide, due to the limited solubility of the compound in aqueous medium. This did not affect enzyme activity in the leaf disc assay. Kuromanin turnover was also determined at a concentration of 0.75 mm for comparison with daidzin, and the reaction was stopped by the addition of 200 μL of methanol containing 4% formic acid. The results were quantified by LC-MS as described below.
LC-MS Analysis
Analytical LC-MS of hydroxynitrile glucosides was carried out using an Agilent 1100 Series liquid chromatograph (Agilent Technologies) fitted with an XTerra MS C18 column (Waters; 3.5 μm, 2.1 × 100 mm; flow rate, 0.2 mL min−1) coupled to a Bruker Esquire 3000+ ion trap mass spectrometer (Bruker Daltonics). The mobile phases were as follows: A, water with HCOOH (0.1%, v/v) and NaCl (50 μm); B, MeCN/water (80%, v/v) with HCOOH (0.1%). The gradient program was as follows: 0 to 4 min, isocratic 2% B; 4 to 10 min, linear gradient 2% to 8% B; 10 to 30 min, linear gradient 8% to 50% B; 30 to 35 min, linear gradient 50% to 100% B; and 35 to 40 min, isocratic 100% B. The mass spectrometer was run in electrospray mode, and positive ions were observed. The HPLC solvent contained NaCl and formic acid to facilitate the identification of adduct ions (and [M+H+]) as described previously (Tattersall et al., 2001; Forslund et al., 2004; Kristensen et al., 2005). Analyses of daidzin and kuromanin were carried out using a Dionex HPLC apparatus with an HPLC pump (Dionex P680), autosampler (Dionex ASI-100), and UV light detector (Dionex UVD340U) with the column oven set to 25°C (Dionex STH585). Separation was performed on a Synergy Fusion column from Phenomenex (150 × 2 mm, 4-μm particles, 0.3 mL/min), and the mobile phases were as follows: A, water with 0.1% formic acid and 2% MeCN (v/v); and B, MeCN/water (50:50, v/v) with 0.1% formic acid. The gradient program for daidzin was as follows: 0 to 2 min, 30% B; 2 to 12 min, linear gradient 0% to 100% B; and 12 to 20 min, 100% B, followed by wash and equilibration. The gradient program for kuromanin was as follows: 0 to 1 min, 0% B; 1 to 8 min, linear gradient 0% to 100% B; and 8 to 15 min, 100% B, followed by wash and equilibration. The flow was passed directly from the UV light detector to the mass spectrometer, a Thermo Finnigan MSQ single quadrupole device operated in electrospray mode with positive ionization (ionization temperature, 365°C; cone voltage, 2.5 kV). Daidzin and its aglucone were detected as the [M+H]+ ion and kuromanin as the [M]+ ion.
Protein Sequence Alignments, Phylogenetic Trees, Protein Sequence Logos, and Prediction of Subcellular Localization and Molecular Weight
Protein sequence alignments were made using ClustalX 1.83 for Windows (http://bips.u-strasbg.fr/fr/Documentation/ClustalX/). ClustalX parameters were as follows. Pairwise alignment parameters: gap opening penalty, 13.50; gap extension penalty, 0.75; protein weight matrix, Gonnet 250. Multiple alignment parameters: gap opening penalty, 15.00; gap extension penalty, 1.00; protein weight matrix, Gonnet series. All gaps were reset before any alignment was run. Alignments were colored using Boxshade (http://www.isrec.isb-sib.ch/ftp-server/boxshade/3.3.1/) compiled for a Windows 32 environment. Neighbor-joining phylogenetic trees were constructed using MEGA 4.0 (Tamura et al., 2007; http://www.megasoftware.net/index.html) with 1,000 bootstrap trials performed. Gaps and missing data were handled via pairwise deletion, and the Poisson substitution model was used to calculate distances. Protein sequence logos were constructed using the Berkeley WebLogo Generator (http://weblogo.berkeley.edu/) using default parameters.
Signal sequence cleavage sites and subcellular localizations were predicted using PSORT (http://psort.ims.u-tokyo.ac.jp/) and TargetP (http://www.cbs.dtu.dk/services/TargetP/).
Molecular weights of the predicted mature sequences of LjBGD2 and LjBGD4 were predicted using the ExPASy Compute pI/Mw tool (http://www.expasy.org/tools/pi_tool.html).
Pairwise amino acid and nucleotide sequence alignments were performed using BioEdit (Hall, 1999) with default settings.
Comparative Modeling of LjBGD2 and LjBGD4
LjBGD2 and LjBGD4 models were built with the homology-modeling program COMPOSER (Blundell et al., 1988) of the Sybyl software (SYBYL). High-resolution crystal structures of TrCBG (1CBG; Barrett et al., 1995), SbDhr1 (1V02; Verdoucq et al., 2004), and Zm-Glu-1 (1e1f; Czjzek et al., 2001) were used as templates to build the structurally conserved regions of LjBGD2 and LjBGD4 (Protein Data Bank codes [Berman et al., 2000] are given in parentheses). Alignments of LjBGD2 and LjBGD4 with TrCBG, SbDhr1, and Zm-Glu-1 to define structurally conserved regions were performed with ClustalX and modified manually. Regions between two structurally conserved regions were defined as loops. Loops were modeled by comparison with TrCBG and SbDhr1. One loop in LjBGD2 (six amino acids long) and three loops in LjBGD4 (three to five amino acids long) could not be modeled by comparison with known β-glucosidase structures and were built manually. To identify backbone conformations that fell outside the allowed areas of the Ramachandran plot, the models were analyzed using PROCHECK (Laskowski et al., 1993) and subsequently optimized. Hydrogen atoms were added to all atoms of the LjBGD2 and LjBGD4 models, and partial atomic charges were calculated with the Pulmann method.
Linamarin, lotaustralin, and rhodiocyanoside A were graphically edited in Sybyl from β-Glc taken from the Glyco-3D monosaccharide database (http://www.cermav.cnrs.fr/glyco3d/index.php). Atom types and charges were chosen according to the PIM parameters for carbohydrate (Imberty et al., 1999), and each compound was docked into the active sites of LjBGD2 and LjBGD4 using COMPOSER. The Glc moieties were positioned in a chair conformation in the glucone-binding pockets of the models using the Zm-Glu-1 structure crystallized as a complex with the inhibitor p-nitrophenyl β-d-thioglucoside (Czjzek et al., 2001) as a template. While the substrate glucone was kept fixed in this defined position, the aglucone conformations were fit into the optimal position of the aglucone-binding sites by several cycles of energy optimization involving the aglucone and the amino acid side chains surrounding the binding site with the use of the Tripos force field (Clark et al., 1989). The active site pocket topologies were visualized using MOLCAD (Waldherr-Teschner et al., 1992), applying lipophilic surface representations. Cartoons showing overall protein conformations and stick representations of the amino acids lining the aglucone-binding pocket were made using the PyMOL molecular visualization system (http://pymol.sourceforge.net/).
Analysis of the Expression Patterns of LjBGD2, LjBGD4, and LjBGD7 in L. japonicus by RT-PCR
LjBGD2 cDNA was amplified using primers BGD2RTfor (5′-CATCTCTTTTATCTTCGATCTGC-3′) and BGD2RTrev (5′-CAGCAAGATGAAAGCAGGATCCTTG-3′). LjBGD4 cDNA was amplified with primers BGD4RTfor (5′-CATCTCTTTTATCTTCAATCTGC-3′) and BGD4RTrev (5′-CCACTAAAACAACCCAACAAGACG-3′), and LjBGD7 cDNA was amplified using primers BGD7RTfor (5′-CATATATTATCACACAAGAACAAGC-3′) and BGD7RTrev (5′-GATATGCTGCGGATGCTGTCCCAA-3′). Primers for the amplification of cDNA encoding the L. japonicus putative actin homolog were those applied by Forslund et al. (2004). L. japonicus cDNA obtained from Forslund et al. (2004) was used as template in PCRs (total volume, 20 μL) performed in Phusion HF PCR buffer (Finnzymes) containing 250 μm of each dNTP, 1.5 mm MgCl2, 0.5 μm of forward and reverse primers, and 0.2 unit of Phusion polymerase (Finnzymes). Thermal cycling parameters were as follows: 98°C for 30 s, followed by 30 (LjBGD2, LjBGD4, and ACTIN) or 35 (LjBGD7) cycles of 98°C for 10 s, 58°C for 30 s, and 72°C for 15 s. RT-PCR products were separated by agarose gel electrophoresis and visualized with ethidium bromide under UV light. The authenticity of the RT-PCR amplicons was verified by DNA sequencing.
Heterologous Expression of LjBGD2 and LjBGD4 in Arabidopsis
The full-length LjBGD2 cDNA was assembled from a genomic bacterial artificial chromosome (BAC) clone and a partial cDNA clone. Nucleotides 1 to 174 corresponding to the first exon were PCR amplified from BAC clone TM0568 (Kazusa DNA Research Institute) using primers 5′-ggtggtggtctgcagATGGCACTCAACACGTTCTTGG-3′ (restriction site underlined) and 5′-TCGCGCACCTTCATACTGGTATGCCGAGGATGCTGTC-3′. Nucleotides 175 to 1,545 corresponding to the remaining LjBGD2 cDNA sequence were amplified using the partial LjBGD2 EST AV423894 (Kazusa DNA Research Institute) as a template with primers 5′-TCCTCGGCATACCAGTATGAAGGTGCGGCAAATAAAGG-3′ and 5′-ccaccaccacccgggCTAATATCTTTTAAGAAAGTTTG-3′. PCR (total volume, 50 μL) was carried out in 10 mm Tris-HCl (pH 8.3), 50 mm KCl, and 2 mm MgSO4 containing 2.5 units of Pwo DNA polymerase (Roche), 50 μm dATP, 50 μm dCTP, 50 μm dGTP, 50 μm dTTP, and 10 pmol of the forward and reverse primers. Thermal cycling parameters were as follows: 94°C for 2 min; followed by 30 cycles of 94°C for 30 s, 55°C for 1 min, 0.2°C s−1 until 45°C, 45°C for 1 min, and 72°C for 30 s (first exon) or 90 s (remaining cDNA); and a final 72°C for 5 min. The partial cDNA sequences were joined by overlap extension PCR using the two outer primers and the two purified PCR products as templates. Thermal cycling parameters were the same as for amplification of LjBGD2 cDNA nucleotides 175 to 1,545. The purified PCR product corresponding to the full-length LjBGD2 cDNA was ligated into the PstI/XmaI sites of pPS48 (Odell et al., 1985; Kay et al., 1987) to yield pPSBGD2, and the nucleotide sequence was verified by DNA sequencing. In pPS48, the cDNA is inserted between the enhanced CaMV 35S promoter and terminator. The 35S∷BGD2 sequence was subsequently ligated into the HindIII site of pCAMBIA2301 to yield pCAMBIABGD2.
The LjBGD4 expression construct was assembled from a genomic BAC clone and a partial cDNA clone. The first intron was included to circumvent problems with PCR amplification and the ability to recover the full-length LjBGD4 cDNA. A HindIII site at nucleotide positions 512 to 517 in the LjBGD4 cDNA was removed by site-directed mutagenesis according to the recommendations from Stratagene to yield pBlueBGD4-3′ using primer 5′-CATTGGGATATGCCCCAAGCaTTGGAAGATGAGTATGG-3′ (changed nucleotide in lowercase) and its complement, with the partial LjBGD4 EST AV425071 (Kazusa DNA Research Institute) as a template to facilitate subsequent cloning steps. The first two exons including the first intron were amplified from BAC clone TM0569 (Kazusa DNA Research Institute) using primers 5′-ggtggtggtctgcagATGGCACTCAACACGTTCTTAG-3′ and 5′-CTATGCGCTCTGCATATTGCACTGAATAGTTGTGGGCGTAG-3′. The remaining cDNA sequence was amplified using pBlueBGD4-3′ as a template and primers 5′-gaagaagaaggatccCTAATATCTTTTGAGAAAGTTCG-3′ and 5′-CTACGCCCACAACTATTCAGTGCAATATGCAGAGCGCATAG-3′. The purified PCR products were joined by overlap extension PCR using the two outer primers. PCR was performed as described above. The obtained full-length PCR amplicon was ligated into the PstI/BamHI sites of pBluescript KS−, and the nucleotide sequence was verified by DNA sequencing. LjBGD4 was subcloned into the EcoRI/BamHI sites of pRT101 (Topfer et al., 1988) to introduce the enhanced CaMV 35S promoter and a poly(A) signal. Finally, the 35S∷BGD4 sequence was subcloned into the HindIII site of pPZP221 (Hajdukiewicz et al., 1994) to yield pPZPBGD4 for transformation into Arabidopsis. At each cloning stage, recombinant plasmids were transformed into Escherichia coli SURE cells (Stratagene). The use of E. coli SURE was required to enable cloning of the LjBGD4 nucleotide sequence, as classical E. coli strains did not allow the recovery of LjBGD4 plasmids. For construction of pCAMBIABGD2, E. coli XL1-Blue cells (Stratagene) were used.
pCAMBIABGD2 and pPZPBGD4 were transformed into Arabidopsis ecotype Columbia via Agrobacterium tumefaciens LBA4404 using the floral dip method (Clough and Bent, 1998), and transformed seeds were selected on Murashige and Skoog medium (Murashige and Skoog, 1962) supplemented with 100 μg/mL kanamycin and gentamycin, respectively.
Localization of β-Glucosidase Activity by in Tissue Staining
Apical leaves of L. japonicus wild type and rosette leaves of approximately 5-week-old Arabidopsis 35S∷LjBGD2, 35S∷LjBGD4, and wild type were embedded in plastic according to the manufacturer's recommendations for Technovit 8100 (Heraeus) with minor alterations. The tissues were dehydrated in a graded series of acetone solutions (25%, 50%, and 100%, 1 h each) and left overnight in the filtration solution. Sections (6 μm for L. japonicus, 10 μm for Arabidopsis) were cut on a Reichert-Jung 2030 rotary microtome. Leaves for 80-μm leaf cross sections were embedded in 5% agarose and cut on a Leica VT 1000S microtome.
In tissue staining for β-glucosidase activity was performed by incubation at 37°C in a solution of 2.6 mm BNG and 1.8 mm Fast Blue BB salt in 50 mm citrate and 100 mm phosphate buffer (pH 5.8). Incubation times were as follows: 15 and 30 min for 6- and 10-μm sections of L. japonicus and Arabidopsis, respectively, and 4 and 15 min (300 rpm) for 80-μm sections of L. japonicus and Arabidopsis, respectively. Assays excluding BNG were included as negative controls. Results were analyzed using a Leica DMR fluorescence microscope fitted with a Leica DC 300F camera.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EU10844 to EU10846.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment of peptide sequences obtained from purified hydroxynitrile glucoside β-glucosidases with full-length LjBGD2 and LjBGD4 polypeptide sequences.
Supplemental Figure S2. Illustration of the degree of conservation of amino acids in plant β-glucosidases by sequence logos.
Supplemental Tree Data S1. Additional phylogenetic trees.
Supplementary Material
Acknowledgments
We thank Dr. Mohammed Saddik Motawia for providing prunasin and for helpful discussions, Dr. Henrik Toft Simonsen for help with LC-MS analyses, and Ph.D. student Sarah Osmani for help with the presentation of protein structure models. We are grateful to Dr. René Mikkelsen for guidance in gel filtration chromatography. Steen Malmmose is sincerely thanked for taking great care of the L. japonicus plants. The Kazusa DNA Research Institute is thanked for providing L. japonicus β-glucosidase sequences.
This work was supported by grants from the Danish National Research Foundation to the Center for Molecular Plant Physiology, from the Villum Kann Rasmussen Foundation to “Pro-Active Plants,” and from the Faculty of Life Sciences, University of Copenhagen, for a Ph.D. stipend to A.V.M.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Søren Bak (bak@life.ku.dk).
The online version of this article contains Web-only data.
References
- Ahn YO, Saino H, Mizutani M, Shimizu B, Sakata K (2007) Vicianin hydrolase is a novel cyanogenic β-glycosidase specific to β-vicianoside (6-O-α-L-arabinopyranosyl-β-D-glucopyranoside) in seeds of Vicia angustifolia. Plant Cell Physiol 48 938–947 [DOI] [PubMed] [Google Scholar]
- Bak S, Feyereisen R (2001) The involvement of two P450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol 127 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36 393–405 [DOI] [PubMed] [Google Scholar]
- Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA (1999) Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J 20 663–671 [DOI] [PubMed] [Google Scholar]
- Bak S, Paquette S, Morant M, Morant AV, Saito S, Bjarnholt N, Zagrobelny M, Jørgensen K, Osmani S, Hamann T, et al (2006) Cyanogenic glucosides: a case study for evolution and application of cytochromes P450. Phytochem Rev 5 309–329 [Google Scholar]
- Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R (2001) CYP83B1, a cytochrome P450 at the metabolic branch paint in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T, Suresh CG, Tolley SP, Dodson EJ, Hughes MA (1995) The crystal structure of a cyanogenic β-glucosidase from white clover, a family 1 glycosyl hydrolase. Structure 3 951–960 [DOI] [PubMed] [Google Scholar]
- Barth C, Jander G (2006) Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J 46 549–562 [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnholt N, Møller BL (2008) Hydroxynitrile glucosides. Phytochemistry (in press) [DOI] [PubMed]
- Bjarnholt N, Rook F, Motawia MS, Cornett C, Jørgensen C, Olsen CE, Jaroszewski JW, Bak S, Møller BL (2008) Diversification of an ancient theme: hydroxynitrile glucosides. Phytochemistry 69 1507–1516 [DOI] [PubMed] [Google Scholar]
- Blundell T, Carney D, Gardner S, Hayes F, Howlin B, Hubbard T, Overington J, Singh DA, Sibanda BL, Sutcliffe M (1988) 18th Hans Krebs Lecture. Knowledge-based protein modeling and design. Eur J Biochem 172 513–520 [DOI] [PubMed] [Google Scholar]
- Bones AM, Rossiter JT (2006) The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 67 1053–1067 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Burmeister WP, Cottaz S, Driguez H, Iori R, Palmieri S, Henrissat B (1997) The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure 5 663–675 [DOI] [PubMed] [Google Scholar]
- Cairns JRK, Champattanachai V, Srisomsap C, Wittman-Liebold B, Thiede B, Svasti J (2000) Sequence and expression of Thai rosewood beta-glucosidase/beta-fucosidase, a family 1 glycosyl hydrolase glycoprotein. J Biochem 128 999–1008 [DOI] [PubMed] [Google Scholar]
- Chen SX, Halkier BA (1999) Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Protein Expr Purif 17 414–420 [DOI] [PubMed] [Google Scholar]
- Chuankhayan P, Hua Y, Svasti J, Sakdarat S, Sullivan PA, Cairns JRK (2005) Purification of an isoflavonoid 7-O-β-apiosyl-glucoside β-glycosidase and its substrates from Dalbergia nigrescens Kurz. Phytochemistry 66 1880–1889 [DOI] [PubMed] [Google Scholar]
- Chuankhayan P, Rimlumduan T, Svasti J, Cairns JRK (2007. a) Hydrolysis of soybean isoflavonoid glycosides by Dalbergia β-glucosidases. J Agric Food Chem 55 2407–2412 [DOI] [PubMed] [Google Scholar]
- Chuankhayan P, Rimlumduan T, Tantanuch W, Kongsaeree PT, Metheenukul P, Svasti J, Jensen ON, Cairns JRK (2007. b) Functional and structural differences between isoflavonoid β-glycosidases from Dalbergia sp. Arch Biochem Biophys 468 205–216 [DOI] [PubMed] [Google Scholar]
- Cicek M, Esen A (1999) Expression of soluble and catalytically active plant (monocot) beta-glucosidases in E. coli. Biotechnol Bioeng 63 392–400 [DOI] [PubMed] [Google Scholar]
- Clark M, Cramer RD, Vanopdenbosch N (1989) Validation of the general purpose Tripos 5.2 force field. J Comput Chem 10 982–1012 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cohen RB, Tsou K, Rutenburg SH, Seligman AM (1952) The colorimetric estimation and histochemical demonstration of β-galactosidase. J Biol Chem 195 239–249 [PubMed] [Google Scholar]
- Conn EE (1993) β-Glycosidases in plants: substrate specificity. In A Esen, ed, β-Glucosidases: Biochemistry and Molecular Biology. American Chemical Society, Washington, DC, pp 15–26
- Czjzek M, Cicek M, Zamboni V, Burmeister WP, Bevan DR, Henrissat B, Esen A (2001) Crystal structure of a monocotyledon (maize ZMGlu1) beta-glucosidase and a model of its complex with p-nitrophenyl beta-D-thioglucoside. Biochem J 354 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhana DP, Ellis BE, Carlson JE (1995) A beta-glucosidase from lodgepole pine xylem specific for the lignin precursor coniferin. Plant Physiol 107 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhana DP, Ellis BE, Carlson JE (1999) cDNA cloning and heterologous expression of coniferin beta-glucosidase. Plant Mol Biol 40 365–372 [DOI] [PubMed] [Google Scholar]
- Dunn MA, Hughes MA, Sharif AL (1988) Synthesis of the cyanogenic beta-glucosidase, linamarase, in white clover. Arch Biochem Biophys 260 561–568 [DOI] [PubMed] [Google Scholar]
- Escamilla-Trevino LL, Chen W, Card ML, Shih MC, Cheng CL, Poulton JE (2006) Arabidopsis thaliana beta-glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry 67 1651–1660 [DOI] [PubMed] [Google Scholar]
- Esen A (1992) Purification and partial characterization of maize (Zea mays L.) β-glucosidase. Plant Physiol 98 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan TWM, Conn EE (1985) Isolation and characterization of two cyanogenic beta-glucosidases from flax seeds. Arch Biochem Biophys 243 361–373 [DOI] [PubMed] [Google Scholar]
- Farag MA, Huhman DV, Dixon RA, Sumner L (2008) Metabolomics reveals novel pathways, differential and elicitor-specific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula cell cultures. Plant Physiol 146 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Huhman DV, Lei Z, Sumner L (2007) Metabolic profiling and systematic identification of flavonoids and isoflavonoids in roots and cell suspension cultures of Medicago truncatula using HPLC–UV–ESI–MS and GC–MS. Phytochemistry 68 342–354 [DOI] [PubMed] [Google Scholar]
- Fia G, Giovani G, Rosi I (2005) Study of beta-glucosidase production by wine-related yeasts during alcoholic fermentation: a new rapid fluorimetric method to determine enzymatic activity. J Appl Microbiol 99 509–517 [DOI] [PubMed] [Google Scholar]
- Forslund K, Morant M, Jørgensen B, Olsen CE, Asamizu E, Sato S, Tabata S, Bak S (2004) Biosynthesis of the nitrile glucosides rhodiocyanoside A and D and the cyanogenic glucosides lotaustralin and linamarin in Lotus japonicus. Plant Physiol 135 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehner M, Conn EE (1987) The linamarin beta-glucosidase in Costa Rican wild lima beans (Phaseolus lunatus L) is apoplastic. Plant Physiol 84 1296–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrehiwot L, Beuselinck PR (2001) Seasonal variations in hydrogen cyanide concentration of three Lotus species. Agron J 93 603–608 [Google Scholar]
- Gerardi C, Blando F, Santino A, Zacheo G (2001) Purification and characterisation of a beta-glucosidase abundantly expressed in ripe sweet cherry (Prunus avium L.) fruit. Plant Sci 160 795–805 [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE (2002) Constraints on effectiveness of cyanogenic glycosides in herbivore defense. J Chem Ecol 28 1301–1313 [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Ades PK, Woodrow IE (2004) Cyanogenesis in Eucalyptus polyanthemos seedlings: heritability, ontogeny and effect of soil nitrogen. Tree Physiol 24 681–688 [DOI] [PubMed] [Google Scholar]
- Gruhnert C, Biehl B, Selmar D (1994) Compartmentation of cyanogenic glucosides and their degrading enzymes. Planta 195 36–42 [Google Scholar]
- Gusmayer S, Brunner H, Schneiderpoetsch HAW, Rudiger W (1994) Avenacosidase from oat: purification, sequence analysis and biochemical characterization of a new member of the BGA family of beta-glucosidases. Plant Mol Biol 26 909–921 [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25 989–994 [DOI] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57 303–333 [DOI] [PubMed] [Google Scholar]
- Halkier BA, Møller BL (1989) Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol 90 1552–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41 95–98 [Google Scholar]
- Hara M, Fujii Y, Sasada Y, Kuboi T (2000) cDNA cloning of radish (Raphanus sativus) myrosinase and tissue-specific expression in root. Plant Cell Physiol 41 1102–1109 [DOI] [PubMed] [Google Scholar]
- Heiss S, Schafer HJ, Haag-Kerwer A, Rausch T (1999) Cloning sulfur assimilation genes of Brassica juncea L.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase. Plant Mol Biol 39 847–857 [DOI] [PubMed] [Google Scholar]
- Hösel W, Conn EE (1982) The aglycone specificity of plant beta-glycosidases. Trends Biochem Sci 7 219–221 [Google Scholar]
- Hösel W, Tober I, Eklund SH, Conn EE (1987) Characterization of beta-glucosidases with high specificity for the cyanogenic glucoside dhurrin in Sorghum bicolor (L) Moench seedlings. Arch Biochem Biophys 252 152–162 [DOI] [PubMed] [Google Scholar]
- Hughes MA (1991) The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity 66 105–115 [Google Scholar]
- Hughes MA (1993) Molecular genetics of plant cyanogenic β-glucosidases. In A Esen, ed, β-Glucosidases: Biochemistry and Molecular Biology. American Chemical Society, Washington, DC, pp 153–169
- Hughes MA, Brown K, Pancoro A, Murray BS, Oxtoby E, Hughes J (1992) A molecular and biochemical analysis of the structure of the cyanogenic β-glucosidase (linamarase) from cassava (Manihot esculenta Crantz). Arch Biochem Biophys 295 273–279 [DOI] [PubMed] [Google Scholar]
- Hughes MA, Dunn MA (1982) Biochemical characterisation of the Li locus, which controls the activity of the cyanogenic β-glucosidase in Trifolium repens L. Plant Mol Biol 1 169–181 [DOI] [PubMed] [Google Scholar]
- Imberty A, Bettler E, Karababa M, Mazeau K, Petrova P, Pérez S (1999) Building sugars: the sweet part of structural biology. In M Vijayan, N Yathindra, eds, Perspectives in Structural Biology. Indian Academy of Sciences and Universities Press, Hyderabad, India, pp 392–409
- Ismail B, Hayes K (2005) β-Glycosidase activity toward different glycosidic forms of isoflavones. J Agric Food Chem 53 4918–4924 [DOI] [PubMed] [Google Scholar]
- Itoh-Nashida T, Hiraiwa M, Uda Y (1987) Purification and properties of beta-D-glucosidase (linamarase) from the butter bean, Phaseolus lunatus. J Biochem 101 847–854 [DOI] [PubMed] [Google Scholar]
- Jenrich R, Trompetter I, Bak S, Olsen CE, Møller BL, Piotrowski M (2007) Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc Natl Acad Sci USA 104 18848–18853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA (1998) Why are so many food plants cyanogenic? Phytochemistry 47 155–162 [DOI] [PubMed] [Google Scholar]
- Jones PR, Møller BL, Høj PB (1999) The UDP-glucose-p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. J Biol Chem 274 35483–35491 [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Bak S, Busk PK, Sørensen C, Olsen CE, Puonti-Kaerlas J, Møller BL (2005. a) Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol 139 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005. b) Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8 280–291 [DOI] [PubMed] [Google Scholar]
- Kahn RA, Bak S, Svendsen I, Halkier BA, Møller BL (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol 115 1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakes P (1985) Linamarase and other beta-glucosidases are present in the cell walls of Trifolium repens L. leaves. Planta 166 156–160 [DOI] [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, McPherson J (1987) Duplication of CaMV-35S promoter sequences creates a strong enhancer for plant genes. Science 236 1299–1302 [DOI] [PubMed] [Google Scholar]
- Keresztessy Z, Brown K, Dunn MA, Hughes MA (2001) Identification of essential active-site residues in the cyanogenic beta-glucosidase (linamarase) from cassava (Manihot esculenta Crantz) by site-directed mutagenesis. Biochem J 353 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztessy Z, Hughes J, Kiss L, Hughes MA (1996) Co-purification from Escherichia coli of a plant beta-glucosidase-glutathione S-transferase fusion protein and the bacterial chaperonin GroEL. Biochem J 314 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketudat Cairns JR, Champattanachai V, Srisomsap C, Wittman-Liebold B, Thiede B, Svasti J (2000) Sequence and expression of Thai Rosewood beta-glucosidase/beta-fucosidase, a family 1 glycosyl hydrolase glycoprotein. J Biochem 128 999–1008 [DOI] [PubMed] [Google Scholar]
- Kim YW, Kang KS, Kim SY, Kim IS (2000) Formation of fibrillar multimers of oat beta-glucosidase isoenzymes is mediated by the As-Glu1 monomer. J Mol Biol 303 831–842 [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim IS (1998) Subunit composition and oligomer stability of oat beta-glucosidase isozymes. Biochim Biophys Acta 1388 457–464 [DOI] [PubMed] [Google Scholar]
- Kristensen C, Morant M, Olsen CE, Ekstrøm CT, Galbraith DW, Møller BL, Bak S (2005) Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc Natl Acad Sci USA 102 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen P, Brzobohaty B, Hohfeld I, Bako L, Melkonian M, Palme K (2000) Developmental regulation of the maize Zm-g60.1 gene encoding a beta-glucosidase located to plastids. Planta 210 407–415 [DOI] [PubMed] [Google Scholar]
- Kuroki GW, Poulton JE (1986) Comparison of kinetic and molecular properties of two forms of amygdalin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys 247 433–439 [DOI] [PubMed] [Google Scholar]
- Kuroki GW, Poulton JE (1987) Isolation and characterization of multiple forms of prunasin hydrolase from black cherry (Prunus serotina Ehrh.) seeds. Arch Biochem Biophys 255 19–26 [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26 283–291 [Google Scholar]
- Leah R, Kigel J, Svendsen I, Mundy J (1995) Biochemical and molecular characterization of a barley seed beta-glucosidase. J Biol Chem 270 15789–15797 [DOI] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126 1109–1120 [DOI] [PubMed] [Google Scholar]
- Li CP, Swain E, Poulton JE (1992) Prunus serotina amygdalin hydrolase and prunasin hydrolase: purification, N-terminal sequencing, and antibody production. Plant Physiol 100 282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maicas S, Mateo JJ (2005) Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl Microbiol Biotechnol 67 322–335 [DOI] [PubMed] [Google Scholar]
- Mewis I, Appel HM, Hom A, Raina R, Schultz JC (2005) Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Nakanishi H, Ema J, Ma SJ, Noguchi E, Inohara-Ochiai M, Fukuchi-Mizutani M, Nakao M, Sakata K (2002) Cloning of beta-primeve-rosidase from tea leaves, a key enzyme in tea aroma formation. Plant Physiol 130 2164–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkpong OE, Yan H, Chism G, Sayre RT (1990) Purification, characterization, and localization of linamarase in cassava. Plant Physiol 93 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller BL, Conn EE (1980) The biosynthesis of cyanogenic glucosides in higher plants: channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (Linn) Moench. J Biol Chem 255 3049–3056 [PubMed] [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen B, Dam W, Olsen CE, Møller BL, Bak S (2007) Lessons learned from metabolic engineering of cyanogenic glucosides. Metabolomics 3 383–398 [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S (2008) β-Glucosidases as detonators of plant chemical defense. Phytochemistry (in press) [DOI] [PubMed]
- Morant M, Bak S, Møller BL, Werck-Reichhart D (2003) Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol 14 151–162 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Naoumkina M, Farag MA, Sumner LW, Tang Y, Liu C-J, Dixon RA (2007) Different mechanisms for phytoalexin induction by pathogen and wound signals in Medicago truncatula. Proc Natl Acad Sci USA 104 17909–17915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KA, Tattersall DB, Jones PR, Møller BL (2008) Metabolon formation in dhurrin biosynthesis. Phytochemistry 69 88–98 [DOI] [PubMed] [Google Scholar]
- Niemeyer HM (1988) Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry 27 3349–3358 [Google Scholar]
- Nikus J, Esen A, Jonsson LMV (2003) Cloning of a plastidic rye (Secale cereale) beta-glucosidase cDNA and its expression in Escherichia coli. Physiol Plant 118 337–345 [Google Scholar]
- Nisius A (1988) The stromacenter in Avena plastids: an aggregation of beta-glucosidase responsible for the activation of oat leaf saponins. Planta 173 474–481 [DOI] [PubMed] [Google Scholar]
- Odell JT, Nagy F, Chua NH (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus-35S promoter. Nature 313 810–812 [DOI] [PubMed] [Google Scholar]
- Olsen KM, Hsu SC, Small LL (2008) Evidence on the molecular basis of the Ac/ac adaptive cyanogenesis polymorphism in white clover (Trifolium repens L.). Genetics (in press) [DOI] [PMC free article] [PubMed]
- Olsen KM, Sutherland BL, Small LL (2007) Molecular evolution of the Li/li chemical defence polymorphism in white clover (Trifolium repens L.). Mol Ecol 16 4180–4193 [DOI] [PubMed] [Google Scholar]
- Piotrowski M, Volmer JJ (2006) Cyanide metabolism in higher plants: cyanoalanine hydratase is a NIT4 homolog. Plant Mol Biol 61 111–122 [DOI] [PubMed] [Google Scholar]
- Pocsi I, Kiss L, Hughes MA, Nanasi P (1989) Kinetic investigation of the substrate-specificity of the cyanogenic beta-D-glucosidase (linamarase) of white clover. Arch Biochem Biophys 272 496–506 [DOI] [PubMed] [Google Scholar]
- Poulton JE (1990) Cyanogenesis in plants. Plant Physiol 94 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton JE, Li CP (1994) Tissue level compartmentation of (R)-amygdalin and amygdalin hydrolase prevents large scale cyanogenesis in undamaged Prunus seeds. Plant Physiol 104 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissler JF, Millar RL (1977) Biochemical evidence for histochemical localization of linamarase activity in Lotus corniculatus infected with Stemphylium loti. Protoplasma 92 57–70 [Google Scholar]
- Sánchez-Pérez R, Jørgensen K, Olsen CE, Dicenta F, Møller BL (2008) Bitterness in almonds. Plant Physiol 146 1040–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Conn EE (1978) Presence of cyanogenic glucoside dhurrin in isolated vacuoles from Sorghum. Plant Physiol 61 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Conn EE, Lin CH, Stocking CR (1977) Subcellular localization of cyanogenic glucoside of Sorghum by autoradiography. Plant Physiol 59 647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B (1988) Mobilization and utilization of cyanogenic glycosides: the linustatin pathway. Plant Physiol 86 711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B, Voigt J (1987) Hevea linamarase: a nonspecific β-glycosidase. Plant Physiol 83 557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Sunyaev S, Loboba A, Shevchenko A, Bork P, Ens W, Standing KG (2001) Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal Chem 73 1917–1926 [DOI] [PubMed] [Google Scholar]
- Sibbesen O, Koch B, Halkier BA, Møller BL (1995) Cytochrome P450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of L-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem 270 3506–3511 [DOI] [PubMed] [Google Scholar]
- Sue M, Ishihara A, Iwamura H (2000. a) Purification and characterization of a beta-glucosidase from rye (Secale cereale L.) seedlings. Plant Sci 155 67–74 [DOI] [PubMed] [Google Scholar]
- Sue M, Ishihara A, Iwamura H (2000. b) Purification and characterization of a hydroxamic acid glucoside beta-glucosidase from wheat (Triticum aestivum L.) seedlings. Planta 210 432–438 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sasaki R, Ogata Y, Nakamura Y, Sakurai N, Kitajima M, Takayama H, Kanaya S, Aoki K, Shibata D, et al (2008) Metabolic profiling of flavonoids in Lotus japonicus using liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. Phytochemistry 69 99–111 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takahashi S, Watanabe R, Fukushima Y, Fujita N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayama T (2006) An isoflavone conjugate-hydrolyzing beta-glucosidase from the roots of soybean (Glycine max) seedlings: purification, gene cloning, phylogenetics, and cellular localization. J Biol Chem 281 30251–30259 [DOI] [PubMed] [Google Scholar]
- Swain E, Li CP, Poulton JE (1992) Tissue and subcellular localization of enzymes catabolizing (R)-amygdalin in mature Prunus serotina seeds. Plant Physiol 100 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Høj PB, Møller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293 1826–1828 [DOI] [PubMed] [Google Scholar]
- Thayer SS, Conn EE (1981) Subcellular localization of dhurrin beta-glucosidase and hydroxynitrile lyase in the mesophyll cells of Sorghum leaf blades. Plant Physiol 67 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till I (1987) Variability of expression of cyanogenesis in white clover (Trifolium repens L). Heredity 59 265–271 [Google Scholar]
- Topfer R, Schell J, Steinbiss HH (1988) Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acids Res 16 8725–8725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoucq L, Czjzek M, Moriniere J, Bevan DR, Esen A (2003) Mutational and structural analysis of aglycone specificity in maize and sorghum beta-glucosidases. J Biol Chem 278 25055–25062 [DOI] [PubMed] [Google Scholar]
- Verdoucq L, Moriniere J, Bevan DR, Esen A, Vasella A, Henrissat B, Czjzek M (2004) Structural determinants of substrate specificity in family 1 beta-glucosidases: novel insights from the crystal structure of sorghum dhurrinase-1, a plant beta-glucosidase with strict specificity, in complex with its natural substrate. J Biol Chem 279 31796–31803 [DOI] [PubMed] [Google Scholar]
- Waldherr-Teschner M, Goetze T, Heiden W, Knoblauch M, Vollhardt H, Brickmann J (1992) MOLCAD: computer aided visualization and manipulation of models in molecular science. In FH Post, AJ Hin, eds, Advances in Scientific Visualization. Springer, Heidelberg, pp 58–67
- Yeoh HH, Woo HC (1992) Cassava leaf beta-glucosidase specificity and inhibition. Phytochemistry 31 2263–2265 [Google Scholar]
- Zagrobelny M, Bak S, Ekstrøm CT, Olsen CE, Møller BL (2007. a) The cyanogenic glucoside composition of Zygaena filipendulae (Lepidoptera: Zygaenidae) as effected by feeding on wild-type and transgenic Lotus populations with variable cyanogenic glucoside profiles. Insect Biochem Mol Biol 37 10–18 [DOI] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Møller BL (2008) Cyanogenesis in plants and arthropods. Phytochemistry 69 1457–1468 [DOI] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Olsen CE, Møller BL (2007. b) Intimate roles for cyanogenic glucosides in the life cycle of Zygaena filipendulae (Lepidoptera, Zygaenidae). Insect Biochem Mol Biol 37 1189–1197 [DOI] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Rasmussen AV, Jørgensen B, Naumann CM, Møller BL (2004) Cyanogenic glucosides and plant-insect interactions. Phytochemistry 65 293–306 [DOI] [PubMed] [Google Scholar]
- Zheng LS, Poulton JE (1995) Temporal and spatial expression of amygdalin hydrolase and (R)-(+)-mandelonitrile lyase in black cherry seeds. Plant Physiol 109 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JM, Hartmann S, Shepherd BK, Poulton JE (2002) Investigation of the microheterogeneity and aglycone specificity-conferring residues of black cherry prunasin hydrolases. Plant Physiol 129 1252–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.