Abstract
The plant family Rosaceae consists of over 100 genera and 3,000 species that include many important fruit, nut, ornamental, and wood crops. Members of this family provide high-value nutritional foods and contribute desirable aesthetic and industrial products. Most rosaceous crops have been enhanced by human intervention through sexual hybridization, asexual propagation, and genetic improvement since ancient times, 4,000 to 5,000 B.C. Modern breeding programs have contributed to the selection and release of numerous cultivars having significant economic impact on the U.S. and world markets. In recent years, the Rosaceae community, both in the United States and internationally, has benefited from newfound organization and collaboration that have hastened progress in developing genetic and genomic resources for representative crops such as apple (Malus spp.), peach (Prunus spp.), and strawberry (Fragaria spp.). These resources, including expressed sequence tags, bacterial artificial chromosome libraries, physical and genetic maps, and molecular markers, combined with genetic transformation protocols and bioinformatics tools, have rendered various rosaceous crops highly amenable to comparative and functional genomics studies. This report serves as a synopsis of the resources and initiatives of the Rosaceae community, recent developments in Rosaceae genomics, and plans to apply newly accumulated knowledge and resources toward breeding and crop improvement.
ROSACEOUS CROPS FOR HUMAN HEALTH AND NUTRITION
Rosaceae, comprised of over 100 genera and 3,000 species, is the third most economically important plant family in temperate regions (Dirlewanger et al., 2002). The United States is a leading producer of almond, apple, plum, peach, pear, raspberry, sour cherry, sweet cherry, and strawberry (in the genera Malus, Pyrus, Prunus, Rubus, and Fragaria; see below). Other nonedible species with almost exclusively ornamental value include rose, hawthorn, potentilla, cotoneaster, and pyracantha. The products of this family are in high demand for their nutritional and aesthetic values, and their cultivation drives regional economies (Fig. 1).
Figure 1.
Representative rosaceous crops exhibiting diversity of fruit types: A, P. persica, peach, fleshy drupe; B, P. armeniaca, apricot, fleshy drupe; C, M. ×domestica, apple, pome; D, F. ×ananassa, strawberry, achenes; E, R. ×hybrida, rose, achene; F, Pyrus communis, pear, pome; G, P. avium, sweet cherry, fleshy drupe; H, P. domestica, plum, fleshy drupe; I, Rubus idaeus, raspberry, drupelets.
The Rosaceous tree genera, including Malus, Pyrus, and Prunus, are predominantly grown for their fruit and originated in regions extending from Asia west to the Caucasus (Vavilov, 1951). The early Malus and Pyrus lineages gave rise to the cultivated apple (Malus ×domestica), considered to be an ancient allopolyploid, with domesticated European and Asian pears representing multiple species. The fruit-producing Prunus also diverged into multiple species. Plums hold the central position within this genus, with numerous species having originated in Europe, Asia, China, and North America (Smartt and Simmonds, 1995). The cherry subgenera evolved in Asia Minor, particularly Iran, Iraq, and Syria (Vavilov, 1951), whereas both peach and apricot have a Chinese center of origin. Pots recovered from cave dwellings in Europe contained cherry pits dated from 5,000 to 4,000 B.C. (Marshall, 1954).
During ancient times, rosaceous fruits populated native human habitats, and fruits from these species were valuable sources of food. Subsequent centuries of selection and domestication produced the dramatic increase in fleshy fruit size that distinguishes today's commercial fruit from their wild relatives.
The economic importance of edible rosaceous crops derives from their flavorful fruits and nuts that provide unique contributions to dietary choices of consumers and overall human health. Rosaceous fruits are consumed in multiple forms, including fresh, dried, juice, and processed products. The variety of flavors, textures, and levels of sweetness and acidity offered by these fruits satisfies diverse consumer tastes and choices. Rosaceae fruits are also a major human dietary source of phytochemicals, such as flavonoids and other phenolic compounds, cyanogenic glucosides, phytoestrogens (Mazur et al., 2000), and phenols that could potentially yield health and disease-fighting advantages (Macheix et al., 1991; Swanson, 1998; Selmar, 1999). l-Ascorbic acid, quercetin, kaempferol, myricetin, p-coumaric acid, gallic acid, and ellagic acid are well-known antioxidants and/or cancer-inhibiting compounds that have been identified in these fruits.
Epidemiological evidence suggests that diets rich in fruit and vegetables significantly reduce cancer risk (Caragay, 1992; Ziegler et al., 1996). In vitro and in vivo studies with animal models provide evidence that fruit and leaf extracts from many Rosaceae species inhibit some cancers or have strong antioxidant activities (Yau et al., 2002). Ellagic acid, present in strawberry, red raspberry, arctic bramble, cloudberry, and other rosaceous berries (Mandal and Stoner, 1990; Häkkinen et al., 1998; Masuda et al., 1999; Häkkinen and Törrönen, 2000; Harris et al., 2001; Cordenunsi et al., 2002), has been shown to affect cell proliferation and apoptosis, suggesting a potential anticancer role. Some strawberry cultivars have the highest concentrations of l-ascorbic acid among all fruits (Haffner and Vestrheim, 1997). Other phenolic compounds belonging to distinct chemical classes, many of which are also potential antioxidants and anticancer agents (Eichholzer et al., 2001; Schieber et al., 2001), have been isolated from rosaceous fruits (Macheix et al., 1991).
Throughout the past century, agricultural research has focused on increasing crop production to feed the growing human population. However, as malnutrition and infectious and nutrition-related diseases remain common, the research focus has begun to shift to include improving the dietary value of different foods and identifying novel compounds with pharmacological properties (Kishore and Shewmaker, 1999). Traditionally, new traits for improvement of crop plants have been primarily derived from their related wild species. However, genomics and bioinformatics advances over the past decade have provided new options for identifying useful compounds in plants and for manipulating encoding genes responsible for their production.
The profitable production of desirable fruits and nuts can be challenging, as rosaceous crop producers must balance stringent quality expectations with yield requirements, cost efficiency, and shipping/marketing constraints. A further challenge is to maintain crop quality following harvest while avoiding loss due to chilling injury disorders, decay, chemical contamination, and overripening. Thus, postharvest and consumer-valued qualities and traits serve as ideal targets for crop product improvement. Complementary to the inescapable economic realities of crop production and distribution, consumer perception of quality characteristics deserves recognition as a primary driving force for cultivar development and establishment of research priorities. Consumers make quality judgments based on sensory perception (including smell, taste, and appearance) as well as perceived nutritional value. The flavor quality of fruits and nuts is determined by many criteria, including firmness, juiciness, sweetness, acidity, aroma, and texture.
Recent consumer interests in purchasing locally produced fruits and vegetables have highlighted the importance of rosaceous fruits and invigorated their production in areas having relative proximity to large population centers. Rosaceous crop products are valued precisely because of their nutritional and aesthetic qualities, thereby offering opportunities for varietal improvement aimed at both problem-solving and market expansion. The impressive gains achieved by conventional breeding notwithstanding, the translation of structural and functional genomics research into breeding tools and broadened research perspectives offers unprecedented opportunities for rapid and sustainable enhancement of value to all stakeholders.
ROSACEAE PHYLOGENY AND DIVERSITY DICTATE THE NEED FOR MULTIPLE MODELS
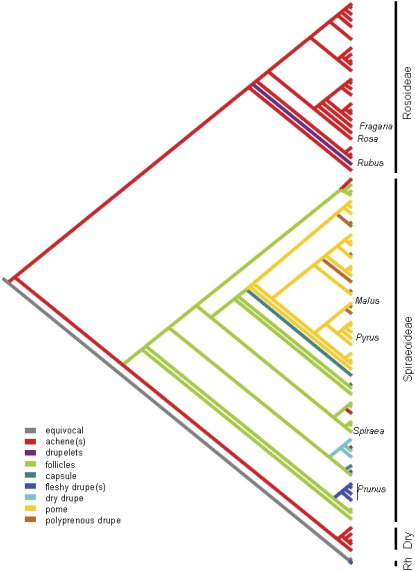
The Rosaceae family has been traditionally divided into four subfamilies grouped by fruit type. These include: Rosoideae (Rosa, Fragaria, Potentilla, and Rubus; fruit, achene; x = 7, 8, or 9), Prunoideae (Prunus; fruit, drupe; x = 8), Spiraeoideae (Spirea; fruit, follicle or capsule; x = 9), and Maloideae (Malus, Pyrus, and Cotoneaster; fruit, pome; x = 17; Potter et al., 2002). Recent phylogenetic analyses of combined sequence data from six nuclear and four chloroplast loci provided strong support for the division of Rosaceae into three subfamilies (Potter et al., 2007; Fig. 2). These include Dryadoideae (including Cercocarpus, Dryas, and Purshia; x = 9), Rosoideae (including Fragaria, Potentilla, Rosa, Rubus, and others; x = 7), and Spiraeoideae (including Kerria, Spiraea, and others; x = 8, 9, 15, or 17). Each of the latter two subfamilies has been further divided into supertribes, tribes, and subtribes. With less emphasis on fruit type, which is clearly homoplasious in the family (Fig. 2), the taxa formerly included within Maloideae and Prunoideae have now been reclassified into Spiraeoideae, with Malus placed in the tribe Pyreae and Prunus in the tribe Amygdaleae (Potter et al., 2007). The pome-bearing members of the family, such as Malus and Pyrus, as well as closely related genera that bear polyprenous drupes (e.g. Crataegus), have been classified in subtribe Pyrinae, which corresponds to the former subfamily Maloideae.
Figure 2.
Phylogenetic tree of Rosaceae, based on the multi-gene analysis by Potter et al. (2007) and modified from their figure 4. Positions of several economically important genera are indicated, and a hypothesis for the evolution of fruit types, based on parsimony optimization of that character, is represented by the different colors of the branches. The three subfamilies recognized by Potter et al. (2007) are shown on the right margin. Dry, Dryadoideae. The tree was rooted with representatives of the family Rhamnaceae (Rh).
Patterns of diversification within the Rosaceae, combined with the need for rapid translation of genomics research into agronomic practice, suggest that multiple rosaceous species must be designated as reference models for acquisition of genomic information. Currently, the best-developed model species for Rosaceae include apple, peach (Prunus persica), and diploid strawberry (Fragaria vesca; Table I). There is no widely accepted model for Dryadoideae, and because there are no major commercial crops within this subfamily, it will not be a focus of this report. Each of the three designated models represents a distant subtaxon within Rosaceae, and each has unique features making it well suited for targeted genomic research. Combined efforts toward developing integrated genetic and genomic resources in three diverse and representative species will advance genomic research in all rosaceous species.
Table I.
Model plant species for Rosaceae genetics and genomic research compared to Arabidopsis
| M. ×domestica | P. persica | F. vesca | A. thaliana | |
|---|---|---|---|---|
| Genome size (Mb/C) | 750 | 280 | 206 | 157 |
| Chromosome no. (2n) | 2n = 2x = 34 (some triploid cultivars) | 2n = 2x = 16 | 2n = 2x = 14 | 2n = 2x = 10 |
| No. of species in the genus | Approximately 35 | Approximately 53 | Approximately 20 | 9 |
| Generation time (seed to seed) | 4–8 years | 3–5 years | 10–16 weeks | 6–8 weeks |
| Life cycle | Perennial | Perennial | Perennial | Annual |
| Seed production/plant | Approximately 700 (5–10 per fruit) | Approximately 300 (1 per fruit) | >2,500 | Approximately 5,000 |
| Plants/m2 | 0.67 | 0.67 | Approximately 100 | 776 |
| Fleshy fruit formation | Pome | Drupe | Receptacle | No |
| Juvenile period | 3–7 years | 1–2 years | None | None |
| Vegetative propagation | Yes, hard and softwood cuttings | Yes, hard and softwood cuttings | Yes, runners and crown divisions | No |
| Self-compatible | No | Yes | Yes, cultivars and model species | Yes |
| Inbreeding depression | Moderate to severe | Moderate | None to moderate | None to moderate |
| Transformation | Tissue culture | Tissue culture | Tissue culture | In planta |
| Transformation efficiency | 80% | <1% | 100% | Approximately 1% |
| ESTs | >300,000 | 85,340 | >45,000 | >1.2 million |
| Genome sequence | Mid-2008 | End of 2008 | Approximately 1% | Yes |
| Physical map | Yes | Yes | In progress | Yes |
| Linkage maps | Yes | Yes | Yes | Yes |
APPLE
Apple, a pome fruit in which the floral receptacle is the fleshy edible tissue, is the most important deciduous tree fruit crop grown in the United States and around the world. The genus Malus has more than 25 species and hybrids; most apple cultivars are diploid (2n = 2x = 34), self-incompatible, and have a juvenile period of 3 to 7 years (Korban and Chen, 1992; Janick et al., 1996).
The apple genome, at 1.54 pg DNA/2C nucleus or 750 Mb per haploid genome complement, is approximately the same size as that of tomato (Solanum lycopersicum; Tatum et al., 2005). The apple molecular map currently has more than 900 markers on 17 linkage groups (Maliepaard et al., 1998; Liebhard et al., 2002, 2003a, 2003b; Xu and Korban, 2002b; Naik et al., 2006). Several efforts are under way to develop a substantial resource of molecular markers for genotyping and marker-assisted selection (MAS; Tartarini et al., 2000; Liebhard et al., 2002, 2003a, 2003b; Silfverberg-Dilworth et al., 2006). Transgenic apple has been obtained via Agrobacterium-mediated transformation of cultivars (Ko et al., 1998; Bolar et al., 2000; Defilippi et al., 2004; Teo et al., 2006), leading to promising transgenic lines with enhanced resistance to serious orchard diseases such as apple scab (Venturia inaequalis) and fire blight (Erwinia amylovora; Ko et al., 2000; Bolar et al., 2001) and lines with suppressed ethylene and volatile esters in the fruit (Defilippi et al., 2004, 2005). Antisense RNA technology was used to induce posttranscriptional gene silencing and double-stranded RNA-mediated posttranscriptional gene silencing used to engineer resistance to crown gall (Escobar et al., 2003). Over 300,000 apple ESTs have been generated from different tissues, conditions, and genotypes (Newcomb et al., 2006). Over 22,600 EST clones from nine apple cDNA libraries, subjected to temperature or drought stress, were clustered and annotated, then used to study gene expression in response to abiotic stress (Wisniewski et al., 2008). Park et al. (2006) analyzed apple EST sequences available in public databases using statistical algorithms to identify genes associated with flavor and aroma components in mature fruit. Sung et al. (1998) generated ESTs from young fruit, peels of mature fruit, and carpels of the Fuji apple and found that most ESTs isolated from young fruit were preferentially expressed in reproductive organs, suggesting their important roles during reproductive organ development.
Two bacterial artificial chromosome (BAC) libraries were constructed (Xu et al., 2001; Xu and Korban, 2002a) and used for map-based positional cloning of a cluster of receptor-like genes within the scab resistance Vf locus in apple (Xu and Korban, 2002a). Other apple BAC libraries (from both fruiting cultivars and rootstocks) were constructed and are being used for gene cloning and mapping (Vinatzer et al., 1998, 2001). Recently, a scaffold physical map was constructed using BAC fingerprinting (Han et al., 2007). A complete genome sequence of the apple is under way, and it will be completed by mid-2008 (R. Velascco, personal communication).
PEACH
Prunus has characteristic stone fruits or drupes in which seeds are encased in a hard, lignified endocarp (the stone), and the edible portion is a juicy mesocarp. Agriculturally important stone fruit species include P. persica (peach, nectarine), Prunus domestica (European or prune plum), Prunus salicina (Japanese plum), Prunus cerasus (sour cherry), Prunus avium (sweet cherry), Prunus armeniaca (apricot), and Prunus amygdalus (almond). The peach karyotype (x = 8) has a clearly identifiable, large submetacentric chromosome and seven smaller chromosomes, two of which are acrocentric (Jelenkovic and Harrington, 1972; Salesses and Mouras, 1977). Little is known about the chromosomal location and organization of gene sequences in peach, but recent fluorescence in situ hybridization in the closely related almond (Prunus dulcis) has enabled detection of individual chromosomes based on length and position of ribosomal DNA genes (Corredor et al., 2004). Peach chromosomal organization is probably similar to that of other Amygdalus species, as crosses between peach and those closely related species (Prunus ferganensis, Prunus mira, Prunus davidiana, Prunus kansuensis, and the cultivated almond) are possible and produce fertile hybrids. Crosses between peach and species of other subgenera (Prunophora and Cerasus), such as apricot (P. armeniaca), myrobalan plum (Prunus cerasifera), European plum (P. domestica), and Japanese plum (P. salicina), are also possible, but fertile hybrids are only occasionally produced (Scorza and Sherman, 1996). However, as predicted by their divergent evolutionary origin, sweet and sour cherry are considered the commercially important Prunus fruit species most distant from peach.
All commercial peach cultivars belong to P. persica, including nectarines, which differ from peach by the absence of pubescence on the fruit surface. This characteristic segregates as a simple trait, and it is presumably controlled by a single gene or a few closely linked genes. Unlike most congeneric species that exhibit gametophytic self-incompatibility, the peach is self-compatible. Breeding cultivars through self-pollination (Miller et al., 1989) combined with recent genetic bottlenecks (Scorza et al., 1985) have resulted in lower genetic variability in peach as compared with other Prunus crops (Byrne, 1990). Peach is the genetic and genomic reference species for Prunus because of its high economic value, self-compatibility allowing for development of F2 progenies, availability of homozygous doubled haploids (Pooler and Scorza, 1997), and possibility of shortening its juvenile period to 1 to 2 years following planting (Scorza and Sherman, 1996). However, one drawback is that peach transformation remains inefficient.
Various Prunus maps connected with anchor markers can be found at the Genome Database for Rosaceae (GDR; see below), including more than 2,000 markers, the backbone of which is the reference Prunus map. This consensus map has been constructed with an interspecific almond × peach F2 population (Texas × Earlygold) and currently has 827 transportable markers covering a total distance of 524 cM (average density of 0.63 cM/marker; Howad et al., 2005). Twenty-eight major morphological, quality, and agronomic characters with simple Mendelian inheritance (Dirlewanger et al., 2004b) and 28 quantitative trait loci (QTLs; Arús et al., 2003), most of them polymorphic within the peach genome, have been placed on the Texas × Earlygold consensus map. The peach doubled haploid Lovell has been selected by the U.S. Department of Energy Joint Genomics Institute's Community Sequencing Program for shotgun sequencing (8× genome coverage), which is currently slated to begin in 2008.
STRAWBERRY
The cultivated strawberry with its accessory fruit comprised of a fleshy receptacle bearing many achenes (the “true” fruit) is genomically complex due to its octoploid (2n = 8x = 56) composition (Davis et al., 2007). Currently, 23 species are recognized in the genus Fragaria, including 13 diploids (2n = 2x = 14), four tetraploids, one hexaploid, and four octoploids (Folta and Davis, 2006). Accessions of the cultivars' two octoploid progenitor species, Fragaria chiloensis and Fragaria virginiana, are valued by strawberry breeders as sources of novel traits, especially pest resistance and abiotic stress tolerance. Because strawberry is a relatively new crop, dating to the 1700s (Darrow, 1966), as few as three introgressive backcrosses can yield selections of cultivar quality (J. Hancock, personal communication).
The current, provisional model of the octoploid strawberry genome constitution is AAA'A'BBB'B' (Bringhurst, 1990). This suggests that the octoploids are diploidized allopolyploids that have descended from four diploid ancestors. Although the diploid ancestry of the octoploid has yet to be definitively established, the leading ancestral candidate diploids are F. vesca and Fragaria iinumae, with Fragaria bucharica and Fragaria mandshurica as additional intriguing possibilities (Folta and Davis, 2006).
One ancestral diploid strawberry, F. vesca, has emerged as an attractive system for both structural and functional genomics due to its many favorable features (Folta and Davis, 2006). Plants are compact enough to be grown on a small scale in a laboratory, easy to propagate vegetatively, self-compatible, and produce many seeds per plant (Darrow, 1966; Table I). F. vesca has one of the smallest genomes among cultivated plants (206 Mb versus 157 Mb in Arabidopsis [Arabidopsis thaliana]; Akiyama et al., 2001) as adjusted by Folta and Davis (2006).
An efficient transformation protocol renders F. vesca amenable to genetic manipulation (El Mansouri et al., 1996; Haymes and Davis, 1998; Alsheikh et al., 2002; Oosumi et al., 2006). The diploid model complements the development of transformation systems for the octoploid cultivated strawberry (Folta and Davis, 2006; Folta and Dhingra, 2006; Mezzetti and Costantini, 2006), allowing direct ingress of transgenes into breeding populations. The availability of robust transformation and reverse genetic systems in strawberry provide excellent resources for the broader Rosaceae genomics community by uniquely complementing the abundant DNA sequence resources available in apple and peach.
Diploid strawberry has several additional advantages over other widely used model species. Unlike the dry siliques of Arabidopsis, the fleshy strawberry fruit allows for studying the molecular mechanisms of fruit development and ripening. Furthermore, strawberry fruit has several unique properties, including nonclimacteric ripening and unusual metabolites that cannot be studied in traditional fruit models, such as tomato.
Recent evidence, based on nucleotide sequence data, has revealed a close phylogenetic relationship between Fragaria and Rosa (Potter et al., 2007), thus suggesting that genomic resources for either genus may be readily applicable to the other. Genomics resources for roses include a BAC library for Rosa rugosa consisting of 27,264 clones, 0.5 pg or 500 Mb, and corresponding to 5.2 × haploid genome equivalents (Kaufmann et al., 2003). This BAC library has been used to develop a fine-scale physical map of the Rdr1 region to assemble a 400-kb tiling path of six BAC clones and covering the Rdr1 locus, which confers resistance to blackspot. Genes involved in ethylene perception, due to their role in flower initiation and senescence, and floral scent, among the most valuable traits in roses, have been of particular interest in rose research. Molecular studies have been conducted to understand ethylene perception and signal transduction pathways in rose by taking advantage of knowledge obtained from alterations in the triple response in ethylene-insensitive mutants in Arabidopsis (Guterman et al., 2002; Lavid et al., 2002). Wang et al. (2004) isolated seven putative 1-aminocyclopropane-1-carboxylate synthase clones from a cDNA library of aging rose petals of Rosa hybrida ‘Kardinal.’ cDNA libraries from petals of the two tetraploid R. hybrida ‘Fragrant Cloud’ and ‘Golden Gate’ have been constructed and over 3,000 cDNA clones sequenced from these libraries. An annotated petal EST database of approximately 2,100 unique genes has been generated and used to create a microarray. Scalliet et al. (2006) explored the evolutionary pathway of scent production in European (Rosa gallica officinalis and Rosa damascena) and Chinese rose (Rosa chinensis and Rosa gigantea) species, both progenitors of the modern hybrid rose, R. hybrida. As genomic resources continue to develop for strawberry and rose, their applicability to each other and other Rosaceae will be more clearly defined.
ROSACEAE GENOMICS
The Rosaceae genomics initiative, like those of other crop groups, exploits and extends the fundamental knowledge generated in the Arabidopsis model system, which has dramatically advanced our understanding of the physiology, biochemistry, genetics, and evolution of plants. Arabidopsis is characterized by its small genome, ease of transformation, rapid regeneration time, and sexual fecundity. As extensive forward and reverse genetic resources are available, this renders Arabidopsis an agile system for exploring fundamental biological questions. The genomic information generated directly from Arabidopsis has proven to translate well to other Brassicaceae. However, Arabidopsis shares only partial functional overlaps with many crop plants in more phylogenetically remote plant families. Here, other suitable research-friendly species have been established as family-specific models, including Medicago truncatula and Lotus japonicus for Fabaceae, rice (Oryza sativa) and Brachypodium for Poaceae, and tomato and potato (Solanum tuberosum) for Solanaceae. For families with valuable crop species, genomics has progressed by selecting a representative model species for the family and focusing research efforts on developing genomic tools for that system. Findings are then translated directly to other related species of agricultural importance.
In recent years, the application of molecular technologies has steadily increased for Rosaceae (Hokanson, 2001). Concerted efforts have been undertaken by the Rosaceae community to develop genomics tools for this economically important family. Coordination within the community led to the establishment of a dedicated bioinformatics portal (GDR) and the production of a national Rosaceae white paper as well as a U.S. Department of Agriculture (USDA) request for proposals that eventually funded 13 new projects to advance Rosaceae genomics (www.rosaceaewhitepaper.com). As these projects have unfolded, the need for developing multiple, subfamily-specific model systems within the Rosaceae has become apparent, focusing on peach (Prunus, Amygdeloideae), apple (Malus, Maloideae), and strawberry (Fragaria, Rosoideae) and their relative community strengths in the areas, respectively, of structural genomics, functional genomics, and reverse genetics. Initial translational genomic efforts have been directed toward developing high-density molecular linkage maps to accelerate breeding through MAS (Dirlewanger et al., 2004b).
Genetic Maps
Linkage mapping in Rosaceae has been conducted on a wide range of germplasm, including most of the important cultivated species. Genetic maps have been developed for different species of Prunus, including peach (Yamamoto et al., 2005; Dirlewanger et al., 2006), almond (Foolad et al., 1995; Viruel et al., 1995; Joobeur et al., 2000), and apricot (Hurtado et al., 2002; Lambert et al., 2004; Dondini et al., 2007). Partial maps for sour cherry (Wang et al., 1998), sweet cherry (Dirlewanger et al., 2004b), and interspecific crosses between peach and other species, e.g. almond (Joobeur et al., 1998; Bliss et al., 2002; Aranzana et al., 2003; Dirlewanger et al., 2004b), P. davidiana (Foulongne et al., 2003), P. cerasifera (Dirlewanger et al., 2004a), and P. ferganensis (Verde et al., 2005), have been developed. Maps for apple (Hemmat et al., 1994; Maliepaard et al., 1998; Liebhard et al., 2003a, 2003b, 2003c; Kenis and Keulemans, 2005; Naik et al., 2006; Silfverberg-Dilworth et al., 2006), pear (Yamamoto et al., 2004), raspberry (Graham et al., 2004), diploid strawberry (Davis and Yu, 1997; Sargent et al., 2004, 2006; Cipriani et al., 2006), cultivated octoploid strawberry (Lerceteau-Kohler et al., 2003; Weebadde et al., 2008), and rose (Debener and Mattiesch, 1999; Debener et al., 2001; Rajapakse et al., 2001; Yan et al., 2005) are also available. Many of these genetic maps were constructed for particular breeding goals using specific mapping populations, resulting in multiple genetic maps of various lengths that vary in degree of saturation and type of markers. Efforts are under way to use high-density molecular markers and anchor loci to consolidate this information into a single genetic consensus or reference map for each species. Characteristic genetic linkage maps for three model rosaceous species (apple, peach, and strawberry) are described in Table II.
Table II.
Most relevant published genetic linkage maps of three model species in Rosaceae
Abbreviations not defined in text: RAPD, randomly amplified polymorphic DNA; STS, sequence-tagged site; CAP, cleaved amplified polymorphism; ISSR, inter simple sequence repeat; SSCP, single-strand conformation polymorphism; NBS, nucleotide-binding site profiling.
| Species | Population | Markers | Linkage Groups | Map Length | Reference(s) |
|---|---|---|---|---|---|
| F. vesca | |||||
| F2 between Baron Solemacher and a clone collected from the wild | 75 RAPDs | 7 | 445 cM | Davis and Yu (1997) | |
| 3 Isozyme loci | |||||
| 2 Morphological traits | |||||
| F2 between F. vesca f. semperflorens and F. nubicola | 68 SSRs | 7 | 448 cM | Sargent et al. (2004) | |
| 1 SCAR | |||||
| 6 Gene-specific markers | |||||
| 3 Morphological traits | |||||
| Additions to above | 109 SSRs and EST-SSRs | 7 | 424 cM | Sargent et al. (2006) | |
| F2 between F. vesca 91.333.2 and Snovit | 66 SSRs | 8 | NA | Cipriani et al. (2006) | |
| BC (F. vesca × [F. vesca × F. viridis]) | 31 SSRs | 7 | NA | Nier et al. (2006) | |
| 2 Gene-specific markers | |||||
| F2 (F. vesca × F. nubicola) and BC1 (F. vesca × F. viridis) | 29 Gene-specific primers | 7 | Added to existing map | Sargent et al. (2007) | |
| Peach | F2 (NC174RL × Pillar) | 83 RAPDs | 15 | NA | Chaparro et al. (1994) |
| 1 Isozyme | |||||
| 4 Phenotypic markers | |||||
| F2 (almond Texas × peach Earlygold) (reference map for Prunus) | 11 Isozymes | 8 | 524 cM | Dirlewanger et al. (2004a); Howad et al. (2005); Joobeur et al. (1998); Aranzana et al. (2003); Lalli et al. (2005) | |
| 361 RFLPs | |||||
| 449 SSRs | |||||
| 5 STSs | |||||
| 42 Resistance-associated genes | |||||
| F2 (dwarf peach 54P455 × almond Padre) | 143 RFLPs | 8 | 1,144 cM | Bliss et al. (2002) | |
| 8 Isozymes | Revision and addition to existing map | ||||
| 4 Phenotypic characters | |||||
| 4 SSRs | |||||
| 1 RAPD | |||||
| 1 CAP | |||||
| F2 (Ferjalou Jalousia × Fantasia) | 82 SSRs | 7 | 621 cM | Dirlewanger et al. (2006) | |
| 37 RFLPs | |||||
| 61 AFLPs | |||||
| 1 Isozyme | |||||
| 6 Phenotypic characters | |||||
| F2 (Akame × Juseitou) | 94 SSRs | 8 | 571 cM | Yamamoto et al. (2005) | |
| 34 AFLPs | |||||
| 24 RAPDs | |||||
| 3 ISSRs | |||||
| 9 Phenotypic characters | |||||
| Lovell × Nemared | 169 AFLPs | 15 | 1,297 cM | Lu et al. (1998) | |
| BC1 (IF7310828 × P. ferganensis) | 78 RFLPs | 8 | 665 cM | Dettori et al. (2001); Verde et al. (2005) | |
| 63 AFLPs | |||||
| 57 SSRs | |||||
| 16 RAPDs | |||||
| 2 Phenotypic characters | |||||
| Apple | F1 Rome Beauty × White Angel | Isozymes | 24 (♀) | 950 cM | Hemmat et al. (1994) |
| RFLP | 21 (♂) | ||||
| F1 Prima × Fiesta | 124 RFLPs | 17 | 842 cM (♀) | Maliepaard et al. (1998) | |
| 133 RAPDs | 984 cM (♂) | ||||
| 17 Isozymes | |||||
| 4 AFLPs | |||||
| 1 SCAR | |||||
| 10 SSRs | |||||
| F1 Fiesta × Discovery | 217 RAPDs | 17 | 914 cM (♀) | Liebhard et al. (2002) | |
| 118 SSR markers | 1,015 cM (♂) | ||||
| Prima × Fiesta | 200 SSRs, 25 AFLPs | 17 | Approximately 800 cM | Liebhard et al. (2003c) | |
| F1 White Angel × Rome Beauty | 41 SSRs | Added to existing map | Hemmat et al. (2003) | ||
| F1 Fiesta × Discovery | 3 CAPS | 14 | Added to existing map | Baldi et al. (2004) | |
| 14 SSCPs | |||||
| F1 Fiesta × Discovery | 149 SSRs (current reference map) | 17 | Added to existing map | Silfverberg-Dilworth et al. (2006) | |
| F1 Braeburn × Telamon | 245 AFLPs | 17 | 1,039 cM (♀) | Kenis and Keulemans (2005) | |
| 19 SSRs | 1,245 cM (♂) | ||||
| Discovery × TN10-8 | 43 NBS markers | 10 | Added to existing map | Calenge et al. (2005) |
Marker-Assisted Breeding
Markers tightly linked to major genes for important traits, such as disease and pest resistances, fruit or nut quality, and self-incompatibility, have been developed in apple (Bus et al., 2000; Tartarini et al., 2000; Huaracha et al., 2004; Tahmatsidou et al., 2006; Gardiner et al., 2007), peach, and strawberry (T. Sjulin, personal communication), and are currently being used for MAS in both public and private breeding programs (Dirlewanger et al., 2004b).
Most rosaceous crops are characterized by long generation cycles and large plant size; hence, the ability to eliminate undesirable progenies in breeding populations through MAS reduces cost and allows breeders to focus on populations comprised of individuals carrying desirable alleles of genes of interest. In peach, Etienne et al. (2002) and Quilot et al. (2004) mapped several major genes and QTLs related to fruit quality in intra- and interspecific peach progenies. Blenda et al. (2006) identified an amplified fragment length polymorphic (AFLP) marker significantly associated with peach tree short life, a complex disease syndrome. This identification could lead to a MAS application in peach. Lalli et al. (2005) generated a resistance map for Prunus with 42 map locations for putative resistance regions distributed among seven of eight linkage groups, and both Lu et al. (1998) and Dirlewanger et al. (2004a) identified markers linked to genes determining root knot nematode resistance. Selection for self-compatibility alleles can be performed in Prunus self-incompatible species using markers based on genes coding for this character (Habu et al., 2006; López et al., 2006). In strawberry, a series of markers has been developed for traits of economic importance, namely, for anthracnose resistance (Lerceteau-Kohler et al., 2003) and seasonal flowering (Albani et al., 2004; Sugimoto et al., 2005). In addition, a sequence-characterized amplified region (SCAR) marker, SCAR R1A, has been linked to locus Rpf1, which confers resistance to a race of Phytophthora fragariae var. fragariae, which is the causal organism of red stele root rot disease in cultivated octoploid strawberry (Haymes et al., 2000).
The development of additional markers for MAS is dependent on the ability to perform genome scans of a progeny from a population segregating for a trait of interest and then validating the trait-marker association(s) in alternate populations. However, in many rosaceous species, a genome scan is currently not possible due to a lack of evenly distributed markers that are polymorphic in the respective breeding population. Thus, marker development remains a top priority in most, if not all, rosaceous crops.
STRUCTURAL GENOMICS
BAC Libraries
Large-insert BAC and cosmid/fosmid libraries have been constructed for peach and apricot (Georgi et al., 2002; Vilanova et al., 2003), apple (Vinatzer et al., 1998; Xu et al., 2001; Xu and Korban, 2002a), strawberry (Davis et al., 2007), rose (Kaufmann et al., 2003), and other rosaceous species.
Physical Maps
Physical maps are important resources for positional cloning, marker development, QTL mapping, and cloning. They serve as useful platforms for whole-genome sequencing efforts. Physical maps are available for several Rosaceae species, including peach and apple. The current physical map of peach is estimated to cover 287.1 Mb of the approximate peach genome size of 290 Mb (Baird et al., 1994), and it is comprised of 1,899 contigs, 239 of which are anchored to eight linkage groups of the Prunus reference map. The peach map is a second generation map (Zhebentyayeva et al., 2006), expanded by high-information-content fingerprinting of additional BAC library clones from the existing BAC libraries of peach. A total of 2,511 markers have been incorporated into the physical framework. Due to the abundance of hybridization data, the initial physical map for peach was biased toward expressed genome regions. According to a conservative estimate, the map covers the entire euchromatic peach genome. This map is publicly available at www.rosaceae.org.
A BAC-based physical map of the apple genome from 74,281 BAC clones representing approximately 10.5× haploid equivalents has been constructed (Han et al., 2007), and a physical map of F. vesca is under construction using a large-insert BAC library (V. Shulaev and A.G. Abbott, personal communication).
Genome Sequencing
Full genome sequencing is an invaluable tool for plant researchers. The Rosaceae community will benefit greatly from sequencing genomes of the representative models for each of the three subfamilies. At present, the largest publicly available genomic sequence resource in Rosaceae is that of F. vesca, from which approximately 1.75 Mb of genomic sequence derived from 50 genomic sites (as fosmid clones) has been deposited in GenBank under accession numbers EU024823 to EU024872. Preliminary analysis of these sequences has revealed a gene density of about one gene per 6 kb and a simple sequence repeat (SSR) density of about one SSR locus per 5 kb (Folta and Davis, 2006). The available Fragaria genomic sequence database will provide a valuable resource in relation to assembly and annotation of other rosaceous genomes.
Peach is a prime candidate for complete sequencing among Rosaceae models, because it has a comparatively small genome and close structural genomic relationship to many other important fruit crops (Dirlewanger et al., 2004b). The full genome sequence of peach will provide information on upstream regions of genes (promoters and enhancers) that are important for gene regulation, aid in identifying genes not discovered through EST sequencing projects, and provide a reference genome for comparative genomics in Rosaceae. The combination of the intrinsic advantages of peach as a model organism, the advent and application of high-throughput technologies for genetic and genomic analyses, and ongoing research collaborations is generating a wealth of genomic information. An initiative to sequence the peach genome was announced by the Joint Genome Institute at the 2007 Animal and Plant Genome Meeting in San Diego. Complete sequencing of the peach genome will provide a road map for future sequencing of other model genomes, including apple, strawberry, and other representatives of the family. A group at the Istituto Agrario di San Michele All'Adige in Italy (R. Velasco, personal communication) is pursuing shotgun sequencing of the apple genome using a combination of Sanger (4× genome coverage) and 454 (7× genome coverage) sequencing.
FUNCTIONAL GENOMICS
Complete sequencing of several plant genomes, including Arabidopsis and rice, has identified tens of thousands of genes. About one-half are of unknown function. A major challenge for genomics is to identify the functions of unassigned genes and use this knowledge to improve economically important agronomic and quality traits in major crops.
ESTs
EST sequencing projects are under way for several rosaceous species. ESTs enable gene and marker discovery, aid genome annotation and gene structure identification, guide single nucleotide polymorphism characterization and aid proteome analysis (Nagaraj et al., 2007). Over 400,000 ESTs are currently available for Rosaceae species via the National Center for Biotechnology Information (NCBI) dbEST and the GDR (www.rosaceae.org). With National Science Foundation (award no. 0321701; Schuyler Korban, principal investigator) funding, over 104,000 5′ end apple EST sequences have been generated, and a 40,000 unigene set has been identified. Another approximately 150,000 5′ end apple ESTs have been deposited in the NCBI by HortResearch in New Zealand (Newcomb et al., 2006). The combined publicly available apple ESTs add up to over 260,000 sequences, resulting in a 17,000 unigene set (NCBI, apple).
EST sequencing projects in peach are being conducted in several laboratories worldwide. Many of these projects have focused on fruit development, so other tissues are underrepresented in the database. All publicly available ESTs for peach have been downloaded from NCBI and housed in GDR. There are currently approximately 87,751 ESTs with a unigene set comprised of 23,721 different sequences. These ESTs have come predominantly from laboratories of the Prunus Genome Mapping consortium, supported by the USDA Initiative for Future Agricultural and Food Systems program (Albert Abbott), ESTree (Carlo Pozzi), and the Chile Consortium (Ariel Orellano).
Approximately 50,000 EST sequences from F. vesca and several thousand from Fragaria ×ananassa have been deposited in GenBank by several contributing laboratories. From F. vesca, cDNA libraries have sampled cold-, heat-, and salt-stressed seedlings (J.P. Slovin and P.D. Rabinowitz, unpublished data), and flower buds (R.L. Brese and T.M. Davis, unpublished data). These sequences have proven invaluable for annotation of Fragaria genomic sequences and as a source of candidate gene and random clones for use in reverse genetics investigations. Nevertheless, when EST sequence resources are considered, development of libraries and strawberry sequencing has lagged behind other major fruit crops and warrants further investment in genomic resource development. Cultivated strawberry represents a potential diverse source of alleles that may be directly relevant to selected traits of interest. Furthermore, an accounting of alleles may further illuminate the ancient diploid contributors to the octoploid strawberry genome.
The GDR development team has assembled and analyzed genera-specific unigenes for approximately 370,000 Rosaceae ESTs available at the GDR Web site (Jung et al., 2008). The analysis includes identification of putative functions by pairwise comparisons against protein datasets, with SSR and single nucleotide polymorphism detection. Sequence comparison with 14 of the major plant EST datasets suggests that up to 14% of the Rosaceae unigenes may be unique to the family. Many of these genes are being functionally characterized in strawberry (K. Folta, personal communication). The availability of full genome sequence is expected to verify these numbers and provide a more refined gene catalog of Rosaceae species.
Reverse and Forward Genetics
Two fundamentally different approaches are currently being used to elucidate gene function. A forward genetics approach begins with the observation of a unique phenotype and seeks to identify the gene(s) responsible for that phenotype. Conversely, reverse genetics begins with a candidate gene and describes the mutant phenotypes that result upon its disruption. Despite the fact that small genomes render forward genetics a potentially useful strategy for gene discovery, currently there are few forward-genetic populations available for rosaceous crops.
One approach to generating mutant phenotypes in plants is to inactivate a gene by inserting foreign DNA via Agrobacterium-mediated transformation (Krysan et al., 1999). This approach can be used for both forward and reverse genetics. An advantage of using insertional mutagenesis to disrupt gene function is that all mutations are tagged by a T-DNA sequence, enabling rapid direct identification of the gene related to the observed phenotype. In plants with completely sequenced genomes, identification of a short sequence of genomic DNA flanking the T-DNA insertion allows unambiguous identification of the mutated gene and linkage of the insertion to a chromosomal location. For plants that have not been completely sequenced, molecular tools such as EST collections and BAC libraries may allow the successful cloning and characterizing of a gene of interest.
Whereas T-DNA mutants may produce loss-of-function phenotypes, comparable studies have utilized a gain-of-function approach through activation tagging (Kardailsky et al., 1999; Weigel et al., 2000). Here, a set of transcriptional enhancers (typically from the cauliflower mosaic virus 35S promoter/enhancer) is integrated into the genome using Agrobacterium-mediated transformation. Upon generally random integration, the enhancers greatly increase the basal transcription rates of nearby promoters. This approach is particularly useful in identifying gene function from members of multigene families where an effective knockout strategy is precluded by genetic redundancy.
F. vesca is an excellent candidate to generate a collection of T-DNA or transposon insertion or activation tagged mutants. With its small genome, F. vesca will require a minimal number of independent mutant lines to cover the complete genome. Based on the approximately 200-Mb genome size of F. vesca and assuming random T-DNA integration and an average gene length of 2 kb, we calculate that 255,000 or 391,000 independent T-DNA transformed lines must be produced to mutate any single gene with 95% or 99% probability, respectively (Krysan et al., 1999). Gene content and spacing in Fragaria are similar to those of Arabidopsis (K.M. Folta and T.M. Davis, unpublished data), suggesting that these goals are realistic. A collection comprised of both T-DNA insertional mutants and AcDs activation tag lines is currently under development, with over 1,000 independently regenerated; these are now being characterized at Virginia Tech. Fewer activation tagged lines will be needed, because one tag may affect several kilobase pairs of gene space. Sequencing flanking regions adjacent to T-DNA integrations will provide resources both for reverse genetics and many markers for future mapping efforts while revealing genes that affect important traits and interesting phenotypes.
RNA Interference
Computational analysis of approximately 120,000 ESTs from apple has identified 10 distinct sequences that can be classified as representatives of seven conserved plant microRNA (miRNA) families, and secondary structure predictions reveal that these sequences possess characteristic fold-back structures of precursor miRNAs (Schaffer et al., 2007). Gleave et al. (2008) analyzed approximately 120,000 M. ×domestica ‘Royal Gala’ ESTs to identify 10 distinct sequences that could be classified into seven conserved plant miRNA families. Three of these miRNAs were expressed constitutively in all tissues tested, whereas others showed more restricted patterns of expression. A candidate gene approach coupled with RNA interference (RNAi) silencing is being used to determine the function of apple ESTs in resistance to the bacterial fire blight disease (Norelli et al., 2007). Bioinformatics is used to identify publicly available apple ESTs either uniquely associated with fire blight-infected apple or similar to Arabidopsis ESTs associated with Pseudomonas syringae pv. tomato infection. An efficient transformation system for apple has been developed in which only a single EST-silencing gene is inserted per transgenic line to select for apple RNAi mutants. The system uses a multi-vector transformation approach and PCR primers developed for pHellsgate8-derived vectors that detect presence of single or multiple EST silencing insertions in RNAi transgenic line and provide a sequencing template for determining the EST contained in the silencing insert. Additional candidate ESTs are currently being selected based upon cDNA suppression subtractive hybridization and cDNA-AFLP analyses (Norelli et al., 2007).
Neither identification of a mutant phenotype nor identification of a gene sequence alone will explain the molecular function of a gene. Modern functional genomics relies heavily on high throughput profiling methodologies like gene expression profiling, proteomics, metabolomics, and bioinformatics for detailed gene characterization. Most importantly, readily available transformation systems provide means for validating gene function in vivo.
Transcriptomics
Apple has the largest collection of ESTs and microarrays and will provide the foundation for rosaceous microarray and gene expression analyses. Several apple microarrays are currently available or under construction and are available on different platforms, including Nimblegen, Invitrogen, and Affymetrix. Pichler et al. (2007) have demonstrated that field microarray experiments provide a viable approach for measuring seasonal changes in gene expression during apple bud development. Teamed with transgenic plants, custom apple arrays have also revealed that ethylene controls substantial production of flavor-associated volatiles (Schaffer et al., 2007), and other studies have investigated early fruit development (Newcomb et al., 2006; Lee et al., 2007). Some of the most fruitful early plant microarray experiments have been performed in strawberry. Using custom arrays, transcripts associated with ripening (Schwab et al., 2001), stress- and auxin-induced gene expression (Aharoni et al., 2002), volatile production (Aharoni et al., 2004), fruit development (Aharoni and O'Connell, 2002), and flavor (Aharoni et al., 2000) have been identified. These seminal studies represent well-designed and highly productive approaches that answered fundamental questions in plant biology. More importantly, research suggests that contemporary studies with larger probe sets may lead to important discoveries for a multitude of plant responses.
Proteomics
Proteomics studies in the family are limited to date. A proteomic approach was applied to study flesh browning in stored Conference pears (Pedreschi et al., 2007) and to identify novel isoforms of Pru av 1, the major cherry allergen in fruit (Reuter et al., 2005). Other studies to investigate the roles of dehydrins, among others, in cold temperature stress responses of peach and apple using proteomic approaches are ongoing (Renaut et al., 2006, 2008).
Metabolomics
Rosaceous plants are extremely rich in specialized metabolites, many of which have documented utility in human health and nutrition. Chemistry and biosynthetic pathways of flavonoids, anthocyanins, and phenolics have been extensively studied in numerous rosaceous fruits with targeted analytical assays. Recent developments in metabolomics allow for global analysis and interrogation of metabolic networks. An untargeted metabolomics approach based on Fourier transform ion cyclotron mass spectrometry was used to study four consecutive stages of strawberry fruit development and identified novel information on the metabolic transition from immature to ripe fruit (Aharoni and O'Connell, 2002). Metabolomic profiles of apple fruit revealed changes provoked by UV-white light irradiation and cold storage in primary and secondary pathways associated with ethylene synthesis, acid metabolism, flavonoid pigment synthesis, and fruit texture (Rudell et al., 2008). Broader use of metabolomics in Rosaceae will speed the discovery of novel gene functions in primary and secondary metabolism and will provide comprehensive data sets necessary to model metabolic networks related to human health-promoting metabolites.
High-Throughput Transformation and Gene Function Validation
High-throughput profiling platforms and bioinformatics data mining generate numerous biological hypotheses about candidate gene functions that must be tested in subsequent experiments. These follow-up experiments rely upon the availability of a robust transformation protocol. Transformation systems have been developed in many rosaceous crops, including apple, peach, rose, and strawberry. Using degenerate primers designed from conserved regions of transcription factor genes, Ban et al. (2007) identified a transcription factor from apple skin responsible for red coloration; its function was verified in transient transformation of apple seedlings as well as in transgenic tobacco (Nicotiana tabacum). A similar candidate gene approach has been used to identify a transcription factor responsible for red flesh and foliage color in apple (Chagné et al., 2007; Espley et al., 2007).
ROSACEAE COMPARATIVE GENOMICS
A phylogenetic view provides a refined basis for the inference of homology, orthology, and functional conservation with well-studied plant species. Common markers between maps constructed from intra- and interspecific populations of Prunus allow for comparisons among genomes of seven species, including peach, almond, apricot, cherry, P. cerasifera, P. davidiana, and P. ferganenesis. All comparisons are essentially syntenic and colinear (Arús et al., 2003; Dirlewanger et al., 2004b), thus strongly suggesting that the Prunus genome is similar within the genus and in agreement with patterns of frequent intercrossability and interspecific hybrid fertility known between many species of this genus. Only two major chromosomal rearrangements, both reciprocal translocations, have been reported. One was reported in the F2 of a cross between Garfi almond and the red leaf peach rootstock Nemared and involved linkage groups 6 and 8 (G6 and G8; Jauregui et al., 2001). This translocation was also found in the cross Akame × Juseitou, where Akame is also a red-leafed genotype (Yamamoto et al., 2005). The other reciprocal translocation was found in a genotype of myrobalan plum (P. cerasifera) and affecting G3 and G5 (Lambert et al., 2004). These results suggested that reciprocal translocations might occur and were maintained within a given Prunus species characterizing monophyletic transects (i.e. red-leafed peaches), although the majority of individuals within a species were within the general Prunus genome configuration.
Pear maps constructed by Dondini et al. (2004), Pierantoni et al. (2004), and Yamamoto et al. (2002, 2004) have sufficient SSRs to enable alignment of all pear and apple linkage groups. Map comparisons suggest that genome organization is conserved between apple and pear.
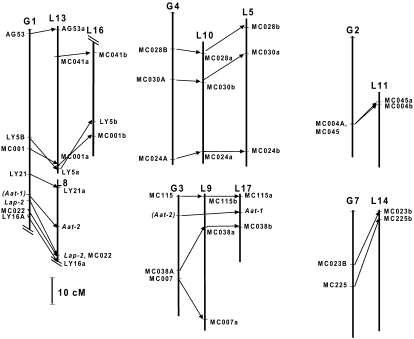
A comparison between apple and peach genomes (Fig. 3) is possible based on 30 common loci (24 random fragment length polymorphisms [RFLPs] and six isozymes) found between the Texas × Earlygold Prunus map and the Prima × Fiesta Malus map (Dirlewanger et al., 2004a). Prunus linkage groups 3 and 4 (G3 and G4) have three or more markers in common with the Malus map; each Prunus group has detected a pair of homoeologous apple groups with the same marker order. Each of two additional Prunus groups (G2 and G7) has two markers in common with one Malus linkage group, and G1 has eight markers in common with Malus. G1 is the longest and most populated linkage group with markers in Texas × Earlygold and most other Prunus maps, suggesting that it corresponds with chromosome 1. Malus does not have a similar long chromosome. Four markers found in the upper part of Prunus G1 correspond to two homoeologous groups of Malus, and the four remaining markers map to a different Malus linkage group. These results indicate that the long Prunus chromosome may be split into two in Malus or in the original species that formed the Malus amphidiploid.
Figure 3.
Map comparison between Prunus (Joobeur et al., 1998) and Malus (Maliepaard et al., 1998). Prunus linkage groups are noted with G and Malus groups as L followed by a number. Only linkage groups with two or more common markers have been included. Markers in parenthesis in Prunus correspond to approximate positions deduced from other maps. Incomplete linkage groups are indicated by two parallel oblique lines.
Results of the diploid strawberry map of F. vesca × F. nubicola (Sargent et al., 2006) indirectly suggest that both genomes are highly conserved as markers mapped on their F2 generate the expected seven linkage groups. Findings by Sargent et al. (2006) indicate that linkage groups found in the octoploid strawberry map can be aligned into seven homoeologous groups, each with four linkage groups. Each homoeologous group corresponds to one of the diploid Fragaria linkage groups. A study between diploid Fragaria and tetraploid Rosa suggests that synteny conservation is high, but chromosomal rearrangements may have occurred between these two genera (markers from two Rosa linkage groups are located in one Fragaria linkage group; M. Rousseau, personal communication).
A comparison between the diploid strawberry and peach genomes based on more than 10 markers per Fragaria linkage group is currently in progress. Results suggest that these two distant genomes still conserve synteny but have undergone reshuffling since their divergence from a common ancestor. Synteny is revealed by considering the eight Prunus linkage groups and noting that five contain most or all markers from only one group of Fragaria and the other three include markers of two groups. Thus, reshuffling must have occurred to a large extent with various fission/fusion, translocation, and inversion events.
The Arabidopsis sequence has been compared with the map positions of: (1) RFLPs mapped in Texas × Earlygold, most of them detected with probes based on Rosaceae or Arabidopsis EST sequences (Dominguez et al., 2003); (2) peach ESTs anchored to the Prunus consensus map (Jung et al., 2006); (3) peach ESTs physically located in Prunus BAC contigs (Jung et al., 2006); (4) complete sequence of one and partial sequences of two peach BAC clones (Georgi et al., 2003); and (5) complete sequence of a single myrobalan plum (P. cerasifera) BAC clone (Claverie et al., 2004b). In all cases, syntenic regions can be found, but these are limited to small DNA fragments. This suggests an overall view of partial genome conservation. The largest Prunus-Arabidopsis conserved region identified by Dominguez et al. (2003) comprised 25 cM in LG2 of Prunus corresponding to 5.4 Mb of Arabidopsis chromosome 5. These results suggest that comparative mapping for gene discovery outside of the family may not be feasible, underscoring the importance of complete genomic characterization of representative species in the family.
A novel marker concept based upon “gene pair” polymorphisms provides a promising approach to comparative genomics (Davis et al., 2008). This concept exploits the small genome sizes and the concomitantly small intergenic distances characteristic of rosaceous species. As demonstrated in F. vesca, the commonality of small (2–5 kb) intergenic distances facilitates the amplification of gene pair PCR products in which a forward primer is targeted to conserved coding sequence in one gene and the reverse primer is similarly targeted in an adjacent gene. By mining the abundance of polymorphism in a respective intergenic region, gene pair PCR products yield conveniently detectable polymorphisms of multiple types, enabling the respective loci to be utilized as robust anchor loci for comparative mapping in the various rosaceous species (Davis et al., 2008).
ROSACEAE BIOINFORMATICS AND DATA INTEGRATION
Several important bioinformatics resources have been developed for various rosaceous crops over the last few years. Sequence databases like ESTree db (www.itb.cnr.it/estree/) with EST information for peach (Lazzari et al., 2005), the F. vesca EST database (FVdbEST, http://www.vbi.vt.edu/∼estap), and a candidate gene database (http://www.bioinfo.wsu.edu/gdr/projects/prunus/abbott/PP_LEa/) and transcript map for peach (Horn et al., 2005) provide important repositories for Rosaceae sequence information and have advanced genomics research in the family.
“Plant Databases: A Needs Assessment White Paper” (Beavis et al., 2005) stressed that access to curated, integrated, and searchable genome databases is essential to fully leverage the wealth of genetic, genomic, and molecular data generated worldwide by plant genome projects. Such data integration gateways have been created for many model species (i.e. The Arabidopsis Information Resource [Rhee et al., 2003]; Oryzabase: An Integrated Biological and Genome Information Database for Rice [Kurata and Yamazaki, 2006]; and MaizeGDB, the community database for maize genetics and genomics data [Lawrence et al., 2007]) and for plant families (i.e. Gramene, A Resource for Comparative Mapping in the Grasses [Jaiswal et al., 2006]; SGN, the Solanaceae Genomics Network [Mueller et al., 2005]; and LIS, the Legume Information System [Gonzales et al., 2005]).
The creation in 2003 of the federally funded GDR (www.bioinfo.wsu.edu/gdr) was an important step toward integrating all Rosaceae structural and functional genomics initiatives (Jung et al., 2004, 2008). Initiated in response to the growing availability of genomic data for peach, GDR now contains comprehensive data for the genetically anchored peach physical map, Rosaceae maps, markers and traits, and all publicly available Rosaceae ESTs. The ESTs are assembled to produce unigene sets of each genus and the entire Rosaceae. Other annotations include putative function, microsatellites, open reading frames, single nucleotide polymorphisms, plant and gene ontology terms, and anchored map position where applicable. Most of the published Rosaceae genetic maps can be viewed and compared through CMap (Ware et al., 2002), the comparative map viewer. The peach physical map can be viewed using WebFPC/WebChrom and also through an integrated GDR map viewer, which provides a graphical interface to the combined genetic, transcriptome, and physical mapping information categorized by taxonomy, tissue type, putative function, and gene ontology. The GDR datasets are available to download or search in batch mode using Web-based BLAST (Altschul et al., 1990) or FASTA (Pearson and Lipman, 1988) servers. Other Web-based tools include a sequence assembly server using CAP3 (Huang and Madan, 1999) and an SSR server for identifying microsatellites and primers in sequence data sets.
The Rosaceae research community consistently uses GDR as a portal for disseminating and mining data, coordinating collaborations and workgroups, and sharing important news and publications. Usage figures show 218,409 visits and 2,070,880 pages accessed between August 1, 2006 and July 31, 2007. GDR will continue to curate, integrate, and visualize new Rosaceae genomics and genetics data as they become available. These will include genetic maps, markers and traits, phenotype and genotype data, apple physical map, microarray data, and genome sequences from apple, peach, and strawberry.
ROSACEAE COMMUNITY EFFORT AND THE FUTURE OF ROSACEAE GENOMICS
The Rosaceae Genomics Initiative (RosIGI, http://www.bioinfo.wsu.edu/gdr/community/international/) was established to link and coordinate cross-Rosaceae genomics efforts internationally. These include EST and genome sequencing, genetic and physical mapping, molecular marker discovery, development of a microarray gene expression analysis platform, forward and reverse genetic tools, and high throughput gene validation. In the United States, these efforts are coordinated by the U.S. Rosaceae Genomics, Genetics, and Breeding Executive Committee. Rather than selecting a single model and focusing all resources on developing a full array of resources and tools for it, the Rosaceae community adopted an alternative strategy of developing family-wide resources and leveraging the most appropriate system to maximize efficiency. This strategy has been effective in other plant and microbial research communities (i.e. legume and Phytophthora). A white paper outlining long- and short-term strategies has been prepared by the community and is available at the GDR Web site (www.rosaceaewhitepaper.com).
BEYOND THE HORIZON
Genomics efforts in the Rosaceae family represent more than simply another set of organism sequences. The accelerating accumulation of genomics-level information in this particular family brings great advantages. Over the past 5 years, while various “revolutionary” genomics tools have come and gone, a validated set of proven microarray platforms, marker development methods, and high-throughput cloning systems has been established. These proven tools are ripe for application in questions applicable to rosaceous plant biology and crop production. New high-throughput sequencing techniques, means for quantitative gene expression analyses, and novel phenotyping platforms are in their infancy and will mature around economically useful species rather than model systems. The crop species within Rosaceae are well positioned to benefit from these emerging technologies.
Being a late arrival to the party has its advantages. The codified Rosaceae community can coalesce around standardized techniques, plant model systems, and computational tools residing in a central database, while strengthening comparative studies and fortifying meaningful meta-analyses. Most importantly, in commitment to the translational genomics paradigm, the Rosaceae genomics community is uniquely poised to incorporate breeders' and growers' input into genomics-level experimental design, bringing greater direct impact to basic science studies while resisting the allure of generating knowledge only for knowledge's sake. With versatile, relevant plant systems, emerging models and a concerted approach, ongoing studies in Rosaceae genomics will contribute to facets of basic plant science while bringing higher-quality products to the consumer with lower environmental impacts.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Vladimir Shulaev (vshulaev@vbi.vt.edu).
Open Access articles can be viewed online without a subscription.
References
- Aharoni A, Giri AP, Verstappen FWA, Bertea CM, Sevenier R, Sun ZK, Jongsma MA, Schwab W, Bouwmeester HJ (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16 3110–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Keizer LCP, Bouwmeester HJ, Sun ZK, Alvarez-Huerta M, Verhoeven HA, Blaas J, van Houwelingen A, De Vos RCH, van der Voet H, et al (2000) Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12 647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Keizer LCP, Van den Broeck HC, Blanco-Portales R, Munoz-Blanco J, Bois G, Smit P, De Vos RCH, O'Connell AP (2002) Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiol 129 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, O'Connell AP (2002) Gene expression analysis of strawberry achene and receptacle maturation using DNA microarrays. J Exp Bot 53 2073–2087 [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Yamamoto Y, Ohmido N, Ohshima M, Fukui K (2001) Estimation of the nuclear DNA content of strawberries (Fragaria spp.) compared with Arabidopsis thaliana by using dual-step flow cytometry. Cytologia (Tokyo) 66 431–436 [Google Scholar]
- Albani MC, Battey NH, Wilkinson MJ (2004) The development of ISSR-derived SCAR markers around the SEASONAL FLOWERING LOCUS (SFL) in Fragaria vesca. Theor Appl Genet 109 571–579 [DOI] [PubMed] [Google Scholar]
- Alsheikh MK, Suso HP, Robson M, Battey NH, Wetten A (2002) Appropriate choice of antibiotic and Agrobacterium strain improves transformation of anti biotic-sensitive Fragaria vesca and F.v. semperflorens. Plant Cell Rep 20 1173–1180 [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215 403–410 [DOI] [PubMed] [Google Scholar]
- Aranzana MJ, Pineda A, Cosson P, Dirlewanger E, Ascasibar J, Cipriani G, Ryder CD, Testolin R, Abbott A, King GJ, et al (2003) A set of simple-sequence repeat (SSR) markers covering the Prunus genome. Theor Appl Genet 106 819–825 [DOI] [PubMed] [Google Scholar]
- Arús P, Howad W, Mnejja M (2003) Marker development and marker-assisted selection in temperate fruit trees. In R Tuberosa, RL Phillips, M Gale, eds, Proceedings of an International Congress: In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution. Avenue Media, Bologna, Italy, pp 309–325
- Baird WV, Estager AS, Wells JK (1994) Estimating nuclear DNA content in peach and related diploid species using laser flow cytometry and DNA hybridization. J Am Soc Hortic Sci 119 1312–1316 [Google Scholar]
- Baldi P, Patocchi A, Zini E, Toller C, Velasco R, Komjanc M (2004) Cloning and linkage mapping of resistance gene homologues in apple. Theor Appl Genet 109 231–239 [DOI] [PubMed] [Google Scholar]
- Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48 958–970 [DOI] [PubMed] [Google Scholar]
- Beavis W, Gessler D, Rhee S, Rokhsar D, Main D, Mueller L, Eva Huala E, Stein L, Lawrence CJ (2005) Plant Biology Databases: A Needs Assessment—White Paper. Gramene. http://www.gramene.org/resources/plant_databases.pdf (June 2, 2008)
- Blenda AV, Wechter WP, Reighard GL, Baird WV, Abbott AG (2006) Development and characterisation of diagnostic AFLP markers in Prunus persica for its response to peach tree short life syndrome. J Hortic Sci Biotechnol 81 281–288 [Google Scholar]
- Bliss FA, Arulsekar S, Foolad MR, Becerra V, Gillen AM, Warburton ML, Dandekar AM, Kocsisne GM, Mydin KK (2002) An expanded genetic linkage map of Prunus based on an interspecific cross between almond and peach. Genome 45 520–529 [DOI] [PubMed] [Google Scholar]
- Bolar JP, Norelli JL, Harman GE, Brown SK, Aldwinckle HS (2001) Synergistic activity of endochitinase and exochitinase from Trichoderma atroviride (T. harzianum) against the pathogenic fungus (Venturia inaequalis) in transgenic apple plants. Transgenic Res 10 533–543 [DOI] [PubMed] [Google Scholar]
- Bolar JP, Norelli JL, Wong KW, Hayes CK, Harman GE, Aldwinckle HS (2000) Expression of endochitinase from Trichoderma harzianum in transgenic apple increases resistance to apple scab and reduces vigor. Phytopathology 90 72–77 [DOI] [PubMed] [Google Scholar]
- Bringhurst RS (1990) Cytogenetics and evolution in American Fragaria. HortScience 25 879–881 [Google Scholar]
- Bus V, Ranatunga C, Gardiner S, Bassett H, Rikkerink E (2000) Marker assisted selection for pest and disease resistance in the New Zealand apple breeding programme. Acta Hortic 538 541–547 [Google Scholar]
- Byrne DH (1990) Isozyme variability in four diploid stone fruits compared with other woody perennial plants. J Hered 81 68–71 [Google Scholar]
- Calenge F, Van der Linden CG, Van de Weg E, Schouten HJ, Van Arkel G, Denance C, Durel CE (2005) Resistance gene analogues identified through the NBS-profiling method map close to major genes and QTL for disease resistance in apple. Theor Appl Genet 110 660–668 [DOI] [PubMed] [Google Scholar]
- Caragay AB (1992) Cancer-preventive foods and ingredients. Food Technol 46 65–68 [Google Scholar]
- Chagné D, Carlisle CM, Blond C, Volz RK, Whitworth CJ, Oraguzie NC, Crowhurst RN, Allan AC, Espley RV, Hellens RP, et al (2007) Mapping a candidate gene (MdMYB10) for red flesh and foliage colour in apple. BMC Genomics 8 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro JX, Werner DJ, Omalley D, Sederoff RR (1994) Targeted mapping and linkage analysis of morphological isozyme, and RAPD markers in peach. Theor Appl Genet 87 805–815 [DOI] [PubMed] [Google Scholar]
- Cipriani G, Pinosa F, Bonoli M, Faedi W (2006) A new set of microsatellite markers for Fragaria species and their application in linkage analysis. J Hortic Sci Biotechnol 81 668–675 [Google Scholar]
- Claverie M, Dirlewanger E, Cosson P, Bosselut N, Lecouls AC, Voisin R, Kleinhentz M, Lafargue B, Caboche M, Chalhoub B, et al (2004. b) High-resolution mapping and chromosome landing at the root-knot nematode resistance locus Ma from Myrobalan plum using a large-insert BAC DNA library. Theor Appl Genet 109 1318–1327 [DOI] [PubMed] [Google Scholar]
- Cordenunsi BR, do Nascimento JRO, Genovese MI, Lajolo FM (2002) Influence of cultivar on quality parameters and chemical composition of strawberry fruits grown in Brazil. J Agric Food Chem 50 2581–2586 [DOI] [PubMed] [Google Scholar]
- Corredor E, Roman M, Garcia E, Perera E, Arús P, Naranjo T (2004) Physical mapping of rDNA genes establishes the karyotype of almond. Ann Appl Biol 144 219–222 [Google Scholar]
- Darrow GM (1966) The strawberry: history, breeding and physiology. Holt, Rinehart and Winston, New York
- Davis TM, Denoyes-Rothan B, Lerceteau-Kohler E (2007) Strawberry. In C Kole, ed, Genome Mapping and Molecular Breeding in Plants IV: Fruits and Nuts. Springer, Berlin, pp 189–206
- Davis TM, Folta KM, Shields M, Zhang Q (2008) Gene pair markers: An innovative tool for comparative linkage mapping. In Proceedings of the 6th North American Strawberry Symposium. North American Strawberry Growers Association, Kemptville, Canada (in press)
- Davis TM, Yu H (1997) A linkage map of the diploid strawberry, Fragaria vesca. J Hered 88 215–221 [Google Scholar]
- Debener T, Mattiesch L (1999) Construction of a genetic linkage map for roses using RAPD and AFLP markers. Theor Appl Genet 99 891–899 [Google Scholar]
- Debener T, Mattiesch L, Vosman B (2001) A molecular marker map for roses. Acta Hortic 547 283–287 [Google Scholar]
- Defilippi BG, Dandekar AM, Kader AA (2004) Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) fruits. J Agric Food Chem 52 5694–5701 [DOI] [PubMed] [Google Scholar]
- Defilippi BG, Kader AA, Dandekar AM (2005) Apple aroma: alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci 168 1199–1210 [Google Scholar]
- Dettori MT, Quarta R, Verde I (2001) A peach linkage map integrating RFLPs, SSRs, RAPDs, and morphological markers. Genome 44 783–790 [PubMed] [Google Scholar]
- Dirlewanger E, Cosson P, Bouderhi K, Renaud C, Capdeville G, Tauzin Y, Laigret F, Moing A (2006) Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet Genomes 3 1–13 [Google Scholar]
- Dirlewanger E, Cosson P, Howad W, Capdeville G, Bosselut N, Claverie M, Voisin R, Poizat C, Lafargue B, Baron O, et al (2004. a) Microsatellite genetic linkage maps of myrobalan plum and an almond-peach hybrid—location of root-knot nematode resistance genes. Theor Appl Genet 109 827–838 [DOI] [PubMed] [Google Scholar]
- Dirlewanger E, Cosson P, Tavaud M, Aranzana MJ, Poizat C, Zanetto A, Arús P, Laigret F (2002) Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor Appl Genet 105 127–138 [DOI] [PubMed] [Google Scholar]
- Dirlewanger E, Graziano E, Joobeur T, Garriga-Caldere F, Cosson P, Howad W, Arús P (2004. b) Comparative mapping and marker-assisted selection in Rosaceae fruit crops. Proc Natl Acad Sci USA 101 9891–9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I, Graziano E, Gebhardt C, Barakat A, Berry S, Arús P, Delseny M, Barnes S (2003) Plant genome archaeology: evidence for conserved ancestral chromosome segments in dicotyledonous plant species. Plant Biotechnol J 1 91–99 [DOI] [PubMed] [Google Scholar]
- Dondini L, Lain O, Geuna F, Banfi R, Gaiotti F, Tartarini S, Bassi D, Testolin R (2007) Development of a new SSR-based linkage map in apricot and analysis of synteny with existing Prunus maps. Tree Genet Genomes 3 239–249 [Google Scholar]
- Dondini L, Pierantoni L, Gaiotti F, Chiondini R, Tartarini S, Bazzi C, Sansavini S (2004) Identifying QTLs for fire-blight resistance via a European pear (Pyrus communis L.) genetic linkage map. Mol Breed 14 407–418 [Google Scholar]
- Eichholzer M, Luthy J, Gutzwiller F, Stahelin HB (2001) The role of folate, antioxidant vitamins and other constituents in fruit and vegetables in the prevention of cardiovascular disease: the epidemiological evidence. Int J Vitam Nutr Res 71 5–17 [DOI] [PubMed] [Google Scholar]
- El Mansouri I, Mercado JA, Valpuesta V, Lopez-Aranda JM, Pliego-Alfaro F, Quesada MA (1996) Shoot regeneration and Agrobacterium-mediated transformation of Fragaria vesca L. Plant Cell Rep 15 642–646 [DOI] [PubMed] [Google Scholar]
- Escobar MA, Civerolo EL, Polito VS, Pinney KA, Dandekar AM (2003) Characterization of oncogene-silenced transgenic plants: implications for Agrobacterium biology and post-transcriptional gene silencing. Mol Plant Pathol 4 57–65 [DOI] [PubMed] [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne C, Rothan C, Moing A, Plomion C, Bodénès C, Svanella-Dumas L, Cosson P, Pronier V, Monet R, Dirlewanger E (2002) Candidate genes and QTLs for sugar and organic acid content in peach [Prunus persica (L.) Batsch]. Theor Appl Genet 105 145–159 [DOI] [PubMed] [Google Scholar]
- Folta KM, Davis TM (2006) Strawberry genes and genomics. Crit Rev Plant Sci 25 399–415 [Google Scholar]
- Folta KM, Dhingra A (2006) Transformation of strawberry: the basis for translational genomics in Rosaceae. In Vitro Cell Dev Biol Plant 42 482–490 [Google Scholar]
- Foolad MR, Arulsekar S, Becerra V, Bliss FA (1995) A genetic map of Prunus based on an interspecific cross between peach and almond. Theor Appl Genet 91 262–269 [DOI] [PubMed] [Google Scholar]
- Foulongne M, Pascal T, Arús P, Kervella J (2003) The potential of Prunus davidiana for introgression into peach [Prunus persica (L.) Batsch] assessed by comparative mapping. Theor Appl Genet 107 227–238 [DOI] [PubMed] [Google Scholar]
- Gardiner S, Bus V, Rusholme R, Chagné D, Rikkerink E (2007) Apple. In C Kole, ed, Genome Mapping and Molecular Breeding, Vol 4. Springer-Verlag, Heidelberg, pp 1–62
- Georgi LL, Wang Y, Reighard GL, Mao L, Wing RA, Abbott AG (2003) Comparison of peach and Arabidopsis genomic sequences: fragmentary conservation of gene neighborhoods. Genome 46 268–276 [DOI] [PubMed] [Google Scholar]
- Georgi LL, Wang Y, Yvergniaux D, Ormsbee T, Inigo M, Reighard G, Abbott AG (2002) Construction of a BAC library and its application to the identification of simple sequence repeats in peach. Prunus persica (L.) Batsch. Theor Appl Genet 105 1151–1158 [DOI] [PubMed] [Google Scholar]
- Gleave AP, Ampomah-Dwamena C, Berthold S, Dejnoprat S, Karunairetnam S, Nain B, Wang YY, Crowhurst RN, MacDiarmid RM (2008) Identification and characterisation of primary microRNAs from apple (Malus domestica cv. Royal Gala) expressed sequence tags. Tree Genet Genomes 4 343–358 [Google Scholar]
- Gonzales MD, Archuleta E, Farmer A, Gajendran K, Grant D, Shoemaker R, Beavis WD, Waugh ME (2005) The Legume Information System (LIS): an integrated information resource for comparative legume biology. Nucleic Acids Res 33 D660–D665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J, Smith K, MacKenzie K, Jorgenson L, Hackett C, Powell W (2004) The construction of a genetic linkage map of red raspberry (Rubus idaeus subsp. idaeus) based on AFLPs, genomic-SSR and EST-SSR markers. Theor Appl Genet 109 740–749 [DOI] [PubMed] [Google Scholar]
- Guterman I, Shalit M, Menda N, Piestun D, Dafny-Yelin M, Shalev G, Bar E, Davydov O, Ovadis M, Emanuel M, et al (2002) Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell 14 2325–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu T, Kishida H, Morikita M, Kitajima A, Yamada T, Tao R (2006) A simple and rapid procedure for the detection of self-compatible individuals in Japanese apricot (Prunus mume Sieb. et Zucc.) using the loop-mediated isothermal amplification (LAMP) method. HortScience 41 1156–1158 [Google Scholar]
- Haffner K, Vestrheim S (1997) Fruit quality of strawberry cultivars. Acta Hortic 439 325–332 [Google Scholar]
- Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR (1998) HPLC method for screening of flavonoids and phenolic acids in berries. J Sci Food Agric 77 543–551 [Google Scholar]
- Häkkinen SH, Törrönen AR (2000) Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: influence of cultivar, cultivation site and technique. Food Res Int 33 517–524 [Google Scholar]
- Han Y, Gasic K, Marron B, Beever JE, Korban SS (2007) A BAC-based physical map of the apple genome. Genomics 89 630–637 [DOI] [PubMed] [Google Scholar]
- Harris GK, Gupta A, Nines RG, Kresty LA, Habib SG, Frankel WL, LaPerle K, Gallaher DD, Schwartz SJ, Stoner GD (2001) Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer 40 125–133 [DOI] [PubMed] [Google Scholar]
- Haymes KM, Davis TM (1998) Agrobacterium mediated transformation of ‘Alpine’ Fragaria vesca, and transmission of transgenes to R1 progeny. Plant Cell Rep 17 279–283 [DOI] [PubMed] [Google Scholar]
- Haymes KM, Van de Weg WE, Arens P, Maas JL, Vosman B, Den Nijs APM (2000) Development of SCAR markers linked to a Phytophthora fragariae resistance gene and their assessment in European and North American strawberry genotypes. J Am Soc Hortic Sci 125 330–339 [Google Scholar]
- Hemmat M, Weeden NF, Brown SK (2003) Mapping and evaluation of Malus × domestica microsatellites in apple and pear. J Am Soc Hortic Sci 128 515–520 [Google Scholar]
- Hemmat M, Weeden NF, Manganaris AG, Lawson DM (1994) Molecular marker linkage map for apple. J Hered 85 4–11 [PubMed] [Google Scholar]
- Hokanson SC (2001) SNiPs, chips, BACs, and YACs: Are small fruits part of the party mix? HortScience 36 859–871 [Google Scholar]
- Horn R, Lecouls AC, Callahan A, Dandekar A, Garay L, McCord P, Howad W, Chan H, Verde I, Main D, et al (2005) Candidate gene database and transcript map for peach, a model species for fruit trees. Theor Appl Genet 110 1419–1428 [DOI] [PubMed] [Google Scholar]
- Howad W, Yamamoto T, Dirlewanger E, Testolin R, Cosson P, Cipriani G, Monforte AJ, Georgi L, Abbott AG, Arús P (2005) Mapping with a few plants: using selective mapping for microsatellite saturation of the Prunus reference map. Genetics 171 1305–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Madan A (1999) CAP3: a DNA sequence assembly program. Genome Res 9 868–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaracha E, Xu M, Korban SS (2004) Narrowing down the region of the Vf locus for scab resistance in apple using AFLP-derived SCARs. Theor Appl Genet 108 274–279 [DOI] [PubMed] [Google Scholar]
- Hurtado MA, Romero C, Vilanova S, Abbott AG, Llacer G, Badenes ML (2002) Genetic linkage maps of two apricot cultivars (Prunus armeniaca L.), and mapping of PPV (sharka) resistance. Theor Appl Genet 105 182–191 [DOI] [PubMed] [Google Scholar]
- Jaiswal P, Ni J, Yap I, Ware D, Spooner W, Youens-Clark K, Ren L, Liang C, Zhao W, Ratnapu K, et al (2006) Gramene: a bird's eye view of cereal genomes. Nucleic Acids Res 34 D717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janick J, Cummins JN, Brown SK, Hemmat M (1996) Apples. In J Janick, JN Moore, eds, Fruit Breeding, Vol I: Tree and Tropical Fruits. John Wiley and Sons, New York, pp 1–77
- Jauregui B, de Vicente MC, Messeguer R, Felipe A, Bonnet A, Salesses G, Arús P (2001) A reciprocal translocation between ‘Garfi’ almond and ‘Nemared’ peach. Theor Appl Genet 102 1169–1176 [Google Scholar]
- Jelenkovic G, Harrington E (1972) Morphology of the pachytene chromosomes in Prunus persica. Can J Genet Cytol 14 317–324 [Google Scholar]
- Joobeur T, Periam N, Vicente MC, King GJ, Arús P (2000) Development of a second generation linkage map for almond using RAPD and SSR markers. Genome 43 649–655 [PubMed] [Google Scholar]
- Joobeur T, Viruel MA, de Vicente MC, Jauregui B, Ballester J, Dettori MT, Verde I, Truco MJ, Messeguer R, Batlle I, et al (1998) Construction of a saturated linkage map for Prunus using an almond x peach F2 progeny. Theor Appl Genet 97 1034–1041 [Google Scholar]
- Jung S, Jesudurai C, Staton M, Du ZD, Ficklin S, Cho IH, Abbott A, Tomkins J, Main D (2004) GDR (Genome Database for Rosaceae): integrated web resources for Rosaceae genomics and genetics research. BMC Bioinformatics 5 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Main D, Staton M, Cho I, Zhebentyayeva T, Arús P, Abbott A (2006) Synteny conservation between the Prunus genome and both the present and ancestral Arabidopsis genomes. BMC Genomics 7 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Staton M, Lee T, Blenda A, Svancara R, Abbott A, Main D (2008) GDR (Genome Database for Rosaceae): integrated web-database for Rosaceae genomics and genetics data. Nucleic Acids Res 36 D1034–D1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Mattiesch L, Lorz H, Debener T (2003) Construction of a BAC library of Rosa rugosa Thunb. and assembly of a contig spanning Rdr1, a gene that confers resistance to blackspot. Mol Genet Genomics 268 666–674 [DOI] [PubMed] [Google Scholar]
- Kenis K, Keulemans J (2005) Genetic linkage maps of two apple cultivars (Malus × domestica Borkh.) based on AFLP and microsatellite markers. Mol Breed 15 205–219 [Google Scholar]
- Kishore GM, Shewmaker C (1999) Biotechnology: enhancing human nutrition in developing and developed worlds. Proc Natl Acad Sci USA 96 5968–5972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K, Brown SK, Norelli JL, Aldwinckle HS (1998) Alterations in nptII and gus expression following micropropagation of transgenic M7 apple rootstock lines. J Am Soc Hortic Sci 123 11–18 [Google Scholar]
- Ko KS, Norelli JL, Reynoird JP, Boresjza-Wysocka E, Brown SK, Aldwinckle HS (2000) Effect of untranslated leader sequence of AMV RNA 4 and signal peptide of pathogenesis-related protein 1b on attacin gene expression, and resistance to fire blight in transgenic apple. Biotechnol Lett 22 373–381 [Google Scholar]
- Korban SS, Chen H (1992) Apples. In F Hammerschlag, R Litz, eds, Biotechnology of Fruit Tree Crops. CAB International, Oxnard, CA, pp 203–227
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata N, Yamazaki Y (2006) Oryzabase. An integrated biological and genome information database for rice. Plant Physiol 140 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli DA, Decroocq V, Blenda AV, Schurdi-Levraud V, Garay L, Le Gall O, Damsteegt V, Reighard GL, Abbott AG (2005) Identification and mapping of resistance gene analogs (RGAs) in Prunus: a resistance map for Prunus. Theor Appl Genet 111 1504–1513 [DOI] [PubMed] [Google Scholar]
- Lambert P, Hagen LS, Arús P, Audergon JM (2004) Genetic linkage maps of two apricot cultivars (Prunus armeniaca L.) compared with the almond Texas x peach Earlygold reference map for Prunus. Theor Appl Genet 108 1120–1130 [DOI] [PubMed] [Google Scholar]
- Lavid N, Wang JH, Shalit M, Guterman I, Bar E, Beuerle T, Menda N, Shafir S, Zamir D, Adam Z, et al (2002) O-Methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol 129 1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CJ, Schaeffer ML, Seigfried TE, Campbell DA, Harper LC (2007) MaizeGDB's new data types, resources and activities. Nucleic Acids Res 35 D895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari B, Caprera A, Vecchietti A, Stella A, Milanesi L, Pozzi C (2005) ESTree db: a tool for peach functional genomics. BMC Bioinformatics (Suppl 4) 6 S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YP, Yu GH, Seo YS, Han SE, Choi YO, Kim D, Mok IG, Kim WT, Sung SK (2007) Microarray analysis of apple gene expression engaged in early fruit development. Plant Cell Rep 26 917–926 [DOI] [PubMed] [Google Scholar]
- Lerceteau-Kohler E, Guerin G, Laigret F, Denoyes-Rothan B (2003) Characterization of mixed disomic and polysomic inheritance in the octoploid strawberry (Fragaria x ananassa) using AFLP mapping. Theor Appl Genet 107 619–628 [DOI] [PubMed] [Google Scholar]
- Liebhard R, Gianfranceschi L, Koller B, Ryder CD, Tarchini R, Van de Weg E, Gessler C (2002) Development and characterisation of 140 new microsatellites in apple (Malus × domestica Borkh.). Mol Breed 10 217–241 [Google Scholar]
- Liebhard R, Kellerhals M, Pfammatter W, Jertmini M, Gessler C (2003. a) Mapping quantitative physiological traits in apple (Malus x domestica Borkh.). Plant Mol Biol 52 511–526 [DOI] [PubMed] [Google Scholar]
- Liebhard R, Koller B, Gianfranceschi L, Gessler C (2003. c) Creating a saturated reference map for the apple (Malus × domestica Borkh.) genome. Theor Appl Genet 106 1497–1508 [DOI] [PubMed] [Google Scholar]
- Liebhard R, Koller B, Patocchi A, Kellerhals M, Pfammatter W, Jermini M, Gessler C (2003. b) Mapping quantitative field resistance against apple scab in a ‘Fiesta’ x ‘Discovery’ progeny. Phytopathology 93 493–501 [DOI] [PubMed] [Google Scholar]
- López M, Vargas FJ, Batlle I (2006) Self-(in)compatibility almond genotypes: a review. Euphytica 150 1–16 [Google Scholar]
- Lu ZX, Sosinski B, Reighard GL, Baird WV, Abbott AG (1998) Construction of a genetic linkage map and identification of AFLP markers for resistance to root-knot nematodes in peach rootstocks. Genome 41 199–207 [Google Scholar]
- Macheix JJ, Sapis JC, Fleuriet A (1991) Phenolic compounds and polyphenoloxidase in relation to browning in grapes and wines. Crit Rev Food Sci Nutr 30 441–486 [DOI] [PubMed] [Google Scholar]
- Maliepaard C, Alston FH, van Arkel G, Brown LM, Chevreau E, Dunemann F, Evans KM, Gardiner S, Guilford P, van Heusden AW, et al (1998) Aligning male and female linkage maps of apple (Malus pumila Mill.) using multi-allelic markers. Theor Appl Genet 97 60–73 [Google Scholar]
- Mandal S, Stoner GD (1990) Inhibition of N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis 11 55–61 [DOI] [PubMed] [Google Scholar]
- Marshall RE (1954) Cherries and Cherry Products, Vol 5. Interscience Publishers Ltd., New York
- Masuda T, Yonemori S, Oyama Y, Takeda Y, Tanaka T, Andoh T, Shinohara A, Nakata M (1999) Evaluation of the antioxidant activity of environmental plants: activity of the leaf extracts from seashore plants. J Agric Food Chem 47 1749–1754 [DOI] [PubMed] [Google Scholar]
- Mazur WM, Uehara M, Wahala K, Adlercreutz H (2000) Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after asingle strawberry-meal in human subjects. Br J Nutr 83 381–387 [PubMed] [Google Scholar]
- Mezzetti B, Costantini E (2006) Strawberry (Fragaria × ananassa). Methods Mol Biol 344 287–295 [DOI] [PubMed] [Google Scholar]
- Miller PJ, Parfitt DE, Weinbaum SA (1989) Outcrossing in peach. HortScience 24 359–360 [Google Scholar]
- Mueller LA, Solow TH, Taylor N, Skwarecki B, Buels R, Binns J, Lin C, Wright MH, Ahrens R, Wang Y, et al (2005) The SOL Genomics Network: a comparative resource for Solanaceae biology and beyond. Plant Physiol 138 1310–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj SH, Gasser RB, Ranganathan S (2007) A hitchhiker's guide to expressed sequence tag (EST) analysis. Brief. Bioinformatics 8 6–21 [DOI] [PubMed] [Google Scholar]
- Naik S, Hampson C, Gasic K, Bakkeren G, Korban SS (2006) Development and linkage mapping of E-STS and RGA markers for functional gene homologues in apple. Genome 49 959–968 [DOI] [PubMed] [Google Scholar]
- Newcomb RD, Crowhurst RN, Gleave AP, Rikkerink EHA, Allan AC, Beuning LL, Bowen JH, Gera E, Jamieson KR, Janssen BJ, et al (2006) Analyses of expressed sequence tags from apple. Plant Physiol 141 147–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nier S, Simpson DW, Tobutt KR, Sargent DJ (2006) A genetic linkage map of an inter-specific diploid Fragaria BC1 mapping population and its comparison with the Fragaria reference map (FV X FN). J Hortic Sci Biotechnol 81 645–650 [Google Scholar]
- Norelli JL, Borejsza-Wysocka E, Baldo AM, Aldwinckle HS, Bassett CL, Farrell RE, Malnoy M, Lalli DA, Korban SS, Gasic K, et al (2007) Functional genomic analysis of apple (Malus) ESTs associated with fire blight (Erwinia amylovora). Phytopathology 97 S185–S185 [Google Scholar]
- Oosumi T, Gruszewski HA, Blischak LA, Baxter AJ, Wadl PA, Shuman JL, Veilleux RE, Shulaev V (2006) High-efficiency transformation of the diploid strawberry (Fragaria vesca) for functional genomics. Planta 223 1219–1230 [DOI] [PubMed] [Google Scholar]
- Park S, Sugimoto N, Larson MD, Beaudry R, van Nocker S (2006) Identification of genes with potential roles in apple fruit development and biochemistry through large-scale statistical analysis of expressed sequence tags. Plant Physiol 141 811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85 2444–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreschi R, Vanstreels E, Carpentier S, Hertog M, Lammertyn J, Robben J, Noben JP, Swennen R, Vanderleyden J, Nicolai BM (2007) Proteomic analysis of core breakdown disorder in Conference pears (Pyrus communis L.). Proteomics 7 2083–2099 [DOI] [PubMed] [Google Scholar]
- Pichler FB, Walton EF, Davy M, Triggs C, Janssen B, Wunsche JN, Putterill J, Schaffer RJ (2007) Relative developmental, environmental, and tree-to-tree variability in buds from field-grown apple trees. Tree Genet Genomes 3 329–339 [Google Scholar]
- Pierantoni L, Cho KH, Shin IS, Chiodini R, Tartarini S, Dondini L, Kang SJ, Sansavini S (2004) Characterisation and transferability of apple SSRs to two European pear F1 populations. Theor Appl Genet 109 1519–1524 [DOI] [PubMed] [Google Scholar]
- Pooler MR, Scorza R (1997) Irradiation and heat affect peach pollen germination and fertility. HortScience 32 290–291 [Google Scholar]
- Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, et al (2007) Phylogeny and classification of Rosaceae. Plant Syst Evol 266 5–43 [Google Scholar]
- Potter D, Gao F, Bortiri PE, Oh SH, Baggett S (2002) Phylogenetic relationships in Rosaceae inferred from chloroplast matK and trnL-trnF nucleotide sequence data. Plant Syst Evol 231 77–89 [Google Scholar]
- Quilot B, Wu BH, Kervella J, Genard M, Foulongne M, Moreau K (2004) QTL analysis of quality traits in an advanced backcross between Prunus persica cultivars and the wild relative species P. davidiana. Theor Appl Genet 109 884–897 [DOI] [PubMed] [Google Scholar]
- Rajapakse S, Byrne DH, Zhang L, Anderson N, Arumuganathan K, Ballard RE (2001) Two genetic linkage maps of tetraploid roses. Theor Appl Genet 103 575–583 [Google Scholar]
- Renaut J, Hausman JF, Bassett C, Artlip T, Cauchie HM, Witters E, Wisniewski M (2008) Quantitative proteomic analysis of short photoperiod and low-temperature responses in bark tissues of peach (Prunus persica L. Batsch). Tree Genet Genomes (in press)
- Renaut J, Hausman JF, Wisniewski ME (2006) Proteomics and low-temperature studies: bridging the gap between gene expression and metabolism. Physiol Plant 126 97–109 [Google Scholar]
- Reuter A, Fortunato D, Garoffo LP, Napolitano L, Scheurer S, Giuffrida MG, Vieths S, Conti AD (2005) Novel isoforms of Pru av 1 with diverging immunoglobulin E binding properties identified by a synergistic combination of molecular biology and proteomics. Proteomics 5 282–289 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Beavis W, Berardini TZ, Chen G, Dixon D, Doyle A, Garcia-Hernandez M, Huala E, Lander G, Montoya M, et al (2003) The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res 31 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudell DR, Mattheis JP, Curry FA (2008) Prestorage ultraviolet-white light irradation alters apple peel metabolome. J Agric Food Chem 56 1138–1147 [DOI] [PubMed] [Google Scholar]
- Salesses G, Mouras A (1977) Use of protoplasts for the study of Prunus chromosomes. Ann Amélior Plant 27 363–368 [Google Scholar]
- Sargent DJ, Clarke J, Simpson DW, Tobutt KR, Arús P, Monfort A, Vilanova S, Denoyes-Rothan B, Rousseau M, Folta KM, et al (2006) An enhanced microsatellite map of diploid Fragaria. Theor Appl Genet 112 1349–1359 [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Davis TM, Tobutt KR, Wilkinson MJ, Battey NH, Simpson DW (2004) A genetic linkage map of microsatellite, gene-specific and morphological markers in diploid Fragaria. Theor Appl Genet 109 1385–1391 [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Rys A, Nier S, Simpson DW, Tobutt KR (2007) The development and mapping of functional markers in Fragaria and their transferability and potential for mapping in other genera. Theor Appl Genet 114 373–384 [DOI] [PubMed] [Google Scholar]
- Scalliet G, Lionnet C, Le Bechec M, Dutron L, Magnard JL, Baudino S, Bergougnoux V, Jullien F, Chambrier P, Vergne P, et al (2006) Role of petal-specific orcinol O-methyltransferases in the evolution of rose scent. Plant Physiol 140 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer RJ, Friel EN, Souleyre EJF, Bolitho K, Thodey K, Ledger S, Bowen JH, Ma JH, Nain B, Cohen D, et al (2007) A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol 144 1899–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of functional compounds: recent developments. Trends Food Sci Technol 12 401–413 [Google Scholar]
- Schwab W, Aharoni A, Raab T, Perez AG, Sanz C (2001) Cytosolic aldolase is a ripening related enzyme in strawberry fruits (Fragaria x ananassa). Phytochemistry 56 407–415 [DOI] [PubMed] [Google Scholar]
- Scorza R, Mehlenbacher SA, Lightner GW (1985) Inbreeding and coancestry of freestone peach cultivars of the eastern United States and implications for peach germplasm improvement. J Am Soc Hortic Sci 110 547–552 [Google Scholar]
- Scorza R, Sherman WB (1996) Peaches. In J Janick, JN Moore, eds, Fruit Breeding, Vol 1. Tree and Tropical Fruits. John Wiley and Sons, New York, pp 325–440
- Selmar D (1999) Cyanide in foods: biology of cyanogenic glucosides and related nutritional problems. In JT Romeo, ed, Phytochemicals in Human Health Protection, Nutrition, and Plant Defense, Vol 33. Kluwer Academic, New York, pp 369–392
- Silfverberg-Dilworth E, Matasci CL, Van de Weg WE, Van Kaauwen MPW, Walser M, Kodde LP, Soglio V, Gianfranceschi L, Durel CE, Costa F, et al (2006) Microsatellite markers spanning the apple (Malus × domestica Borkh.) genome. Tree Genet Genomes 2 202–224 [Google Scholar]
- Smartt J, Simmonds NW (1995) Evolution of Crop Plants, Ed 2. Longman Scientific and Technical, Harlow, UK
- Sugimoto T, Tamaki K, Matsumoto J, Yamamoto Y, Shiwaku K, Watanabe K (2005) Detection of RAPD markers linked to the everbearing gene in Japanese cultivated strawberry. Plant Breed 124 498–501 [Google Scholar]
- Sung SK, Jeong DH, Nam JM, Kim SH, Kim SR, An G (1998) Expressed sequence tags of fruits, peels, and carpels and analysis of mRNA expression levels of the tagged cDNAs of fruits from the Fuji apple. Mol Cells 8 565–577 [PubMed] [Google Scholar]
- Swanson CA (1998) Vegetables, fruits, and cancer risk: the role of phytochemicals. In WR Bidlack, ST Omaye, MS Meskin, DKW Topham, eds, Phytochemicals. A New Paradigm. CRC Press L.L.C., Boca Raton, FL, pp 1–12
- Tahmatsidou V, O'Sullivan J, Cassells AC, Voyiatzis D, Paroussi G (2006) Comparison of AMF and PGPR inoculants for the suppression of Verticillium wilt of strawberry (Fragaria × ananassa cv. Selva). Appl Soil Ecol 32 316–324 [Google Scholar]
- Tartarini S, Sansavini S, Vinatzer B, Gennari FCD (2000) Efficiency of marker assisted selection (MAS) for the Vf scab resistance gene. Acta Hortic 538 549–552 [Google Scholar]
- Tatum TC, Stepanovic S, Biradar DP, Rayburn AL, Korban SS (2005) Variation in nuclear DNA content in Malus species and cultivated apples. Genome 48 924–930 [DOI] [PubMed] [Google Scholar]
- Teo G, Suziki Y, Uratsu SL, Lampinen B, Ormonde N, Hu WK, DeJong TM, Dandekar AM (2006) Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality. Proc Natl Acad Sci USA 103 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilov NI (1951) The origin, variation, immunity and breeding of cultivated plants. Selected writings of N.I. Vavilov, translated from Russian by K. Starr Chester. Chron Bot 13 1–366 [Google Scholar]
- Verde I, Lauria M, Dettori M, Vendramin E, Balconi C, Micali S, Wang Y, Marrazzo MT, Cipriani G, Hartings H, et al (2005) Microsatellite and AFLP markers in the Prunus persica [L. (Batsch)] × P. ferganensis BC1 linkage map: saturation and coverage improvement. Theor Appl Genet 111 1013–1021 [DOI] [PubMed] [Google Scholar]
- Vilanova S, Romero C, Abernathy D, Abbott AG, Burgos L, Llacer G, Badenes ML (2003) Construction and application of a bacterial artificial chromosome (BAC) library of Prunus armeniaca L. for the identification of clones linked to the self-incompatibility locus. Mol Genet Genomics 269 685–691 [DOI] [PubMed] [Google Scholar]
- Vinatzer BA, Patocchi A, Gianfranceschi L, Tartarini S, Zhang HB, Gessler C, Sansavini S (2001) Apple contains receptor-like genes homologous to the Cladosporium fulvum resistance gene family of tomato with a cluster of genes cosegregating with Vf apple scab resistance. Mol Plant Microbe Interact 14 508–515 [DOI] [PubMed] [Google Scholar]
- Vinatzer BA, Zhang HB, Sansavini S (1998) Construction and characterization of a bacterial artificial chromosome library of apple. Theor Appl Genet 97 1183–1190 [Google Scholar]
- Viruel MA, Messeguer R, Devicente MC, Garciamas J, Puigdomenech P, Vargas F, Arús P (1995) A linkage map with RFLP and isozyme markers for almond. Theor Appl Genet 91 964–971 [DOI] [PubMed] [Google Scholar]
- Wang D, Fan J, Ranu RS (2004) Cloning and expression of 1-aminocyclopropane-1-carboxylate synthase cDNA from rosa (Rosa x hybrida). Plant Cell Rep 22 422–429 [DOI] [PubMed] [Google Scholar]
- Wang D, Karle R, Brettin TS, Iezzoni AF (1998) Genetic linkage map in sour cherry using RFLP markers. Theor Appl Genet 97 1217–1224 [Google Scholar]
- Ware DH, Jaiswal P, Ni J, Yap IV, Pan X, Clark KY, Teytelman L, Schmidt SC, Zhao W, Chang K, et al (2002) Gramene, a tool for grass genomics. Plant Physiol 130 1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weebadde CK, Wang D, Finn CE, Lewers KS, Luby JJ, Bushakra J, Sjulin TM, Hancock JF (2008) Using a linkage mapping approach to identify QTL for day-neutrality in the octoploid strawberry. Plant Breed 127 94–101 [Google Scholar]
- Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM, et al (2000) Activation tagging in Arabidopsis. Plant Physiol 122 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M, Bassett C, Norelli J, Macarisin D, Artlip T, Gasic K, Korban S (2008) Expressed sequence tag analysis of the response of apple (Malus × domestica ‘Royal Gala’) to low temperature and water deficit. Physiol Plant 133 298–317 [DOI] [PubMed] [Google Scholar]
- Xu M, Song J, Cheng Z, Jiang J, Korban SS (2001) A bacterial artificial chromosome (BAC) library of Malus floribunda 821 and contig construction for positional cloning of the apple scab resistance gene Vf. Genome 44 1104–1113 [PubMed] [Google Scholar]
- Xu ML, Korban SS (2002. a) A cluster of four receptor-like genes resides in the Vf locus that confers resistance to apple scab disease. Genetics 162 1995–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ML, Korban SS (2002. b) AFLP-derived SCARs facilitate construction of a 1.1 Mb sequence-ready map of a region that spans the Vf locus in the apple genome. Plant Mol Biol 50 803–818 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kimura T, Saito T, Kotobuki K, Matsuta N, Liebhard R, Gessler C, van de Weg WE, Hayashi T (2004) Genetic linkage maps of Japanese and European pears aligned to the apple consensus map. Acta Hortic 663 51–56 [Google Scholar]
- Yamamoto T, Kimura T, Shoda M, Imai T, Saito T, Sawamura Y, Kotobuki K, Hayashi T, Matsuta N (2002) Genetic linkage maps constructed by using an interspecific cross between Japanese and European pears. Theor Appl Genet 106 9–18 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yamaguchi M, Hayashi T (2005) An integrated genetic linkage map of peach by SSR, STS, AFLP and RAPD. J Jpn Soc Hort Sci 74 204–213 [Google Scholar]
- Yan Z, Denneboom C, Hattendorf A, Dolstra O, Debener T, Stam P, Visser PB (2005) Construction of an integrated map of rose with AFLP, SSR, PK, RGA, RFLP, SCAR and morphological markers. Theor Appl Genet 110 766–777 [DOI] [PubMed] [Google Scholar]
- Yau MH, Che CT, Liang SM, Kong YC, Fong WP (2002) An aqueous extract of Rubus chingii fruits protects primary rat hepatocytes against tert-butyl hydroperoxide induced oxidative stress. Life Sci 72 329–338 [DOI] [PubMed] [Google Scholar]
- Zhebentyayeva TN, Horn R, Mook J, Lecouls A, Georgi L, Abbott AG, Reighard GL, Swire-Clark G, Baird WV (2006) A physical framework for the peach genome. Acta Hortic 713 83–88
- Ziegler RG, Mayne ST, Swanson CA (1996) Nutrition and lung cancer. Cancer Causes Control 7 157–177 [DOI] [PubMed] [Google Scholar]