Abstract
Plants are increasingly being employed to clean up environmental pollutants such as heavy metals; however, a major limitation of phytoremediation is the inability of plants to mineralize most organic pollutants. A key component of organic pollutants is halogenated aliphatic compounds that include 1,2-dichloroethane (1,2-DCA). Although plants lack the enzymatic activity required to metabolize this compound, two bacterial enzymes, haloalkane dehalogenase (DhlA) and haloacid dehalogenase (DhlB) from the bacterium Xanthobacter autotrophicus GJ10, have the ability to dehalogenate a range of halogenated aliphatics, including 1,2-DCA. We have engineered the dhlA and dhlB genes into tobacco (Nicotiana tabacum ‘Xanthi’) plants and used 1,2-DCA as a model substrate to demonstrate the ability of the transgenic tobacco to remediate a range of halogenated, aliphatic hydrocarbons. DhlA converts 1,2-DCA to 2-chloroethanol, which is then metabolized to the phytotoxic 2-chloroacetaldehyde, then chloroacetic acid, by endogenous plant alcohol dehydrogenase and aldehyde dehydrogenase activities, respectively. Chloroacetic acid is dehalogenated by DhlB to produce the glyoxylate cycle intermediate glycolate. Plants expressing only DhlA produced phytotoxic levels of chlorinated intermediates and died, while plants expressing DhlA together with DhlB thrived at levels of 1,2-DCA that were toxic to DhlA-expressing plants. This represents a significant advance in the development of a low-cost phytoremediation approach toward the clean-up of halogenated organic pollutants from contaminated soil and groundwater.
Two major groups of halogenated, aliphatic compounds are haloalkanes, which are used as chemical intermediates and solvents, and haloalkanoic acids, which are additionally used as herbicides and disinfectants. Many of these compounds have high chemical stability, which, although desirable for industrial applications, also means that they can be recalcitrant to degradation in the environment. The haloalkane 1,2-dichloroethane (1,2-DCA) is used almost exclusively as an intermediate in the synthesis of vinyl chloride, a known carcinogen, but smaller amounts of 1,2-DCA are used in the production of vinylidene chloride, 1,1,1-trichloroethane, trichloroethene, tetrachloroethene, ziridines, and ethylene diamines and in chlorinated solvents (U.S. Agency for Toxic Substances and Disease, 2001). Over 14.5 million metric tons of 1,2-DCA were produced in the United States alone in 1994 (U.S. International Trade Commission, 1995) and, with a global capacity for vinyl chloride monomer of 35 million metric tons in 2005, production continues. 1,2-DCA is listed as a priority pollutant and probable human carcinogen by the U.S. Environmental Protection Agency (EPA). Although 1,2-DCA degradation has been demonstrated in soils, incomplete biodegradation of 1,2-DCA produces highly toxic vinyl chloride (Nobre and Nobre, 2004). 1,2-DCA can also be bioactivated to toxic compounds in fish and mammals (Inskeep et al., 1986; Koga et al., 1986; Guengerich et al., 1987; Cmarik et al., 1992). Furthermore, the water solubility and low sorption coefficient properties mean that 1,2-DCA leaches rapidly into groundwater, where degradation rates are low (Barbash and Reinhard, 1989); the half-life of 1,2-DCA in the simulated hypoxic conditions of groundwater is 23 years (Barbash and Reinhard, 1989). 1,2-DCA can then concentrate in aquifers, with reported levels of 10 mg/L (Nobre and Nobre, 2004), 300 mg/L (Dyer et al., 2000), and up to 2,500 mg/L (Hunkeler et al., 2005). Thus, 1,2-DCA needs to be rapidly removed from soil and groundwater to prevent both bioactivation and accumulation in aquifers.
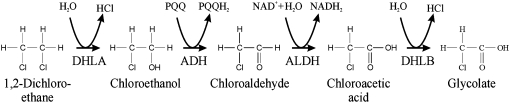
Higher plants, such as hybrid poplars (Populus spp.), have been successfully used to treat soil and groundwater contaminated with chlorinated solvents (Gordon et al., 1998; Hong et al., 2001; Wang et al., 2004) and can extract pollutants from soils or water alongside root uptake of nutrients. However, no detectable haloalkane dehalogenating activity has been found in tobacco (Nicotiana tabacum), Arabidopsis (Arabidopsis thaliana), oil seed rape (Brassica napus), rice (Oryza sativa), maize (Zea mays), or barley (Hordeum vulgare; Naested et al., 1999), and it is likely that plants have no, or only limited, ability to dehalogenate this compound. Bacteria have been isolated that have the ability to break down halogenated aliphatic compounds such as 1,2-DCA (Janssen et al., 1989; van den Wijngaard et al., 1992; Hage and Hartmans 1999). In Xanthobacter autotrophicus GJ10, 1,2-DCA is initially dehalogenated by the substitution of one of the two terminal chlorine atoms by a hydroxyl group to form chloroethanol. This reaction is catalyzed by the enzyme haloalkane dehalogenase (DhlA; Janssen et al., 1985, 1989). The structure and substrate specificities of DhlA have been well characterized (Rozeboom et al., 1988; Franken et al., 1991), and DhlA has activity on a range of halogenated aliphatic compounds (Holloway et al., 1998). Chloroethanol is then converted to the haloalkanoic acid chloroacetic acid (CAA) by two sequential dehydrogenation steps catalyzed by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH; Janssen et al., 1985; Fig. 1). Many industrial applications use CAA, and it is listed by the EPA as a hazardous air pollutant. It is potentially highly toxic to the bacterium but is converted to glycolate by the action of l-haloacid dehalogenase (DhlB; van der Ploeg et al., 1991). The rate at which X. autotrophicus can degrade 1,2-DCA is limited by the rate at which the chloroacetaldehyde and CAA are metabolized, as these intermediates are more toxic than 1,2-DCA and are utilized poorly (Baptista et al., 2006). At concentrations above 5 mm 1,2-DCA, the bacteria produce extracellular polysaccharides as a protective barrier to reduce uptake (van den Wijngaard et al., 1993), limiting their use for in situ bioremediation.
Figure 1.
Catabolic pathway for the degradation of 1,2-DCA in X. autotrophicus GJ10.
Naested et al. (1999) developed a negative selectable marker system by transforming dhlA into Arabidopsis. Plants expressing DhlA were able to convert 1,2-DCA to more toxic intermediates. ADH null plants were used to demonstrate that chloroacetaldehyde is likely to be the most phytotoxic intermediate in the pathway (Naested et al., 1999); Chloro- and fluoroacetaldehydes are converted via the tricarboxylic acid cycle enzyme citrate synthase (Peters et al., 1953) to chloro- and fluorocitrate, potent inhibitors of aconitase (Morrison and Peters, 1954).
Here, we demonstrate that the introduction of both DhlA and DhlB, in combination with endogenous ADH and ALDH activities, into tobacco plants can create a complete pathway for the degradation of 1,2-DCA.
RESULTS
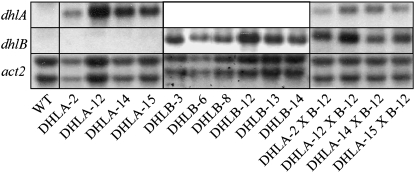
Four DHLA and six DHLB, homozygous, transformed T3 tobacco lines were produced and characterized. Both genes were expressed using the cauliflower mosaic virus 35S near-constitutive promoter. Northern analysis of tobacco leaves revealed a range of transgene expression levels, with lines DHLA-12 and DHLB-12 exhibiting the highest transgene expression levels (Fig. 2). Quantification of DhlA activity in tobacco leaves of T2 DHLA-10 and DHLA-16 lines using 1,2-DCA as substrate gave dehalogenase activity 10- and 7-fold higher, respectively, than the background levels detected in boiled samples of wild type (Table I). Both ADH and ALDH activities were detected in the roots and shoots of liquid culture-grown tobacco. In wild-type tobacco shoots, the level of ADH activity was twice as high as that in roots (9.2 ± 4.4 and 4.2 ± 1.7 μmol min1 mg−1 fresh weight, respectively), whereas ALDH activity was similar in both roots (26.8 ± 5.6 μmol min1 mg−1) and shoots (27.4 ± 11.4 μmol min1 mg−1).
Figure 2.
Northern-blot analysis of leaf RNA from 8-week-old wild-type (WT), T3 DHLA, T3 DHLB, and F1 DHLA × DHLB tobacco plants showing expression of dhlA, dhlB, and the constitutively expressed actin gene (act2).
Table I.
DhlA activity in tobacco leaf extracts from wild-type and T2 dhlA-expressing transgenic tobacco plants
Results are the mean ± sd from three replicates.
| Sample | Activity |
|---|---|
| μmol min−1 mg protein−1 | |
| Wild type | 0.0050 ± 0.0004 |
| Wild type (boiled) | 0.0052 ± 0.0012 |
| DHLA-10A | 0.0363 ± 0.0012 |
| DHLA-10A (boiled) | 0.0049 ± 0.0011 |
| DHLA-16A | 0.0506 ± 0.0018 |
| DHLA-16A (boiled) | 0.0044 ± 0.0012 |
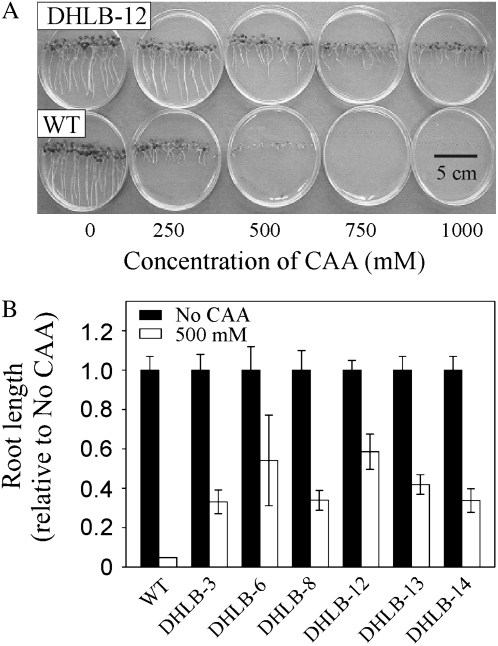
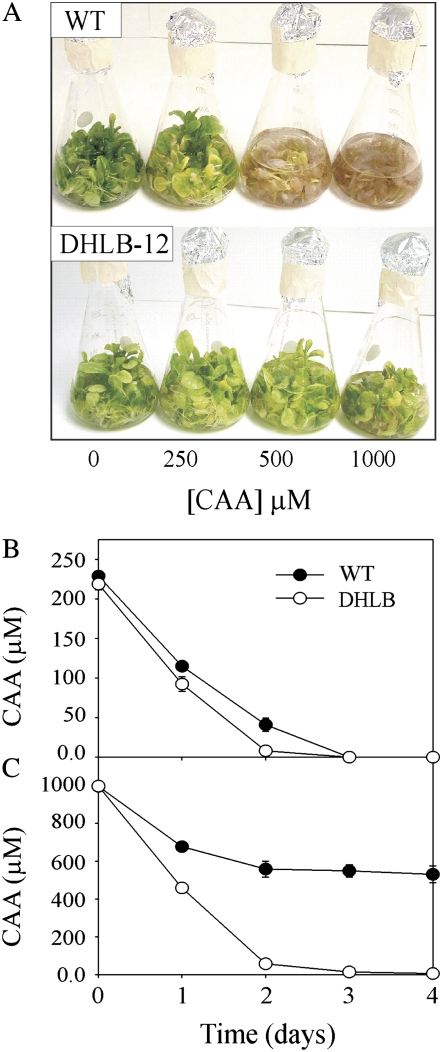
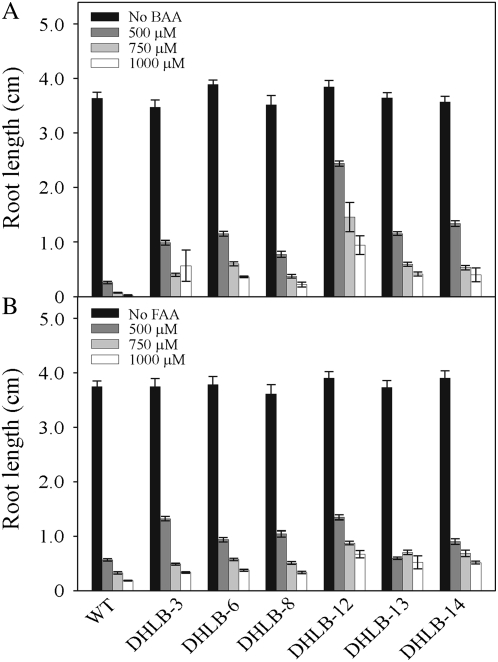
In the presence of CAA, seedlings of the DHLB lines tolerated higher concentrations than wild-type seedlings (shown in Fig. 3A for DHLB-12) with all six lines having significantly longer roots than wild type (Fig. 3B). When DHLB-12 plants were grown in liquid culture containing 250, 500, or 1,000 μm CAA, they tolerated all concentrations tested remaining green, while wild-type plants died at concentrations above 250 μm CAA (Fig. 4A). Figure 4B shows that both wild-type and DHLB-12 plants removed all the 250 μm CAA from media within 3 d; however, after this time, the wild-type plants had yellowing and necrotic regions on the leaves, while DHLB lines remained green. In media containing 1,000 μm CAA, wild-type plants died within 48 h of exposure, while DHLB-12 plants removed all the CAA (Fig. 4) and continued to accumulate biomass during the course of the experiment (data not shown). All six DHLB lines also exhibited enhanced root growth in the presence of 500, 750, or 1,000 μm bromoacetic acid (BAA) and, to a lesser extent, fluoroacetic acid (FAA), with DHLA-12 showing the greatest level of resistance to these compounds (Fig. 5).
Figure 3.
Growth of T3 DHLB lines in the presence of CAA. A and B, Appearance (A) and root length (B) of 14-d-old seedlings on CAA. Results are mean ± se of 20 seedlings from each of three plates.
Figure 4.
Growth of wild type and T3 DHLB-12 in liquid culture. A to C, Plants 5 d after exposure to CAA (A) and levels of CAA in the media of plants dosed with 250 μm (B) and 1,000 μm (C) CAA. Results are mean ± se of three replicate flasks.
Figure 5.
Root length of 14-d-old wild-type (WT) and T3 DHLB seedlings grown on media containing BAA (A) or FAA (B). Results are mean ± se of 20 14-d-old seedlings from each of three plates.
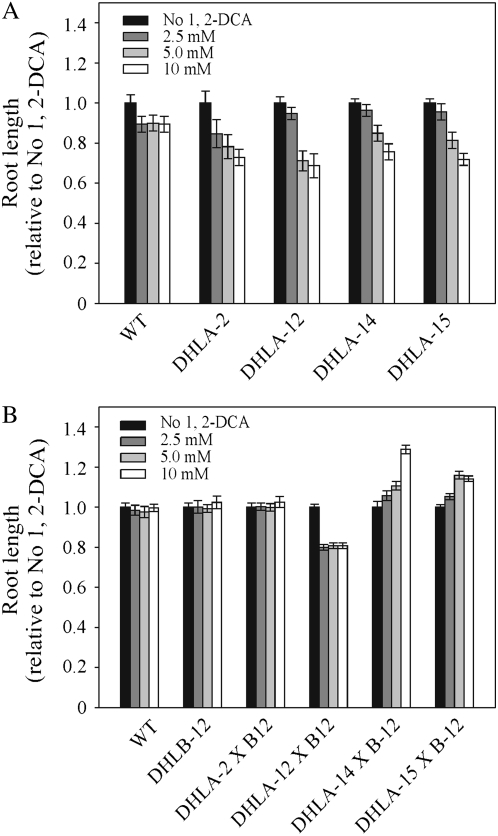
Roots of seedlings expressing only DhlA were significantly shorter than either wild-type or DhlB-expressing seedlings when grown on media containing 2.5, 5, or 10 mm 1,2-DCA. Although the northern analysis revealed a range of dhlA expression levels, the roots of all four DHLA lines exhibited a similar response to increasing concentrations of 1,2-DCA (Fig. 6). When line DHLA-12 was grown in liquid culture containing 10, 25, or 50 mm, 1,2-DCA, the plants died within 48 h at all concentrations, while wild-type and DHLB lines remained green and appeared healthy at 10 and 25 mm 1,2-DCA (Fig. 7A).
Figure 6.
Root length of 14-d-old seedlings on 1,2-DCA. A, Wild type (WT) and T3 DHLA lines. B, Wild type (WT), T3 DHLB, and F1 DHLA × DHLB lines. Results are mean ± se of 20 seedlings from each of three plates.
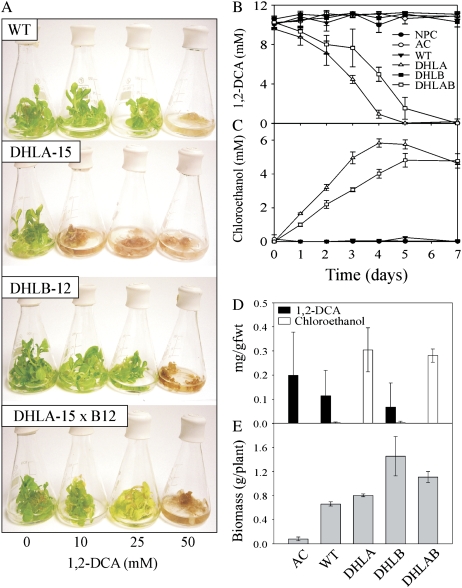
Figure 7.
Growth of wild type (WT), T3 DHLA, T3 DHLB, and F1 DHLA × DHLB lines in liquid culture in the presence of 1,2-DCA. A, Plants 5 d after exposure to 1,2-DCA. B and C, Levels of 1,2-DCA (B) and chloroethanol (C) in the media of liquid culture-grown plants dosed with 10 mm 1,2-DCA. D and E, Levels of 1,2-DCA and chloroethanol in plant tissues (D) and biomass of plants dosed with 10 mm 1,2-DCA (E). AC, Autoclaved wild type. Results are mean ± se of three replicate flasks.
Line DHLB-12, which exhibited the highest level of gene expression and resistance to CAA, was independently crossed with the four DHLA lines. The presence of both transgenes in the F1 progeny was confirmed by PCR (results not shown). Northern analysis of leaves from F1 plants revealed reduced levels of dhlA transcript, while levels of dhlB transcript were similar to parental levels (Fig. 2).
When the double DHLAB lines were grown on media containing 1,2-DCA, the root lengths of DHLA-2 × DHLB-12 and DHLA-12 × DHLB-12 lines, like wild type and DHLB-12, did not alter with increasing 1,2-DCA concentration, although line DHLA-12 × DHLB-12 did show an unexplained, uniform decrease in root length in the presence of 1,2-DCA. The root lengths of DHLA-14 × DHLB-12 and DHLA-15 × DHLB-12 lines increased with increasing concentration of 1,2-DCA. At 10 mm 1,2-DCA, the root lengths of DHLA-14 × DHLB-12 and DHLA-15 × DHLB-12 seedlings were 129% and 114% longer than wild-type roots (Fig. 6B). To test the ability of the double DHLAB lines to perform the complete detoxification of 1,2-DCA, DHLA-15 × DHLB-12, parental lines and wild-type plants were grown in liquid culture containing 0, 10, 25, and 50 mm 1,2-DCA. After 5 d, Figure 7A shows that the DHLA-15 plants were more susceptible to 1,2-DCA toxicity than wild type and died at all concentrations tested, while the wild-type, DHLB-12, and DHLA-15 × DHLB-12 plants remained green and appeared healthy at 10 and 25 mm 1,2-DCA. At 50 mm 1,2-DCA, all plants died.
Additional experiments were performed using sealed Mininert vials containing 10 mm 1,2-DCA to monitor the levels of 1,2-DCA and production of the intermediates chloroethanol and chloroacetaldehyde. The level of 1,2-DCA in the media of the no plant control, wild type, and DHLB-12-containing vials did not significantly alter during the course of the experiment (Fig. 7B). In contrast, both the DHLA-15 and DHLA-15 × DHLB-12-expressing lines removed all the 1,2-DCA from the media, with the concomitant production of chloroethanol. No chloroethanol was detected in the media of the no plant control, wild type, or DHLB-12 lines (Fig. 7C). The tissues of both DHLA-15 and DHLA-15 × DHLB-12 lines contained similar levels of chloroethanol (Fig. 7D), but DHLA-15 plants died while DHLA-15 × DHLB-12 plants remained green and appeared healthy. After 7 d, the biomass of the DHLA-15 × DHLB-12 plants was significantly greater than for wild-type or DHLA-15 plants (Fig. 7E).
DISCUSSION
No detectable DhlA activity has been observed in tobacco, Arabidopsis, oil seed rape, rice, maize, or barley using 1,2-DCA or 1,2-dibromoethane as substrate (Naested et al., 1999). Here, DhlA and DhlB activities have been successfully conferred to tobacco plants by the expression of the dhlA and dhlB genes from X. autotrophicus GJ10 (Fig. 2). Significant DhlA activity was detected in the DHLA lines (lines 10 and 16) and, as predicted by Naested et al. (1999), the expression of DhlA alone conferred increased sensitivity toward 1,2-DCA. This is due to the conversion by DhlA of 1,2-DCA to the more toxic chloroalcohol and chloroaldehyde intermediates. Studies of bacterial systems suggest that chloroacetaldehyde is the more toxic of the two compounds (Janssen et al., 1994). When ADH null Arabidopsis plants, which are unable to convert chloroethanol to chloroacetaldehyde, were grown in the presence of 12 mm chloroethanol for 2 d, no toxic effects were observed, while toxicity was observed in DHLA lines under the same conditions (Naested et al., 1999).
Endogenous activities of ADH and ALDH, the enzymes required to complete the pathway for the catabolism of 1,2-DCA by bridging the gap between DhlA and DhlB activities, were found to be expressed in both root and shoots of liquid culture-grown tobacco plants. Although we were unable to measure DhlB activity in extracts of the DHLB lines, the increased resistance of these lines to CAA, BAA, and FAA (Figs. 3–5) indicates that the DhlB enzyme is active in this plant tissue. The reduced resistance to FAA suggests that the smaller fluoride ions bind less effectively in the active site of DhlB than the bromide or chloride ions.
When producing the double DHLAB lines, to prevent the build-up of toxic intermediates due to limiting DhlB activity, line DHLB-12 was selected to cross with the DHLA lines, as this line exhibited both the highest level of gene expression (Fig. 2) and tolerance to CAA (Fig. 3). Northern analysis of the expression levels of dhlA and dhlB in leaves from F1 plants relative to the expression levels of actin, used here as an indicator of constitutive expression, revealed reduced levels of dhlA transcript compared to parental expression levels, although levels of dhlB transcript in the double transgenic lines were similar to parental levels (Fig. 2). This suggests that the DHLAB lines had correspondingly higher levels of DhlB activity than DhlA activity and may have reduced the possibility that limited DhlB activity contributed to an accumulation of toxic intermediates.
All four DHLAB lines showed increased tolerance to 1,2-DCA compared to their DHLA-only parental lines, indicating that the complete pathway from 1,2-DCA to glycolate was active in these lines (Fig. 6). Lines DHLA-14 × DHLB-12 and DHLA-15 × DHLB-12 exhibited increased root length with increasing 1,2-DCA concentration, and this may have been due to the utilization of glycolate from the metabolism of 1,2-DCA as an energy source for growth.
Our experiments using Mininert vials containing 10 mm 1,2-DCA showed that while the tissues of both DHLA-15 and DHLA-15 × DHLB-12 lines contained similar levels of chloroethanol (Fig. 7D), the DHLA-15 plants died while DHLA-15 × DHLB-12 plants remained green and appeared healthy. This implies that the toxicity was due to chloroacetaldehyde and that the cellular toxic threshold was below the quantification limit of the gas chromatography-mass spectrometry for chloroacetaldehyde, which was 3 μm.
In addition to haloacetic acids, DhlA also has activity with a range of mono- and dihalogenated methanes, ethanes, propanes, and butanes (Keuning et al., 1985), and DhlB dehalogenates dichloroacetate, mono- and dibromoacetate, and 2-chloropropionate (van der Ploeg et al., 1991). Many of these aliphatic halogenated compounds are ranked in the top 100 of the U.S. Agency for Toxic Substances and Disease Registry's list of priority hazardous substances (2003) and classed as pollutants by the EPA. Conventional remediation technologies to remove pollutants from soil and groundwater can be prohibitively expensive and produce hazardous by-products. Here, we present the early development of a potentially cost-effective and efficient alternative remediation strategy for aliphatic halogenated hydrocarbons. The levels of these pollutants are highest at industrial production sites, but low sorption coefficients and high volatilities restrict the levels of DCA in soil to within the range of uptake demonstrated by our model system. Our results demonstrate the potential practical applicability of this phytoremediation approach for the clean-up of contaminated sites.
MATERIALS AND METHODS
Expression of dhlA and dhlB in Tobacco
The dhlA and dhlB genes were cloned into the binary vector system, pART27 (Gleave, 1992), under the control of the cauliflower mosaic virus 35S promoter and ocs terminator to produce the vectors pART27-dhlA and pART27-dhB. Agrobacterium-mediated floral dipping was used to transform these vectors into tobacco (Nicotiana tabacum ‘Xanthi’). Northern-blot analysis was performed using RNA from 8-week-old leaf tissue. Homology to the constitutively expressed Arabidopsis (Arabidopsis thaliana) act2 gene sequence (National Center for Biotechnology Information accession no. U41998) was used as a loading control.
Measurement of ADH, ALDH, and DhlA Activity
The ADH assays were performed according to Freeling and Schwartz (1973) with modifications by Xie and Wu (1989). ALDH activity was determined according to op den Camp and Kuhlemeier (1997). A colorimetric assay was used to assay DhlA activity based on the decrease of the pH in a weakly buffered media (Holloway et al., 1998).
Liquid Culture Experiments
Sterilized tobacco seeds were germinated and grown aseptically in 250-mL conical flasks containing 100 mL of one-half-strength Murashige and Skoog (Murashige and Skoog, 1962) medium. The seedlings were grown on a rotary shaker (100 rpm) at 24°C, 16-h photoperiod with 240 μmol m−2 s−1 white light for 14 d. The seedlings were weighed and equal amounts of biomass transferred to flasks containing 1,2-DCA or CAA in sterile water. The experiments in Figures 4 and 7A were carried out using flasks sealed with rubber bungs. To monitor the absorption of 1,2-DCA to the flask environment, no-plant control flasks were included, and measurements of 1,2-DCA demonstrated that equilibrium was reached after 3 h. The results presented in Figure 7, B to E, used vials sealed with Mininert valves that did not react with the 1,2-DCA.The headspace within the Mininert vials was not sampled, but results of no-plant control vials demonstrated that volatilization to the Mininert headspace was not significant.
Extraction and Analysis of 1,2-DCA, CAA, and Intermediates
Levels of CAA (Fig. 4, B and C) in the liquid culture medium were determined by ion chromatography (DX-180 Dionex) using a Dionex ASII column (250-mm × 4-mm i.d.). Eluate used was 40% (v/v) methanol, 1 mm NaOH for 5 min, increasing to 2 mm for 10 min, then 40 mm for 5 min. The simultaneous determination of 1,2-DCA and metabolites (Fig. 7, B–D) was performed using headspace chromatography. In a 2-mL glass vial, 20 μL of esterizing reagent (H2O:MeOH:H2SO4, 6:5:1) was added to 10 μL of sample taken from the media. The vials were sealed with magnetic crimp-caps, shaken at 85°C for 10 min, then 1 mL headspace injected into a liner containing Tenax TA adsorbent held at 10°C for 0.5 min. Sample was desorbed from the liner onto the separation column by a 12°C/s temperature ramp to 245°C using a 2.5-mL/min He constant flow at a 20:1 split ratio. Separation was achieved on a CP-Wax 52CB 25-m × 0.32-mm i.d. × 1.2-μm film thickness capillary column, held at 70°C for 2 min then ramped at 20°C/min to 200°C. Ions were detected in positive electron impact mode using a Leco Pegasus IV gas chromatography-time of flight-mass spectrometry scanning over the range 20 to 500 m/z at 10 spectra/s. Toluene was used as the internal standard for the quantification of 1,2-DCA and 2,2,2-trichloroethanol for the metabolites; internal standards were added with the esterizing reagents. Quantification limits achieved were: 2 μm, 1,2-DCA; 49 μm, chloroethanol; 110 μm, chloroacetaldehyde; 3 μm, CAA.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers M26950 and M81691.
This work was supported by Consejo Nacional de Ciencias y Tecnología Mexico (to G.L.M.-B.) and by EMBO (to F.G.-H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Neil C. Bruce (ncb5@york.ac.uk).
Open Access articles can be viewed online without a subscription.
References
- Baptista II, Peeva LG, Zhou NY, Leak DJ, Mantalaris A, Livingston AG (2006) Stability and performance of Xanthobacter autotrophicus GJ10 during 1,2-dichloroethane biodegradation. Appl Environ Microbiol 72 4411–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash JE, Reinhard M (1989) Abiotic dehalogenation of 1,2-dichloroethane and 1,2-dibromoethane in aqueous solution containing hydrogen sulfide. Environ Sci Technol 23 1349–1357 [Google Scholar]
- Cmarik JL, Humphreys WG, Bruner KL, Lloyd RS, Tibbetts C, Guengerich FP (1992) Mutation spectrum and sequence alkylation selectivity resulting from modification of bacteriophage M13mp18 DNA with S-(2-chloroethyl)glutathione. Evidence for a role of S-(2-N7-guanyl)ethyl)glutathione as a mutagenic lesion formed from ethylene dibromide. J Biol Chem 267 6672–6679 [PubMed] [Google Scholar]
- Dyer M, Van Heiningen E, Gerritse J (2000) In situ bioremediation of 1,2-dichloroethane under anaerobic conditions. Geotech Geol Eng 18 313–334 [Google Scholar]
- Franken SM, Rozeboom HJ, Kalk KH, Dijkstra BW (1991) Crystal structure of haloalkane dehalogenase: an enzyme to detoxify halogenated alkanes. EMBO J 10 1297–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M, Schwartz D (1973) Genetic relationships between the multiple alcohol dehydrogenases of maize. Biochem Genet 8 27–36 [DOI] [PubMed] [Google Scholar]
- Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20 1203–1207 [DOI] [PubMed] [Google Scholar]
- Gordon M, Choe N, Duffy J, Ekuan G, Heilman P, Muiznieks I, Ruszaj M, Shurtleff BB, Strand S, Wilmoth J, et al (1998) Phytoremediation of trichloroethylene with hybrid poplars. Environ Health Perspect 106 1001–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Peterson LA, Cmarik JL, Koga N, Inskeep PB (1987) Activation of dihaloalkanes by glutathione conjugation and formation of DNA adducts. Environ Health Perspect 76 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage JC, Hartmans S (1999) Monooxygenase-mediated 1,2-dichloroethane degradation by Pseudomonas sp. strain DCA1. Appl Environ Microbiol 65 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P, Knoke KL, Trevors JT, Lee H (1998) Alteration of the substrate range of haloalkane dehalogenase by site-directed mutagenesis. Biotechnol Bioeng 59 520–523 [PubMed] [Google Scholar]
- Hong MS, Farmayan WF, Dortch IJ, Chiang CY, McMillan SK, Schnoor JL (2001) Phytoremediation of MTBE from a groundwater plume. Environ Sci Technol 35 1231–1239 [DOI] [PubMed] [Google Scholar]
- Hunkeler D, Aravena R, Berry-Spark K, Cox E (2005) Assessment of degradation pathways in an aquifer with mixed chlorinated hydrocarbon contamination using stable isotope analysis. Environ Sci Technol 39 5975–5981 [DOI] [PubMed] [Google Scholar]
- Inskeep PB, Koga N, Cmarik JL, Guengerich FP (1986) Covalent binding of 1,2-dihaloalkanes to DNA and stability of the major DNA adduct, S-[2-(N7-guanyl)ethyl]glutathione. Cancer Res 46 2839–2844 [PubMed] [Google Scholar]
- Janssen DB, Pries F, van der Ploeg J, Kazemier B, Terpstra P, Witholt B (1989) Cloning of 1,2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J Bacteriol 171 6791–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen DB, Scheper A, Dijkhuizen L, Witholt B (1985) Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol 49 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen DB, van der Ploeg JR, Pries F (1994) Genetics and biochemistry of 1,2-dichloroethane degradation. Biodegradation 5 249–257 [DOI] [PubMed] [Google Scholar]
- Keuning S, Janssen DB, Witholt B (1985) Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol 163 635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga N, Inskeep PB, Harris TM, Guengerich FP (1986) S-[2-(N7-guanyl)ethyl]glutathione, the major DNA adduct formed from 1,2-dibromoethane. Biochemistry 25 2192–2198 [DOI] [PubMed] [Google Scholar]
- Morrison JF, Peters RA (1954) Biochemistry of fluoroacetate poisoning: the effect of fluorocitrate on purified aconitase. Biochem J 56 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15 473–496 [Google Scholar]
- Naested H, Fennema M, Hao L, Andersen M, Janssen DB, Mundy J (1999) A bacterial haloalkane dehalogenase gene as a negative selectable marker in Arabidopsis. Plant J 18 571–576 [DOI] [PubMed] [Google Scholar]
- Nobre RC, Nobre MM (2004) Natural attenuation of chlorinated organics in a shallow sand aquifer. J Hazard Mater 110 129–137 [DOI] [PubMed] [Google Scholar]
- op den Camp RG, Kuhlemeier C (1997) Aldehyde dehydrogenase in tobacco pollen. Plant Mol Biol 35 355–365 [DOI] [PubMed] [Google Scholar]
- Peters R, Wakelin RW, Buffa P, Thomas LC (1953) Biochemistry of fluoroacetate poisoning: the isolation and some properties of the fluorotricarboxylic acid inhibitor of citrate metabolism. Proc R Soc Lond B Biol Sci 140 497–507 [DOI] [PubMed] [Google Scholar]
- Rozeboom HJ, Kingma J, Janssen DB, Dijkstra BW (1988) Crystallization of haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Mol Biol 200 611–612 [DOI] [PubMed] [Google Scholar]
- U.S. Agency for Toxic Substances and Disease (2001) Toxicological Profile for 1,2-Dichloroethane. U.S. Agency for Toxic Substances and Disease Registry, Atlanta
- U.S. International Trade Commission (1995) Synthetic Organic Chemicals, United States Production and Sales. U.S. International Trade Commission, Washington, DC
- van den Wijngaard AJ, van der Kamp KW, van der Ploeg J, Pries F, Kazemier B, Janssen DB (1992) Degradation of 1,2-dichloroethane by Ancylobacter aquaticus and other facultative methylotrophs. Appl Environ Microbiol 58 976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard AJ, van der Kleij RG, Doornweerd RE, Janssen DB (1993) Influence of organic nutrients and cocultures on the competitive behavior of 1,2-dichloroethane-degrading bacteria. Appl Environ Microbiol 59 3400–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg J, van Hall G, Janssen DB (1991) Characterization of the haloacid dehalogenase from Xanthobacter autotrophicus GJ10 and sequencing of the dhlB gene. J Bacteriol 173 7925–7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dossett MP, Gordon MP, Strand SE (2004) Fate of carbon tetrachloride during phytoremediation with poplar under controlled field conditions. Environ Sci Technol 38 5744–5749 [DOI] [PubMed] [Google Scholar]
- Xie Y, Wu R (1989) Rice alcohol dehydrogenase genes: anaerobic induction, organ specific expression and characterization of cDNA clones. Plant Mol Biol 13 53–68 [DOI] [PubMed] [Google Scholar]