Abstract
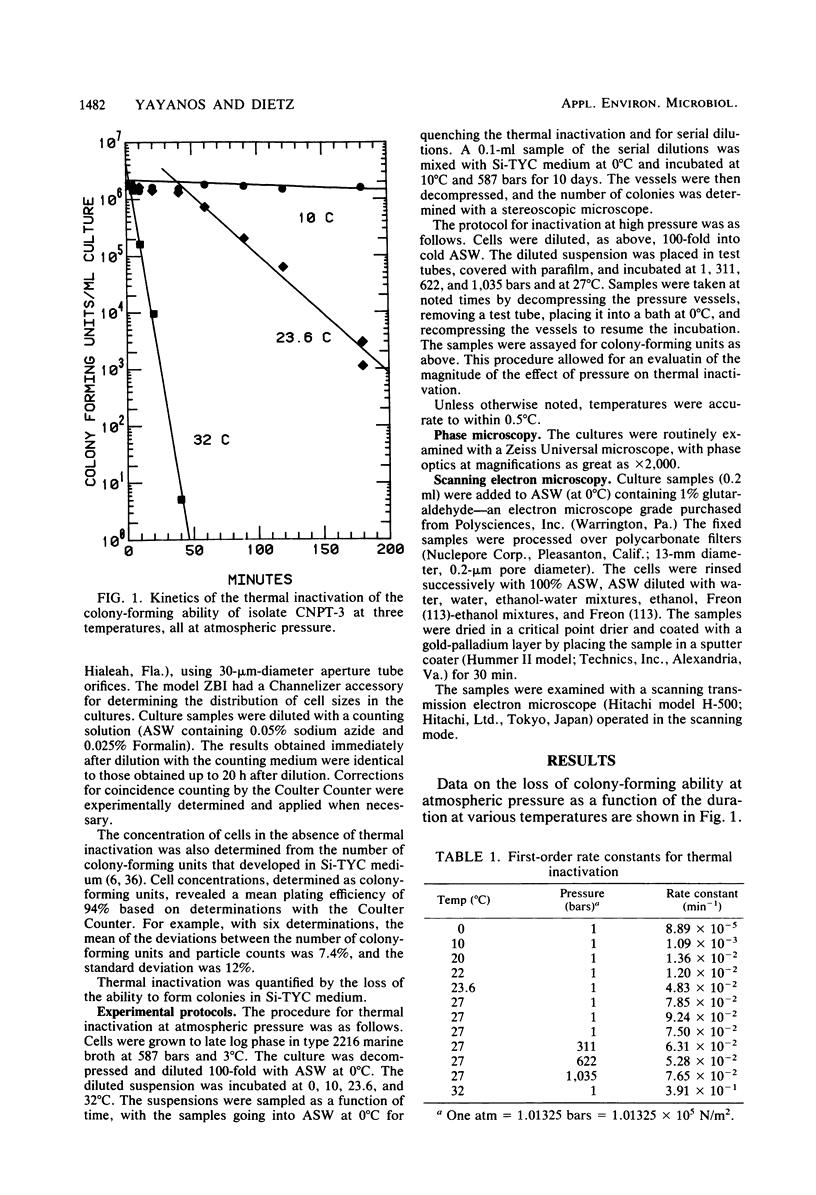
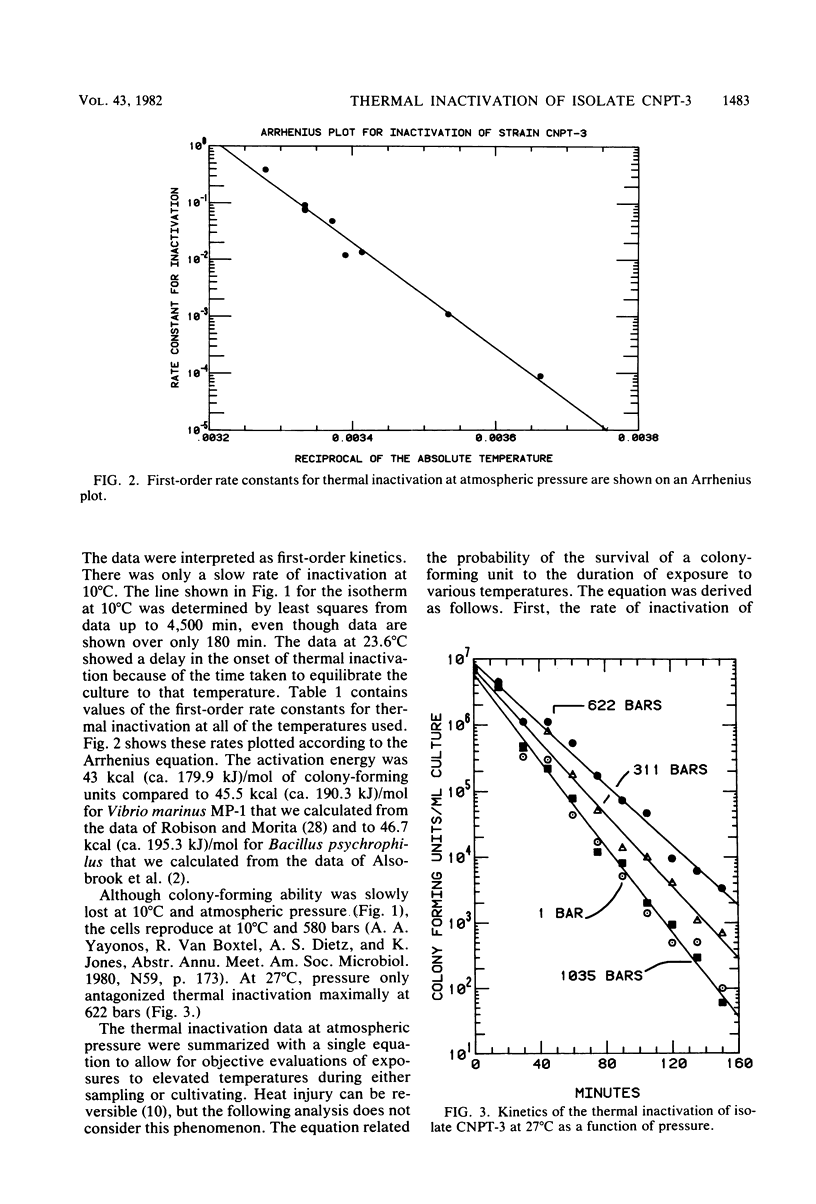
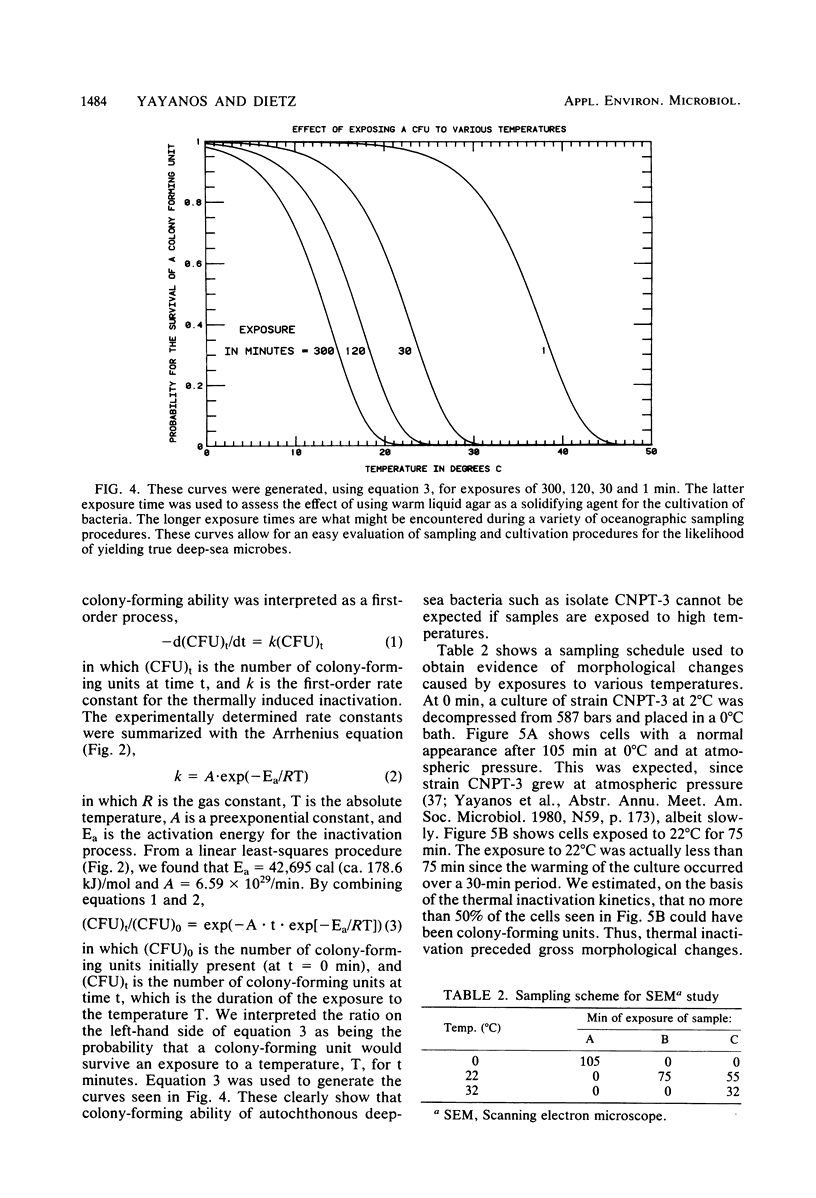
The barophilic deep-sea bacterium, isolate CNPT-3, was inactivated by exposures to temperatures between 10 and 32°C at atmospheric pressure. Inactivation in samples from warmed cell suspensions was measured as the loss of colonyforming ability (CFA) at 10°C and 587 bars. At atmospheric pressure, there was a slow loss of CFA even at 10°C. The loss of CFA was rapid above 20°C and only slightly affected by high pressures. The first-order rate constants for thermal inactivation fit the Arrhenius equation with an activation energy of 43 kcal (ca. 179.9 kJ)/mol. Light microscopy and scanning transmission electron microscopy revealed morphological changes due to warming of the cells. The changes ensued the loss of CFA. The results supported the hypothesis from an earlier work that indigenous (autochthonous) deep-sea bacteria from cold deep seas are both barophilic and psychrophilic. If ultimately sustained, these characteristics may be useful in designing experiments to assess the relative importance of the autochthonous and allochthonous bacteria in the deep sea. The data were used to evaluate how barophilic bacteria may have been missed in many investigations because of warming of the cells during sample retrieval from the sea or during cultivation in the laboratory. The evaluation revealed the need for temperature and pressure data during retrieval of samples and cultivation in the laboratory. Most deep-ocean microbiology may be possible with thermally insulated equipment for retrieval from the sea and with high-pressure vessels for laboratory incubations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsobrook D., Larkin J. M., Sega M. W. Effect of temperature on the cellular integrity of Bacillus psychrophilus. Can J Microbiol. 1972 Nov;18(11):1671–1678. doi: 10.1139/m72-259. [DOI] [PubMed] [Google Scholar]
- Arcuri E. J., Ehrlich H. L. Influence of hydrostatic pressure on the effects of the heavy metal cations of manganese, copper, cobalt, and nickel on the growth of three deep-sea bacterial isolates. Appl Environ Microbiol. 1977 Feb;33(2):282–288. doi: 10.1128/aem.33.2.282-288.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz A. S., Yayanos A. A. Silica gel media for isolating and studying bacteria under hydrostatic pressure. Appl Environ Microbiol. 1978 Dec;36(6):966–968. doi: 10.1128/aem.36.6.966-968.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter R. A., Colwell R. R., Chapman G. B. Morphology and round body fermation in Vibrio marinus. J Bacteriol. 1969 Jul;99(1):326–335. doi: 10.1128/jb.99.1.326-335.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. P., Haight R. D. Reversible heat injury in the marine psychrophilic bacterium Vibrio marinus MP-1. Can J Microbiol. 1973 May;19(5):557–561. doi: 10.1139/m73-092. [DOI] [PubMed] [Google Scholar]
- Inniss W. E. Interaction of temperature and psychrophilic microorganisms. Annu Rev Microbiol. 1975;29:445–465. doi: 10.1146/annurev.mi.29.100175.002305. [DOI] [PubMed] [Google Scholar]
- Jannasch H. J., Wirsen C. O., Taylor C. D. Undecompressed microbial populations from the deep sea. Appl Environ Microbiol. 1976 Sep;32(3):360–367. doi: 10.1128/aem.32.3.360-367.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O. Microbial life in the deep sea. Sci Am. 1977 Jun;236(6):42–52. doi: 10.1038/scientificamerican0677-42. [DOI] [PubMed] [Google Scholar]
- Krieg N. R. Biology of the chemoheterotrophic spirilla. Bacteriol Rev. 1976 Mar;40(1):55–115. doi: 10.1128/br.40.1.55-115.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Bender G. R. Isolation of a variant of Streptococcus faecalis with enhanced barotolerance. Can J Microbiol. 1980 Mar;26(3):371–376. doi: 10.1139/m80-060. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. High-pressure microbial physiology. Adv Microb Physiol. 1976;14(11):159–241. doi: 10.1016/s0065-2911(08)60228-3. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Microbial life in the deep sea. Can J Microbiol. 1980 Dec;26(12):1375–1385. doi: 10.1139/m80-230. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Metcalf E. S. Conversion of mesophilic to psychrophilic bacteria. Science. 1968 Dec 13;162(3859):1288–1289. doi: 10.1126/science.162.3859.1288. [DOI] [PubMed] [Google Scholar]
- Pope D. H., Smith W. P., Orgrinic M. A., Landau J. V. Protein synthesis at 680 atm: is it related to environmental origin, physiological type, or taxonomic group? Appl Environ Microbiol. 1976 Jun;31(6):1001–1002. doi: 10.1128/aem.31.6.1001-1002.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Colwell R. R. Heterotrophic activity of deep-sea sediment bacteria. Appl Microbiol. 1975 Oct;30(4):639–649. doi: 10.1128/am.30.4.639-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Walker J. D., Colwell R. R. Deep-sea bacteria: growth and utilization of n-hexadecane at in situ temperature and pressure. Can J Microbiol. 1975 May;21(5):682–687. doi: 10.1139/m75-098. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Yayanos A. A., Colwell R. R. Metabolic activities of the intestinal microflora of a deep-sea invertebrate. Appl Environ Microbiol. 1976 Jan;31(1):46–48. doi: 10.1128/aem.31.1.46-48.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., VAN Boxtel R. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science. 1979 Aug 24;205(4408):808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., Van Boxtel R. Obligately barophilic bacterium from the Mariana trench. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5212–5215. doi: 10.1073/pnas.78.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOBELL C. E., MORITA R. Y. Barophilic bacteria in some deep sea sediments. J Bacteriol. 1957 Apr;73(4):563–568. doi: 10.1128/jb.73.4.563-568.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. Bacterial Life at the Bottom of the Philippine Trench. Science. 1952 May 9;115(2993):507–508. doi: 10.1126/science.115.2993.507. [DOI] [PubMed] [Google Scholar]