Abstract
PURPOSE
A spliced variant of the human glucocorticoid receptor GRβ has been implicated in glucocorticoid responsiveness in glaucoma. Over-expression of the FK506-binding immunophilin FKBP51 also causes a generalized state of glucocorticoid resistance. In the present study, the roles of FKBP51 in the nuclear transport of GRβ and glucocorticoid responsiveness were investigated.
METHODS
Human trabecular meshwork cells (GTM3 and TM5) and HeLa cells were treated with dexamethasone (DEX) and FK506 and transfected with GRβ and FKBP51 expression vectors. Coimmunoprecipitation and Western blot analyses were performed to study interactions of FKBP51 and FKBP52 with GRα, GRβ, Hsp90, or dynein. The cells were transfected with a GRE-luciferase reporter to evaluate the effects of DEX and FK506 and the overexpression of GRβ and FKBP51 on glucocorticoid-mediated gene expression.
RESULTS
FKBP51 was involved in constitutive nuclear transport of both GRα and -β in the absence of ligands. FKBP52 appeared to be solely responsible for the nuclear transport of ligand-activated GRα. DEX stimulated the translocation of GRα but not GRβ. Overexpression of either GRβ or FKBP51 stimulated GRβ translocation and reduced DEX-induced luciferase in HeLa cells. FK506 did not alter DEX-induced translocation of GRα. However, FK506 increased the association of FKBP51 with GRβ and stimulated DEX-induced translocation of GRβ in normal TM cells, but not in glaucoma TM cells. Increased nuclear GRβ significantly inhibited glucocorticoid responsiveness in TM cells.
CONCLUSIONS
Nuclear transport of GRβ represents a novel mechanism through which FKBP51 alters GC sensitivity. GRβ and FKBP51 may be responsible for increased responsiveness in steroid-induced ocular hypertensive individuals as well as in patients with glaucoma.
Glucocorticoids have diverse pleiotropic effects, ranging from altering metabolism and behavior to regulating inflammation and the immune response.1–3 The glucocorticoid receptor (GR) belongs to the superfamily of nuclear hormone receptors.4,5 In humans, alternative splicing of the GR gene generates two receptor isoforms termed glucocorticoid receptor-α (GRα) and -β (GRβ), which differ only at their carboxyl terminus.4,5 GRα functions as a ligand-dependent transcription factor that regulates diverse effects of glucocorticoids.6,7 Clinically, the actions of exogenous glucocorticoids used in the treatment of a wide variety of diseases, including allergic and autoimmune diseases, are achieved by binding to and activating GRα. In contrast, the GRβ does not bind glucocorticoids and lacks transcriptional activity.8,9 However, GRβ suppresses GRα activity9–12 and has been implicated in several glucocorticoid resistance diseases, including asthma, arthritis, and inflammatory bowel disease.13
In the eye, glucocorticoids have long been associated with the development of elevated intraocular pressure (IOP) and glaucoma.14 The elevated IOP associated with glaucoma and induced by glucocorticoids is due to increased aqueous outflow resistance in the trabecular meshwork (TM), a reticulated tissue located at the corneal–iridial junction that regulates aqueous outflow resistance. Whereas topical ocular administration of glucocorticoids causes measurably increased IOP in approximately 30% to 40% of the general population,15 a greater percentage of patients with primary open-angle glaucoma (POAG),16,17 and their descendants15,18 develop elevated IOP. Our laboratory has worked to elucidate the factors responsible for enhanced glucocorticoid responsiveness in glaucoma. In previous studies, we demonstrated relatively lower levels of GRβ in glaucomatous TM cell lines than in normal TM cell lines.19 GRβ acts as a dominant negative inhibitor in glucocorticoid action in regulation of gene transcription regulation and in suppression of phagocytotic function in TM.19,20 These data support a role for GRβ in the increased glucocorticoid responsiveness in patients with glaucoma and may explain the differences in steroid sensitivity among normal individuals and patients with glaucoma.21
In the classic paradigm of glucocorticoid action, ligand-induced nuclear translocation of GRα regulates gene transcription. In the absence of ligand, GRα resides in the cytoplasm as a multiprotein heterocomplex that contains heat shock protein (Hsp)90, Hsp70, and one of the immunophilins, such as the FK506-binding proteins FKBP51 and FKBP52.22–24 FKBP51 and FKBP52 possess peptidyl-prolyl cis/trans isomerase (PPIase) domains that bind immunosuppressant drugs such as FK50625,26 and tetratricopeptide repeat (TPR) domains, which form binding sites for Hsp90.27 FKBP52 and FKBP51 share approximately 75% sequence similarity and an overall similar architecture. However, diverse orientations between different domains may be responsible for differences in Hsp90 binding and microtubule motor protein dynein association between these two proteins.28–31 Both FKBP51 and FKBP52 are found in mature GRα complexes. Hormone binding appears to induce switching of FKBP51 for FKBP52 GRα complex, and the GRα-Hsp90-FKBP52 heterocomplex recruits dynein, which shuttles the complex along microtubular tracks toward the nucleus. GRα is released into the nucleus,32–35 where it regulates the expression of target genes in concert with other transcription factors.36,37 We therefore posed the question as to whether the highly homologous FKBP51 also can shuttle GRα into the nucleus without ligand binding to the receptor, because FKBP51 directly interacts with Hsp90 and dynein.31 Moreover, the transport mechanism responsible for moving the non–ligand-binding isoform GRβ into the nucleus to inhibit GRα activity is largely unknown.
GRβ is present in both the cytoplasm and the nucleus11,38,39 and can complex with Hsp90.11 Previously, we demonstrated that Hsp90 was an essential molecular chaperone for the nuclear transport of GRβ. Inhibiting Hsp90 activity led to the exclusion of GRβ from the nucleus and subsequent GRβ degradation in proteasomes.39 However, the identity and potential role of immunophilins in GRβ translocation is currently unknown.
Of note, increased expression of FKBP51 causes glucocorticoid resistance in many New World primates.40,41 Indeed, many studies have shown that FKBP51 can inhibit FKBP52-mediated expression of hormone-dependent reporter and endogenous genes.27,31,41–43 Furthermore, the expression of FKBP51 is increased by glucocorticoids,44–46 suggesting that the regulation of FKBP51 levels represent a possible feedback mechanism for inhibiting prolonged glucocorticoid responses. The mechanism responsible for FKBP51 overexpression causing glucocorticoid resistance has focused exclusively on the GRα isoform. Increased expression of FKBP51 causes a lower hormone binding affinity to GRα,41–43,47 delaying the translocation and activation of GRα.31 Even at a maximum ligand concentration, overexpression of FKBP51 significantly decreases the maximum induction of GRα activity,31 suggesting FKBP51 involvement in additional steps in the glucocorticoid signaling pathway. GRβ and FKBP51 may concomitantly inhibit GRα activity through a novel mechanism involving FKBP51-mediated potentiation of GRβ nuclear translocation.
In the present study, we investigated the differential regulation of GRα and -β nuclear transport by FKBP51 and FKBP52 in trabecular meshwork (TM) cells. We found that FKBP51, but not FKBP52, is involved in constitutive nuclear transport of GRα and -β in the absence of ligand. The enhanced nuclear transport of GRβ by either overexpression of FKBP51 or treatment with FK506 reduced glucocorticoid responsiveness in both TM cells and HeLa cells. Chaperoning nuclear transport of GRβ may represent a novel mechanism through which FKBP51 causes glucocorticoid resistance. The differential distribution patterns of FKBP51 in glaucomatous TM cells could disrupt the constitutive nuclear import of GRβ and contribute to the low nuclear expression of GRβ and the increased responsiveness to glucocorticoids.
METHODS
Materials and Antibodies
Dexamethasone (DEX) and FK506 were obtained from Sigma-Aldrich Corp (St. Louis, MO). 17-AAG (17-allylamino, 17-demethoxygeldanamycin, a geldanamycin derivative) was the kind gift of Thomas Mueller (Kosan Biosciences, Inc.; Hayward, CA). Polyclonal anti-GRβ and polyclonal anti-FKBP51 antibodies were purchased from Affinity Bioreagents (Golden, CO). Monoclonal anti-FKBP51 antibody was obtained from BD-Transduction Laboratories (Franklin Lakes, NJ). Anti-GRα antibody and monoclonal antibodies to Hsp90, histone1 and β-tubulin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FKBP52 antibody was purchased from Calbiochem (San Diego, CA). Anti-dynein intermediate chain antibody was purchased from Chemicon International, Inc (Temecula, CA). Alexa Fluor 633 goat anti-rabbit IgG, Alexa Fluor 488 goat anti-mouse IgG, and DAPI (4′, 6′-diamidino-2-phenylindole) were from Invitrogen-Molecular Probes (Eugene, OR).
Cell Lines and Cell Culture
The human transformed normal NTM-5 cell line was derived from a donor with no reported history of glaucoma, and a human glaucoma GTM-3 cell line was generated from a donor with documented history of glaucoma.48 HeLa cells were purchased from ATCC (Manassas, VA). The cells were cultured in 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin and streptomycin, and glutamate (Invitrogen-Gibco, Grand Island, NY). Confluent cells were used in most studies. For transfection experiments or 24-hour drug treatment, the cells were treated when subconfluent as specified in each experiment, to achieve complete confluence at the end of the experiment.
Plasmid and Transfections
The GRβ expression vector pCMX-hGRβ has been described previously.19 An FKBP51 expression vector (a gift from Rein Theo, Max Planck Institute of Psychiatry, Germany) was cloned downstream of the CMV promoter in the pRK5MCS vector.31 A mercury luciferase reporter, pGRE-Luc, was purchased from BD-Clontech (Palo Alto, CA). The cells were seeded on 100-mm culture dishes for immunoprecipitation or 12-well plates for luciferase assays. When the cells were approximately 60% confluent, they were transfected with the control pCMX, pCMX-hGRβ, or pCMV-FKBP51 expression vector, with or without cotransfection of pGRE-Luc with a lipophilic transfection reagent (Lipofectamine; BD Biosciences, San Jose, CA), in a ratio of 1 µg of DNA to 1.5 µL of transfection reagent in serum-free medium. Ten micrograms of expression vector or 1.5 µg of expression vector with 0.4 µg of pGRE-Luc per well was used in a 100-mm dish or 12-well plate, respectively. The cells were transfected for 9 hours, followed by a posttransfection incubation in serum-containing medium for 24 hours. For the luciferase assay, the cells were switched to serum-free medium and treated with vehicle or 100 nM DEX for another 24 hours.
Immunoblotting and Coimmunoprecipitation
Cytoplasmic and nuclear fractions or whole-cell lysates were isolated as described previously. We have shown that this method clearly separates the nuclear from the cytosolic fractions.19,34 Immunoprecipitation of 100 µg cytoplasmic or 50 µg nuclear fractions was performed with 4 µg of anti-FKBP51 or anti-FKBP52 antibodies, followed by a Western immunoblot analysis with anti-GRα, anti-GRβ, anti-Hsp90, or anti-dynein antibodies.
Luciferase Assays
The cells were transfected with the mercury luciferase reporter, pGRE-Luc (BD-Clontech), as described earlier, followed by 100 nM DEX treatment for 24 hours. Cell lysates were prepared, and luciferase assays were performed with a luminometer.19 Luciferase activity was normalized with 1 µg of protein for each sample.
Immunofluorescence Studies
Cells grown on glass coverslips were fixed in 4% paraformaldehyde for 30 minutes, permeabilized in 0.2% Triton X-100 for 15 minutes, and incubated in 0.2 M glycine for 30 minutes. After the reaction was blocked for 20 minutes with 5% bovine serum albumin+5% normal goat serum, these cells were incubated overnight at 4°C with polyclonal anti-FKBP51 + monoclonal anti-Hsp90 antibodies. Subsequently, the cells were treated for 1 hour with Alexa Fluor 633 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG. To visualize the nuclei, the cells were incubated for 10 minutes with DAPI. Confocal immunofluorescence microscopy was then performed (model LSM-410; Carl Zeiss Meditec, Inc., Dublin, CA).
Statistic Analyses
Where appropriate, for multiple group comparisons, results were analyzed with one-way ANOVA followed by a multiple-comparison test (Tukey). Differences between the results obtained from studying two different groups of subjects were analyzed with the two-sample Mann-Whitney t-test. Significance was defined as P ≤ 0.05.
RESULTS
Interaction of FKBP51 and FKBP52 with GRα and GRβ
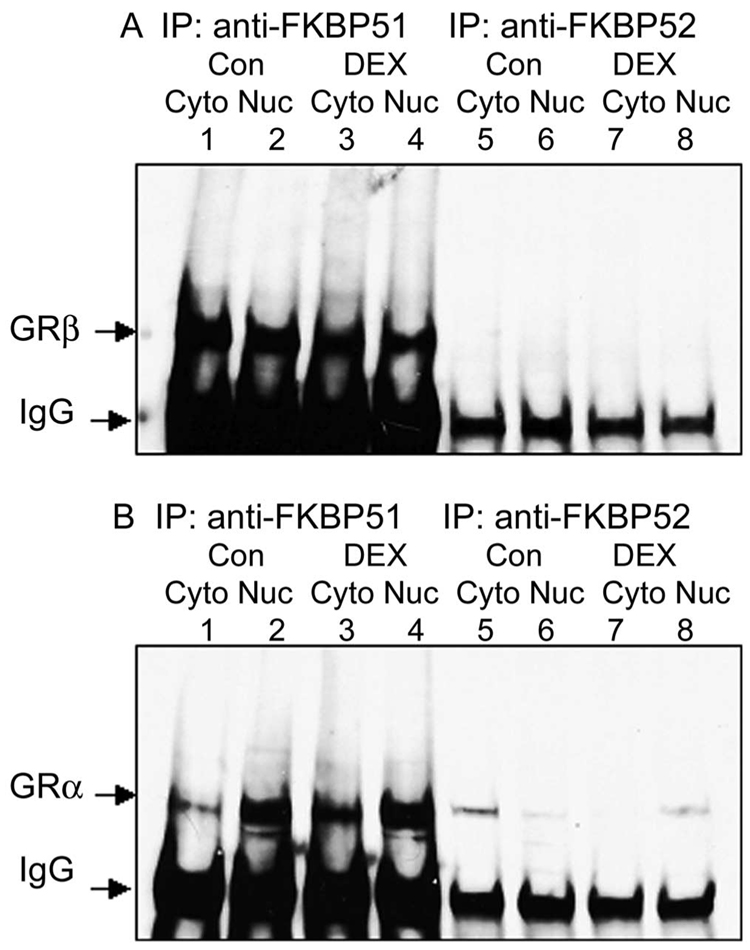
Previous studies have shown that cytoplasmic GRα is complexed with heat shock proteins and immunophilins.22–24,49 We previously reported that Hsp90 was an essential molecular chaperone for nuclear transport of GRβ,39 and we wanted to know whether any immunophilin was also associated with the GRβ complex. Cytoplasmic and nuclear fractions were isolated from NTM-5 cells treated with vehicle (control) or 100 nM DEX for 2 hours. We have previously reported that the technique used clearly separates these fractions in NTM-5 cells.19,20,39 Immunoprecipitation was performed with anti-FKBP51 or anti-FKBP52 antibodies followed by Western immunoblotting with anti-GRβ antibody (Fig. 1A). Anti-FKBP51 antibody pulled down both GRα and GRβ (Fig. 1A, lanes 1–4). In contrast, anti-FKBP52 antibody did not coimmunoprecipitate GRβ (Fig. 1A, lanes 5–8), indicating that FKBP51, but not FKBP52, complexed with GRβ. The GRβ-FKBP51 complex existed in both the cytoplasmic and nuclear fractions in the absence of DEX, and this association was not regulated by DEX treatment, suggesting that FKBP51 is involved in constitutive nuclear transport of GRβ in NTM-5 cells, independent of a glucocorticoid signal.
FIGURE 1.
Coimmunoprecipitation of FKBP51 with both GRα and -β and of FKBP52 with only GRα in NTM-5 cells. NTM-5 cells were cultured in 10% FBS-DMEM to complete confluence, shifted to serum-free DMEM, and treated with vehicle (ethanol) or 100 nM DEX for 2 hours. The cytoplasmic (Cyto) and nuclear (Nuc) fractions were isolated. Anti-FKBP51 or anti-FKBP52 immunoprecipitates of cytoplasmic (100 µg) and nuclear (50 µg) proteins were subjected to electrophoresis in SDS-polyacrylamide gradient gels followed by Western immunoblot analysis with anti-GRα or anti-GRβ antibodies. (A) Coimmunoprecipitation with anti-FKBP51 or anti-FKBP52 antibodies, and Western immunoblot analysis with a specific anti-GRβ antibody. GRβ was detected as a 90-kDa protein band. (B) Coimmunoprecipitation with anti-FKBP51 or anti-FKBP52 antibodies and Western immunoblot analysis with a polyclonal GRα antibody. GRα was detected as a 95-kDa protein band. The experiment was performed independently three or more times.
We also examined the immunophilin associated with GRα. Immunoprecipitation with anti-FKBP51 or anti-FKBP52 antibody and subsequent immunoblot analyses showed that both FKBP51 and FKBP52 complexed with GRα (Fig. 1B). As expected, the FKBP52-GRα complex was located predominantly in the cytoplasmic fraction in the absence of ligand, and DEX treatment shifted the GRα-FKBP52 complex into the nuclear fraction (Fig. 1B, lanes 5–8). The decreased density of the GRα protein band reflected a downregulation of the GRα-FKBP52 complex after 2 hours of DEX treatment. In contrast, the FKBP51-GRα was present in both the cytoplasmic and nuclear fractions in the absence of ligand, and DEX treatment did not alter the subcellular levels or distribution of FKBP51-GRα complex (Fig. 1B, lanes 1–4). The distribution pattern of FKBP51-GRα appeared similar to that of FKBP51-GRβ without dependency on DEX, but distinct from FKBP52-GRα, which was regulated by hormone treatment, indicating different mechanisms of GRα and GRβ translocation.
FKBP51 Association with Hsp90 and Dynein in NTM-5 Cells
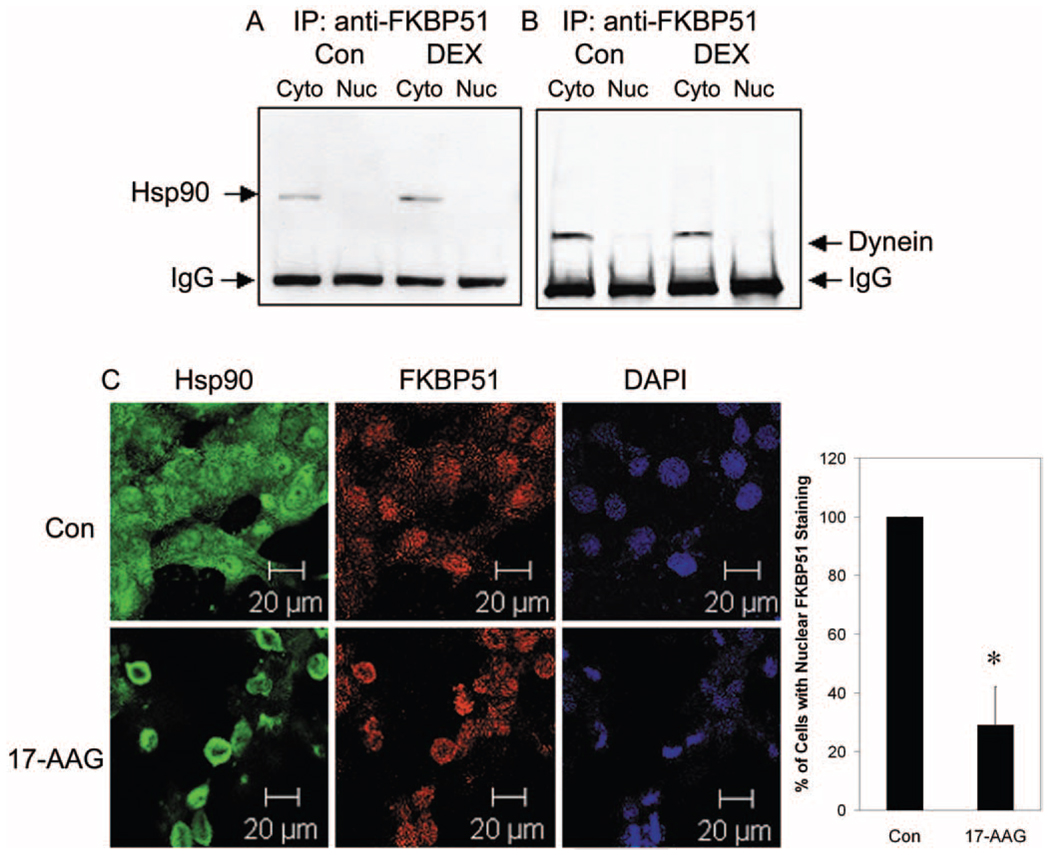
The selective association of GRβ with FKBP51 suggests a possible chaperone role of FKBP51 in GRβ translocation. To characterize the potential role of FKBP51 in nuclear translocation, the association of FKBP51 with Hsp90 and the microtubular retrograde motor protein dynein was investigated. Cytoplasmic and nuclear fractions were isolated from NTM-5 cells. Coimmunoprecipitation with anti-FKBP51 antibody followed by Western immunoblot analysis with anti-Hsp90 detected Hsp90 in the cytoplasmic fractions (Fig. 2A). DEX treatment did not alter this subcellular distribution. Western immunoblot analysis of the anti-FKBP51-immunoprecipitated proteins also detected dynein in the cytoplasmic fractions that likewise was not regulated by DEX treatment (Fig. 2B). These results demonstrate that FKBP51 formed a complex with both Hsp90 and dynein, essential characteristics for immunophilin chaperone function, and suggested that microtubules are involved in the nuclear transport of the FKBP51-Hsp90-GRβ complex in NTM-5 cells.
FIGURE 2.
Immunoprecipitation and colocalization of FKBP51 with Hsp90 and dynein in NTM-5 cells. Confluent NTM-5 cells were treated with vehicle (ethanol) or 100 nM DEX for 30 minutes. The cytoplasmic (Cyto) and nuclear (Nuc) fractions were isolated. Cytoplasmic (100 µg) and nuclear (50 µg) proteins were subjected to coimmunoprecipitation with anti-FKBP51 antibody and proteins resolved by electrophoresis in SDS-polyacrylamide 4% to 15% gradient gels followed by Western immunoblot analysis with (A) anti-Hsp90 antibody or (B) anti-dynein antibody. (C) Subconfluent (80%–90% confluence) NTM-5 cells were treated with either vehicle control (ethanol) or 1 µM 17-AAG (a specific Hsp90 inhibitor) for 24 hours. The cells were incubated with primary mouse anti-Hsp90 antibody and rabbit anti-FKBP51 antibodies, followed by Alexa Fluor 488 goat anti-mouse and Alexa Fluor 633 goat anti-rabbit secondary antibodies. Confocal microscopy was used to detect Hsp90 (green), FKBP51 (red), and DAPI nuclear staining (blue), as labeled. The ratio of the number of cells with nuclear FKBP51 staining versus the total number of cells imaged is represented as the mean percentage ± SEM of results. *P < 0.05 17-AAG vs. vehicle control by t-test; n = 3. Scale bars, 20 µm.
Previously, we reported that Hsp90 was a molecular chaperone for nuclear transport of GRβ and that an Hsp90 inhibitor, 17-AAG, blocked the activity of Hsp90 and consequently excluded GRβ from the nucleus.39 If FKBP51 and Hsp90 work as cochaperones for GRβ, then inhibition of Hsp90 should block FKBP51 entry into the nucleus. To examine this possibility directly, we treated NTM-5 cells with 1 µM of 17-AAG, and subsequently subcellular expression of FKBP51 was investigated by confocal microscopy, to visualize cellular FKBP51 distribution. 17-AAG treatment for 24 hours completely blocked the nuclear localization of Hsp90 (Fig. 2C). This treatment concomitantly blocked the nuclear distribution of FKBP51, with 29% of imaged cells showing nuclear staining of FKBP51 with the treatment of 17-AAG, compared with 100% nuclear distribution of FKBP51 in all imaged cells with the vehicle treatment (Fig. 2C). Therefore, blocking Hsp90 activity inhibited the trafficking of both Hsp90 and FKBP51 between subcellular compartments.
Overexpression of FKBP51 on the Nuclear Transport of GRβ
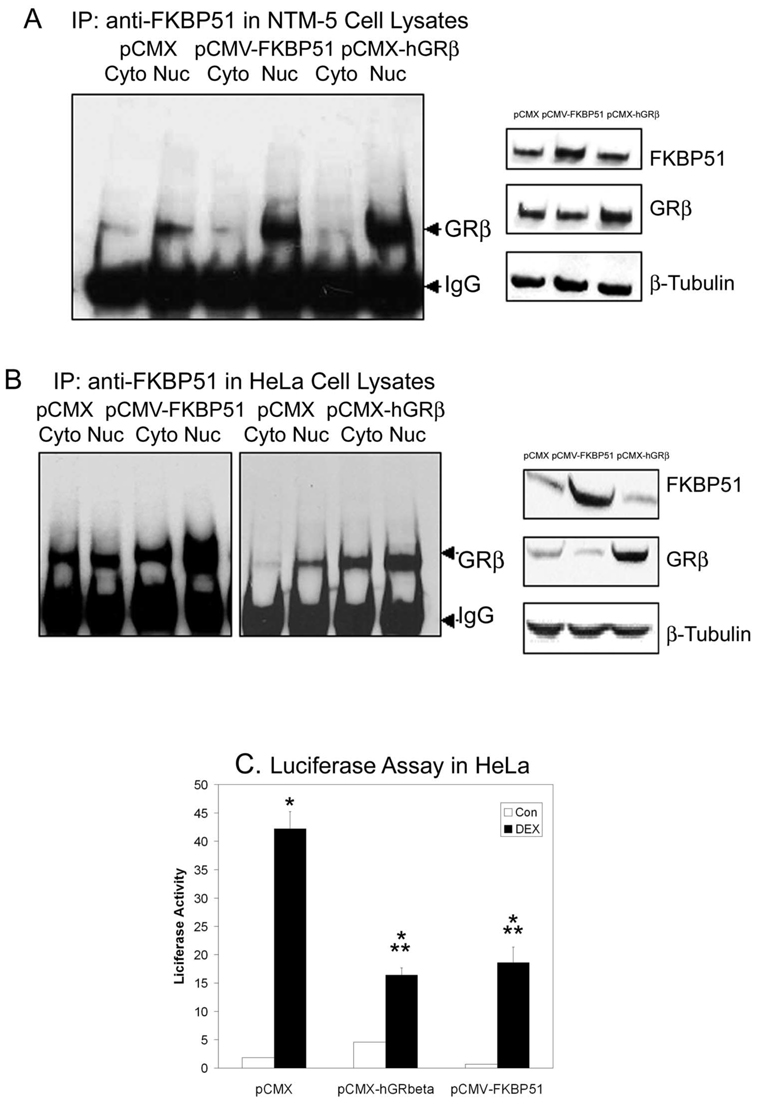
To support our contention that FKBP51 serves as a chaperone for GRβ nuclear translocation, we examined whether alteration of FKBP51 expression regulates GRβ transport. We over-expressed FKBP51 by transfecting NTM-5 cells with the FKBP51 expression construct pCMV-FKBP51 and then compared the nuclear distribution of the FKBP51-GRβ complex among transfected and control cells by using immunoprecipitation and subsequent Western immunoblot analysis. Induction of GRβ expression by transfecting with a GRβ expression vector pCMX-hGRβ was used as a positive control for increased accumulation of GRβ in the nucleus. Approximately 40% NTM-5 cells were typically transfected with pCMX-hGRβ or pCMV-FKBP51. FKBP51 or GRβ expression was increased in lysates from NTM-5 cells transfected with FKBP51 or GRβ expression vectors, respectively (Fig. 3A, right). The GRβ-FKBP51 complex existed in both cytoplasm and nuclear fractions. Overexpression of FKBP51 increased the amount of GRβ complex in the nucleus compared with empty vector–transfected cells (Fig. 3A, left). Increased expression of GRβ followed by immunoprecipitation with anti-FKBP51 also resulted in increased nuclear GRβ (Fig. 3A, left). These results further support the chaperone role of FKBP51 in the nuclear translocation of GRβ.
FIGURE 3.
Effects of FKBP51 overexpression on the nuclear transport of GRβ in NTM-5 and HeLa cells and on DEX-induced luciferase activity in HeLa cells. (A) Left: NTM-5 cells (~60% confluent) were transiently transfected with the empty vector pCMX, pCMV-FKBP51, or pCMX-hGRβ, as labeled. After posttransfection incubation for 24 hours, when the cell were completely confluent, cytosolic (100 µg) and nuclear (50 µg) fractions were prepared and subjected to coimmunoprecipitation with anti-FKBP51 antibody and SDS-PAGE, followed by Western immunoblot analysis with anti-GRβ antibody. Right: Western blot analysis for FKBP51 or GRβ in whole-cell lysates was used as a control for protein expression after transfection of pCMX, pCMV-FKBP51, or pCMX-hGRβ. (B) Left: HeLa cells (~60% confluent) were transiently transfected with pCMX, pCMV-FKBP51, or pCMX-hGRβ. After 24 hours of posttransfection incubation, cytosol and nuclear fractions were subjected to coimmunoprecipitation with anti-FKBP51 antibody and Western immunoblot analysis with anti-GRβ antibody. Right: Western immunoblot analysis for FKBP51 or GRβ in whole-cell lysates was used as a control for protein expression after transfection of pCMX, pCMV-FKBP51, or pCMX-hGRβ. (C) HeLa cells were cotransfected with 0.4 µg of glucocorticoid-responsive mercury luciferase reporter pGRE-Luc or 1.5 µg of empty vector pCMX, pCMV-FKBP51, or pCMX-hGRβ in 12-well culture slides. After a 24-hour incubation in complete medium, the cells were shifted to serum-free medium and treated with control vehicle or 100 nM DEX for an additional 24 hours. They were harvested, and luciferase activity was determined. Data are plotted as the x-fold change from each basal activation of vehicle treatment and represent the mean ± SEM of results in three independent experiments (*P < 0.05 pCMX+DEX versus pCMX+vehicle; pCMV-FKBP51+DEX versus pCMV-FKBP51+vehicle; pCMX-hGRβ+DEX versus pCMX-hGRβ+vehicle and **P < 0.05 pCMV-FKBP51+DEX versus pCMX+DEX; pCMX-hGRβ+DEX versus pCMX+DEX; one-way ANOVA).
We further substantiated the chaperone function of FKBP51 in the nuclear transport of GRβ by using a different cell line. HeLa cells were chosen for these experiments because they express both GRα and -β and are frequently used for GR studies. HeLa cells were transiently transfected with pCMV-FKBP51 or pCMX-hGRβ expression vectors, and Western immunoblot analysis of whole-cell lysates detected increased expression of FKBP51 and GRβ, respectively (Fig. 3B, right). Immunoprecipitation with anti-FKBP51 also coprecipitated GRβ in both cytoplasmic and nuclear fractions in control (pCMX transfected) cells. Overexpression of FKBP51 increased levels of the GRβ-FKBP51 complex in both cytoplasmic and nuclear fractions (Fig. 3B, left). Likewise, transfection of GRβ increased the association of the GRβ-FKBP51 complex in both the cytoplasm and the nucleus (Fig. 3B, left panel). These results are consistent with the finding in NTM-5 cells and further support the general concept that FKBP51 serves as an essential chaperone for the nuclear translocation of GRβ.
We have reported that increased GRβ expression negatively regulates glucocorticoid responses in TM cells.19,20 To determine whether this also occurs in HeLa cells, we overexpressed FKBP51 or GRβ and tested for glucocorticoid responsiveness. HeLa cells were transiently cotransfected with pGRE-Luc reporter vector and control, FKBP51, or GRβ expression vectors followed by glucocorticoid challenge. DEX treatment significantly increased the luciferase activity in control (PCMX transfected) cells (P < 0.05). Increased GRβ or FKBP51 expression significantly inhibited this DEX luciferase induction by 61% (P < 0.05) and 56% (P < 0.05), respectively (Fig. 3C).
Differential Effects of FK506 on FKBP51-Chaperoned Nuclear Translocation of GRβ in Normal NTM-5 and Glaucomatous GTM-3 Cells
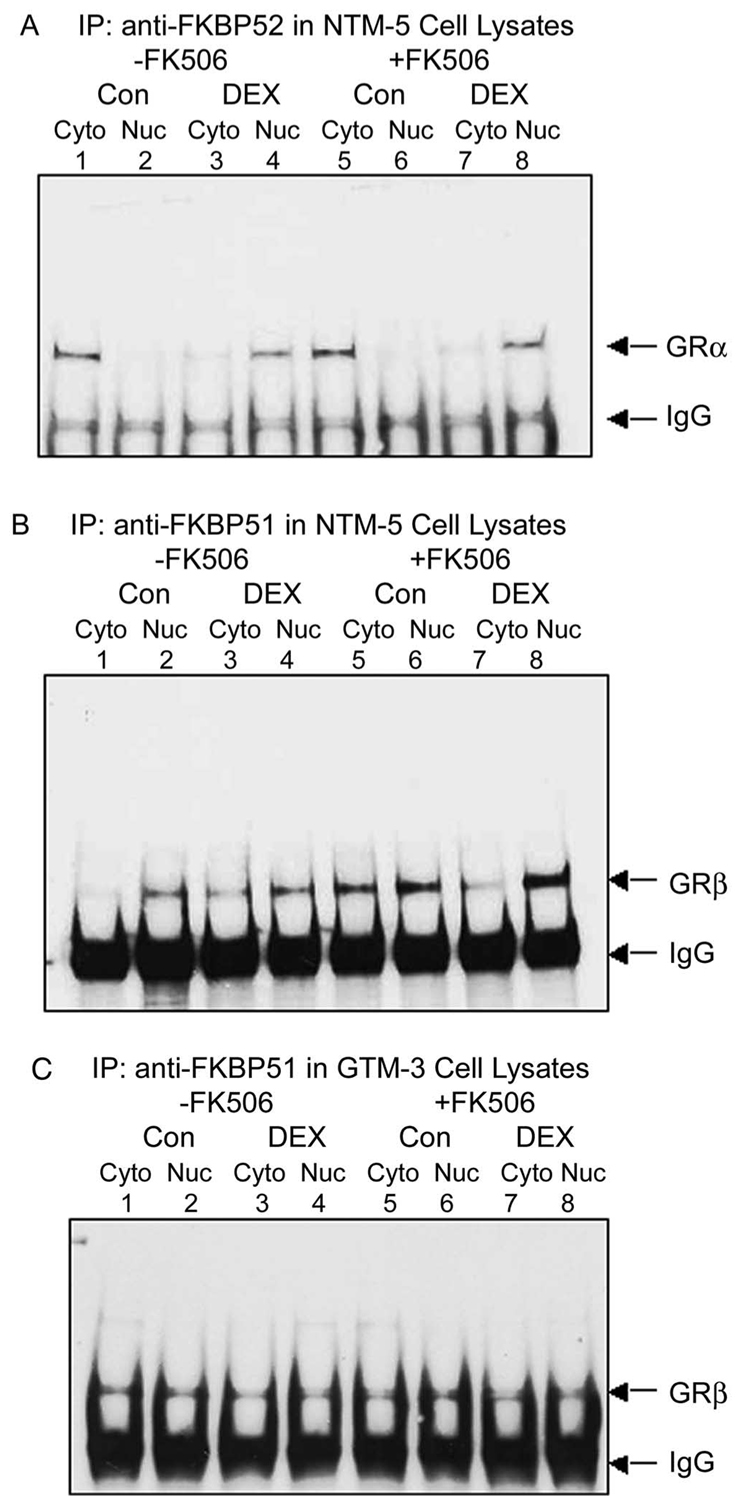
FK506 has diverse effects on GRα activity, including potentiation, no effect, and even inhibitory action.50–54 It differentially binds to FKBP51 and FKBP52, and its diverse effects on glucocorticoid activity may be determined by relative cellular levels of FKBP51 and FKBP52.22,55 Therefore, we examined the effect of FK506 on FKBP51- and FKBP52-chaperoned nuclear transport of GRα and GRβ in TM cells. NTM-5 cells were pretreated with 1 µM FK506 or vehicle control for 1 hour, followed by additional vehicle or DEX treatment for another 30 minutes. As seen earlier, GRα coprecipitated with FKBP52 in the cytoplasmic fraction in the absence of DEX, and DEX treatment caused the translocation of GRα-FKBP52 from the cytoplasm to the nucleus (Fig. 4A, lanes 1–4). FK506 treatment did not change the amount and cellular distribution of the GRα-FKBP52 complex and did not alter DEX-induced nuclear translocation of this complex (Fig. 4A, lanes 5–8). This is consistent with previous reports that FK506 binding to FKBP52 did not cause the activation or dissociation of GRα complex.55,56 In contrast, FKBP51 complexed with GRβ in both the cytoplasm and the nucleus, and DEX treatment had no effect on this complex (Fig. 4B, lanes 1–4). FK506 treatment increased the association of GRβ-FKBP51 in both the cytoplasmic and nuclear fractions. Of interest, in the presence of FK506, DEX treatment resulted in a rapid translocation of GRβ-FKBP51 complex from the cytoplasm to the nucleus in NTM-5 cells (Fig. 4B, lanes 5–8).
FIGURE 4.
Differential effects of FK506 on FKBP51-chaperoned nuclear translocation of GRβ in normal NTM-5 and glaucomatous GTM-3 cells. Confluent NTM-5 and GTM-3 cells were pretreated with vehicle or 1 µM FK506 for 1 hour and then subjected to additional vehicle control or 100 nM DEX treatment, as labeled, for another 30 minutes. The cytosol (100 µg) and nuclear (50 µg) fraction proteins were prepared and resolved in 4% to 15% gradient SDS-polyacrylamide gels after immunoprecipitation. (A) Coimmunoprecipitation with anti-FKBP52 antibody followed by Western immunoblot analysis to detect GRα in NTM-5 cells. (B) Coimmunoprecipitation with anti-FKBP51 antibody followed by Western immunoblot analysis to detect GRβ in NTM-5 cells. (C) Coimmunoprecipitation with anti-FKBP51 antibody followed by Western immunoblot analysis to detect GRβ in GTM-3 cells. The presented data are representative of the results of three independent experiments.
We have shown that glaucomatous GTM-3 cells had low nuclear expression of GRβ than did normal NTM-5 cells.19 We speculated that differential GRβ transport may contribute to this low expression in glaucomatous TM cells. In this study, GTM-3 cells were similarly pretreated with FK506 or vehicle for 1 hour, followed by additional vehicle or DEX treatment for another 30 minutes. GRβ coprecipitated with FKBP51 in both the cytoplasmic and nuclear fractions. DEX treatment did not regulate the transport of this complex between subcellular compartments (Fig. 4C, lanes 1–4). In contrast to NTM-5 cells, FK506, with or without concomitant DEX treatment, did not regulate the association of FKBP51 with GRβ in GTM-3 cells (Fig. 4C, lanes 5–8).
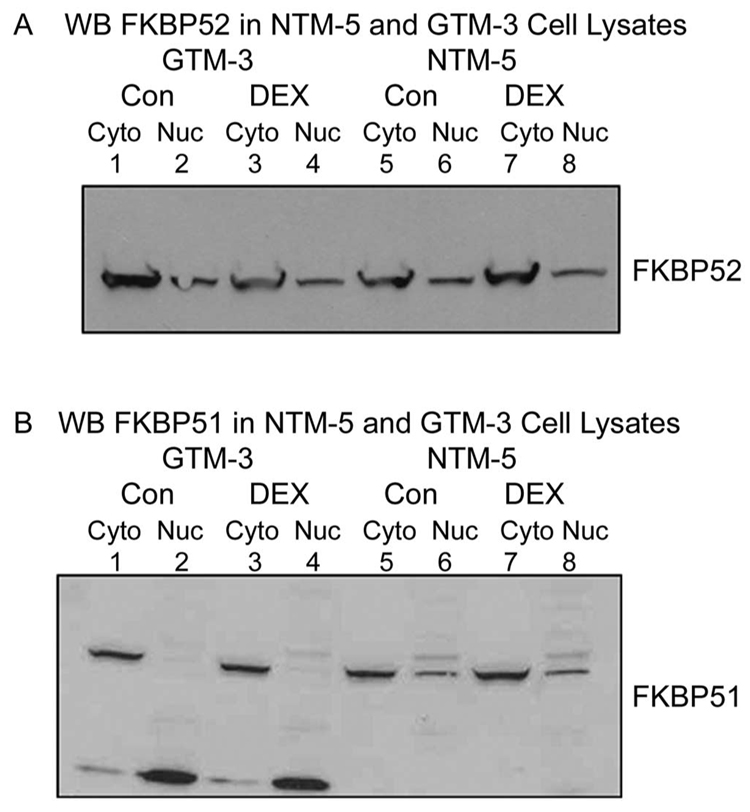
Differential Subcellular Distribution of FKBP51 in Normal NTM-5 and Glaucomatous GTM-3 Cells
The distribution of GRβ19 and sensitivity to FK506 differ between NTM-5 and GTM-3 cells, and so we investigated whether the expression profiles of FKBP51 also differ between NTM-5 and GTM-3 cells. In GTM-3 cells, FKBP51 was present only in the cytoplasmic fractions without detectable FKBP51 (50 kDa) bands in the nuclear fractions, and DEX treatment for 30 minutes did not alter this subcellular distribution (Fig. 5B, lanes 1–4). However, a lower molecular weight immunoreactive band (~20 kDa) was observed in both cytoplasmic and nuclear fractions and was more prominent in the nuclear fractions. FKBP51 was found in both the cytoplasm and nuclear fractions of NTM-5 cells and its distribution was not regulated by DEX treatment (Fig. 5B, lanes 5–8). In contrast, FKBP52 was distributed in the cytoplasm and the nucleus in both normal NTM-5 and glaucomatous GTM-3 cells (Fig. 5A). The differences in FKBP51 subcellular distribution between GTM-3 and NTM-5 cells may account for the different distribution of GRβ in these cells, as well as the differential effects of FK506 on GRβ nuclear transport.
FIGURE 5.
Differential subcellular distribution of FKBP51 in normal NTM-5 and glaucomatous GTM-3 cells. Confluent NTM-5 and GTM-3 cells were treated with vehicle (control) or 100 nM DEX treatment for 30 minutes. The cytosol (100 µg) and nuclear (50 µg) fraction proteins were prepared and resolved on 4% to 15% gradient SDS-polyacrylamide gels. (A) Western immunoblot analysis with anti-FKBP52 antibody to detect the subcellular expression of FKBP52 under control or DEX treatment in GTM-3 and NTM-5 cells, as indicated. (B) Western immunoblot analysis with anti-FKBP51 antibody to detect the subcellular expression of FKBP51 under control or DEX treatment in GTM-3 and NTM-5 cells. The presented data are representative of three independent experiments.
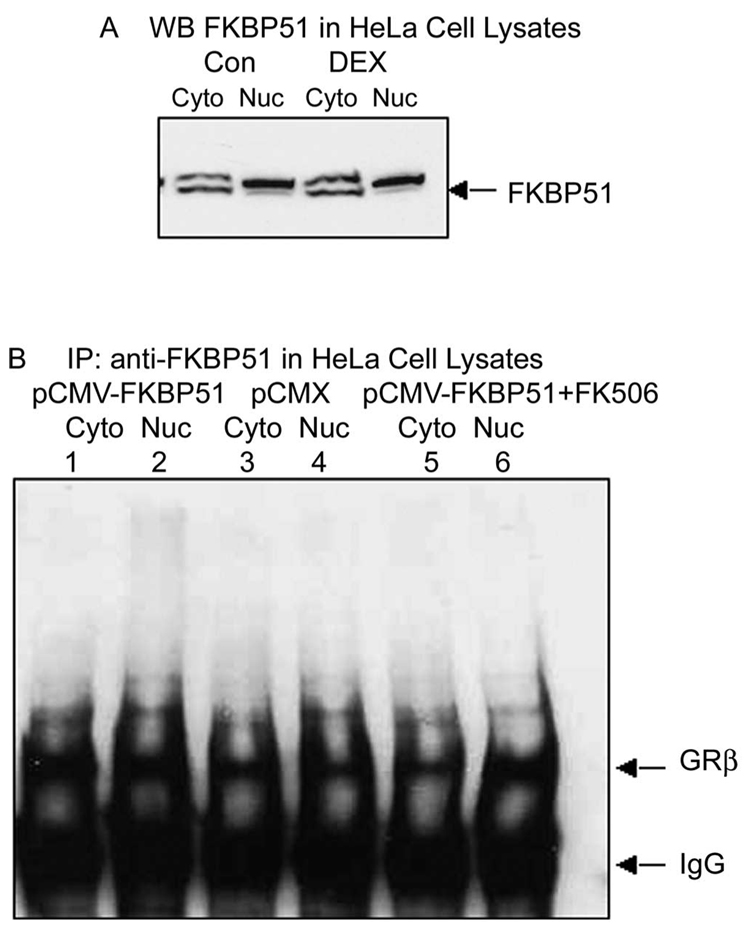
Effect of FK506 on GRβ Nuclear Transport in HeLa Cells
The correlation of the subcellular expression profile of FKBP51 with effects of FK506 on the chaperone activity of FKBP51 was also evaluated in HeLa cells. Western blot analysis revealed a cytoplasmic and nuclear distribution of FKBP51, similar to NTM-5 cells, and this distribution was not regulated by DEX treatment (Fig. 6A). Anti-FKBP51 coprecipitated GRβ in both cytoplasmic and nuclear fractions in control (pCMX transfected) cells (Fig. 6B, lanes 3, 4). Overexpression of FKBP51 increased the GRβ-FKBP51 complex in both the cytoplasmic and nuclear fractions (Fig. 6B, lanes 1, 2). FK506 treatment of FKBP51 transfected cells enhanced the translocation of GRβ from the cytoplasm to the nucleus (Fig. 6B, lanes 5, 6). These results are consistent with the findings in NTM-5 cells.
FIGURE 6.
Subcellular distribution of FKBP51 and effects of FK506 on FKBP51-chaperoned nuclear translocation of GRβ HeLa cells. (A) Confluent HeLa cells were treated with vehicle (control) or 100 nM DEX treatment for 30 minutes. The cytosol (100 µg) and nuclear (50 µg) fraction proteins were prepared and resolved on 4% to 15% SDS-polyacrylamide gradient gels. Western immunoblot analysis was performed to detect the subcellular distribution of FKBP51. (B) Subconfluent HeLa cells were transfected with either control (pCMX) or pCMX-FKBP51 plasmids and then treated with control or 1 µM FK506 for 1 hour before additional vehicle control or 100 nM DEX treatment, as labeled, for another 30 minutes. The cytosol (100 µg) and nuclear (50 µg) proteins were coimmunoprecipitated with anti-FKBP51 antibody followed by Western immunoblot analysis, to detect the effects of overexpression of FKBP51 or FK506 on subcellular distribution of GRβ. The data are representative of results of three independent experiments.
Effect of FK506 on DEX-Induced Luciferase Activity in NTM-5 and GTM-3 Cells
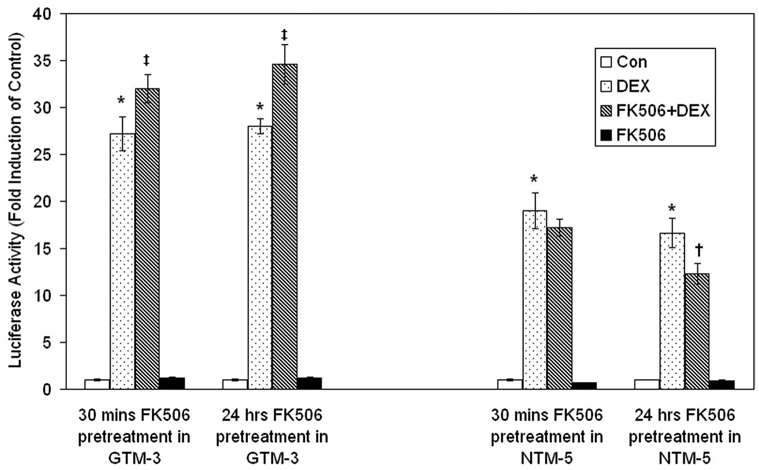
FK506 differentially regulated the FKBP51-chaperoned nuclear transport of GRβ in NTM-5 and GTM-3 cells, particularly in the presence of DEX. We therefore compared the effect of FK506 on DEX-induced gene expression in NTM-5 and GTM-3 cells to evaluate whether FKBP51-mediated nuclear transport of GRβ could alter glucocorticoid responsiveness. NTM-5 and GTM-3 cells were transiently transfected with a pGRE-Luc reporter construct and pretreated with FK506 30 minutes or 24 hours before treatment with DEX for 30 minutes. In GTM-3 cells, DEX caused a 27-fold induction of luciferase activity (P < 0.05), and FK506 pretreatment significantly enhanced this DEX response (32-fold induction after 30 minutes and 35-fold induction after 24 hours, P < 0.05; Fig. 7). In contrast, DEX treatment of NTM-5 cells resulted a 16- to 19-fold induction of luciferase activity (P < 0.05) and pretreatment with FK506 for 30 minutes modestly inhibited this DEX induction, while 24-hour FK506 pretreatment significantly reduced the DEX-induced luciferase activity (P < 0.05; Fig. 7 as labeled). The distinct effects of FK506 on DEX-induced luciferase activity between GTM-3 and NTM-5 cells reinforce the roles of FKBP51 and GRβ in regulation of glucocorticoid responsiveness in these cells.
FIGURE 7.
Effect of FK506 on DEX induction of reporter gene luciferase activity in normal NTM-5 and glaucomatous GTM-3 cells. GTM-3 and NTM-5 cells (~60% confluent) were transfected with 0.4 µg of glucocorticoid-responsive mercury luciferase reporter pGRE-Luc in 12-well culture plates. After posttransfection incubation, GTM-3 and NTM-5 cells were pretreated with control or 1 µM FK506 for either 30 minutes or 24 hours, and the cells were treated with vehicle (control) or 100 nM DEX for another 24 hours. Cell lysate luciferase activity was determined. Data are plotted as the x-fold change from control vehicle treatment and represent the mean ± SEM of results from three independent experiments (*P < 0.05 DEX versus vehicle control in both GTM-3 and NTM-5 cells; ‡P < 0.05 FK506+DEX versus DEX treatment in GTM-3 cells; †P < 0.05 FK506+DEX versus DEX treatment in NTM-5 cells; one way ANOVA; n = 3).
DISCUSSION
A great deal is known about the glucocorticoid response regulated by GRα. In contrast, less is understood about the mechanisms responsible for glucocorticoid resistance or enhanced glucocorticoid responsiveness in some physiological conditions and disease states.13,19,41,46 Several different mechanisms have been independently proposed to explain the cause of glucocorticoid resistance, including relative levels of GRβ expression,13,19,57–59 relative abundance of FKBP51,40,41 and expression of structurally different isoforms of GRα.60,61 In the present study, we explored the potential role of FKBP51 and FKBP52 in regulating nuclear translocation of GRα and GRβ. We have shown that immunophilin FKBP51 is a molecular chaperone for nuclear translocation of GRβ. This FKBP51-mediated transport of GRβ to the nucleus decreased glucocorticoid responsiveness (i.e., caused glucocorticoid resistance). These conclusions are drawn based on the following findings: (1) FKBP51 interacted with GRβ, Hsp90, dynein, and required Hsp90 activity for its chaperone function; (2) overexpression of FKBP51 increased nuclear accumulation of GRβ and reduced responsiveness to a glucocorticoid regulated reporter gene; (3) FK506 facilitated FKBP51-chaperoned nuclear transport of GRβ in NTM-5 cells and reduced the DEX-induction of luciferase, but FK506 had little effect on FKBP51-mediated GRβ translocation in GTM-3 cells and potentiated DEX induction of luciferase.
Unlike GRα and other steroid receptors, less is known about the nuclear translocation of GRβ. We have reported that Hsp90 serves as a chaperone for GRβ in TM cells,39 but other components may also be involved in this process. In the present study, FKBP51 also served as a chaperone for GRβ in TM cells. We detected the protein–protein interactions of FKBP51 with GRβ, Hsp90, and dynein. Inhibition of Hsp90 activity blocked the nuclear import of FKBP51. Similar in some ways to the previously reported transportosome concept for GRα,24 the heterocomplex involving Hsp90, FKBP51, and dynein guides GRβ along cytoskeletal tracts to the nucleus. Of interest, the interaction of FKBP51 with GRβ differed from the interaction of FKBP51 with Hsp90 or dynein, as the FKBP51-GRβ complex was present in both cytoplasm and nucleus, whereas the FKBP51-Hsp90 or FKBP51-dynein complexes were found only in the cytoplasm. We have reported that the Hsp90–GRβ complex resides in the cytoplasm.39 The association of FKBP51 with GRβ persisted in the nucleus even though Hsp90 and dynein dissociated from GRβ receptor. Based on these findings, we propose the following: FKBP51 binds to GRβ or the GRβ–Hsp90 complex in the cytoplasm and recruits the microtubule motor protein dynein to move this heteromeric complex through the cytoplasm to the nucleus along a microtubule track. On reaching the nucleus, Hsp90 and dynein disassociate from the complex, releasing GRβ-FKBP51 into the nucleus where GRβ can inhibit GRα transcriptional activity. How FKBP51 directly interacts with GRα is unknown. Different regions of FKBP52 can determine its association with GRα, Hsp90, and dynein.62 FKBP51 is composed of two FKBP domains that bind immunosuppressant drugs such as FK50625,26 and TPR domains, which form binding sites for Hsp90.27 The arrangement of these domains allows possible interactions with some Hsp90 client proteins.28–30
We also found that the association of FKBP51 with GRα and -β was ligand independent and was not regulated by ligand treatment. This result suggests that FKBP51 is involved in constitutive, ligand-independent transport of both GRα and -β through the cytoplasmic compartment to the nucleus. In contrast, FKBP52 appears to be solely responsible for the nuclear transport of ligand-activated GRα. How FKBP51 and FKBP52 distinctly regulate the transport of GRα is not known. Although FKBP52 and FKBP51 share approximately 75% amino acid sequence similarity and similar architecture domains,28 the differences in orientation between the FK1 and FK2 domains in FKBP51 and FKBP52 can impact Hsp90 binding and dynein association,28–30 which could account for the different functions of FKBP51 and FKBP52 on GRα and -β translocation.
FK506 has diverse effects on GRα activity, with reports of potentiation, no effect, and even inhibitory action.50–54 FK506 differentially binds to FKBP51 and FKBP52, and its diverse effects on glucocorticoid activity may be determined by relative cellular levels of FKBP51 and FKBP52.22,55 Our data showed that FK506 had differential effects on GRβ nuclear transport in individual cell lines, which could also account for the diverse effects of FK506 on glucocorticoid activity reported in literature. FK506 differentially regulated the nuclear translocation of GRβ in normal NTM-5 and glaucomatous GTM-3 cells. FK506 potentiated the nuclear import of GRβ in NTM-5 cells, but had little effect in GTM-3 cells. Even more interesting is our finding that in the presence of FK506, DEX caused a rapid translocation of FKBP51-GRβ from the cytoplasm to the nucleus. GRβ has been reported to lack ligand-binding activity. The sequence of GRβ is identical with GRα through amino acid 727, where they diverge and have unique C termini. The C-terminal 50 amino acids of GRα encode the glucocorticoid binding domain, whereas the C-terminal 15 amino acids of GRβ lack a glucocorticoid binding domain.8,9 However, a recent study has shown that GRβ is capable of binding RU486, an anti-progestin and glucocorticoid antagonist, but not any of the numerous other steroids tested.63 RU486 binding caused the nuclear translocation of GRβ. Therefore, our unexpected finding of DEX-induced nuclear translocation of GRβ in the presence of FK506 could be due to an FK506-induced conformation change in FKBP51 or by other protein–protein interactions involving Hsp90 and/or GRβ.
The concomitant effects of FKBP51 on nuclear transport of GRβ and on inhibition of glucocorticoid responses suggest a novel pathway for FKBP51 to suppress glucocorticoid responses. FKBP51 chaperones the transport of GRβ through the cytoplasm into the nucleus where GRβ can antagonize transcriptionally active GRα. To prove this concept, we had to determine the relationship between the GRβ chaperone function of FKBP51 and the inhibitory activity of FKBP51 on the GRα-mediated glucocorticoid response. FK506 differentially regulated the FKBP51-chaperoned nuclear transport of GRβ in NTM-5 and GTM-3 cells, particularly in the presence of DEX, so comparing the effects of FK506 on DEX responses between NTM-5 and GTM-3 cells represented a unique opportunity to test this novel mechanism. Indeed, we detected differential effects of FK506 on DEX-induced luciferase between NTM-5 and GTM-3 cells with a reduction in DEX luciferase response by FK506 pretreatment in NTM-5 cells but a potentiation in DEX luciferase activity by FK506 in GTM-3. These differing effects on DEX-induced gene expression correlated nicely with FK506 effects on GRβ nuclear translocation. FK506 facilitated the nuclear transport of GRβ in NTM-5 cells, but FK506 failed to enhance the transport of GRβ in GTM-3 cells. The range of reduction or potentiation by FK506 of the DEX-induced reporter gene was relevant to the length of time of pretreatment of FK506: Longer pretreatment resulted in greater effects, further supporting a role for FK506 effects on the nuclear import of GRβ. These data indicate that FKBP51 suppresses glucocorticoid response by enhancing the transport of GRβ.
In agreement with our present study in TM cells, FK506 has diverse effects on glucocorticoid responses in other cell types, including potentiation, no effect, or inhibitory action.50–54 Furthermore, the FK506 effect on GRα was differentially regulated by the timing of glucocorticoid and FK506 treatment, leading to enhanced or inhibited glucocorticoid activity.54 The finding that FK506 enhanced glucocorticoid responsiveness by causing FKBP51 release and enhanced FKBP52 association with the GRα heterocomplex40,42,54,64 may not explain the diversity of the effects of FK506. In the present study, FK506 enhanced nuclear transport of GRβ, which could negatively regulate GRα activity. This novel mechanism of action should be considered when interpreting the actions of FK506 on glucocorticoid activities, as the FKBP51 dissociated from the GRα complex could facilitate GRβ nuclear translocation. Therefore, the effect of FK506 on GR activity may depend on the preexisting balance of cellular FKBP51 and FKBP52 levels.
Western blot analysis detected both cytoplasmic and nuclear distributions of FKBP51 protein in NTM-5 cells but solely cytoplasmic expression of FKBP51 in GTM-3 cells. Immunoblot analysis also detected a much smaller immunoreactive protein band predominantly in the nucleus in GTM-3 cells, which may be a degradation product of FKBP51. It is possible that amino acid or structure differences within its functional domains are responsible for the different distribution pattern of FKBP51 in GTM-3 cells, because potency-related sequence differences have been reported in squirrel monkey and human FKBP51.40,41 Previously, we reported that glaucomatous TM cell lines (including GTM-3) had lower levels of GRβ than did normal TM cell lines (including NTM-5).19 It is possible that FKBP51 structural differences in GTM-3 could affect its interaction with GRβ and Hsp90 complex, disrupt the constitutive nuclear transport of GRβ, and consequently decrease the amount of nuclear GRβ that we saw previously. It is also possible that FK506 interacts with FKBP51 in GTM-3 differently and fails to modulate FKBP51 chaperone function, as seen in this study. GRβ acts as a dominant negative regulator of GRα function. Increased expression of GRβ has been reported in several GC-resistant diseases including asthma,30 rheumatoid arthritis,25 and inflammatory bowel diseases.31 Higher efficiency of chaperoning nuclear transport of GRβ by FKBP51 in normal TM cells should increase the accumulation of GRβ in the nucleus and make them more resistant to the ocular hypertensive effects of GCs. Conversely, the deficiency in the chaperone function of FKBP51 in glaucomatous TM cells could result in the low cellular expression of GRβ and contribute to the elevated IOP in response to glucocorticoids in patients with glaucoma.
In summary, FKBP51 was identified as an essential molecular chaperone for the translocation of GRβ from the cytoplasm to the nucleus. This novel pathway involves FKBP51-mediated translocation of GRβ, which can antagonize the transcriptional activity of activated GRα, inhibiting cellular glucocorticoid responsiveness. The lack of nuclear entry of FKBP51 results in inefficient nuclear transport of GRβ and may contribute to the low expression of GRβ and enhanced glucocorticoid responsiveness. The roles of GRβ and FKBP51 in regulating glucocorticoid responsiveness in TM cells may provide a molecular explanation for the development of ocular hypertension and increased glucocorticoid sensitivity in individuals with POAG.
Acknowledgments
The authors thank Allan Shepard (Alcon Research, Ltd.) for help in generating the GRβ expression construct pCMX-hGRβ.
Supported by National Eye Institute Grants EY11979 and EY016242 and Alcon Research, Ltd.
Footnotes
Disclosure: X. Zhang, Alcon Research, Ltd. (E, F); A.F. Clark, Alcon Research, Ltd. (E, F); T. Yorio, Alcon Research, Ltd. (F), Kosan Biosciences, Inc. (F)
References
- 1.Kimberly RP. Glucocorticoids Curr Opin Rheumatol. 1994;6:273–280. doi: 10.1097/00002281-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 3.Schmid W, Cole TJ, Blendy JA, Schutz G. Molecular genetic analysis of glucocorticoid signalling in development. J Steroid Biochem Mol Biol. 1995;53:33–35. doi: 10.1016/0960-0760(95)00038-2. [DOI] [PubMed] [Google Scholar]
- 4.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson-Jurica MA, Schrader WT, O’Malley BW. Steroid receptor family: structure and functions. Endocr Rev. 1990;11:201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 8.Hollenberg SM, Weinberger C, Ong ES, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform: expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 10.Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform: specificity and mechanisms of action. J Biol Chem. 1999;274:27857–27866. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- 12.Yudt MR, Jewell CM, Bienstock RJ, Cidlowski JA. Molecular origins for the dominant negative function of human glucocorticoid receptor beta. Mol Cell Biol. 2003;23:4319–4330. doi: 10.1128/MCB.23.12.4319-4330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung DY, Chrousos GP. Is there a role for glucocorticoid receptor beta in glucocorticoid-dependent asthmatics? Am J Respir Crit Care Med. 2000;162:1–3. doi: 10.1164/ajrccm.162.1.9911032a. [DOI] [PubMed] [Google Scholar]
- 14.Clark AF, Morrison JC. Corticosteroid glaucoma. In: Morrison JC, Pollack IP, editors. Glaucoma: Science and Practice. New York: Thieme Medical Publisher; 2002. pp. 197–206. [Google Scholar]
- 15.Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Fed Proc. 1965;24:1274–1278. [PubMed] [Google Scholar]
- 16.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- 17.Becker B, Hahn KA. Topical corticosteroids and heredity in primary open-angle glaucoma. Am J Ophthalmol. 1964;57:543–551. doi: 10.1016/0002-9394(64)92500-0. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett JD, Woolley TW, Adams CM. Identification of high intraocular pressure responders to topical ophthalmic corticosteroids. J Ocul Pharmacol. 1993;9:35–45. doi: 10.1089/jop.1993.9.35. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005;46:4607–4616. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007;84:275–284. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 22.Pratt WB, Czar MJ, Stancato LF, Owens JK. The hsp56 immunophilin component of steroid receptor heterocomplexes: could this be the elusive nuclear localization signal-binding protein? J Steroid Biochem Mol Biol. 1993;46:269–279. doi: 10.1016/0960-0760(93)90216-j. [DOI] [PubMed] [Google Scholar]
- 23.Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- 24.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 25.Dolinski K, Muir S, Cardenas M, Heitman J. All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:13093–13098. doi: 10.1073/pnas.94.24.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 27.Riggs DL, Roberts PJ, Chirillo SC, et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu B, Li P, Liu Y, et al. 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Natl Acad Sci USA. 2004;101:8348–8353. doi: 10.1073/pnas.0305969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–17394. doi: 10.1074/jbc.M300955200. [DOI] [PubMed] [Google Scholar]
- 30.Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci USA. 2003;100:868–873. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 32.Czar MJ, Lyons RH, Welsh MJ, Renoir JM, Pratt WB. Evidence that the FK506-binding immunophilin heat shock protein 56 is required for trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus. Mol Endocrinol. 1995;9:1549–1560. doi: 10.1210/mend.9.11.8584032. [DOI] [PubMed] [Google Scholar]
- 33.Perrot-Applanat M, Lescop P, Milgrom E. The cytoskeleton and the cellular traffic of the progesterone receptor. J Cell Biol. 1992;119:337–348. doi: 10.1083/jcb.119.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200. [DOI] [PubMed] [Google Scholar]
- 35.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 36.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 37.Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 38.Gougat C, Jaffuel D, Gagliardo R, et al. Overexpression of the human glucocorticoid receptor alpha and beta isoforms inhibits AP-1 and NF-kappaB activities hormone independently. J Mol Med. 2002;80:309–318. doi: 10.1007/s00109-001-0302-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Clark AF, Yorio T. Heat shock protein 90 is an essential molecular chaperone for nuclear transport of glucocorticoid receptor beta. Invest Ophthalmol Vis Sci. 2006;47:700–708. doi: 10.1167/iovs.05-0697. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- 41.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three new world primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 42.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 43.Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure-function analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005;146:3194–3201. doi: 10.1210/en.2005-0027. [DOI] [PubMed] [Google Scholar]
- 44.Baughman G, Wiederrecht GJ, Campbell NF, Martin MM, Bourgeois S. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15:4395–4402. doi: 10.1128/mcb.15.8.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489. [DOI] [PubMed] [Google Scholar]
- 46.Scammell JG. Steroid resistance in the squirrel monkey: an old subject revisited. ILAR J. 2000;41:19–25. doi: 10.1093/ilar.41.1.19. [DOI] [PubMed] [Google Scholar]
- 47.Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- 49.Ratajczak T, Carrello A, Mark PJ, et al. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–13192. [PubMed] [Google Scholar]
- 50.Ning YM, Sanchez ER. Potentiation of glucocorticoid receptor-mediated gene expression by the immunophilin ligands FK506 and rapamycin. J Biol Chem. 1993;268:6073–6076. [PubMed] [Google Scholar]
- 51.Renoir JM, Mercier-Bodard C, Hoffmann K, et al. Cyclosporin A potentiates the dexamethasone-induced mouse mammary tumor virus-chloramphenicol acetyltransferase activity in LMCAT cells: a possible role for different heat shock protein-binding immunophilins in glucocorticosteroid receptor-mediated gene expression. Proc Natl Acad Sci USA. 1995;92:4977–4981. doi: 10.1073/pnas.92.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutchison KA, Scherrer LC, Czar MJ, et al. FK506 binding to the 56-kilodalton immunophilin (Hsp56) in the glucocorticoid receptor heterocomplex has no effect on receptor folding or function. Biochemistry. 1993;32:3953–3957. doi: 10.1021/bi00066a015. [DOI] [PubMed] [Google Scholar]
- 53.Sheppard KE, Cyclosporin A. FK506 are potent activators of proopiomelanocortin-derived peptide secretion without affecting corticotrope glucocorticoid receptor function. J Neuroendocrinol. 1995;7:833–840. doi: 10.1111/j.1365-2826.1995.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 54.Croxtall JD, Paul-Clark M, Van Hal PT. Differential modulation of glucocorticoid action by FK506 in A549 cells. Biochem J. 2003;376:285–290. doi: 10.1042/BJ20030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256:1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 57.Derijk RH, Schaaf MJ, Turner G, et al. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28:2383–2388. [PubMed] [Google Scholar]
- 58.Orii F, Ashida T, Nomura M, et al. Quantitative analysis for human glucocorticoid receptor alpha/beta mRNA in IBD. Biochem Biophys Res Commun. 2002;296:1286–1294. doi: 10.1016/s0006-291x(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 59.Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000;105:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- 60.Patel PD, Lyons DM, Zhang Z, Ngo H, Schatzberg AF. Impaired transactivation of the glucocorticoid receptor cloned from the guyanese squirrel monkey. J Steroid Biochem Mol Biol. 2000;72:115–123. doi: 10.1016/s0960-0760(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 61.Her S, Patel PD, Schatzberg AF, Lyons DM. Mutations in squirrel monkey glucocorticoid receptor impair nuclear translocation. J Steroid Biochem Mol Biol. 2005;94:319–326. doi: 10.1016/j.jsbmb.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Silverstein AM, Galigniana MD, Kanelakis KC, Radanyi C, Renoir JM, Pratt WB. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem. 1999;274:36980–36986. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 63.Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies TH, Ning YM, Sanchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]