Abstract
Gene linkage and association studies have implicated the region of chromosome 10q containing the calcyon locus with attention deficit hyperactivity disorder (ADHD), bipolar disorder, and schizophrenia susceptibility. In addition, levels of calcyon protein and transcripts are also significantly increased in postmortem tissue from schizophrenic brains. But whether altered calcyon expression might be part of the disease etiology or merely a patho-physiological side effect is not known. To begin to address this issue, we generated a transgenic mouse line (CalOE) using the human calcyon cDNA in which calcyon expression is up-regulated in a number of forebrain structures including the hippocampus, prefrontal cortex, striatum, and amygdala. Compared to control littermates, the CalOE mice display a range of abnormal behaviors including spontaneous hyperactivity, reduced anxiety, and/or impaired restraint (harm avoidance) that would indicate that calcyon up-regulation leads to deficits in control over behavioral output.
Keywords: ADHD, schizophrenia, disease model, anxiety, impulsivity, novelty seeking
Introduction
Calcyon is a single transmembrane protein expressed in brain [1;2] that has been linked to neuropsychiatric disorders such as schizophrenia in which expression of this protein is up-regulated in the prefrontal cortex (PFC) and thalamus [3–6]. Recent molecular studies indicate that calcyon primarily interacts with clathrin light chain and enhances clathrin-mediated endocytosis (CME) [7]. But, whether or how altered calcyon expression might influence behavior in not known.
Polymorphisms in the calcyon gene are associated with attention deficit hyperactivity disorder (ADHD) [8]. Additionally, a genome wide linkage scan for loci influencing in ADHD reported that the strength of association of a marker at 10q26, the cytogenetic position of the calcyon locus, significantly exceeded that of the DA transporter (DAT1), and the D4R (DRD4) [9]. Like severe neuro-psychiatric disorders including schizophrenia and bipolar disorder, family, twin and adoption studies suggest that ADHD exhibits significant heritability, but the mode of transmission appears to be complex involving multiple susceptibility alleles, as well as environmental factors such as stress [10]. The challenge of pinpointing risk alleles for ADHD is further complicated by the likelihood that susceptibility involves inheriting alterations in multiple genes, and perhaps that different genes are causally related to different “subtypes” of the disorder. For example, inattention and impulsivity appear to be independent characteristics, and possibly two dissociable subtypes, of ADHD [11;12]. Thus, although some genes may affect specific aspects of ADHD, others may be responsible for the common characteristics of the disorder. However, a major gap in the study of ADHD is a lack of transgenic animal models that fulfill a number of common criteria for modeling the disorder. Animal models are valuable in that they afford an intact system in which to study disease mechanisms at systems, neural, and molecular levels.
Here, we report the initial behavioral characterization of transgenic mice with up-regulated calcyon expression in forebrain. These studies indicate that over-expression in forebrain contributes to hyperactivity, increased exploratory activity, impaired restraint, and/or reduced anxiety. Given the genetic evidence for potential ADHD susceptibility alleles at the calcyon locus, our data suggest that the calcyon transgenic mice mimic some key behavioral features of ADHD.
Methods
Production of calcyon over-expressing mice
The linearized TRE-FLAG-hCalcyon (TRE-Cal) mini-gene was microinjected into pronuclei of fertilized F2 oocytes (resulting from a cross between CBA and C57Bl/6) by the Medical College of Georgia (MCG) transgenic core facility. Embryos were subsequently transplanted into the oviducts of pseudopregnant foster mothers, and progeny screened for germline transmission. Prior to carrying out the present studies, both the TRE-Cal and the αCamKII-tTA mice [13] (gift of Dr. T. Abel, U. Penn) were backcrossed to C57Bl/6 for eight generations. CalOE (TRE-Cal/tTA) double transgenic mice result from mating of TRE-Cal and αCamKII-tTA mice.
Immunohistochemistry and Imaging
Fresh frozen half brains of each genotype were set together in a block of tissue freezing medium, sliced at 20 micron-thick coronal sections on a cryostat, captured on slides and kept at −80 °C until staining. Six slides from each block were selected from the prefrontal cortex, striatum and the medial portion of the dorsal hippocampus. The tissue was fixed in 2% paraformaldehyde at 7.4 pH for 5 minutes, washed in 2XSSC (pH 7.0), followed by 50:50% acetone-methanol at 4°C for 5 minutes. The tissue was washed in 2XSSC+0.05% Twin-20 and quenched in 2XSSC+1% H2O2 for 15 min. After blocking with TSA blocking buffer (Perkin Elmer Life Sciences, Emeryville, CA), the slides were incubated with rabbit anti-calcyon antibody (1:100, Lezcano et al., 2000) overnight at 4°C. Incubation with the anti-rabbit biotinylated secondary anti-rabbit antibody (1:200, Vector Labs, Burlingam, CA) for 2 hrs at room temperature was followed by amplification with the Avidin+Biotin system (Vector Labs) for 45 minutes. The staining was visualized using the TSA fluorescence system CY3 (PerkinElmer Life Sciences). Additional slides were double labeled for calcyon/NeuN by first staining for calcyon and then incubating with mouse monoclonal biotin conjugated anti-NeuN antibody (1:1,000, Chemicon, Billerica, MA) overnight. The avidin-biotin amplified signal was revealed with CY5. The nuclei were conterstained with Sytox-green (Molecular Probes, Eugene, OR). No staining was detected in the absence of the primary or secondary antibodies.
Image stacks were collected with either a 10x or 20x objective on a fluorescent microscope equipped with an Apotome system (Zeiss, Thorwood, NY) from the medial prefrontal cortex (prelimbic area), the dorsal hippocampus, dorsal striatum and basolateral amygdala. Each region was identified on the basis of cytoarchitectonics and relative position to prominent landmarks, such as the midline, lateral ventricles, external capsule and rhinal fissue. Intensity of excitation illumination was set separately for each region and kept constant for all brain sections on a slide, ensuring that sections from the different genotypes were imaged with equal settings. The researcher performing the imaging was unaware of the genotype to which each brain section belonged.
Behavioral apparatus and testing
All behavioral tests were conducted by experimenters blind to genotype. Testing rooms were equipped with white noise generators (San Diego Instruments, San Diego, CA) set to provide a constant background level of 70 dB and ambient lighting of approximately 25–30 Lux (lumen/m2). Test subjects were housed in rooms with reverse light-dark cycle lighting (lights on/off, 6 am/6 pm), and handled daily for several minutes (each) for at least one week prior to experimentation. Animals were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments. All procedures employed during this study were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
Mouse open field activity monitors (27.9 cm × 27.9 cm, Med Associates St Albans, VT) were used to measure locomotor activity. The following parameters were recorded for the 30 min test session: horizontal activity (horizontal photobeam breaks or counts), number of stereotypical movements, and vertical activity (vertical photobeam breaks).
In the light/dark box exploration test, the open field activity monitors described above were fitted with dark box inserts (which are opaque to visible light) to cover one-half of the open field area thus separating the apparatus into two zones of equal area (i.e., a brightly lit zone and a darkened zone). Desk lamps located above the activity monitors were used to provide an illumination level of approximately 1000 lux in the brightly lit zone, whereas the illumination level in the darkened zone was approximately 5 lux. The time spent and distance traveled in the light and dark zones of the apparatus were recorded for the 5 min test session.
The elevated plus maze consisted of two orthogonal closed (15 × 6 × 30 cm) and open (1 × 6 × 30 cm) arms forming a cross, with a quadrangular (6 × 6 cm) area located at the intersection. The maze was placed 50 cm above the floor and was made of black plastic. Each test lasted 5 min, and was initiated by placing a mouse on the center facing an open arm. The behavior of the animal was recorded with a video camera and later scored by two independent observers each blind to genotype. The following behaviors were scored: open and closed arm entries, time spent and distance traveled in the closed and open arms as well as in the center area, extension of head over the edge of the open arms, and exploration of open arm ends.
Statistical analyses
Statistical analyses were performed using Origin 6.0 (Microcal Software, Northampton, MA) or SigmaStat 2.03 (SPSS Inc., Chicago, IL). Paired t-test or two-way analysis of variance (with repeated measures when indicated) was used for all genotype comparisons. A Student Newman Keuls multiple comparison procedure was used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05.
Results
Calcyon Transgenic Mice
Mice transgenic for the TRE-FLAG-hCalcyon (TRE-Cal) mini-gene containing the human calcyon open reading frame (fig. 1A,B) were mated with mice carrying the tetracycline transactivator (tTA) transgene driven by the αCamKII promoter [13]. The ability of αCamKII driven tTA to induce expression of TRE-FLAG-hcalcyon was tested by immunocytochemistry using affinity purified antibodies that recognize human, but not rodent calcyon [3;7;14;15]. Antibody labeling of brain sections indicated that the calcyon transgene is expressed in the TRE-Cal/tTA double transgenic mice (CalOE), but not in the tTA or TRE-Cal single transgenic animals (fig. 1C, D). For example, robust calcyon transgene expression was detected in hippocampus (fig. 1C, D), prefrontal cortex, amygdala and striatum (fig. 2).
Figure 1. Calcyon Transgenic Mice.
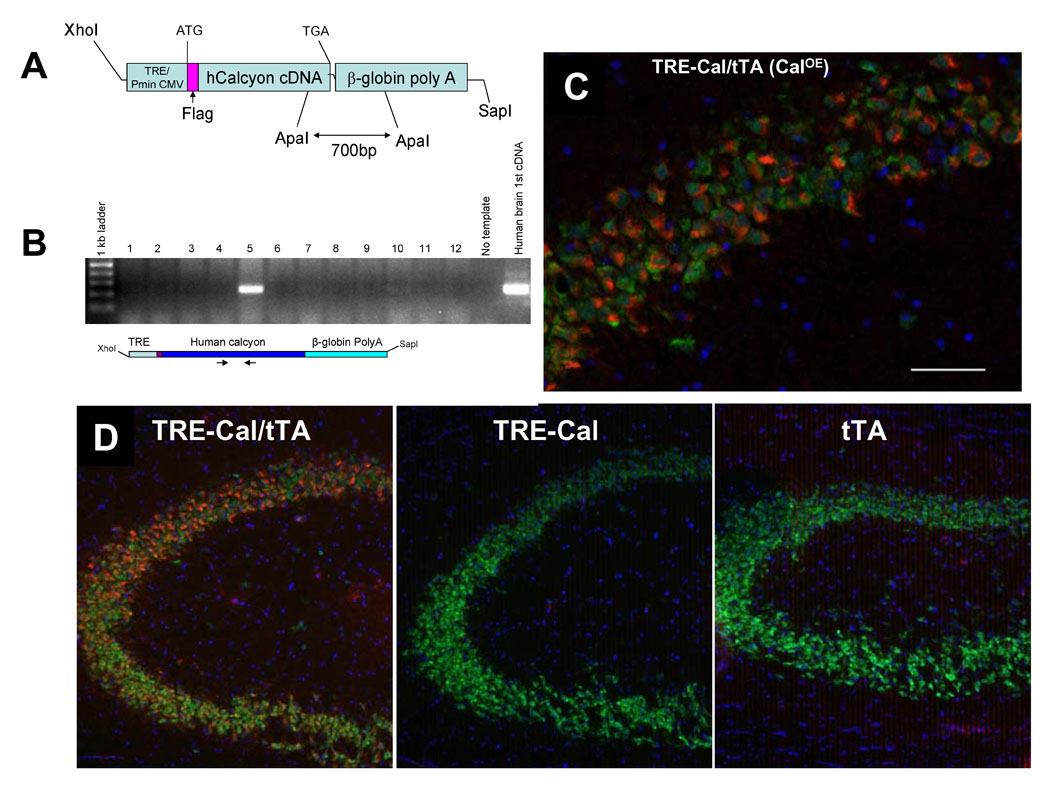
A. Map of TRE-FLAG-hcalcyon transgene construct. The calcyon transgene contains the tetracycline transactivator (tTA) binding site (TRE) and an RNA polymerase II transcriptional start site of the cytomegalovirus IE promoter upstream of the FLAG epitope fused to full-length human calcyon cDNA, followed by a β-globin polyadenylation sequence. B. PCR screen of tail DNA to identify transgenic progeny. C. Representative image from a 1.7-micron thick optical section through the CA1 region of the hippocampus of TRE-Cal/tTA (CalOE) mouse expressing human calcyon immunolabeled for calcyon (red) and NeuN (green). Nuclei (blue) were stained with a DNA-specific dye (Sytox Green). Note that calcyon is expressed in neurons (NeuN-positive cells). Scale bar is 50 micrometers. D. Immunoloabeling of hCalcyon is not detected in hippocampus of either the TRE-Cal or CamKII-tTA (tTA) single transgenic mice.
Figure 2. Calcyon transgene expression is observed in the prefrontal cortex striatum and amygdala of CalOE mice.
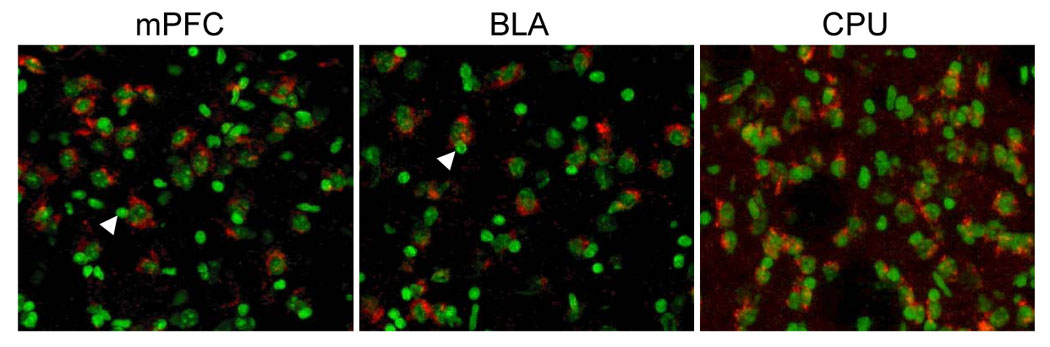
Human calcyon (red) is clearly detected in the cytoplasm around neuronal nuclei (green) of the forebrain, including the medial prefrontal cortex (mPFC), the basolateral amygdala (BLA), and the dorsal striatum (CPU). Note that glia-like nuclei which appear small and uniformly stained (white arrowheads; [27]), do not express the calcyon transgene. Each image represents 180×150 µm of tissue.
Behavioral Analyses of the CalOE mice
We subjected male CalOE mice (n=12 to 13) and tTA control littermates (n=10), 3 to 4 months old, to a battery of tests for anxiety and exploratory activity, to investigate the effects of calcyon gene up-regulation on behavior. The same cohorts of mice were used in all experiments. Neither cohort exhibited gross motor problems. However, the performance of the CalOE mice significantly differed from that of the tTA mice on a variety of measures, many of which are consistent with reduced anxiety, hyperactivity, impulsivity, or impaired restraint.
Exploratory locomotion including horizontal movement, rearing and stereotypy were assessed in an open field. CalOE mice showed greater total distance traveled and greater exploration of the center of the open field (p<0.05 for both comparisons, fig. 3A). Although the CalOE mice displayed higher exploratory activity measured by horizontal photo-beam breaks (fig. 3B), a repeated measures ANOVA across 5 min time bins demonstrated only a trend for genotype (F (1, 21)= 2.9, p=0.10). In addition, the CalOE and tTA mice did not differ with respect to stereoptypic movement, rearing or sniffing activity (data not shown). These results suggest that the trend in increased exploratory activity is likely due to decreased anxiety. The higher number of beam breaks by the CalOE mice in each 5 minute bin in the open field test potentially also includes a response to novelty. However, if the hyperactivity detected were solely triggered by novelty, one might expect to see significant differences the beam break data especially in the initial time periods after introduction into the test environment. This would seem not to be the case since the differences in the first two bins (the 0–5 and 5–10 min time periods) are not significant (by t-tests), and only are in the 10 to 15 min bin (shown in fig. 3B). Interestingly, no differences between genotypes were detected in habituation over time (repeated measures ANOVA, main effect F (5, 105) = 19.49, p<0.0001; interaction between genotype and locomotion was not significant, p=0.16), suggesting that CalOE mice retained some control over exploratory locomotion.
Figure 3. CalOE mice display increased basal locomotor activity.
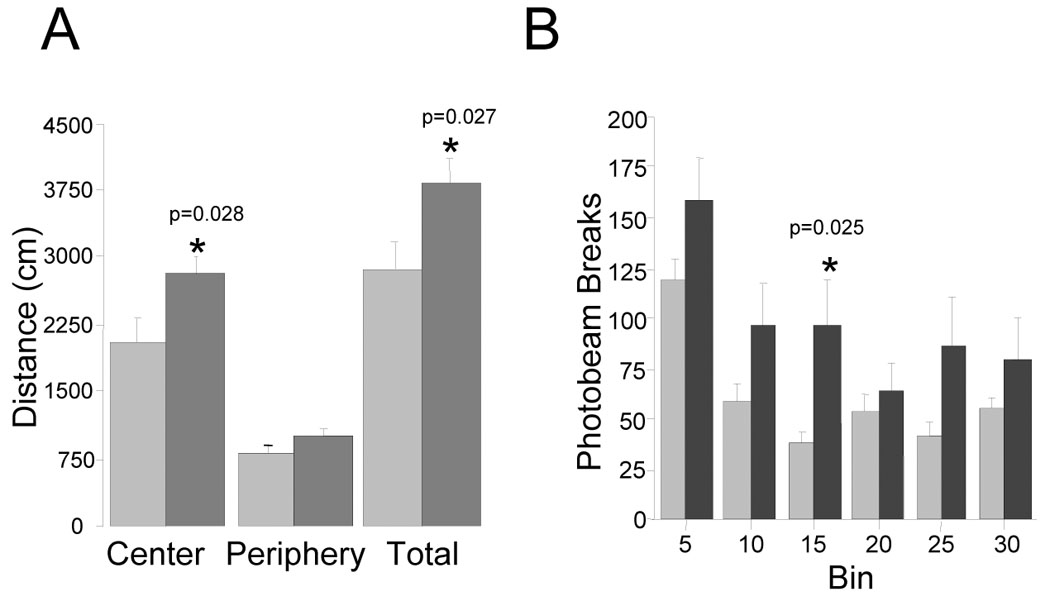
A. Bars show cumulative distance traveled (cm) per zone and in entire box (total) during the 30 min open field test session by the CalOE (dark bars) and tTA (light bars) mice. B. Average ambulatory counts (horizontal photo beam breaks) measured per 5 min segment (bin). *p<0.05; **p<0.01 based on paired t-test comparison of tTA and CalOE values.
To further examine the hypothesis that CalOE mice are less anxious than littermates, we tested all mice on two tasks that are sensitive to the degree of anxiety in rodents- the light/dark box test and the elevated plus maze. The light/dark box test detects anxiety levels in animals because it relies on the natural tendency of rodents to prefer darker environments [16]. Normal or control animals spend more time in the dark field of the test box. In contrast, animals that are less anxious or perhaps more impulsive spend more time in the open field of the test apparatus than controls. The CalOE mice spent significantly more time in the lit side of the box than the control tTA littermates (p=0.01, t-test), as well as significantly less time in the dark portion of the box (p<0.01) (fig. 4), consistent with the hypothesis that they are less anxious. However, the latency to dark times did not differ between genotypes (p=0.5) (data not shown).
Figure 4. CalOE mice exhibit increased exploratory activity and reduced anxiety.
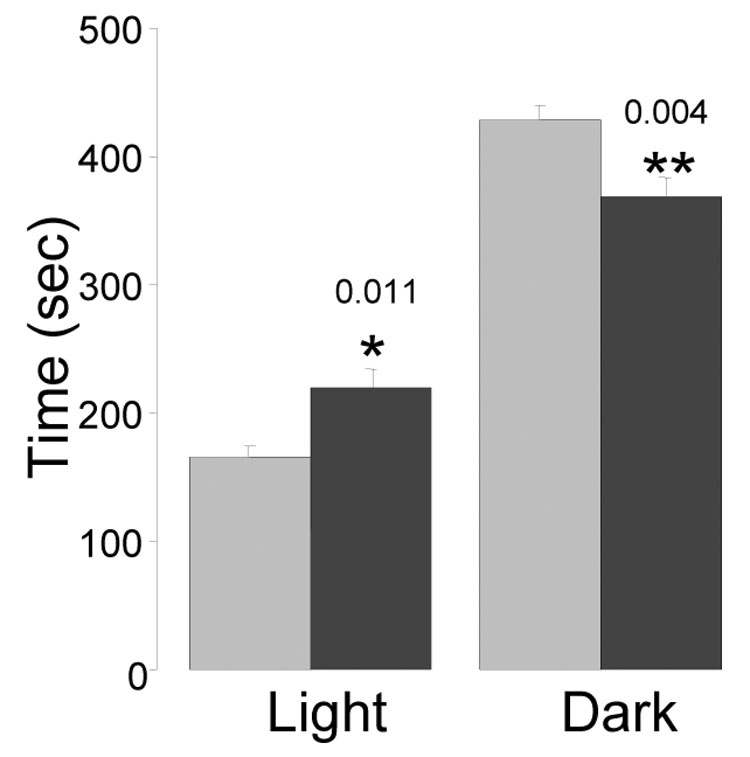
A. Bars show mean time (sec) spent in the light and dark areas during the 10 min light-dark box test by the CalOE (dark bars) and tTA (light bars) mice. *p<0.05 and **p<0.01, paired t-test.
CalOE mice also showed a behavioral pattern consistent with reduced anxiety in the elevated plus maze, where the combination of height, luminosity and open space is assumed to induce unconditioned anxiety in the rodent [17]. Entries and time spent in the closed arm of the elevated plus maze are positively correlated with anxiety, whereas the converse is true for open arm entry and time [18]. Like open arm entry, head dipping and end exploration negatively correlate with anxiety [18;19], but are considered to be more ‘ethologically-derived’ measures [19]. Compared to the tTA animals, CalOE mice entered more frequently the open arms of the maze and explored them more extensively, as measured by time spent and distance traveled in the open arms (for all comparisons p<0.05, fig. 5). We also quantified the exploratory activity of the mice by measuring head dipping and end exploration (fig. 5B). The CalOE mice tended to engage in riskier behavior than the tTA mice as indicated by the greater number of head dips (p=0.052), and exploration of the ends of the open arms (p=0.060) (fig. 5B). Compared to tTA mice, CalOE mice also exhibited higher overall exploratory activity in the elevated plus maze as measured by the total distance traveled during the test period (fig. 5C). Collectively, these data suggest that CalOE animals display reduced anxiety and/or greater activity in situations that would typically cause most animals to perceive danger.
Figure 5. CalOE mice exhibit increased exploratory activity as well as reduced anxiety.
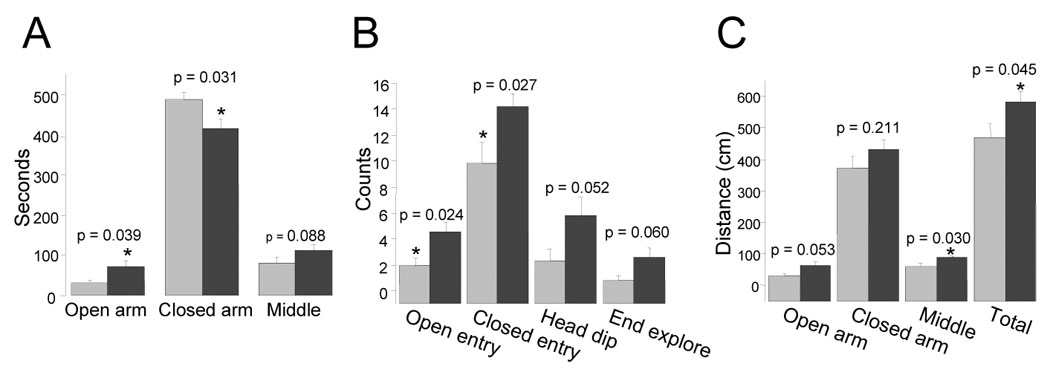
A. Bars show the mean and error bars the SEM of time spent (sec) in each area of the elevated plus maze by the tTA (light bars) and CalOE (dark bars) mice during a 10 min test session. B. Exploratory activity data show the mean number of open and closed arm entries, of times the subjects head extended over the edge of the open arm (Head dip) or times the subject traveled to the end of the open arm (End explore). C. Mean distance traveled in each area of the maze individually and combined (Total). *p<0.05 and **p<0.01, paired t-test.
Discussion
The CalOE mice displayed spontaneous hyperactivity, reduced anxiety and increased exploratory activity during open field exploration, light/dark box preference and elevated plus maze testing. Hence, these studies suggest that increased expression of calcyon in forebrain potentially alters neural processes involved in the regulation of locomotor activity, anxiety and/or harm avoidance. Calcyon up-regulation was detected in mice transgenic for both the TRE-hCal and αCaMKII-tTA transgenes, but not in TRE-Cal mice indicating that the tTA transcriptional activator is necessary for expression of the calcyon transgene in brain. Consistent with this idea, the calcyon transgene was expressed in hippocampus, striatum, PFC and amydala of the double transgenic mice (TRE-Cal/tTA) was similar to other TRE-transgenes driven by the αCamKII-tTA transgene [20;21]. As the TRE-hCal and αCaMKII-tTA transgenes were both extensively backcrossed to C57Bl/6, it seems unlikely that genetic background could account for the behavioral differences between the CalOE and tTA mice. Alternatively, the behavioral deficits detected in the CalOE mice might have arisen as the result of a compensatory mechanism linked to over-expression of calcyon during development. Another potential explanation relates to expression of the human isoform of calcyon in mouse brain. Despite the high degree of sequence similarity between the human and rodent proteins, and their functional similarities, at least with respect to endocytosis in vitro ([7;22] and unpublished data), the current studies cannot formally exclude this caveat. However, the discovery of elevated endogenous calcyon gene expression in forebrain of the spontaneous hyperactive rat [23] raises the possibility that calcyon up-regulation, albeit of the human isoform, directly contributes to the behavioral abnormalities detected in the CalOE mice.
The CalOE mice exhibited elevated horizontal locomotor activity compared to tTA mice throughout the entire period of activity monitoring in the open field test consistent with the idea that the double transgenic CalOE mice are innately hyperactive. The CalOE mice also showed increased exploratory and locomotor activity in the light-dark box and elevated plus maze. Overall these behaviors seem to point to possible underlying abnormalities in movement control circuits. As such, the data also indicate that the CalOE mice perform similarly to animal models for diseases of cognitive dysfunction such as schizophrenia and ADHD in that these models typically show increased spontaneous basal locomotor activity [24;25].
Hyperactivity can also result from decreased anxiety during situations that should normally be threatening. Indeed, CalOE mice showed an overall behavioral pattern consistent with reduced anxiety on three tasks that measure anxiety induced by one or a combination of anxiogenic factors for rodents, such as light, open space and height. CalOE mice spent more time in the center/open area of an unfamiliar environment, more time in the light part of an apparatus that had a ‘safe’/dark compartment, and on the open arms on the elevated plus maze. These behaviors are also consistent with impulsivity or impaired restraint potentially resulting from reduced control over behavioral output [25]. While the data indicate motor control deficits, further studies are necessary to test whether the abnormalities might extend to executive functions such as attention or working memory.
Some explanations for hyperactivity in rodents stress deficits in prefrontal response inhibition, indicating that the vulnerable pathway might include projections from PFC to dorsal striatum. Consistent with this possibility, the calcyon transgene is expressed in PFC. However, other circuit based explanations for the hyperactive phenotype of the CalOE mice can also be advanced based on the expression of the transgene in ventral striatum (i.e., the nucleus accumbens (NAcc)), hippocampus and the amygdala. For example, similar behaviors could result from deficits in the circuit linking NAcc to PFC, and involve alterations in the reward system. Alternatively, calcyon up-regulation in NAcc could lead to altered excitability of medium spiny neurons in NAcc, and the gating of PFC inputs by hippocampal and amygdalar projections might be abnormal [26]. This latter explanation might also account for the reduced anxiety detected in the CalOE mice. Given the apparent activity and anxiety-related abnormalities reported here, the CalOE transgenic mice could prove useful for better understanding the development of motor and emotional control mechanisms.
Acknowledgements
P50 MH068789 (McCormick, PI, Co-PI Project I, CB); MCG Neuroscience Training Grant T32 NS045543 (HTD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Oakman SA, Meador-Woodruff JH. Calcyon transcript expression in macaque brain. J Comp Neurol. 2004;468:264–276. doi: 10.1002/cne.10993. [DOI] [PubMed] [Google Scholar]
- 2.Zelenin S, Aperia A, Diaz HR. Calcyon in the rat brain: cloning of cDNA and expression of mRNA. J Comp Neurol. 2002;446:37–45. doi: 10.1002/cne.10198. [DOI] [PubMed] [Google Scholar]
- 3.Koh PO, Bergson C, Undie AS, Goldman-Rakic PS, Lidow MS. Up-regulation of the D1 dopamine receptor-interacting protein, calcyon, in patients with schizophrenia. Arch Gen Psychiatry. 2003;60:311–319. doi: 10.1001/archpsyc.60.3.311. [DOI] [PubMed] [Google Scholar]
- 4.Bai J, He F, Novikova SI, Undie AS, Dracheva S, Haroutunian V, Lidow MS. Abnormalities in the dopamine system in schizophrenia may lie in altered levels of dopamine receptor-interacting proteins. Biol Psychiatry. 2004;56:427–440. doi: 10.1016/j.biopsych.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Clinton SM, Ibrahim HM, Frey KA, Davis KL, Haroutunian V, Meador-Woodruff JH. Dopaminergic abnormalities in select thalamic nuclei in schizophrenia: involvement of the intracellular signal integrating proteins calcyon and spinophilin. Am J Psychiatry. 2005;162:1859–1871. doi: 10.1176/appi.ajp.162.10.1859. [DOI] [PubMed] [Google Scholar]
- 6.Baracskay KL, Haroutunian V, Meador-Woodruff JH. Dopamine receptor signaling molecules are altered in elderly schizophrenic cortex. Synapse. 2006;60:271–279. doi: 10.1002/syn.20292. [DOI] [PubMed] [Google Scholar]
- 7.Xiao J, Dai R, Negyessy L, Bergson C. Calcyon, a novel partner of clathrin light chain, stimulates clathrin-mediated endocytosis. J Biol Chem. 2006;281:15182–15193. doi: 10.1074/jbc.M600265200. [DOI] [PubMed] [Google Scholar]
- 8.Laurin N, Misener VL, Crosbie J, Ickowicz A, Pathare T, Roberts W, Malone M, Tannock R, Schachar R, Kennedy JL, Barr CL. Association of the calcyon gene (DRD1IP) with attention deficit/hyperactivity disorder. Mol Psychiatry. 2005;10:1117–1125. doi: 10.1038/sj.mp.4001737. [DOI] [PubMed] [Google Scholar]
- 9.Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, Newbury DF, Crawford LR, Palmer CG, Woodward JA, Del'Homme M, Cantwell DP, Nelson SF, Monaco AP, Smalley SL. A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. Am J Hum Genet. 2002;70:1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 11.Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- 12.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin Psychol Rev. 2006 doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayford M, Abel T, Kandel ER. Transgenic approaches to cognition. Curr Opin Neurobiol. 1995;5:141–148. doi: 10.1016/0959-4388(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 14.Lezcano N, Mrzljak L, Eubanks S, Levenson R, Goldman-Rakic P, Bergson C. Dual signaling regulated by calcyon, a D1 dopamine receptor interacting protein. Science. 2000;287:1660–1664. doi: 10.1126/science.287.5458.1660. [DOI] [PubMed] [Google Scholar]
- 15.Lezcano N, Mrzljak L, Levenson R, Bergson C. Retraction. Science. 2006;314:1681. doi: 10.1126/science.314.5806.1681b. [DOI] [PubMed] [Google Scholar]
- 16.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 17.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 18.Anseloni VC, He F, Novikova SI, Turnbach RM, Lidow IA, Ennis M, Lidow MS. Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience. 2005;131:635–645. doi: 10.1016/j.neuroscience.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Wall PM, Messier C. Ethological confirmatory factor analysis of anxiety-like behaviour in the murine elevated plus-maze. Behav Brain Res. 2000;114:199–212. doi: 10.1016/s0166-4328(00)00229-1. [DOI] [PubMed] [Google Scholar]
- 20.Mansuy IM, Winder DG, Moallem TM, Osman M, Mayford M, Hawkins RD, Kandel ER. Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron. 1998;21:257–265. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 21.Krestel HE, Mayford M, Seeburg PH, Sprengel R. A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res. 2001;29:E39. doi: 10.1093/nar/29.7.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai R, Bergson C. Structure and expression of the murine calcyon gene. Gene. 2003;311:111–117. doi: 10.1016/s0378-1119(03)00564-x. [DOI] [PubMed] [Google Scholar]
- 23.Heijtz RD, Alexeyenko A, Castellanos FX. Calcyon mRNA expression in the frontalstriatal circuitry and its relationship to vesicular processes and ADHD. Behav Brain Funct. 2007;3:33. doi: 10.1186/1744-9081-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 25.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 27.Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer T, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces Arc, a plasticity-related immediate-early gene, only in calcium/calmoduline-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]


