Abstract
Experimental autoimmune uveitis (EAU) induced by immunization of animals with retinal Ags is a model for human uveitis. The immunosuppressive cytokine IL-10 regulates EAU susceptibility and may be a factor in genetic resistance to EAU. To further elucidate the regulatory role of endogenous IL-10 in the mouse model of EAU, we examined transgenic (Tg) mice expressing IL-10 either in activated T cells (inducible) or in macrophages (constitutive). These IL-10-Tg mice and non-Tg wild-type controls were immunized with a uveitogenic regimen of the retinal Ag interphotoreceptor retinoid-binding protein. Constitutive expression of IL-10 in macrophages abrogated disease and reduced Ag-specific immunological responses. These mice had detectable levels of IL-10 in sera and in ocular extracts. In contrast, expression of IL-10 in activated T cells only partially protected from EAU and marginally reduced Ag-specific responses. All IL-10-Tg lines showed suppression of Ag-specific effector cytokines. APC from Tg mice constitutively expressing IL-10 in macrophages exhibited decreased ability to prime naive T cells, however, Ag presentation to already primed T cells was not compromised. Importantly, IL-10-Tg mice that received interphotoreceptor retinoid-binding protein-specific uveitogenic T cells from wild-type donors were protected from EAU. We suggest that constitutively produced endogenous IL-10 ameliorates the development of EAU by suppressing de novo priming of Ag-specific T cells and inhibiting the recruitment and/or function of inflammatory leukocytes, rather than by inhibiting local Ag presentation within the eye.
Uveitis has been reported to affect ∼150,000 persons annually in the U.S. (1), and if untreated can lead to blindness. Experimental autoimmune uveitis (EAU)4 is an autoimmune disease model that represents human uveitis. EAU is induced by immunization of susceptible animals with purified retinal proteins or their fragments (2, 3). EAU is a T cell-mediated disease in which Th1- and Th17-type effector T cells and proinflammatory cytokines play a prominent role (4–7, 31).
The anti-inflammatory cytokine IL-10 inhibits activation and effector function of T cells and monocytes/macrophages. It modulates the expression of cytokines and chemokines that mediate inflammatory responses by recruiting various cell types. It also inhibits the expression of MHC class II and costimulatory molecules on monocytes/macrophages, and down-regulation of these molecules affects the T cell-activating ability of APC. Furthermore, IL-10 plays a key role in differentiation and function of some T regulatory cells, which are important in controlling immune responses and tolerance in vivo (8).
IL-10 regulates many aspects of the immune and inflammatory responses (9), and its critical role in protection against autoimmunity has been highlighted by in vivo studies on animal models. Importantly, IL-10-deficient mice spontaneously develop chronic inflammatory bowel disease. In the experimental autoimmune encephalomyelitis (EAE) model IL-10-deficient mice are more susceptible, while IL-10 transgenic (Tg) mice were more resistant than wild-type (WT) controls (8).
In addition, previous data from our laboratory suggested that endogenous IL-10 regulates EAU susceptibility and may even be a factor in genetic resistance to EAU (10, 11). Interestingly, basal levels of mRNA encoding IL-10 or an IL-10-like molecule correlated with resistance to EAU in a series of rat strains (10). Treatment with rIL-10 ameliorated development of EAU in mice, whereas neutralization of endogenous IL-10 exacerbated EAU scores and delayed disease resolution (11). Importantly, IL-10 is able to inhibit fully differentiated uveitogenic Th1 cells, as represented by a long-term uveitogenic T cell line, which is impervious even to the inhibitory effects of TGF-β (12). Thus, endogenous IL-10 seems to be important in regulating both the induction and the effector phase of EAU.
The present study was designed to examine whether increased endogenous production of IL-10 by targeted overexpression in hematopoietic cells would reduce susceptibility to EAU, and what kind of overexpression is optimal. We compared three strains of mice in which transgenic expression of IL-10 was directed to hematopoietic cells: two strains expressing IL-10 in activated T cells (inducible), one under the human IL-2 promoter/enhancer and the other under the mouse CD2 enhancer and Pμ promoter (IL-2/IL-10 or CD2/IL-10, respectively) (13, 14), and a third strain expressing IL-10 in macrophages (constitutive) under the human CD68 promoter/enhancer (CD68/IL-10) (15). Mice transgenic for IL-10 were partially to completely protected from development of EAU: CD68/IL-10 > CD2/IL-10 > IL-2/IL-10. Further studies demonstrated that not only priming of Ag-specific T cells, but also the development of EAU after the T cells have already been primed, could be inhibited by endogenously produced IL-10. These results support the notion that targeted overexpression of IL-10 may provide a potential therapeutic approach to ocular autoimmunity.
Materials and Methods
Animals
Generation of three lines of IL-10-Tg mice has been published previously (13–15). In this study, we used heterozygous IL-10-Tg mice and their non-Tg littermates on the (C57BL/6 × FVB/N) F1 background bred in-house, except for the experiments in Fig. 2 in which we used homozygous IL-2/IL-10 Tg mice on the C57BL/6 background. OT-II TCR-Tg mice (16) specific for OVA323−339 were purchased from The Jackson Laboratory. Mice were housed under specific pathogen-free conditions and their care and use was in compliance with Institutional guidelines and guidelines of the Association for Research in Vision and Ophthalmology.
FIGURE 2.
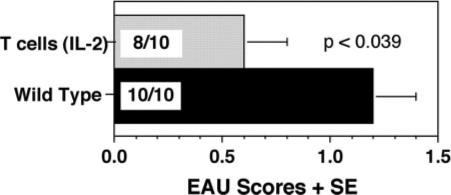
Genetic background and/or gene dose effect of IL-10 in IL-2/IL-10-Tg mice. Homozygous IL-2/IL-10-Tg and WT mice on the C57BL/6 background were immunized with IRBP (150 μg) and injected with PTX (0.5 μg). Eyes were harvested 21 days later. Note significant protection from disease in IL-2/IL-10-Tg mice compared with WT controls. EAU scores are average of two experiments.
Reagents
Interphotoreceptor retinoid-binding protein (IRBP) was prepared from bovine retinas as described (17, 18). IRBP fractions were pooled, dialyzed against PBS, aliquoted, and stored at −80°C until used (2, 17–19). Pertussis toxin (PTX), α-methyl-D-mannopyranoside (α-MMP), and CFA were purchased from Sigma-Aldrich. The anti-CD3 Ab (145−2C11) was purchased from BD Pharmingen. Mycobacterium tuberculosis strain H37RA was from Difco.
Immunization
To induce EAU, mice were immunized s.c. in the thighs and base of the tail with 150 μg IRBP in 200 μl of emulsion with CFA/PBS (1:1, v/v) that had been supplemented with M. tuberculosis to a final concentration of 2.5 mg/ml. PTX (0.2−0.5 μg in 100 μl) was injected i.p. as an additional adjuvant at the time of immunization (2, 19).
Histopathology and EAU scoring
Three wk after immunization, eyes were enucleated, fixed in 4% phosphate buffered glutaraldehyde for 1 h, and stored in 10% phosphate buffered formaldehyde until processing. Tissues were embedded in methacrylate. In brief, 4−6 μm sections were cut through the pupillary-optic nerve plane, and were stained with H&E. Eight to ten sections cut at different planes were examined for each eye in a masked fashion by an ophthalmic pathologist (C.C.C.). The severity of EAU was scored on an arbitrary scale of 0 to 4 in half-point increments, according to a semiquantitative system described earlier (2, 19, 20).
Ag-specific immunological responses
Delayed-type hypersensitivity (DTH) responses to IRBP were evaluated by the ear swelling assay (21). For Ag-specific lymphocyte proliferation and cytokine production, spleen and draining lymph nodes (LNs; inguinal and iliac) of individual mice (4−5 per group) were collected 3 wk after immunization. Cells were pooled within the group and were stimulated with graded concentrations of Ag in triplicate cultures of 0.2 ml, essentially as described (22). Proliferation of LN cells was determined by [3H]thymidine incorporation. Cytokine levels were determined in splenocyte culture supernatants 48 h after Ag-stimulation using the Pierce multiplex Search-Light technology (23).
Flow cytometry
Single cell suspensions were prepared from spleens of naive IL-10-Tg and non-Tg mice. RBC were lysed in ACK lysing buffer (Invitrogen Life Technologies). Fc receptors were blocked with anti-CD16/32 Ab (clone 2.4G2) and cells were stained with the following anti-mouse Abs: FITC-H-2q (KH114), PE-H-2Kb (AF6−88.5), Biotin-I-Aq (KH116), FITC-I-Ab (AF6−120.1), PerCP-Cy5.5-CD45R/B220 (RA3−6B2), allophycocyanin-CD3 (145−2C11), and PE-Streptavidin (BD Biosciences). Cells were acquired on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences), and data were analyzed using FlowJo software (TreeStar).
Spontaneous and anti-CD3-driven IL-10 production
Splenocytes from naive IL-10-Tg and non-Tg mice were harvested and processed into single cell suspensions. Cells were cultured in 96-well flat-bottom plates at 0.5 × 106 cells/200 μl/well with or without anti-CD3 Ab (1 μg/ml). Supernatants were collected after 48 h and IL-10 levels were measured by ELISA (R&D Systems).
Assay for in vitro Ag-specific T cell priming
Spleens were collected from naive OT-II mice were processed into single cell suspensions. T cells were isolated using anti-CD90 microbeads and AutoMACS (Miltenyi Biotec). The purity of the T cells was 94%, as analyzed by flow cytometry with FITC-anti-CD3 and PE-anti-CD4 Abs (BD Biosciences, data not shown). T cells (5 × 104/well) were stimulated with graded concentrations (0−20 μg/ml) of OVA323−339 peptide (Peptides International) in the presence of splenocytes irradiated at 3000 Rads from naive CD68/IL-10-Tg mice or their WT littermates.
Ag presentation to effector T cells
For in vivo Ag presentation assay by the local adoptive transfer of IRBP-specific T cells into the ear pinna, WT mice were immunized with 150 μg IRBP in CFA and 0.2 μg PTX. After 10 days, T cells from draining LNs and spleens were purified by negative selection using T cell enrichment columns (R&D Systems). Peritoneal exudate cells (PEC) were induced in naive CD68/IL-10 or WT mice by i.p. injection of 3% thioglycollate. Three days later, PEC were harvested by peritoneal lavage and pulsed (or not) with 100 μg/ml IRBP for 18−22 h in the presence of 2 mg/ml α-MMP in RPMI 1640 medium containing 1% normal mouse serum. Cell suspensions of 106 purified T cells and 5 × 105 PEC in 10 μl were injected into the ear pinna (right ear, Ag-pulsed; left ear, unpulsed) of syngeneic WT mice. Ear thickness was measured before injection and 48 h after injection. For in vitro Ag presentation to primed T cells, T cells from IRBP-immunized WT mice were purified using T cell enrichment columns, and were stimulated with IRBP in the presence of irradiated splenic APC from naive CD68/IL-10 Tg or WT mice. Proliferation was analyzed as described above.
Adoptive transfer of uveitogenic T cells
Donor WT mice were immunized with 150 μg IRBP in CFA and 0.2 μg of PTX. After 12−14 days, draining LNs and spleens were harvested, pooled, and single cell suspension was prepared. T cells were purified using T cell enrichment columns and were stimulated with 30 μg/ml IRBP in DMEM supplemented with 1% normal mouse serum and 2 mg/ml α-MMP in the presence of irradiated splenocytes from naive WT mice for 72 h. Cells were washed and counted, and 10 × 106 cells were injected i.p. into recipient mice. Animals were monitored for development of disease by funduscopy. On day 14, 1 wk after the onset of the disease, eyes were harvested for the evaluation of EAU by histopathology as described above.
Preparation of ocular extracts
Eyes from naive or immunized mice (day 21) were collected. After removal of the lenses, four eyes from each genotype were pooled and minced into small pieces in 200 μl PBS with proteinase inhibitor mixture (Calbiochem). The tissues were briefly sonicated. All procedures were conducted on ice. The soluble fraction was collected after high-speed centrifugation and stored at −80°C for cytokine assay by multiplex ELISA.
Statistical analysis, reproducibility, and data presentation
Experiments were repeated at least twice. Response patterns were highly reproducible. Disease severity for each animal was calculated as average of both eyes. Statistical analysis of EAU scores was performed by frequency analysis, using Snedecor and Cochran's test for linear trend in proportions (24). Analyses of DTH, lymphocyte proliferation, and cytokine levels were performed by independent t test. Probability values ≤0.05 were considered statistically significant (*) and ≤0.005 were considered highly significant (#).
Results
IL-10-Tg mice are less susceptible to EAU induction
To examine the effect of endogenous overexpression of IL-10 on EAU susceptibility, three different strains of IL-10-Tg mice were used: two strains in which IL-10 is inducibly expressed in activated T cells under control of the IL-2 promoter/enhancer (13) or the Pμ promoter/CD2 enhancer (14) (IL-2/IL-10 or CD2/IL-10, respectively), and the third strain constitutively expressing IL-10 in macrophages under control of the CD68 promoter/enhancer (15) (CD68/IL-10). The IL-2/IL-10 strain was on the C57BL/6 background, whereas CD2/IL-10 and CD68/IL-10 strains were on the FVB/N background, which carries the rd gene and lacks the photoreceptor layer in eye that is the target of EAU. To bring all the strains onto the same genetic background, and to correct the lack of target tissue in FVB/N, we crossed the C57BL/6 Tg with FVB/N WT and vice versa, and used (C57BL/6 × FVB/N) F1 heterozygous Tg and WT mice. Mice were immunized with IRBP using the standard uveitogenic protocol described in Materials and Methods. All three strains of IL-10-Tg mice exhibited lower EAU scores than WT controls. Histopathology of IRBP immunized IL-10-Tg mice compared with WT mice is shown in Fig. 1a. The strongest protection was observed in CD68/IL-10 mice and intermediate in CD2/IL-10 mice. Protection in IL-2/IL-10 mice usually did not attain statistical significance (Fig. 1b). However, in contrast to the heterozygous F1 IL-2/IL-10 mice, homozygous C57BL/6 IL-2/IL-10 animals were significantly protected from EAU (Fig. 2), suggesting presence of a gene-dose effect of IL-10, and/or possible modifying effects of genetic background differences.
FIGURE 1.
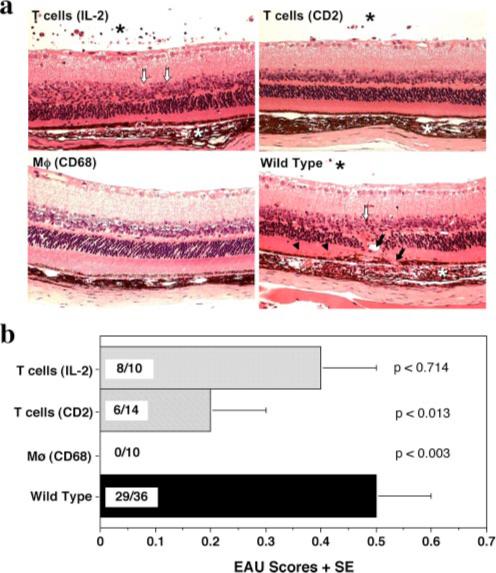
Abrogation of EAU in IL-10-Tg mice. IL-10-Tg and non-Tg mice on the (C57BL/6 × FVB/N) F1 background were immunized with 150 μg of IRBP and injected with 0.5 μg of PTX. Eyes were harvested 3 wk later. a, EAU histopathology in IL-10-Tg and non-Tg littermates. Note normal appearance of retina in CD68/IL-10-Tg mouse vs inflammatory cells in choroid and vitreous (*), photoreceptor outer segment shortening and nuclear layer disorganization (open arrows), choroidal neovascularization (arrows) and subretinal hemorrhages (arrowheads) in non-Tg, IL-2/IL-10- or CD2/IL-10-Tg mouse. b, EAU scores. The average + SE is shown as the EAU score within a group. Incidence is shown within each bar as EAU positive/total mice in the group. These data are a composite of at least two experiments. p values compared with WT are indicated in the graph.
Ag-specific immunological responses are decreased in IL-10-Tg mice
We examined the effect of IL-10 overexpression on Ag-specific adaptive immune responses in IL-10 Tg and WT mice 21 days after immunization with a uveitogenic protocol of IRBP in CFA. The DTH response of mice challenged with IRBP into the ear pinna 48 h earlier was assessed, and LN and spleen cells were collected for analysis of proliferation and Ag-specific cytokine production. Compared with non-Tg controls, all three IL-10-Tg strains, including the IL-2/IL-10, displayed a significantly reduced DTH to IRBP with the same hierarchy as that observed in protection from EAU: CD68/IL-10 > CD2/IL-10 > IL-2/IL-10 (Fig. 3a). Ag-specific proliferation of cells from draining LNs (inguinal and iliac) was slightly reduced at all concentrations of IRBP. The reduction was statistically significant only at 10 μg/ml in IL-2/ IL-10 and CD2/IL-10 strains, while it was statistically significant at all concentrations in CD68/IL-10 strains (Fig. 3b). Cytokine profiles of splenocytes from the same IL-10-Tg mice showed reduced levels of Ag-specific secretion of IFN-γ, TNF-α, IL-12, IL-4, IL-5, IL-6, and IL-17 (Fig. 3c). In contrast, IL-10 content in supernatants was increased compared with non-Tg controls (Fig. 3c). The most prominent effect was observed in CD68/IL-10-Tg mice.
FIGURE 3.
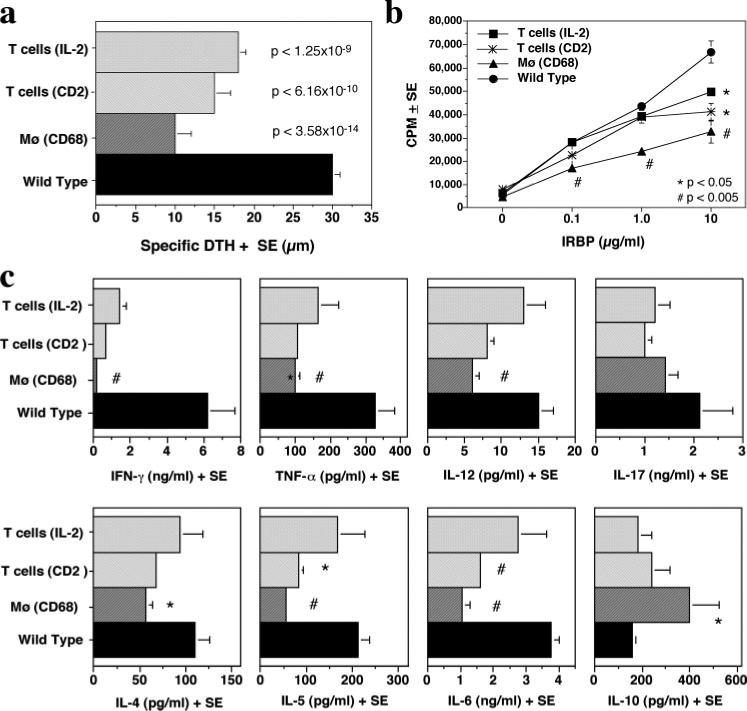
Ag-specific immunological responses in IL-10-Tg mice. a, DTH response. Mice immunized with IRBP and PTX were ear challenged on day 19 with 10 μg IRBP in PBS, and the ear swelling was measured 48 h later. The data are a composite of two experiments. IL-2/IL-10, n = 10; CD2/IL-10, n = 9; CD68/IL-10, n = 10; WT, n = 30. p values compared with WT are indicated in the graph. b, LN cell proliferation. Draining LNs collected on day 21 after immunization were pooled within each group. Cells were stimulated with graded concentrations of IRBP (0−10 μg/ml) in triplicates and were pulsed with [3H]thymidine for the last 18 h of the 60 h culture. Proliferation is represented as mean count per minutes (cpm) ± SE. The data are from a representative experiment of two. c, Agspecific cytokine profiles. Supernatants were collected from 48 h cultures of splenocytes stimulated with 10 μg/ml IRBP and were assayed for the indicated cytokines. Shown is a representative experiment of two. Statistically significant differences compared with WT are indicated as *, significant (p < 0.05) or #, highly significant (p < 0.005).
MHC expression is not altered in the (FVB/N × B6) F1 mice
MHC class II is a susceptibility locus for EAU (2). Because IL-10 Tg mice were F1 progeny of C57BL/6 × FVB/N, these mice were expected to express both H-2b and H-2q MHC molecules on the surface of APC. To exclude the possibility of uneven expression of the parental MHC molecules that could affect expression of disease independently of IL-10, we tested MHC class I and class II expression on the surface of splenocytes from IL-10-Tg mice and WT controls by flow cytometry. MHC class I and class II expression was equivalent in all the F1 strains irrespective of their IL-10-Tg genotype and was slightly lower than that of the parental strains (Fig. 4, see overlaid histograms). However, this is unlikely to affect the comparability of the data because the F1 strains are being compared among themselves. Proportions of T cells and B cells in spleens of IL-10-Tg mice were similar to non-Tg controls (data not shown).
FIGURE 4.
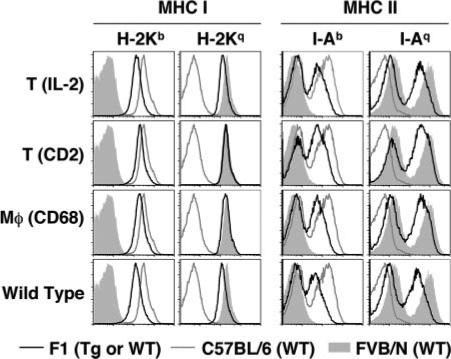
MHC class I and II staining of splenocytes from IL-10 Tg and non-Tg mice. Cells were gated on the lymphocyte population. MHC class I (H-2Kb and H-2Kq) or MHC class II (I-Ab and I-Aq) levels are shown as histograms (black line) overlaid to WT FVB/N (filled histogram) or C57BL/6 (gray line) parental strains. Shown is a representative experiment of two, each using two randomly selected individuals per genotype.
Spontaneous and anti-CD3-induced IL-10 production
To examine the basal and stimulated levels of IL-10 as an indication of the milieu in which priming of T cells takes place in IL-10-Tg mice, we cultured naive splenocytes (0.5 × 106/well) from IL-10-Tg mice and WT controls with or without soluble anti-CD3 Ab (1.0 μg/ml). When cells were incubated for 48 h in complete culture medium without any stimulants, low levels of IL-10 were detected in supernatants from spleens of IL-2/IL-10-Tg, CD2/IL-10-Tg, and their WT controls, which hovered around the detection limit of the ELISA (10 pg/ml). In contrast, significantly higher levels of IL-10 were detected in the supernatants of CD68/IL-10-Tg splenocytes (Fig. 5a). In splenocytes incubated in the presence of soluble anti-CD3 Ab, the levels of IL-10 in IL-2/IL-10-Tg and CD68/IL-10-Tg mice were only slightly increased over WT controls. In contrast, IL-10 production by splenocytes of CD2/IL-10-Tg mice was 3−4 times higher than WT or other genotypes (Fig. 5b). Thus, the highest constitutive IL-10 production was detected in the CD68/IL-10 mice, whereas the highest induced IL-10 production was observed in CD2/IL-10 mice. It should be mentioned that we previously observed that the CD2/Pμ promoter/enhancer combination in CD2/IL-10-Tg mice is very robust and can surpass the IL-2/IL-2 or CD68/CD68 promoter/enhancer combinations in producing IL-10 once these T cells are activated (P. Murray, unpublished observations).
FIGURE 5.
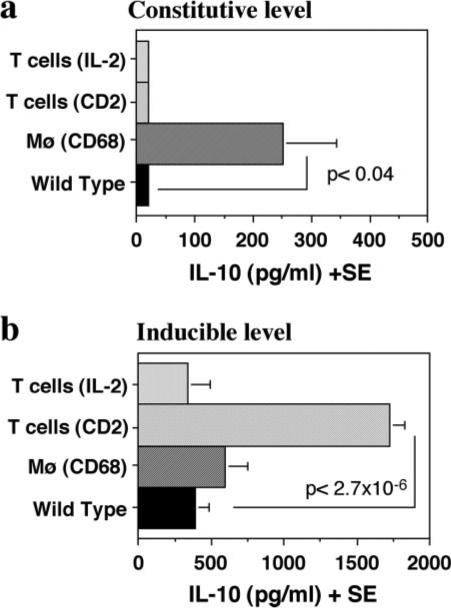
Spontaneous vs induced levels of IL-10. Splenocytes from naive IL-10-Tg and non-Tg mice were cultured with or without soluble anti-CD3 Ab and supernatants were collected at 48 h to measure IL-10 levels. The data are average of three separate experiments. Statistical significance is calculated using independent t test. a, Spontaneous levels of IL-10. Supernatants from unstimulated cultures were used. b, Induced levels of IL-10. Supernatants from anti-CD3-stimulated culture (1.0 μg/ml) were used. Statistically significant differences compared with WT controls are indicated.
Ocular and systemic IL-10 expression is elevated in CD68/IL-10-Tg mice
To further investigate the correlation of systemic and local IL-10 levels in vivo with the Ag-specific immunological responses in EAU, IL-10 levels in sera and in ocular extracts of IL-10-Tg mice were examined. Constitutive expression of IL-10 in macrophages resulted in elevated basal IL-10 levels systemically, as measured in the serum, and locally, as measured in the eyes (Fig. 6a). In contrast, both ocular and systemic levels of IL-10 in naive IL-2/IL-10-or CD2/IL-10-Tg mice were comparable to those in WT mice (Fig. 6a). However, when mice were immunized to induce EAU, the ocular and systemic levels of IL-10 were increased in CD2/IL-10-Tg mice (Fig. 6b), in agreement with the enhanced IL-10 production in splenocytes upon anti-CD3 stimulation (Fig. 5b). The ocular and systemic IL-10 levels of CD68/IL-10-Tg mice after immunization were also elevated compared with those of WT mice (Fig. 6b), likely as a result of activation of monocyte-macrophages by inflammatory mediators. In contrast, proinflammatory cytokines including IL-1β and IL-6 were high in eyes of WT mice that developed EAU compared with eyes of EAU-protected CD68/IL-10 mice (data not shown).
FIGURE 6.
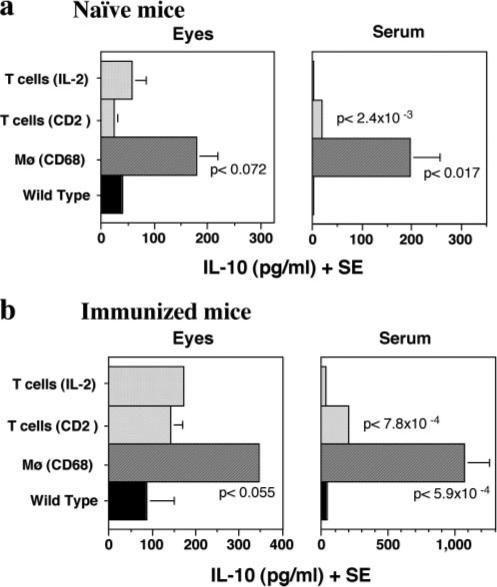
IL-10 levels in ocular extracts and sera of naive and immunized IL-10-Tg mice. a, IL-10 levels in naive mice. b, IL-10 levels in IRBP-immunized mice on day 21 post immunization. Data are a composite of at least two independent experiments. p values compared with WT controls are indicated in the graphs.
The IL-10-Tg environment inhibits effector mechanisms of disease
Because CD68/IL-10 Tg mice exhibited a high basal intraocular level of IL-10, we investigated whether transgenic expression of IL-10 would affect effector mechanisms of disease, where T cells primed in the periphery must infiltrate the eye and orchestrate the sequence of events that culminates in clinical uveitis. For this, we used adoptive transfer of effector T cells, that had been primed in a normal milieu, into IL-10 Tg recipients. Uveitogenic effector T cells prepared from IRBP-immunized WT mice were activated for 3 days in vitro with IRBP and were adoptively transferred into naive IL-10-Tg or WT recipients. EAU scores were determined by histopathology at day 14 posttransfer. All three strains of IL-10-Tg mice were significantly protected from EAU compared with WT recipients (Fig. 7). These data indicate that despite the presence of already primed uveitogenic effector T cells, endogenously produced IL-10 plays a suppressive role in development of EAU.
FIGURE 7.
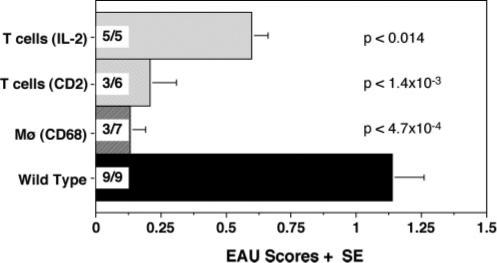
Adoptive transfer of uveitogenic effector T cells into IL-10-Tg mice. Donor WT (C57BL/6 × FVB/N) F1 mice were immunized with 150 μg IRBP and 0.2 μg PTX. Two weeks later, cells from spleens and draining lymph nodes were harvested and stimulated in vitro for 72 h with 30 μg/ml IRBP in the presence of irradiated WT splenocytes. Activated T cells were injected i.p. into IL-10-Tg or WT mice. Eyes were collected histopathological evaluation of EAU 14 days after transfer. Incidence is shown within each bar as EAU positive/total mice in the group. Data are a composite of two experiments. Statistical significances are shown in the graph.
The CD68/IL-10-Tg environment causes reduced T cell priming but does not affect Ag presentation to primed T cells
To address the mechanism by which high endogenous levels of IL-10 prevent both the induction, and the expression of EAU, we examined the effects of the IL-10 Tg environment on effector T cell priming and function. Of the three IL-10-Tg strains, only the CD68/IL-10 mice express IL-10 in cells that potentially can serve as APC. Therefore, we tested the ability of APC from these mice to prime naive T cells from OT-II mice, that express a TCR specific for OVA peptide 323−339. Purified T cells from naive OT-II mice were stimulated with OVA323−339 in the presence of irradiated splenocytes from IL-10-Tg or WT mice. The data showed a significantly reduced proliferative response to OVA323−339 presented by APC from CD68/IL-10 mice compared with those from WT controls at the concentration of 0.2 μg/ml (Fig. 8a). However, at higher OVA323−339 concentrations, proliferation of T cells with CD68/IL-10-Tg APC reached the same level to that with WT APC, indicating that impaired priming caused by endogenous IL-10 production could be overcome by an increased level of TCR occupancy.
FIGURE 8.
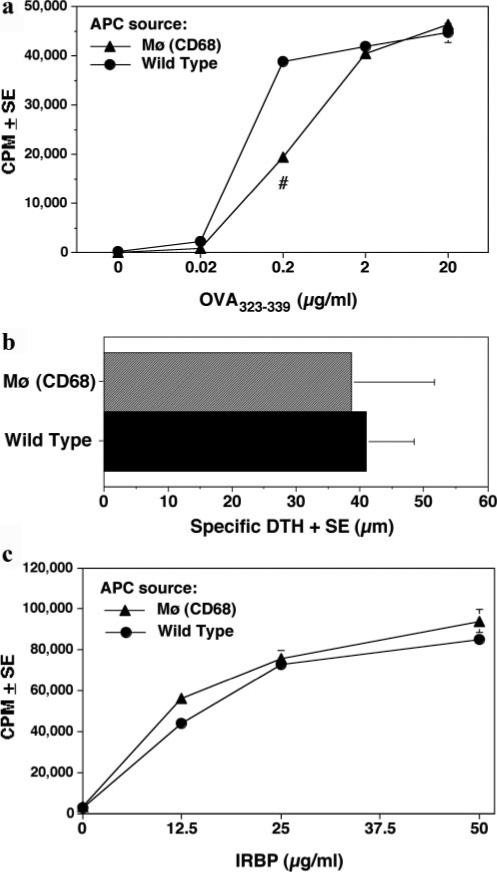
Ag presenting function of IL-10 Tg APC to naive and primed T cells. a, Naive T cells from OT-II mice were stimulated in the presence of APC from IL-10-Tg or WT F1 mice with various concentrations of OVA peptide. Cells were incubated for 60 h and were pulsed with [3H]thymidine for the last 18 h. Proliferation is represented as mean cpm ± SE. #, p < ( 0.005. b, Local adoptive transfer of WT T cells and IRBP-pulsed APC (PEC) from CD68/IL-10-Tg or WT mice 48 h posttransfer (n = 5 per group). c, In vitro proliferation of T cells from IRBP-immunized WT mice to various concentrations of IRBP (0−50 μg/ml) in the presence of irradiated APC from spleens of either naive CD68/IL-10-Tg or WT mice. Proliferation assay was done as in a. All data are representative of three independent experiments. No statistical significance in b or c.
Expression of EAU requires local Ag recognition by effector T cells that infiltrate the eye (25). Compared with naive T cells, Ag-experienced T cells have a lower threshold for activation. To address how efficiently CD68/IL-10 APC present the Ag to T cells that have already been primed, we performed two assays: an in vivo local adoptive transfer assay, and an in vitro proliferation recall response. For the in vivo local adoptive transfer assay, IRBP-primed WT T cells were mixed with IRBP-pulsed (or unpulsed) APC from either CD68/IL-10-Tg mice or WT controls, and were injected into the ear pinna of WT recipients. As shown in Fig. 8b, the specific DTH at 48 h following the local adoptive transfer was not significantly different between CD68/IL-10 APC and WT APC recipients. For the in vitro recall proliferation assay, T cells from IRBP-primed WT donors were stimulated with IRBP presented by either CD68/IL-10 APC or WT APC. As in the in vivo assay, the primed T cells responded similarly to IRBP presented by either CD68/IL-10 APC or WT APC (Fig. 8c). Together, these results support the conclusion that under the conditions used in this study, IL-10 Tg APC can productively present Ag to primed T cells.
Discussion
In the present study, we demonstrate that transgenically expressed IL-10 can decrease susceptibility to EAU by interfering with priming, and moreover, can inhibit the ability of already primed effector T cells, that were generated in a non-Tg donor, to elicit disease in an IL-10-Tg host. The mechanisms of protection operate at the afferent level, through inhibition of de novo priming of uveitogenic effectors, and at the efferent level, most likely through inhibition of inflammatory cell recruitment and/or function.
The present study confirms and extends our previous observations on the role of systemic IL-10 in EAU (11). Systemic administration of rIL-10 during the first week after uveitogenic immunization inhibits induction of EAU and lowers effector responses, without skewing the Th1/Th2 effector response. Similarly to the situation in constitutively expressing CD68/IL-10 Tg mice, this is most likely an afferent effect due to inhibition of T cell priming, as IL-10 inhibits APC function (26). In the Tg mice where IL-10 is expressed in T cells, IL-10 production can only occur after that T cells have already been activated, so that inhibition sets in at a later phase. This might in part explain the more efficient protection in CD68/IL-10-Tg mice, where IL-10 is expressed constitutively. A second mechanism appears to operate at the efferent stage of the disease. Neutralization of endogenous IL-10 in WT mice prevents resolution of EAU (11). IL-10 Tg mice that have high levels of endogenous IL-10 are resistant to EAU induced by T cells that have been primed in a normal milieu, i.e., the IL-10 Tg environment is able to inhibit disease-relevant effector function(s). This could involve elevated systemic as well as local IL-10. The target cells of such inhibition could be macrophages themselves as well as T cells (27, 28). Both cell types are major components of the inflammatory cell infiltrate recruited into the eye during EAU (20).
Although the respective contributions to protection from EAU of systemic IL-10 from local IL-10 production in vivo cannot be clearly dissected, a suppressive effect on the disease by locally produced IL-10 is supported by two prior studies. De Kozak et al. applied ocular gene therapy with live cells or an adeno-associated virus construct engineered to express IL-10 (29, 30). Such forced local expression of IL-10 protects mice from EAU and is likely to represent local inhibitory effects on effector cells entering the ocular environment. Interestingly, our earlier study shows that basal expression of IL-10 or an IL-10-like molecule in the eye of five strains correlates with their resistance to EAU (10). These data suggest bioeffectiveness of locally present IL-10 in regulating development of the disease.
What might be the local mechanisms by which EAU is decreased in the IL-10-Tg recipients that received uveitogenic effector T cells? In our previous publication, we showed that infiltration of inflammatory cells into the eye following adoptive transfer of (fluorescently labeled) activated uveitogenic T cells is bi-phasic: in the first 24 h, small numbers of uveitogenic T cells enter the eye, which are only detectable by examining the entire isolated retina (25). Recognition of Ag in situ is thought to take place at that time. The second phase, which follows 48 h later, involves massive infiltration of recruited host cells and induction of pathology (25). Although CD68/IL-10 Tg mice express basal levels of IL-10 locally in the eyes, our data suggest that local Ag recognition may not be affected. However, we have not excluded effects on functions other than proliferation that may be involved in pathogenesis. We hypothesize that initial IL-10-producing macrophages recruited into the eye from the circulation produce more local IL-10 and prevent development of disease. In this scenario, the inflammation appears to be arrested early, when obvious pathology is not detectable. Processes potentially affected could be activation of vascular endothelium causing reduced recruitment of inflammatory leukocytes from the circulation as well as inhibition of the function of any recruited leukocytes.
The mechanisms leading to the differences in EAU susceptibility in the different IL-10-Tg strains are likely to be as complex as are the cellular interactions involved in the process of induction and expression of disease, and additional studies are needed to unravel them fully. Nevertheless, the present data demonstrate the ability of endogenously produced IL-10 not only to reduce effector T cell priming but also to control disease at an efferent stage. Our study may thus support a rationale for using IL-10 gene transfer into hematopoietic cells as a therapeutic approach to uveitis.
Acknowledgments
We thank Dr. I. Suffia for helpful comments and help with experimental procedures, L. Xu for technical assistance, and the NEI Histology Core Facility for processing the histology slides.
Footnotes
This study was supported by National Institutes of Health intramural funding.
Abbreviations used in this paper: EAU, experimental autoimmune uveitis; EAE, experimental autoimmune encephalomyelitis; Tg, transgenic; WT, wild type; IRBP, interphotoreceptor retinoid-binding protein; PTX, pertussis toxin; α-MMP, α-methyl-D-mannopyranoside; LN, lymph node; DTH, delayed-type hypersensitivity; PEC, peritoneal exudate cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol. Med. 2004;102:395–419. doi: 10.1385/1-59259-805-6:395. [DOI] [PubMed] [Google Scholar]
- 3.Gery I, Nussenblatt RB, Chan CC, Caspi RR. The Molecular Pathology of Autoimmune Diseases. Taylor and Francis; New York, NY: 2002. Autoimmune diseases of the eye. pp. 978–998. [Google Scholar]
- 4.Caspi RR. Mechanisms underlying autoimmune uveitis. Drug Discov. Today: Dis. Mech. 2006;3:199–206. [Google Scholar]
- 5.Tang J, Zhu W, Silver PB, Su SB, Chan CC, Caspi RR. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J. Immunol. 2007;178:5578–5587. doi: 10.4049/jimmunol.178.9.5578. [DOI] [PubMed] [Google Scholar]
- 6.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 7.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest. Ophthalmol. Visual Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 9.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Sun B, Sun SH, Chan CC, Caspi RR. Evaluation of in vivo cytokine expression in EAU-susceptible and resistant rats: a role for IL-10 in resistance? Exp. Eye Res. 2000;70:493–502. doi: 10.1006/exer.1999.0808. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo LV, Xu H, Chan CC, Wiggert B, Caspi RR. IL-10 has a protective role in experimental autoimmune uveoretinitis. Int. Immunol. 1998;10:807–814. doi: 10.1093/intimm/10.6.807. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Silver PB, Tarrant TK, Chan CC, Caspi RR. TGF-β inhibits activation and uveitogenicity of primary but not of fully polarized retinal antigen-specific memory-effector T cells. Invest. Ophthalmol. Visual Sci. 2003;44:4805–4812. doi: 10.1167/iovs.02-0843. [DOI] [PubMed] [Google Scholar]
- 13.Hagenbaugh A, Sharma S, Dubinett SM, Wei SH, Aranda R, Cheroutre H, Fowell DJ, Binder S, Tsao B, Locksley RM, et al. Altered immune responses in interleukin 10 transgenic mice. J. Exp. Med. 1997;185:2101–2110. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J. Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 15.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J. Immunol. 2002;168:3402–3411. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 16.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 17.Pepperberg DR, Okajima TL, Ripps H, Chader GJ, Wiggert B. Functional properties of interphotoreceptor retinoid-binding protein. Photochem. Photobiol. 1991;54:1057–1060. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 18.Pepperberg DR, Okajima TL, Wiggert B, Ripps H, Crouch RK, Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP): molecular biology and physiological role in the visual cycle of rhodopsin. Mol. Neurobiol. 1993;7:61–85. doi: 10.1007/BF02780609. [DOI] [PubMed] [Google Scholar]
- 19.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevech EM, Strober W, editors. Current Protocols in Immunology. John Wiley and Sons; New York: 2003. p. 15.16. [DOI] [PubMed] [Google Scholar]
- 20.Chan CC, Caspi RR, Ni M, Leake WC, Wiggert B, Chader GJ, Nussenblatt RB. Pathology of experimental autoimmune uveoretinitis in mice. J. Autoimmun. 1990;3:247–255. doi: 10.1016/0896-8411(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal RK, Chan CC, Wiggert B, Caspi RR. Pregnancy ameliorates induction and expression of experimental autoimmune uveitis. J. Immunol. 1999;162:2648–2654. [PubMed] [Google Scholar]
- 22.Bagenstose LM, Agarwal RK, Silver PB, Harlan DM, Hoffmann SC, Kampen RL, Chan CC, Caspi RR. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J. Immunol. 2005;175:124–130. doi: 10.4049/jimmunol.175.1.124. [DOI] [PubMed] [Google Scholar]
- 23.Moody MD, Van Arsdell SW, Murphy KP, Orencole SF, Burns C. Array-based ELISAs for high-throughput analysis of human cytokines. BioTechniques. 2001;31:186–190. 192–184. doi: 10.2144/01311dd03. [DOI] [PubMed] [Google Scholar]
- 24.W Snedecor, G., Cochran WG. Statistical Methods. Iowa State University Press; Ames, IA: 1967. [Google Scholar]
- 25.A. Prendergast, R., Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest. Ophthalmol. Visual Sci. 1998;39:754–762. [PubMed] [Google Scholar]
- 26.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 27.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 28.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells: specific inhibition of IL-2 production and proliferation. J. Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 29.Verwaerde C, Naud MC, Delanoye A, Wood M, Thillaye-Goldenberg B, Auriault C, de Kozak Y. Ocular transfer of retinal glial cells transduced ex vivo with adenovirus expressing viral IL-10 or CTLA4-Ig inhibits experimental autoimmune uveoretinitis. Gene Ther. 2003;10:1970–1981. doi: 10.1038/sj.gt.3302101. [DOI] [PubMed] [Google Scholar]
- 30.Smith JR, Verwaerde C, Rolling F, Naud MC, Delanoye A, Thillaye-Goldenberg B, Apparailly F, de Kozak Y. Tetracyclineinducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum. Gene Ther. 2005;16:1037–1046. doi: 10.1089/hum.2005.16.1037. [DOI] [PubMed] [Google Scholar]
- 31.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman E, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 2008 doi: 10.1084/jem.20071258. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


