SUMMARY
Mutations in human Retinol Dehydrogenase 12 (RDH12) are known to cause photoreceptor cell death but the physiological function of RDH12 in photoreceptors remains poorly understood. In vitro, RDH12 recognizes both retinoids and medium-chain aldehydes as substrates. Our previous study suggested that RDH12 protects cells against toxic levels of retinaldehyde and retinoic acid [Lee et al., J. Biol. Chem. 282 (2007) 35621–35628]. Here, we investigated whether RDH12 can also protect cells against highly reactive medium-chain aldehydes. Analysis of cell survival demonstrated that RDH12 was protective against nonanal but not against 4-hydroxynonenal. At high concentrations, nonanal inhibited the activity of RDH12 towards retinaldehyde, suggesting that nonanal was metabolized by RDH12. 4-Hydroxynonenal did not inhibit the RDH12 retinaldehyde reductase activity, but it strongly inhibited the activities of lecithin:retinol acyl transferase and aldehyde dehydrogenase, resulting in decreased levels of retinyl esters and retinoic acid and accumulation of unesterified retinol. Thus, the results of this study showed that RDH12 is more effective in protection against retinaldehyde than against medium-chain aldehydes, and that medium-chain aldehydes, especially 4-hydroxynonenal, severely disrupt cellular retinoid homeostasis. Together, these findings provide a new insight into the effects of lipid peroxidation products and the impact of oxidative stress on retinoid metabolism.
Keywords: retinoic acid, retinaldehyde, retinol, oxidoreductase, nonanal, 4-hydroxynonenal
INTRODUCTION
Human Retinol Dehydrogenase 12 (RDH12) is an NADP+-dependent oxidoreductase that acts exclusively in the reductive direction in the cells [1]. Kinetic analysis suggests that RDH12 recognizes retinaldehyde as well as medium-chain aldehydes as substrates [2]. Photoreceptors, which are the main site of RDH12 expression in human tissues [2], contain high levels of both types of substrates for RDH12. All-trans-retinaldehyde is produced by photoisomerization of 11-cis-retinaldehyde covalently bound to rod and cone opsins and can theoretically reach concentrations as high as 3 mM depending on ambient light intensity [3]. Photoreceptors are also extremely rich in unsaturated docosahexaenoic acid (22:6, n - 3) [4, 5], which can readily undergo peroxidic oxidation to medium-chain aldehydes. Under the conditions of constant illumination and high oxygen pressure, concentrations of these aldehydes in photoreceptors can reach millimolar range [6].
Both medium-chain aldehydes and retinoids exert potent biological activities that can impact photoreceptor viability. For example, one of the most abundant and toxic medium-chain aldehydes, 4-hydroxynonenal (4-HNE), combines spontaneously with glutathione and with cysteine, histidine, and lysine residues in cellular proteins, causing a variety of cytotoxic and genotoxic effects [7]. High levels of all-trans-retinaldehyde are also cytotoxic and, in addition, can give rise to bioactive all-trans-retinoic acid [8], which was shown to accelerate photoreceptor cell death by apoptosis [9, 10].
Mutations in RDH12 have been linked to severe early onset macular degeneration [11–14], but the molecular basis of photoreceptor cell death in patients carrying mutations in RDH12 has not yet been resolved. Our recent study suggested that RDH12 may function to protect photoreceptors against toxic levels of all-trans-retinaldehyde and all-trans-retinoic acid [1]. Whether RDH12 can also play a role in protection of cells against medium-chain aldehydes remains unknown. Here, we investigated the contribution of RDH12 to cell protection against medium chain aldehydes and examined the effects of medium chain aldehydes on RDH12 retinaldehyde reductase activity and retinoid metabolism. The results of this study shed new light on the function of RDH12 and the impact of lipid peroxidation products on the overall retinoid homeostasis.
MATERIALS AND METHODS
Cell Viability Assay
HEK293 cells seeded in a 24-well plate were transiently transfected with RDH12/pIRESneo or empty pIRESneo plasmid (Clontech, Mountain View, CA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Construction of RDH12/pIRESneo vector and Western blot analysis of RDH12 expression in the cells was described previously [1]. Twenty-four hours after transfection, fresh media containing various concentrations (0–200 µM) of nonanal or 4-HNE (four-replicate wells for each concentration) was added. Cells were incubated with aldehydes for indicated time intervals. Cell viability was assessed using a modified MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide) (Sigma-Aldrich) assay [15]. Medium containing 0.5 mg/ml MTT solution was added to each well and plates were incubated at 37 °C for 2 h. The medium was decanted, and the reaction was stopped by the addition of 0.5 ml dimethyl sulfoxide. Plates were incubated at 37 °C for several minutes, and 0.2 ml of the solubilized formazan solution was transferred to a 96-well plate. The absorbance was read at 595 nm using an ELISA plate reader.
Analysis of RDH12 Activity in HEK293 Cells
HEK293 cells plated in 35 mm dishes were transiently transfected with RDH12/pIRESneo or empty pIRESneo plasmid as described above. Two days after transfection, the cells were incubated with 10 µM all-trans-retinaldehyde (Sigma-Aldrich, St. Louis, MO) in the presence of 4-HNE or nonanal (100 or 200 µM). 4-HNE and nonanal were added to cell culture media from ethanol stock solutions; the concentration of ethanol in the media did not exceed 1%. Retinoids were extracted into hexane from media and cells under red light and separated by normal phase high performance liquid chromatography (HPLC) using Waters Alliance Separation Module and 2996 Photodiode Array Detector (Waters Corp., Milford, MA, USA) [2]. Peaks were identified by comparison to retention times of retinoid standards and evaluation of wavelength maxima and quantified as described previously [2].
Inhibition of Lecithin:Retinol Acyltransferase Activity
Human lecithin:retinol acyltransferase (LRAT) was expressed in Sf9 cells as described elsewhere [16] and the microsomal preparation of LRAT was obtained by differential centrifugation [16]. The activity of LRAT in the presence of varied concentrations of 4-HNE or nonanal was determined by monitoring the formation of retinyl esters from all-trans-retinol and dipalmitoylphosphatidylcholine (DPPC). The reactions were carried out in 90 mM KH2PO4 (pH 7.4) and 40 mM KCl at 37 °C for 15 min and stopped by the addition of methanol. Retinoids were extracted twice with 4 ml of hexane and analyzed by normal phase HPLC as described previously [16]. The IC50 value for the inhibition of LRAT activity was calculated using GraFit (Erithacus Software Ltd., Horley, Surrey, U.K.).
RT-PCR Analysis of ALDH1A1 and ALDH1A2 Expression in HEK293 cells
Total RNA was isolated from HEK293 cells using RNeasy Mini Kit (Qiagen Inc. Valencia, CA). One µg of total RNA was used to perform reverse transcription (RT) according to the manufacturer’s protocol (Promega Inc. Madison, WI). The amplification of cDNA was performed for 1 cycle at 94 °C for 4 min followed by 35 cycles at 94 °C for 40 sec, 60 °C for 40 sec, 72 °C for 40 sec and 1 cycle at 72 °C for 10 min. The PCR primers were designed to span an intron according to GenBank sequences of human ALDH1A1 and ALDH1A2, and were as follows: ALDH1A1, forward 5'- CCG CAA GAC AGG CTT TTC AGA -3' and reverse 5'- AAG ATG CCA CGT GGA GAG CAG -3'; ALDH1A2, forward 5'- GTG GAA CGG GAC AGG GCA GT -3' and reverse 5'- CTT TCG GCC CAG TCC TTT GC - 3'. The RT-PCR products were analyzed by agarose (1.5%) gel electrophoresis with ethidium bromide staining. The expected size of PCR product was 429 bp and 856 bp for ALDH1A1 and ALDH1A2, respectively.
Inhibition of Retinaldehyde Dehydrogenase Activity
Purified recombinant human aldehyde dehydrogenase ALDH1A1 and the corresponding cDNA expression vector were a kind gift from Dr. Henry Weiner (Purdue University). The activity of ALDH1A1 in the presence of varied concentrations of nonanal and 4-HNE was determined by monitoring the formation of retinoic acid from all-trans-retinaldehyde using HPLC as described above. The reactions were carried out in 90 mM KH2PO4 (pH 7.4) and 40 mM KCl at 37 °C for 15 min and stopped by the addition of methanol. The IC50 values for inhibition of ALDH1A1 retinaldehyde dehydrogenase activity were calculated using GraFit.
Statistical Analysis
Statistical significance was determined by a two-tailed unpaired Student's t test using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). p<0.05 was considered statistically significant.
RESULTS
To determine whether RDH12 plays a role in cell protection against lipid-derived aldehydes, we selected two medium chain aldehydes, nonanal and 4-HNE, known to be substrates for RDH12 in vitro [2]. HEK293 cells transfected with either RDH12/pIRESneo or empty vector (mock-transfected) were incubated with different concentrations of 4-HNE or nonanal and cell viability was determined using MTT assay. Expression levels of RDH12 in the cells were monitored by Western blot analysis (Fig. 1 A). There was no difference in survival of RDH12-expressing cells versus RDH12-lacking cells upon exposure to 4-HNE (Fig. 1 B). However, a statistically significant (P<0.05) increase in survival of RDH12- expressing cells was observed after incubation of cells with high concentrations (150–200 µM) of nonanal (Fig. 1 C, D). Thus, RDH12 protected the cells against nonanal-induced toxicity but was ineffective against 4-HNE, suggesting that, in living cells, RDH12 was able to metabolize and eliminate nonanal, but not 4-HNE.
Figure 1. Role of RDH12 in protection against nonanal- or 4-HNE-induced cell death.
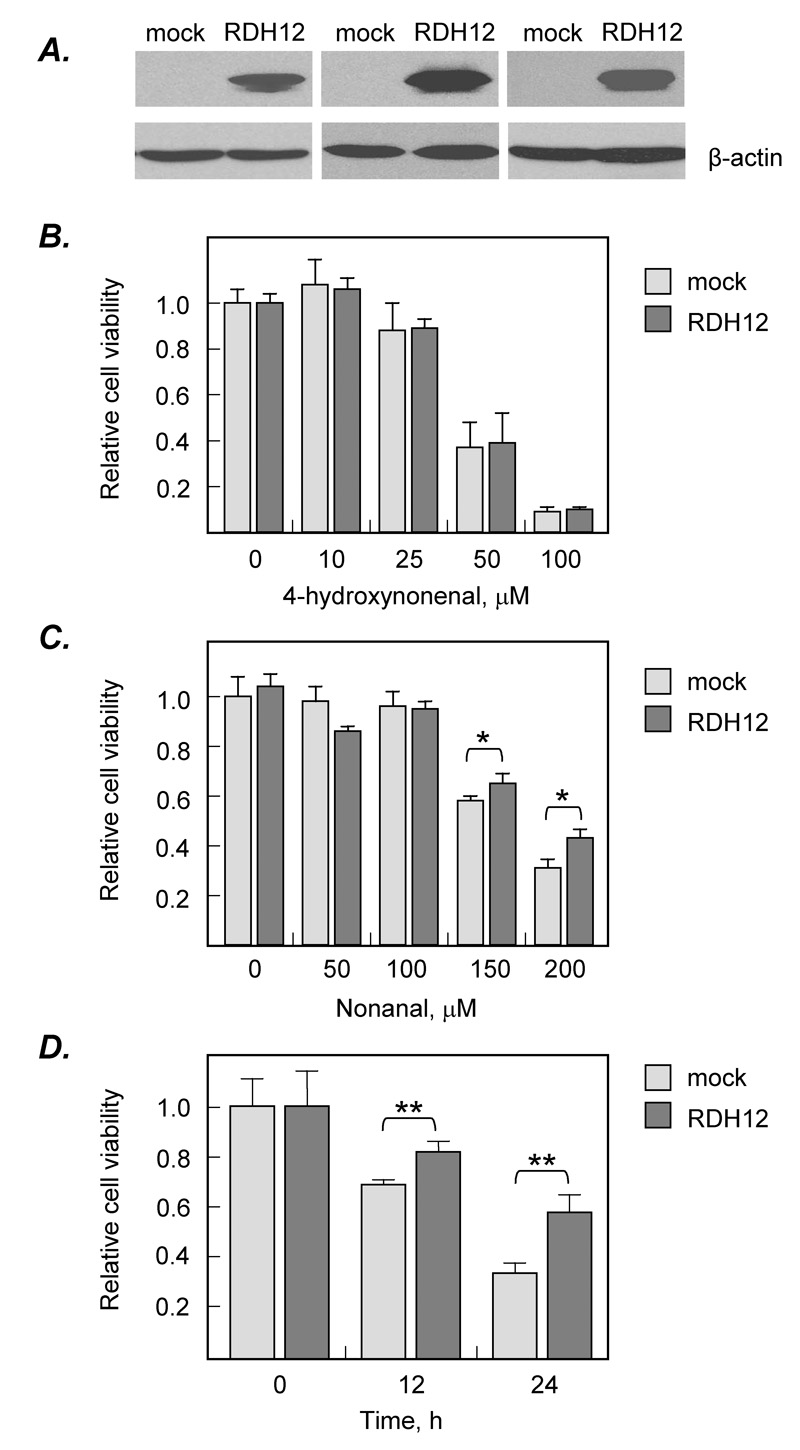
A, Western blot analysis of RDH12 expression. HEK293 cells were transiently transfected with empty vector (mock) or an expression construct for RDH12 (RDH12). RDH12 protein was detected using rabbit polyclonal antibodies against RDH12 (1:10,000 dilution) [1]. The blots were re-stained for β-actin using anti-β-actin monoclonal antibody (Sigma-Aldrich) at a 1:5,000 dilution to control for protein loading.
B–D, MTT assays. RDH12-transfected cells (RDH12) and mock-transfected cells (mock) were incubated with varied concentrations of 4-hydroxynonenal (B) or nonanal (C) for 24 h; or with a fixed concentration of nonanal (200 µM) for different time intervals (D). Cell viability was determined by MTT assay and the optical density (OD) was monitored at 595 nm using ELISA plate reader. Data are mean ± SD of four independent experiments. Statistical analysis was done using two-tailed unpaired Student’s t test comparing RDH12-transfected cells to mock-transfected cells.
To obtain further evidence that nonanal was utilized as a substrate by RDH12 in the cells, we examined the effect of nonanal on RDH12 activity toward retinaldehyde. Nonanal was added to RDH12/pIRESneo-transfected and mock-transfected cells together with retinaldehyde and the products of retinaldehyde metabolism were analyzed by HPLC after extraction from cells and medium. The presence of nonanal in cell culture medium resulted in significantly decreased production of retinol from retinaldehyde in the cells expressing RDH12 (1.5-fold at 100 µM, p=0.004) but not in mock-transfected cells (Fig. 2), suggesting that nonanal caused a decrease in RDH12 retinaldehyde reductase activity. The extent of inhibition by nonanal was concentration-dependent: 200 µM nonanal exhibited a 1.5-fold greater inhibition than 100 µM nonanal (Fig. 2). The inhibition of RDH12 retinaldehyde reductase activity by nonanal was consistent with nonanal acting as an alternative substrate for RDH12. Nonanal also inhibited the biosynthesis of retinoic acid but had little or no effect on retinyl esters (Fig. 2).
Figure 2. Effect of nonanal on RDH12 activity and retinoid metabolism.
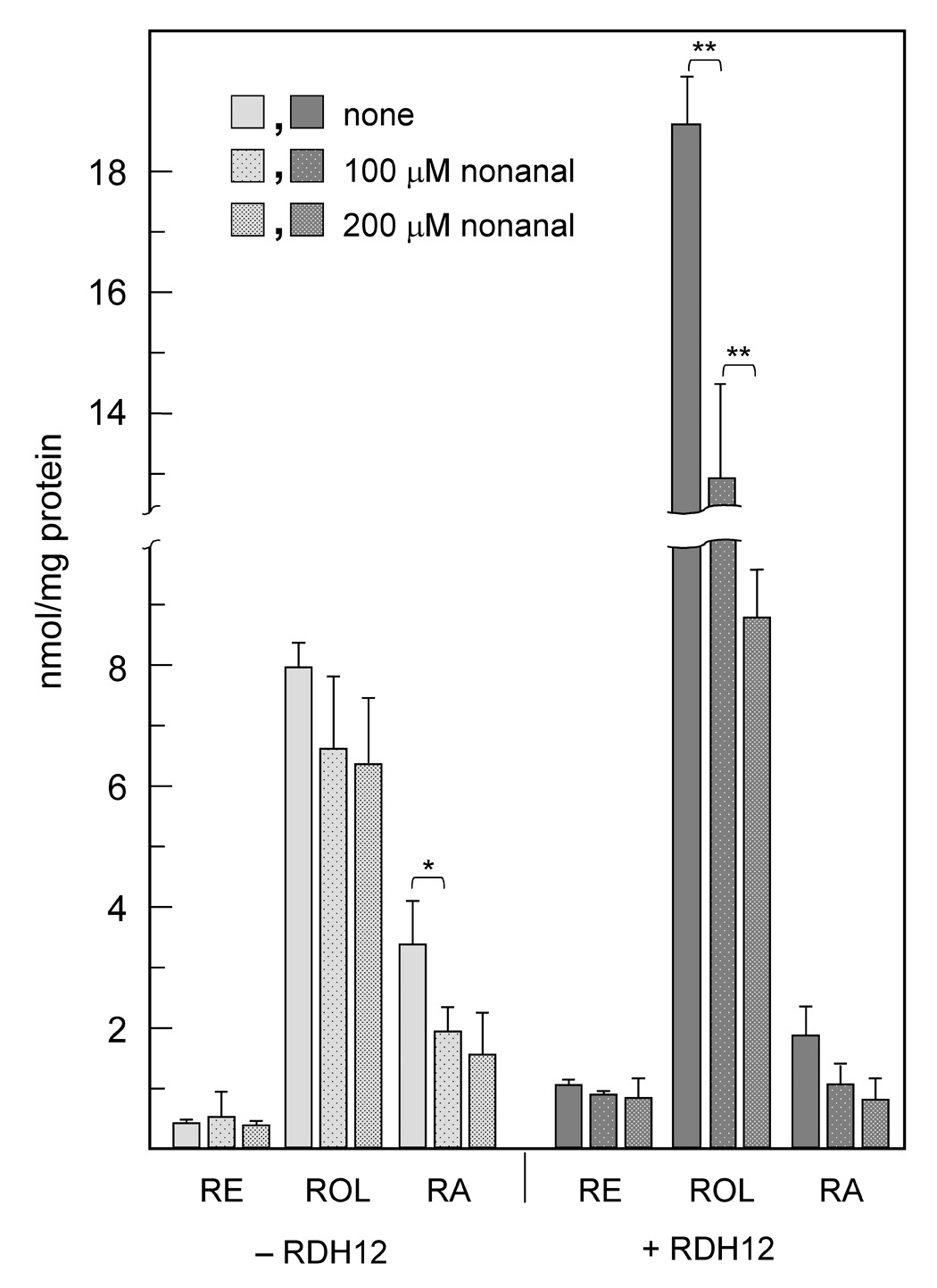
RDH12-transfected cells (+RDH12) and mock-transfected cells (−RDH12) were incubated with 10 µM all-trans-retinaldehyde for 3 h in the absence (none) or presence of nonanal. The products of retinaldehyde metabolism were analyzed by normal phase HPLC as described in Materials and Methods. Data shown represent combined retinoids extracted from medium and cells. RE, retinyl esters; ROL, retinol; RA, retinoic acid. Data are mean ± SD of five independent experiments. Statistical analysis was done using two-tailed unpaired Student's t test. *, p < 0.05; **, p < 0.01.
In contrast to nonanal, 4-HNE had a dramatic effect on the overall retinoid metabolism in the cells but did not appear to affect the RDH12 activity. The presence of 4-HNE in cell culture medium resulted in an increased rather than a decreased production of retinol from retinaldehyde (1.3-fold, p=0.02) (Fig. 3). At the same time, 4-HNE caused a significant drop in the cellular levels of retinoic acid and retinyl esters in both RDH12-transfected and mock-transfected cells (3–4–fold and 3–fold, respectively, at 100 µM, p<0.001) (Fig. 3), suggesting that 4-HNE inhibits the activities of endogenous retinol esterases and retinaldehyde dehydrogenases.
Figure 3. Effect of 4-HNE on RDH12 activity and retinoid metabolism.
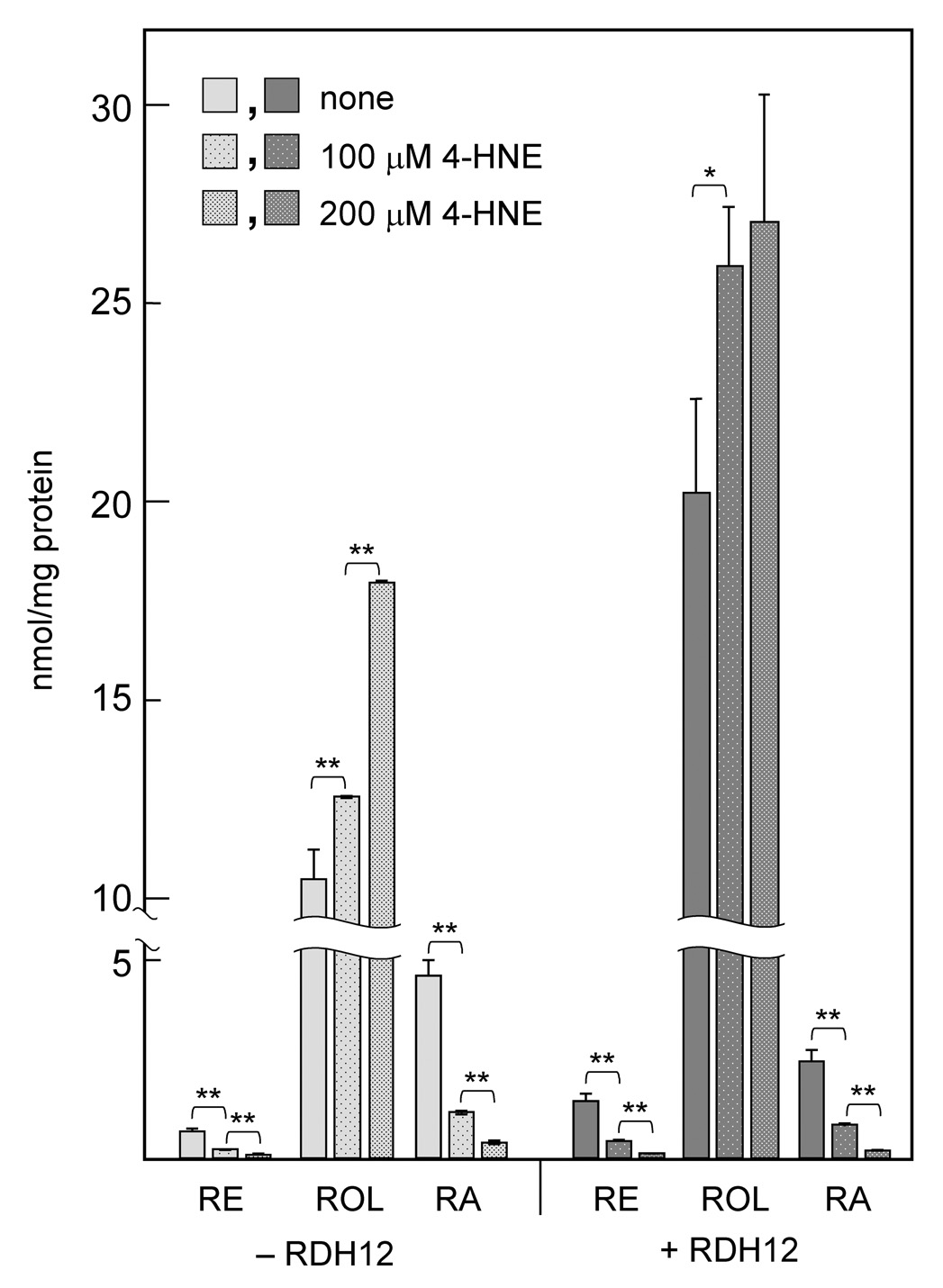
RDH12-transfected cells (+RDH12) and mock-transfected cells (−RDH12) were incubated with 10 µM all-trans-retinaldehyde for 3 h in the absence (none) or presence of 4-HNE. The products of retinaldehyde metabolism were analyzed by normal phase HPLC. Abbreviations are as in Figure 2. Data are mean ± SD of three independent experiments. Statistical analysis was done using two-tailed unpaired Student's t test. *, p < 0.05; **, p < 0.01.
To obtain direct evidence that 4-HNE inhibits the biosynthesis of retinyl esters, we examined the effect of 4-HNE on the activity of a major retinol-esterifying enzyme, LRAT, known to be present in HEK293 cells [17]. LRAT was expressed in Sf9 cells using the Baculovirus expression system and Sf9 microsomes containing the recombinant LRAT were isolated for activity assays. Analysis of LRAT activity in the presence of increasing concentrations of 4-HNE showed that 4-HNE inhibited LRAT activity with the IC50 value of 37 µM, reducing the formation of retinyl esters to ~25% at 200 µM (Fig. 4). In comparison, nonanal was a much weaker inhibitor and did not decrease the activity of LRAT below 70% at the highest concentration used (200 µM) (Fig. 4). Strong inhibition of LRAT activity by 4- HNE and the lack of significant inhibition by nonanal in vitro were in agreement with the effects of 4-HNE and nonanal on retinyl ester biosynthesis in the cells (Fig. 2 and Fig. 3).
Figure 4. Inhibition of LRAT activity by nonanal and 4-HNE.
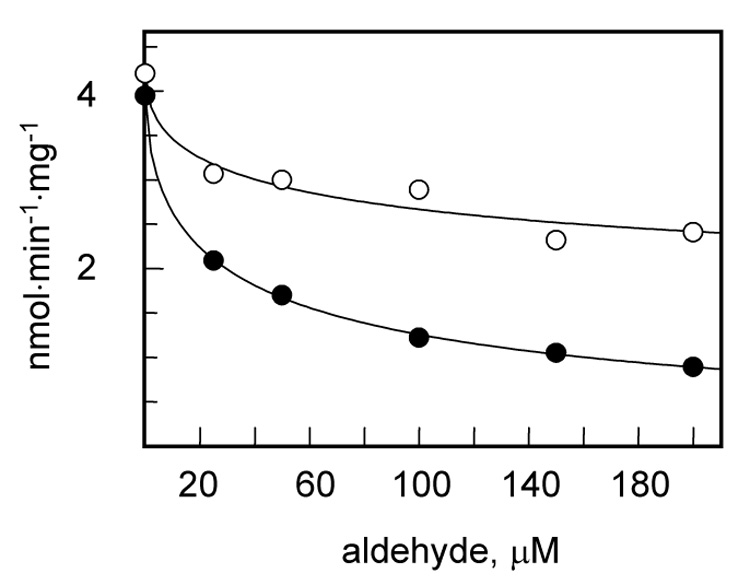
LRAT-containing microsomes were incubated with retinol (2 µM) and DPPC (200 µM) in the presence of 0–200 µM nonanal (○) or 4-HNE (●). The formation of retinyl esters was monitored by normal phase HPLC. Data shown are representative of three independent experiments.
To determine whether medium chain aldehydes inhibit the activity of retinaldehyde dehydrogenase, we examined which retinoid-active aldehyde dehydrogenases are expressed in HEK293 cells. RT-PCR analysis showed that HEK293 cells express both human cytosolic ALDH1A1 (also known as RALDH1) [18, 19] and ALDH1A2 (known as RALDH2) [20] (Fig. 5 A). One of the enzymes, ALDH1A1, was chosen as a model for investigation of the effect of aldehydes on its retinaldehyde dehydrogenase activity.
Figure 5. Inhibition of ALDH activity by nonanal and 4-HNE.
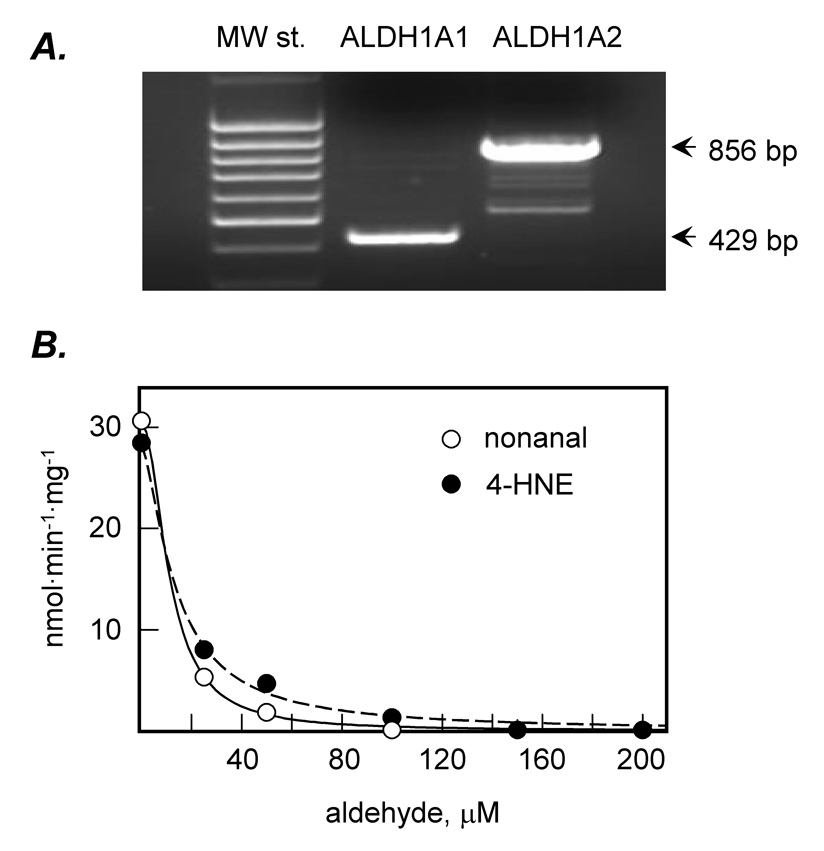
A, RT-PCR analysis of ALDH1A1 and ALDH1A2 expression in HEK293 cells. MW st., molecular weight standards. B, Purified human ALDH1A1 was incubated with retinaldehyde (5 µM) and NAD+ (1 mM) in the presence of 0–200 µM nonanal or 4-HNE. The amount of produced retinoic acid was determined by HPLC as described under Materials and Methods. Data shown are representative of three independent experiments.
Recombinant human His6-tagged ALDH1A1 was expressed in E. coli and purified using Ni2+-affinity chromatography [21]. ALDH1A1 was incubated with retinaldehyde in the presence of increasing concentrations of nonanal or 4-HNE and the formation of retinoic acid was analyzed by HPLC. Both nonanal and 4-HNE strongly inhibited ALDH1A1-catalyzed oxidation of retinaldehyde with the IC50 values of 11 µM and 14 µM, respectively (Fig. 5 B). Inhibition of ALDH1A1 activity by nonanal and 4-HNE was in agreement with the reduced levels of retinoic acid in RDH12-transfected and mock-transfected cells treated with the medium-chain aldehydes.
DISCUSSION
The results of this study demonstrate that RDH12 can protect cells against nonanal-associated toxicity but not against 4-HNE because, in contrast to 4-HNE, nonanal appears to be metabolized by RDH12 in the cells. These results correlate well with the in vitro catalytic properties of RDH12. Catalytic efficiency of RDH12 for the reduction of nonanal is ~30-fold greater than that for the reduction of 4-HNE [2]. While 4-HNE does not appear to inhibit the retinaldehyde reductase activity of RDH12, it profoundly affects the overall retinoid homeostasis in the cells by inhibiting the retinol esterification and retinoic acid biosynthesis. The finding that 4-HNE inhibits the activities of both ALDH1A1 and LRAT offers an explanation for the surprising increase in retinol levels observed in the cells incubated with 4-HNE: inhibition of retinaldehyde oxidation to retinoic acid by ALDH results in an increased flux of retinaldehyde through retinaldehyde reductases and, therefore, an increased biosynthesis of retinol, whereas the inhibition of LRAT activity further raises the levels of cellular retinol by blocking its conversion to retinyl esters (Fig. 6).
Figure 6. Retinoid metabolic pathways.
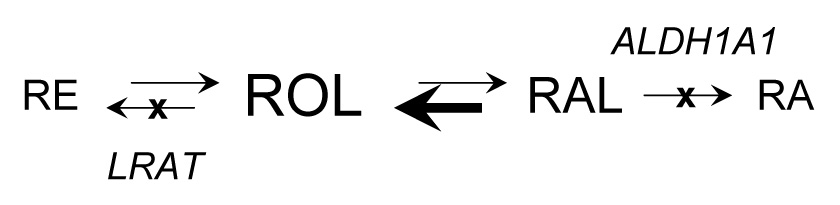
RE, retinyl esters; ROL, retinol; RAL, retinaldehyde; RA, retinoic acid. The arrows pointing from ROL to RE and from RAL to RA are crossed (X) to indicate the inhibition of LRAT and ALDH1A1 activities by 4-HNE.
The inhibition of retinaldehyde oxidation to retinoic acid in tissue homogenates by 4-HNE has been observed by others [22, 23], but the inhibitory effect of 4-HNE on retinol esterification, to our knowledge, has not been reported previously. This finding may offer a new insight into the molecular mechanism of liver retinyl ester depletion in alcoholic liver disease. Ethanol-induced depletion of hepatic vitamin A stores is one of the characteristic features of alcoholic liver injury, both in experimental animals and in humans [reviewed in ref. 24–ref. 26]. Several mechanisms have been suggested to explain this phenomenon, including increased mobilization of vitamin A from the liver and enhanced catabolism of vitamin A in the liver and other organs [24–26]. However, alcohol consumption is also associated with enhanced lipid peroxidation, protein carbonyl formation, production of reactive oxygen species (ROS), and decreases in hepatic antioxidant defense, especially GSH [reviewed in ref. 27]. Our observation that 4-HNE strongly inhibits LRAT activity suggests that the oxidative stress may contribute to the depletion of retinyl esters in alcoholic liver disease, because LRAT is believed to be the major enzyme responsible for esterification of retinol in liver [28]. Although some researchers found no difference in the activity of LRAT in ethanol-fed versus control rats [29], others reported that the relative amounts of unesterified retinol were significantly increased in the ethanol-fed rats [30], consistent with our results.
Although our data suggest that RDH12 can contribute to cell protection against nonanal, quantitatively nonanal produced as a result of microsomal membrane peroxidation is a minor product compared to 4-HNE, which represents more than 95% of the total unsaturated aldehydes [31–33]. Considering that 4-HNE is much more abundant than nonanal and that RDH12 is not effective for protection against 4-HNE, it is not surprising that knockout mice that lack RDH12 do not appear to have increased levels of lipid peroxidation products in the retina [34]. It is well established that there are multiple pathways for detoxification of 4-HNE. In most cells, conjugation of 4-HNE with glutathione and oxidation to 4-hydroxynonenoic acid are the two major routes of 4-HNE elimination [35–39]. 4-HNE-GST conjugate can be further reduced to 1,4-dihydroxy-2-nonene-GST and this reaction appears to be catalyzed primarily by the cytosolic aldose reductase [40, 41]. The product of direct NADPH-dependent reduction of 4-HNE, unconjugated 1,4-dihydroxy-2-nonene, which could be potentially produced by RDH12, constitutes only about ~10–13% of all 4-HNE metabolites [38, 39]. Based on these estimates, the contribution of RDH12 to detoxification of 4-HNE, if any, is rather limited.
Together, these observations suggest that retinaldehyde, not the lipid peroxidation products, is the primary target of RDH12 in photoreceptors, reinforcing the role of RDH12 in retinoid metabolism. The finding that 4-HNE strongly inhibits the biosynthesis of retinyl esters and retinoic acid suggests that oxidative stress and lipid peroxidation can have deleterious consequences for the overall retinoid homeostasis in the cells.
Acknowledgements
We would like to thank Dr. Henry Weiner in the Department of Biochemistry at Purdue University, West Lafayette, IN, for sharing ALDH1A1 expression plasmid as well as purified ALDH1A1. This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grant AA12153 to N.Y.K.
Abbreviations
- RDH12
retinol dehydrogenase 12
- 4-HNE
4-hydroxynonenal
- LRAT
lecithin:retinol acyltransferase
- ALDH1A1
aldehyde dehydrogenase 1 family, member A1
- GSH
glutathione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lee SA, Belyaeva OV, Popov IK, Kedishvili NY. Overproduction of bioactive retinoic acid in cells expressing disease-associated mutants of retinol dehydrogenase 12. J. Biol. Chem. 2007;282:35621–35628. doi: 10.1074/jbc.M706372200. [DOI] [PubMed] [Google Scholar]
- 2.Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44:7035–7047. doi: 10.1021/bi050226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saari JC. Retinoids in photosensitive systems. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. New York: Raven Press; 1994. pp. 351–385. [Google Scholar]
- 4.Bazan NG. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. vol. 8. New York: Raven Press; 1990. pp. 1–24. [Google Scholar]
- 5.Anderson RE, Maude MB, McClellan M, Matthes MT, Yasumura D, LaVail MM. Low docosahexaenoic acid levels in rod outer segments of rats with P23H and S334ter rhodopsin mutations. Mol. Vision. 2002;8:351–358. [PubMed] [Google Scholar]
- 6.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993;57:779S–786S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- 8.Mangelsdorf D, Umesono K, Evans RM. The Retinoid Receptors. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. New York: Raven Press; 1994. pp. 319–350. [Google Scholar]
- 9.Smith SB, Bora N, McCool D, Kutty G, Wong P, Kutty RK, Wiggert B. Photoreceptor cells in the vitiligo mouse die by apoptosis. TRPM- 2/clusterin expression is increased in the neural retina and in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1995;36:2193–2201. [PubMed] [Google Scholar]
- 10.Soderpalm AK, Fox DA, Karlsson JO, van Veen T. Retinoic Acid Produces Rod Photoreceptor Selective Apoptosis in Developing Mammalian Retina. Invest. Ophthalmol. Vis. Sci. 2000;41:937–947. [PubMed] [Google Scholar]
- 11.Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, Wissinger B, Nurnberg P, Gal A. Nat. Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 12.Thompson DA, Janecke AR, Lange J, Feathers KL, McHenry CL, Stockton DW, Rammesmayer G, Lupski JR, Antinolo G, Ayuso C, Baiget M, Gouras P, Heckenlively JR, den Hollander A, Jacobson SG, Lewis RA, Sieving PA, Wissinger B, Yzer S, Zrenner E, Utermann G, Gal A. Retinal degeneration associated with RDH12 mutations results from decreased 11-cis-retinal synthesis due to disruption of the visual cycle. Human Mol. Genet. 2005;14:3865–3875. doi: 10.1093/hmg/ddi411. [DOI] [PubMed] [Google Scholar]
- 13.Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, Rozet JM. Retinal dehydrogenase 12 (RDH12) mutations in Leber congenital amaurosis. Am. J. Hum. Genet. 2004;75:639–646. doi: 10.1086/424889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W, Gerth C, Maeda A, Lodowski DT, Van Der Kraak L, Saperstein DA, Héon E, Palczewski K. Novel RDH12 mutations associated with Leber congenital amaurosis and cone-rod dystrophy: biochemical and clinical evaluations. Vision Res. 2007;47:2055–2066. doi: 10.1016/j.visres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 16.Gallego O, Belyaeva OV, Porte S, Ruiz FX, Stetsenko AV, Shabrova EV, Kostereva NV, Farres J, Pares X, Kedishvili NY. Comparative functional analysis of human medium-chain dehydrogenases, short-chain dehydrogenases/reductases and aldo-keto reductases with retinoids. Biochem. J. 2006;399:101–109. doi: 10.1042/BJ20051988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Moiseyev G, Wu BX, Ma JX, Crouch RK. Visual cycle retinoid processing proteins are present in HEK293S cells. Vision Res. 2003;43:3037–3044. doi: 10.1016/j.visres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Bhat PV, Samaha H. Kinetic properties of the human liver cytosolic aldehyde dehydrogenase for retinal isomers. Biochem. Pharmacol. 1999 57;:195–197. doi: 10.1016/s0006-2952(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 19.Stewart MJ, Malek K, Crabb DW. Distribution of messenger RNAs for aldehyde dehydrogenase 1, aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human tissues. J. Investig. Med. 1996;44:42–46. [PubMed] [Google Scholar]
- 20.Ono Y, Fukuhara N, Yoshie O. TAL1 and LIM-only proteins synergistically induce retinaldehyde dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia by acting as cofactors for GATA3. Molec. Cell. Biol. 1998;18:6939–6950. doi: 10.1128/mcb.18.12.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho KK, Hurley TD, Weiner H. Selective alteration of the rate-limiting step in cytosolic aldehyde dehydrogenase through random mutagenesis. Biochemistry. 2006;45:9445–9453. doi: 10.1021/bi060718c. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Juchau MR. Inhibition of embryonic retinoic acid synthesis by aldehydes of lipid peroxidation and prevention of inhibition by reduced glutathione and glutathione S-transferases. Free Radic. Biol. Med. 1998;24:408–417. doi: 10.1016/s0891-5849(97)00272-4. [DOI] [PubMed] [Google Scholar]
- 23.Khalighi M, Brzezinski MR, Chen H, Juchau MR. Inhibition of human prenatal biosynthesis of all-trans-retinoic acid by ethanol, ethanol metabolites, and products of lipid peroxidation reactions: a possible role for CYP2E1. Biochem. Pharmacol. 1999;57:811–821. doi: 10.1016/s0006-2952(98)00362-1. [DOI] [PubMed] [Google Scholar]
- 24.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am. J. Clin. Nutr. 1999;69:1071–1085. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- 26.Wang XD. Alcohol, vitamin A, and cancer. Alcohol. 2005;35:251–258. doi: 10.1016/j.alcohol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 28.O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J. Biol. Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Chung J, Seitz HK, Russell RM, Wang XD. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol. Clin. Exp. Res. 2002;26:1703–1709. doi: 10.1097/01.ALC.0000037135.09289.69. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen M, Blomhoff R, Helgerud P, Solberg LA, Berg T, Norum KR. Retinol and retinyl esters in parenchymal and nonparenchymal rat liver cell fractions after long-term administration of ethanol. J. Lipid. Res. 1985;26:1112–1119. [PubMed] [Google Scholar]
- 31.Esterbauer H, Zollner H, Schaur R. Aldehydes formed by lipid peroxidation : mechanisms of formation, occurrence, and determination. In: Vigo-Pelfrey C, editor. Membrane Lipid Peroxidation. vol. III. Boca Raton, FL: CRC Press; 1990. pp. 240–268. [Google Scholar]
- 32.Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–286. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 33.Mlakar A, Spiteller G. Previously unknown aldehydic lipid peroxidation compounds of arachidonic acid. Chem. Phys. Lipids. 1996;79:47–53. doi: 10.1016/0009-3084(95)02506-5. [DOI] [PubMed] [Google Scholar]
- 34.Kurth I, Thompson DA, Ruther K, Feathers KL, Chrispell JD, Schroth J, McHenry CL, Schweizer M, Skosyrski S, Gal A, Hubner CA. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol. Cell. Biol. 2007;27:1370–1379. doi: 10.1128/MCB.01486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luckey SW, Petersen DR. Metabolism of 4-hydroxynonenal by rat Kupffer cells. Arch. Biochem. Biophys. 2001;389:77–83. doi: 10.1006/abbi.2001.2307. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava S, Chandra A, Wang LF, Seifert WE, Jr., DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2- nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava S. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radical Biol. Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 38.Hartley DP, Ruth JA, Petersen DR. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch. Biochem. Biophys. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- 39.Aldini G, Granata P, Marinello C, Beretta G, Carini M, Facino RM. Effects of UVB radiation on 4-hydroxy-2-trans-nonenal metabolism and toxicity in human keratinocytes. Chem. Res. Toxicol. 2007;20:416–423. doi: 10.1021/tx0601657. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava S, Chandra A, Wang LF, Seifert WE, Jr., DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choudhary S, Srivastava S, Xiao T, Andley UP, Srivastava SK, Ansari NH. Metabolism of lipid derived aldehyde, 4-hydroxynonenal in human lens epithelial cells and rat lens. Invest. Ophthalmol. Vis. Sci. 2003;44:2675–2682. doi: 10.1167/iovs.02-0965. [DOI] [PubMed] [Google Scholar]


