Abstract
Members of the serpin (serine proteinase inhibitor) superfamily play a central role in the control of inflammatory, coagulation, and fibrinolytic cascades. Point mutations that cause abnormal conformational transitions in these proteins can trigger disease. Recent work has defined three pathways by which these conformers cause tissue damage. Here, we describe how these three mechanisms can be integrated into a new model of the pathogenesis of emphysema caused by mutations in the serpin α1-antitrypsin.
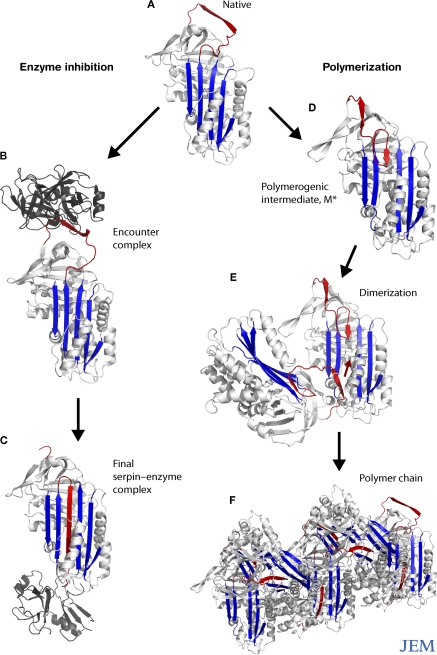
Serpins are found in most branches of life, including animals, plants, viruses, and prokaryotes. They are critical in the control of numerous proteolytic cascades in man, including those involved in inflammation (α1-antitrypsin and α1-antichymotrypsin), thrombosis (antithrombin, heparin cofactor II, and protease nexin-1), and the complement cascade (C1-inhibitor). Serpins are defined by at least 30% sequence identity with the archetypal member α1-antitrypsin and a common structural fold that is based on a five-stranded β-sheet and mobile-reactive center loop. The binding of target proteinases to the reactive loop springs a remarkable “mousetrap”-like mechanism that flips the enzyme from the apex to the base of the serpin (Fig. 1). This is associated with insertion of the reactive loop as an extra strand in β-sheet A and inactivation of the catalytic triad of the target protease (1). This mechanism provides specific and irreversible inhibition of enzymes, but the dramatic movement of the protein also renders serpins vulnerable to aberrant conformational transitions caused by point mutations. Many members of the serpin superfamily can be affected by these mutations, which cause a diverse range of diseases, including cirrhosis, dementia, thrombosis, angio-edema, and emphysema. We have termed this group of diseases the serpinopathies, based on their common molecular mechanism (2). There is now a wealth of information on the structural transitions that underlie the serpinopathies, but much less is known about the cellular and tissue response to the aberrant protein conformers that cause disease. Here, we will discuss three distinct mechanisms: cell death due to intracellular accumulation of mutant serpin, uncontrolled proteolytic activity due to loss of serpin function, and tissue damage due to extracellular deposition of mutant serpins.
Figure 1.
Conformational transitions of the serpins. The reactive loop of α1-antitrypsin (A, red) binds to neutrophil elastase (B, dark gray), triggering a conformational change that flips the enzyme from the apex to the base of the serpin (C). This is associated with insertion of the reactive loop of the target proteinase as an extra strand into β-sheet A (blue) and inactivation of the catalytic triad of the protease. Point mutations subvert this mechanism and cause aberrant conformational transitions (D) and the formation of polymers (E and F).
Intracellular accumulation of mutant serpins: mechanism 1
Insights into the toxicity caused by the intracellular accumulation of mutant serpins largely comes from work on α1-antitrypsin deficiency, the best characterized of the serpinopathies. α1-antitrypsin is synthesized in the liver and is constitutively released into the circulation where it protects tissues against damage from the neutrophil enzyme elastase. The Z mutant of α1-antitrypsin is found in 4% of the north European population and causes some of the newly synthesized protein to misfold and form ordered polymers (Fig. 1) that are retained within the endoplasmic reticulum (ER) of hepatocytes (3, 4). The resulting protein overload predisposes individuals homozygous for the Z mutation to neonatal hepatitis, cirrhosis, and hepatocellular carcinoma. Other mutants of α1-antitrypsin (Δ51Phe, Ser53Phe) also cause the protein to form ordered polymers and create a similar degree of protein overload. These mutations probably also predispose to liver disease, but their rarity makes it difficult to undertake detailed epidemiological studies. The S (Glu264Val) and I (Arg39Cys) mutants of α1-antitrypsin form polymers much less readily than the Z mutant and thus can be cleared by the normal disposal pathways within the cell. These mutants are not associated with liver disease unless they are inherited along with the more severe Z allele. Thus, there is a threshold effect whereby the mutation must cause sufficient accumulation of polymers to cause disease.
Mutants of the brain-specific serpin neuroserpin also form ordered polymers that accumulate within the ER of neurons. These mutations cause an autosomal-dominant dementia known as familial encephalopathy with neuroserpin inclusion bodies (FENIB) (5). Similar to α1-antitrypsin mutants, the neuroserpin mutants display a striking genotype–phenotype correlation: more severe mutations lead to faster polymer formation, more inclusions, and disease onset at a younger age.
How intracellular accumulation of mutant α1-antitrypsin and neuroserpin polymers causes the cell death and inflammation that characterize cirrhosis and FENIB is not completely clear. The intracellular pathways that respond to mutant serpins are now being elucidated. A majority of the mutated protein fails to fold and is degraded by the proteasome (6), and 10–15% of the protein folds normally and is secreted into the plasma (in the case of Z α1-antitrypsin). However, a small proportion of the mutant protein folds into ordered polymers, some of which are degraded by autophagy (7) and the remainder of which accumulates within the ER. Accumulation of the mutant protein in the ER activates the transcription factor nuclear factor κB (NF-κB) and caspases (8–10), thereby inducing inflammation and apoptosis. The mechanism that prevents the exit of polymers from the ER and the consequent signaling that activates NF-κB and caspases has yet to be elucidated.
Overactivity of proteolytic cascades: mechanism 2
Not all pathogenic serpin mutations result in the build up of toxic polymers. In some cases, it is the absence of functional protein that causes disease. This is exemplified by naturally occurring mutations in the plasma proteins C1-inhibitor, antithrombin, and α1-antichymotrypsin, which control the complement, coagulation, and inflammatory cascades, respectively. These mutations destabilize the protein's architecture, allowing the formation of unstable intermediates and inactive polymers within the ER of hepatocytes. However, as these proteins are less abundant (synthesized at 10% the rate of α1-antitrypsin) and the mutations are usually heterozygous, the aberrant protein can be effectively cleared by degradative pathways and does not form toxic inclusions. However, intrahepatic clearance of these mutant proteins leads to reduced secretion and a lack of functional protein in the circulation. C1-inhibitor deficiency results in uncontrolled activity of the complement cascade and angio-edema; antithrombin deficiency causes thrombosis; and α1-antichymotrypsin deficiency renders tissues vulnerable to proteolytic attack, which can lead to inflammation and chronic obstructive pulmonary disease.
A mutation in the serpin heparin cofactor II, which normally inhibits coagulation, is associated with plasma deficiency of the protein, but so far has not been shown to cause disease (11). This mutation is of particular interest as it is analogous to the Z allele that causes polymerization of α1-antitrypsin. The same mutation in the Drosophila serpin necrotic causes temperature-dependent polymerization and inactivation of the protein (12). We therefore predict that episodes of fever will precipitate the inactivation of unstable serpins and thus exacerbate the tissue damage of the serpinopathies.
Tissue damage caused by extracellular deposits: mechanism 3
Many serpins are synthesized in the liver and released into the circulation. Although mutation-induced polymerization often results in the retention of the protein within hepatocytes, some mutant protein can traffic through the secretory pathway and reach the circulation or local tissues. This protein often retains function as a proteinase inhibitor but still carries the mutation and thus the propensity to form polymers once secreted. Indeed, polymers of α1-antitrypsin, antithrombin, and C1-inhibitor have been identified in the plasma of individuals with mutations in these proteins (13–15). Moreover, polymers of neuroserpin predominate in the culture media of cells transfected with mutants of neuroserpin (16). It is clearly important to consider whether extracellular polymers are inactive bystanders or whether they can themselves exacerbate the tissue damage of the serpinopathies.
Recent work has shown that polymers of α1-antitrypsin are chemotactic for neutrophils in vitro and cause a neutrophil influx when instilled into the lungs of mice (17–19). In contrast, the monomeric protein has little effect on the migration of neutrophils in vitro or in vivo. The influx of inflammatory cells in mice treated with polymers is not mediated by chemokines, but appears to be a direct effect of α1-antitrypsin polymers on neutrophils. Neutrophil chemotaxis caused by systemic polymers may explain the well-recognized association between vascular disease associated with the presence of antibodies against neutrophil cytoplasmic components and the Z allele of α1-antitrypsin (20). In this case, polymers may cause the aberrant leukocyte migration that underlies the arteritis and capillaritis that causes organ damage. The inflammatory properties of polymers may also play a role in other serpinopathies. For example, the presence of neuroserpin polymers at the neuronal synapse may cause local inflammation, thus contributing to neuronal dysfunction and disruption of synaptic plasticity in FENIB. Inflammation caused by the tissue deposition of C1-inhibitor polymers may exacerbate the vascular permeability that characterizes angio-edema.
α1-Antitrypsin polymers, inflammation, and emphysema
It is clear that all three mechanisms of serpin-mediated tissue damage may contribute to the pathogenesis of disease, although their contribution is likely to vary between serpins and serpinopathies. The interaction of the different pathways is perhaps best characterized for the type of emphysema that is associated with α1-antitrypsin deficiency. This accounts for 1–3% of all cases of chronic obstructive pulmonary disease. Individuals who are homozygous for the Z mutation typically develop panlobular emphysema in their 40s and 50s (or 30s in those who smoke). For many years, this type of emphysema was largely attributed to the lack of effective antiproteinase activity and control of inflammation within the lung (mechanism 2). However, the recognition that other pathways contribute to tissue damage in the serpinopathies requires a reconsideration of the pathogenesis of disease.
In emphysema, noxious stimuli such as cigarette smoke are thought to trigger the initial inflammation within the lung. A chemotactic gradient of interleukin (IL)-8 and leukotriene-B4 recruits large numbers of macrophages and neutrophils from capillaries into the small airways and alveoli (Fig. 2). To reach the alveoli, the cells must migrate through an interstitial space that contains elastin, proteoglycans, and collagen, and then through the junctions between epithelial cells (21, 22). In patients with emphysema, neutrophils are initially concentrated in the centrilobular regions of the lung parenchyma where they release serine and cathepsin proteinases, which degrade elastin and other structural proteins and thus contribute to disease (23). Degraded elastin fragments themselves act as chemoattractants and recruit additional inflammatory cells into the lungs (24, 25). Emphysema is further exacerbated by free radicals contained in cigarette smoke (1017 per puff) and superoxide released by activated neutrophils, which damage proteins, lipids, and DNA (26) and cause cell death.
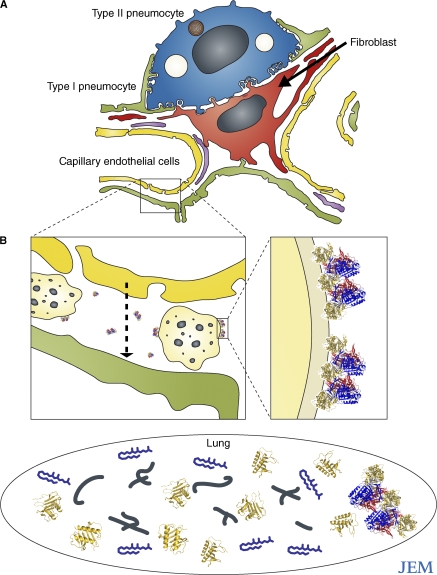
Figure 2.
The localization of α1-antitrypsin polymers and neutrophils in the lung interstitium. (A) The alveolar wall is composed of structural type I (green) and type II (blue) pneumocytes (reference 22). These are separated from the capillary endothelium (yellow) by interstitial matrix that is maintained by fibroblasts (red) and also contains macrophages. (B) Neutrophils (light yellow) migrate through the interstitium (arrow) into the lung (black oval) in response to inflammatory mediators, such as LTB4 (blue), IL-8 (gold), and elastin fragments (black). Interstitial polymers (inset, repeating units of blue, red, and yellow) bind to neutrophils and cause them to degranulate, thus amplifying and accelerating tissue destruction in emphysema.
As the major inhibitor of neutrophil elastase, α1-antitrypsin is critical for defense against such proteolytic lung damage. α1-Antitrypsin enters the lung by passive diffusion from the circulation and is also secreted locally by macrophages and bronchial and alveolar epithelial cells. In individuals homozygous for the Z mutation, however, all of the α1-antitrypsin in the lungs has the propensity to form polymers, regardless of its source. Indeed, polymers of α1-antitrypsin are found in lavage (18, 27) and tissue sections (19) from the lungs of these individuals. Deposits of polymers are particularly prominent around capillaries (consistent with a circulating source of polymers) and epithelial cells (consistent with local synthesis). The greatest resistance to diffusion is the interstitium, where Z α1-antitrypsin is concentrated and where it forms polymers. The chemotactic property of α1-antitrypsin polymers can then trap neutrophils within the interstitium as they migrate from the vascular space to the alveolar compartment in response to chemokines (Fig. 2). Once neutrophils are retained within the interstitium, the polymers cause the cells to adhere, degranulate, and release proteolytic enzymes (17), thereby maximizing damage to the extracellular matrix and spreading the focus of inflammation throughout the lobules of the lung.
The destructive effects of neutrophil-derived proteolytic enzymes are amplified in individuals with α1-antitrypsin deficiency, as the small amount of monomeric Z α1-antitrypsin that is present is less efficient at inhibiting neutrophil elastase. The activity of α1-antitrypsin is likely to be further reduced by oxidation of the key 358Met residue by superoxide radicals. In addition to exacerbating proteolysis, the lack of active α1-antitrypsin leads to uncontrolled activation of intracellular caspase-3 and hence alveolar cell apoptosis and emphysema (28).
Thus, there is evidence that both uncontrolled proteolytic activity due to loss of α1-antitrypsin function (mechanism 2) and tissue damage due to extracellular deposition of Z α1-antitrypsin polymers (mechanism 3) contribute to the pathogenesis of emphysema. Although less well investigated, cell death and inflammation caused by intracellular accumulation of Z α1-antitrypsin (mechanism 1) is also likely to contribute to tissue damage in the alveolus and bronchial epithelium. As in the liver, the intracellular production of Z α1-antitrypsin may activate NF-κB signaling cascades in lung cells (8, 9), which could increase the production of inflammatory mediators and further amplify neutrophil recruitment and tissue damage. The chronic activation of NF-κB is also likely to accelerate apoptosis within all alveolar cells, which would help explain the widespread destruction of the alveoli and the panlobular distribution of this type of emphysema.
Novel therapies for α1-antitrypsin deficiency
Although the relative importance of each of the three types of tissue damage remains to be clarified, this integrated model of emphysema associated with α1-antitrypsin deficiency suggests some novel therapeutic strategies.
Small molecule inhibitors have been developed that block the polymerization of Z α1-antitrypsin in vitro and clear protein aggregates in cell models of disease (29). However, the current generation of small molecules also inactivates α1-antitrypsin as a proteinase inhibitor. Thus, although these drugs may help reduce the risk of liver disease associated with α1-antitrypsin deficiency, they would be predicted to exacerbate emphysema. It is therefore important to develop refined versions of these molecules that block polymerization of the protein without affecting its inhibitory activity. Such drugs could be administered directly into the lung to prevent polymerization and so ameliorate the inflammatory response.
Small molecules that block the binding of polymers to neutrophils would be an alternative approach. This strategy would block the ability of polymers to activate neutrophils and thus restore the normal migration pathway of neutrophils from the circulation to the alveoli. Such an approach would also prevent polymers from amplifying the inflammatory response.
Strategies that target the downstream effects, rather than the polymer itself, provide a third possibility. Given the central role of free radicals in emphysema, antioxidant agents are required to reduce oxidative stress within the lung. Although the current generation of antioxidants is not sufficiently potent, they may be effective if coadministered with agents that block polymer formation and/or the interaction of polymers with neutrophils. Finally, inhibition of the inflammatory response by blocking intracellular (NF-κB–dependent) and extracellular pathways that are activated by polymers might also be a successful approach to ameliorating the lung disease associated with α1-antitrypsin deficiency.
Acknowledgments
We are very grateful to Jim Hogg (University of British Columbia) for advice on neutrophil trafficking and the participants at the Alpha-1 Foundation meeting in Miami 2007, whose suggestions helped us to develop this commentary. We thank Andrew Purkiss (Birkbeck College, University of London) for his assistance with modeling α1-antitrypsin in the M* conformation.
This work is supported by the Medical Research Council (UK) and Papworth NHS Trust. B.G. is a Wellcome Trust Intermediate Clinical Fellow.
References
- 1.Huntington, J.A., R.J. Read, and R.W. Carrell. 2000. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 407:923–926. [DOI] [PubMed] [Google Scholar]
- 2.Lomas, D.A., and R. Mahadeva. 2002. Alpha-1-antitrypsin polymerisation and the serpinopathies: pathobiology and prospects for therapy. J. Clin. Invest. 110:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomas, D.A., D.L. Evans, J.T. Finch, and R.W. Carrell. 1992. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 357:605–607. [DOI] [PubMed] [Google Scholar]
- 4.Lomas, D.A. 2006. The selective advantage of α1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 173:1072–1077. [DOI] [PubMed] [Google Scholar]
- 5.Davis, R.L., A.E. Shrimpton, P.D. Holohan, C. Bradshaw, D. Feiglin, P. Sonderegger, J. Kinter, L.M. Becker, F. Lacbawan, D. Krasnewich, et al. 1999. Familial dementia caused by polymerisation of mutant neuroserpin. Nature. 401:376–379. [DOI] [PubMed] [Google Scholar]
- 6.Teckman, J.H., J. Burrows, T. Hidvegi, B. Schmidt, P.D. Hale, and D.H. Perlmutter. 2001. The proteasome participates in degradation of mutant α1-antitrypsin Z in the endoplasmic reticulum of hepatoma-derived hepatocytes. J. Biol. Chem. 276:44865–44872. [DOI] [PubMed] [Google Scholar]
- 7.Kamimoto, T., S. Shoji, T. Hidvegi, N. Mizushima, K. Umebayashi, D.H. Perlmutter, and T. Yoshimori. 2006. Intracellular inclusions containing mutant alpha1-antitrypsin Z are propagated in the absence of autophagic activity. J. Biol. Chem. 281:4467–4476. [DOI] [PubMed] [Google Scholar]
- 8.Lawless, M.W., C.M. Greene, A. Mulgrew, C.C. Taggert, S.J. O'Neill, and N.G. McElvaney. 2004. Activation of endoplasmic reticulum-specific stress responses associated with the conformational disease Z α1-antitrypsin deficiency. J. Immunol. 172:5722–5726. [DOI] [PubMed] [Google Scholar]
- 9.Hidvegi, T., B.Z. Schmidt, P. Hale, and D.H. Perlmutter. 2005. Accumulation of mutant alpha1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and BAP31 but not the unfolded protein response. J. Biol. Chem. 280:39002–39015. [DOI] [PubMed] [Google Scholar]
- 10.Davies, M.J., and D.A. Lomas. 2008. The molecular aetiology of the serpinopathies. Int. J. Biochem. Cell Biol. 40:1273–1286. [DOI] [PubMed] [Google Scholar]
- 11.Corral, J., J. Aznar, R. Gonzalez-Conejero, P. Villa, A. Minano, A. Vaya, R.W. Carrell, J.A. Huntington, and V. Vicente. 2004. Homozygous deficiency of heparin cofactor II: relevance of P17 glutamate residue in serpins, relationship with conformational diseases, and role in thrombosis. Circulation. 110:1303–1307. [DOI] [PubMed] [Google Scholar]
- 12.Green, C., G. Brown, T.R. Dafforn, J.M. Reichhart, T. Morley, D.A. Lomas, and D. Gubb. 2003. Drosophila necrotic mutations mirror disease-associated variants of human serpins. Development. 130:1473–1478. [DOI] [PubMed] [Google Scholar]
- 13.Lomas, D.A., P.R. Elliott, S.K. Sidhar, R.C. Foreman, J.T. Finch, D.W. Cox, J.C. Whisstock, and R.W. Carrell. 1995. Alpha1-antitrypsin Mmalton (52Phe deleted) forms loop-sheet polymers in vivo: evidence for the C sheet mechanism of polymerisation. J. Biol. Chem. 270:16864–16870. [DOI] [PubMed] [Google Scholar]
- 14.Eldering, E., E. Verpy, D. Roem, T. Meo, and M. Tosi. 1995. COOH-terminal substitutions in the serpin C1 inhibitor that cause loop overinsertion and subsequent multimerization. J. Biol. Chem. 270:2579–2587. [DOI] [PubMed] [Google Scholar]
- 15.Picard, V., M.-D. Dautzenberg, B.O. Villoutreix, G. Orliaguet, M. Alhenc-Gelas, and M. Aiach. 2003. Antithrombin Phe229Leu: a new homozygous variant leading to spontaneous antithrombin polymerisation in vivo associated with severe childhood thrombosis. Blood. 102:919–925. [DOI] [PubMed] [Google Scholar]
- 16.Miranda, E., K. Römisch, and D.A. Lomas. 2004. Mutants of neuroserpin that cause dementia accumulate as polymers within the endoplasmic reticulum. J. Biol. Chem. 279:28283–28291. [DOI] [PubMed] [Google Scholar]
- 17.Parmar, J.S., R. Mahadeva, B.J. Reed, N. Farahi, K. Cadwallader, D. Bilton, E.R. Chilvers, and D.A. Lomas. 2002. Polymers of α1-antitrypsin are chemotactic for human neutrophils: a new paradigm for the pathogenesis of emphysema. Am. J. Respir. Cell Mol. Biol. 26:723–730. [DOI] [PubMed] [Google Scholar]
- 18.Mulgrew, A.T., C.C. Taggart, M.W. Lawless, C.M. Greene, M.L. Brantly, S.J. O'Neill, and N.G. McElvaney. 2004. Z α1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest. 125:1952–1957. [DOI] [PubMed] [Google Scholar]
- 19.Mahadeva, R., C. Atkinson, J. Li, S. Stewart, S. Janciauskiene, D.G. Kelley, J. Parmar, R. Pitman, S.D. Shapiro, and D.A. Lomas. 2005. Polymers of Z α1-antitrypsin co-localise with neutrophils in emphysematous alveoli and are chemotactic in vivo. Am. J. Pathol. 166:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esnault, V.L.M., A. Testa, M. Audrain, C. Rogé, M. Hamidou, J.H. Barrier, R. Sesboüé, J.-P. Martin, and P. Lesavre. 1993. Alpha1-antitrypsin genetic polymorphism in ANCA-positive systemic vasculitis. Kidney Int. 43:1329–1332. [DOI] [PubMed] [Google Scholar]
- 21.Damiano, V.V., A. Cohen, A.L. Tsang, G. Batra, and R. Petersen. 1980. A morphologic study of the influx of neutrophils into dog lung alveoli after lavage with sterile saline. Am. J. Pathol. 100:349–364. [PMC free article] [PubMed] [Google Scholar]
- 22.Burns, A.R., C.W. Smith, and D.C. Walker. 2003. Unique structural features that influence neutrophil emigration into the lung. Physiol. Rev. 83:309–336. [DOI] [PubMed] [Google Scholar]
- 23.Damiano, V.V., A. Tsang, U. Kucich, W.R. Abrams, J. Rosenbloom, P. Kimbel, M. Fallahnejad, and G. Weinbaum. 1986. Immunolocalization of elastase in human emphysematous lungs. J. Clin. Invest. 78:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senior, R.M., G.L. Griffin, R.P. Mecham, D.S. Wrenn, K. Prasad, and D.W. Urry. 1984. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J. Cell Biol. 99:870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghton, A.M., P.A. Quintero, D.L. Perkins, D.K. Kobayashi, D.G. Kelley, L.A. Marconcini, R.P. Mecham, R.M. Senior, and S.D. Shapiro. 2006. Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 116:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacNee, W. 2005. Oxidants and COPD. Curr. Drug Targets Inflamm. Allergy. 4:627–641. [DOI] [PubMed] [Google Scholar]
- 27.Elliott, P.R., D. Bilton, and D.A. Lomas. 1998. Lung polymers in Z α1-antitrypsin related emphysema. Am. J. Respir. Cell Mol. Biol. 18:670–674. [DOI] [PubMed] [Google Scholar]
- 28.Petrache, I., I. Fijalkowska, T.R. Medler, J. Skirball, P. Cruz, L. Zhen, H.I. Petrache, T. Flottee, and R.M. Tuder. 2006. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am. J. Pathol. 169:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallya, M., R.L. Phillips, S.A. Saldanha, B. Gooptu, S.C. Leigh Brown, D.J. Termine, A.M. Shirvani, Y. Wu, R.N. Sifers, R. Abagyan, and D.A. Lomas. 2007. Small molecules block the polymerisation of Z α1-antitrypsin and increase the clearance of intracellular aggregates. J. Med. Chem. 50:5357–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]