Abstract
Cutaneous immune responses must be tightly controlled to prevent unwanted inflammation in response to innocuous antigens, while maintaining the ability to combat skin-tropic pathogens. Foxp3+ regulatory T (T reg) cells are potent immune regulators and are found at high frequency in both human and mouse skin. Although T reg cells migrate to the skin and can dampen immune responses during experimentally induced inflammation or infection, the importance of cutaneous T reg cells for maintaining normal immune homeostasis in the skin has not been addressed. To selectively block T reg cell function in the skin, we restored the T reg cell compartment in Foxp3-deficient scurfy mice with cells whose ability to migrate to the skin was impaired because of targeted mutation of α-1,3-fucosyltransferase VII (Fut7). Although Fut7-deficient T reg cells were present at normal frequency and could function in all other tissues examined, these animals rapidly developed severe cutaneous inflammation. Thus, skin-resident T reg cell are essential for maintaining normal immune homeostasis at this site.
Tolerance against self- and harmless environmental antigens involves several levels of immune regulation. Whereas deletional mechanisms attempt to purge the B and T cell repertoire of self-reactive specificities, antigen-specific suppression of autoimmunity by CD4+Foxp3+ regulatory (T reg) T cells also plays a critical role in maintaining self-tolerance. The importance of T reg cells is highlighted by the severe autoimmunity in humans and mice rendered T reg cell–deficient because of lesions in the FOXP3 gene, which in mice can be prevented by adoptive transfer of purified T reg cells (1–3). In addition, neonatal or adult ablation of T reg cells, or adoptive transfer of T reg cell–depleted CD4+ T cells to immunodeficient mice rapidly triggers severe autoimmune or inflammatory disease (4–6). Collectively, these data demonstrate that T reg cells are required for both the establishment and maintenance of self-tolerance in vivo.
T reg cells can be subdivided based on expression of homing receptors thought to direct their migration to lymphoid versus nonlymphoid tissues (7). However, the relative contributions of the various T reg cell subsets to the maintenance of self-tolerance are not well defined. Several reports have demonstrated that T reg cells function within nonlymphoid sites to restrict T cell responses to foreign antigens during experimentally induced acute inflammation or infection (8–11). In addition, we have shown that mice whose T reg cells lack the chemokine receptor CCR4 develop spontaneous inflammatory disease in skin and lungs (12). However, although CCR4 helps direct T cell migration to the skin and lung airways, it may also facilitate interactions between T reg cells and antigen-presenting cells within secondary lymphoid tissues. Thus, the inflammatory disease in these animals is likely the result of impaired T reg cell function in both lymphoid and nonlymphoid organs, and the importance of nonlymphoid T reg cells in maintaining tolerance in the absence of a strong inflammatory stimulus has not been adequately addressed.
Surface expression of functional E- and P-selectin ligands (E-/P-lig) is required for optimal migration of effector T cells to inflamed skin (13). Interestingly, a high percentage of circulating human and mouse T reg cells express E- and/or P-lig, and Foxp3+ T reg cells make up a large fraction of the CD4+ T cells in normal skin from both humans and mice (12, 14). This suggests that cutaneous T reg cells may have an important immunoregulatory function at this site, even in the absence of any infection or inflammatory response. The carbohydrate determinants of E- and P-selectin binding are generated by the α-1,3-fucosyltransferase VII (FuT7) enzyme (15). Therefore, we used FuT7-deficient mice to determine the importance of T reg cell migration to the skin for their ability to prevent cutaneous inflammatory disease. We found that after transfer into T reg cell–deficient scurfy (sf) mice, T reg cells up-regulated expression of cutaneous homing receptors such as E- and P-lig and migrated to the skin. Loss of FuT7 dramatically reduced T reg cell accumulation in the skin, and resulted in selective onset of severe cutaneous inflammation. Thus, our results reveal the importance of correct T reg cell positioning in the skin for the maintenance of immune homeostasis and prevention of spontaneous autoimmune and inflammatory disease.
RESULTS AND DISCUSSION
T reg cells up-regulate skin homing receptors after transfer into sf mice
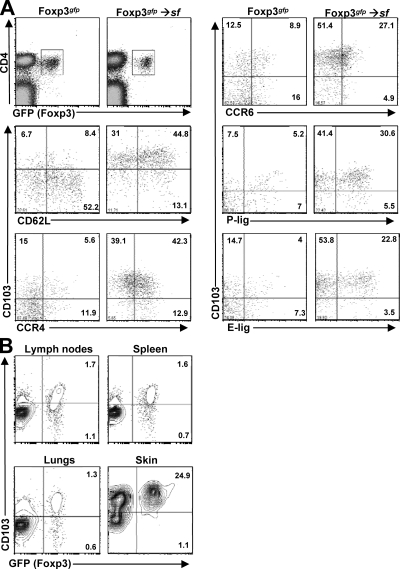
In sf mice, T reg cell differentiation and function is blocked because of a 2-bp insertion in the foxp3 gene, which causes a frameshift and premature translational termination. As a result, these mice succumb to rapid and fatal multiorgan autoimmunity that predominantly affects the skin, liver, and lungs (16). This inflammation can be prevented by adoptive transfer of CD4+CD25+ T reg cells, which undergo extensive expansion to fill their unique homeostatic “niche” (3). In WT mice, T reg cells are distributed throughout both lymphoid and nonlymphoid tissues (12). Therefore, we examined T reg cell homing receptor expression and tissue-distribution 4 wk after transfer into 1–3-d-old neonatal sf mice. To facilitate T reg cell identification, we transferred T reg cells purified from mice carrying a Foxp3gfp “knock-in” allele (17). When compared with T reg cells from age-matched WT mice, we found that T reg cells recovered from the LNs and spleens of the recipient animals were enriched in cells expressing adhesion and chemoattractant receptors such as CCR4, CCR6, CD103, E-lig, and P-lig, that are associated with T cell migration to the skin and other nonlymphoid sites (Fig. 1 A). Accordingly, the transferred Foxp3+ T reg cell were found in both lymphoid and nonlymphoid tissues of the recipient animals, including the LNs, spleen, intestine, liver, lungs and skin (Fig. 1 B and not depicted). Moreover, as in WT mice (12), the transferred T reg cells made up a particularly large percentage of the CD4+ T cells in the skin (Fig. 1 B).
Figure 1.
T reg cells transferred into sf mice up-regulate cutaneous homing receptors and accumulate in the skin. (A) Homing receptor expression in LNs and spleen 4 wk after transfer of T reg cells into a neonatal sf mouse was compared with that in an 8-wk-old Foxp3gfp mouse by flow cytometry. Numbers indicate the frequency of positive cells among the Foxp3+ T reg cells. Plots are gated on total live cells (top left) or CD4+Foxp3+ cells (all other graphs). (B) Flow cytometry analysis of CD103 and Foxp3 expression by gated CD4+ T cells isolated from the indicated tissues 4 wk after T reg cell transfer into neonatal sf mice. Data are representative of three independent experiments.
Consistent with previous reports (18), analysis of CFSE-labeled WT T reg cells indicated that after transfer, these cells undergo extensive proliferation in the LNs, but not the spleen, of recipient sf mice (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20072594/DC1). As early as day 6, most labeled T reg cells had fully diluted their CFSE, and in particular, CD103+ “effector memory” T reg cells were exclusively found in the CFSElow fraction. T reg cell migration to nonlymphoid sites is associated with their expression of CD103 (7). To determine if the high percentage of CD103+ T reg cells in recipient animals is caused by selective outgrowth of this subset, or instead is the result of up-regulation of CD103 expression by CD103− cells, we sorted CD103+ and CD103− T reg cells from CD45.1+ or CD45.2+ donors, and cotransferred them into neonatal sf recipients. We found that the CD103− “naive-like” T reg cells expanded nearly as well as their CD103+ counterparts, and rapidly up-regulated CD103 and other nonlymphoid tissue homing receptors (Fig. S1 B and not depicted). Collectively, these results demonstrate that after transfer into neonatal sf mice, Foxp3+ T reg cells undergo robust expansion and phenotypic differentiation, resulting in their distribution throughout both lymphoid and nonlymphoid tissues. This is consistent with data from our laboratory and others demonstrating that OVA-specific T reg cells up-regulate cutaneous homing receptors after antigen-recognition in the peripheral LNs (12, 19, 20), and suggests that T reg cell activity in nonlymphoid tissues may be important for their ability to prevent fatal autoimmunity after transfer into neonatal sf mice.
Characterization of FuT7−/−-T reg cells
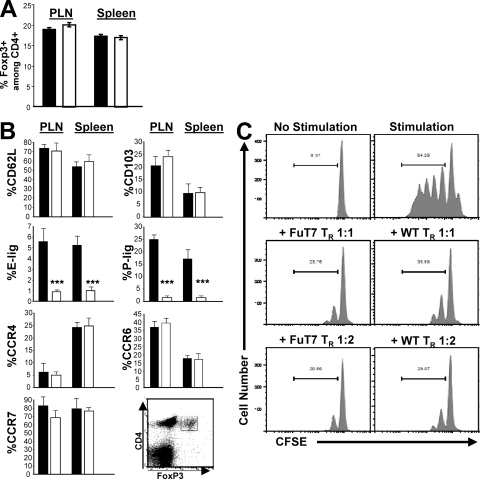
The relative abundance of Foxp3+ T reg cells in both human and mouse skin suggests that this may be a location in which these cells receive critical survival and/or homeostatic signals. Indeed, IL-15 and dermal fibroblasts can induce the proliferation of human cutaneous T reg cells in vitro (21). Leukocyte expression of E- and P-lig is severely diminished in FuT7−/− mice, and as a result, T cell migration to the skin during cutaneous inflammatory responses or infection is almost completely abolished in these animals (15, 22). Therefore, to determine if loss of FuT7 impacts T reg cell homeostasis and differentiation, we compared T reg cell abundance and phenotype in age-matched WT and FuT7−/− mice. In the spleen and LNs, total lymphocyte numbers (not depicted) and the frequency of Foxp3+ T reg cells (Fig. 2 A) were indistinguishable in WT and FuT7−/− mice. FuT7−/−-T reg cells also exhibited normal expression of various T reg cell–associated cell surface markers, such as CD25 and GITR (unpublished data). As expected, expression of E- and P-lig was strongly and significantly reduced in the FuT7−/−-T reg cells. However, the percentages of FuT7−/−-T reg cells expressing other lymphoid and nonlymphoid homing receptors such as CD62L, CCR7, CD103, CCR4, and CCR6 were not significantly different than those observed in WT animals (Fig. 2 B). In addition, the surface levels of each of these markers were unchanged in FuT7−/− mice (unpublished data). Finally, we compared the ability of WT- and FuT7−/−-T reg cells to suppress the proliferation of CFSE-labeled effector T cells in vitro. Consistent with a previous study analyzing the function of T cell receptor-transgenic T reg cells (9), WT- and FuT7−/−-T reg cells were equally effective in this assay (Fig. 2 C). Therefore, in the absence of FuT7-dependent expression of E- and P-lig, there are no gross impairments in T reg cell differentiation, maintenance, or function.
Figure 2.
Phenotypic and functional characterization of FuT7−/−-T reg cells. (A) Frequency of Foxp3+ T reg cells among CD4+ T cells in peripheral LNs and spleens of 8-wk-old WT (shaded bars) or FuT7−/− (open bars) mice. (B) Frequency of CD4+Foxp3+ T reg cells expressing the indicated homing receptors in peripheral LNs (PLN) or spleens of WT and FuT7−/− mice. Data in A and B are the mean and SD of values from three age-matched mice/group. Significance was measured using two-tailed, unpaired Student's t tests. *** indicates P < 0.0001; P > 0.05 for all other comparisons. (C) Proliferation of CD4+CD25− effector T cells as measured by CFSE dilution after 96 h of culture with CD4-depleted splenocytes in the presence or absence of anti-CD3 and -CD28 antibodies (top) and the indicated ratio of WT- or FuT7−/−-T reg cells (middle and bottom). Representative results of one out of two independent experiments.
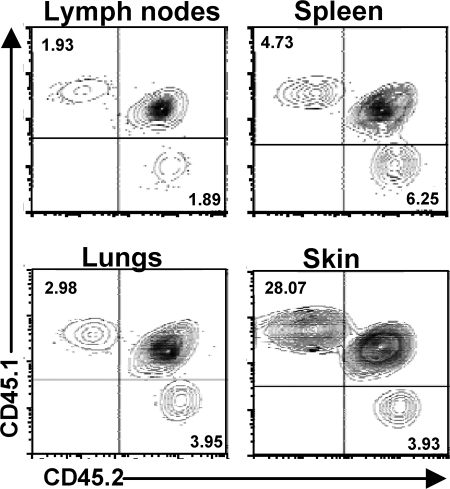
The requirement for FuT7 in T cell migration to the skin has been primarily demonstrated in settings of experimentally induced inflammation, during which both E- and P-selectin are strongly expressed by vascular endothelial cells in the dermis (23, 24). However, E-selectin is also expressed constitutively (albeit at low levels) in the microvascular beds of the skin, and almost all T cells isolated from human skin are E-lig+, suggesting a role for this receptor pair in T cell migration to the skin under homeostatic conditions as well (25, 26). Indeed, the frequency of CD4+CD25+ cells was significantly reduced in the skin of FuT7−/− mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20072594/DC1). To directly compare the ability of WT and FuT7−/− T reg cells to migrate to the skin, we cotransferred equal numbers of CD4+CD25+ T reg cells isolated from CD45.1+ WT (not Foxp3gfp) and CD45.2+ FuT7−/− mice into neonatal sf mice, and assessed the tissue distribution of the transferred cells 4–6 wk later. In these experiments, the recipients were heterozygous for CD45.1 and CD45.2, and thus endogenous CD4+ T cells in these mice were CD45.1+CD45.2+. Importantly, the recipient animals remained healthy and displayed no histological signs of the inflammatory disease or dermatitis typical of sf mice (unpublished data). After transfer, both WT- and FuT7−/−-T reg cells expanded and were present at roughly equivalent percentages in most lymphoid and nonlymphoid tissues analyzed, including the spleen, peripheral and mesenteric LNs, lungs and lung airways, intestinal epithelium, and intestinal lamina propria (Fig. 3 and not depicted). This confirms that FuT7 is not required for T reg cell expansion and maintenance in vivo, even when placed in competition with WT cells. In contrast, relative to the cotransferred WT cells, the accumulation of FuT7−/−-T reg cells in the skin was impaired by 5–10-fold (Fig. 3). Thus, FuT7-dependent expression of P- and E-lig is necessary for optimal T reg cell migration to the skin, even in the absence of any overt cutaneous inflammation or infection.
Figure 3.
Accumulation of FuT7−/−-T reg cells in the skin is selectively impaired. WT- (CD45.1+) and FuT7−/−- (CD45.2+) T reg cells were transferred into 2-d-old sf mice (CD45.1+CD45.2+), and tissue distribution was analyzed 6 wk later by flow cytometry analysis of CD45.1 and CD45.2 expression by gated CD4+ T cells isolated from the indicated tissues. Representative results of one out of three independent experiments.
Inhibiting T reg cell migration to the skin results in tissue-specific inflammatory disease
sf mice spontaneously develop multiorgan autoinflammatory disease that is fatal by 3–4 wk of age. The skin is severely affected, displaying lymphohistiocytic infiltration of the dermis, along with epidermal thickening and erosion (16). Combined with the high frequency of Foxp3+ T reg cells among CD4+ T cells in normal skin, this suggests that T reg cell–mediated immunosuppression is particularly important for maintenance of cutaneous self-tolerance. However, it is not clear if T reg cell migration to the skin is actually important for this protection, or if instead T reg cells function mainly to block the initiation of aberrant immune responses in the skin draining LNs.
The importance of T reg cell migration to nonlymphoid tissues has primarily been demonstrated during strong immune responses to foreign antigens after immunization, infection, or transplantation (8, 9, 27). In these settings, T reg cells are believed to restrain the responses of effector T cells, preventing collateral tissue damage and inflammation. For example, during delayed-type hypersensitivity responses mediated by OVA-specific TH1 cells, cutaneous T reg cells had no effect on the extent of footpad swelling after OVA injection, but instead promoted more rapid resolution of the inflammation (9). In contrast, T reg cells were able to inhibit development of spontaneous colitis mediated by CD4+CD45RBhi T cells in RAG-deficient mice even when their ability to access the intestines was severely impaired, and this is likely caused by their ability to inhibit the initial priming and differentiation of colitogenic T cells specific for intestinal antigens within secondary lymphoid tissues (28). Thus, it is not clear if T reg cell migration to nonlymphoid sites is required to maintain tolerance in the absence of any strong inflammatory response or infection.
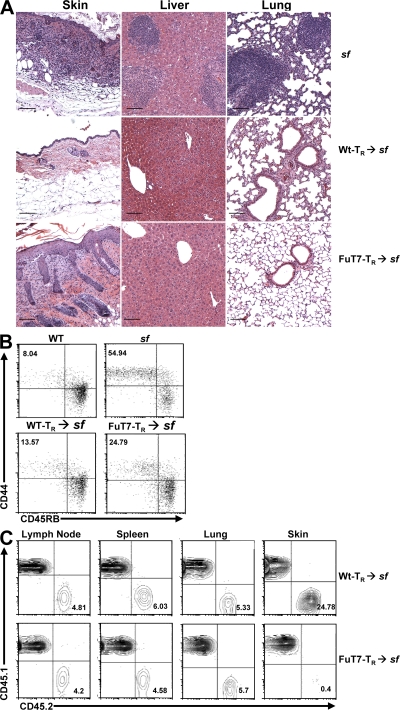
To determine if cutaneous T reg cells are required for immune homeostasis in the skin, we compared the ability of WT and FuT7−/−-T reg cells to prevent cutaneous inflammation after transfer into neonatal sf mice. In these experiments, we did not add any additional inflammatory stimuli or foreign antigens. Thus, unlike previous reports examining T reg cells in responses to highly immunogenic foreign antigens, our system examined the ability of T reg cells to control the initiation and progression of immune responses directed against innocuous self- and environmental-antigens, against which there are normally no strong inflammatory responses. Upon transfer, both WT- and FuT7−/−-T reg cells effectively prevented the early development of autoinflammatory disease observed in unmanipulated sf mice (unpublished data). However, ∼35 d after transfer, all recipients of FuT7−/−-T reg cells developed visible dermatitis that rapidly progressed to include multiple focal lesions. Histologically, the affected skin resembled that of sf mice, with dense leukocytic infiltration of the dermis accompanied by epidermal thickening and erosion (Fig. 4 A). Importantly, recipients of FuT7−/−-T reg cells were completely protected from the autoimmune hepatitis that occurs in sf mice, and there was only limited and sporadic inflammation observed in the lungs of these animals. As expected, WT-T reg cells prevented inflammation in all tissues analyzed for at least 5 mo after transfer.
Figure 4.
Blocking T reg cell migration to the skin results in cutaneous inflammation. (A) Photomicrographs of hematoxylin and eosin–stained sections from skin, liver, and lung of sf mice left untreated (top; 21 d old) or given WT- (middle; 53 d old) or FuT7−/−- (bottom; 35 d old) T reg cells. Bars, 100 μm. (B) Flow cytometry analysis of CD44 and CD45RB expression by gated CD4+Foxp3− cells from pooled LNs and spleens of age-matched WT and sf mice (top; 21 d old) or sf mice given WT- or FuT7−/−-T reg cells (bottom; 35 d old). Numbers indicate the frequency of activated CD44high/CD45RBlow cells. (C) Flow cytometry analysis of CD45.1 and CD45.2 expression by gated CD4+ T cells isolated from the indicated tissues of CD45.1+sf mice given CD45.2+ WT- or FuT7−/−-T reg cells 5 wk before. Percentages indicate the frequency of CD45.2+ T reg cells among CD4+ cells. Data are representative of three to six experiments.
Compared with sf mice given WT-T reg cells, recipients of FuT7−/−-T reg cells displayed mild lymphadenopathy in the skin-draining LNs, associated with a twofold increase in the frequency of CD44hiCD45RBlo effector memory CD4+ T cells in the LNs and spleen (Fig. 4 B and not depicted). However, the frequency of effector memory T cells was much lower than that seen in untreated sf mice. Moreover, whereas the frequencies of FuT7−/−-T reg cells in the peripheral LN, spleen, and lungs were similar to those in mice receiving WT cells, the FuT7−/−-T reg cells were almost completely absent from the inflamed skin (Fig. 4 C). Collectively, these data indicate that although FuT7−/−-T reg cells were maintained at normal numbers and could function in the skin-draining LNs and other peripheral tissues, their inability to access the skin itself resulted in dysregulated cutaneous immune responses and severe inflammation selectively at this site.
The skin is a complex immunological organ that is continuously exposed to antigens from the external environment and its own microbial flora. The consequences of dysregulated cutaneous immune responses can be dire, and include common inflammatory and allergic diseases such as psoriasis, pemphigus vulgaris, scleroderma, and atopic dermatitis. Our data demonstrate that the constitutive recirculation of T reg cells through the skin is severely impaired in the absence of FuT7, and that blocking T reg cell migration to the skin results in severe tissue-specific inflammatory disease. Thus, we define a prominent role for cutaneous T reg cells in maintaining normal immune homeostasis and tolerance at this site. These results underscore the importance of further defining the signals that direct T reg cell expression of cutaneous homing receptors and promote their migration to the skin, delineating the suppressive mechanisms used by T reg cells to control inflammatory responses in the skin, and understanding the specificity and homeostasis of cutaneous T reg cells.
MATERIALS AND METHODS
Mice.
sf mice (B6.Cg-Foxp3sf/J) were obtained from The Jackson Laboratory and bred to B6.SJL mice (Taconic Farms) to generate CD45.1+ animals. FuT7−/− mice and Foxp3gfp mice have been previously described (15, 17). All experiments were performed in accordance with institutional guidelines with approval from the Benaroya Research Institute Institutional Animal Care and Use Committee.
T reg cell purification and injection.
CD4+ T cells from pooled spleens and LNs were enriched using the mouse CD4+ isolation kit from Invitrogen according to the manufacturer's protocol. The CD4+ cells were subsequently labeled with anti-CD25-PE antibody (Miltenyi Biotech), and CD4+CD25+ cells were enriched using anti-PE magnetic beads (Miltenyi Biotech). The resulting cells were routinely ∼90% CD4+CD25+ as measured by flow cytometry, and normalized such that 106 CD4+CD25+ were given to 1–3-d-old sf mice by i.p. injection. In some experiments, CD25+ cells were further sorted into CD103+ or CD103− subsets by flow cytometry, and 0.5–1 × 106 cells from each subset were injected i.p. into neonatal sf mice. For tracking, cells were sorted from either CD45.1+ or CD45.2+ congenic mice.
Cell isolation and flow cytometry.
Lymphocytes were obtained from spleen and LNs by mechanical disruption. For lymphocyte isolation from skin and lungs, tissues were finely minced with scissors and digested with 0.14 U/ml Liberase Blendzyme 2 and 100 μg/ml DNase I (Roche) in plain RPMI, while being stirred at 37°C for 20 min. Released cells were filtered through a 70-μm strainer. This was repeated 2 times, pooling all released cells. All antibodies were obtained from eBioscience except for anti-CCR6, which was purchased from R&D Systems. Expression of functional E- and P-lig, CCR4, and CCR7 was measured by staining with ligand fusion proteins and secondary anti-huIgM and -huIgG antibodies purchased from Jackson Immunoresearch Laboratories, as previously described (12). In some experiments, intracellular Foxp3 was measured using anti-Foxp3 antibody (eBioscience) according to the manufactures protocol.
T cell proliferation assay.
The in vitro suppression assay was performed as previously described (12). In brief, CD4+CD25+ and CD4+CD25− T cells were isolated from either WT or FuT7−/− mice as indicated. CD25− cells were labeled with CFSE and co-cultured with CD4-depleted splenocytes for 96 h in the presence of 2 μg anti-CD28 and 4 μg anti-CD3 antibody in the presence or absence of the indicated ratio of CD25+ T reg cells.
Histology.
Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. 5-μm sections were stained with hematoxylin and eosin.
Online supplemental material.
Fig. S1 shows that T reg cells proliferate and up-regulate CD103 after transfer into neonatal sf mice. Fig. S2 shows that the frequency of CD4+CD25+ cells is significantly decreased in the skin of FuT7−/− mice. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20072594/DC1.
Supplementary Material
Acknowledgments
We thank Drs. Alexander Rudensky and John Lowe for providing mice; Dr. Jessica Hamerman for comments on the manuscript; Mary Beauchamp and Ashley Basset for histology processing and staining; Dr. K. Arumuganathan for flow cytometry assistance and cell sorting; and Matt Warren for administrative assistance.
This work was supported by grants to D.J. Campbell from the National Institutes of Health (DK072295, AI067750, and AI069889) and from the Department of Defense (USAMRAA W81XWH-07-0246). J.C. Dudda was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft.
The authors have no conflicting financial interests.
References
- 1.Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. [DOI] [PubMed] [Google Scholar]
- 2.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 4.Kim, J.M., J.P. Rasmussen, and A.Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197. [DOI] [PubMed] [Google Scholar]
- 5.Powrie, F., M.W. Leach, S. Mauze, L.B. Caddle, and R.L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 6.Lahl, K., C. Loddenkemper, C. Drouin, J. Freyer, J. Arnason, G. Eberl, A. Hamann, H. Wagner, J. Huehn, and T. Sparwasser. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huehn, J., K. Siegmund, J. C. Lehmann, C. Siewert, U. Haubold, M. Feuerer, G. F. Debes, J. Lauber, O. Frey, G. K. Przybylski, U. Niesner, M. de la Rosa, C.A. Schmidt, R. Brauer, J. Buer, A. Scheffold, and A. Hamann. 2004. Developmental Stage, Phenotype, and Migration Distinguish Naive- and Effector/Memory-like CD4+ Regulatory T Cells. J. Exp. Med. 199:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, I., L. Wang, A.D. Wells, M.E. Dorf, E. Ozkaynak, and W.W. Hancock. 2005. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegmund, K., M. Feuerer, C. Siewert, S. Ghani, U. Haubold, A. Dankof, V. Krenn, M.P. Schon, A. Scheffold, J.B. Lowe, et al. 2005. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 106:3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suffia, I., S.K. Reckling, G. Salay, and Y. Belkaid. 2005. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 174:5444–5455. [DOI] [PubMed] [Google Scholar]
- 11.Yurchenko, E., M. Tritt, V. Hay, E.M. Shevach, Y. Belkaid, and C.A. Piccirillo. 2006. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 203:2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sather, B.D., P. Treuting, N. Perdue, M. Miazgowicz, J.D. Fontenot, A.Y. Rudensky, and D.J. Campbell. 2007. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 204:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tietz, W., Y. Allemand, E. Borges, D. von Laer, R. Hallmann, D. Vestweber, and A. Hamann. 1998. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 161:963–970. [PubMed] [Google Scholar]
- 14.Hirahara, K., L. Liu, R.A. Clark, K. Yamanaka, R.C. Fuhlbrigge, and T.S. Kupper. 2006. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 177:4488–4494. [DOI] [PubMed] [Google Scholar]
- 15.Maly, P., A. Thall, B. Petryniak, C.E. Rogers, P.L. Smith, R.M. Marks, R.J. Kelly, K.M. Gersten, G. Cheng, T.L. Saunders, et al. 1996. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 86:643–653. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey, V.L., J.E. Wilkinson, and L.B. Russell. 1991. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 17.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 18.Bayer, A.L., A. Yu, D. Adeegbe, and T.R. Malek. 2005. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J. Exp. Med. 201:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, J.H., S.G. Kang, and C.H. Kim. 2007. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J. Immunol. 178:301–311. [DOI] [PubMed] [Google Scholar]
- 20.Siewert, C., A. Menning, J. Dudda, K. Siegmund, U. Lauer, S. Floess, D.J. Campbell, A. Hamann, and J. Huehn. 2007. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 37:978–989. [DOI] [PubMed] [Google Scholar]
- 21.Clark, R.A., and T.S. Kupper. 2007. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 109:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdmann, I., E.P. Scheidegger, F.K. Koch, L. Heinzerling, B. Odermatt, G. Burg, J.B. Lowe, and T.M. Kundig. 2002. Fucosyltransferase VII-deficient mice with defective E-, P-, and L-selectin ligands show impaired CD4+ and CD8+ T cell migration into the skin, but normal extravasation into visceral organs. J. Immunol. 168:2139–2146. [DOI] [PubMed] [Google Scholar]
- 23.Cotran, R.S., M.A. Gimbrone Jr., M.P. Bevilacqua, D.L. Mendrick, and J.S. Pober. 1986. Induction and detection of a human endothelial activation antigen in vivo. J. Exp. Med. 164:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harari, O.A., J.F. McHale, D. Marshall, S. Ahmed, D. Brown, P.W. Askenase, and D.O. Haskard. 1999. Endothelial cell E- and P-selectin up-regulation in murine contact sensitivity is prolonged by distinct mechanisms occurring in sequence. J. Immunol. 163:6860–6866. [PubMed] [Google Scholar]
- 25.Clark, R.A., B. Chong, N. Mirchandani, N.K. Brinster, K. Yamanaka, R.K. Dowgiert, and T.S. Kupper. 2006. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176:4431–4439. [DOI] [PubMed] [Google Scholar]
- 26.Ley, K., and G.S. Kansas. 2004. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4:325–335. [DOI] [PubMed] [Google Scholar]
- 27.Chen, Z., A.E. Herman, M. Matos, D. Mathis, and C. Benoist. 2005. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denning, T.L., G. Kim, and M. Kronenberg. 2005. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J. Immunol. 174:7487–7491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.