Abstract
Transforming growth factor β–activated kinase 1 (TAK1), a member of the MAPKKK family, is a key mediator of proinflammatory and stress signals. Activation of TAK1 by proinflammatory cytokines and T and B cell receptors induces the nuclear localization of nuclear factor κB (NF-κB) and the activation of c-Jun N-terminal kinase (JNK)/AP1 and P38, which play important roles in mediating inflammation, immune responses, T and B cell activation, and epithelial cell survival. Here, we report that TAK1 is critical for the survival of both hematopoietic cells and hepatocytes. Deletion of TAK1 results in bone marrow (BM) and liver failure in mice due to the massive apoptotic death of hematopoietic cells and hepatocytes. Hematopoietic stem cells and progenitors were among those hematopoietic cells affected by TAK1 deletion–induced cell death. This apoptotic cell death is autonomous, as demonstrated by reciprocal BM transplantation. Deletion of TAK1 resulted in the inactivation of both JNK and NF-κB signaling, as well as the down-regulation of expression of prosurvival genes.
A proper level of apoptosis is required for the maintenance of normal hematopoietic homeostasis by removing aged or abnormal cells. The balance between proapoptotic and antiapoptotic mechanisms is tightly controlled in hematopoietic cells, including hematopoietic stem cells (HSCs) and progenitor cells (HSC/Ps) by intrinsic signals that are subject to regulation by signals emanating from the BM microenvironment (1–3). Hematopoietic cytokines such as stem cell factor, IL-3, and thrombopoietin provide a survival signal for hematopoietic cells (4, 5) by inducing the activation of PI3K/AKT (6) and NF-κB (7) signaling, and by up-regulating antiapoptotic genes, such as members of the Bcl2 family (1–3). Proinflammatory cytokines, including TNF-α (8) and IL-1β (9), as well as Fas ligand (10), all mediate dual signals for HSC/P functions. A negative signal induces programmed cell death and/or differentiation, whereas a positive signal promotes proliferation and survival. Disruption of this balance will result in hematopoietic disorders, including BM failure and leukemia (1–3).
TGF-β–activated kinase 1 (TAK1) is a key mediator of stress and proinflammatory signals (11). The proinflammatory cytokines induce both proapoptotic and antiapoptotic signals in their target cells. TAK1 mediates the prosurvival signal of the proinflammatory cytokines by inducing the nuclear localization of NF-κB and the activation of c-Jun N-terminal kinases (JNKs)/AP1 (11), whereas the proapoptotic signal is mediated by the activation of the caspase cascade (12). Studies have demonstrated that TAK1-transduced signals from TCRs and B cell receptors, as well as antigen stimulation, play a role in T and B cell activation and survival, T regulatory cell development, and B cell–mediated innate immunity (13–16). The role of TAK1 in hematopoiesis is still not fully understood. Although previous studies suggested that proinflammatory cytokines might play a negative role in hematopoiesis and contribute to some of the BM failure syndromes (17, 18), recent studies demonstrate that HSC/Ps are resistant to proinflammatory and stress signal–induced apoptosis (10, 19). We propose that TAK1 is expressed and activated in hematopoietic cells, including HSC/Ps, thus protecting them from apoptosis.
The TAK1-null phenotype is lethal early in embryonic development (11, 13), impeding exploration of the role of TAK1 in adult hematopoiesis. To investigate whether TAK1 plays a role in the regulation of normal hematopoietic homeostasis, we generated inducible TAK1 knockout mice. We found that inducing the deletion of TAK1 in adult mice results in BM and liver failure due to massive cell-autonomous apoptotic death of hematopoietic cells, including HSC/Ps, and hepatocytes. Further study demonstrated that TAK1 mediates the survival signal in HSC/Ps via activation of JNK/AP1 and NF-κB signal pathways and up-regulation of survival gene expression.
RESULTS
TAK1 expression and activity in hematopoietic cells
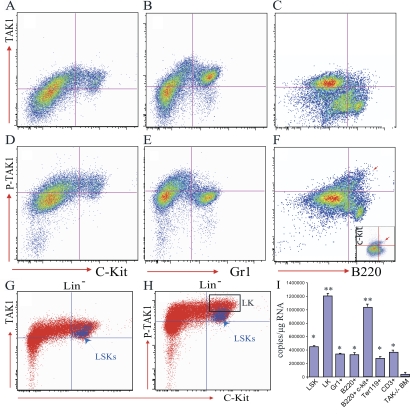
To study whether TAK1 is involved in normal hematopoiesis, we first examined whether TAK1 is expressed in hematopoietic cells by using quantitative RT-PCR assays. TAK1 knockout BM cells were used as a negative control. We found that TAK1 RNA is expressed in all lineages of hematopoietic cells, including HSCs (lineage−Sca1+c-kit+ cells [LSKs]), committed hematopoietic progenitors (CPs) (lineage−Sca1−c-kit+ cells [LKs]), mature myeloid cells (Gr1+), B cells (B220+), B cell progenitors (B220+c-kit+), nucleated erythroid cells (Ter119+), and T cells (CD3+). Its level of expression is also increased in progenitors including CPs and B cell progenitors (Fig. 1 I). Furthermore, by using a flow cytometry–based intracellular protein detection assay, we found that the expression of TAK1 protein in c-kit+ HSC/Ps (including HSCs and CPs) and Gr1+ myeloid cells was higher than that in B220+ B cells (Fig. 1, A–C); yet, TAK1 activity (indicated by p-TAK1 levels) (20) is higher in c-kit+ HSC/Ps than in differentiated Gr1+ myeloid cells and B220+ B lymphocytes (Fig. 1, D–F). We further separated HSCs and CPs by sca1 and c-kit staining followed by lineage depletion and found that, although TAK1 protein levels were comparable in HSCs and CPs, p-TAK1 levels were higher in CPs than in HSCs (Fig. 1, G and H). Interestingly, we also found that among B220+ cells, B cell progenitors displayed higher levels of TAK1 activity (Fig. 1 F).
Figure 1.
TAK1 is expressed and activated in hematopoietic cells, including HSC/Ps. BM cells were collected into lyse/fix buffer immediately after the mice were killed to fix the BM cells and lyse the RBCs simultaneously. BM-nucleated cells were washed three times with PBS containing 5% FBS to completely remove all traces of the lyse/fix buffer. The cells were stained with cell surface markers as indicated and then permeabilized using permeabilization buffer for TAK1 and p-TAK1 staining. (A–C) High level of TAK1 protein expression in c-kit+ hematopoietic cells and Gr1+ myeloid cells, but low expression in B220+ B cells. (D–F) TAK activity (shown here by p-TAK1 levels) is higher in c-kit+ hematopoietic cells (including B220+c-kit+ B cell progenitors, red arrow in F). (G and H) No obvious difference in TAK1 expression in LSK-HSC/Ps and LK-CPs, but increased TAK1 activity in LK-CPs compared with LSK-HSC/Ps. (I) TAK1 is expressed in all lineages of hematopoietic cells, including HSC/Ps, as shown by quantitative RT-PCR. BM cells from TAK1 knockout mice (4 d after mutation induction) were used as negative controls. Compared with differentiated cells, including Gr1+ myeloid cells, B220+ B cells, Ter119+-nucleated erythoid cells, and CD3+ T cells, TAK1 RNA expression was significantly higher in LK-CPs and B cell progenitors (B220+c-kit+). Both * and ** are P < 0.01. *, compared with TAK1−/− BM cells; **, compared with mature hematopoietic cells, including Gr1+, B220+, and CD3+ cells.
Generation of inducible TAK1−/− mice
To study whether TAK1 plays a role in hematopoiesis, we generated inducible TAK1 knockout mice (Mx1Cre+TAK1fx/fx, TAK1−/− hereafter) by crossing TAK1fx/fx mice with Mx1Cre mice. The second exon of the TAK1 gene (which encodes the enzyme's catalytic domain) is flanked with two loxp sites in the TAK1fx/fx mice (13) and thus can be deleted by Cre-mediated recombination. Mx1Cre mice are an interferon-inducible Cre mouse line in which Cre expression can be induced at any time after birth by injecting the mice with the interferon inducer polyinosinic:polycytidylic acid (polyI:C) (21). Mx1Cre-mediated recombination of loxp sites is very efficient in hematopoietic cells from the BM, spleen, and thymus, including HSC/Ps as well as liver cells (21). By proper mating, we generated TAK1−/−, heterozygous (Mx1Cre+TAK1fx/+, TAK1+/− hereafter), and WT control mice (Mx1Cre−TAK1fx/fx or Mx1Cre−TAK1fx/+, WT hereafter) in the same litter. Therefore, all of the TAK1−/− mice analyzed had both TAK1+/− and WT littermate controls. At 6–8 wk of age, the mice (including TAK1+/− and WT controls) were injected with polyI:C either once or three times (once every other day) to induce the TAK1 deletion. To control for the possibility that polyI:C injection itself might affect hematopoiesis, at least one mouse in each litter was maintained without injection and served as noninjected control. All mice were checked daily for phenotypic changes.
Liver failure in inducible TAK1 knockout mice due to increased hepatocyte apoptosis
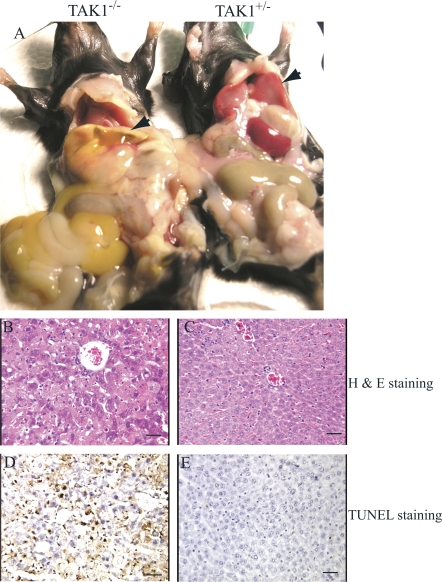
We found that all the TAK1−/− mice died within 8–10 d after the first polyI:C injection, regardless of whether they were injected once or three times; however, the TAK1+/− mice showed no significant defects. By careful dissection of the mice, we found that all of the TAK1−/− mice had both jaundice and ascites (Fig. 2 A). The livers of the knockout mice were relatively smaller and stiff, and they appeared a pale, jaundiced color. Destruction of normal liver histological structure was evident upon microscopic analysis. The nuclei of >30–50% of the hepatocytes were condensed because of apoptosis, which was demonstrated by terminal deoxynucleotidyltransferase-mediated UTP end-labeling (TUNEL) staining (Fig. 2, B–E). Although, >30% of the mice showed intestinal, urinary bladder, and/or gallbladder hemorrhaging (not depicted), we believe that acute liver failure, as a consequence of massive hepatocyte apoptosis, was the major cause of mortality among the TAK1−/− mice. In support of this notion, we observed that the TAK1−/− recipient mice that received normal BM did not show any signs of bleeding but did show evidence of significant hepatocyte apoptosis (not depicted). These mice also died 8–10 d after their first polyI:C injection. This also suggests that the bleeding observed in the TAK1−/− mice was not due to the liver defects and was not the cause of mortality.
Figure 2.
Hepatocyte apoptosis and liver failure in TAK1−/− mice.TAK1−/− mice were killed on day 8 after the first polyI:C injection. (A) Both jaundice and ascites were observed in TAK1−/− mice. Livers of the TAK1−/− mice were pale and jaundiced (left specimen, arrowhead) compared with TAK1+/− control (right specimen, arrow). Nuclear condensation due to apoptosis was observed in >30–50% of hepatocytes of TAK1−/− mice as shown by hematoxylin and eosin staining of liver sections (B), which is confirmed by TUNEL staining (D). Liver sections from TAK1+/− mice were used as controls. (C and E) Bars, 100 μm.
BM failure in TAK1 knockout mice due to the apoptotic loss of hematopoietic cells
To investigate whether TAK1 deletion affects hematopoiesis, we first examined white blood cell (WBC) counts, platelet number (plt), RBC counts, and hemoglobin (Hb) concentrations at 8 d after the first polyI:C injection. We found that both WBCs and plt were significantly reduced in the TAK1−/− mice (Fig. 3, A and B). We believe that the decrease in plt counts was the reason for the bleeding seen in multiple organs in the TAK1−/− mice because their plt counts were <200 × 109/ml. Human patients showing such severe thrombocytopenia typically present with severe bleeding. However, RBCs and Hb were slightly lower in TAK1−/− than in WT and TAK1+/− controls (not depicted). These data suggest that TAK1−/− results in a pancytopenic effect.
Figure 3.
Pancytopenia and BM failure in TAK1−/− mice. Peripheral blood, BM, spleens, and thymuses were collected on day 8 after the first polyI:C injection. WBC count (A), plt in peripheral blood (B), and nucleated cell number in the BM (C), spleen (D), and thymus (E) were measured in TAK1−/−, TAK1+/−, and WT mice, as well as in noninjected control mice. BM sections from TAK1+/− (F) and TAK1−/− (G) mice at day 8 after the first polyI:C injection were stained with hematoxylin and eosin. BM sections from TAK1+/− (H) and TAK1−/− (I) mice at day 4 after the first polyI:C injection were stained with TUNEL to display the apoptotic cells (indicated by red color staining). Bars, 100 μm. *, P < 0.05; **, P < 0.01.
To study whether the pancytopenia in TAK1−/− mice was due to BM hematopoietic defects, we collected BM, spleens, and thymuses from TAK1−/− and control mice at day 8 after the first polyI:C injection. The total nucleated cell number (TNC) of BM, spleens, and thymuses were analyzed. We found that the TNC of BM, spleens, and thymuses was significantly decreased in TAK1−/− mice compared with TAK1+/− and WT control mice, as well as noninjected control mice (Fig. 3, C–E, and Fig. S1, which is available at http://www.jem.org/cgi/content/full/jem.20080297/DC1). Although the TNCs in the BM, spleens, and thymuses in the polyI:C-injected groups were more diverse from mouse to mouse, shown by the relatively larger standard deviations, no significant differences were observed between TAK1+/−and WT, the latter being comparable to noninjected control mice. This indicates that heterozygous TAK1 deletion did not affect hematopoiesis in the mice. It also suggests that polyI:C injection itself likely contributes negligibly to the phenotypes we observed in the TAK1−/− mice. This notion was supported by cell surface staining and flow cytometric analysis. We found that there were no significant differences among TAK1+/−, WT, and noninjected control mice in the percentage of myeloid cells and T and B lymphocytes in all hematopoietic tissues examined including the peripheral blood, BM, and spleen. However, in TAK1−/− mice, significant increases in T cell percentage were consistently observed in the peripheral blood, BM, and spleen (the absolute T cell number in all these tissues was still dramatically lower than controls; Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20080297/DC1). We believe that the increase in T cell percentage in the TAK1−/− mice has nothing to do with thymic T cell production because the thymus gland in TAK1−/− mice was very small. Only 1–4 × 106 TNCs could be obtained from each TAK1−/− mouse thymus, which was 1/20 to 1/100 of the number of TNCs obtained from each control mouse thymus.
Histological sectioning showed that BM from TAK1−/− mice was hypocellular and BM structure was destroyed, similar to the myelodepletion phenotype seen in mice after high-dose chemotherapy or irradiation (Fig. 3, E and G). These data suggest that the TAK1−/− mice had BM failure. In situ TUNEL staining demonstrated that the BM failure of the TAK1−/− mice was due to the massive apoptotic death of hematopoietic cells (Fig. 3 I).
Because no significant difference was observed between TAK+/− and WT mice, and similar to TAK1−/− mice, TAK1+/− mice also express Cre after polyI:C injection, we considered TAK1+/− mice to be better controls than WT mice. Therefore we used TAK1+/− mice as controls in most of our subsequent studies.
HSC/Ps are among those hematopoietic cells affected by TAK1 deletion–induced apoptosis in TAK1−/− mice
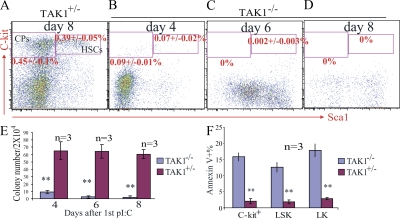
To investigate whether HSC/Ps were affected in TAK1−/− mice, we first examined HSC/P numbers by cell surface marker staining and flow cytometric analysis. We found that both LSK-HSCs and LK-CPs were significantly reduced in TAK1−/− mice (Fig. 4, A–D). The obvious reduction in HSCs and CPs could be observed at day 4 after the first dose of polyI:C injection (Fig. 4 B). There were almost no HSCs nor CPs detectable at day 8. The reduction in HSC/Ps in TAK1−/− mice was confirmed by CFU assay (Fig. 4 E). We found that the CFU number (counted at day 12 after seeding, which reflects the number of functional HSC/Ps) was significantly reduced in TAK1−/− BM. Upon further analysis using annexin V staining, we found a three- to fivefold increase in apoptosis (shown as annexin V+) in the c-kit+ population (consisting of HSCs and CPs) from TAK1−/− mice compared with control mice at 16 h after a single polyI:C injection. Upon further analysis by separating HSCs from CPs within the c-kit+ population, we found apoptosis among both of these cell types to be significantly increased (Fig. 4 F) compared with such cells taken from control mice.
Figure 4.
HSC/Ps are affected by TAK1 deletion–induced apoptotic cell death in TAK1−/− mice. BM was collected from TAK1+/− and TAK1−/− mice on days 4, 6, and 8 after the first polyI:C injection. After lysis of RBCs, BM-nucleated cells were stained with cell surface markers and analyzed by flow cytometry for HSCs and CPs. Progressive loss of HSCs and CPs in TAK1−/− mouse BM is shown by lineage marker and Sca1 c-kit staining (B–D). Day 8 TAK1+/− BM cells were used as a control (A). (E) Progressive reduction of colony-forming ability of TAK1−/− BM cells after TAK1 deletion. (F) BM cells were collected from TAK1−/− and TAK1+/− mice 16 h after a single polyI:C injection, and apoptosis was checked by annexin V staining. *, P < 0.05; **, P < 0.01.
Hematopoietic cells of TAK1−/− mice show intrinsic defects
Previously, we have found that Mx1Cre can mediate gene deletion in both hematopoietic and BM stromal cells (22). To address whether the defects of hematopoietic cells in TAK1−/− mice were intrinsic to the cells, reciprocal BM transplantations were performed. We transplanted TAK1−/− BM cells (before deletion was induced) into lethally irradiated syngeneic WT recipients. Because in these mice the BM microenvironment is normal, we were able to test the function of TAK1−/− hematopoietic cells within this normal BM microenvironment. 1 mo after transplantation, the recipients received polyI:C injections every other day for a total of three injections and were killed for analysis on day 8 after the first injection. We found pancytopenia and BM failure in the recipients that had received the TAK1−/− BM; however, the TAK1+/− BM recipients were normal (Fig. 5, A, B, and E–H). This suggests that the hematopoietic cells from TAK1−/− mice have cell-intrinsic defects that prohibit their survival in a normal BM microenvironment. We also transplanted BM from WT mice into the lethally irradiated TAK1−/− recipients (before deletion induction). 1 mo after transplantation, the recipients were given three polyI:C injections, once every other day, and the livers, peripheral blood, spleens, and BM were harvested for analysis on day 8 after the last injection. We found significant apoptosis of hepatocytes in all TAK1−/− recipients (not depicted). However, the normal BM hematopoiesis in TAK1−/− recipients suggested that the TAK1 deletion BM microenvironment has a normalizing hematopoietic supportive function (Fig. 5, C and D). Consistent with this observation, we found that plt counts and Hb concentrations in the TAK1−/− recipients were comparable to those of the TAK1+/− recipients (Fig. 5, E–H). Furthermore, we found that the livers of the TAK1−/− recipients that had received WT BM had obvious myeloid cell infiltration (not depicted). The WBC counts, neutrophil percentages, and neutrophil/lymphocyte ratios were significantly increased in the TAK1−/− recipients compared with the TAK1+/− recipients (Fig. 5 E and not depicted). We believe that the increase in WBC counts and neutrophils as well as the myeloid infiltration of liver tissue in the TAK1−/− recipients were due to inflammatory reactions induced by hepatocyte apoptosis.
Figure 5.
Cell-autonomous defects of TAK1−/− HSC/Ps. Reciprocal transplantation assay was performed by transplantation of TAK1−/− BM (before mutation was induced) into lethally irradiated WT mice (A) and, reciprocally, WT BM into lethally irradiated TAK1−/− mice (before mutation was induced; C). TAK1 deletion was induced 1 mo after transplantation by injecting the recipient mice with polyI:C every other day for a total of three injections. TAK1+/− mice were used as respective controls in this experiment (B and D). On day 8 after the first polyI:C injection, recipient mice were killed for analysis. BM sections from the recipients showed that TAK1−/− HSC/Ps failed to grow in the WT BM environment (A), whereas the TAK1−/− BM microenvironment was able to support the growth of WT HSC/Ps (C). (E–H) Significant reduction of WBC counts and plt in peripheral blood, and nucleated cells in the BM and spleen in WT recipients that received TAK1−/− BM. (E) Obvious increase in WBC counts in TAK1−/− recipients that received WT BM. (I and J) Equal numbers of TAK1−/− BM cells (CD45.2+, before mutation was induced) were mixed with WT (CD45.1+) BM cells and transplanted into lethally irradiated WT (CD45.1+) recipients. 6 wk after transplantation, TAK1 mutation was induced by injecting the recipient mice with polyI:C every other day for a total of three injections. TAK1+/− BM cells were transplanted in parallel as a control. The contribution of the TAK1−/− BM HSC/Ps to hematopoiesis in the recipient mice (shown as CD45.2+ percentage in peripheral blood) was significantly lower than that of control BM HSC/Ps. Data shown in I and J were analyzed on day 15 after the first polyI:C injection. *, P < 0.05; **, P < 0.01.
DISCUSSION
TAK1 was originally identified as a key regulator of TGF-β/bone morphogenic protein signals (23). It is known to play an important role in early embryonic development (13). TAK1-null mice die at embryonic day 11.5 with significant vascular defects, reminiscent of the mouse phenotype in which the TGF-β1 receptors ALK-1 and endoglin have been knocked out. Moreover, TAK1 overexpression can compensate for the TGFβ1 receptor knockout phenotype (24). However, in vitro biochemical and in vivo genetic studies suggest that TAK1 is also a significant mediator of inflammatory cytokine signals (11).
Mouse embryonic fibroblasts from TAK1-null mice exhibit dramatically impaired NF-κB and JNK activation through TNFR-1, IL-1R, and Toll-like receptors. These cells are also highly sensitive to TNF-α–induced apoptosis (11). TAK1 has also been found to be critical for keratinocyte survival. Keratinocytes with TAK1 deletions are inactivated for NF-κB and JNK, and are sensitive to TNF-α–induced apoptosis (25).
Our data suggest that TAK1 might mediate a general survival signal for many tissue cells, although the sensitivity of different types of cells to TAK1 deletion varies. In our inducible TAK1 knockout mice, massive apoptotic cell death was observed in hematopoietic cells in the BM, spleen, and thymus, as well as in hepatocytes, where Cre-mediated TAK1 deletion occurred. We also found that in hematopoietic cells and hepatocytes, TAK1 stimulated a survival signal also mediated by up-regulating the expression of survival genes via the IKKκ–NF-κB and JNK–AP1 signaling pathways (not depicted).
It has been reported that NF-κB mediates a very important survival signal to protect cells from TNF-α–induced apoptosis. Inactivation of NF-κB by gene mutation or pharmacological inhibition sensitizes such cells to TNF-α–induced apoptosis. However, NF-κB knockout mice (Mx1Cre-mediated polyI:C-inducible knockout, the same system we used in our present studies) developed normally under normal husbandry without differences in gross anatomy, histological organization, or hepatic function (26). In contrast to the acute liver failure of TAK1−/− mice, the syndrome of hepatic failure resulting from hepatocyte apoptosis seen in NF-κB knockout mice occurred only when the mice were treated with TNF-α (26). Moreover, as opposed to the BM failure phenotype of our TAK1−/− mice, which was secondary to cell-autonomous apoptosis, the mice with IKKα/NF-κB signal inactivation showed stress-related B and T cell depletion, whereas HSC/Ps remained unaffected in this condition (27). In fact, the myeloid cells of NF-κB–inactivated mice show a high proliferative index and multiple tissue inflammation reactions due to autocrine secretion of inflammatory cytokines (such as TNF-α) (27). This suggests that IKKβ/NF-κB signaling is not essential for the survival of HSC/Ps and myeloid cells.
In TAK1−/− HSC/Ps, in addition to the inactivation of NF-κB signals, JNK signals were also inactivated. The role of JNK signaling is cell context dependent. Both proapoptotic and prosurvival effects of JNK signals have been reported (28). The role of JNK in HSC/Ps has not yet been addressed. We predict that both JNK and NF-κB signals are required for HSC/P survival in mice. This needs to be further confirmed by conditional compound knockout mouse studies.
Studies using tissue-specific TAK1 knockout mice have demonstrated that TAK1, mediating B cell receptor signaling and antigen responses in B cells and TCR signaling in T cells, is essential for B cell–mediated innate immunity and both peripheral T cell and T-regular cell activation, survival, and function (13–16). However, in these knockout mice, significant reductions of nucleated cells in the thymus and of B cells in the BM and spleen were not observed (13–16). In our inducible TAK1−/− mice, we found significant reductions in the numbers of all lineages of hematopoietic cells, including myeloid cells and B cells and T cells in the spleen and BM. We also found a dramatic reduction in thymus size due to decreased numbers of thymocytes (Fig. 3). We speculate that c-kit+ progenitors might possibly rely more on TAK1 for their survival, and that these were not targeted in the B cell– and T cell–specific knockout mice. In support of this idea, our data showed higher TAK1 activity in HSC/Ps and B cell progenitors than mature myeloid and B cells. But further study will be needed to establish this definitively. Whether cells with higher TAK1 activity are more sensitive to TAK1 deletion–induced apoptosis than cells with lower TAK1 activity will require further detailed investigation.
Both JNK and NF-κB have been found to be activated in many malignant disorders and inflammatory diseases (29, 30). We predict that TAK1, which is upstream of JNK and NF-κB, might also be involved in these disorders. Further study may also reveal whether the BM failure phenotype seen in our TAK1−/− mice might also be involved in some BM failure syndromes such as aplastic anemia. Further examination of TAK1 expression profiles and activities in clinical samples could be expected to provide new insights into the important roles TAK1 might play in these diseases.
MATERIALS AND METHODS
Generation of inducible TAK1 knockout (TAK1−/−) mice.
TAK1fx/fx mice were described previously (13). Exon-2 of the TAK1 gene was flanked by two loxp sites. Mx1Cre mice were purchased from JAX Laboratories (21). All mice were maintained according to the standards set forth in the National Institutes of Health Guidelines for the Care and Use of Animals in the animal facility at Loyola University Medical Center in a CD57B6 background. All experiments performed on animals were approved in advance by Loyola University Institutional Animal Care and Use Committee (protocol no. 108832). By crossing Mx1Cre mice with TAK1fx/fx mice and then backcrossing, we generated interferon-inducible TAK1 knockout mice, including TAK1−/−, TAK1+/− (heterozygous, Mx1Cre+TAK1fx/+) and WT (WT, MX1Cre−TAK1fx/fx or MX1Cre−TAK1fx/+) animals in the same litter, so that all the TAK1−/− mice analyzed had proper controls. 6–8 wk after birth, one mouse from each litter was maintained without injection as noninjected control, and all other mice, including TAK1+/− and WT controls, were injected with 5 μg/g body weight of polyI:C for either a single injection or every other day for a total of three injections to induce TAK1 deletions.
Mouse hematopoietic phenotype analysis.
Mice were killed at the indicated time points to collect peripheral blood, spleen, thymus, and BM. Peripheral blood was analyzed for WBC counts, plt, RBC counts, and Hb concentration by using Hemavet 950FS (Drew Scientific Inc.). After lysis of RBCs, nucleated cells from peripheral blood, spleen, thymus, and BM were counted and further stained with cell surface markers for phenotype analysis by using flow cytometry as described previously (22). All the fluorescent antibodies used in flow cytometric analysis were purchased from eBioscience.
Reciprocal BM transplantation.
To test the role of TAK1 in HSC/Ps, BM from either TAK1−/− or TAK1+/− mice (before induction of TAK1 deletion) was transplanted into lethally irradiated WT mice. Each recipient mouse received 5 × 106 nucleated BM cells. 6 wk after transplantation, the recipients were injected with polyI:C every other day for a total of three injections. Hematopoietic phenotypes were analyzed on day 8 after the first injection. To test the role of TAK1 on the function of the BM microenvironment, BM from WT mice was transplanted into lethally irradiated TAK1−/− and TAK1+/− recipient mice (before mutation was induced). 6 wk later, TAK1 deletion was induced by three polyI:C injections. Both liver and hematopoietic phenotypes were analyzed on day 8 after the first injection.
Competitive BM transplantation.
Equal numbers of TAK1−/− (CD45.2+, before induction of TAK1 deletion) and WT (CD45.1+) mouse BM cells were mixed and transplanted into lethally irradiated WT (CD45.1+) recipient mice. Control TAK1+/− mice were transplanted in the same manner. 6 wk after transplantation, the contribution of donor HSC/Ps to hematopoiesis in recipients was assessed by analyzing the CD45.2+ cell percentage in the peripheral blood of recipients. As expected, both TAK1−/− and TAK1+/− HSC/Ps contributed close to 50% of hematopoietic cells in the recipients (TAK1−/− HSC/Ps contribute ∼46.8% ± 5.6%, whereas TAK1+/− HSC/Ps contribute ∼51.6% ± 6.1%). Recipients were next injected with three polyI:C injections as described above, and the contributions of TAK1−/− HSC/Ps to hematopoiesis in recipient mice were evaluated again on day 15 after the first injection.
Apoptosis analysis.
Hepatocyte apoptosis was analyzed by counting the percentage of hepatocytes with small, condensed nuclear and TUNEL+ staining. TUNEL staining was performed using the DeadEnd Colorimetric TUNEL System (Promega) according to the protocol supplied. To analyze apoptosis in BM HSC/Ps, BM was collected from TAK1−/− and control mice at 15–18 h after a single polyI:C injection. BM cells were collected into lyse/fix buffer (BD Biosciences) for not more than 10 min to fix the nucleated cells and lyse the RBCs simultaneously. After two washes with cold PBS/2% FBS, the nucleated cells were adjusted to a concentration of 5 × 106/ml in 1× Binding Buffer (BD Biosciences) and aliquotted into 5-ml staining tubes, 100 μl of cells per tube. Cells were then stained with allophycocyanin (APC)–c-kit and FITC–annexin V (BD Biosciences) or PECy5.5-lineage+ markers (including Gr1, B220, Ter119, CD3, and CD8), PE-Sca1, APC–c-kit, and FITC–annexin V for 20 min at room temperature. After two washes with 1× Binding Buffer, cells were analyzed by flow cytometry for annexin V+ cell percentage in different cell populations.
Intracellular protein analysis.
BM cells were collected into lyse/fix buffer (BD Biosciences) to simultaneously fix the nucleated cells and lyse the RBCs. After two washes with cold PBS/2% FBS, BM cells were stained with cell surface markers as indicated for 30 min. Cells were then permeabilized using a fixation/permeabilization kit (BD Biosciences) for 20 min at room temperature. Cells were then washed with wash buffer twice and stained with antibodies against intracellular proteins, followed by APC-conjugated secondary antibody staining. The levels of intracellular proteins were detected by flow cytometry comparing the APC intensity in different populations of the BM cells. Rabbit anti-TAK1, rabbit anti–p-TAK1, rabbit anti–p-NF-κB P65, rabbit anti–p-P38 (Thr180/Tyr182), and rabbit p-JNK (Thr183/Tyr185) antibodies were purchased from Cell Signaling Technology.
RT-PCR to detect gene expression.
RNA was extracted using RNeasy Plus Mini kit (QIAGEN). cDNA was prepared using SuperScript First-Strand Synthesis System (Invitrogen). Quantitative PCR was performed by using SYBR Green PCR master (Applied Biosystems). Each sample was a mixture of LSK-HSCs from three mice with the same phenotype. Triplicate RT-PCRs were performed.
Statistical analyses.
t tests were performed to assess the significance of changes in TAK1−/− group data compared with both TAK1+/− and WT groups.
Online supplemental material.
Fig. S1 shows reduction of BM-nucleated cell numbers in TAK1−/− mice after the induction of TAK1 deletion. Fig. S2 shows analyses of the percentage of mature hematopoietic cells in the TAK1−/− and littermate control mice. Figs. S1 and S2 are available at http://www.jem.org/cgi/content/full/jem.20080297/DC1.
Supplementary Material
Acknowledgments
We appreciate the excellent animal care services provided by the staff of the Department of Comparative Medicine at Loyola University Medical Center. Ms. Patricia Simms helped with flow cytometric sorting of HSCs and progenitors, as well as in the analysis of flow cytometry data. We appreciate the ongoing professional collaborations of Drs. Nancy Zeleznik-Le and Andrew Dingwall, whose scientific suggestions and discussions improved the present studies.
P. Breslin is supported in part by a grant from the Jimmy Burns Foundation.
The authors have no conflicting financial interests.
Abbreviations used: APC, allophycocyanin; CP, committed hematopoietic progenitor; Hb, hemoglobin; HSC, hematopoietic stem cell; HSC/P, HSC and progenitor cell; JNK, c-Jun N-terminal kinase; LK, lineage−Sca1−c-kit+ cell; LSK, lineage−Sca1+c-kit+ cell; plt, platelet number; polyI:C, polyinosinic:polycytidylic acid; TAK1, TGF-β–activated kinase 1; TNC, total nucleated cell number; TUNEL, terminal deoxynucleotidyltransferase-mediated UTP end-labeling; WBC, white blood cell.
M. Tang, X. Wei, and Y. Guo contributed equally to this paper.
X. Wei's present address is Henan Tumor Hospital, 127 Dongming Road, Zhengzhou 450008, China.
Y. Guo's present address is Dept. of Respiratory and Molecular Biology, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200127, China.
References
- 1.Orelio, C., and E. Dzierzak. 2007. Bcl-2 expression and apoptosis in the regulation of hematopoietic stem cells. Leuk. Lymphoma. 48:16–24. [DOI] [PubMed] [Google Scholar]
- 2.Motoyama, N., F. Wang, K.A. Roth, H. Sawa, K. Nakayama, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, S. Fujii, et al. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 267:1506–1510. [DOI] [PubMed] [Google Scholar]
- 3.Opferman, J.T., H. Iwasaki, C.C. Ong, H. Suh, S. Mizuno, K. Akashi, and S.J. Korsmeyer. 2005. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 307:1101–1104. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, J.E., K. Bhalla, and R. Hoffman. 1994. Effects of interleukin-3 and c-kit ligand on the survival of various classes of human hematopoietic progenitor cells. Blood. 83:1507–1514. [PubMed] [Google Scholar]
- 5.Kimura, S., A.W. Roberts, D. Metcalf, and W.S. Alexander. 1998. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc. Natl. Acad. Sci. USA. 95:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blume-Jensen, P., R. Janknecht, and T. Hunter. 1998. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 8:779–782. [DOI] [PubMed] [Google Scholar]
- 7.Kerzic, P.J., D.W. Pyatt, J.H. Zheng, S.A. Gross, A. Le, and R.D. Irons. 2003. Inhibition of NF-kappaB by hydroquinone sensitizes human bone marrow progenitor cells to TNF-alpha-induced apoptosis. Toxicology. 187:127–137. [DOI] [PubMed] [Google Scholar]
- 8.Rusten, L.S., F.W. Jacobsen, W. Lesslauer, H. Loetscher, E.B. Smeland, and S.E. Jacobsen. 1994. Bifunctional effects of tumor necrosis factor alpha (TNF alpha) on the growth of mature and primitive human hematopoietic progenitor cells: involvement of p55 and p75 TNF receptors. Blood. 83:3152–3159. [PubMed] [Google Scholar]
- 9.Hangoc, G., D.E. Williams, J.H. Falkenburg, and H.E. Broxmeyer. 1989. Influence of IL-1 alpha and -1 beta on the survival of human bone marrow cells responding to hematopoietic colony-stimulating factors. J. Immunol. 142:4329–4334. [PubMed] [Google Scholar]
- 10.Pearl-Yafe, M., J. Stein, E.S. Yolcu, D.L. Farkas, H. Shirwan, I. Yaniv, and N. Askenasy. 2007. Fas transduces dual apoptotic and trophic signals in hematopoietic progenitors. Stem Cells. 25:1448–1455. [DOI] [PubMed] [Google Scholar]
- 11.Shim, J.H., C. Xiao, A.E. Paschal, S.T. Bailey, P. Rao, M.S. Hayden, K.Y. Lee, C. Bussey, M. Steckel, N. Tanaka, et al. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19:2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallach, D. 1997. Cell death induction by TNF: a matter of self control. Trends Biochem. Sci. 22:107–109. [DOI] [PubMed] [Google Scholar]
- 13.Sato, S., H. Sanjo, K. Takeda, J. Ninomiya-Tsuji, M. Yamamoto, T. Kawai, K. Matsumoto, O. Takeuchi, and S. Akira. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6:1087–1095. [DOI] [PubMed] [Google Scholar]
- 14.Liu, H.H., M. Xie, M.D. Schneider, and Z.J. Chen. 2006. Essential role of TAK1 in thymocyte development and activation. Proc. Natl. Acad. Sci. USA. 103:11677–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato, S., H. Sanjo, T. Tsujimura, J. Ninomiya-Tsuji, M. Yamamoto, T. Kawai, O. Takeuchi, and S. Akira. 2006. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int. Immunol. 18:1405–1411. [DOI] [PubMed] [Google Scholar]
- 16.Wan, Y.Y., H. Chi, M. Xie, M.D. Schneider, and R.A. Flavell. 2006. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat. Immunol. 7:851–858. [DOI] [PubMed] [Google Scholar]
- 17.Mori, T., T. Nishimura, Y. Ikeda, T. Hotta, H. Yagita, and K. Ando. 1998. Involvement of Fas-mediated apoptosis in the hematopoietic progenitor cells of graft-versus-host reaction-associated myelosuppression. Blood. 92:101–107. [PubMed] [Google Scholar]
- 18.Dufour, C., A. Corcione, J. Svahn, R. Haupt, V. Poggi, A.N. Beka'ssy, R. Scime, A. Pistorio, and V. Pistoia. 2003. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 102:2053–2059. [DOI] [PubMed] [Google Scholar]
- 19.Pearl-Yafe, M., E.S. Yolcu, J. Stein, O. Kaplan, H. Shirwan, I. Yaniv, and N. Askenasy. 2007. Expression of Fas and Fas-ligand in donor hematopoietic stem and progenitor cells is dissociated from the sensitivity to apoptosis. Exp. Hematol. 35:1601–1612. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai, H., H. Miyoshi, J. Mizukami, and T. Sugita. 2000. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 474:141–145. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn, R., F. Schwenk, M. Aguet, and K. Rajewsky. 1995. Inducible gene targeting in mice. Science. 269:1427–1429. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, J., C. Niu, L. Ye, H. Huang, X. He, W.G. Tong, J. Ross, J. Haug, T. Johnson, J.Q. Feng, et al. 2003. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 425:836–841. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi, K., K. Shirakabe, H. Shibuya, K. Irie, I. Oishi, N. Ueno, T. Taniguchi, E. Nishida, and K. Matsumoto. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 270:2008–2011. [DOI] [PubMed] [Google Scholar]
- 24.Jadrich, J.L., M.B. O'Connor, and E. Coucouvanis. 2006. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 133:1529–1541. [DOI] [PubMed] [Google Scholar]
- 25.Omori, E., K. Matsumoto, H. Sanjo, S. Sato, S. Akira, R.C. Smart, and J. Ninomiya-Tsuji. 2006. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J. Biol. Chem. 281:19610–19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisler, F., H. Algul, S. Paxian, and R.M. Schmid. 2007. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 132:2489–2503. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz, B.H., M.L. Scott, S.R. Cherry, R.T. Bronson, and D. Baltimore. 1997. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity. 6:765–772. [DOI] [PubMed] [Google Scholar]
- 28.Liu, J., and A. Lin. 2005. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 15:36–42. [DOI] [PubMed] [Google Scholar]
- 29.Guzman, M.L., S.J. Neering, D. Upchurch, B. Grimes, D.S. Howard, D.A. Rizzieri, S.M. Luger, and C.T. Jordan. 2001. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 98:2301–2307. [DOI] [PubMed] [Google Scholar]
- 30.Hartman, A.D., A. Wilson-Weekes, A. Suvannasankha, G.S. Burgess, C.A. Phillips, K.J. Hincher, L.D. Cripe, and H.S. Boswell. 2006. Constitutive c-jun N-terminal kinase activity in acute myeloid leukemia derives from Flt3 and affects survival and proliferation. Exp. Hematol. 34:1360–1376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.