Abstract
Deficits in brain reward function during nicotine withdrawal may serve as an important substrate for negative reinforcement that contributes to the persistence of the tobacco habit in human smokers. The ability to assess withdrawal-associated reward deficits in genetically modified mice may facilitate understanding of the neurobiological mechanisms of nicotine dependence. Here, we assessed the effects of nicotine withdrawal on brain reward function in mice, as measured by intracranial self-stimulation (ICSS) thresholds. Male C57BL6 mice were trained in a discrete-trial current-threshold ICSS procedure until stable reward thresholds were obtained. Mice then received experimenter-administered saline or nicotine (2 mg/kg/injection salt; × 4 daily) injections for 7 consecutive days, and ICSS thresholds assessed for 3 days after cessation of injections. Thresholds were unaltered in nicotine- and saline-treated mice after cessation of injections, indicating that this treatment regimen was not sufficient to induce withdrawal-associated reward deficits. Next, mice were implanted subcutaneously with osmotic minipumps delivering a constant daily amount of saline or nicotine (24 mg/kg/day; free-base), with pumps surgically removed 13 days later. The nicotinic receptor antagonist mecamylamine (2 mg/kg) elevated ICSS thresholds in nicotine- but not saline-treated mice when administered 8–10 days after pump implantation. Similarly, reward thresholds were elevated in nicotine-treated mice 12–72 h after minipump removal. These data demonstrate that antagonist-precipitated or spontaneous withdrawal from nicotine delivered via osmotic minipumps induced reward deficits in mice. Further, these findings highlight the potential utility of the ICSS procedure for assessing this important affective component of nicotine withdrawal in genetically modified mice.
Keywords: Nicotine, C57BL6 mice, intracranial self-stimulation, withdrawal, reward, addiction, dependence, mouse
Addiction to tobacco smoking may depend not only on the positive reinforcing and hedonic actions of nicotine, but also on escape from the aversive consequences of nicotine withdrawal (Doherty et al., 1995; George et al., 2007; Kenny and Markou, 2001). Indeed, prolonged nicotine exposure results in the development of nicotine dependence, and smoking cessation elicits an aversive withdrawal syndrome in human smokers that can be directly attributed to a reduction of nicotine intake (Hughes et al., 1991; Shiffman and Jarvik, 1976). Thus, nicotine replacement therapy, of which nicotine gum and nicotine patch are examples, can reduce the occurrence of withdrawal symptoms in abstinent smokers (Fagerstrom et al., 1993; Molander et al., 2000; Schneider and Jarvik, 1984). Conversely, reduction of the nicotine content in smoked tobacco can induce a withdrawal syndrome in smokers accompanied by a significant reduction in plasma nicotine levels (West et al., 1984). Importantly, the duration and severity of withdrawal may predict relapse in abstinent human smokers (Piasecki et al., 1998; Piasecki et al., 2003; Piasecki et al., 2000). Further, the efficacy of nicotine replacement therapy, at least in certain individuals (Fagerstrom, 1988; Sachs and Leischow, 1991), is related to prevention of the onset and reduction in the duration of nicotine withdrawal. Hence, an understanding of the mechanisms of nicotine withdrawal may facilitate the development of new and possibly novel therapeutics to aid smoking cessation efforts.
The ability to assess components of the nicotine withdrawal syndrome in genetically modified mice with altered expression of targeted genes offers a promising approach to identify and understand neurobiological substrates contributing to the persistence of the tobacco habit in smokers. Many studies have assessed ‘physical’ or somatic signs of nicotine withdrawal in mice. For example, Isola and colleagues have shown that spontaneous or mecamylamine-precipitated withdrawal from chronic nicotine injections resulted in increased expression of somatic withdrawal signs (rearing, jumping, shakes, abdominal constrictions, chewing, scratching, facial tremor) in mice (Isola et al., 1999). Similarly, spontaneous and antagonist-precipitated withdrawal from nicotine delivered via osmotic minipump (24–48 mg/kg/day free-base; 7–60 days continuous treatment) also increased somatic withdrawal signs in mice (Damaj et al., 2003; Kota et al., 2007). Importantly, somatic withdrawal signs were increased by a similar magnitude in wild-type mice and in mice with null mutations in β2 nicotinic acetylcholine receptor (nAChR) subunits (β2−/− mice) during mecamylamine-precipitated withdrawal from nicotine delivered via osmotic minipump (Besson et al., 2006; Jackson et al., 2008). Similarly, the α7-selective nAChR antagonist methyllycaconitine also increased somatic withdrawal signs in wild-type and β2−/− mice by a similar magnitude in mice treated chronically with nicotine via osmotic minipump (Jackson et al., 2008; Salas et al., 2004). However, under similar treatment conditions, somatic withdrawal signs were diminished in α5−/−, α7−/− and β4−/− mice relative to wildtype mice (Jackson et al., 2008; Salas et al., 2007; Salas et al., 2004). Thus, it is likely that α5, α7 and β4 but not β2 subunits are components of the nAChRs that regulate the development of physical dependence on nicotine, and the expression of somatic signs during withdrawal. Taken together, the above observations demonstrate that mice display a robust somatic syndrome during spontaneous or antagonist-precipitated nicotine withdrawal, and highlight the utility of genetically modified mice for identifying targets for the actions of nicotine that regulate nicotine dependence processes.
Importantly, accumulating evidence suggests that affective components of withdrawal may play a more important role than somatic aspects in the maintenance of dependence to drugs of abuse, including nicotine (George et al., 2007; Kenny and Markou, 2001; Markou et al., 1998). Consistent with an affective component to nicotine withdrawal in mice, increased anxiety-like (Costall et al., 1989; Damaj et al., 2003; Jonkman et al., 2005) and depression-like behavior (Mannucci et al., 2006) behaviors have been observed in mice undergoing withdrawal from nicotine delivered via osmotic minipump or repeated daily injections. Importantly, affective signs of nicotine withdrawal, reflected in increased anxiety-related behavior or induction of a conditioned place aversion, were absent in β2−/− mice but were unaltered in α5−/− and α7−/− mice compared with wildtype controls (Jackson et al., 2008). Further, the deficits in fear conditioning typically observed in mice undergoing withdrawal from nicotine were intact in α7−/− mice, but were greatly diminished in β2−/− mice (Portugal et al., 2008). Thus, β2-containing nAChRs, but not α5- or α7-containing nAChRs may contribute to affective components of the nicotine withdrawal syndrome. In line with these observations is the fact that varenicline (Chantix), a federally approved pharmacological aid for smoking cessation that is efficacious in preventing smoking relapse, acts as a partial agonist at α4β2* nAChRs and can attenuate affective components of the nicotine withdrawal syndrome in smokers (Gonzales et al., 2006; Jorenby et al., 2006; West et al., 2007). However, it is important to note that Stapleton and colleagues recently published data suggesting that varenicline can increase short-term rates of cessation in smokers without altering the severity of adverse mood during abstinence (Stapleton et al., 2008). Taken together, the above data highlight the potential utility of genetically modified mice for identifying the neurobiological mechanisms that regulate affective components of the nicotine withdrawal syndrome.
Withdrawal from nicotine and other major drugs of abuse has been shown to decrease brain reward function, reflected in elevated intracranial self-stimulation (ICSS) thresholds in rats (Epping-Jordan et al., 1998; Kenny and Markou, 2005; Markou and Koob, 1991; Watkins et al., 2000). Such reward deficits are considered a particularly important affective component of withdrawal that maintains drug-taking behavior (Koob and Le Moal, 2005). Indeed, emerging evidence suggests that withdrawal-associated reward deficits, detected as elevated ICSS thresholds, may represent an important substrate for negative reinforcement that facilitates the development and maintenance of compulsive drug seeking behaviors (Ahmed et al., 2002; Kenny, 2007; Koob et al., 2004). Thus, an understanding of the mechanisms by which chronic nicotine exposure induces plasticity in brain reward systems that results in the expression of reward deficits during withdrawal may provide important insights into the persistence of the tobacco habit in human smokers. In particular, the ability to assess nicotine withdrawal-associated reward deficits in genetically modified mice may provide a powerful tool to investigate affective aspects of nicotine dependence. The aim of the present study was to establish conditions sufficient to observe nicotine withdrawal-associated reward deficits in mice trained in a discrete-trial current-threshold ICSS procedure. We employed treatment regimens previously shown to elicit the expression of somatic or affective components of nicotine withdrawal in mice. Specifically, we examined the effects of spontaneous withdrawal from chronic experimenter-delivered nicotine injections on ICSS threshold in C56BL6 mice. In addition, we also assessed the effects of mecamylamine-precipitated and spontaneous withdrawal from chronic nicotine treatment delivered via osmotic minipumps on ICSS thresholds in C57BL6 mice.
MATERIALS AND METHODS
Subjects
16 male C57BL6/J mice aged approximately 6 weeks at the start of the experiment were used. Mice were obtained from The Jackson Laboratory and were housed in groups of 2–4 per cage. Mice were maintained in a temperature-controlled vivarium under a 12-h light/dark cycle (lights off at 12:00 PM). Animals were tested during the dark portion of the light/dark cycle, except for the spontaneous nicotine withdrawal experiment when mice were tested at time points according to the experimental design. Studies were approved by the Institutional Animal Care and Use Committee of Scripps Florida, and animals were treated in accordance with the guidelines of the National Institutes of Health regarding the principles of animal care.
Drugs
(−)-Nicotine hydrogen tartrate salt and mecamylamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Drugs were prepared immediately before each administration. For systemic administration, drugs were dissolved in sterile physiological saline and administered by subcutaneous (SC; nicotine) or intraperitoneal (IP; mecamylamine) injection, in a volume of 10 ml/kg body weight. Unless otherwise stated, all drug doses refer to the salt form.
Apparatus
Intracranial self-stimulation training and testing took place in 12 Plexiglas operant chambers (21.6 × 17.8 × 12.7 cm) (MED Associates, St. Albans, VT). The floors of the operant chambers were constructed of parallel aluminum rods spaced 0.75 cm apart. One wall contained a metal wheel manipulandum. The wheel (width of 3.8 cm, response registered for every 90 degrees of rotation) extended 1.5 cm out of the wall. Each testing chamber was enclosed within a light- and sound-attenuated chamber of interior dimensions (55.9 × 38.1 × 40.6 cm). Intracranial stimulation was delivered by constant current stimulators (PHM-152/2 Dual Programmable ICSS Stimulator; Med Associates). Subjects were connected to the stimulation circuit through flexible bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (model SL2C; Plastics One) mounted above the chamber. The stimulation parameters, data collection, and all test session functions were controlled by a microcomputer.
Placement of ICSS stimulating electrodes
Mice were anesthetized by inhalation of 1 to 3% isoflurane in oxygen and positioned instereotaxic frame (Kopf Instruments, Tujunga, CA). The skull was exposed and stainless steel bipolar electrodes (6 mm in length) were implanted into the posterior lateral hypothalamus (AP: −0.5 mm from bregma; ML: ±1.3 mm; DV: −5.0 mm from skull surface; flat-skull position) (Paxinos and Franklin, 2001). Four skull screws together with the application of dental acrylic held the electrode in place for the duration of the experiment. Animals were allowed to recover from surgery for at least 7 days before training in the ICSS paradigm commenced. Mice continued to be housed 2–4 per cage after recovery from surgery. Once trained successfully in the ICSS procedure we did not lose any mice due to loss of cap/electrode assemblies.
ICSS Reward Threshold Procedure
Mice were trained to respond according to a modification of the discrete-trial current threshold procedure of Kornetsky and Esposito (1979). Briefly, a trial was initiated by the delivery of a non-contingent electrical stimulus. This electrical reinforcer had a train duration of 500 ms and consisted of 0.1 ms square wave pulses that were delivered at a frequency of 50 to 100 Hz. The frequency of the stimulation was selected for individual animals so that current-intensity thresholds of each subject were within 50 to 300 μA, and thus allowed both threshold elevations and lowerings to be detected. This frequency for each mouse was held constant throughout the experiment. A one-quarter turn of the wheel manipulandum within 7.5 s of the delivery of the non-contingent electrical stimulation resulted in the delivery of an electrical stimulus identical in all parameters to the non-contingent stimulus that initiated the trial. After a variable intertrial interval (7.5–12.5 s, average of 10 s), another trial was initiated with the delivery of a non-contingent electrical stimulus. Failure to respond to the non-contingent stimulus within 7.5 s resulted in the onset of the inter-trial interval. Responding during the inter-trial interval reset the inter-trial interval and thereby delayed the onset of the next trial. Current levels were varied in alternating descending and ascending series. A set of five trials was presented for each current intensity. Current intensities were altered in 5 μA steps. In each testing session, four alternating descending and ascending series were presented. The threshold for each series was defined as the midpoint between three consecutive current intensities that yielded “positive scores” (animals responded for at least three of the five trials) and two consecutive current intensities that yielded “negative scores” (animals did not respond for three or more of the five trials). The overall threshold of the session was defined as the mean of the thresholds for the four individual series. Each testing session was approximately 45 min in duration. The time between the onset of the non-contingent stimulus and a positive response was recorded as the response latency. The response latency for each test session was defined as the mean response latency of all trials during which a positive response occurred. After establishment of stable ICSS reward thresholds (defined as ≤10% variation in thresholds over a 3 day period), mice were tested in the ICSS procedure once daily except for the spontaneous nicotine withdrawal experiments when mice were tested at time points according to the experimental design.
Spontaneous withdrawal from nicotine delivered via daily injections
Mice with stable ICSS thresholds were allocated to two groups such that there was no difference in mean thresholds between groups. One group received four daily injections of nicotine (2 mg/kg salt, SC) delivered approximately 2–3 h apart and the other group received injections of saline at the same time-points, starting at approximately 10:00 h each day for 7 consecutive days. This intermittent nicotine treatment schedule was chosen to recapitulate the chronic intermittent nature of nicotine consumption by human tobacco addicts, and is similar to that previously shown to elicit withdrawal responses upon cessation of the nicotine injections (Biala et al., 2005; Isola et al., 1999; Mannucci et al., 2006). ICSS thresholds continued to be assessed once each day during treatments, approximately 1 h after the first daily nicotine or saline injection. All mice had ICSS thresholds assessed 12, 24, and 48 h after completion of the chronic treatment regimen.
Osmotic minipump surgery
Seven days after completion of the experimenter-administered injection regimen described above, mice were reallocated to two groups such that there was no difference in mean ICSS thresholds between groups, and with mice previously treated with saline or nicotine counterbalanced between both groups. Mice were anesthetized by inhalation of 1 to 3% isoflurane in oxygen and prepared with Alzet osmotic minipumps [model 2004 (28 day); Alza, Palo Alto, CA] placed subcutaneously on the back of the animal parallel to the spine. Pumps were filled with either sterile saline (n=9) or nicotine salt solution (n=7). The concentration of the nicotine salt solution was adjusted according to animal body weight, resulting in delivery of 24 mg/kg/day free-base. This dose was chosen based on previous studies demonstrating that it induced nicotine dependence in mice, and the expression of antagonist-precipitated or spontaneous somatic withdrawal signs (Damaj et al., 2003; Salas et al., 2004). After minipump implantation (or removal), the surgical wound was closed with 9 mm stainless steel wound clips (BD Biosciences Primary Care Diagnostics, Sparks, MD) and treated with topical antibiotic (Bacitracin) ointment.
Mecamylamine-precipitated withdrawal from nicotine delivered via osmotic minipumps
Eight to 10 days after minipump implantation all mice received a systemic injection of mecamylamine (2 mg/kg, IP). Mice were then immediately placed into the ICSS chambers, and post-injection ICSS thresholds were assessed.
Spontaneous withdrawal from nicotine delivered via osmotic minipumps
Osmotic minipumps were surgically removed from nicotine-treated mice (n=7) or corresponding control mice (n=9; mice prepared with saline-containing minipumps) on day 13 after minipump implantation. All mice were then tested in the ICSS procedure at 12, 24, 36, 48, 72 and 96 h after the removal of osmotic minipumps. These time points were chosen based on the time course of ICSS threshold elevations previously observed in rats undergoing spontaneous nicotine withdrawal after removal of nicotine-delivering osmotic minipumps (Epping-Jordan et al., 1998; Kenny et al., 2003; Watkins et al., 2000), and based on the time-course of somatic withdrawal signs observed in mice undergoing spontaneous nicotine withdrawal (Damaj et al., 2003; Isola et al., 1999).
Statistical Analyses
Mean absolute thresholds and response latencies (±S.E.M.) prior to any drug treatment are presented for groups of mice in the results section. For all experiments, percentage change of reward thresholds was calculated by expressing the absolute threshold scores obtained during the baseline period (the three days immediately prior to mecamylamine-precipitated or spontaneous withdrawal), and during mecamylamine-precipitated or spontaneous withdrawal period as a percentage of the mean reward thresholds obtained on the three days immediately prior to the baseline period, minus 100. Percentage change of reward threshold data was analyzed by two-factor repeated-measures analyses of variance (ANOVA), with Treatment (baseline or mecamylamine) or Time as within-subjects factors and Pump (nicotine or saline) as the between-subjects factor. To assess the overall magnitude of withdrawal, the ‘area under the curve’ (AUC) for threshold data obtained during spontaneous nicotine withdrawal across the multiple time-points was calculated. AUC data for saline- and nicotine-treated mice were analyzed by unpaired t-test. For all experiments, response latency data were analyzed in the same manner as the threshold data described above. Following significant main effects in ANOVAs experimental groups were compared by Bonferroni post-tests. In all cases, the level of significance was set at 0.05. All statistical analyses and AUC calculations were performed using GraphPad Prism software.
RESULTS
Spontaneous withdrawal from nicotine delivered via daily injections
Mean absolute thresholds in saline- and nicotine-treated mice prior to any drug treatment were 144.6 ± 21.9 and 151.3 ± 27.1 μA, respectively. As seen in Fig. 1, reward thresholds were unaltered in saline-treated or nicotine-treated mice at each time-point after cessation of experimenter-delivered injections; Pump (F(1,42)=0.64, p=0.43); Time (F(2,42)=0.11, p=0.9); Pump × Time interaction (F(2,42)=0.54, p=0.6).
Figure 1.
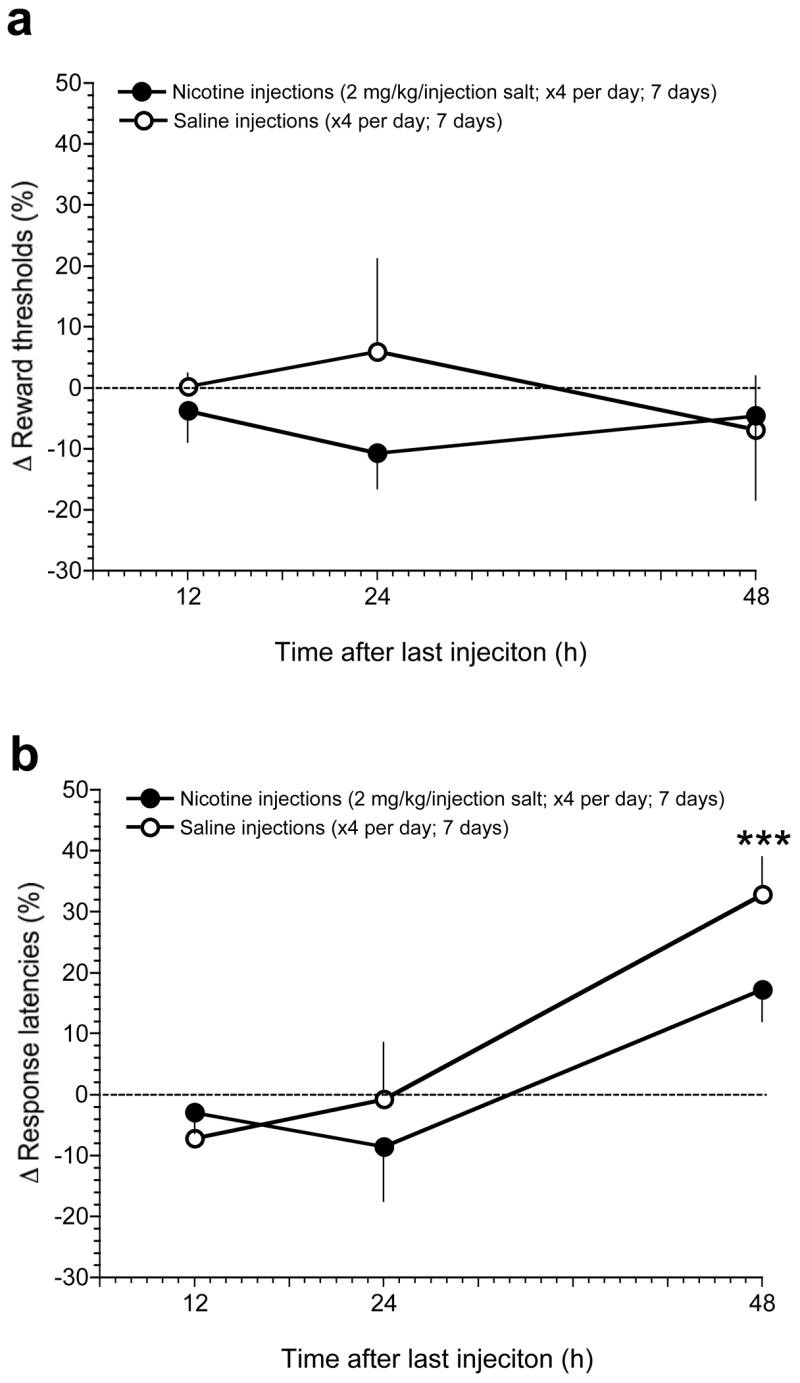
Effects of spontaneous withdrawal from experimenter-administered nicotine injections on ICSS thresholds in mice. Mice received four daily injections of saline or nicotine (2 mg/kg salt, SC) delivered 2–3 h apart starting at approximately 10:00 am each day for 7 consecutive days. A, data are expressed as mean (±S.E.M.) percentage change of reward thresholds in mice assessed 12, 24 and 48 h after their last injection. B, data are expressed as mean (±S.E.M.) percentage change of response latencies in mice assessed 12, 24 and 48 h after their last injection. *** P < 0.001, main effect of Time after last injection in two-way repeated measures ANOVA (see Results).
Mean absolute response latencies in saline-treated and nicotine-treated mice prior to any drug treatment were 3.09 ± 0.25 and 3.22 ± 0.26 sec, respectively. As seen in Fig. 1, percentage change in response latencies was unaltered in saline-treated or nicotine-treated mice at 12 and 24 h after cessation of experimenter-delivered injections. However, response latencies were increased similarly in nicotine- and saline-treated mice 48 h after cessation of injections: Time (F(2,42)=8.9, p=0.001); Pump (F(1,42)=0.92, p=0.34); Time × Pump interaction (F(2,42)=0.75, p=0.5).
Mecamylamine-precipitated withdrawal from nicotine delivered via osmotic minipumps
As shown in Fig. 2A, the nAChR antagonist mecamylamine (2 mg/kg) precipitated significant elevations of ICSS thresholds compared with baseline thresholds in nicotine-treated but not saline-treated mice: Pump (F(1,28)=12.6, p<0.005); Treatment (F(1,28)=6.0, p<0.05); Pump × Treatment interaction (F(1,28)=5.3, p<0.05). Bonferroni post-tests demonstrated that mecamylamine significantly elevated reward thresholds in nicotine-treated mice compared with baseline reward thresholds (p<0.001; see Fig. 2A).
Figure 2.
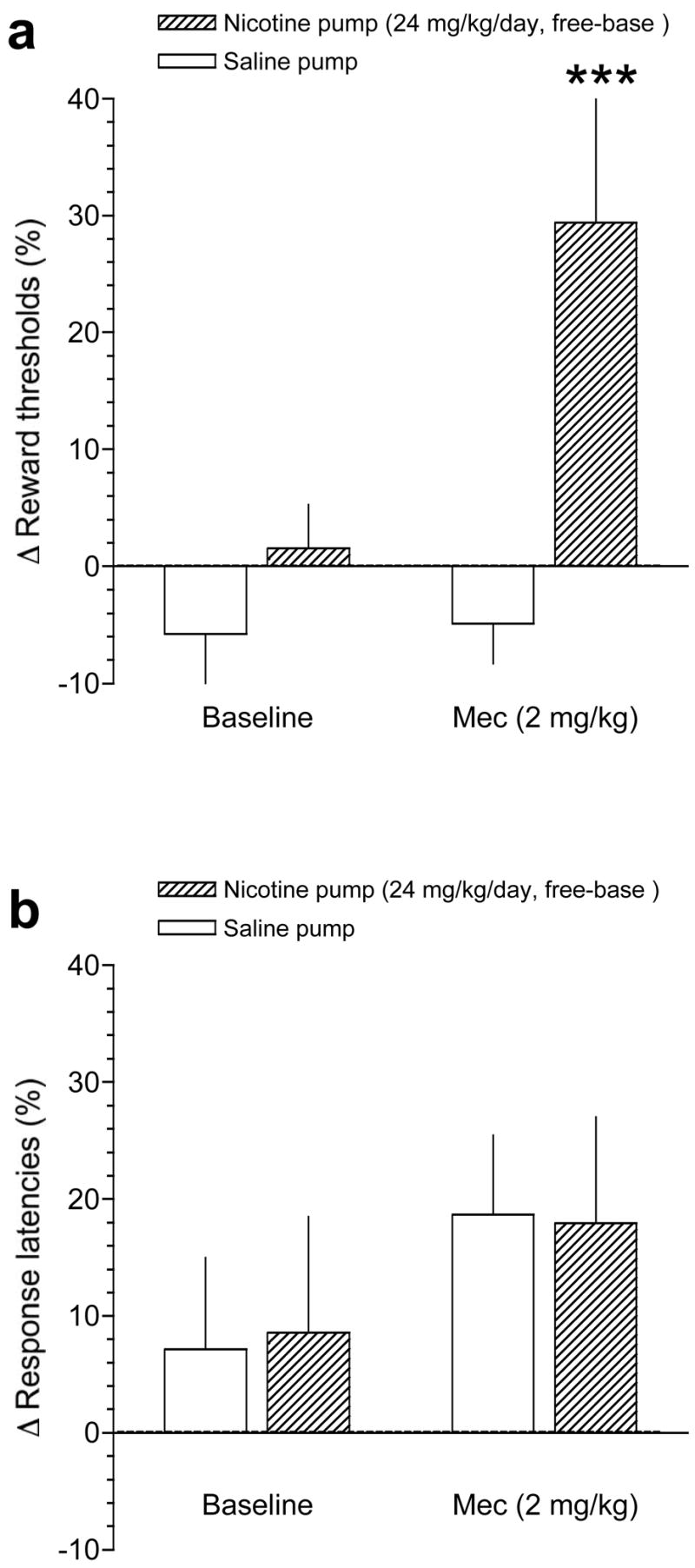
Effects of precipitated withdrawal from nicotine delivered via osmotic minipump on ICSS thresholds in mice. A, data are expressed as mean (±S.E.M.) percentage change of reward thresholds during the baseline period (see Methods) or after administration of mecamylamine (2 mg/kg, IP), in mice receiving chronic saline or nicotine (24 mg/kg/day, free-base) delivered via osmotic minipump. B, data are expressed as mean (±S.E.M.) percentage change of response latencies during the baseline period (see Methods) or after administration of mecamylamine (2 mg/kg, IP), in mice receiving chronic saline or nicotine (24 mg/kg/day, free-base) delivered via osmotic minipump. *** P < 0.001, different from control (saline-treated) mice after administration of mecamylamine, Bonferroni post-test after significant interaction effect in two-way repeated-measures ANOVA.
As shown in Fig. 2B, mecamylamine had no statistically significant effects on response latencies in saline-treated or nicotine-treated mice, although there was a non-significant trend for mecamylamine to increase response latencies similarly in both groups of mice: Pump (F(1,28)=0.01, p=0.97); Treatment (F(1,28)=1.6, p=0.2); Pump × Treatment interaction (F(1,28)=0.02, p=0.9).
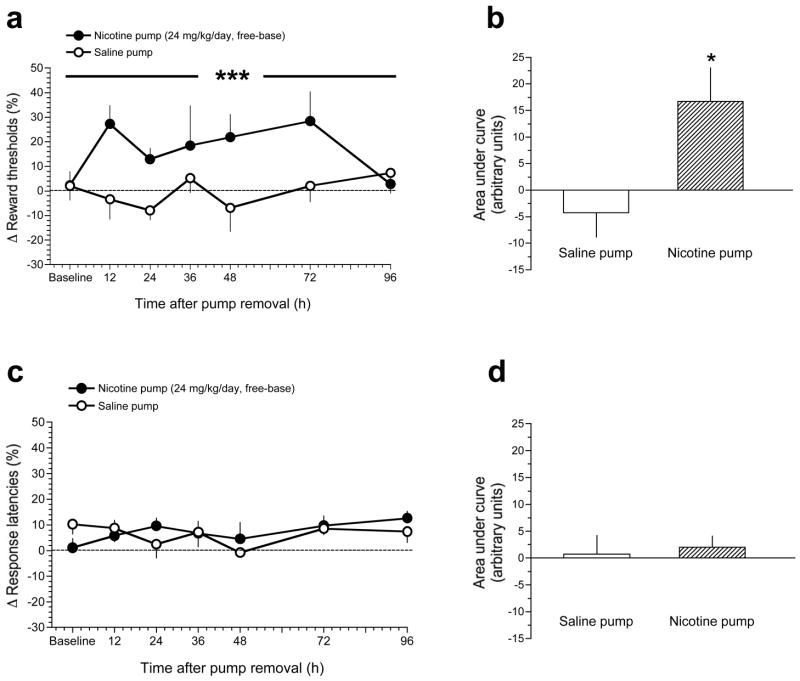
Spontaneous withdrawal from nicotine delivered via osmotic minipumps
Spontaneous withdrawal from nicotine delivered via osmotic minipump significantly elevated reward thresholds compared with mice treated with saline-delivering minipumps: Pump (F(6,98)=14.5, p<0.0005); Time (F(6,98)=0.8, p=0.8); Pump × Time interaction (F(6,98)=1.5, p=0.2); Fig. 3A. To assess the total magnitude of reward threshold elevation during spontaneous withdrawal, the AUC for threshold data obtained across the multiple time-points of spontaneous withdrawal was calculated, and compared between saline- and nicotine-treated mice. As seen in Fig. 3B, AUC for reward threshold data was significantly greater in nicotine-treated mice compared with controls (p<0.05, unpaired t-test).
Figure 3.
Effects of spontaneous withdrawal from nicotine delivered via osmotic minipump on ICSS thresholds in mice. A, data are expressed as mean (±S.E.M.) percentage change of reward thresholds in mice during the baseline period (see Methods) and after surgical removal of osmotic minipumps delivering saline or nicotine (24 mg/kg/day, free-base), assessed 12, 24, 36, 48, 72 and 96 h after pump removal. B, reward threshold data presented in panel A for saline- and nicotine-treated mice are expressed as mean (±S.E.M.) area under the curve (AUC). C, data are expressed as mean (±S.E.M.) percentage change of response latencies in mice during the baseline period (see Methods) and after surgical removal of osmotic minipumps delivering saline or nicotine (24 mg/kg/day, free-base), assessed 12, 24, 36, 48, 72 and 96 h after pump removal. D, response latency data presented in panel C for saline- and nicotine-treated mice are expressed as mean (±S.E.M.) AUC. *** P < 0.001, different from saline-treated mice, main effect of Pump in two-way repeated measures ANOVA (see Results). * P < 0.05, different from saline-treated mice, unpaired t-test.
Mecamylamine had no statistically significant effects on response latencies in saline- or nicotine-treated mice at any time-point after removal or minipumps: Pump (F(1,28)=0.01, p=0.97); Treatment (F(1,28)=1.6, p=0.2); Pump × Treatment interaction (F(1,28)=0.02, p=0.9); see Fig. 3C. Similarly, AUC analyses demonstrated that there were no statistically significant differences in response latency data between saline- and nicotine-treated mice (p = 0.4; Fig. 3D).
DISCUSSION
Accumulating evidence suggests that avoidance and alleviation of the reward deficits associated with nicotine withdrawal may provide a crucial source of motivation that contributes to the persistence of the tobacco habit in smokers (George et al., 2007; Kenny and Markou, 2001; Markou et al., 1998). Over recent years, nicotine withdrawal has been shown to diminish brain reward function in rats, as measured by elevated ICSS thresholds (Bruijnzeel et al., 2006; Epping-Jordan et al., 1998; Kenny and Markou, 2005; Watkins et al., 2000). The data presented here demonstrate that mecamylamine-precipitated or spontaneous withdrawal from chronic nicotine treatment, delivered via osmotic minipumps, was associated with elevated ICSS thresholds in C57BL6 mice. These data support the notion that reward deficits during nicotine withdrawal occur across species, and are not restricted to rats. Further, this study establishes experimental conditions suitable to assess reward deficits during nicotine withdrawal in genetically modified mice, an advance that may facilitate understanding of the neurobiological mechanisms of nicotine dependence and withdrawal processes.
Many variants of the ICSS procedures have been developed for use in laboratory animals (Wise, 1996). The threshold measure that has been most widely used primarily in mice so far is the rate–intensity procedure, which involves the generation of a stimulation input–response output function, see (Carlezon and Chartoff, 2007). In this ICSS procedure, rate–intensity curves are generated by allowing mice to perform an operant response, typically to press a lever or turn a wheel, to obtain ICSS of different intensities. The ICSS intensity that elicits 50% of the asymptotic maximal rate of responding is usually termed the ICSS threshold in this procedure. Acute administration of drugs of abuse such as cocaine (Gilliss et al., 2002), morphine (Elmer et al., 2005) or amphetamine (Cazala, 1976; Elmer et al., 2005) lowers ICSS thresholds in mice as detected in this procedure. However, rate-intensity measures of ICSS thresholds may be sensitive to disruptions in motor performance and/or sensory processes that affect rates of responding, without necessarily altering brain reinforcement systems. This may be of particular concern when assessing ICSS thresholds in genetically modified mice in which deletion of particular genes may have secondary effects on performance of rate-dependent behavioral variables without directly altering the function of brain reward circuits or their response to drugs of abuse. An alternative method of obtaining the ICSS threshold, successfully utilized in rats and employed in the present studies is the discrete-trial threshold procedure. This discrete-trial threshold procedure is a modification of the classical psychophysical method of limits and provides a rate-independent current-intensity threshold measure (Huston-Lyons and Kornetsky, 1992; Kornetsky and Esposito, 1979; Markou and Koob, 1992). In the discrete-trial procedure utilized in the present studies, the current intensity was varied between trials, and the ICSS threshold is defined as the current intensity at which the animal responds approximately 50% of the time. Above this threshold intensity the animal responds more frequently, and below this intensity the animal responds less frequently. Similar to rate-intensity measures of ICSS thresholds, administration of drugs of abuse lowers ICSS thresholds in the discrete-trial procedure in rats (Harrison et al., 2002). Most recently, cocaine was shown to lower ICSS thresholds in mice using this discrete-trial current-threshold procedure (Gill et al., 2004). A major advantage of this rate-free threshold procedure is that it is less sensitive than rate-dependent measures to performance-altering effects of pharmacological or genetic manipulations, which may be a concern when testing genetically modified mouse strains in rate-dependent behavioral tasks. The present data demonstrate that withdrawal from nicotine elevates ICSS thresholds in C57BL6 mice in the discrete-trial current-intensity threshold procedure by a magnitude and with a duration of effect similar to that previously reported in rats (Epping-Jordan et al., 1998; Kenny et al., 2003; Watkins et al., 2000). Importantly, C57BL6 is a strain onto which many lines of genetically modified mice are commonly backcrossed, and may be particularly sensitive to the behavioral actions of nicotine (Damaj et al., 2003; Grabus et al., 2006; Robinson et al., 1996; Stolerman et al., 1999).
In the present study we have shown that withdrawal from nicotine delivered via osmotic minipumps, but not from experimenter-administered nicotine injections (2 mg/kg salt; × 4 injections per day), decreased brain reward function in mice. Importantly, the amount of nicotine mice received via experimenter-delivered injections (8 mg/kg/day salt; ~2.6 mg/kg/day free-base; 7 days exposure) was far less than that delivered by osmotic minipumps (24 mg/kg/day; 13 days exposure). In addition, mice receiving nicotine through minipumps were exposed to the drug continuously during the 13 days of treatment, whereas the experimenter-treated mice were exposed to the drug in a far more pulsatile manner. Hence, the absence of elevated ICSS thresholds in mice undergoing withdrawal from experimenter-administered nicotine injections may reflect an insufficient level of nicotine exposure to induce nicotine dependence and the expression of withdrawal-associated reward deficits, and that higher nicotine doses or periods of nicotine exposure greater than 7 days may have been necessary to induce nicotine dependence. However, it should be noted that withdrawal from experimenter-administered nicotine injections under treatment conditions similar to those used here has been reported to induce the expression of somatic and affective components of nicotine withdrawal in mice (Besson et al., 2006; Mannucci et al., 2006). Therefore, it is likely that brain reward systems are relatively resistant to the effects of experimenter-administered nicotine injections delivered under the treatment conditions utilized in this study, and that the greater levels of nicotine exposure achieved using osmotic minipumps are necessary to induce dependence-like adaptations in brain reward circuitry in mice that results in the expression of withdrawal-associated reward deficits. It is curious to note that response latencies, considered a measure of operant performance in the ICSS procedure, were increased similarly in saline- and nicotine-treated mice 48 h after cessation of chronic experimenter-delivered injections (Fig. 1). The reasons for the increased response latencies at this time-point are unclear. Like most other procedures used to assess motivated behavior in rodents, ICSS thresholds assessed under the present rate-independent ICSS procedure requires motor output, rendering it at least partly sensitive to baseline differences in operant performance in mutant mice. Importantly, however, the increased response latencies in mice noted above was independent of any modification in brain reward function, as ICSS thresholds were unaltered in either treatment group at the same time-point. This observation supports the notion that reward- and performance-dependent variables may be at least somewhat dissociable in mice using a discrete-trial current-threshold ICSS procedure, highlighting the utility of this procedure for assessing ICSS thresholds in strains of genetically modified mice in which constitutive deficits in operant performance may occur.
In summary, spontaneous or antagonist-precipitated nicotine withdrawal is associated with decreased brain reward function in mice, reflected in elevated ICSS thresholds. Hence, the ICSS procedure described here may serve as an important tool to analyze the mechanisms by which nicotine impacts brain reward circuitry in genetically modified mice, and thereby provide novel insights into the molecular mechanisms of nicotine dependence and withdrawal processes.
Acknowledgments
Supported by the National Institute on Drug Abuse (DA020686; PJK), and The James and Esther King Biomedical Research Program, Florida Department of Health (07KN-06; PJK). The authors are grateful to Dr. Conan Kornetsky for expert guidance in the surgical preparation and training of mice in the ICSS procedure, and for help with programming of ICSS chambers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: Beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Biala G, Budzynska B, Kruk M. Naloxone precipitates nicotine abstinence syndrome and attenuates nicotine-induced antinociception in mice. Pharmacol Rep. 2005;57:755–760. [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF Receptors Prevents the Deficit in Brain Reward Function Associated with Precipitated Nicotine Withdrawal in Rats. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Cazala P. Effects of d- and l-amphetamine on dorsal and ventral hypothalamic self-stimulation in three inbred strains of mice. Pharmacol Biochem Behav. 1976;5:505–510. doi: 10.1016/0091-3057(76)90259-8. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berl) 2005;182:33–44. doi: 10.1007/s00213-005-0051-2. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Efficacy of nicotine chewing gum: a review. Prog Clin Biol Res. 1988;261:109–128. [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG, Lunell E. Effectiveness of nicotine patch and nicotine gum as individual versus combined treatments for tobacco withdrawal symptoms. Psychopharmacology (Berl) 1993;111:271–277. doi: 10.1007/BF02244941. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill BM, Knapp CM, Kornetsky C. The effects of cocaine on the rate independent brain stimulation reward threshold in the mouse. Pharmacol Biochem Behav. 2004;79:165–170. doi: 10.1016/j.pbb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gilliss B, Malanga CJ, Pieper JO, Carlezon WA., Jr Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology (Berl) 2002;163:238–248. doi: 10.1007/s00213-002-1153-8. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–196. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol. 2005;516:40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Mannucci C, Tedesco M, Bellomo M, Caputi AP, Calapai G. Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochem Int. 2006;49:481–486. doi: 10.1016/j.neuint.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Molander L, Lunell E, Fagerstrom KO. Reduction of tobacco withdrawal symptoms with a sublingual nicotine tablet: a placebo controlled study. Nicotine Tob Res. 2000;2:187–191. doi: 10.1080/713688123. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; 2001. [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112:14–27. [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 1996;124:332–339. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- Sachs DP, Leischow SJ. Pharmacologic approaches to smoking cessation. Clin Chest Med. 1991;12:769–791. [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME. Time course of smoking withdrawal symptoms as a function of nicotine replacement. Psychopharmacology (Berl) 1984;82:143–144. doi: 10.1007/BF00426399. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- West RJ, Russell MA, Jarvis MJ, Feyerabend C. Does switching to an ultra-low nicotine cigarette induce nicotine withdrawal effects? Psychopharmacology (Berl) 1984;84:120–123. doi: 10.1007/BF00432039. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Ann Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]