Abstract
Otosclerosis is a complex disease that results in a common form of conductive hearing loss due to impaired mobility of the stapes. Stapedial motion becomes compromised secondary to invasion of otosclerotic foci into the stapedio-vestibular joint. Although environmental factors and genetic causes have been implicated in this process, the pathogenesis of otosclerosis remains poorly understood. To identify molecular contributors to otosclerosis we completed a microarray study of otosclerotic stapedial footplates. Stapes footplate samples from otosclerosis and control patients were used in the analysis. One-hundred-and-ten genes were found to be differentially expressed in otosclerosis samples. Ontological analysis of differentially expressed genes in otosclerosis provides evidence for the involvement of a number of pathways in the disease process that include interleukin signaling, inflammation and signal transduction, suggesting that aberrant regulation of these pathways leads to abnormal bone remodeling. Functional analyses of genes from this study will enhance our understanding of the pathogenesis of this disease.
Keywords: Otosclerosis, Stapes, Microarray, Gene expression
1. Introduction
Otosclerosis is a common form of conductive hearing loss. It is characterized by sclerosis of the inner ear bony labyrinth due to abnormal bone remodeling in the otic capsule, which normally undergoes very little bone remodeling after development (Frisch et al., 2000). Bone remodeling is a highly regulated process that involves bone resorption by osteoclasts and bone formation by osteoblasts (for review of bone remodeling see (Cohen, 2006)). Inappropriate regulation of either of these processes can lead to bony abnormalities. As a result, increased formation of bone at otosclerotic foci in the otic capsule can reflect either over activity of osteoblasts or reduced activity of osteoclasts.
Both clinical and histological forms of otosclerosis are recognized. Clinical otosclerosis is characterized by the encroachment of otosclerotic foci into the stapedio-vestibular joint with subsequent compromise of free motion of the stapes, leading to a conductive hearing loss (Schuknecht et al., 1985). The prevalence of clinical otosclerosis is about 0.2 to 1% in the Caucasian population, with lower prevalences rates reported in black, Asian and Native American populations (Altmann et al., 1967; Gordon, 1989). Age-of-onset varies from 15 to 40 years, with the average age-of-onset falling in the third decade (Menger et al., 2003). Long-term follow up suggests that approximately 10% of clinical otosclerosis patients ultimately develop sensorineural hearing loss (Browning et al., 1984; Ramsay et al., 1994). In contrast to clinical otosclerosis, histological otosclerosis is present in 2.5 to 12% of the Caucasian population. Histological otosclerosis does not have a clinical manifestation and can be diagnosed only by analysis of temporal bone sections (Declau et al., 2007). It is not known whether bone remodeling in clinical and histological otosclerosis is caused by the same trigger.
The cause of otosclerosis remains unknown. Environmental factors like measles virus have been implicated in its pathogenesis (McKenna et al., 1990; Niedermeyer et al., 1994) and genetic factors are supported by the identification of seven discrete genetic loci in families segregating autosomal dominant otosclerosis (Bel Hadj Ali et al., 2008; Brownstein et al., 2006; Chen et al., 2002; Thys et al., 2007a; Tomek et al., 1998; Van Den Bogaert et al., 2004; Van Den Bogaert et al., 2001). However, none of the causative genes at these loci has been identified.
In addition to linkage analyses, association of several genes with otosclerosis has been reported. Association between COL1A1 and otosclerosis has been reported although with controversial results (Chen et al., 2007; McKenna et al., 1998; Rodriguez et al., 2004). Another more robust study showed that there is an under-representation of an active variant of TGFB1 in otosclerosis patients, which may inhibit osteoclast differentiation and initiation of aberrant bone remodeling in the otic capsule (Thys et al., 2007b). Association of two bone morphogenetic proteins, BMP2 and BMP4, has also been reported (Schrauwen et al., 2007). In aggregate, these data provide compelling evidence that otosclerosis is a genetically heterogeneous disease, although our understanding of the pathways involved remains speculative; the possibility of environmental triggers remains unanswered.
One approach to identify molecular contributors to otosclerosis is through microarray analyses of gene expression profiles in diseased tissue. In this study, we performed microarray analysis using total RNA from stapes footplates of otosclerosis patients undergoing stapedectomy; control stapes footplates were removed from patients undergoing translabyrinthine surgery for vestibular schwannomas. We report a number of differentially expressed genes in otosclerosis patients that may serve as candidates for further genetic screens and functional analyses to help uncover the genetic components that contribute to this disease.
2. Methods
2.1. Tissue Samples
Stapes tissue samples from nine otosclerosis patients were surgically removed during stapedectomy and immediately placed in liquid nitrogen. This limited number reflects the fact that the preferred surgical treatment of otosclerosis now involves laser-assisted stapedotomy rather than stapedectomy, making the collection of large numbers of pathological specimens very unlikely. Control stapes were taken from seven patients undergoing translabyrinthine surgery for vestibular schwannomas; harvest conditions were similar and none of these specimens had evidence of otosclerotic foci. All patients gave informed consent prior to collection of samples. The sex, age, ethnicity, or medical history of patients who donated samples for this study was not disclosed.
2.2. RNA Isolation and Preparation
Stapes footplate samples were separated from the stapes suprastructure using a dissecting microscope and then disrupted and homogenized using a tissue-tearer (Biospec Products, Inc.) in 1 ml QIAzol Lysis Reagent. Total RNA was extracted using RNeasy Lipid Tissue Mini Kit (Qiagen) following the manufacturer’s protocol. Quality and quantity of total RNA was determined using a NanoDrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies).
Two rounds of linear RNA amplification were performed on each individual RNA sample using the Applied Biosystems NanoAmp™ RT-IVT Labeling Kit (Applied Biosystems, Inc.). 50 ng of total RNA from each stapes footplate sample were used for the first round of amplification without digoxigenin (DIG)-UTP labeling. After purification and quantification, 120 ng of amplified cRNA was used for a second round of amplification followed by DIG-UTP labeling. Quantity and quality of labeled amplified cRNA was determined prior to array hybridization. RNA amplification and labeling were performed in triplicate for each individual stapes footplate RNA sample.
2.3 Reproducibility of Arrays
To determine the effect of two rounds of RNA amplification between technical replicates, reproducibility of microarray results between replicates was tested. Two arrays from independently amplified and labeled cRNA from the same biological sample were analyzed to determine whether two rounds of amplification of RNA would lead to different results for the same biological sample. This procedure was completed in duplicate using two different biological samples. Analysis of signal intensity between probes of the four arrays showed a correlation coefficient above 97%, indicating high intra-sample reproducibility across arrays after two rounds of RNA amplification.
2.4. Microarray Analysis
Microarray analysis was performed using the Applied Biosystems Human Genome Survey Microarray v2.0 (Applied Biosystems, Inc.). 10 ug of each DIG-UTP labeled cRNA sample from the second round of amplification was hybridized to an Applied Biosystems Human Genome Survey Microarray v2.0 chip at 55°C for 16 hours. All nine otosclerosis samples and all seven control samples were hybridized in triplicate for a total of 48 arrays. Chemiluminescence detection and image acquisition were performed using the Applied Biosystems Chemiluminescence Detection Kit on the Applied Biosystems 1700 Chemiluminescent Microarray Analyzer following the manufacturer’s specifications. Images were auto-gridded and the chemiluminescent signals were quantified, background subtracted, and finally spot- and spatially-normalized using the Applied Biosystems 1700 Chemiluminescence Microarray Analyzer software v1.1.1.
2.5. Statistical Analysis and Gene Ontology Analysis
Array data from each array were quantile normalized and filtered based on the quality flags for the raw data. Detection threshold for probes on the array was set as a signal-to-noise ratio (S/N) > 3 and quality flag <5000. Using this standard, 45% and 47% of the 32,878 probes on the array were detected, representing 47% and 49% of the total 29,098 genes represented on the array for patient and control samples respectively. After filtering, the statistical significance of the fold change of each transcript between the patient and control data was determined by Student’s t-test using log2 transformed data. For multiple test correction, Storey’s q-value false discovery rate (FDR) method was used to control the expected value of the ratio of the number of false-positives and the total number of genes called as significant (Storey et al., 2003). Significantly differentially expressed genes were determined using a false discovery rate (q-value) of 5%, and a fold change greater that 2.0.
To determine the pathways, molecular functions, and biological processes represented in otosclerosis, differentially expressed genes were analyzed using the PANTHER Classification System (Applied Biosystems, http://www.pantherdb.org/) (Thomas et al., 2003).
2.6. Sequencing
Significant genes from the microarray analysis located in the OTSC3 locus were sequenced in the original family used to identify this locus. Sequencing was performed using Big Dye terminator chemistry version 1.1. Sequences of exons and splice site junctions were analyzed using Sequencher 4.5.
2.7. Real-Time PCR
TaqMan® Gene Expression Assays for 7 genes (CDKN1A, LTB, ATP1B3, PF4, IBSP, LDLR, CCL2) were used to validate microarray results on first-round amplified RNA from each sample used for microarray analysis. First-round amplified RNA was used due to the limited quantity of original RNA used for the microarray analysis. Real-time PCR was performed on the ABI PRISM 7500 Sequence Detection System (Applied Biosystems) using standard conditions (95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute). Human beta glucuronidase (GUSB) was used as the endogenous control.
3. Results
Comparison of transcript expression in three technical replicates of sample and control stapes footplate RNA samples showed that 110 genes were differentially expressed in patients compared with control samples. Of these 110 genes, 92 were upregulated and 18 were down regulated (Table 1). Of the 92 genes that were upregulated in otosclerosis, 70 were known genes, 1 was a pseudogene, 2 had a predicted protein function assigned by PANTHER, and 19 were genes of unknown function. Of the down-regulated genes, 13 were known genes, 1 was a pseudogene, 1 had a predicted protein function assigned by PANTHER, and 3 were genes of unknown function.
Table 1.
Up- and down-regulated genes in otosclerosis patients. Fold change and q-value are based on microarray analysis.
|
Upregulated Genes | ||||
|---|---|---|---|---|
| Gene | Gene Function | Panther Pathway | Fold Change | q-value |
| ADAMTS4 | disintegrin-like and metalloprotease with thrombospondin type 1 motif, 4 | 9.249 | 0.0029 | |
| APOLD1 | apolipoprotein L domain containing 1 | 2.832 | 0.0395 | |
| ARG2 | arginase, type II | 2.930 | 0.0095 | |
| ARL11 | ADP-ribosylation factor-like 11 | 4.653 | 0.0213 | |
| ARMC5 | armadillo repeat containing 5 | 2.203 | 0.0249 | |
| ATP1B3† | ATPase, Na+/K+ transporting, beta 3 polypeptide | 2.320 | 0.0094 | |
| ATXN7L2 | ataxin 7-like 2 | 2.773 | 0.0024 | |
| BC070289 | hypothetical protein | 3.245 | 0.0029 | |
| BTNL8 | butyrophilin-like 8 | 6.409 | 0.0081 | |
| C11orf75 | chromosome 11 open reading frame 75 | 2.078 | 0.0249 | |
| C13orf33 | chromosome 13 open reading frame 33 | 2.473 | 0.0009 | |
| C1orf21 | chromosome 1 open reading frame 21 | 2.168 | 0.0126 | |
| CCL2† | chemokine (C-C motif) ligand 2 | Inflammation | 6.157 | 0.0024 |
| CCL4 | chemokine (C-C motif) ligand 4 | Inflammation | 3.651 | 0.0029 |
| CDKN1A† | cyclin-dependent kinase inhibitor 1A (p21, Cip1) | Interleukin signaling, p53 signaling | 4.252 | 0.0117 |
| CHST11 | carbohydrate (chondroitin 4) sulfotransferase 11 | 2.227 | 0.0117 | |
| CLEC1B | C-type lectin domain family 1, member B | 3.243 | 0.0129 | |
| CLEC4E | C-type lectin domain family 4, member E | 3.490 | 0.0182 | |
| CRISPLD2 | cysteine-rich secretory protein LCCL domain containing 2 | 2.348 | 0.0351 | |
| DKFZp451A211 | Homo sapiens DKFZp451A211 protein | 7.286 | 0.0095 | |
| EDN2 | endothelin 2 | Endothelin signaling | 3.267 | 0.0423 |
| FAM129C | family with sequence similarity 129, member C | 2.036 | 0.0259 | |
| FLJ16165 | hypothetical protein | 2.001 | 0.0117 | |
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | 7.941 | 0.0178 | |
| FPR1 | formyl peptide receptor 1 | Inflammation | 2.572 | 0.0095 |
| GIYD2, GIYD1, SULT1A3 | sulfotransferase family, cytosolic, 1A, phenol- preferring, member 3 | 2.628 | 0.0156 | |
| GPR158 | G protein-coupled receptor 158 | 3.962 | 0.0095 | |
| HBA2, HBA1 | hemoglobin, alpha 2; hemoglobin, alpha 1 | 2.460 | 0.0029 | |
| HBD | hemoglobin, delta | 4.088 | 0.0057 | |
| HBEGF | heparin-binding EGF-like growth factor | EGF receptor signaling | 3.200 | 0.0204 |
| HBG1, HBG2 | hemoglobin, gamma | 3.507 | 0.0092 | |
| HYAL1 | hyaluronoglucosaminidase 1 | 3.095 | 0.0095 | |
| IGSF9B | immunoglobulin superfamily, member 9B | 2.468 | 0.0259 | |
| IL1R2 | interleukin 1 receptor, type II | 3.942 | 0.0019 | |
| IL6 | interleukin 6 (interferon, beta2) | Interleukin signaling | 6.088 | 0.0474 |
| IL8RA | interleukin 8 receptor, alpha | Inflammation, Interleukin signaling | 2.457 | 0.0009 |
| IL8RB | interleukin 8 receptor, beta | Inflammation, Interleukin signaling | 7.904 | 0.0009 |
| ITGAX | integrin, alpha X | Integrin signaling | 2.010 | 0.0098 |
| ITPKC | inositol 1,4,5-trisphosphate 3-kinase C | 3.381 | 0.0201 | |
| KCNJ15 | potassium inwardly-rectifying channel, subfamily J, member 15 | 3.991 | 0.0374 | |
| KIAA1539 | hypothetical protein | 2.365 | 0.0117 | |
| KIAA1622 | HEAT-like repeat-containing protein | 2.948 | 0.0432 | |
| LDLR† | low density lipoprotein receptor | Alzheimer disease-presenilin pathway | 4.031 | 0.0305 |
| LILRA1, LILRB1 | leukocyte immunoglobulin-like receptor, subfamily A (with TM domain), member 1 | 3.734 | 0.0223 | |
| LILRB3, LILRB2 | leukocyte immunoglobulin-like receptor, subfamily B | 3.651 | 0.0095 | |
| LOC387763 | hypothetical protein | 5.873 | 0.0043 | |
| LTB† | lymphotoxin beta (TNF superfamily, member 3) | Apoptosis | 2.785 | 0.0162 |
| MT1A | metallothionein 1A | 2.610 | 0.0095 | |
| MT1B | metallothionein 1B | 2.290 | 0.0104 | |
| MT1E | metallothionein 1E | 2.386 | 0.0098 | |
| MT1J | metallothionein 1J | 3.801 | 0.0060 | |
| MT1L | metallothionein 1L | 2.289 | 0.0121 | |
| MT1M | metallothionein 1M | 2.743 | 0.0273 | |
| MT2A | metallothionein 2A | 2.642 | 0.0024 | |
| MTHFD2 | methylene tetrahydrofolate dehydrogenase (NAD+ dependent), methenyltetrahydrofolate cyclohydrolase | 2.747 | 0.0474 | |
| MYC | v-myc myelocytomatosis viral oncogene homolog | Interleukin signaling, Oxidative stress response, p53 signaling, WNT signaling, PDGF signaling | 3.128 | 0.0110 |
| MYEOV | myeloma overexpressed gene | 4.286 | 0.0076 | |
| NFIL3 | nuclear factor, interleukin 3 regulated | 2.331 | 0.0095 | |
| NPBWR2 | neuropeptides B/W receptor 2 | 2.338 | 0.0320 | |
| NRN1 | neuritin 1 | 2.121 | 0.0206 | |
| OVOL1 | ovo-like 1(Drosophila) | 2.185 | 0.0314 | |
| OR7E47P | olfactory receptor, family 7, subfamily E, member 47 pseudogene | 9.883 | 0.0420 | |
| PF4†,§ | platelet factor 4 (chemokine (C-X-C motif) ligand 4) | Inflammation | 12.163 | 0.0117 |
| PLEC1 | plectin 1, intermediate filament binding protein | 3.390 | 0.0009 | |
| PPBP | pro-platelet basic protein (chemokine (C-X-C motif) ligand 7) | 3.972 | 0.0001 | |
| PRPH2 | peripherin 2 | 2.271 | 0.0249 | |
| PSMD9 | proteasome (prosome, macropain) 26S subunit, non-ATPase, 9 | Ubiquitin proteasome pathway | 2.055 | 0.0098 |
| RGC32 | response gene to complement 32 | 2.966 | 0.0161 | |
| S100A8 | S100 calcium binding protein A8 (calgranulin A) | 2.938 | 0.0425 | |
| SERTAD1 | SERTA domain containing 1 | 2.175 | 0.0242 | |
| SLA | Src-like-adaptor | 2.045 | 0.0347 | |
| SLC39A14 | solute carrier family 39 (zinc transporter), member 14 | 5.292 | 0.0264 | |
| STATH | statherin | 6.455 | 0.0095 | |
| TBC1D10B | TBC1 domain family, member 10B | 2.082 | 0.0156 | |
| TIPARP | TCDD-inducible poly(ADP-ribose) polymerase | 2.341 | 0.0089 | |
| TNFAIP8L3 | tumor necrosis factor, alpha-induced protein 8-like 3 | 2.520 | 0.0003 | |
| TNFRSF10C | tumor necrosis factor receptor superfamily, member 10c, decoy without an intracellular domain | Apoptosis, p53 signaling | 2.314 | 0.0020 |
| TREML1 | triggering receptor expressed on myeloid cells-like 1 | 2.515 | 0.0460 | |
| TSHR | thyroid stimulating hormone receptor | 2.767 | 0.0223 | |
| TUBB6 | tubulin, beta 6 | Huntington disease, Cytoskeletal regulation by Rho GTPase, Hedgehog signaling pathway | 2.143 | 0.0273 |
| ZKSCAN1 | zinc finger with KRAB and SCAN domains 1 | 3.601 | 0.0497 | |
| unassigned | chr6: 4083845-4105692 | 2.099 | 0.0447 | |
| unassigned | chr22:23689553-23690241 | 2.216 | 0.0447 | |
| unassigned | chr19:33316188-33318797 | 2.227 | 0.0264 | |
| unassigned | chr3:9362200-9366861 | 2.239 | 0.0035 | |
| unassigned | chr6: 170835774-170849263 | 2.351 | 0.0474 | |
| unassigned | chr13:23061887-23063001 | 2.381 | 0.0490 | |
| unassigned | chr4:185449989-185450691 | 2.487 | 0.0060 | |
| unassigned | chr4:72073747-72074717 | 2.945 | 0.0104 | |
| unassigned | chr1:210416660-210417060 | 3.012 | 0.0026 | |
| unassigned | chr5:74422818-74423714 | 3.248 | 0.0117 | |
| unassigned | chr16:41179572-41181020 | 5.996 | 0.0038 | |
|
Down-regulated Genes | ||||
| Gene | Gene Function | Panther Pathway | Fold Change | q-value |
|
| ||||
| CASR | calcium-sensing receptor | 2.556 | 0.0447 | |
| CHST9 | carbohydrate (N-acetylgalactosamine 4-0) sulfotransferase 9 | 2.550 | 0.0223 | |
| DUSP16 | dual specificity phosphatase 16 | Oxidative stress response | 3.733 | 0.0440 |
| FLJ4394 | hypothetical protein | 8.370 | 0.0387 | |
| FOXE1 | forkhead box E1 (thyroid transcription factor 2) | Interleukin signaling, Insulin/IGF pathway, PI3 kinase pathway, TGF-β signaling | 2.062 | 0.0009 |
| IBSP†,§ | integrin-binding sialoprotein (bone sialoprotein) | 6.221 | 0.0319 | |
| IFIT1 | interferon-induced protein with tetratricopeptide repeats 1 | 2.112 | 0.0095 | |
| KBTBD10 | kelch repeat and BTB (POZ) domain containing 10 | 2.666 | 0.0262 | |
| KRT19 | keratin 19 | 2.485 | 0.0110 | |
| LOC124220 | similar to common salivary protein | 3.761 | 0.0009 | |
| LOC160313 | keratin 19 pseudogene | 2.655 | 0.0117 | |
| NDP | Norrie disease (pseudoglioma) | 3.031 | 0.0294 | |
| OTX1 | orthodenticle homolog 1 (Drosophila) | 3.560 | 0.0232 | |
| SOX2 | SRY (sex determining region Y)-box 2 | 2.433 | 0.0009 | |
| SPRY1 | sprouty homolog 1, antagonist of FGF signaling (Drosophila) | EGF receptor signaling, FGF signaling | 2.781 | 0.0365 |
| TAF2 | TAF2 RNA polymerase II, TATA box binding protein (TBP)-associated factor | Transcription regulation by bZIP transcription factor, General transcription regulation | 2.039 | 0.0327 |
| unassigned | chr11:69182960-69190468 | 3.955 | 0.0209 | |
| unassigned | chr16:18863672-18878591 | 2.016 | 0.0162 | |
Tested via real-time PCR
Validated via real-time PCR
Pathway analysis of the differentially expressed genes using the PANTHER Classification System showed pathways possibly affected in otosclerosis (Figure 1, Table 1). The top pathways include interleukin signaling and inflammation, suggesting an increased inflammatory response in the otosclerosis patients. Other pathways suggested to be involved in otosclerosis in this analysis include p53 signaling, the apoptotic pathway, and two other pathways that have not been implicated in otosclerosis, EGF receptor signaling, and oxidative stress response.
Figure 1.
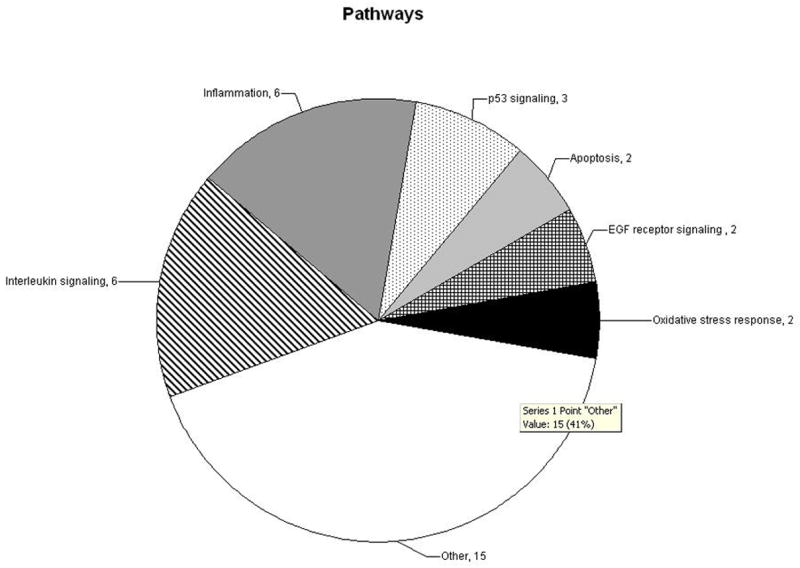
Pathways represented by differentially expressed genes. Differentially expressed genes were parsed through the PANTHER Classification System to determine pathways over-represented in otosclerosis patients. Values represent the number of genes present in each pathway.
Gene ontology analysis of differentially expressed genes using the PANTHER Classification System showed many genes involved in a number of biological processes and molecular functions (Figure 2). The top biological processes represented were signal transduction, immunity and defense, cell structure and motility, and nucleic acid metabolism. The top molecular functions represented included receptors, transcription factors, and signaling molecules. Some of the genes are involved in multiple pathways and molecular or biological processes, which are represented in the analysis.
Figure 2.
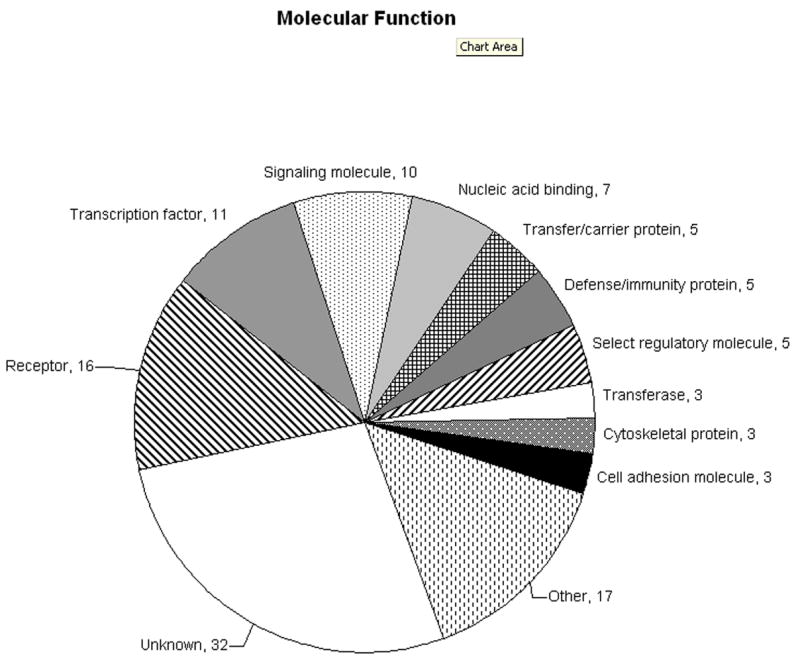
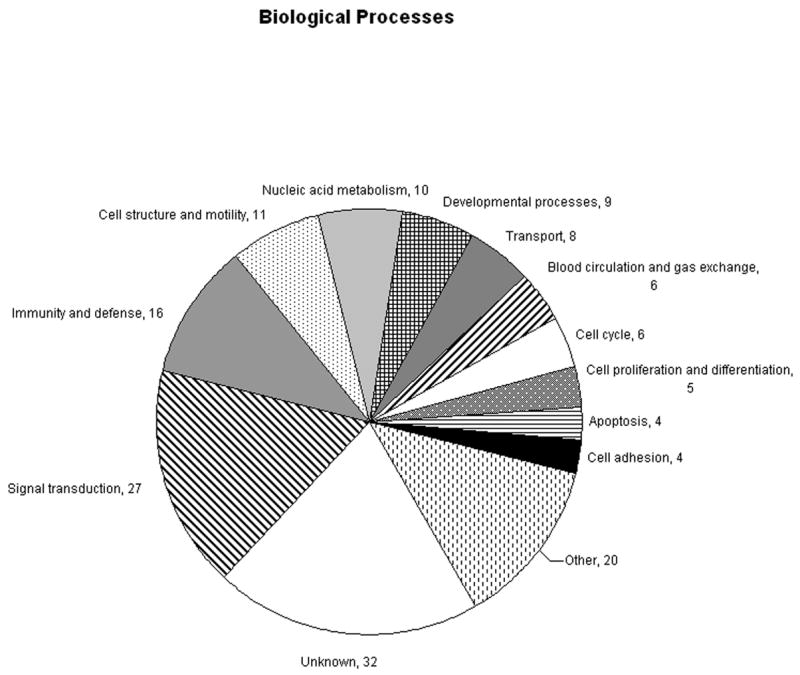
A. Molecular functions represented by differentially expressed genes in otosclerosis. B. Biological processes represented by differentially expressed genes in otosclerosis. Values represent the number of genes in each group. Genes assigned multiple molecular functions or biological processes by PANTHER are included.
The gene with the largest fold change PF4 was upregulated 12.16 fold in otosclerosis patients in microarray analysis and upregulated 2.46 fold after analysis by real-time PCR. The gene encoding the bone sialoprotein, IBSP, was the most down-regulated gene; it was down-regulated 6.22 fold in microarray analysis and showed a similar fold change after real-time PCR analysis. Five other genes were analyzed by real-time PCR but did not validate the microarray results. These genes include LDLR, CCL2, and three other genes located in two of the seven previously mapped OTSC loci: CDKN1A and LTB in the OTSC3 locus, and ATP1B3 in the OTSC5 locus. Both CDKN1A and LTB were sequenced in individuals from the original family mapped to OTSC3; however, no mutations were found in the coding region, 5′ and 3′ untranslated regions, or splice sites.
4. Discussion
Gene ontology analysis of differentially expressed genes in otosclerosis patients provides evidence that a number of pathways may be involved in otosclerosis. The presence of several differentially expressed genes in two pathways, interleukin signaling and inflammation, suggests that an inflammatory response may be a contributing factor in otosclerosis. This association is consistent with data predicting a potential role for measles virus infection and is also consistent with a genetic contribution based on the differential gene expression we observed. For example, gene ontology analysis showed that many of the genes are involved in signal transduction, suggesting that aberrant regulation of different signal transduction pathways may lead to abnormal bone remodeling in otosclerosis.
Genes that have been previously described as differentially expressed in otosclerosis were not detected in this study. In the study by Lehnerdt et al. otospongiotic stage stapes footplates showed increased expression of BMP-2, −4, and −7 (Lehnerdt et al., 2007). In studies by Karosi et al. TNF-alpha has been noted as expressed in otosclerotic stapes; expression was associated with detection of measles virus RNA (Karosi et al., 2005). This group has also shown that osteoprotegerin expression is decreased in otosclerotic stapes footplates that are positive for measles virus and TNF-alpha expression (Karosi et al., 2006). Decreased parathyroid hormone-parathyroid hormone related peptide receptor expression has also been documented in otosclerotic stapes samples (Grayeli et al., 1999). Failure to identify these genes in this current study could be due to the limited amount of samples available for this study or to the complex nature of the disease.
Microarray analysis identified CDKN1A, which encodes the cyclin dependent kinase inhibitor p21, and LTB, which encodes the TNF superfamily member lymphotoxin beta, located in the OTSC3 interval and ATP1B3, which encodes the beta subunit of the Na/K ATPase, located in the OTSC5 interval. However, these candidate genes could not be validated via real-time PCR analysis on the same samples used for the initial microarray analysis. This suggests that nonlinear amplification during RNA amplification may have occurred. Unexpectedly, two pseudogenes were identified as differentially expressed between otosclerosis patients and controls in the microarray analysis (Table 1). Real-time PCR was not performed on these two transcripts to determine whether this result is valid and whether these transcripts are specific to the otic capsule.
It is noteworthy that two genes found to be differentially expressed and validated by real-time PCR relate to transforming growth factor beta (TGF-β) signaling. TGF-β signaling plays a role in the regulation of bone remodeling by stimulating osteoblast chemotaxis, proliferation and differentiation. The presence of TGF-β1 at low levels primes osteoclast precursors for osteoclast differentiation, and at higher levels inhibits osteoclastogenesis (Karst et al., 2004). A recent case/control association study has shown that there is a variant of TGFB1 that is under-represented in patients with otosclerosis as compared to control subjects in Belgian-Dutch and French populations (Thys et al., 2007b). Identification of an association of two bone morphogenetic proteins, BMP2 and BMP4, with otosclerosis lends additional support to a role for TGF-β signaling in the disease process (Schrauwen et al., 2007).
Two genes that were differentially expressed in both microarray and real-time PCR analysis have been shown to be affected by TGF-β1 signaling. Platelet factor 4 (PF4), selectively inhibits binding of TGF-β1 to the type I TGF-β1 receptor (Whitson et al., 1991). Increased expression of PF4 may be inhibiting bone resorption by down-regulating signaling through the TGF-β pathway, which primes osteoclast precursor cells to undergo osteoclast differentiation. TGF-β has also been shown to regulate expression of the IBSP homolog in rat (Ogata et al., 1997). Increased TGF-β signaling results in increased expression of IBSP. Down-regulation of IBSP in otosclerosis patients could be due to decreased TGF-β1 signaling. However, IBSP can also be negatively regulated by the transcription factor AP-1, which is composed of jun and fos heterodimers (Yamauchi et al., 1996). A member of the fos family of AP-1 transcription factors, FOSB, was also upregulated in otosclerosis patients, suggesting increased expression of FOSB in otosclerotic lesions may be responsible for the decrease in expression of IBSP.
In summary, microarray analysis of stapes footplate samples from otosclerosis patients has identified differentially expressed genes and defined a number of biological pathways that are predicted to play a role in the pathogenesis of otosclerosis. These data provide a rational basis for the in depth study of specific genes and in complement with additional genetic association studies will lead to a better understanding of this disease.
Acknowledgments
We would like to thank all of the patients who made this research possible. This research was supported in part by National Institutes of Health grant R01DC05218 (R. J. H. S. and G. V. C.).
Abbreviations
- OTSC
otosclerosis
- PF4
platelet factor 4
- IBSP
bone sialoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann F, Glasgold A, Macduff JP. The incidence of otosclerosis as related to race and sex. Ann Otol Rhinol Laryngol. 1967;76:377–92. doi: 10.1177/000348946707600207. [DOI] [PubMed] [Google Scholar]
- Bel Hadj Ali I, Thys M, Beltaief N, Schrauwen I, Hilgert N, Vanderstraeten K, Dieltjens N, Mnif E, Hachicha S, Besbes G, Ben Arab S, Van Camp G. Hum Genet. 2008. A new locus for otosclerosis, OTSC8, maps to the pericentromeric region of chromosome 9. [DOI] [PubMed] [Google Scholar]
- Browning GG, Gatehouse S. Sensorineural hearing loss in stapedial otosclerosis. Ann Otol Rhinol Laryngol. 1984;93:13–16. doi: 10.1177/000348948409300104. [DOI] [PubMed] [Google Scholar]
- Brownstein Z, Goldfarb A, Levi H, Frydman M, Avraham KB. Chromosomal mapping and phenotypic characterization of hereditary otosclerosis linked to the OTSC4 locus. Arch Otolaryngol Head Neck Surg. 2006;132:416–24. doi: 10.1001/archotol.132.4.416. [DOI] [PubMed] [Google Scholar]
- Chen W, Meyer N, McKenna M, Pfister M, McBride D, Jr, Fukushima K, Thys M, Camp G, Smith R. Single-nucleotide polymorphisms in the COL1A1 regulatory regions are associated with otosclerosis. Clin Genet. 2007;71:406–14. doi: 10.1111/j.1399-0004.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Campbell CA, Green GE, Van Den Bogaert K, Komodikis C, Manolidis LS, Aconomou E, Kyamides Y, Christodoulou K, Faghel C, Giguere CM, Alford RL, Manolidis S, Van Camp G, Smith RJ. Linkage of otosclerosis to a third locus (OTSC3) on human chromosome 6p21.3–22.3. J Med Genet. 2002;39:473–7. doi: 10.1136/jmg.39.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- Declau F, van Spaendonck M, Timmermans JP, Michaels L, Liang J, Qiu JP, van de Heyning P. Prevalence of histologic otosclerosis: an unbiased temporal bone study in Caucasians. Adv Otorhinolaryngol. 2007;65:6–16. doi: 10.1159/000098663. [DOI] [PubMed] [Google Scholar]
- Frisch T, Sorensen MS, Overgaard S, Bretlau P. Estimation of volume referent bone turnover in the otic capsule after sequential point labeling. Ann Otol Rhinol Laryngol. 2000;109:33–9. doi: 10.1177/000348940010900106. [DOI] [PubMed] [Google Scholar]
- Gordon MA. The genetics of otosclerosis: a review. Am J Otol. 1989;10:426–38. doi: 10.1097/00129492-198911000-00003. [DOI] [PubMed] [Google Scholar]
- Grayeli AB, Sterkers O, Roulleau P, Elbaz P, Ferrary E, Silve C. Parathyroid hormone-parathyroid hormone-related peptide receptor expression and function in otosclerosis. Am J Physiol. 1999;277:E1005–12. doi: 10.1152/ajpendo.1999.277.6.E1005. [DOI] [PubMed] [Google Scholar]
- Karosi T, Konya J, Szabo LZ, Pytel J, Jori J, Szalmas A, Sziklai I. Codetection of measles virus and tumor necrosis factor-alpha mRNA in otosclerotic stapes footplates. Laryngoscope. 2005;115:1291–7. doi: 10.1097/01.MLG.0000165462.35495.DF. [DOI] [PubMed] [Google Scholar]
- Karosi T, Jokay I, Konya J, Szabo LZ, Pytel J, Jori J, Szalmas A, Sziklai I. Detection of osteoprotegerin and TNF-alpha mRNA in ankylotic Stapes footplates in connection with measles virus positivity. Laryngoscope. 2006;116:1427–33. doi: 10.1097/01.mlg.0000225928.35838.e5. [DOI] [PubMed] [Google Scholar]
- Karst M, Gorny G, Galvin RJ, Oursler MJ. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J Cell Physiol. 2004;200:99–106. doi: 10.1002/jcp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnerdt G, Unkel C, Metz KA, Jahnke K, Neumann A. Immunohistochemical evidence of BMP-2, β4 and β7 activity in otospongiosis. Acta Otolaryngol. 2007:1–5. doi: 10.1080/00016480701299659. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Mills BG. Ultrastructural and immunohistochemical evidence of measles virus in active otosclerosis. Acta Otolaryngol Suppl. 1990;470:130–9. discussion 139–40. [PubMed] [Google Scholar]
- McKenna MJ, Kristiansen AG, Bartley ML, Rogus JJ, Haines JL. Association of COL1A1 and otosclerosis: evidence for a shared genetic etiology with mild osteogenesis imperfecta. Am J Otol. 1998;19:604–10. [PubMed] [Google Scholar]
- Menger DJ, Tange RA. The aetiology of otosclerosis: a review of the literature. Clin Otolaryngol Allied Sci. 2003;28:112–20. doi: 10.1046/j.1365-2273.2003.00675.x. [DOI] [PubMed] [Google Scholar]
- Niedermeyer H, Arnold W, Neubert WJ, Hofler H. Evidence of measles virus RNA in otosclerotic tissue. ORL J Otorhinolaryngol Relat Spec. 1994;56:130–2. doi: 10.1159/000276627. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Niisato N, Furuyama S, Cheifetz S, Kim RH, Sugiya H, Sodek J. Transforming growth factor-beta 1 regulation of bone sialoprotein gene transcription: identification of a TGF-beta activation element in the rat BSP gene promoter. J Cell Biochem. 1997;65:501–12. doi: 10.1002/(sici)1097-4644(19970615)65:4<501::aid-jcb6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ramsay HA, Linthicum FH., Jr Mixed hearing loss in otosclerosis: indication for long-term follow-up. Am J Otol. 1994;15:536–9. [PubMed] [Google Scholar]
- Rodriguez L, Rodriguez S, Hermida J, Frade C, Sande E, Visedo G, Martin C, Zapata C. Proposed association between the COL1A1 and COL1A2 genes and otosclerosis is not supported by a case-control study in Spain. Am J Med Genet A. 2004;128:19–22. doi: 10.1002/ajmg.a.30074. [DOI] [PubMed] [Google Scholar]
- Schrauwen I, Thys M, Vanderstraeten K, Fransen E, Dieltjens N, Huyghe JR, Ealy M, Claustres M, Cremers CR, Dhooge I, Declau F, Van de Heyning P, Vincent R, Somers T, Offeciers E, Smith RJ, Van Camp G. Association of Bone Morphogenetic Proteins With Otosclerosis. J Bone Miner Res. 2007 doi: 10.1359/JBMR.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF, Barber W. Histologic variants in otosclerosis. Laryngoscope. 1985;95:1307–17. doi: 10.1288/00005537-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thys M, Van Den Bogaert K, Iliadou V, Vanderstraeten K, Dieltjens N, Schrauwen I, Chen W, Eleftheriades N, Grigoriadou M, Pauw RJ, Cremers CR, Smith RJ, Petersen MB, Van Camp G. A seventh locus for otosclerosis, OTSC7, maps to chromosome 6q13–16.1. Eur J Hum Genet. 2007a doi: 10.1038/sj.ejhg.5201761. [DOI] [PubMed] [Google Scholar]
- Thys M, Schrauwen I, Vanderstraeten K, Janssens K, Dieltjens N, Van Den Bogaert K, Fransen E, Chen W, Ealy M, Claustres M, Cremers CR, Dhooge I, Declau F, Claes J, Van de Heyning P, Vincent R, Somers T, Offeciers E, Smith RJ, Van Camp G. The coding polymorphism T263I in TGF-{beta}1 is associated with otosclerosis in two independent populations. Hum Mol Genet. 2007b doi: 10.1093/hmg/ddm150. [DOI] [PubMed] [Google Scholar]
- Tomek MS, Brown MR, Mani SR, Ramesh A, Srisailapathy CR, Coucke P, Zbar RI, Bell AM, McGuirt WT, Fukushima K, Willems PJ, Van Camp G, Smith RJ. Localization of a gene for otosclerosis to chromosome 15q25-q26. Hum Mol Genet. 1998;7:285–90. doi: 10.1093/hmg/7.2.285. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaert K, De Leenheer EM, Chen W, Lee Y, Nurnberg P, Pennings RJ, Vanderstraeten K, Thys M, Cremers CW, Smith RJ, Van Camp G. A fifth locus for otosclerosis, OTSC5, maps to chromosome 3q22–24. J Med Genet. 2004;41:450–3. doi: 10.1136/jmg.2004.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bogaert K, Govaerts PJ, Schatteman I, Brown MR, Caethoven G, Offeciers FE, Somers T, Declau F, Coucke P, Van de Heyning P, Smith RJ, Van Camp G. A second gene for otosclerosis, OTSC2, maps to chromosome 7q34–36. Am J Hum Genet. 2001;68:495–500. doi: 10.1086/318185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson RH, Jr, Wong WL, Itakura K. Platelet factor 4 selectively inhibits binding of TGF-beta 1 to the type I TGF-beta 1 receptor. J Cell Biochem. 1991;47:31–42. doi: 10.1002/jcb.240470105. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Ogata Y, Kim RH, Li JJ, Freedman LP, Sodek J. AP-1 regulation of the rat bone sialoprotein gene transcription is mediated through a TPA response element within a glucocorticoid response unit in the gene promoter. Matrix Biol. 1996;15:119–30. doi: 10.1016/s0945-053x(96)90153-5. [DOI] [PubMed] [Google Scholar]


