Abstract
Background
Correct pacemaker (PM) diagnosis of paroxysmal atrial tachyarrhythmias is crucial for their prevention and intervention with specific atrial pacing programmes. The PM mode switch to only ventricular pacing after detection of atrial tachyarrhythmias is often used as the parameter to quantify the ‘burden’ of atrial tachyarrhythmias.
Objectives
This review addresses potential errors in the detection and diagnosis of atrial tachyarrhythmias, sometimes resulting in incorrect mode switches. The interpretation of PM-stored data of patients with atrial tachyarrhythmias and the results of trials of pace prevention and intervention can be better appreciated with more insight into the technical options and pitfalls.
Results
Literature and clinical experience demonstrate that the correctness of PM-derived diagnosis of atrial tachyarrhythmias depends on 1) the sensitivity setting to detect the onset and perpetuation of atrial tachyarrhythmias frequently characterised by variable and low-voltage signals, 2) the rejection of far-field R wave sensing by the atrial sense amplifier, 3) the facility for verification of mode switches by a high-quality intracardiac registration of the nonmodified atrial electrogram. The configuration of the atrial lead also contributes to the diagnostic performance of the PM.
Conclusion
Not only pacing algorithms and diverse technical PM features but also the atrial lead configuration are currently the limiting factors to the fully reliable, automated detection and diagnosis of atrial tachyarrhythmias. If these technical shortcomings can be improved, better signal processing will result. Then atrial pacing to prevent or suppress atrial tachyarrhythmias will be more justified. (Neth Heart J 2008;16:201-10.)
Keywords: atrial fibrillation, pacing, mode switch, pacemaker leads, sensing, atrial overdrive, pace prevention, far-field R wave
Until now antiarrhythmic drugs remain the first step in the treatment strategy for atrial fibrillation (AF), tachycardia (AT) and flutter (AFL). When successive drug failures or severe side effects of drugs emerge, selected patients can benefit from radiofrequency (RF) catheter ablative or surgical procedures.1-9 For patients with conventional reasons for chronic cardiac pacing in whom also paroxysmal atrial tachyarrhythmias (PAT) prevail or other reasons exist to avoid invasive therapies for PAT, specific atrial pacing programmes delivered by implanted pacemakers can be switched on. Often pacing will be combined with antiarrhythmic drugs to treat PAT. In the foregoing, prevention or suppression of PAT is addressed. If immediate termination of PAT is the option, in-hospital DC cardioversion or emergency drug intervention is needed because the atrial defibrillator is currently not being used due to the patient’s discomfort and chest pain despite low-energy shocks.10
In the past decade manufacturers of implantable cardiac devices have made impressive efforts and spent impressive budgets on designing technical facilities and algorithms to prevent or suppress PAT. These technical developments led to the initiation of clinical studies to evaluate the efficacy and safety of these pacemaker (PM) programmes. The efforts accumulated in the development of increased storage of the intrinsic and paced rhythm, an improved signal recognition and sophisticated, individually adaptable atrial sensing and pacing programmes.11-13 The expanded PM memory offered more insight into the natural course of PAT, and also displayed the relevance of the mode switch to DDI or VVI pacing in PAT as a marker of correct detection.11,14,15 All together, these technical developments promised to become an attractive alternative treatment for PAT, thus justifying primary PM implantation when drug treatment of PAT fails.
However, not only literature but also our daily clinical experience shows disappointing or no results regarding the prevention or suppression of PAT with diverse pacing algorithms.16-21 These disappointing results must be attributed to PM and lead properties that do not meet the requirements of correct detection and/or pacing when PAT emerge. Furthermore, the PM-delivered information about the type, incidence and timing of PAT can strongly mislead the cardiologist and the cardiac device technician.14,16,22
This review discusses a number of technical reasons why PM prevention or suppression of PAT can fail. The cardiologist’s awareness of these potential errors can guide a tailored programming of specific pacing treatment to prevent or suppress PAT in already implanted PMs. Second, knowledge of technical options and risks of these pacing methods can prevent unneeded PM implants. Both objectives preserve the safety of the patient suffering from paroxysmal PAT who is considered a candidate for atrial pacing therapy.
Definitions of atrial fibrillation burden
Before discussing the technical aspects, we must first consider the term ‘AF burden’ because this term has been used differently in various device and pharmacological studies. Sometimes AF burden is defined as number and duration of ‘patients complaints of AF’. Page et al. showed that asymptomatic AF occurred six times more frequently than symptomatic AF.23 The large-scale SOPAT study revealed that 50% of the transtelephonic AF recordings occurred without any symptoms.24 Furthermore, this study showed that transtelephonic recordings were associated with a large intra-patient variability in AF symptoms, and that symptomatic and asymptomatic AF can occur in the same patient.24 These observations undermine the validity of AF symptoms as a marker of the incidence and duration of atrial fibrillation.25
The AF burden calculated from the ECG recordings in terms of total time in AF during a specific period can be called the ‘electrocardiographic AF burden’ (EAFB). The EAFB can be further subdivided into total time in AF, the number of AF (re)occurrences in a specific period or duration of the AF-free period until the recurrence of AF, or a combination of these. The PM memory could be the ideal tool to quantify the EAFB because of its implanted Holter facilities and because it is more accurate than ‘symptomatic AF burden’ or the AF burden based on Holter monitoring in a restricted time span as AF tends to vary significantly over time.26,27
Pacemaker automated detection of atrial tachyarrhythmias
A review of the automated PM options and facilities to detect and diagnose AF, AT and AFL includes comments on the appropriateness, correctness and optimal programming of their detection and diagnosis. Furthermore, to estimate the reliability of automated PM detection and diagnosis the results of signal processing need to be compared with the ‘golden standard’ of the diagnosis of AF, AT and AFL. For this purpose 24-hour Holter recordings, (episodic) transtelephonic monitoring and implantable loop recordings are available to compare the performance of automated PM diagnosis of various PAT. Studies have shown that the total duration of the electrocardiographic AF burden measured with a PM memory is highly reliable during a prolonged period of time, but not the number of AF episodes and their duration.14,22.
Programming of the atrial sensitivity
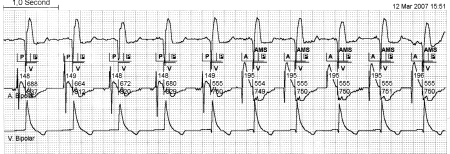
For the correct detection of atrial tachyarrhythmias, the programming of the atrial sensitivity is crucial and should preferably be <0.2 mV in bipolar sensing. Programming of a low sensitivity, e.g. sensing >1 mV, will introduce intermittent AF undersensing as the amplitudes of AF signals vary continuously in amplitude and will lead to false calculation of the frequency and duration of AF episodes (figure 1). Figure 1 shows a continuous strip from the PM memory. The electrogram (EGM) is derived from the atrial bipolar lead and clearly demonstrates the change in voltage of AF. When the amplitudes of the atrial fibrillatory waves remain very small, continuous undersensing will occur. In principle this sensing failure does not harm patients as they will only be unnecessarily paced in the atrium without capture, and the ventricular on demand pacing still provides an adequate rhythm.
Figure 1.
Periodical undersensing of atrial fibrillation. The automatic mode switch (AMS) in the beginning of the strip is due to AF. An exit of the AMS is caused by undersensing of the fibrillatory waves in the atrial sense channel. Reengagement of AMS is caused by sensing the increasing voltage of AF again. This periodical undersensing of AF results in multiple incorrect mode switches during AF, although AF is continuously present.
Figure abbreviations
A=atrial paced, P=atrial sensed, P in black box=sensed atrial activity in the refractory period, V=ventricular paced, R=ventricular sensed, AMS=automatic mode switch.
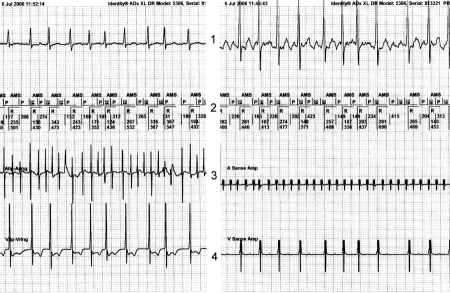
When atrial overdrive pacing algorithms are engaged and low sensitivity is programmed for atrial signals (for example >0.5 mV) inadvertent DDD pacing with a high rate can occur. Because some of the atrial fibrillatory waves are sensed but the majority are not, the sensed atrial waves will be interpreted by the PM as atrial premature beats: this sequence can initiate an atrial overdrive algorithm (figures 2A and B). In the type of pacemaker in figure 2 (St. Jude Affinity), an atrial overdrive algorithm is triggered by the appearance of frequent atrial electrical activity with a higher rate than the atrial paced interval. It can be concluded that mainly undersensing of AF occurs in this tracing and only some of the atrial fibrillatory waves are sensed by the pacemaker. The pacemaker interprets this as frequent atrial premature beats during atrial asystole. This will trigger the atrial overdrive algorithm, although (the undersensed) AF is present. On the other hand, programming of a very high sensitivity (<0.2 mV) to optimise the detection of AF with low amplitudes can promote the sensing of unwanted signals as myopotentials, external noise and far-field ventricular conducted or paced R waves (FFRW).
Figure 2.
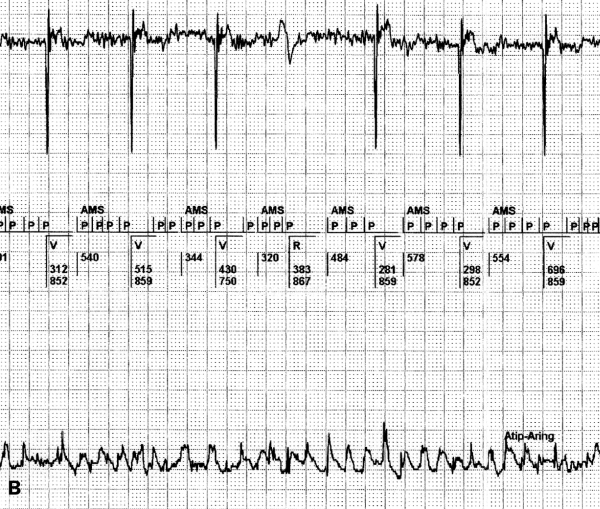
A) Permanent high ventricular rate due to the atrial overdrive algorithm. The surface ECG shows a ventricular paced rhythm of 130 beats/min. The broad band 0-100 Hz registration of the EGM derived from the bipolar atrial lead clearly depicts AF. During the high atrial and ventricular paced rhythm, several atrial sensed events occur (arrows). These atrial events trigger the atrial overdrive mode. B) This tracing is from the same patient, when atrial sensitivity is programmed at 0.1mV. Atrial fibrillation is now sensed properly, resulting in an AMS.
Far-field R wave sensing in the atrial channel
Sensing of FFRW occurs when the electrical activity from the ventricular activation is sensed by the atrial channel. Literature shows that the most frequent causes of incorrect detection of atrial tachyarrhythmias are the occurrence of short runs of atrial premature beats and secondly, intermittent FFRW sensing by the atrial channel.14,18,28-30
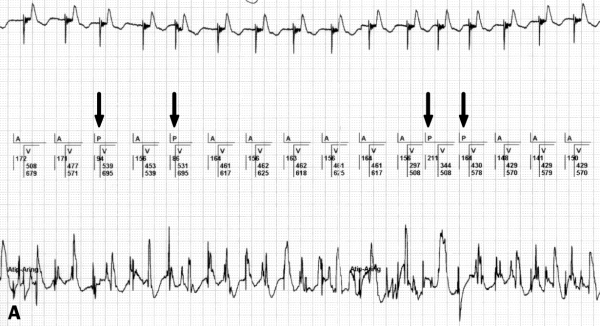
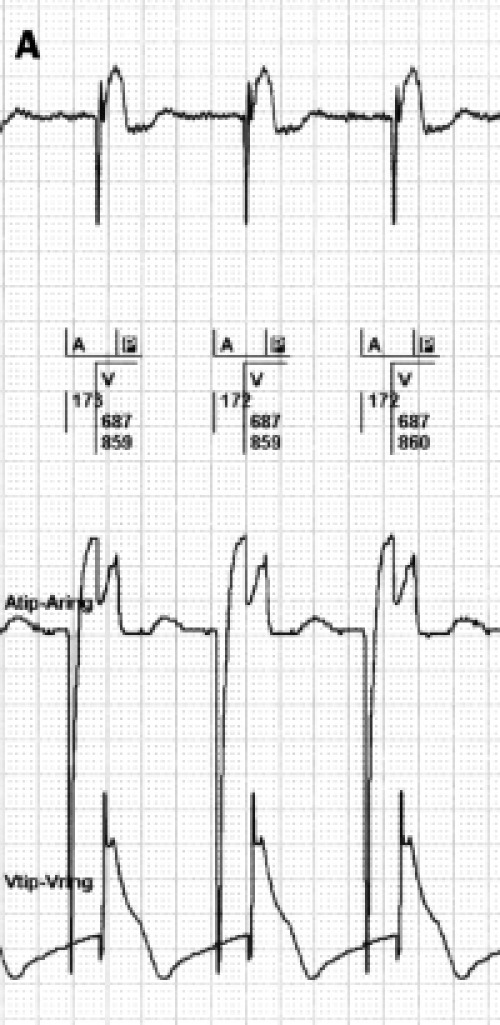
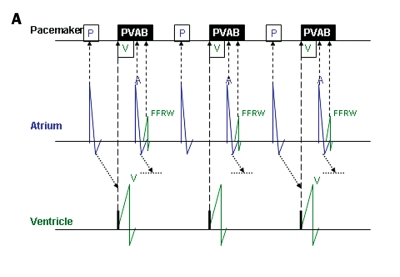
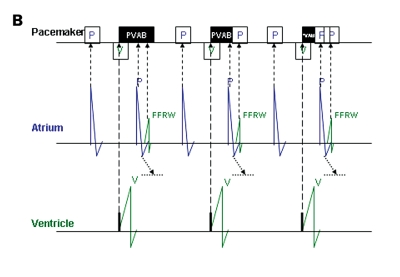
FFRW sensing occurs when a deflection is detected in the programmed AV interval or after postventricular atrial blanking (PVAB) (figure 3). FFRW sensing can occur prior to a ventricular paced event during a fusion beat where the intrinsic ventricular wave front is sensed by the atrial channel before the ventricular channel paces.31 However, in the majority of cases an FFRW will be sensed during the postventricular atrial refractory period (PVARP). When the FFRW occurs after the PVAB but within the PVARP, the FFRW will be counted as an atrial event. However, as the FFRW falls within the PVARP, the FFRW will not act as a sensed atrial event that tracks ventricular pacing after the AV delay. This double counting in the atrium can result in high rate counting and as a consequence mode switching from DDDR to VVIR or DDIR. Figure 4 displays an example of this type of double counting and thus false calculation of atrial tachyarrhythmias causing a mode switch.
Figure 3.
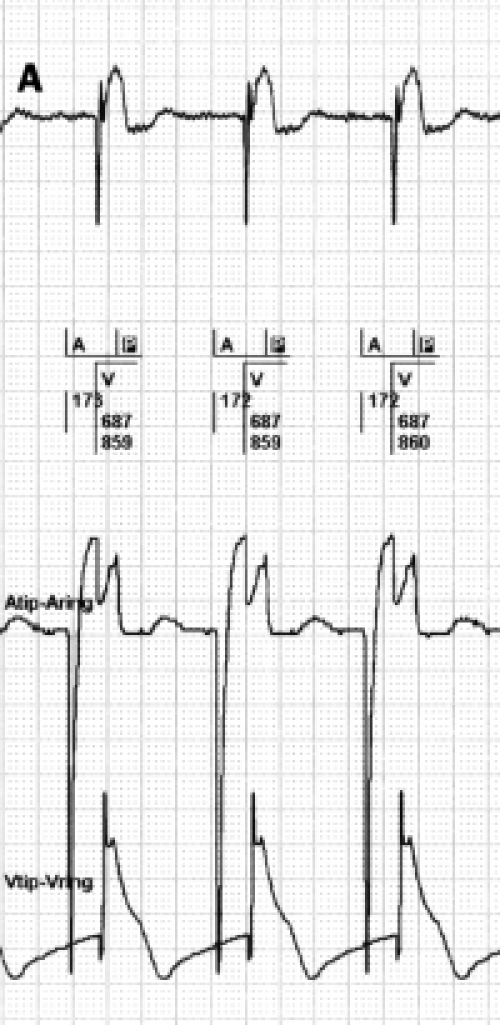
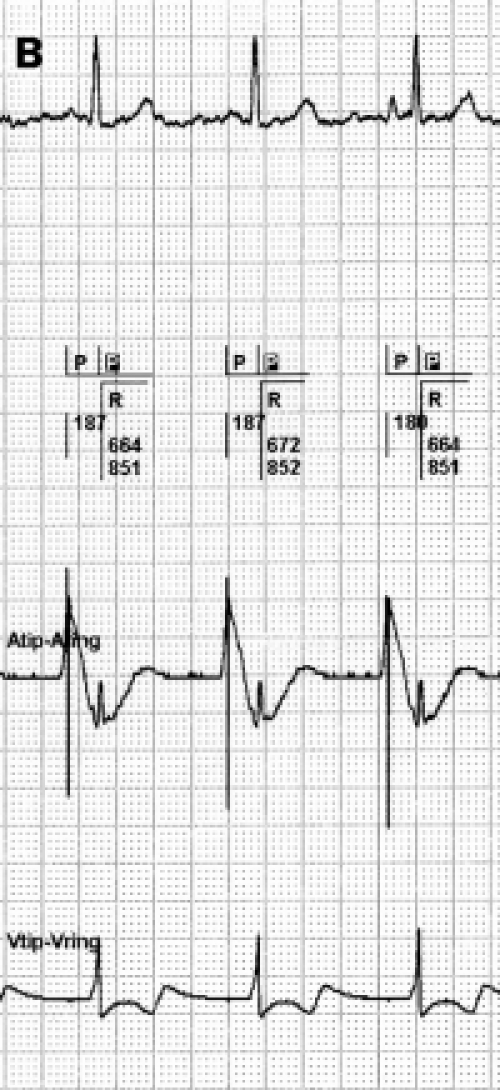
A paced far-field R wave (FFRW) (left panel) and sensed FFRW (right panel). The top channel depicts the surface ECG. The second channel depicts the interpretation of the rhythm by the pacemaker. The third channel is the atrial EGM, the fourth channel the ventricular EGM. Sensing of the paced FFRW in the atrial channel in the left panel occurs after a ventricular paced event (P in black box). This will cause counting of two instead of one event in the atrial channel. When a normal conducted ventricular depolarisation occurs (right panel), it depends on the timing of sensing whether the ventricular electrode or the atrial electrode will sense this event first. Usually the ventricular lead senses first, but the atrial lead can sense the ventricular depolarisation before the ventricular electrode, as is the case in the right panel. When blanking of the FFRW is selected, post- and pre-ventricular blanking should be considered.
Figure 4.
Mode switch caused by FFRW sensing. The upper channel depicts a surface ECG with a sensed atrial rhythm and a paced ventricular rhythm. The second channel shows the interpretation by the pacemaker of the atrial and ventricular rhythm. The third channel is the atrial EGM, the fourth channel the ventricular EGM. The AMS to the DDI mode is caused by a high atrial event counting due to adding up of atrial paced events and FFRW sensing.
To prevent sensing of FFRWs the atrial sensitivity should be programmed at such a level that FFRWs are no longer detected by the atrial sense amplifier but the P wave is still correctly sensed.32 In practice, the atrial sensitivity should be programmed at 1 mV or even higher as the FFRW signals often have average values of slightly less than 1 mV.33 Unfortunately, this sensitivity level is inadequate for correct sensing of AF, where the atrial sensing level should be programmed to 0.2 mV or even lower. Several options to avoid FFRW sensing while programming the atrial sensing <0.2 mV are available:
Programming the postventricular atrial blanking (PVAB) period that is ‘fixed’ and extends beyond the time interval when the ventricular depolarisation is sensed in the atrial channel can be helpful. This time interval is generally <125 ms after the onset of the paced or intrinsic ventricular beat and can be seen on the EGM. Alternatively, if this EGM is not available the atrial marker channel offers the opportunity to programme the PVAB duration individually33 when the sensing level is programmed at its lowest level (figure 5). When the PVAB is programmed, and AF, AT or AFL occur, the effect of the PVAB time results in a slightly shorter duration of the sensed atrial arrhythmia. This shortcoming is not of major concern as AF is sensed properly and a mode switch will occur provided the sensitivity setting is set at the proper value.
Figure 5.
The effect of extension of the postventricular atrial blanking (PVAB). The PVAB is extended from 100 (right panel) to 125 msec (left panel). This obscures the FFRW from the atrial sensor as the PVAB is an absolute blanking for atrial sensing. The result of this programming is the prevention of a falsely high atrial rate count by adding together of the atrial paced events and the FFRW.
Recognition of the FFRW by timing is another possibility to prevent a mode switch due to double counting. Any event in the atrial sense channel at the same fixed time interval after a ventricular sense is marked as an FFRW of the normally conducted ventricular depolarisation. With ventricular pacing an event shortly after the pace and emerging at a fixed interval will be considered an FFRW. This recognition programme could implement a shorter blanking period of ventricular signals for the atrial PM channel. This blanking period should be calculated by the PM because the time of the onset of FFRW is patient dependent.
Far-field R wave sensing in atrial tachycardia and flutter
When an atrial tachycardia arises, the postventricular atrial blanking period (PVAB) does not only obscure the FFRW in the atrial channel, but also every second atrial beat of a tachycardia. This event is defined as the ‘2:1 lock-in phenomenon’ (figure 6A and B).34 PVAB to prevent FFRW sensing also prevents the sensing of every second atrial beat, causing the 2:1 lock-in phenomenon during the atrial tachycardia, resulting in a high ventricular paced rate when normal AV conduction is not present. During atrial tachycardia a mode switch will not occur due to this mechanism, if the upper rate limit is programmed at a higher rate than half of the AT/AFL rate. This 2:1 lock-in phenomenon delays the detection of the onset of AT/AFL and therefore delays antitachycardia pacing algorithms in becoming operational immediately after the onset of AT/AFL. In these cases AFL can deteriorate into AF before antitachycardia pacing therapy can effectively be applied as the atrial tachycardia is not detected correctly (figure 7). Accurate measurement of the duration of an AT by the pacemaker is not only influenced by the programming of the ATDR rate but also by the duration of the PVAB. In comparison with AT, a more accurate scoring of AF time is observed in the same strip (figure 7).
Figure 6.
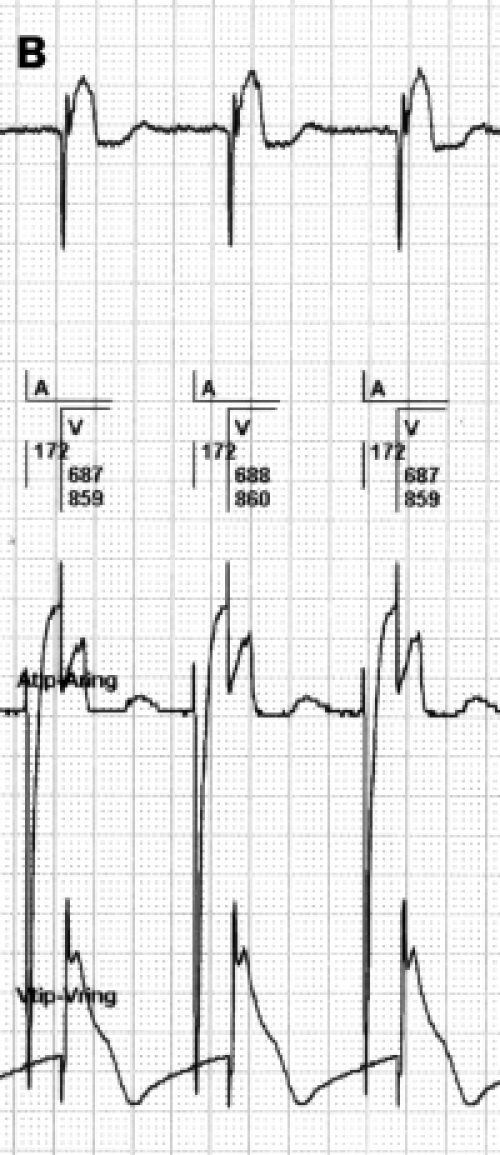
A) 2:1 lock in phenomenon during atrial tachycardia (AT) by the postventricular atrial blanking (PVAB). B) Effect of programming a different PVAB duration (black box). Programming a PVAB to prevent FFRW sensing is causing the 2:1 lock-in phenomenon of AT in the first and long PVAB. The shorter PVAB in the second sequence shows double counting in the atrial counter due to FFRW sensing. Only a very short PVAB as programmed in the third sequence will allow sensing of AT; however, the FFRW is sensed as well. This will cause an appropriate mode switch, but not the correct diagnosis of an AT.
Figure 7.
Sensing during atrial tachycardia (AT) and atrial fibrillation (AF). The EGM is derived from the atrial lead in bipolar setting with a sensitivity of 0.4 mV. AT detection rate (ATDR) was set at 180 beats/min and PVAB at 125 msec. This continuous strip shows an AT of 210 beats/min. As every second atrial complex is hidden from the pacemaker (PM) counter during PVAB, only half of the atrial complexes are recognised by the PM. This results in an atrial sense (P) of 105 beats/min and a ventricular pace (V) of 105 beats/min. At the end of the first strip and the start of the second, the AT deteriorates into AF. The high frequency of the irregular atrial activity is sensed immediately (black P and white P in black box). The PM is triggered into an AMS.
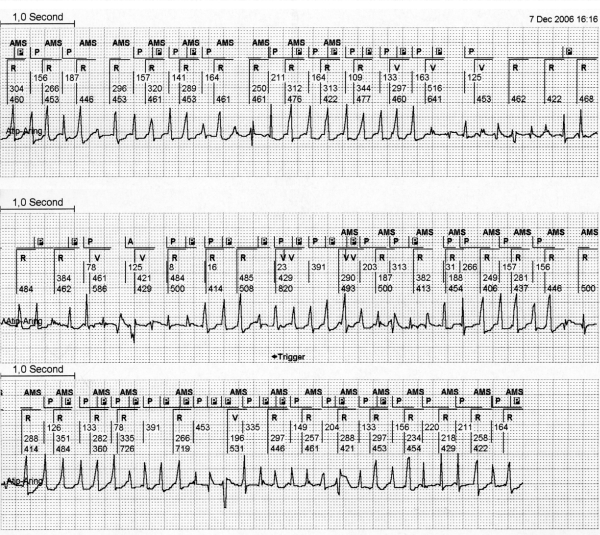
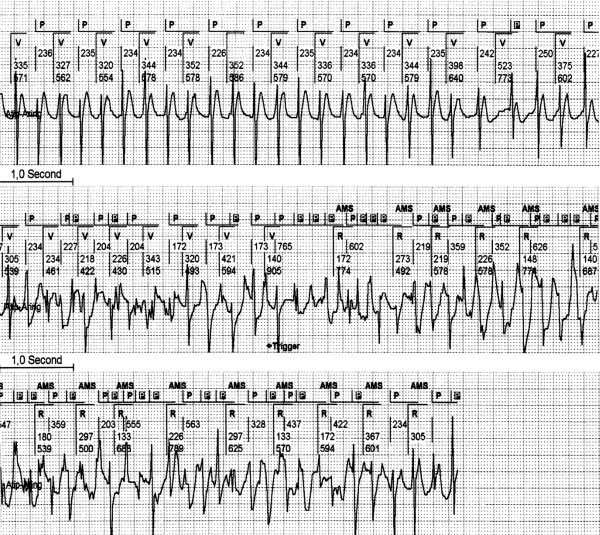
Most of the current pacemakers are provided with a search system for the ‘2:1 lock in phenomenon’ and will engage a mode switch eventually. However, during this mode switch to DDI, a 2:1 AV conduction of the AT/AFL will often occur. In that case the PVAB after a sensed ventricular complex will also blank every second atrial beat of the atrial tachycardia. The PM counter will assume an atrial rhythm at half its real rate and resumes to a normal DDDR mode. This makes the pacemaker switch mode time and again. As a consequence many mode switches can occur during a period of atrial tachycardia. This sequence is also a clear example of reduced reliability of the mode switch as the parameter to quantify AT/AFL episodes (figure 8). The programming of a very short PVAB permits the sensing of atrial deflections shortly after a ventricular depolarisation but this approach provokes unwanted FFRW sensing. The effects of the programming of various durations of PVAB during atrial tachycardia are depicted in figure 6B. These examples emphasise that technical solutions for avoiding FFRW sensing are strongly needed to optimise the PM diagnosis of atrial arrhythmias.
Figure 8.
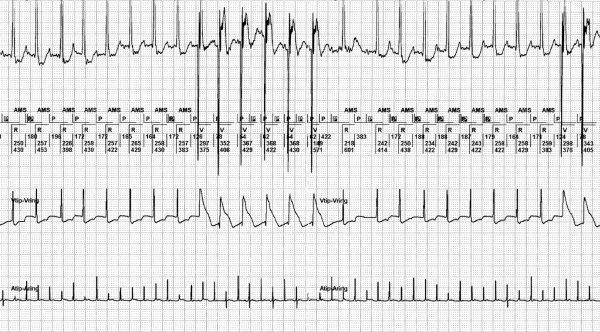
During atrial tachycardia, repeated automatic mode switch (AMS) due to a second atrial depolarisation during the postventricular atrial blanking (PVAB), the 2:1 lock in phenomenon. This EGM shows in the first channel a surface ECG strip. The second channel shows the interpretation of the rhythm by the pacemaker (PM). The third channel shows the ventricular EGM and the fourth channel the atrial EGM derived from the atrial lead in bipolar setting with a sensitivity of 0.6 mV. In the first part of the strip, half of the atrial depolarisation occurs during the PVAB (which is set to 60 msec in this example) and is therefore not detected by the PM. The PM switches back from AMS to normal DDD mode. During automatic ventricular threshold search directly after the AMS a short PV interval is seen. After the AMS exit, atrial deflections fall outside the PVAB and the PM returns to AMS. This sequence was seen time and again in this patient.
Dedicated lead and pacemaker for diagnosing atrial arrhythmias
The atrial lead
An implantable lead specially designed to reject FFRW sensing can overcome the majority of pitfalls in the detection of atrial arrhythmias including correct sensing of AFL waves and AF with its variable (low) voltages. Atrial leads with a dipole spacing >10 mm are not suitable for correctly discriminating between FFRW and real atrial signals. A shorter inter-electrode distance with a shorter ‘dipole’ can greatly reduce the sensing of FFRW and myopotentials because of the shorter ‘antennal function’.35 In animals and during human pacemaker implants36 we compared the sensing characteristics of a new atrial lead with a 1.2 mm tipto- ring spacing to an atrial lead with a dipole distance of 9 mm. In the former the voltage of the sensed P wave showed only a trend towards a lower value in the limb leads. Although the dipole distance is a major factor in FFRW rejection, the electrode material and the size of the anodal surface also contribute to appropriate FFRW rejection.36 It is emphasised that a further reduction in the dipole distance and dimension can cause a stiffer distal portion of the lead that is more prone to dislocation and perforation of the atrial wall. Further experimental and human pacing studies on lead technology are needed to select the optimal atrial lead configuration.
The pacemaker
Crucial components of sensing are sampling frequency and the sensing amplifier; both handle the original intracardiac signals in two different ways. First, the sample frequency determines the shape and amplitude of the signal (figure 9A). When the sampling frequency is low, the shape and amplitude of the original intracardiac signal is determined by a low number of sampling moments; this can result in a distorted signal before it is processed by the amplifier. Secondly, the processing of the signal is performed by the sense amplifier that augments the distorted signal and thereby changes the shape and amplitude of the original signal again. The result is a signal that clearly deviates from the original configuration (figure 9B).
Figures 9A and B.
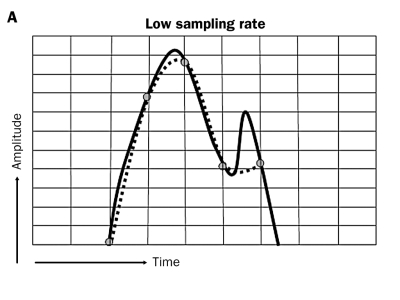
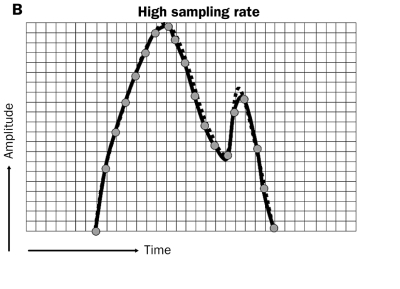
Low and high sampling rate. Low sampling rate can alter the waveform of the intracardiac depolarisation as the infrequent sampling (grey dots with dotted line) will not allow some fast deflections to be recognised (see last sharp deflection in 9B). A high sampling rate allows accurate recognition of the form of high frequency intracardiac signals.
It is understandable that a high sampling frequency of intracardiac signals promotes the correctness of the automated classification of these signals and allows automated recognition of the form of FFRW signals. In the near future more attention should be directed to the technique of high frequency signal detection because nowadays sampling rates are too low to discriminate signal configuration properly.37,38 Finally, digital processing of incoming signals permits grouping of atrial, ventricular and FFRW events and promotes better discrimination between true P waves, atrial arrhythmias and inadvertent signals. After a short learning period, these PM algorithms can adapt continuously to new wave forms and discriminate between various types of atrial tachyarrhythmias.37,38
Verifying automated detection of atrial tachycardia
For the diagnosis of atrial signals, the PM utilises the electrogram (EMG) transmitted by the atrial lead. Atrial signals are modified by the atrial amplifier (see above). Verification of the atrial signals by the operator requires a high-quality EGM stored in the PM memory. To facilitate the storage of a high-quality EGM, the wide bandpass filter of the telemetry circuit appears to be a better tool than the current bandpass of the sensing circuit because the wider bandpass of the telemetry circuit delivers a more detailed EGM which is not modified by the atrial sense amplifier (figure 10). Often the EMG presented by the PM offers much more information than the routine 12-lead ECG. On the other hand the quality of the EGM derived from the atrial sense amplifier and the EMG produced by the broad bandpass filter of the telemetry circuit can strongly differ; this difference can mislead the operator because the atrial arrhythmia can not be classified correctly (figure 11). To improve the diagnostic power of the next PM generation it is proposed to incorporate the broad bandpass filter in the sensing circuits of the atrial port. This technical improvement will obviously provide more detailed information for the discriminative process of atrial events. Furthermore, the memory capacity of the pacemaker should be extended to such a degree that it enables the storage of high-quality EGMs of all onsets and offsets of episodes of atrial tachyarrhythmias. This makes it possible to verify manually the reason why the mode switch related to AF, AT or AFL is automatically switched on.
Figure 10.
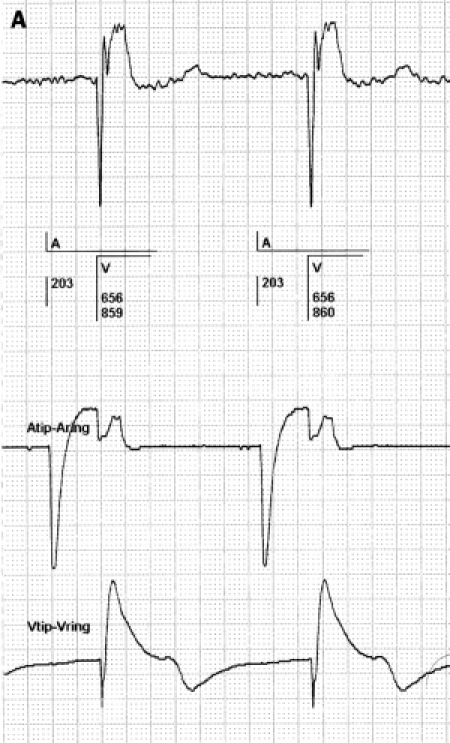
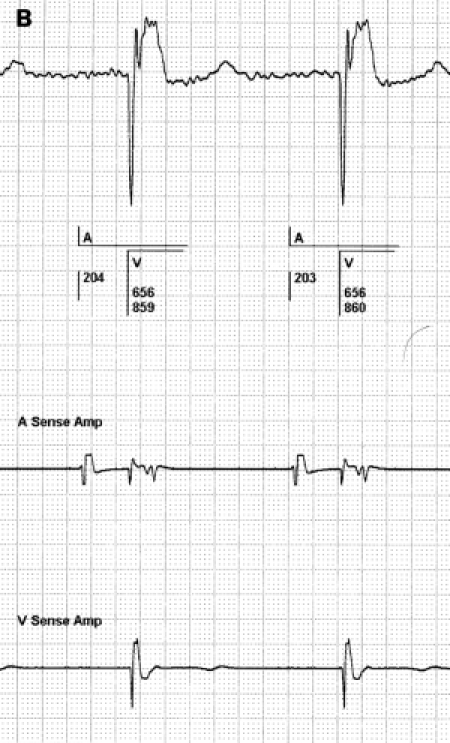
Difference between a high bandpass filtered signal (left panel) and the same signal when processed by the atrial sense amplifier (right panel). The left and right strip are from the same patient. Both show the bipolar EGM derived from the tip and ring of the atrial electrode. The left strip shows that a bandpass filter of approximately 1-100 Hz from the telemetry circuit is far wider than that of the sensing circuit used by the PM. The right strip shows the EGM after being processed by the atrial sense amplifier. The distortion of the original signal is clearly seen in the right panel. This could lead to both undersensing and oversensing of a signal. All counting data and voltage measurements by the PM are derived from signals of the amplifier in the right panel.
Figure 11.
Difference between atrial signal processing by high bandpass telemetry filter (left panel) and the atrial sense amplifier (right panel). The distortion of the original high bandpass filtering by the atrial amplifier is clearly visible. Top: surface ECG. Second strip: PM marker channel. Third strip: atrial EGM. Left is derived from the bipolar atrial electrode (1-100Hz), right has passed the atrial amplifier. Bottom: ventricular EGM as derived from the bipolar ventricular lead.
Decision making using pacemaker-derived data of atrial tachyarrhythmias
The atrial electrograms stored in the PM memory have clearly improved our knowledge of atrial tachyarrhythmias. However, due to the technical shortcomings14,29 described above, the (re)programming of the PM to detect atrial tachyarrhythmias and afterwards to select algorithms for atrial pacing intervention of atrial tachyarrhythmias cannot only rely on the screen or printed output of the diagnostic counters of pacemakers. A manual inspection and comparison of the electrograms stored in the PM memory with the figures, tables and histograms of the counters is still unavoidable. Amongst other reasons, these technical shortcomings impair the widespread use of atrial pacing algorithms in the treatment of atrial tachycardias despite promising case reports or favourable shortterm results of trials in selected patients.39
Conclusions and perspectives
This review of the detection and classification of atrial tachyarrhythmias shows that many technical pitfalls undermine the quality of the diagnostic PM algorithms and settings. Programming of very high atrial sensitivity is nowadays needed to optimise detection of the low and often variable voltages of atrial tachyarrhythmias with a bipolar lead in the right atrium. New atrial lead technology, with rejection of far-field signals without reduction of near-field sensing, seems mandatory.36 To improve atrial sensing the atrial sensing amplifier should be fed with high-quality signals at a high sampling rate and the configuration and technical characteristics of the implanted atrial lead should be optimised. This new technology facilitates the precise detection of near-field (atrial) signals and the rejection of FFRW signals. In addition, increased memory capacity is needed for the storage of all EGMs triggered by the initiation of AF, AFL or AT, comparable to the ICD capacity. This requires new compression and digital techniques without signal deformation. Until now, visual verification of the PM interpretation of atrial tachyarrhythmias is still mandatory before clinical decision making can fully rely on PM-derived counter data. As long as the detection of regular ATs remains imperfect, 14,29 pace interventions for AF, AFL and AT remain tentative options and can hardly be advocated for daily practice. The appreciation of pace intervention and prevention trials in literature is challenging, as pacemaker diagnostics programming should be tailored to the individual patient and verified thereafter. Major improvement in pacemaker diagnostics is mandatory before pace intervention in atrial arrhythmias can be put to the test in large-scale trials in our pacemaker patients.
References
- 1.Haissaguerre M, Shah DC, Jais P, Hocini M, Yamane T, Deisenhofer I, et al. Mapping-guided ablation of pulmonary veins to cure atrial fibrillation. Am J Cardiol 2000;86:K9-K19. [DOI] [PubMed] [Google Scholar]
- 2.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327-34. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Rosanio S. Evolution of non-pharmacological curative therapy for atrial fibrillation. Where do we stand today? Int J Cardiol 2003;88:135-42. [DOI] [PubMed] [Google Scholar]
- 4.Jessurun ER, van Hemel NM, Defauw JA, Stofmeel MA, Kelder JC, de la Riviere AB, et al. Results of maze surgery for lone paroxysmal atrial fibrillation. Circulation 2000;101:1559-67. [DOI] [PubMed] [Google Scholar]
- 5.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation 2000;102:2619-28. [DOI] [PubMed] [Google Scholar]
- 6.Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 2004;351:2373-83. [DOI] [PubMed] [Google Scholar]
- 7.Cox JL. Evolving applications of the maze procedure for atrial fibrillation. Ann Thorac Surg 1993;55:578-80. [DOI] [PubMed] [Google Scholar]
- 8.Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 1991;101:584-92. [PubMed] [Google Scholar]
- 9.Cox JL, Schuessler RB, D’Agostino HJ Jr, Stone CM, Chang BC, Cain ME, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83. [PubMed] [Google Scholar]
- 10.Wellens HJ, Lau CP, Luderitz B, Akhtar M, Waldo AL, Camm AJ, et al. Atrioverter: an implantable device for the treatment of atrial fibrillation. Circulation 1998;98:1651-6. [DOI] [PubMed] [Google Scholar]
- 11.Israel CW, Gronefeld G, Li YG, Hohnloser SH. Usefulness of atrial pacing for prevention and termination of atrial tachyarrhythmias in a patient with persistent atrial fibrillation. Pacing Clin Electrophysiol 2002;25:1527-9. [DOI] [PubMed] [Google Scholar]
- 12.Carlson MD, Ip J, Messenger J, Beau S, Kalbfleisch S, Gervais P, et al. A new pacemaker algorithm for the treatment of atrial fibrillation: results of the Atrial Dynamic Overdrive Pacing Trial (ADOPT). J Am Coll Cardiol 2003;42:627-33. [DOI] [PubMed] [Google Scholar]
- 13.Padeletti L, Purerfellner H, Adler SW, Waller TJ, Harvey M, Horvitz L, et al. Combined efficacy of atrial septal lead placement and atrial pacing algorithms for prevention of paroxysmal atrial tachyarrhythmia. J Cardiovasc Electrophysiol 2003;14:1189-95. [DOI] [PubMed] [Google Scholar]
- 14.Nowak B, McMeekin J, Knops M, Wille B, Schroder E, Moro C, et al. Validation of dual-chamber pacemaker diagnostic data using dual-channel stored electrograms. Pacing Clin Electrophysiol 2005; 28:620-9. [DOI] [PubMed] [Google Scholar]
- 15.Schuchert A, Lepage S, Ostrander JJ, Bos RJ, Gwechenberger M, Nicholls A, et al. Automatic analysis of pacemaker diagnostic data in the identification of atrial tachyarrhythmias in patients with no prior history of them. Europace 2005;7:242-7. [DOI] [PubMed] [Google Scholar]
- 16.Israel CW. Analysis of mode switching algorithms in dual chamber pacemakers. Pacing Clin Electrophysiol 2002;25:380-93. [DOI] [PubMed] [Google Scholar]
- 17.Israel CW, Gronefeld G, Ehrlich JR, Hohnloser SH. Suppression of atrial tachyarrhythmias by pacing. J Cardiovasc Electrophysiol 2002;13:S31-S39. [DOI] [PubMed] [Google Scholar]
- 18.Israel CW, Hugl B, Unterberg C, Lawo T, Kennis I, Hettrick D, et al. Pace-termination and pacing for prevention of atrial tachyarrhythmias: results from a multicenter study with an implantable device for atrial therapy. J Cardiovasc Electrophysiol 2001;12:1121-8. [DOI] [PubMed] [Google Scholar]
- 19.Padeletti L, Pieragnoli P, Ciapetti C, Colella A, Musilli N, Porciani MC, et al. Randomized crossover comparison of right atrial appendage pacing versus interatrial septum pacing for prevention of paroxysmal atrial fibrillation in patients with sinus bradycardia. Am Heart J 2001;142:1047-55. [DOI] [PubMed] [Google Scholar]
- 20.de Voogt W, van Hemel N, de Vusser P, Mairesse GH, Van Mechelen R, Koistinen J, et al. No evidence of automatic atrial overdrive pacing efficacy on reduction of paroxysmal atrial fibrillation. Europace 2007;9:798-804. [DOI] [PubMed] [Google Scholar]
- 21.Camm AJ, Sulke N, Edvardsson N, Ritter P, Albers BA, Ruiter JH, et al. Conventional and dedicated atrial overdrive pacing for the prevention of paroxysmal atrial fibrillation: the AFTherapy study. Europace 2007;9:1110-8. [DOI] [PubMed] [Google Scholar]
- 22.de Voogt WG, van Hemel NM, van de Bos AA, Koistinen J, Fast JH. Verification of pacemaker automatic mode switching for the detection of atrial fibrillation and atrial tachycardia with Holter recording. Europace 2006;8:950-61. [DOI] [PubMed] [Google Scholar]
- 23.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994;89:224-7. [DOI] [PubMed] [Google Scholar]
- 24.Patten M, Maas R, Bauer P, Luderitz B, Sonntag F, Dluzniewski M, et al. Suppression of paroxysmal atrial tachyarrhythmias – results of the SOPAT trial. Eur Heart J 2004;25:1395-404. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 2006;3:1445-52. [DOI] [PubMed] [Google Scholar]
- 26.Israel CW, Ehrlich JR, Gronefeld G, Klesius A, Lawo T, Lemke B, et al. Prevalence, characteristics and clinical implications of regular atrial tachyarrhythmias in patients with atrial fibrillation: insights from a study using a new implantable device. J Am Coll Cardiol 2001;38:355-63. [DOI] [PubMed] [Google Scholar]
- 27.Padeletti L, Santini M, Boriani G, Botto G, Capucci A, Gulizia M, et al. Temporal variability of atrial tachyarrhythmia burden in bradycardia-tachycardia syndrome patients. Eur Heart J 2005;26: 165-72. [DOI] [PubMed] [Google Scholar]
- 28.Israel CW, Neubauer H, Ossowski A, Hohnloser SH. Why did mode switching occur? Pacing Clin Electrophysiol 2000;23:1422-4. [DOI] [PubMed] [Google Scholar]
- 29.Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol 2004;27:983-92. [DOI] [PubMed] [Google Scholar]
- 30.Nowak B, Kracker S, Rippin G, Horstick G, Vincent A, Geil S, et al. Effect of the atrial blanking time on the detection of atrial fibrillation in dual chamber pacing. Pacing Clin Electrophysiol 2001;24:496-9. [DOI] [PubMed] [Google Scholar]
- 31.Fitts SM, Hill MR, Mehra R, Gillis AM. High rate atrial tachyarrhythmia detections in implantable pulse generators: low incidence of false-positive detections. The PA Clinical Trial Investigators. Pacing Clin Electrophysiol 2000;23:1080-6. [DOI] [PubMed] [Google Scholar]
- 32.Barold SS. Far-field R wave sensing causing prolongation of the atrial escape interval of DDD pacemakers with atrial-based lower rate timing. Pacing Clin Electrophysiol. 2003;26:2188-91. [DOI] [PubMed] [Google Scholar]
- 33.De Voogt WG, Van Mechelen R, van den Bos AA, Scheffer M, van Hemel NM, Levine PA. Electrical characteristics of low atrial septum pacing compared with right atrial appendage pacing. Europace 2005;7:60-6. [DOI] [PubMed] [Google Scholar]
- 34.Goethals M, Timmermans W, Geelen P, Backers J, Brugada P. Mode switching failure during atrial flutter: the ‘2:1 lock-in’ phenomenon. Europace 2003;5:95-102. [DOI] [PubMed] [Google Scholar]
- 35.Ruiter JH, de Voogt W.G., Binner J, Brunekreeft J, van Rooijen H. High atrial sensitivity: assessment of the incidence of far field Rwave and myopotential sensing (abstract). Pacing Clin Electrophysiol 1999;19:187 A [Google Scholar]
- 36.de Voogt W, van Hemel N, Willems A, Visser J, Chitre Y, Bornzin G, et al. Far-field R-wave reduction with a novel lead design: experimental and human results. Pacing Clin Electrophysiol 2005;28:782-8. [DOI] [PubMed] [Google Scholar]
- 37.van Hemel NM, Wohlgemuth P, Engbers JG, Lawo T, Nebaznivy J, Taborsky M, et al. Form analysis using digital signal processing reliably discriminates far-field R waves from P waves. Pacing Clin Electrophysiol 2004;27:1615-24. [DOI] [PubMed] [Google Scholar]
- 38.Lewalter T, Tuininga Y, Frohlig G, Remerie S, Eberhardt F, Schmidt J, et al. Morphology-enhanced atrial event classification improves sensing in pacemakers. Pacing Clin Electrophysiol 2007;30:1455-63. [DOI] [PubMed] [Google Scholar]
- 39.Shlevkov N, Yang A, Schrickel JW, Schwab JO, Bielik H, Lickfett L, et al. Role of high frequency atrial pacing for the termination of acute atrial fibrillation and atypical atrial flutter. Pacing Clin Electrophysiol 2007;30:322-32. [DOI] [PubMed] [Google Scholar]