Abstract
This study evaluated, for the first time, the selection of antibiotic resistance in fecal Escherichia coli, a potential reservoir of genes of resistance, during the prolonged exposure to fluoroquinolones after the implantation of a local drug delivery system (LDDS) in a swine model. Fourteen pigs were randomly assigned to group IM (5 mg/kg/day of intramuscular enrofloxacin — EFX) or LD (surgical implantation of EFX-polymethyl-methacrylate perifemoral implants). Blood samples were collected daily for determination of plasma EFX and ciprofloxacin (CFX) concentrations. Fecal samples were collected daily to determine the E. coli counts and the susceptibility patterns of its isolates as evaluated by antibiotic disk diffusion tests. In both groups, EFX administration significantly reduced the bacterial counts after 2 days. During recolonization, the bacterial counts remained lower than baseline in group IM but not significantly, and almost reached pre-treatment levels in group LD. Susceptibility to EFX, CFX, and nalidixic acid of recolonizing E. coli in LD pigs slightly decreased but remained within the limit of “susceptible” isolates. In contrast, quinolone susceptibility of recolonizing E. coli in IM pigs dropped dramatically (P < 0.0001). In addition, intramuscular exposure to fluoroquinolones significantly decreased the susceptibility of E. coli to ampicillin and trimethoprim-sulfamethoxazole (P < 0.05). In conclusion, the use of a dosing regimen that minimized the intestinal output of fluoroquinolones also minimized the selection of resistance to several classes of antibiotics. This could represent another advantage of LDDS usage compared to long-lasting systemic administration of fluoroquinolones.
Résumé
Pour la première fois une étude a évalué la sélection de résistance aux antibiotiques chez des isolats fécaux d’Escherichia coli, un réservoir potentiel de gènes de résistance, durant une exposition prolongée aux fluoroquinolones après l’implantation d’un système d’administration locale de médicaments (LDDS) dans un modèle porcin. Quatorze porcs ont été assignés au hasard au groupe IM (5 mg/kg/jour d’enrofloxacin [EFX] par voie intramusculaire) ou LD (mise en place chirurgicale d’un implant péri-fémoral d’EFX-polyméthyl-méthacrylate). Des échantillons sanguins ont été prélevés quotidiennement afin de déterminer les concentrations plasmatiques d’EFX et de ciprofloxacin (CFX). Des échantillons de fèces ont été collectés quotidiennement afin de dénombrer les E. coli et déterminer les patrons de sensibilité des isolats tels qu’évalués par la méthode de diffusion en gélose. Dans les deux groupes, l’administration d’EFX a réduit significativement les comptes bactériens après 2 jours. Au cours de la recolonisation, les comptes sont demeurés inférieurs de manière non-significative à la valeur de base dans le groupe IM, et ont presque atteint les niveaux prétraitement dans le groupe LD. Chez les porcs LD la sensibilité à l’EFX, CFX et l’acide nalidixique des E. coli recolonisant a diminué légèrement mais est demeurée à l’intérieur de la limite pour les isolats «sensibles». À l’opposé, chez les porcs du groupe IM la sensibilité aux quinolones des E. coli recolonisant a chuté dramatiquement (P < 0,0001). De plus, l’exposition intramusculaire aux fluoroquinolones a diminué de manière significative la sensibilité de E. coli à l’ampicilline et au trimethroprime-sulfaméthoxazole (P < 0,05). En conclusion, l’utilisation d’un régime doseur qui minimise l’excrétion intestinale de fluoroquinolones minimise également la sélection de résistance à plusieurs classes d’antibiotiques. Ceci pourrait représenter un autre avantage de l’utilisation de LDDS comparativement à l’administration systémique de fluoroquinolones à longue action.
(Traduit par Docteur Serge Messier)
Introduction
Musculoskeletal infections can be difficult to treat with systemic antimicrobials because of their limited penetration into the devascularized tissues and biofilms that may be present at the infection site (1,2). The current therapeutic approach for such infections includes an aggressive surgical debridement, removal or change of fixation devices without impairing bone stability, soft tissue coverage, and long-term systemic antibiotic therapy, with a success rate varying between 80% and 90% (3,4). To improve this treatment response, local drug delivery systems (LDDS) have been developed, to allow a sustained high-level local antibiotic concentration at the infection site (1,2,5). Although elution characteristics can vary greatly among different antibiotic and LDDS combinations, the systemic absorption of the chosen antibiotic is generally poor, which leads to sustained low plasma drug concentrations and minimal risk of systemic toxicity (2,5,6). The systemically absorbed antibiotic, however, may provide concentrations within the mutant selection window (MSW) of various bacteria, a theory that has been proposed to explain how exposure to antibiotics leads to the selective enrichment of resistant cell subpopulations (7–11). In the case of veterinary fluoroquinolones, the risk of resistance selection may be significant, because P-glycoprotein has been shown in vivo to contribute to the intestinal excretion of this class of antibiotics (12). Hence, parenterally administered fluoroquinolones may exert a selective pressure on the intestinal microbiota, including Escherichia coli and other bacterial species with zoonosis potential.
While several studies have investigated the potential role of LDDS in the selection of antibiotic resistance among pathogens (13–20), none to our knowledge has evaluated the impact of the prolonged systemic antibiotic concentrations near MSW on commensal flora. Resistance selection among pathogenic bacteria is of concern as it increases the risk of infections that are refractory to classical treatments, which limits the therapeutic possibilities of practitioners. Moreover, the selection of resistance among commensal flora is equally of concern because of its insidious role as a reservoir of genes of resistance (21,22). Many reports in human and veterinary medicine have highlighted the potential for these resistant commensal bacteria to transfer resistance genes to any incoming pathogen or to themselves, leading to the possibility of infection (23–26).
The objective of this study was to investigate the selection of bacterial resistance in the fecal E. coli flora of a swine model during local administration of enrofloxacin (EFX) by a LDDS, and to compare the results to intramuscular (IM) EFX injections. It was hypothesized that the EFX-loaded LDDS used in this model would lead to plasma drug concentrations of EFX that would be low enough to avoid any bacterial resistance selection in the fecal flora, as opposed to that from the IM administration of EFX.
Materials and methods
Animals and housing conditions
Fourteen, 4–8-week-old healthy pigs (13.6 ± 5.9 kg) were used that were known not to have had previous exposure to antibiotics. These pigs were randomly assigned to 2 equal groups: IM (intramuscular EFX), and LD [local delivery with EFX-loaded polymethyl-methacrylate (PMMA) implants]. The pigs were housed individually in separate pens, and did not have any direct contact with pigs in the other group. The pigs were fed twice daily with an antibiotic-free commercial pig feed, and had unlimited access to water. Pigs were allowed to acclimatize for 4 d before experiment initiation on day 1. Operators always went through a rigorous disinfection procedure before entering and exiting the unit and between manipulations to avoid cross-contamination. The protocol was in agreement with the Canadian Council on Animal Care guidelines and was approved by the Institutional Animal Care and Use Committee of the Université de Montréal.
Drugs and implants
An injectable solution of enrofloxacin (Baytril; Bayer, Toronto, Ontario), 100 mg/mL, was used for EFX administration. The EFX-loaded PMMA implants were prepared in a sterile fashion by adding 6 g of EFX powder (Sigma-Aldrich, Oakville, Ontario) to 40 g of powdered polymer (Surgical Simplex P Radiopaque Bone Cement; Howmedica, Limerick, Ireland). The combination was homogenized, the liquid monomer was added, and the soft EFX-PMMA mixture was poured into 3-mL syringe barrels. Plungers were then inserted and depressed to the 0.7 mL mark to expel air and excess cement (27). The cement in the syringe was allowed to harden to produce 500 mg cylindrical EFX-PMMA implants that were 9 mm in diameter, 7 mm long, and contained ~65 mg EFX.
Experimental procedures
On day 7, pigs received IM injections of azaperone [2 mg/kg body weight (BW)], ketamine (8 mg/kg BW), and hydromorphone (0.1 mg/kg BW). Anaesthesia was induced using a face mask delivering 1–3% isoflurane in 100% O2, and then continued via an endotracheal tube. No antibiotic prophylaxis was applied because this would interfere with the study’s objective. An indwelling catheter was placed in the left external jugular vein for serial blood collection. In the LD group, the lateral aspect of the left femur was approached to place 7 peri-diaphyseal EFX-PMMA implants/10 kg BW.
From days 7 to 21, pigs in the IM group received IM injections of EFX at a dose of 5 mg/kg BW, q24h, adjusted twice a week based on increase in body weight. Pigs in the LD group received no other antibiotic after their peri-femoral EFX-PMMA implants.
In all pigs, blood was collected in EDTA tubes on 8 occasions during the first 32 h of treatment (from day 7 to day 8), and then was collected once daily between days 9 and 21, before the morning EFX IM injections. Plasma was harvested after centrifugation and stored at −70°C pending analysis.
Fecal samples (20 g) were collected once daily from fresh feces or rectally. Samples taken between days 1 and 6 were used to determine the baseline conditions, and samples taken between days 7 and 21 were used to document the time-course of the E. coli population size and antibiotic susceptibility of isolates during exposure to EFX. After collection, all samples were kept at 4°C until homogenized and plated (within the next 6 h).
At day 21, all pigs were euthanized by barbiturate overdose injected intravenously. For practical reasons, experiments were divided into 2 separate time blocks, such that 2 subgroups of 3 or 4 pigs from each treatment group were studied simultaneously.
Plasma enrofloxacin and ciprofloxacin concentrations
A liquid chromatography, electrospray-tandem mass spectrometry (LC-ESI/MS/MS) method was developed to measure the plasma concentrations of EFX and its active metabolite ciprofloxacin (CFX) simultaneously. Briefly, the EFX and CFX were extracted by protein precipitation from plasma. One hundred microliters of plasma sample were mixed with 500 μL of acetonitrile fortified with 250 ng/mL of reserpine (internal standard). The samples were centrifuged at 12 000 g for 10 min and 10 μL of supernatant was injected into the HPLC system, fitted with a Waters Symmetry C18 150 × 3.9 mm column (3.5 μm particule size). The mobile phase was a 80:20 mixture of acetonitrile and 0.5% formic acid in water and the flow rate was 0.8 mL/min. The analytes were measured by mass spectrometry using a PESciex API 3+ instrument equipped with an electrospray ion source (ESI). The analyte responses were measured in selected reaction monitoring mode (SRM) using mass transitions of m/z 360→316 (EFX), m/z 332→245 (CFX), and m/z 609→174 (reserpine). The quantification was based on the peak-area ratios of each analyte with the internal standard, and the calibration curve was constructed using a linear regression model (weighted 1/concentration). The lower limit of detection of the method was 2 μg/L for both analytes, with overall accuracies and precisions of 100.3%, 10.5% for EFX and 98.7%, 8.4% for CFX.
Microbiological analyses
The daily fecal Escherichia coli counts were performed according to standard procedures on MacConkey agar. For each sample, 20 g of feces were homogenized in a sterile bag by hand mixing for 1 min, and 5 g were diluted in sterile saline (1:10). The direct plating method (DPM) for detection of antimicrobial-resistant E. coli was carried out (28). Each dilution (100 μL) was plated onto a MacConkey Agar plate (Quelab Laboratories, Montreal, Quebec) to yield isolated colonies. Bacterial growth was examined after overnight incubation at 37°C for lactose-fermenting colonies with a morphological appearance typical of E. coli. For each fecal sample, 3 such colonies were selected at random and purified on 5% sheep blood agar (Quelab Laboratories) and confirmed with triple iron sugar agar, citrate agar, indole and urease tests (29). One out of 10 isolates with atypical morphology or with typical biochemical patterns of E. coli was further characterized on API20E galleries (Biomérieux, Hazelwood, Missouri, USA) (30). The antimicrobial susceptibility of E. coli isolates was determined using the disk diffusion method, standardized according to the Clinical and Laboratory Standards Institute guidelines (31). The following antibiotics were used: EFX, CFX, nalidixic acid, ceftiofur, amoxicillin, ampicillin, streptomycin, gentamicin, apramycin, trimethoprim-sulfamethoxazole (TMS), florfenicol, and tetracycline. After overnight incubation at 37°C for 16 to 18 h, the inhibition zone diameters (mm) of each antibiotic were measured using a standard ruler.
Pharmacokinetic and statistical analyses
The time-course of plasma EFX and CFX concentrations in pigs dosed IM or with the LDDS were subject to statistical moment PK analyses using WinNonlin 5.1 standard edition (Pharsight, Palo Alto, California, USA) (32). The linear trapezoid algorithm was applied to estimate the areas under the concentration (AUC) and first moment curves (AUMC) associated with the first IM dosing, and with the LDDS implantation. Extrapolation to infinity of AUC and AUMC was performed with standard formulas after estimation of the terminal decay slope (λz). The following PK parameters were calculated: apparent systemic clearance (CL/F) and volume of distribution (V/F), mean residence time (MRT), and half-life of the terminal decay slope (t1/2 − λz). In addition, the overall systemic exposure to EFX and CFX was estimated with the measured AUC for the whole study period.
Statistical analyses were performed with the SAS system, version 9.1 (SAS Institute, Cary, North Carolina, USA), using the 0.05 level of significance for all analyses. All daily fecal E. coli counts were log10 transformed to normalize their distributions and include in the analysis. In each pig, 1 to 4 samples were randomly chosen per period for susceptibility testing, depending on the duration of each period and the presence of recovered colonies. Three colonies were tested per sample and averaged. Inhibition zone diameters were also log10 transformed, and the assumptions of normality necessary to perform a parametric analysis were fulfilled. The data are still presented without transformation [mean and standard error (Sx̄)] in the text or figures. Accounting for the diameter of the antimicrobial disks, the minimal value for reported inhibition zone diameter was 6 mm. The time-course of the size of E. coli population and the antibiotic susceptibility of their isolates was divided in 4 periods, based on the observed fecal E. coli counts: (1) baseline, days 1 to 6, (2) antibiotic first exposure effect, day 7 to time of undetectable bacterial count, (3) bactericidal equilibrium, duration of undetectable bacterial counts, and (4) regrowth equilibrium, time of bacterial resurgence to day 21. The effect of EFX dosing route on the duration of periods 2 and 3 was assessed with a survival analysis, using the log-rank test at the 0.05 α-level to focus on the late differences in E. coli recovery (33). Linear mixed-effect models for repeated measures, with time periods 1, 2, and 4 as a within-subject factor, treatment as a between-subject factor, and pig within treatment as random variable, were used to assess the changes in E. coli count and inhibition diameters over time. The time × treatment interaction was also assessed as a fixed effect. Afterwards, least-square means were used to examine differences in the time-course of the size of the bacterial population and of the inhibition zone diameters, as well as the differences recorded for each treatment group. Results are presented as least-square means ± standard errors unless otherwise specified. Pearson correlation coefficients were determined between antimicrobials.
Results
Pigs gained 6.57 ± 2.85 kg during the experiment and none demonstrated any major systemic or local adverse reactions.
Plasma enrofloxacin and ciprofloxacin concentrations
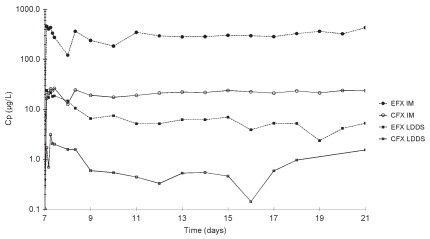
Mean plasma EFX and CFX concentrations in groups LD and IM are presented in Figure 1, and selected PK parameters are presented in Table I. Plasma EFX concentrations in LD pigs were on average 4.5% as high as those in IM pigs, and plasma CFX concentrations were on average 8.5% as high as those of EFX in each treatment group. Plasma EFX concentration peaked at similar times in both treatment groups, but its decay in LD pigs was clearly biphasic, with a sharp decrease during the 2 d following tmax, and a slope with 8.76-day half-life that lasted for the rest of the experiment (Table I). In both treatment groups, the time-course of plasma CFX concentrations was similar to that of EFX but the plasma CFX concentrations were below the quantification limit in some LD pigs.
Figure 1.
Mean plasma enrofloxacin and ciprofloxacin concentrations in IM and LD pigs.
Fecal Escherichia coli counts
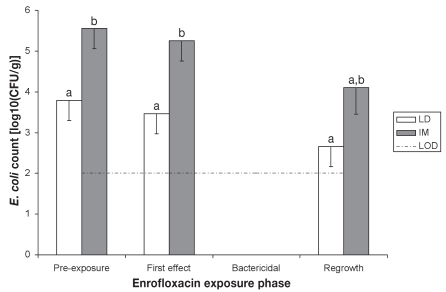
The course of fecal E. coli counts is depicted in Figure 2. During the baseline period (from days 1 to 6), this bacterial population averaged 103.79 ± 100.42 and 105.55 ± 100.42 CFU/g in LD and IM pigs, respectively, which was significantly different (P = 0.019). The administration of EFX, either intramuscularly (IM) or locally (LD), markedly reduced the size of the fecal E. coli population during the next 2 days (first exposure effect period), resulting at its end in counts below the limit of detection of 100 CFU/g. The duration of the first effect period was not significantly different between groups IM and LD (2.43 ± 0.48 d and 1.71 ± 0.18 d, respectively, survival analysis P = 0.17). However, the duration of the bactericidal equilibrium in LD pigs was significantly less than in IM pigs: 4.57 ± 0.72 d versus 8 ± 1.66 d, respectively (survival analysis, P = 0.037). Finally, the fecal E. coli counts during the days of the regrowth equilibrium period were lower (but not significantly lower) than those of baseline values in group IM (P = 0.0837), and almost reached pre-treatment levels in group LD (P = 0.1116). Though not statistically significant, the number of pigs per group for which bacterial counts remained below the detection level during the entire experiment differed between group LD (0/7 pigs) and IM (3/7 pigs).
Figure 2.
Evolution of the fecal Escherichia coli population size (least-square means, and standard errors).
LOD — lower limit of bacterial detection.
a,b — least-square means with different superscripts significantly differ
Escherichia coli susceptibility to EFX simultaneously
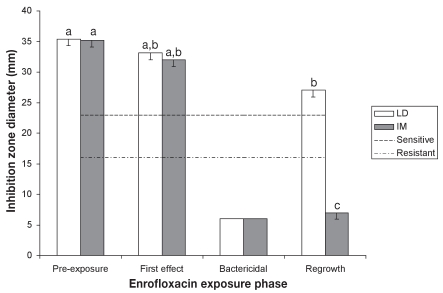
The results of fecal E. coli isolates susceptibility to EFX, as estimated from growth inhibition zone diameters, are presented in Figure 3. Significant effects of the groups and time periods were identified (P < 0.001). In group LD, susceptibilities during period 2 were not significantly different from baseline values (35.4 ± 1.1 mm, P = 0.6474), but reached statistical significance in period 4 (P = 0.05). However, the isolates remained susceptible to EFX according to CLSI guidelines. In group IM, EFX susceptibility in period 2 was not statistically different from baseline (35.2 ± 1.1 mm; P = 0.494), but dropped dramatically during period 4 (P < 0.0001). The E. coli isolates recovered from IM pigs during the regrowth phase were resistant to EFX. The significant time × treatment interaction on the susceptibility of E. coli isolates to EFX was restricted to period 4 (P < 0.0001).
Figure 3.
Evolution of Escherichia coli susceptibility to enrofloxacin (least-square means, and standard errors).
a,b,c — least-square means with different superscripts or subscripts significantly differ (statistical analysis was performed on log transformed values)
Escherichia coli susceptibility to other quinolones
The results of fecal E. coli isolates susceptibility to CFX and nalidixic acid were similar to those described for EFX for both groups. Significant effects of treatment, time, and treatment × time were recorded (P < 0.0001 for both drugs). Compared with baseline values, the susceptibility of E. coli isolates to CFX and nalidixic acid in the LD pigs experienced no significant variation at period 2 (P > 0.78), but significant decreases were recorded at period 4 (P ≤ 0.025). The average inhibition zone diameters of CFX and nalidixic acid for the latter isolates, however, were still > 21 mm and > 18 mm, respectively, indicating that they were still susceptible. Similar findings were recorded for IM pigs: baseline inhibition zone diameters were 38 ± 1.1 mm for CFX and 26.4 ± 1.1 mm for nalidixic acid, within-group differences for period 2 were not statistically significant (P ≥ 0.352), but significant decreases were recorded at period 4 (7 ± 1.1 mm and 7 ± 1.1 mm respectively, with P < 0.0001 for both). The statistically significant time × treatment interaction was limited to inhibition zone diameter differences for the regrowth period (P < 0.0001). Pearson correlation coefficients were excellent among all 3 fluoroquinolones tested: 0.98 between EFX and CFX, 0.96 between EFX and nalidixic acid, and 0.95 between CFX and nalidixic acid.
Escherichia coli susceptibility to other antibiotics
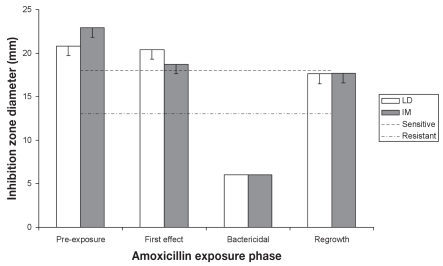
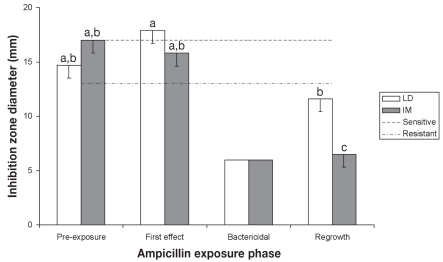
The results of fecal E. coli isolates susceptibility to amoxicillin and ampicillin are presented in Figures 4 and 5, respectively. Susceptibility for amoxicillin and ampicillin were subject to a significant time effect (P = 0.0207 and P ≤ 0.0003, respectively). In the LD and IM pigs, no significant difference in amoxicillin inhibition zone diameters was recorded for exposure periods 1, 2, and 4 (P > 0.1313 and P > 0.0611, respectively). In contrast, ampicillin susceptibility of E. coli isolates recovered from IM pigs decreased at period 4 (P < 0.0005) while ampicillin inhibition zone diameters from LD pigs decreased slightly, but significantly, during period 4 compared with period 2 (P = 0.0357). Pearson correlation coefficients were 0.29 between EFX and amoxicillin, and 0.56 between EFX and ampicillin.
Figure 4.
Evolution of Escherichia coli susceptibility to amoxicillin — least-square means and standard errors (statistical analysis was performed on log transformed values).
Figure 5.
Evolution of Escherichia coli susceptibility to ampicillin (least-square means, and standard errors).
a,b,c — least-square means with different superscripts or subscripts significantly differ (statistical analysis was performed on log transformed values)
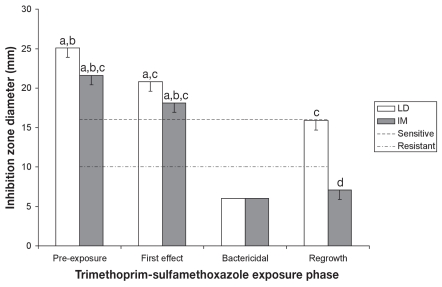
The results of fecal E. coli isolates susceptibility to TMS are presented in Figure 6. Treatment, time and treatment × time effects were also found for TMS (P < 0.02). Inhibition zone diameters for the E. coli isolates recovered from period 4 were significantly smaller than those of isolates from period 1 (P = 0.0412 for LD and P = 0.0003 for IM), and the difference between periods 2 and 4 was significant for IM pigs (P = 0.0016). The treatment × time interaction was limited to period 4, where isolates from LD pigs had inhibition zone diameters close to baseline values, as opposed to those from IM pigs, in which inhibition zone diameters were mostly less than baseline values (P < 0.0023). Pearson correlation coefficient between EFX and TMS was 0.78.
Figure 6.
Evolution of Escherichia coli susceptibility to trimethoprim-sulfamethoxazole — least-square means, and standard errors.
a,b,c,d — least-square means with different superscripts or subscripts significantly differ (statistical analysis was performed on log transformed values)
Susceptibility to ceftiofur, gentamicin, streptomycin, tetracycline, apramycin, and florfenicol were also tested, and no significant variations were observed for these antibiotics. Excellent Pearson correlation coefficients were found between amoxicillin and ceftiofur (0.87) or ampicillin (0.86).
Discussion
During the last decades, LDDS have gained popularity in the prophylaxis and treatment of human and animal osteomyelitis and infected articular prosthesis. However, there has been more and more concern about their ability to select antibiotic resistance in pathogenic bacteria (13–17), a problem that is favored by the production of biofilms on the surface of surgical implants (18–20,34).
The focus in this study was on the effect of prolonged low systemic antibiotic concentrations following a LDDS implantation on the E. coli population from the commensal fecal flora, which to our knowledge has never been investigated.
Our LDDS was designed to achieve 2 objectives: 1) to achieve low systemic antibiotic concentrations over a prolonged period of time, and 2) to mimic clinical situations where, most of the time, clinicians design and shape their own LDDS implants according to bacterial susceptibility and room available for implantation. Enrofloxacin and PMMA are clinically relevant choices for this purpose: EFX is a fluoroquinolone efficient against staphylococci and gram-negative bacteria, which are the main infectious agents implicated in post-traumatic osteomyelitis; PMMA is still considered the LDDS matrix gold standard until an affordable and convenient resorbable matrix becomes available (1,3,35–37). Moreover, low serum EFX concentrations were measured throughout the study with our EFX-PMMA LDDS, similar to most LDDS described in the literature (35,37). It is known that the material used as a matrix, the shape, porosity and volume of the implants, as well as the nature and concentration of the chosen antibiotic all influence the elution properties of LDDS (5,6,27,38–40). The plasma EFX concentrations measured after implantation typically followed a biphasic pattern with a quick release during the first 24 h, likely resulting from solvation of the fraction of antibiotic that is loosely adhered to the implant surface. Afterwards, elution from deeper layers may have sustained the plasma EFX concentration. In vitro and local in vivo elution studies and microscopic porosity evaluation could have been performed to 1) more precisely describe the characteristics of our EFX-PMMA LDDS, and 2) verify that our LDDS was providing higher concentrations at the site of interest than the IM route for the treatment duration. Various studies have reported systemic or local in vivo elution characteristics of different LDDS and have compared them to local concentrations obtained by systemic antibiotic administration (6,38,40). Adams et al (6) reported good granulation tissue and bone CFX concentrations (above break point sensitivity level) 28 d after peritibial implantation of CFX impregnated PMMA beads (6 g CFX/40 g PMMA powder).
Resistance to fluoroquinolones can occur mainly by 3 mechanisms: 1) decreased permeability of the bacterial cell wall caused by alterations of the hydrophilic pores (outer membrane proteins), 2) an efflux pump, which actively transports the fluoroquinolone molecule out of the cell as it approaches or passes through the bacterial membrane, and 3) mutations in the genes encoding the GyrA and GyrB enzyme subunits of DNA gyrase and the ParC and ParE subunits of topoisomerase IV, thus altering the quinolone molecule’s binding site (14,35,41,42). The genetic determinants associated with our recorded changes in antibiotic susceptibility patterns will be reported in a separate communication. For resistant mutants to develop in the environment, favorable events like an exposition of the bacterial population to sub-bactericidal concentrations of the incriminated antibiotic, a sufficient energy metabolism to support a plasmid acquisition and a positive biologic balance of a mutation, or both, are required, among other possibilities. The mutant selection window (MSW) is an antimicrobial concentration range extending from the minimal concentration required to block the growth of wild-type bacteria up to that required to inhibit the growth of the least susceptible, single-step mutant (7,8). It has been demonstrated that placing antimicrobial concentrations inside the MSW enriches resistant mutant subpopulations selectively, whereas placing concentrations above the upper boundary of the window, also called the mutant prevention concentration, restricts selective enrichment (7,8). We observed a temporary but significant first antibiotic exposure effect of our LDDS on the total E. coli count from the 2nd to the 4th day post-implantation. This effect could be explained by the EFX peak release (26.4 ± 2.5 μg/L) reached in the first 3 to 4 h, which is near the E. coli minimum inhibitory concentration (MIC) (30–125 μg/L) (35). Through intestinal excretion of EFX via P-glycoproteins, this initial burst effect could have had a temporary impact on the fecal flora. However, only a slight change in EFX isolates susceptibility of the E. coli population was observed after implantation of the LDDS. This could be explained by the very short duration of the systemic peak EFX concentration following LDDS implantation, presumably not enough to allow a sustained selection. Although a slight decrease in susceptibility was observed during the period of bactericidal equilibrium, it stayed largely above the “intermediate” sensitivity threshold. Even if this may not seem clinically relevant, these results demonstrate that a change in the E. coli isolates EFX susceptibility did occur, and that the use of a LDDS should be well justified, especially for prophylaxis where excessive usage happens more often.
In contrast, the susceptible E. coli population was completely replaced by presumably highly resistant variants during IM administration of EFX. This result was expected, and was in agreement with those of Wiuff et al (43). The EFX resistant E. coli isolates were most likely selected from pre-existing mutants initially present in low frequencies (43). This would mean that IM administration of 5 mg/kg BW EFX led, for some of the pigs, to fecal concentrations above the MSW (the 3 pigs for which the E. coli population was completely and durably depleted), whereas for others it presumably led to concentrations inside the MSW (the 4 pigs for which the E. coli isolates population recovered with resistant strains) (9–11). Although a mild decrease in susceptibility to various antibiotics was found after the use of our LDDS, this change was dramatically more important during the IM administration of EFX. For similar or better efficacy for treating severe bone infections, where antibiotics are mandatory, LDDS seem to be more advantageous than the IM route of administration for the antimicrobial resistance selection of resident microflora (2,4,37). Furthermore, based on comparisons of resistance selection between the IM and oral administrations of EFX previously published, this may also be more advantageous than the oral route of administration (43). All pigs were euthanized at the end of the study, preventing any follow-up of the susceptibility pattern of the E. coli population after stopping EFX IM administration. However, this has been studied by Wiuff et al (43) who reported that the resistant phenotype of fecal E. coli would persist and predominate for at least 2 wk or even longer after only 3 consecutive days of oral or IM EFX administration.
It is known that resistance to 1 quinolone frequently results in resistance to all (35), as seen in our study with CFX and nalidixic acid. However, some significant variations of E. coli susceptibility to other classes of antibiotics tested during EFX administration were observed. Some mutations that confer resistance to the fluoroquinolones, via alterations in permeability or activation of the efflux pump can confer resistance to other antimicrobial agents; however, previously reported “cross-resistances” during fluoroquinolone administration concerned the cephalosporins and tetracyclines (35). To our knowledge, this is the first report of ampicillin and TMS resistance development induced by the administration of EFX. As previously reported, the mechanisms implicated in these “cross-resistances” could potentially concern the marRAB regulon, responsible for the number of porines in the external membrane or the AcrAB-TolC efflux pump system in the cytoplasmic membrane (41). We were surprised by the discrepancies of bacterial susceptibilities between amoxicillin and ampicillin during period 4, as these 2 antibiotics belong in the same class and are often used interchangeably. Although this could potentially result from a type α statistical error, it could also be explained by a difference in their respective affinity for the previously mentioned porines or efflux pump. Another hypothesis could be that, in fluoroquinolone-resistant mutants, modification of penicillin-binding protein (PBP) with reduced drug affinity or reduced bacterial permeability has occurred (44). Given the different affinities for PBPs between ampicillin (PBPs 2 and 3) and amoxicillin (PBPs 1A and 2), modification of one particular PBP could theoretically affect bacterial susceptibility for one penicillin but not the other (45). However, these hypotheses need to be confirmed. Our next communication will report the precise nature of the mutations involved in the mechanism of resistance to assess their relative location/interactions with those involved in resistance against fluoroquinolones.
The IM and local administration of EFX logically resulted in a marked difference in the systemic exposure to this antibiotic and its metabolite CFX, as estimated by the area under the curve (AUC; Figure 1, Table I). This resulted in a difference in the exposure of the fecal flora to both EFX and CFX, but because none of these drugs was administered intravenously, it is not possible at this stage to assess their absorption, metabolism, and disposition pharmacokinetics, or their respective contributions to the selection of fecal resistant bacteria. However, it was felt that having a “clinical-based” model with different overall EFX doses (which is inherent to the specific mode of action of the LDDS and its administration route) would be more representative and clinically relevant than a “pharmacological-based” model where both IM and local overall doses would have been equal. Moreover, the dosing regimens herein revealed that the mutant selection window has a lower boundary in vivo, both in terms of amount and duration of drug exposure. A negative control group was not used in our experimental design because the rate of fluoroquinolone resistance mutation in E. coli was expected to be low in group LDDS and not detectable in the absence of selection pressure. Moreover, given the highly controlled environment of the pigs during the study (room temperature and humidity, light cycle, disinfection procedure, etc.) we felt that having each individual being its own negative control would be valuable and more accurate. Since the difference in baseline E. coli counts between both groups was statistically significant, a higher probability of mutation could have theoretically biased group IM and affected our results. Genetic mutations responsible for antimicrobial resistance, however, are random events occurring at very low frequencies (10−6 to 10−10 for the fluoroquinolones), which suggests that the risk of bias in our study was negligible (46). Finally, we are aware that conclusions regarding the rate of exposure of fecal E. coli to EFX and CFX produced with our implants are difficult to transpose to other LDDS. As previously mentioned, each combination of antibiotic and matrix presents specific elution properties that may vary greatly, and additionally be subject to host effects. Moreover, fluoroquinolone resistance usually occurs through stepwise accumulation of point mutations, which is quite different from most other resistances mainly caused by onestep acquisition of a resistance gene (14,35,41,42). Future research is warranted on other antibiotic/matrix associations to confirm our observations and increase our understanding of this topic.
Table 1.
Selected pharmacokinetic parameters in groups LD and IM
| Enrofloxacin
|
Ciprofloxacin
|
||||
|---|---|---|---|---|---|
| Parameter | Units | LD | IM | LD | IM |
| AUC0–24 h | h · (μg/L) | — | 6344 ± 3625 | 451.8 ± 239.4 | |
| AUC0–336 h | h · (μg/L) | 2100 ± 229 | 90079 ± 51277 | ||
| λza | /h | 0.005 ± 0.003 | 0.081 ± 0.046 | ||
| Cmax | μg/L | 26.4 ± 2.5 | 499.7 ± 348.6 | 5.1 ± 3.5 | 27.5 ± 6.6 |
| tmaxa | h | 3.4 ± 1.9 | 3.3 ± 2.1 | 13.3 ± 11.9 | 8.3 ± 2.0 |
| MRT | h | 301.28 ± 100.79 | 19.38 ± 5.96 | ||
| t1/2 − λzb | h | 210.16 | 11.97 | ||
| CL/F | L/(h · kg) | 0.0017 ± 0.0003 | 0.0007 ± 0.0003 | ||
| V/F | L/kg | 0.514 ± 0.065 | 0.015 ± 0.012 | ||
Normal distribution.
Harmonic mean and jack-knife estimate of standard deviation.
AUC0–24h — Area under the curve for the first 24 h; AUC0–336h — Area under the curve for the entire project; λz — terminal decay slope; Cmax — maximal concentration; tmax — time to Cmax; MRT — mean residence time; CL/F — apparent systemic clearance; V/F — volume of distribution.
This study evaluated, for the first time, the development of antibiotic resistance in E. coli isolates from the commensal flora, indicator microorganism and potential reservoir of genes of resistance, following the use of a LDDS. We showed that the implantation of EFX-loaded PMMA implants has minimal potential to select for antimicrobial resistance within the studied fecal E. coli isolates in a swine model. Although implantation of our LDDS led to a temporary decrease in the total count of the fecal E. coli population, the intestines were rapidly recolonized by a population of E. coli with a very slightly decreased antimicrobial susceptibility pattern. Even if the changes in susceptibility were minor, this finding dictates proper justification of LDDS usage for prophylaxis. Also, the largely inferior variations in susceptibility for group LD versus IM could represent another advantage of LDDS usage compared to long-lasting systemic administration of fluoroquinolones. Finally, further studies implying other antibiotic-matrix combinations and flora would be interesting to complement our observations.
Acknowledgments
This study was supported by Santé Canada and the Fondation du XXIIIe Congrès Mondial Vétérinaire.
The authors thank Guy Beauchamp for assistance in the statistical tests, Mrs. Marianne Turgeon-Plouffe and Valérie Normand for technical assistance, and the Laboratoire d’Hygiène Vétérinaire et Alimentaire (Agriculture et Agroalimentaire Canada, St-Hyacinthe) for use of their animal research facilities.
References
- 1.Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for the local delivery of antibiotics in bone infections. Drugs. 2000;59:1223–1232. doi: 10.2165/00003495-200059060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Streppa HK, Singer MJ, Budsberg SC. Applications of local antimicrobial delivery systems in veterinary medicine. J Am Vet Med Assoc. 2001;219:40–48. doi: 10.2460/javma.2001.219.40. [DOI] [PubMed] [Google Scholar]
- 3.Braden TD. Posttraumatic osteomyelitis. Vet Clin North Am Small Anim Pract. 1991;21:781–811. doi: 10.1016/s0195-5616(91)50084-x. [DOI] [PubMed] [Google Scholar]
- 4.Mader JT, Ortiz M, Calhoun JH. Update on the diagnosis and management of osteomyelitis. Clin Podiatr Med Surg. 1996;13:701–724. [PubMed] [Google Scholar]
- 5.Henry SL, Galloway KP. Local antibacterial therapy for the management of orthopaedic infections. Pharmacokinetic considerations. Clin Pharmacokinet. 1995;29:36–45. doi: 10.2165/00003088-199529010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. [PubMed] [Google Scholar]
- 7.Drlica K. The mutant selection window and antimicrobial resistance. J Antimicrob Chemother. 2003;52:11–17. doi: 10.1093/jac/dkg269. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutants: A general strategy derived from fluoroquinolone studies. Clin Infect Dis. 2001;33 (Suppl 3):S147–S156. doi: 10.1086/321841. [DOI] [PubMed] [Google Scholar]
- 9.Linde HJ, Lehn N. Mutant prevention concentration of nalidixic acid, ciprofloxacin, clinafloxacin, levofloxacin, norfloxacin, ofloxacin, sparfloxacin or trovafloxacin for Escherichia coli under different growth conditions. J Antimicrob Chemother. 2004;53:252–257. doi: 10.1093/jac/dkh036. [DOI] [PubMed] [Google Scholar]
- 10.Olofsson SK, Marcusson LL, Komp Lindgren P, Hughes D, Cars O. Selection of ciprofloxacin resistance in Escherichia coli in an in vitro kinetic model: Relation between drug exposure and mutant prevention concentration. J Antimicrob Chemother. 2006;57:1116–1121. doi: 10.1093/jac/dkl135. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali F, Manfreda G. Mutant prevention concentration of ciprofloxacin and enrofloxacin against Escherichia coli, Salmonella Typhimurium and Pseudomonas aeruginosa. Vet Microbiol. 2007;119:304–310. doi: 10.1016/j.vetmic.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Cavet ME, West M, Simmons NL. Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1997;121:1567–1578. doi: 10.1038/sj.bjp.0701302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanzen L, Walder M. Antibiotic resistance of coagulase-negative staphylococci in an orthopaedic department. J Hosp Infect. 1988;12:103–108. doi: 10.1016/0195-6701(88)90132-6. [DOI] [PubMed] [Google Scholar]
- 14.Weber FA, Lautenbach EE. Revision of infected total hip arthroplasty. Clin Orthop Relat Res. 1986;211:108–115. [PubMed] [Google Scholar]
- 15.Kendall RW, Duncan CP, Smith JA, Ngui-Yen JH. Persistence of bacteria on antibiotic loaded acrylic depots. A reason for caution. Clin Orthop Relat Res. 1996;329:273–280. doi: 10.1097/00003086-199608000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Thomes B, Murray P, Bouchier-Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg Br. 2002;84:758–760. doi: 10.1302/0301-620x.84b5.11907. [DOI] [PubMed] [Google Scholar]
- 17.van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand. 2001;72:557–571. doi: 10.1080/000164701317268978. [DOI] [PubMed] [Google Scholar]
- 18.Arciola CR, Campoccia D, Montanaro L. Effects on antibiotic resistance of Staphylococcus epidermidis following adhesion to polymethylmethacrylate and to silicone surfaces. Biomaterials. 2002;23:1495–1502. doi: 10.1016/s0142-9612(01)00275-7. [DOI] [PubMed] [Google Scholar]
- 19.Arizono T, Oga M, Sugioka Y. Increased resistance of bacteria after adherence to polymethyl methacrylate. An in vitro study. Acta Orthop Scand. 1992;63:661–664. doi: 10.1080/17453679209169731. [DOI] [PubMed] [Google Scholar]
- 20.Naylor PT, Myrvik QN, Gristina A. Antibiotic resistance of biomaterial-adherent coagulase-negative and coagulase-positive staphylococci. Clin Orthop Relat Res. 1990;261:126–133. [PubMed] [Google Scholar]
- 21.Sørum H, Sunde M. Resistance to antibiotics in the normal flora of animals. Vet Res. 2001;32:227–241. doi: 10.1051/vetres:2001121. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson I, Sjolund M, Torell E, et al. Bacteria with increased mutation frequency and antibiotic resistance are enriched in the commensal flora of patients with high antibiotic usage. J Antimicrob Chemother. 2003;52:645–650. doi: 10.1093/jac/dkg427. [DOI] [PubMed] [Google Scholar]
- 23.Amyes SG, Tait S, Thomson CJ, et al. The incidence of antibiotic resistance in aerobic faecal flora in south India. J Antimicrob Chemother. 1992;29:415–425. doi: 10.1093/jac/29.4.415. [DOI] [PubMed] [Google Scholar]
- 24.Shanahan PM, Wylie BA, Adrian PV, Koornhof HJ, Thomson CJ, Amyes SG. The prevalence of antimicrobial resistance in human faecal flora in South Africa. Epidemiol Infect. 1993;111:221–228. doi: 10.1017/s0950268800056922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunde M, Fossum K, Solberg A, Sørum H. Antibiotic resistance in Escherichia coli of the normal intestinal flora of swine. Microb Drug Resist. 1998;4:289–299. doi: 10.1089/mdr.1998.4.289. [DOI] [PubMed] [Google Scholar]
- 26.Thomson CJ. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: A 10-year perspective. J Antimicrob Chemother. 1999;43 (Suppl A):31–40. doi: 10.1093/jac/43.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 27.Ethell MT, Bennett RA, Brown MP, Merritt K, Davidson JS, Tran T. In vitro elution of gentamicin, amikacin, and ceftiofur from polymethylmethacrylate and hydroxyapatite cement. Vet Surg. 2000;29:375–382. doi: 10.1053/jvet.2000.7535. [DOI] [PubMed] [Google Scholar]
- 28.Bartoloni A, Bartalesi F, Mantella A, Dell’Amico E, Roselli M, Strohmeyer M, et al. High prevalence of acquired antimicrobial resistance unrelated to heavy antimicrobial consumption. J Infect Dis. 2004;189:1291–1294. doi: 10.1086/382191. [DOI] [PubMed] [Google Scholar]
- 29.Lidin-Janson G, Kaijser B, Lincoln K, Olling S, Wedel H. The homogeneity of the faecal coliform flora of normal school-girls, characterized by serological and biochemical properties. Med Microbiol Immunol. 1978;164:247–253. doi: 10.1007/BF02125493. [DOI] [PubMed] [Google Scholar]
- 30.Kronvall G, Larsson M, Borén C, et al. Extended antimicrobial resistance screening of the dominant faecal Escherichia coli and of rare resistant clones. Int J Antimicrob Agents. 2005;26:473–478. doi: 10.1016/j.ijantimicag.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. CLSI, Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests of Bacteria Isolated from Animals; approved standard M31-S1. Villanova, Pennsylvania, 2004.
- 32.Yamaoka K, Nakagawa T, Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]
- 33.SAS Online doc® 9.1.3. SAS Institute Inc. [Last accessed May 10, 2008]; Cary, North Carolina, USA [page on the Internet] Available from http://support.sas.com/documentation/onlinedoc/91pdf/index_913.html.
- 34.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 35.Walker RD. Fluoroquinolones. In: Prescott JF, Baggot JD, Walker RD, editors. Antimicrobial Therapy in Veterinary Medicine. 3. Ames, Iowa: Iowa State Univ Pr; 2000. pp. 315–338. [Google Scholar]
- 36.Cariou M, Boulouis HJ, Moissonnier P. Inclusion of marbofloxacin in PMMA orthopaedic cement: An in vitro experimental study. Vet Comp Orthop Traumatol. 2006;19:106–109. [PubMed] [Google Scholar]
- 37.Huneault LM, Lussier B, Dubreuil P, Chouinard L, Désévaux C. Prevention and treatment of experimental osteomyelitis in dogs with ciprofloxacin-loaded crosslinked high amylose starch implants. J Orthop Res. 2004;22:1351–1357. doi: 10.1016/j.orthres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 38.DiMaio FR, O’Halloran JJ, Quale JM. In vitro elution of ciprofloxacin from polymethylmethacrylate cement beads. J Orthop Res. 1994;12:79–82. doi: 10.1002/jor.1100120110. [DOI] [PubMed] [Google Scholar]
- 39.Ramos JR, Howard RD, Pleasant RS, et al. Elution of metronidazole and gentamicin from polymethylmethacrylate beads. Vet Surg. 2003;32:251–261. doi: 10.1053/jvet.2003.50024. [DOI] [PubMed] [Google Scholar]
- 40.Anguita-Alonso P, Rouse MS, Piper KE, Jacofsky DJ, Osmon DR, Patel R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin Orthop Relat Res. 2006;445:239–244. doi: 10.1097/01.blo.0000201167.90313.40. [DOI] [PubMed] [Google Scholar]
- 41.Hooper DC. Mechanisms of fluoroquinolone resistance. Drug Resist Updat. 1999;2:38–55. doi: 10.1054/drup.1998.0068. [DOI] [PubMed] [Google Scholar]
- 42.Piddock LJ. Mechanisms of fluoroquinolone resistance: An update 1994–1998. Drugs. 1999;58 (Suppl 2):11–18. doi: 10.2165/00003495-199958002-00003. [DOI] [PubMed] [Google Scholar]
- 43.Wiuff C, Lykkesfeldt J, Svendsen O, Aarestrup FM. The effects of oral and intramuscular administration and dose escalation of enrofloxacin on the selection of quinolone resistance among Salmonella and coliforms in pigs. Res Vet Sci. 2003;75:185–193. doi: 10.1016/s0034-5288(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 44.Prescott JF. Beta-lactam antibiotics: Penam penicillins. In: Prescott JF, Baggot JD, Walker RD, editors. Antimicrobial Therapy in Veterinary Medicine. 3. Ames, Iowa: Iowa State Univ Pr; 2000. pp. 105–133. [Google Scholar]
- 45.van Heijenoort J. Mécanismes moléculaires de la bactéricidie: Beta-lactamines. In: Courvalin P, Drugeon H, Flandrois J-P, Goldstein F, editors. Bactéricidie. Aspects théoriques et thérapeutiques. Paris, France: Editions Maloine; 1990. pp. 13–22. [Google Scholar]
- 46.Acar JF, Goldstein FW. Trends in bacterial resistance to fluoro-quinolones. Clin Infect Dis. 1997;24 (Suppl 1):S67–S73. doi: 10.1093/clinids/24.supplement_1.s67. [DOI] [PubMed] [Google Scholar]