Abstract
The purpose of this study was to determine the pharmacokinetics of tramadol and the active metabolite mono-O-desmethyltramadol (M1) in 6 healthy male mixed breed dogs following intravenous injection of tramadol at 3 different dose levels. Verification of the metabolism to the active metabolite M1, to which most of the analgesic activity of this agent is attributed to, was a primary goal. Quantification of the parent compound and the M1 metabolite was performed using gas chromatography. Pharmacodynamic evaluations were performed at the time of patient sampling and included assessment of sedation, and evaluation for depression of heart and respiratory rates. This study confirmed that while these dogs were able to produce the active M1 metabolite following intravenous administration of tramadol, the M1 concentrations were lower than previously reported in research beagles. Adverse effects were minimal, with mild dose-related sedation in all dogs and nausea in 1 dog. Analgesia was not documented with the method of assessment used in this study. Tramadol may be useful in canine patients, but additional studies in the canine population are required to more accurately determine the effective clinical use of the drug in dogs and quantification of M1 concentrations in a wider population of patients.
Résumé
Le but de la présente étude était de déterminer les pharmacocinétiques du tramadol et du métabolite actif mono-O-desméthyltramadol (M1) chez six chiens mâles croisés en santé après injection intraveineuse de tramadol à trois dosages différents. Une vérification du métabolisme jusqu’au métabolite actif M1, auquel on attribue la majeure partie de l’activité analgésique de cet agent, était l’objectif primaire. La quantification du composé parent et du métabolite M1 était faite par chromatographie en phase gazeuse. Les évaluations pharmacodynamiques ont été effectuées au moment de la prise d’échantillon et incluaient une appréciation de la sédation et une évaluation de la dépression des rythmes cardiaque et respiratoire. Cette étude a confirmé que bien que les chiens utilisés étaient en mesure de produire le métabolite M1 actif suite à l’injection intraveineuse de tramadol, les concentrations de M1 étaient inférieures à celles précédemment rapportées chez des Beagle de recherche. Les effets indésirables étaient limités, avec une sédation reliée aux doses légères chez tous les chiens et de la nausée chez un chien. L’analgésie n’était pas documentée avec la méthode d’évaluation utilisée dans la présente étude. Le tramadol peut être utile chez les patients canins, mais des études supplémentaires dans la population canine sont requises afin de mieux déterminer l’utilisation clinique efficace de cette drogue chez les chiens et de quantifier les concentrations de M1 dans une population élargie de patients.
(Traduit par Docteur Serge Messier)
Introduction
Control of chronic painful conditions is becoming an increasingly important part of veterinary medicine. Many of the available analgesics are considered effective for a variety of painful conditions in dogs; however, their use may be limited for various reasons. The development of unwanted side effects are a major factor that limits their use, particularly in chronically painful conditions.
Tramadol has only recently gained significant attention as an analgesic in dogs despite its having been used in humans in Germany since 1977 and in North America since the mid 1980’s (1). Tramadol has been used as an analgesic in a variety of human conditions (2,3) and has been demonstrated to be as effective as morphine for moderate pain, but less effective for severe acute pain (4). One advantage of tramadol for chronic pain treatment over many other opioids is the absence of strict regulatory measures with regards to its use (4). In addition, tramadol in humans appears to have less potential for abuse, gastrointestinal side effects (5,6) and respiratory depression (7) than conventional opioids.
Tramadol is a centrally acting synthetic 4-phenyl-piperidine analogue of codeine (4). It proposedly has 2 complementary modes of action: 1) binding of the parent compound (tramadol) and the mono-O-desmethyltramadol (M1) metabolite to the mu opioid receptor; and 2) inhibition of norepinephrine and serotonin reuptake in the central nervous system. Since endogenous norepinephrine and serotonin are involved in central pain modulation, these properties may thus enhance the analgesic effects of tramadol produced by its opioid binding activity.
The evidence for mechanisms other than those due to pure opioid effects is supported by studies using yohimibine, a nonspecific alpha-2-antagonist. Yohimibine administration decreases some of tramadol’s antinociceptive activity, while with the concurrent administration of naloxone, a specific opioid antagonistic drug, will almost completely abolish the analgesic effects of tramadol (8). In addition, ondansetron, a selective (serotonin) 5HT3 antagonist, also reduces the analgesic effects of tramadol (9).
Tramadol exists as a racemic mixture and there are differences in opioid receptor binding, monoamine reuptake inhibition, and metabolism between the 2 enantiomers (10). The (+) enantiomer has higher affinity for the mu receptor and preferentially inhibits serotonin uptake and enhances release. The (−) enantiomer preferentially inhibits norepinephrine reuptake (4). The 2 enantiomers of tramadol act synergistically to provide analgesia. Studies evaluating the enantiomers of tramadol have demonstrated that the (+) enantiomer provides similar analgesia to that of the racemic tramadol and superior analgesia compared with the (−) enantiomer. However, racemic tramadol provides an improved tolerability profile compared with the (+) enantiomer (3).
This binary mechanism of action of tramadol may explain the reduced potential for abuse as well as less significant respiratory depression and other adverse effects typically attributed to traditional opioids. This may also explain why tramadol has been reported to be effective in chronic pain conditions that have opioid resistance (11).
Hepatic metabolism of tramadol in humans is by the CYP2D6 isoenzyme of the cytochrome p450 system and produces the active M1 metabolite. This metabolite has 2 to 4 times the analgesic potency of the parent compound and 4 to 200 times greater affinity for the mu opioid receptor (4). This metabolite also exists as a racemic mixture: the (+) enantiomer having affinity for the mu opioid receptor, and the (−) enantiomer having affinity for adrenergic receptors. Studies in rats showed that administration of the (−) enantiomer of M1 metabolite alone resulted in no antinociceptive activity; however, it was capable of potentiating the antinociceptive effects elicited by (+) M1 enantiomer (12).
Metabolism of tramadol occurs in the liver through 2 main metabolic pathways to form N- and O-demethylated compounds (phase 1 reactions); the O-demethylated metabolites are conjugated further (phase 2 reactions). The M1 metabolite arises from the phase 1 reaction. Phase 2 reactions form the sulfates and glucoronides of M1 (2). In humans, hepatic impairment will result in decreased metabolism of the parent compound and the active metabolite, resulting in a greater area under the plasma concentration curve and prolongation of elimination half-life. In humans, elimination half-life increases with renal insufficiency (4) as tramadol and its metabolites are primarily excreted via the kidneys (90%) with the remaining 10% being excreted via feces (3).
The pharmacokinetics of tramadol have been studied in 6 research beagles, but the pharmacodynamics were not investigated. This study was able to demonstrate the presence of the active metabolite, M1. In addition to the production of the active metabolite, this study was able to demonstrate the importance of pharmacokinetic studies in veterinary patients (13). The pharmacokinetic data of this study was able to document a rapid elimination half-life, which would dictate more frequent dosage intervals in canine patients than those used in humans if proven as an effective agent for control of painful conditions.
Despite this lack of pharmacokinetic and pharmacodynamic data in veterinary patients this drug has become increasingly popular for pain management. Tramadol has most recently been used in veterinary medicine as oral formulations made by compounding pharmacies. This drug, if proven an effective analgesic agent with reliable metabolism to the active metabolite, may prove useful in those patients that do not tolerate the most commonly used class of medications to control chronic pain (nonsteroidal anti-inflammatories) and for adjunctive pain when discharged from the hospital following surgical procedures.
The purpose of this study was to extend the observations on the pharmacokinetics of tramadol and the active M1 metabolite following intravenous injection of tramadol at 3 different dose levels in healthy, mixed breed dogs. Quantification of the serum levels of the M1 metabolite was of primary interest, as this metabolite is mainly responsible for the analgesic activities of this drug in humans. Reliable metabolism to M1 is important; however, formulations made by compounding pharmacies must be evaluated for their bioavailability and effects on drug levels and also if they correlate with effective therapeutic concentrations. Pharmacodynamic effects including sedation, and effects on heart and respiratory rates were also assessed, since the absence of unwanted effects on these systems in human medicine is regarded as one of tramadol’s major advantages (3).
Materials and methods
Animals
The subjects of the study were 6 male, mixed breed dogs that were approximately 3 to 5 years old and had a body weight (BW) between 22 and 32 kg (mean 28.8; s = +/− 3.6). The dogs were considered healthy based on physical examination, complete blood (cell) count, serum biochemistry, and urine specific gravity both before initiation and after completion of the study. The dogs were kept in an approved animal care facility before and during the trial. The study was approved by the local University Animal Care Committee in accordance with Canadian Council of Animal Care guidelines.
Procedure
Tramadol was administered to each dog at 3 different doses: 1, 2, and 4 mg/kg IV. The tramadol was prepared from the pure dry substance (Grünenthal GmbH, Aachen, Germany) by diluting in sterile 0.9% saline to a concentration of 50 mg/mL; it was then stored in multidose vials. Dogs were dosed in random order using a Latin square design at each dose level. A Latin square design was used to avoid the influence of enzyme induction as a contributing factor with serum drug levels at subsequent dosages in the study. Tramadol was administered as a bolus injection through an aseptically placed 22 gauge, 3/4 in catheter (Surflo, Terumo Medical Corporation, Elkton, Maryland, USA) in a cephalic vein, followed by 3 mL of 0.9% sterile saline. Each pharmacokinetic and pharmacodynamic study was performed a minimum of 1 wk apart in each dog, in order to decrease the risk of metabolism induction interfering with metabolite concentrations measured and pharmacokinetic data obtained.
Sample collection
Pharmacokinetic analysis
An 18 gauge, 12-in jugular catheter (Angiocath, Becton Dickinson Vascular Access, Sandy, Utah, USA) was placed in each dog approximately 1/2 h prior to each study period. Following injection of tramadol into the cephalic vein catheter, 3-mL blood samples were taken from the jugular catheter at time 0, 1, 2, 5, 10, 20, 40, and 60 min and at 2, 4, 6, 12, and 24 h. Prior to removing blood samples for pharmacokinetic analysis, a 3-mL sample of blood was removed from the jugular catheter and discarded. Catheters were flushed with 3 mL of sterile saline following each sample collection. Samples were placed in ethylenediamine tetra-acetic acid (EDTA) vacutainers (BD Vacutainer; Becton Dickinson, Franklin Lanes, New Jersey, USA) and immediately placed on ice. Samples were labelled so that analysis of the plasma was performed by the laboratory technicians blinded to the study groups and time points. Blood samples were centrifuged for 8 min at 5000 rpm, the plasma was removed and placed in Eppendorf tubes and stored at −20°C until analysis.
Pharmacodynamic analysis
All dogs were assessed by the 2 principal investigators at times 0, 5, 10, 20, 30, and 60 min, and at 2, 4, 6, 12, and 24 h following tramadol injection. The initial assessment included pulse, respiration rate, and sedation score; all parameters were assessed before blood sampling was initiated. The sedation scores were based on a scale of 0 to 4 (Table I), and were assigned by the 2 principal investigators, who were not blinded to the treatment protocol.
Table I.
Sedation scores for assessing sedation following intravenous tramadol
| Sedation score | Description of behaviors |
|---|---|
| 0 | No sedation |
| 1 | Slight sedation |
| 2 | Sedated, animal calmer, will sit down, decreased activity |
| 3 | Sedated, notably calmer, animal will lie down, still responds to external stimulus |
| 4 | Very sedate, animal sleeping |
Sample analysis
Analysis was performed within 6 mo of sampling. Tramadol has been reported to retain its potency up to 1 y in human plasma when stored at −20°C (14).
Tramadol and its M1 metabolite were measured using a modified published gas chromatography (GC) method (15). Modification included a precolumn derivatization of M1 with diazoethane, as well as the use of a ß-cyclodextrin column as the chiral stationary phase and hydrogen as the carrier gas. In addition, methyl-tert-butyl-ether was used instead of n-hexane in the 1st step of the 3-stage extraction of the serum samples. Assay validation was performed using pure preparations of tramadol and M1, which were used as internal standards. Sensitivity limit evaluation of the assay was performed daily prior to analysis. The lower limit of quantification (LLOQ) was 9.8 ng/mL plasma for tramadol and varied from 9.8 or 19.7 ng/mL for the M1 metabolite.
Pharmacokinetic analysis
Pharmacokinetic analyses of tramadol and the M1 metabolite were performed using a pharmacokinetic computer software program (WinNonlin Ver. 2.0; Pharsight, Mountain View, California, USA). A weight factor of (1/y2) was used for all pharmacokinetic calculations. Values for total body clearance (Cl), volume of distribution (Vd), area under the plasma concentration curve (AUC), plasma distribution half-life α (α T1/2), plasma clearance half-life β (β T1/2), intercept of the distribution phase (A), intercept of the elimination phase (B), rate constant associated with distribution (alpha), and rate constant associated with elimination (beta) were derived.
Statistical analysis
Sedation scores were described as medians and interquartile ranges (IQR), and as descriptive and nonparametric data were evaluated using a Kruskal-Wallis test. This non-parametric testing was also used to assess AUC’s for the 3 dosing intervals. A non-parametric test was used since data from 1 dog was not used thus making the groups evaluated statistically of unequal size. The level of significance was taken at P < 0.05.
Results
Pharmacokinetic studies
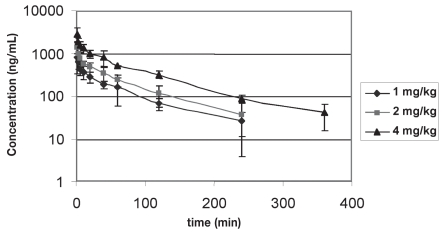
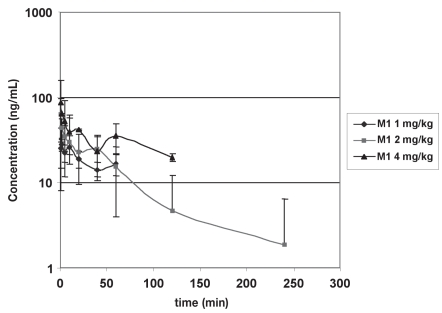
Plasma concentrations from dog 4 receiving the 4 mg/kg dose were not included in subsequent pharmacokinetic analysis. The concentrations measured in this dog were dramatically different from other subjects leading to a concern of analysis or dosing error. Mean and standard deviations for tramadol and M1 concentrations over the 3 dose ranges are shown in Figures 1 and 2; MI concentrations in the individual subjects are shown in Table II.
Figure 1.
Mean + /− standard deviation tramadol plasma concentrations following 3 intravenous dosages of tramadol: 1 (n = 6), 2 (n = 6), and 4 (n = 5) mg/kg.
Figure 2.
Mean +/− standard deviation M1 plasma concentrations following 3 intravenous dosages of tramadol M1: 1 (n = 6), 2 (n = 6), and 4 (n = 2) mg/kg.
Table II.
Individual subjects and concentrations of M1 at time intervals over the 3 dosage ranges
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Dose = 1 mg/kg | ||||||
| Time (min) | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL |
| 1 | 24.2 | 31.3 | 0 | 0 | 48.2 | 23.3 |
| 2 | 25.6 | 23 | n.s | 0 | 34.5 | 28.4 |
| 5 | 18.9 | 18.8 | 22.6 | 0 | 30.5 | 20.2 |
| 10 | 38.9 | 16 | 18.7 | 136.1 | 30.7 | 24.1 |
| 20 | 20.8 | 15.4 | 13.4 | 0 | 24 | 21.2 |
| 40 | 14.5 | 14.4 | 10.2 | 0 | 18.9 | 11.6 |
| 60 | 15.6 | 11.2 | 0 | 0 | 14.9 | 41.9 |
| 120 | 0 | 0 | 0 | 0 | 0 | 0 |
| 240 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dose = 2 mg/kg | ||||||
| Time (min) | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL |
| 1 | 40.4 | 43.1 | 59.6 | 50.3 | 53.4 | 126.7 |
| 2 | 31.1 | 32.5 | 58.1 | 36.1 | 56.2 | 46.7 |
| 5 | 19.4 | 35.1 | 52.9 | 28.3 | 32.8 | 38.1 |
| 10 | 26.2 | 31 | 29.3 | 0 | 32.7 | 29.1 |
| 20 | 29.3 | 29.7 | — | 21.9 | 33.4 | 0 |
| 40 | 17.5 | 24.1 | 24.5 | 14.5 | 42.8 | 14.8 |
| 60 | 21 | 20.7 | 16.8 | 11.6 | 20.6 | 0 |
| 120 | 10.9 | 17.2 | 0 | 0 | 0 | 0 |
| 240 | 0 | 11.1 | 0 | 0 | 0 | 0 |
| Dose = 4 mg/kg | ||||||
| Time (min) | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL |
| 1 | 0 | 0 | 35.3 | 0 | 135.1 | 0 |
| 2 | 0 | 0 | — | 0 | 64.4 | 0 |
| 5 | 78.3 | 0 | 23.4 | 0 | 80.4 | 0 |
| 10 | 0 | 0 | 26.4 | 0 | 51.1 | 0 |
| 20 | 0 | 0 | 41.7 | 0 | 40.8 | 0 |
| 40 | 0 | 0 | 15 | 0 | 30.9 | 0 |
| 60 | 0 | 0 | 25.4 | 0 | 45 | 0 |
| 120 | 0 | 0 | 21 | 0 | 18.2 | 0 |
| 240 | 0 | 0 | 0 | 0 | 0 | 0 |
Mean and standard deviations were determined for pharmacokinetic parameters based on plasma tramadol concentrations over the 3 dosages (Table III). Plasma concentrations of tramadol increased with increasing dosages administered. The Kruskal-Wallis statistic for AUC medians was 12.94 with a P-value of 0.002. This indicated a difference in medians between the 3 dosages: 1 mg/kg [median 492.23 (ng × h)/mL with an IQR of 392.3 to 574.9]; 2 mg/kg (median 775.8; IQR, 554.7 to 1072.9); and 4 mg/kg (median 1883.13; IQR, 1755.4 to 2013.3). Post hoc comparison determined that the 1 and 4 mg/kg dosages had statistically different AUC’s (P < 0.05).
Table III.
Mean +/− standard deviation of tramadol following 2 intravenous dosages: 1, 2, and 4 mg/kg
| IV dosage
|
|||
|---|---|---|---|
| Variable | 1 mg/kg | 2 mg/kg | 4 mg/kg |
| A | 820.28 +/− 922.07 | 1665.5 +/− 1713.9 | 4664.0 +/− 6992.0 |
| B | 225.15 +/− 69.45 | 510.46 +/− 140.01 | 858.11 +/− 328.8 |
| Alpha | 0.21 +/− 0.24 | 0.5 +/− 0.47 | 0.51 +/− 0.67 |
| Beta | 0.01 +/− 0.0 | 0.01 +/− 5.16 × 10−3 | 0.01 +/− 0.0 |
| Vd (mL/kg) | 3.23 +/− 0.69 | 3.35 +/− 0.6 | 3.42 +/− 0.47 |
| Cl (mL/min/kg) | 36.15 +/− 10.03 | 45.12 +/− 15.59 | 35.58 +/− 2.96 |
| α T1/2 (h) | 0.13 +/− 0.1 | 0.07 +/− 0.07 | 0.12 +/− 0.12 |
| β; T1/2 (h) | 1.28 +/− 0.32 | 1.04 +/− 0.42 | 1.36 +/− 0.36 |
| AUC (ng/h)/mL/L | 492.23 +/− 141.75 | 820.35 +/− 297.45 | 1884.1 +/− 155.31 |
A — intercept of the distribution phase; B — intercept of the elimination phase; Alpha — distribution rate constant; Beta — elimination rate constant; Vd — volume of distribution; Cl — clearance; α(α T1/2) — plasma distribution half-life; β(β T1/2) — plasma clearance half-life; AUC — area under the plasma concentration curve.
Tramadol concentrations best fit a two-compartment model for all dogs in this study. The M1 metabolite was detected in all dogs following intravenous dosages of tramadol, verifying that dogs are capable of metabolizing this drug into this metabolite. In many instances, however, the plasma concentrations of the M1 metabolite were close to the lower limit of quantification of the assay and the M1 metabolite could only be reliably detected in 2 of the dogs (dogs 3 and 5) following the highest (4 mg/kg) intravenous dose (Table III). Of the 2 dogs receiving the 4 mg/kg dosage with measurable concentrations of M1 at the 4 mg/kg dosage, only 1 had levels that positively correlated with increased dose. So, although this study verified that animals are capable of producing the active M1 metabolite, accurate representative pharmacokinetic modelling could not be performed consistently in dogs of this study due to the low basal concentrations of M1 measured. Plasma concentrations of the M1 metabolite were close to the lower limit of quantification of the assay in most instances.
Pharmacodynamic studies
Dog 4 developed nausea and increased salivation following each dose of tramadol. This did not appear to correlate with any differences in this subject’s tramadol or M1 plasma concentrations.
Sedation scores increased with increasing doses of intravenous tramadol. No sedation was seen in any dog for more than 2 h following tramadol administration; therefore, statistical analysis only included sedation scores for the first 4 h of observation. Descriptive statistics were used to describe sedation scores: median (25, 75 IQ) 1 mg/kg: 0.58 (0, 1.16 IQ), 2 mg/kg: 1.0, (0.83, 1.16 IQ), and 4 mg/kg: 1.16 (0.58, 2.34 IQ). The differences of sedation score medians during the 4-h period between the 3 doses were not statistically significant.
There was no depression of heart or respiratory rates following intravenous administration of tramadol at all dosage ranges. Throughout the assessment period femoral pulses remained strong and synchronous (data not shown).
One dog developed an acute splenic torsion early in the study unrelated to the treatment or the study and was euthanized. This dog was replaced by a similar dog.
No animals showed any significant changes in hematology or blood biochemistry following the 3 dosing programs.
Discussion
Tramadol is a centrally acting analgesic agent that is widely used in human medicine. It has a complex mode of action involving opioid, norepinephrine, and serotonin receptors, through the different actions of its 2 enantiomers (2) and primary metabolite. Prior to the recent study of Kukanich and Papich (13) there was no information available on the pharmacokinetics of tramadol in dogs despite increasing interest and use of this drug in veterinary medicine.
Kukanich and Papich (13) determined that some pharmacokinetic parameters of tramadol were significantly different from the data previously reported for humans. In beagles, tramadol was found to have a distribution half-life of 0.32 h and an elimination half-life of 1.80 h following intravenous administration (4.4 mg/kg), and an elimination half-life of 1.71 h following oral administration. Pharmacokinetic studies performed in humans showed an elimination half-life following oral administration of 5.5 h (3). This rapid elimination rate for tramadol in dogs was confirmed in our study with elimination half-life over the 3 dosage ranges (1, 2, and 4 mg/kg) being 1.28, 2.04, and 1.36 h, respectively. This rapid elimination rate has implications when designing dosage regimens in dogs and may result in failure of analgesia if canine dosage regimens are based on human studies. Dogs will require more frequent doses to maintain adequate therapeutic drug concentrations.
This study confirmed that dogs are able to produce the active M1 metabolite of tramadol, but the levels of M1 detected were low and near the lower limits of quantification of the assay (9.8 to 19.7 ng/mL). The M1 metabolite has also been shown to have a more rapid elimination phase in canines (2.18 +/− 0.55 h) compared with humans (6.7 h) (13). The low levels of M1 metabolite detected and their proximity to the LLOQ of the assay did not allow for evaluation of pharmacokinetic parameters of the M1 metabolite in this study.
A study following the administration of tramadol in 4 rats and 2 dogs with subsequent analysis revealed a total of 26 metabolites (16).
The study by Kukanich and Papich (13) investigated 1 intravenous dose of tramadol at 4.4 mg/kg, and M1 concentrations were found to be higher than those described herein. In our study, only 2 dogs had detectable M1 at the 4 mg/kg dose. Both the M1 levels at this dose in dogs and the resultant M1 concentrations in all subjects at the lower doses of tramadol administered resulted in lower M1 values than those measured in the previous study. Since the M1 metabolite is a significant contributor to the analgesic effects of tramadol, this may significantly limit its usefulness as an analgesic in dogs. Another study evaluating metabolites in 2 beagles also found low levels of M1 (16).
In humans, the production of the active M1 metabolite is through the CYP2D6 isoenzyme. This isoenzyme demonstrates extensive genetic polymorphism in humans (17) and has been reported to be deficient in approximately 8% of Caucasians (3) and in people of African descent. In 10 Nigerian adult subjects, 96% of tramadol was excreted unchanged in the urine following oral administration (18).
Studies comparing analgesic effects of tramadol in humans show that extensive metabolizers have statistically lower AUCs for tramadol (+) and (−) and higher M1 (+) AUCs than poor metabolizers (17). In fact, in some patient populations, M1 in poor metabolizers may be virtually undetectable (17). In experimental pain studies investigating analgesic effects in laboratory animals, tramadol appeared to be a better analgesic in those animals that are extensive metabolizers via CYP2D6 (19). The analgesic effect of tramadol in effective metabolizers is due to both the mu receptor affinity of the (+) M1 enantiomer and activation of monoaminergic antinociceptive pathways induced by the two enantiomers of tramadol. In poor metabolizers the analgesic effects appear to be predominantly due to the effect on the monoaminergic pathway (17).
The enzyme for tramadol metabolism in dogs is unknown. Larger populations of dogs need to be evaluated to assess the true prevalence of the active metabolite. This may help determine if like in human populations, there are differences in metabolism, which could have implications for using this drug as an analgesic agent. Pharmacogenetics is an expanding field, and a few CYP metabolic enzyme pathways have been shown to be polymorphic in dogs. Polymorphism exists for CYP2B11 affecting propofol metabolism (20,21), and CYP2B11 and celecoxib metabolism in dogs (22). CYP2C41 has also been shown to be polymorphic in dogs (23).
The volume of distribution of tramadol has been reported to be 2.7 L/kg in humans (4). The volume of distribution in this study was 3.42 +/− 0.47 L/kg, which is similar to the volume of distribution of 3.01 +/− 0.45 L/kg found by Kukanich and Papich (13). This illustrates that tramadol has a high volume of distribution consistent with high affinity for tissues. In addition, the rates of clearance (mL/min/kg) determined in this study (35.58 +/− 0.47 mL/min/kg) were similar to the rate of clearance reported in the previous study (54.63 +/− 8.19 mL/min/kg) (13).
Most veterinary drugs are administered as a dose per body weight; however, it would be more accurate to dose based on body surface area. In this study, the dogs were heavier than those in the study of Kukanich and Papich (13), but the differences in body surface area for the body weight range would not significantly alter the amounts given or the pharmacokinetics since the data showed they were not dose-dependent.
Administration of tramadol appeared to be safe at all of the doses administered, with very few adverse effects noted. Most dogs showed a trend of increasing sedation score with increasing dose, and there was no depression in respiratory or heart rates. Lack of respiratory depression is considered an important advantage over morphine in analgesia in humans following laparoscopy and thoracotomy as it helps preserve respiratory function (7). A study in dogs evaluating control of postoperative pain following tramadol administration also evaluated end-tidal CO2, oxygen saturation, and blood gas analysis and there was no evidence of respiratory depression based on these data (24). Tramadol is most commonly used at this time in awake veterinary patients for adjunctive control of postoperative pain and chronic painful conditions. Although it is important that tramadol does not cause evidence of respiratory depression in anesthetized patients this is not a significant problem when opiates are used in patients requiring analgesia who are awake.
Dog 4 showed nausea (salivation and retching) at all dosage levels. In humans, nausea and vomiting are particularly likely after rapid intravenous administration, similar to the bolus technique used in this study. To prevent these effects in humans, it is recommended that intravenous tramadol be administered slowly over 1 to 2 min (4). It is possible that dog 4 was more sensitive to this adverse side effect of tramadol and that a slower infusion would not have resulted in these adverse reactions. Gastrointestinal side effects are less common with tramadol than morphine in humans (3,6).
Studies evaluating the effectiveness of tramadol, as an effective analgesic agent in a large number and variety of dogs, should be performed before it is widely recommended for use as an analgesic agent. This is of particular importance in the face of 2 small studies that demonstrated different levels of the M1 metabolite which is responsible for most of the analgesic properties.
Only 1 study in dogs has evaluated tramadol and compared its clinical analgesic activity and effectiveness to morphine. This was performed in a population of dogs undergoing surgery to resolve pyometra (24). Animals were assessed for pain based on heart and respiratory rates, arterial blood pressure, and previously reported descriptive criteria and visual analogue scales to assess pain. Cortisol and catecholamine levels were measured in addition. This study reported that a 2 mg/kg dose of tramadol produced comparable analgesia to morphine (0.2 mg/kg). The investigators of this study felt that they were able to demonstrate effective analgesia in the tramadol group (24). It would have been of interest to determine levels of the active M1 metabolite levels in the canine patients in this study.
The present study confirmed that mixed breed dogs have a similar pharmacokinetic profile for tramadol as that previously described in beagles (13); however, lower levels of the active M1 metabolite were detected herein. This may reflect differences in the metabolisms of the study population. Larger studies evaluating a diverse population of canine subjects should be undertaken to determine the true prevalence of the active metabolite. In addition, the analgesic activity of this drug in the face of M1 levels should be assessed in controlled clinical situations to determine appropriate dosing regimens. Although not a primary focus of this study, some pharmacodynamic parameters including sedation score, respiratory rates, and heart rates were evaluated at 3 dosage levels. There was some sedation noted in increasing levels at the 3 dosage increments; however, the differences were not statistically significant. There was no evidence of depression of heart or respiration rates in the doses that were used in this study.
Acknowledgments
The authors thank Grünenthal laboratories for supplying the tramadol as dry substance. In addition, we thank Katharina Kaiser for performing the analysis of the samples.
Footnotes
This paper was part of Dr. McMillan’s Masters of veterinary science requirements. The project was funded by the Companion Animal Health Fund of the Western College of Veterinary Medicine and the interprovincial graduate student fund.
References
- 1.Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCL. Am J Med. 1996;101(S1a):47–53. doi: 10.1016/s0002-9343(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 2.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 3.Scott LJ, Perry CM. Tramadol. A review of its use in perioperative pain. Drugs. 2000;60:139–176. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lewis KS, Han NH. Tramadol: A centrally acting analgesic. Am J Health-Syst Pharm. 1996;54:643–652. doi: 10.1093/ajhp/54.6.643. [DOI] [PubMed] [Google Scholar]
- 5.Wilder Smith CH, Hill L, Wilkins J, et al. Effects of morphine and tramadol on somatic and visceral sensory function and gastrointestinal motility after abdominal surgery. Anesthesiology. 1999;91:639–647. doi: 10.1097/00000542-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Crighton IM, Martin PH, Hobbs GJ, et al. A comparison of effects of intravenous tramadol, codeine, and morphine on gastric emptying in human volunteers. Anesth Analg. 1998;87:445–449. doi: 10.1097/00000539-199808000-00040. [DOI] [PubMed] [Google Scholar]
- 7.Turker G, Goren S, Bayram S, et al. Comparison of lumbar epidural tramadol and lumbar epidural morphine for pain relief after thoracotomy: A repeated-dose study. J Cardiothorac Vasc Anesth. 2005;19:468–474. doi: 10.1053/j.jvca.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kayser V, Besson JM, Guilbaud G. Evidence for a noradrenergic component in the antinociceptive effect of the analgesic agent tramadol in an animal model of clinical pain, the arthritic rat. Eur J Phar. 1992;224:83–88. doi: 10.1016/0014-2999(92)94822-d. [DOI] [PubMed] [Google Scholar]
- 9.DeWitte JL, Schoenmaekers B, Sessler DI, et al. The efficacy of tramadol is impaired by concurrent administration of ondansetron. Anesth Analg. 2002;92:1319–1321. doi: 10.1097/00000539-200105000-00045. [DOI] [PubMed] [Google Scholar]
- 10.Dayer P, Collart L, Desmeules J. The pharmacology of tramadol. Drugs. 1994;47:3–7. doi: 10.2165/00003495-199400471-00003. [DOI] [PubMed] [Google Scholar]
- 11.Raffa RB. Pharmacology of oral combination analgesics: Rational therapy for pain. J Clin Pharm Ther. 2001;26:257–264. doi: 10.1046/j.1365-2710.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 12.Garrido MJ, Valle M, Campanero MA, et al. Modeling of the in vivo antinociceptive interaction between an opioid agonist, (+) O–desmethyltramadol, and a monoamine reuptake inhibitor, (−)-O-desmethyltramadol, in rats. J Pharmacol Exp Ther. 2000;295:352–359. [PubMed] [Google Scholar]
- 13.Kukanich B, Papich MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Therap. 2004;27:239–246. doi: 10.1111/j.1365-2885.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 14.Gan SH, Ismail R. Method development and validation of a high-performance liquid chromatographic method for tramadol in human plasma using solid — phase extraction. J Chromat B Analyt Technol Biomed Sci. 2002;772:123–129. doi: 10.1016/s1570-0232(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 15.Becker R, Lintz W. Determination of tramadol in human serum by capillary gas chromatography with nitrogen-selective detection. J Chromatogr. 1986;377:213–220. doi: 10.1016/s0378-4347(00)80776-8. [DOI] [PubMed] [Google Scholar]
- 16.Wu WN, Mckown LA, Gauthier AD, et al. Metabolism of the analgesic drug, tramadol hydrochloride, in rat and dog. Xenobiotica. 2001;31:423–441. doi: 10.1080/00498250110057378. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen L, Arendt-Nielsen L, Brosen K, et al. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther. 1996;60:636–644. doi: 10.1016/S0009-9236(96)90211-8. [DOI] [PubMed] [Google Scholar]
- 18.Ogunleye DS. Investigation of racial variations in the metabolism of tramadol. Eur J Drug Metab Pharmacokinet. 2001;26:95–98. doi: 10.1007/BF03190382. [DOI] [PubMed] [Google Scholar]
- 19.Garrido MJ, Sayar O, Segura C, et al. Pharmacokinetic/Pharmacodynamic modelling of the antinociceptive effects of (+) tramadol in the rat:role of the cytochrome p450 activity. J Pharmacol Exp Ther. 2003;305:710–718. doi: 10.1124/jpet.102.047779. [DOI] [PubMed] [Google Scholar]
- 20.Kraus BL, Greenblatt DJ, Venkatakrishnan K. Evidence for propofol hydroxylation by cytochrome P4502B11. Xenobiotica. 2000;30:575–588. doi: 10.1080/004982500406417. [DOI] [PubMed] [Google Scholar]
- 21.Court MH, Hay Kraus BL, Hill DW, et al. Propofol hydroxylation by dog liver microsomes: Assay development and dog breed differences. Drug Met Disp. 1999;27:1293–1299. [PubMed] [Google Scholar]
- 22.Paulson SK, Engel L, Reitz B, et al. Evidence for polymorphism in the canine metabolism of the cyclooxygenase 2 inhibitor, celecoxib. Drug Met Disp. 1999;27:1133–1142. [PubMed] [Google Scholar]
- 23.Blaisdell J, Goldstein JA, Bai SA. Isolation of a new canine cytochrome P450 CDNA from the cytochrome P450 2C subfamily (CYP2C41) and evidence for polymorphic differences in its expression. Drug Met Disp. 1998;26:278–283. [PubMed] [Google Scholar]
- 24.Mastrocinque S, Fantoni D. A comparism of preoperative tramadol and morphine for the control of early postoperative pain in canine ovariohysterectomy. Vet Anaesth Analg. 2003;30:220–228. doi: 10.1046/j.1467-2995.2003.00090.x. [DOI] [PubMed] [Google Scholar]