Abstract
The purpose of this study was to investigate the effects of a medetomidine–midazolam combination on some neurohormonal and metabolic variables in healthy cats. Five cats were used repeatedly in each of 5 groups, which were injected intramuscularly with physiological saline solution (control), 0.5 mg/kg of midazolam, 40 μg/kg of medetomidine, 80 μg/kg of medetomidine, and 40 μg/kg of medetomidine plus 0.5 mg/kg of midazolam. Blood samples were taken 10 times over 24 h from a catheter introduced into the jugular vein. Plasma concentrations of glucose, insulin, glucagon, cortisol, nonesterified fatty acids (NEFAs), norepinephrine, and epinephrine were determined. In addition, the duration of lateral recumbency, rectal temperature, heart rate, and respiratory rate were examined. The combination of medetomidine and midazolam enhanced the duration of lateral recumbency and reduced the hyperglycemia induced by medetomidine alone. Recovery from hypoinsulinemia induced by the medetomidine–midazolam combination tended to be more rapid than when the same dose of medetomidine was used alone. The decrease in plasma norepinephrine levels induced by medetomidine alone was diminished by the addition of midazolam. Midazolam alone did not significantly change the plasma glucose, insulin, glucagon, cortisol, epinephrine, or NEFA concentration, but increased the norepinephrine concentration. This study revealed that the combination of medetomidine and midazolam produces minimal neurohormonal and metabolic changes when compared with medetomidine alone in cats.
Résumé
Cette étude avait pour but d’étudier les effets d’une combinaison medetomidine-midazolam sur quelques variables neurohormonales et métaboliques chez des chats en santé. Cinq chats ont été utilisés de manière répétée dans chacun de 5 groupes traités comme suit : saline physiologique (témoin), 0,5 mg/kg midazolam, 40 μg/kg medetomidine, 80 μg/kg medetomidine, et 40 μg/kg medetomidine et 0,5 mg/kg de midazolam par voie intramusculaire. Des échantillons sanguins ont été prélevés 10 fois pendant une période de 24 h à partir d’un cathéter introduit dans la veine jugulaire. Les concentrations plasmatiques de glucose, d’insuline, de glucagon, de cortisol, d’acides gras non-estérifiés (NEFA), de noradrénaline, et d’adrénaline ont été déterminées. De plus, on nota la durée du décubitus latéral, la température rectale, ainsi que les rythmes cardiaques et respiratoires. La combinaison de medetomidine et de midazolam a augmenté la durée du décubitus latéral et a réduit l’hyperglycémie causée par l’administration de medetomidine seule. La réversion de l’hypo-insulinémie causée par la combinaison medetomidine-midazolam avait tendance à être plus rapide comparativement à la même dose de medetomidine administrée seule. La diminution des niveaux plasmatiques de noradrénaline induite par l’administration de medetomidine seule était réduite par l’ajout de midazolam à la medetomidine. L’administration de midazolam uniquement n’a pas modifié de manière significative les concentrations plasmatiques de glucose, d’insuline, de glucagon, de cortisol, d’adrénaline et de NEFA, mais augmenta la concentration de noradrénaline. Cette étude a révélé que la combinaison de medetomidine et de midazolam ne causait que des changements neurohormonaux et métaboliques mineurs lorsque comparé à l’administration de medetomidine seule chez les chats.
(Traduit par Docteur Serge Messier)
Introduction
Medetomidine, a selective α2-adrenoceptor agonist, is widely used for sedation, analgesia, and muscle relaxation in veterinary medecine (1). However, it induces undesirable effects such as hyperglycemia, hypoinsulinemia, emesis, and bradyarrhythmias in dogs and cats (2–5). The medetomidine-induced hyperglycemia is much greater in cats than in dogs (5); there are no reports indicating why this is so. In feline practice, diabetes mellitus, hyperthyroidism, and hypertension are often observed. Such disorders greatly influence feline metabolic and neurohormonal function. Therefore, the metabolic and neurohormonal effects of sedatives should be understood for appropriate use of these agents in cats. Both the hyperglycemia induced by α2-adrenoceptor agonists and diabetes mellitus are accompanied by hypoinsulinemia. Thus, medetomidine-induced hyperglycemia may affect feline metabolism in a manner similar to diabetes mellitus. Recently, we studied the neurohormonal and metabolic effects of medetomidine at different dosages in cats and found that this agent induces hyperglycemia and hypoinsulinemia and inhibits catecholamine release and lipolysis (5). An adequate sedative effect is needed for various purposes in feline practice. For example, cats must be immobile for ophthalmologic or otologic examinations and treatments. Treatment of wounds and biopsy also need to be done under good sedation. High doses of medetomidine increase the sedative effect but also produce larger metabolic and neurohormonal effects such as hyperglycemia in cats (5). Therefore, medetomidine could be used in combination with other drugs to obtain adequate sedation with minimal neurohormonal and metabolic effects.
Midazolam, a water-soluble benzodiazepine, is used as an anxiolytic in humans (6). Alone, midazolam does not typically produce sedation in healthy cats, but it induces ataxia, restlessness, and abnormal behavior such that the animals are more difficult to approach and restrain, and it does not induce profound sedation (7). A combination of medetomidine and midazolam has been reported to enhance the sedative and analgesic actions of the individual drugs in rats (8) and pigs (9) and to produce deep sedation in dogs (10). This combination has been reported to greatly reduce the dose of sodium thiopental and propofol needed for anesthesia induction in dogs (11). The influence of a medetomidine–midazolam combination on cardiopulmonary function and on the electroencephalogram has been reported for dogs and sheep (12–14). However, to the best of our knowledge, there are no reports on the neurohormonal and metabolic effects of medetomidine–midazolam in cats. As previously reported for laboratory pigs (15), midazolam in combination with medetomidine may reduce the hyperglycemic effect in cats induced by medetomidine alone.
To evaluate the advantages of a medetomidine–midazolam combination in cats, we investigated the effects of these agents alone or in combination on several neurohormonal and metabolic variables. The study was designed to model clinical conditions. The clinically recommended dose of medetomidine as a sedative–analgesic in cats has been reported to be 0.04 to 0.08 mg/kg given intramuscularly (IM) (16). A previous study revealed that administration of 0.05 and 0.5 mg/kg of midazolam intraveneously (IV) after 3 mg/kg of ketamine had beneficial effects on the behavioral response in cats (7), causing a greater proportion of cats to assume lateral recumbency, with the head down, compared with ketamine alone. In addition, doses of midazolam of 0.5 mg/kg or greater reduced muscle rigidity in cats, compared with ketamine alone, and greatly diminished the nociceptive response (17). Doses of 40 μg/kg of medetomidine and 0.5 mg/kg of midazolam were chosen, therefore, for the combination.
Materials and methods
Animals
Five healthy mixed-breed cats (2 intact males, 2 intact females, and 1 neutered male), with a mean age of 4.8 [standard deviation (s) ± 3.2] y and a mean weight of 5.1 (s ± 0.8) kg were used. The cats were housed in the laboratory for at least 1 mo before the study initiation and were fed a standard commercial dry food. Routine hematologic examination before the study showed all values to be within normal physiological ranges (18). The experimental protocol was approved by the Animal Research Committee of Tottori University, Tottori, Japan.
Experimental protocol
The 5 cats were used repeatedly in each of 5 groups according to a randomized Latin square crossover design. There was at least 1 wk between treatments for each cat. The treatments were physiological saline solution (2.0 mL per animal) (control); 0.5 mg/kg of midazolam hydrochloride (Dormicum; Astellas Pharma, Tokyo, Japan) (MID0.5); 40 or 80 μg/kg of medetomidine hydrochloride (Domitor; Meiji Seika Kaisha, Tokyo, Japan) (MED40 and MED80, respectively); and 40 μg/kg of medetomidine hydrochloride plus 0.5 mg/kg midazolam hydrochloride (MED40–MID0.5) mixed in a syringe. The drugs were administered IM. As the α2-adrenoceptor agonists have often been given by IM injection (19), this route was preferred in our study. The quadriceps or semi-membranosus muscle was used for injection.
The cats were fasted for 12 h before injection of each agent. Food and water were also withheld during each test period, and offered again 1 h after the last blood sampling of the day. During the sampling period, each cat was kept in a room with the air temperature set at 25°C.
Instrumentation and sample collection
One day before the experiment, a 17-gauge central venous catheter (EXCV catheter kit; Tyco Healthcare Japan, Tokyo, Japan) was introduced into the jugular vein under general anesthesia. Before placement of the catheter, 6.6 to 8.8 mg/kg of propofol (Rapinovet; Schering-Plough Animal Health, Osaka, Japan) was administered IV until adequate anesthesia was induced. Anesthesia was maintained with a constant infusion of 0.22 to 0.44 mg/kg per minute of propofol. Lidocaine spray (Xylocaine pump spray 8%; Astra Zeneca, Osaka, Japan) was used to assist with local analgesia at the catheterization site. The catheter was flushed with 1.5 mL of heparinized physiological saline solution, capped, and fixed. The cat was then placed in an individual cage to rest overnight. Blood samples (approximately 20 mL in total per treatment period) were taken from the catheter at 0 (baseline), and 0.25, 0.5, 1, 2, 3, 4, 5, 6, and 24 h after injection of the test agent(s). After every sampling, the cat was put back into the cage. Packed cell volume and the other routine hematologic parameters were monitored throughout each sampling period. After each sampling period, the catheter was removed and the cat allowed to recover.
Measurements of behavioral responses and physical parameters
The cats were observed for behavioral and visible effects such as sedation, excitation, and vomiting for 6 h after injection of each test agent. The time from initiation of lateral recumbency until recovery to the prone position by itself was measured as a behavioral response to the sedative effect.
After every blood sampling, other technicians held the cat and immediately measured physical parameters. Heart rate was monitored with a stethoscope. Respiratory rate was monitored by movements of the thorax. Rectal temperature was measured with a digital thermometer.
Sample processing and analysis
Of the 2.0 mL of blood collected at each sampling, 0.5 mL was mixed with aprotinin (Trasylol; Bayer Pharmaceuticals, Leverksen, Germany) for glucagon measurement, and the remaining 1.5 mL was mixed with ethylene diamine tetraacetic acid (EDTA) for other measurements. Both samples were centrifuged immediately at 4°C; until the plasma was then separated and frozen at −80°C until analysis for concentrations of glucose, insulin, cortisol, catecholamines (epinephrine and norepinephrine), glucagon, and nonesterified fatty acids (NEFAs).
Glucose and NEFA concentrations were determined by enzyme assay with the use of commercially available kits (Glucose CII-test Wako and NEFA C-test Wako; Wako Junyakukogyo, Osaka, Japan). Glucose was analyzed by the mutarotase–glucose oxidase method, and NEFAs were analyzed by the acyl-coenzyme A (CoA) synthetase-acyl-CoA oxidase method. The intra-assay coefficient of variation (CV) with these kits was < 2% for glucose and < 3% for NEFAs, and the limits of quantification were 700 mg/dL and 2 mmol/L, respectively. Measurement was by means of a spectrophotometer (Auto Sipper Photometer U-1080; Hitachi, Tokyo, Japan).
Insulin and glucagon concentrations were measured by double-antibody radioimmunoassay (RIA) with the use of commercially available kits (I-AJ16; Eiken Chemical Company, Tokyo, Japan; and Glucagon kit Daiichi, TFB Stock Company, Tokyo, Japan). The intra-assay CV with insulin kit was < 10%. The intra- and inter-assay CVs with the glucagon kit were 2.6% to 5.3% and 2.4% to 3.6%, respectively. The limits of detection and quantification were 5 to 320 μU/mL for insulin and 15.6 to 4000 pg/mL for glucagon.
Cortisol was measured by single-antibody RIA with the use of a commercially available kit (Gamma Coat Cortisol; Nihon Sheering, Chiba, Japan). The intra-assay CV was 3.5% to 5.0% and the inter-assay CV 4.2% to 8.7%. The limits of detection and quantification were 0.23 to 60 μg/dL.
Catecholamines were extracted on activated alumina according to the method described by Bouloux et al (20) and measured by high-performance liquid chromatography (LaChrom; Hitachi) and an electrochemical detector (Coulochem II; ESA, Chelmsford, Massachusetts, USA). As an internal standard, 3,4-dihydroxybenzylamine (DHBA; Sigma Chemical Company, St. Louis, Missouri, USA) was used. The percentage recovery of authentic DHBA standard was 64% to 77%.
Data evaluation
All data obtained were analyzed together with Prism statistical software (Version 4; GraphPad Software, San Diego, California, USA). One-way analysis of variance (ANOVA) for repeated measures was used to examine the time effect within each group, and 1-way ANOVA was used to study group effect at each time point. When a significant difference was found, the Tukey test was used to compare the means.
The area under the curve (AUC) was calculated for each biochemical variable. The AUC was measured by calculating the sum of the trapezoids formed by the data points and the x-axis. The level of significance in all tests was set at P < 0.05.
Results
Behavioral responses and physical parameters
Profound sedation was not observed in the MID0.5 group; however, excitement-like behaviors such as ataxia, salivation, and restlessness were observed. In the MED40, MED80, and MED40–MID0.5 groups, all the cats showed profound sedation and, subsequently, lateral recumbency. Vomiting was observed before profound sedation in all cats given medetomidine. The mean duration of lateral recumbency was 49 [standard error (Sx̄) ± 11] min in the MED40 group, 126 ± 14 min in the MED80 group, and 88 ± 10 min in MED40–MID0.5 group. The duration was significantly longer in the combination group than in the MED40 group (P < 0.05), but there was no significant difference in duration between the combination group and the MED80 group.
No significant change in rectal temperature was found in the control and MED0.5 groups, but the temperature decreased significantly (P < 0.05) at 1 to 5 h after administration in the other 3 groups (Table I). The decrease in the MED40–MID0.5 group tended to be greater than that in the MED40 group and smaller than that in the MED80 group.
Table I.
Rectal temperature, heart rate, and respiratory rate after the administration of physiological saline (control) or sedative agents to 5 cats
| Time after administration (h) (mean ± standard deviation)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | 0 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 24 |
| Rectal temperature (°C) | Control | 38.4 ± 0.4 | 38.7 ± 0.7 | 39.0 ± 0.8 | 38.9 ± 0.8 | 38.8 ± 0.8 | 38.7 ± 0.8 | 38.5 ± 0.7 | 38.7 ± 0.6 | 38.7 ± 0.5 | 38.4 ± 0.8 |
| MID0.5 | 38.8 ± 0.7 | 39.3 ± 0.3 | 39.0 ± 0.2 | 39.1 ± 0.5 | 39.0 ± 0.4 | 39.0 ± 0.3 | 38.8 ± 0.6 | 38.6 ± 0.7 | 38.6 ± 0.3 | 38.3 ± 0.4 | |
| MED40 | 38.8 ± 0.5 | 38.8 ± 0.7 | 38.8 ± 0.6 | 37.5 ± 0.6a | 36.5 ± 0.6a | 36.0 ± 0.9a | 36.4 ± 1.3a | 37.5 ± 1.5 | 38.0 ± 1.3 | 38.8 ± 0.4 | |
| MED80 | 38.5 ± 0.6 | 38.6 ± 0.8 | 38.2 ± 0.7 | 37.3 ± 0.8 | 35.6 ± 0.8a | 34.5 ± 1.0a | 35.3 ± 0.7a | 36.3 ± 1.4a | 36.7 ± 1.4 | 38.4 ± 0.7 | |
| MED40–MID0.5 | 38.4 ± 0.7 | 38.3 ± 0.9 | 38.2 ± 0.6 | 37.2 ± 0.8a | 35.9 ± 1.2a | 35.4 ± 1.1a | 36.4 ± 0.9 | 38.0 ± 0.9 | 38.8 ± 0.6 | 38.7 ± 0.5 | |
| Heart rate (beats/min) | Control | 178 ± 46 | 193 ± 32 | 186 ± 23 | 190 ± 45 | 178 ± 32 | 175 ± 56 | 169 ± 41 | 191 ± 32 | 200 ± 25 | 194 ± 38 |
| MID0.5 | 179 ± 30 | 225 ± 16 | 200 ± 32 | 206 ± 41 | 227 ± 43 | 193 ± 29 | 212 ± 22 | 216 ± 21 | 191 ± 19 | 190 ± 32 | |
| MED40 | 201 ± 31 | 121 ± 29a | 131 ± 27a | 112 ± 23a | 103 ± 8a | 162 ± 51 | 184 ± 51 | 180 ± 45 | 174 ± 37 | 211 ± 5 | |
| MED80 | 184 ± 49 | 109 ± 32a | 111 ± 25a | 100 ± 11a | 95 ± 13a | 107 ± 32a | 167 ± 40 | 188 ± 40 | 190 ± 42 | 218 ± 23 | |
| MED40–MID0.5 | 191 ± 43 | 112 ± 17a | 107 ± 17a | 102 ± 17a | 115 ± 37a | 155 ± 52 | 190 ± 39 | 205 ± 24 | 196 ± 44 | 211 ± 29 | |
| Respiratory rate (breaths/min) | Control | 53 ± 11 | 74 ± 31 | 64 ± 19 | 66 ± 14 | 64 ± 34 | 52 ± 15 | 58 ± 14 | 60 ± 24 | 57 ± 12 | 53 ± 16 |
| MID0.5 | 68 ± 28 | 68 ± 19 | 42 ± 16 | 54 ± 17 | 57 ± 12 | 60 ± 11 | 50 ± 10 | 58 ± 23 | 61 ± 19 | 56 ± 26 | |
| MED40 | 55 ± 17 | 49 ± 10 | 41 ± 8 | 40 ± 9 | 30 ± 6a | 34 ± 7a | 34 ± 5a | 41 ± 8 | 44 ± 9 | 52 ± 14 | |
| MED80 | 53 ± 17 | 53 ± 12 | 47 ± 8 | 40 ± 8 | 31 ± 10 | 35 ± 7 | 38 ± 9 | 46 ± 23 | 42 ± 11 | 47 ± 5 | |
| MED40–MID0.5 | 66 ± 37 | 66 ± 7 | 48 ± 10 | 38 ± 6 | 38 ± 9 | 37 ± 12 | 46 ± 17 | 60 ± 9 | 64 ± 20 | 55 ± 27 | |
MID0.5 — midazolam, 0.5 mg/kg; MED40 — medetomidine, 40 μg/kg; MED80 — medetomidine, 80 μg/kg.
Significantly different from the baseline (0) value (P < 0.05).
Heart rate decreased significantly (P < 0.05) at 0.25 to 2 h in the MED40 and MED40–MID0.5 groups, and at 0.25 to 3 h in the MED80 group (Table I). There were no significant differences in heart rate between the MED40–MID0.5 and MED40 groups.
In the MID0.5 group, the respiratory rate tended to decrease at 0.5 h, but not significantly (Table I). In the MED40 group, the respiratory rate decreased significantly (P < 0.05) at 2 to 4 h after administration. A similar, but not significant, tendency was found in the MED80 group. In the MED40–MID0.5 group the respiratory rate also tended to decrease, and it recovered more rapidly than in the MED40 group.
Glucose
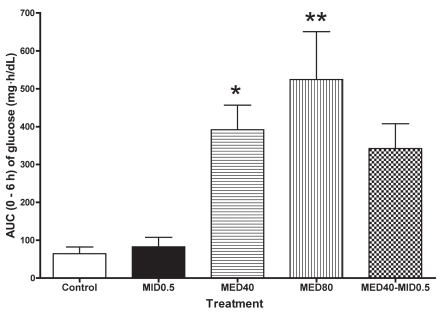
The plasma glucose concentration increased in all the groups receiving medetomidine; there was no significant change from baseline in the control and MID0.5 groups (Table II). The increase was significant at 1 and 2 h after administration of MED40; the mean maximum value was 225 ± 72 [mean ± standard deviation (s)] mg/dL at 2 h. In the MED80 group, the increase was significant at 1 to 3 h, and the maximum value was 267 ± 99 mg/dL at 2 h. Although the glucose value tended to increase at 1 to 2 h in the MED40–MID0.5 group, no significant change was found. The AUC for 0 to 6 h in the MED40 and MED80 groups was significantly greater than that in the control group (Figure 1). The AUC for the combination group tended to be greater than that of the control group, but not significantly so. There was no significant difference in AUC value between the MID0.5 and control groups.
Table II.
Plasma glucose, insulin, and nonesterified fatty acid (NEFA) values after administration of the test agents
| Time after administration (h) (mean ± standard deviation)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | 0 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 24 |
| Glucose (mg/dL) | Control | 118 ± 17 | 115 ± 25 | 112 ± 29 | 111 ± 25 | 107 ± 18 | 130 ± 43 | 107 ± 23 | 109 ± 19 | 103 ± 17 | 126 ± 38 |
| MID0.5 | 99 ± 11 | 99 ± 31 | 123 ± 22 | 106 ± 14 | 106 ± 6 | 105 ± 12 | 111 ± 21 | 109 ± 17 | 105 ± 15 | 106 ± 10 | |
| MED40 | 105 ± 20 | 139 ± 41 | 184 ± 71 | 224 ± 77a | 225 ± 72a | 173 ± 44 | 99 ± 37 | 85 ± 24 | 82 ± 18 | 100 ± 20 | |
| MED80 | 106 ± 16 | 155 ± 87 | 198 ± 94 | 242 ± 104a | 267 ± 99a | 234 ± 66a | 136 ± 70 | 83 ± 20 | 78 ± 13 | 94 ± 15 | |
| MED40–MID0.5 | 119 ± 39 | 147 ± 54 | 173 ± 67 | 201 ± 73 | 196 ± 77 | 140 ± 37 | 78 ± 9 | 80 ± 7 | 83 ± 2 | 92 ± 14 | |
| Insulin (μU/mL) | Control | 8.1 ± 5.9 | 8.8 ± 5.4 | 8.4 ± 5.9 | 7.8 ± 5.0 | 6.7 ± 6.4 | 10.0 ± 7.3 | 7.0 ± 5.0 | 7.4 ± 6.2 | 6.3 ± 5.4 | 9.0 ± 4.7 |
| MID0.5 | 8.4 ± 7.1 | 7.5 ± 6.8 | 9.7 ± 7.0 | 9.6 ± 6.5 | 7.8 ± 7.3 | 7.7 ± 6.0 | 6.0 ± 5.6 | 6.5 ± 5.5 | 8.2 ± 5.5 | 7.1 ± 5.3 | |
| MED40 | 8.4 ± 7.0 | 5.8 ± 6.6 | 6.2 ± 6.4 | 5.6 ± 6.8 | 8.2 ± 9.6 | 13.9 ± 10.8 | 9.7 ± 8.0 | 7.7 ± 6.4 | 8.0 ± 6.3 | 8.3 ± 5.5 | |
| MED80 | 7.0 ± 6.4 | 5.8 ± 5.5 | 5.2 ± 6.3a | 5.4 ± 6.3 | 7.0 ± 7.6 | 11.0 ± 15.5 | 10.6 ± 7.8 | 11.5 ± 12.7 | 9.9 ± 8.3 | 9.9 ± 6.9 | |
| MED40–MID0.5 | 11.6 ± 6.5 | 6.2 ± 6.3 | 5.9 ± 6.0 | 4.4 ± 5.6a | 10.1 ± 5.0 | 16.7 ± 10.4a | 8.1 ± 7.2a | 11.0 ± 8.2 | 9.4 ± 9.4 | 9.1 ± 6.4 | |
| NEFA (mmol/L) | Control | 594 ± 207 | 670 ± 132 | 616 ± 143 | 508 ± 132 | 591 ± 184 | 569 ± 389 | 519 ± 158 | 578 ± 131 | 676 ± 148 | 539 ± 208 |
| MID0.5 | 544 ± 360 | 336 ± 239 | 441 ± 376 | 506 ± 430 | 655 ± 404 | 715 ± 349 | 753 ± 343 | 691 ± 310 | 732 ± 314 | 617 ± 275 | |
| MED40 | 471 ± 213 | 336 ± 126 | 239 ± 83a | 169 ± 83a | 147 ± 94a | 165 ± 101a | 290 ± 210 | 634 ± 364 | 838 ± 407 | 535 ± 138 | |
| MED80 | 411 ± 156 | 247 ± 105 | 164 ± 158 | 113 ± 119a | 81 ± 56a | 112 ± 86a | 311 ± 231 | 399 ± 390 | 515 ± 340 | 574 ± 180 | |
| MED40–MID0.5 | 473 ± 234 | 253 ± 170 | 192 ± 143a | 164 ± 105a | 94 ± 81a | 215 ± 220 | 379 ± 256 | 604 ± 317 | 779 ± 354 | 678 ± 413 | |
MID0.5 — midazolam, 0.5 mg/kg; MED40 — medetomidine, 40 μg/kg; MED80 — medetomidine, 80 μg/kg.
Significantly different from the baseline (0) value (P < 0.05).
Figure 1.
Area-under-the-curve (AUC) data from 0 to 6 h for the plasma glucose concentration in 5 cats after administration of physiological saline (control), midazolam, 0.5 mg/kg (MID0.5), medetomidine, 40 or 80 μg/kg (MED40 and MED80), or a combination of these sedative agents. Means are shown along with vertical bars indicating the standard error. *significantly different from the control value at P < 0.05; **significantly different from the control at P < 0.01.
Insulin
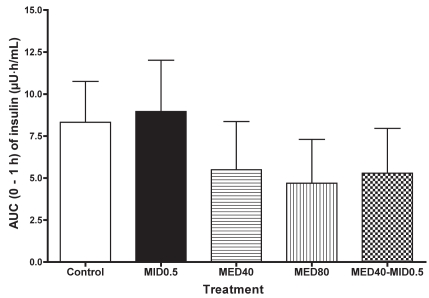
The plasma insulin concentration in the MID0.5 group did not significantly change, but in the MED40 and MED80 groups the concentration tended to decrease at 0.25 to 1 h after administration and then increase over the baseline value (Table II). In the MED40–MID0.5 group, the concentration decreased significantly at 1 h (P < 0.05) and subsequently increased significantly (P < 0.05) at 3 h after administration. The mean AUC for 0 to 6 h did not significantly differ between the groups. However, the mean AUC for 0 to 1 h tended to be lower in the MED40, MED80, and MED40–MID0.5 groups than in the control group (Figure 2).
Figure 2.
For the plasma insulin concentration the AUC data for 0 to 1 h are plotted.
Glucagon
The plasma glucagon concentration did not significantly change in the MID0.5 and control groups. In the MED40 and MED40–MID0.5 groups, the concentration tended to increase at 0.25 h and then decrease at 2 or 3 h after administration, but not significantly so (data not shown). In the MED80 group, the concentration tended to decrease at 1 to 2 h after administration, but also not significantly so. The AUC data for 0 to 6 h did not reveal significant differences between the groups.
Cortisol
In the MID0.5 group, the plasma cortisol concentration tended to increase at 0.5 h after administration and then decrease gradually. In the MED40, MED80, and MED40-MID0.5 groups, it tended to decrease slightly at 1 h after administration, but not significantly so (data not shown). Throughout the sampling period, the concentration in the control group did not change significantly. The mean AUC at 0 to 6 h did not differ significantly among the groups.
Nonesterified fatty acids
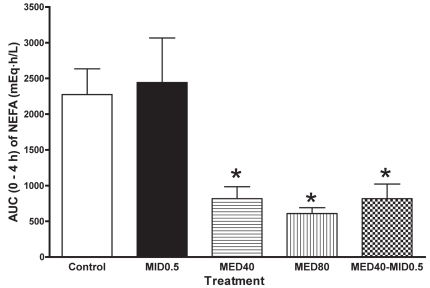
In the MED40, MED80, and MED40–MID0.5 groups, the plasma NEFA concentration decreased significantly (P < 0.05) at 0.5 to 3 h after administration (Table II). In the MID0.5 group the concentration tended to decrease transiently at 0.25 h after administration and then increase gradually. The mean AUC at 0 to 4 h was lower (P < 0.05) in the MED40, MED80, and MED40–MID0.5 groups than in the control group (Figure 3).
Figure 3.
For the plasma nonesterfied fatty acid (NEFA) concentration, the AUC data from 0 to 4 h are plotted. *significantly different from the control value at P < 0.05.
Norepinephrine
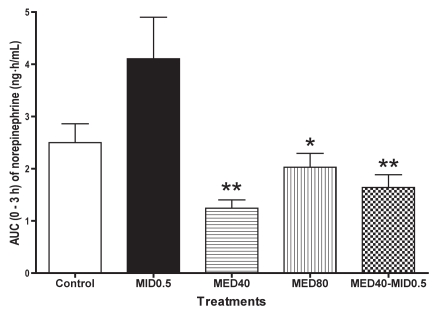
In the control group, there was no significant change in the plasma norepinephrine concentration throughout the sampling period. In the MID0.5 group the concentration increased significantly (P < 0.05) at 0.5 h after administration and also tended to increase at 2 h (Table III). In the MED40 group, the concentration decreased significantly (P < 0.05) at 0.25 to 1 h after administration, and in MED80 group it decreased significantly (P < 0.05) at 0.25 to 3 h. The changes in the MED40–MID0.5 group were similar to those in the MED40 and MED80 groups, but not significantly so. The mean AUC for 0 to 3 h was significantly lower in the MED40 (P < 0.01), MED80 (P < 0.05), and MED40–MID0.5 (P < 0.01) groups than in the MID0.5 group (Figure 4); the value was greater in the MED40–MID0.5 group than in the MED40 group, but not significantly so.
Table III.
Plasma norepinephrine and epinephrine values after administration of the test agents
| Time after administration (h) (mean ± standard deviation)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | 0 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | 24 |
| Norepinephrine (ng/mL) | Control | 0.94 ± 0.36 | 0.78 ± 0.26 | 0.81 ± 0.28 | 0.83 ± 0.28 | 0.82 ± 0.26 | 0.89 ± 0.38 | 0.88 ± 0.28 | 1.00 ± 0.30 | 1.09 ± 0.16 | 1.23 ± 0.34 |
| MID0.5 | 0.70 ± 0.11 | 0.98 ± 0.56 | 3.11 ± 2.64a | 0.92 ± 0.54 | 1.43 ± 1.02 | 0.90 ± 0.16 | 0.84 ± 0.13 | 0.73 ± 0.16 | 1.00 ± 0.17 | 1.00 ± 0.17 | |
| MED40 | 0.91 ± 0.29 | 0.27 ± 0.15a | 0.29 ± 0.12a | 0.30 ± 0.13a | 0.47 ± 0.09 | 0.52 ± 0.31 | 0.82 ± 0.20 | 0.87 ± 0.37 | 0.96 ± 0.41 | 1.07 ± 0.31 | |
| MED80 | 1.30 ± 0.20 | 0.64 ± 0.27a | 0.67 ± 0.16a | 0.69 ± 0.24a | 0.60 ± 0.19a | 0.69 ± 0.27a | 0.76 ± 0.27 | 0.80 ± 0.33 | 1.22 ± 0.29 | 1.52 ± 0.60 | |
| MED40–MID0.5 | 0.89 ± 0.22 | 0.50 ± 0.17 | 0.44 ± 0.23 | 0.45 ± 0.30 | 0.55 ± 0.16 | 0.82 ± 0.29 | 0.96 ± 0.37 | 1.00 ± 0.58 | 1.00 ± 0.40 | 1.41 ± 0.78 | |
| Epinephrine (ng/mL) | Control | 0.70 ± 0.27 | 0.54 ± 0.14 | 0.52 ± 0.13 | 0.60 ± 0.16 | 0.69 ± 0.36 | 0.55 ± 0.10 | 0.55 ± 0.11 | 0.62 ± 0.15 | 0.63 ± 0.13 | 0.64 ± 0.18 |
| MID0.5 | 0.48 ± 0.09 | 0.72 ± 0.27 | 0.68 ± 0.35 | 0.71 ± 0.49 | 0.75 ± 0.40 | 0.61 ± 0.07 | 0.65 ± 0.13 | 0.47 ± 0.22 | 0.64 ± 0.13 | 0.56 ± 0.14 | |
| MED40 | 0.63 ± 0.15 | 0.41 ± 0.18 | 0.46 ± 0.26 | 0.54 ± 0.30 | 0.45 ± 0.14 | 0.58 ± 0.24 | 0.66 ± 0.20 | 0.65 ± 0.35 | 0.55 ± 0.26 | 0.63 ± 0.28 | |
| MED80 | 0.78 ± 0.41 | 0.71 ± 0.49 | 0.60 ± 0.23 | 0.58 ± 0.32 | 0.51 ± 0.13 | 0.67 ± 0.24 | 0.74 ± 0.24 | 0.68 ± 0.23 | 1.28 ± 0.48 | 0.81 ± 0.26 | |
| MED40–MID0.5 | 0.75 ± 0.23 | 0.68 ± 0.22 | 0.47 ± 0.14 | 0.52 ± 0.15 | 0.61 ± 0.20 | 0.72 ± 0.32 | 0.90 ± 0.20 | 0.70 ± 0.07 | 0.65 ± 0.14 | 0.74 ± 0.16 | |
MID0.5 — midazolam, 0.5 mg/kg; MED40 — medetomidine, 40 μg/kg; MED80 — medetomidine, 80 μg/kg.
Significantly different from the baseline (0) value (P < 0.05).
Figure 4.
For the plasma norepinehrine concentration, the AUC data for 0 to 3 h are plotted. *significantly different from the MID0.5 value at P < 0.05. **significantly different from the MID0.5 value at P < 0.01.
Epinephrine
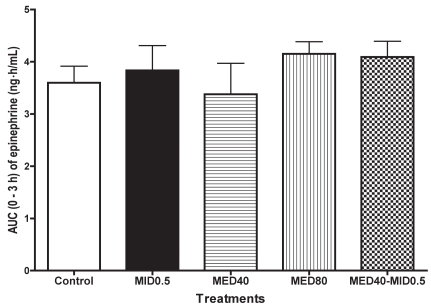
There was no significant change in the plasma epinephrine concentration in any group throughout the smapling period (Table III). However, the concentration tended to decrease from baseline at 0.25 h after administration in MED40 group, at 0.25 to 0.5 h in the MED40–MID0.5 group, and at 0.25 to 2 h in the MED80 group. In contrast, in the MID0.5 group, the concentration tended to increase at 0.25 to 2 h after administration. There were no significant differences in AUC data for 0 to 3 h among the groups (Figure 5).
Figure 5.
For the plasma epinephrine concentration, the AUC data for 0 to 3 h data are plotted.
Discussion
In this study, midazolam in combination with medetomidine significantly prolonged the lateral recumbency induced by medetomidine in cats. Despite the longer period of immobility, the addition of midazolam did not significantly worsen the adverse effects such as hypothermia, bradyarrhythmia, and bradypnea induced by medetomidine alone.
Our previous study revealed that in cats medetomidine induces hyperglycemia and accompanying hypoinsulinemia. This hyperglycemic effect may limit the use of medetomidine in cats with metabolic and neurohormonal problems such as diabetes mellitus, ketosis, and glycosuria. The current study examined if adding midazolam could influence the hyperglycemic effect of medetomidine. Indeed, although the medetomidine–midazolam combination tended to induce hyperglycemia, the tendency was not significant, whereas both doses of medetomidine given alone induced apparent hyperglycemia. Midazolam given alone did not produce a significant change in plasma glucose concentration. These results indicate that a MED40–MID0.5 combination can reduce the hyperglycemic effect induced by the same dose of medetomidine alone. This reduction may be due to interactions between the α2-adrenoceptors and benzodiazepine receptors or the γ-aminobutyric acid (GABA) receptors in the neurohormonal mechanisms related to hyperglycemia and hypoinsulinemia. However, the precise mechanism is unknown. This effect of a medetomidine–midazolam combination on hyperglycemia was also reported for laboratory pigs (15). To our knowledge, this is the first report that a medetomidine–midazolam combination can reduce the increase in blood glucose concentration induced by medetomidine alone in cats.
In this study, the decrease in plasma insulin concentration did not differ significantly between the MED40–MID0.5 and MID40 groups. However, recovery from the induced hypoinsulinemia tended to be more rapid in the MED40–MID0.5 group than in the MED40 group. There are conflicting reports on the effect of midazolam alone on insulin secretion from islet cells. Desborough et al (21) have reported that the use of midazolam was associated with a decrease in insulin secretion during upper abdominal surgery and a decrease in blood glucose levels in humans. Desborough et al (22) also reported that midazolam did not directly inhibit glucose-stimulated insulin secretion from an in vitro rat islet preparation. In contrast, Cuparencu et al (23) reported that midazolam increased the plasma insulin level in streptozotocin-induced diabetes in rats. In our study, midazolam alone did not significantly affect the plasma insulin concentration in cats. On the other hand, an enhancement of the binding of GABA to low-affinity receptor sites may give rise to many of the in vivo actions of the benzodiazepines (24), and GABA has been reported to inhibit insulin secretion from β cells at high concentrations of glucose (25,26), and to inhibit the release of glucagon from α cells (27). These actions may also influence the plasma glucose concentration in cats given a medetomidine–midazolam combination. Our results also indicate that the changes in glucagon concentrations induced by the MED40–MID0.5 combination did not differ from those induced by MED40 alone. In addition, no significant differences in glucose concentration were found between the MID0.5 and control groups. Therefore, it seems that the dose of midazolam used in this study did not affect the plasma glucagon and insulin levels.
Medetomidine and xylazine have been reported to inhibit norepinephrine release in cats (5) and dogs (3). The finding in the present study that MED40 and MED80 inhibit norepinephrine release in cats is in agreement. The medetomidine–midazolam combination tended to reduce the medetomidine-induced inhibition of norepinephrine release. This might be due in part to the effect of midazolam on norepinephrine release, because midazolam alone increased plasma norepinephrine levels. In contrast, midazolam did not significantly change plasma norepinephrine concentrations in humans (28). In our study, however, it is unknown whether the increase in norepinephrine concentration was induced by a pharmacologic effect of midazolam or by excitement-like behavior associated with midazolam administration. This is the first report that midazolam increases the plasma norepinephrine concentration in cats.
Medetomidine, midazolam, and their combination did not significantly change the plasma epinephrine concentration of the cats in this study. The result with midazolam agrees with a previous report on humans (28). Several studies, however, have reported that medetomidine reduces the plasma epinephrine concentration in cats (5) and dogs (3). However, medetomidine has also been reported not to affect the epinephrine level in humans (29). Thus, the effect of medetomidine on the plasma epinephrine concentration is controversial.
In this study, there were no significant changes in the plasma cortisol concentration in any group. Our results were similar to previous findings (5). In addition, no significant differences were found among the MED40, MED80, and MED40–MID0.5 groups. Therefore, these results indicate that the addition of midazolam does not affect the plasma cortisol concentration of cats given medetomidine.
The plasma NEFA concentration has been reported to be reduced by medetomidine in cats (5). This response may be due to the suppression of lipolysis mediated by α2-adrenoceptors (30,31). The results of this study confirm the previous finding that medetomidine decreased plasma NEFA levels (5). The changes in plasma NEFA concentration in the MED40–MID0.5 group, however, were similar to those in the MED40 group in this study. Therefore, our results indicate that the addition of midazolam does not alter the inhibition of lipolysis induced by medetomidine in cats.
In conclusion, a medetomidine–midazolam combination reduced the hyperglycemia induced by medetomidine in cats. The decrease in plasma norepinephrine concentration induced by medetomidine alone was diminished by the addition of midazolam. Midazolam alone increased the plasma norepinephrine level, but did not change the glucose, insulin, glucagon, cortisol, epinephrine, or NEFA concentration. Therefore, this study has revealed that a medetomidine– midazolam combination produces minimal neurohormonal and metabolic changes without greater adverse effects than with medetomidine alone in healthy cats.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (18580316; to Y. Hikasa) and by the Scholarship Donation Fund from Meiji Seika, Tokyo, Japan (to Y. Hikasa). The authors thank Dr. Takashi Takeuchi for his valuable advice.
References
- 1.Greene SA. Pros and cons of using alpha-2 agonists in small animal anesthesia practice. Clin Tech Small Anim Pract. 1999;14:10–14. doi: 10.1016/s1096-2867(99)80022-x. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi K, Nishimura R, Yamaki A, et al. Cardiopulmonary effects of medetomidine, medetomidine–midazolam and medetomidine– midazolam–atipamezole in dogs. J Vet Med Sci. 1995;57:99–104. doi: 10.1292/jvms.57.99. [DOI] [PubMed] [Google Scholar]
- 3.Ambrisko TD, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can J Vet Res. 2002;66:42–49. [PMC free article] [PubMed] [Google Scholar]
- 4.Lamont LA, Bulmer BJ, Grimm KA, Tranquilli WJ, Sisson DD. Cardiopulmonary evaluation of the use of medetomidine hydrochloride in cats. Am J Vet Res. 2001;62:1745–1749. doi: 10.2460/ajvr.2001.62.1745. [DOI] [PubMed] [Google Scholar]
- 5.Kanda T, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in healthy cats. Can J Vet Res. 2008;72:278–286. [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CR, Sarnquist FH, Canup CA, Pedley TA. Clinical, electroencephalographic, and pharmacokinetic studies of a water-soluble benzodiazepine, midazolam maleate. Anesthesiology. 1979;50:467–470. doi: 10.1097/00000542-197905000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Ilkiw JE, Suter CM, Farver TB, McNeal D, Steffey EP. The behaviour of healthy awake cats following intravenous and intramuscular administration of midazolam. J Vet Pharmacol Ther. 1996;19:205–216. doi: 10.1111/j.1365-2885.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Salonen M, Reid K, Maze M. Synergistic interaction between alpha 2-adrenergic agonists and benzodiazepines in rats. Anesthesiology. 1992;76:1004–1011. doi: 10.1097/00000542-199206000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura R, Kim H, Matsunaga S, et al. Sedative effect induced by a combination of medetomidine and midazolam in pigs. J Vet Med Sci. 1993;55:717–722. doi: 10.1292/jvms.55.717. [DOI] [PubMed] [Google Scholar]
- 10.Itamoto K, Hikasa Y, Sakonjyu I, Itoh H, Kakuta T, Takase K. Anaesthetic and cardiopulmonary effects of balanced anaesthesia with medetomidine–midazolam and butorphanol in dogs. J Vet Med A Physiol Pathol Clin Med. 2000;47:411–420. doi: 10.1046/j.1439-0442.2000.00302.x. [DOI] [PubMed] [Google Scholar]
- 11.Kojima K, Nishimura R, Mutoh T, Hong SH, Mochizuki M, Sasaki N. Effects of medetomidine–midazolam, acepromazine–butorphanol, and midazolam–butorphanol on induction dose of thiopental and propofol and on cardiopulmonary changes in dogs. Am J Vet Res. 2002;63:1671–1679. doi: 10.2460/ajvr.2002.63.1671. [DOI] [PubMed] [Google Scholar]
- 12.Raekallio M, Tulamo RM, Valtamo T. Medetomidine–midazolam sedation in sheep. Acta Vet Scand. 1998;39:127–134. doi: 10.1186/BF03547814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima K, Nishimura R, Mutoh T, et al. Comparison of sedative effects of medetomidine–midazolam, acepromazine–butorphanol and midazolam-butorphanol in dogs. Zentralbl Veterinarmed A. 1999;46:141–148. doi: 10.1046/j.1439-0442.1999.00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Itamoto K, Taura Y, Wada N, et al. Quantitative electroencephalography of medetomidine, medetomidine-midazolam and medetomidine-midazolam-butorphanol in dogs. J Vet Med A Physiol Pathol Clin Med. 2002;49:169–172. doi: 10.1046/j.1439-0442.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura R, Kim HY, Matsunaga S, et al. Effects of medetomidine-midazolam on plasma glucose and insulin concentrations in laboratory pigs. J Vet Med Sci. 1994;56:559–561. doi: 10.1292/jvms.56.559. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J. 2003;44:885–897. [PMC free article] [PubMed] [Google Scholar]
- 17.Ilkiw JE, Suter CM, McNeal D, Farver TB, Steffey EP. The effect of intravenous administration of variable-dose midazolam after fixed-dose ketamine in healthy awake cats. J Vet Pharmacol Ther. 1996;19:217–224. doi: 10.1111/j.1365-2885.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 18.Jain NC. The cat: Normal hematology with comments on response to disease. In: Jain NC, editor. Schalm’s Veterinary Hematology. 4. Philadelphia: Lea & Febiger; 1986. pp. 126–139. [Google Scholar]
- 19.Nilsfors L, Garmer L, Adolfsson A. Sedative and analgesic effects of medetomidine in dogs — an open clinical study. Acta Vet Scand Suppl. 1989;85:155–159. [PubMed] [Google Scholar]
- 20.Bouloux P, Perrett D, Besser GM. Methodological considerations in the determination of plasma catecholamines by high-performance liquid chromatography with electrochemical detection. Ann Clin Biochem. 1985;22(pt 2):194–203. doi: 10.1177/000456328502200217. [DOI] [PubMed] [Google Scholar]
- 21.Desborough JP, Hall GM, Hart GR, Burrin JM. Midazolam modifies pancreatic and anterior pituitary hormone secretion during upper abdominal surgery. Br J Anaesth. 1991;67:390–396. doi: 10.1093/bja/67.4.390. [DOI] [PubMed] [Google Scholar]
- 22.Desborough JP, Jones PM, Persaud SJ, Howell SL. Effects of midazolam on insulin secretion from isolated rat pancreatic islets of Langerhans. Br J Anaesth. 1993;70:221–222. doi: 10.1093/bja/70.2.221. [DOI] [PubMed] [Google Scholar]
- 23.Cuparencu B, Horák J, Orbai P, Horák A, Lenghel A. The effects of the intraperitoneal administration of midazolam on blood glucose level and serum lipids in streptozotocin-induced diabetes in rats. Acta Physiol Hung. 1997–1998;85:83–88. [PubMed] [Google Scholar]
- 24.Skerritt JH, Johnston GA. Enhancement of GABA binding by benzodiazepines and related anxiolytics. Eur J Pharmacol. 1983;89:193–198. doi: 10.1016/0014-2999(83)90494-6. [DOI] [PubMed] [Google Scholar]
- 25.Brice NL, Varadi A, Ashcroft SJ, Molnar E. Metabotropic glutamate and GABA(B) receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2002;45:242–252. doi: 10.1007/s00125-001-0750-0. [DOI] [PubMed] [Google Scholar]
- 26.Dong H, Kumar M, Zhang Y, et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia. 2006;49:697–705. doi: 10.1007/s00125-005-0123-1. [DOI] [PubMed] [Google Scholar]
- 27.Rorsman P, Berggren PO, Bokvist K, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 28.Kong KL, Willatts SM, Prys-Roberts C, Harvey JT, Gorman S. Plasma catecholamine concentration during sedation in ventilated patients requiring intensive therapy. Intensive Care Med. 1990;16:171–174. doi: 10.1007/BF01724797. [DOI] [PubMed] [Google Scholar]
- 29.Kallio A, Salonen M, Forssell H, Scheinin H, Scheinin M, Tuominen J. Medetomidine premedication in dental surgery — a double-blind cross-over study with a new alpha 2-adrenoceptor agonist. Acta Anaesthesiol Scand. 1990;34:171–175. doi: 10.1111/j.1399-6576.1990.tb03065.x. [DOI] [PubMed] [Google Scholar]
- 30.Taouis M, Berlan M, Montastruc P, Lafontan M. Mechanism of the lipid-mobilizing effect of alpha-2 adrenergic antagonists in the dog. J Pharmacol Exp Ther. 1988;247:1172–1180. [PubMed] [Google Scholar]
- 31.Vikman HL, Savola JM, Raasmaja A, Ohisalo JJ. Alpha 2A-adrenergic regulation of cyclic AMP accumulation and lipolysis in human omental and subcutaneous adipocytes. Int J Obes Relat Metab Disord. 1996;20:185–189. [PubMed] [Google Scholar]