Abstract
Aseptic meningitis (AM) is a disease that causes grave clinical signs such as intensive neck pain, fever, and lethargy. The severity of this disease is reflected in the fact that affected animals require long-term, and in chronic cases, lifelong therapy with corticosteroids. A number of dogs must be euthanized because of therapeutic failure. In recent years, the Norwegian population of Nova Scotia duck tolling retrievers has experienced an increase in individuals with AM. The aim of the present study was to investigate the prevalence of AM and to pursue the suspicion of hereditary factors influencing an accumulation of AM cases in the breed. Using the Norwegian Kennel Club registery, a random sample (362 dogs) stratified by year of birth was drawn from the total population born from 1994 to 2003 (1525 individuals). The owners were contacted and questioned about clinical signs of AM in their dogs. Subsequently, the practising veterinarians and the breeders of positive responders were contacted in order to confirm a clinical diagnosis of AM and to identify possible affected family members. Pedigrees of AM positive individuals and affected relatives were investigated. The study estimated a prevalence of AM of 2.5%. For all affected dogs, it was possible to trace the pedigree of both parents of affected dogs back to a specific founder dog. The genealogical investigation strongly indicates that genetic factors are involved in the etiology of the disease.
Résumé
La méningite aseptique (AM) est une maladie qui cause des signes cliniques sévères tels que douleur nucale intense, fièvre et léthargie. La sévérité de cette condition est manifeste par le fait que les animaux atteints requièrent une thérapie de longue durée avec des corticostéroïdes, et dans les cas chroniques une thérapie à vie. Plusieurs chiens doivent être euthanasiés suite à un échec thérapeutique. Au cours des dernières années, on a noté une augmentation du nombre d’individus présentant une AM au sein de la population norvégienne des Duck Tolling Retriever de Nouvelle-Écosse. La présente étude visait à déterminer la prévalence d’AM et vérifier l’hypothèse du rôle de facteurs héréditaires dans l’accumulation de cas d’AM au sein de la race. À l’aide du registre du Club Canin Norvégien, un échantillon aléatoire (362 chiens), stratifié en fonction de l’année de naissance, a été généré de la population totale née entre 1994 et 2003 (1525 individus). Les propriétaires ont été contactés et questionnés sur les signes cliniques d’AM chez leur chien. Par la suite, les vétérinaires praticiens et les éleveurs ayant répondu positivement ont été contactés afin de confirmer un diagnostic clinique d’AM et d’identifier des membres de la famille possiblement atteints. Les pedigrees des individus positifs pour AM et les membres affectés de leur famille ont été étudiés. L’étude a estimé une prévalence d’AM de 2,5 %. Pour tous les chiens atteints, il a été possible de retracer le pedigree des deux parents des chiens affectés jusqu’à un ancêtre spécifique. L’enquête généalogique indique fortement que des facteurs génétiques sont impliqués dans l’étiologie de cette maladie.
(Traduit par Docteur Serge Messier)
Introduction
Aseptic meningitis (AM) is a non-infectious inflammatory condition characterized by histiocytic to lymphocytic periarterial inflammation, fibrinoid necrosis of the tunica media, vascular trombosis of vessel walls of the meninges, and massive periarterial accumulation of inflammatory cells (1–11). It has been shown that affected dogs have an overrepresentation of B lymphocytes in meningeal lesions and in peripheral blood, whereas T lymphocytes are the only lymphocytic population found in inflamed arteries (12). Aseptic meningitis is typically seen in medium- to large-breed dogs that are 7 to 16 months old (13–15). There is no apparent sex-predilection (2,14). The disease has been reported in Bernese mountain dogs, boxers, and beagles (2–8,10,16–20). The etiology of aseptic meningitis is unknown, but immune-mediated mechanisms appear to be involved (1–5,7,8,10,15–18,21). Excessive immunoglobulin (IgA) production is associated with the pathogenesis of the condition (8,9,17,22–24). It has been suggested that repeated injections of live multivalent vaccines may trigger the disease and that estrous may influence the onset of the disease in bitches (13,16). Two clinical forms are recognized: the typical acute condition characterized by severe neck pain, fever, depression, anorexia, pain when opening the mouth, hunched back, reluctance to move, and a stiff stilted gait, with the cerebrospinal fluid showing a significant neutrophilic pleocytosis and an elevated protein concentration (7–9,15–18,21,23,24); and the more protracted form with severe neurological signs such as ataxia, paresis tetra-paresis or paraplegia, mild to moderate mixed cell pleocytosis, and possible protein elevation. A peripheral neutrophilia may be apparent upon a complete blood (cell) count (CBC) (1,2,4,5,7,8,14,16–21,24). Affected dogs may also have polyarthritis (16,25,26). In a substantial number of cases, corticosteroids have a good effect on the clinical signs, which is why this condition has also been called steroid-responsive meningitis. However, approximately 50% of affected dogs have recurrence of clinical signs following discontinuation of corticosteroid therapy and lifelong medication may be needed to avoid relapse (13). Over the last 5 years an increasing number of AM cases have appeared in Norwegian Nova Scotia duck tolling retrievers (NSDTRs). The Norwegian population of NSDTRs predominantly originated from dogs imported from Sweden in the mid to late eighties. Over time, dogs from other European countries and Canada were brought into the Norwegian population. The first Norwegian litter was born in 1989 and the population has increased quickly. According to the Norwegian Kennel Club (NKC) register, 65 dogs were born in 1994, 106 in 1995, 142 in 1996, and from 1997 to 2003 the number of dogs born per year has fluctuated between 129 and 221. The aim of the present study was to investigate if AM represents a growing problem in the Norwegian population of NSDTRs, and the possibility of a familial predisposition.
Materials and methods
The work was carried out at the Norwegian Veterinary School, Oslo, Norway and at the Department of Small Animal Clinical Sciences, Faculty of Life Sciences, University of Copenhagen, Denmark.
Study design and description of the population
As the purpose of the study was to investigate if AM represents a growing problem in Norwegian NSDTR, the study design was aimed at disclosing if there had been a history of typical clinical signs associated with this disease in previous populations. The study, therefore, was designed as a retrospective cohort study and conducted as a telephone interview of a representative number of NSDTR owners drawn from the reference population. This was followed by contacts with the practising veterinarians of possible positive individuals, in order to confirm the information given by the owners and exclude other diseases that may also express similar clinical signs.
Sampling of study population
A random sample, stratified by year of birth, was drawn from the reference population constituting a total of 1525 dogs registered in the NKC from January 1, 1994 to December 31, 2003. At the time of the study, none of the dogs was younger than 1-year old. Data comprising registration number, pedigree, date of birth, name and sex of the dog, and the name, address, and telephone number of the owner was obtained from the NKC central registry. Dogs are registered chronologically by date of birth in this registry, which contains information on all purebred dogs registered in Norway.
Sample size
A precision (L) of the prevalence of 2% was considered appropriate for this study. The prevalence (P) was expected to be around 5%, based on the authors’ best guess. According to this, a sample (n) of 362 dogs was needed to achieve the desired precision. The calculations were carried out as follows.
A preliminary sample (n*) is calculated.
P = estimated prevalence = 0.05
L2 = precision to the power of 2 = 0.022
This formula is based on the assumption that the population is infinite. In a finite population, such as the present, a smaller sample is needed. The following formula, therefore, corrects for this by taking into account that the entire population (N) consisted of 1525 dogs:
In order to obtain a sample that mirrored the unevenness in births per year, the sample was drawn as follows: all 1525 dogs were ranked according to date of birth. The sampling was conducted in 2 rounds; both in which the first dog was selected randomly. After this, every 4th dog was selected in the 1st round, and every 11th dog in the 2nd round. If a dog had already been selected in the 1st round, then the next dog was selected. Both living and deceased dogs born January 1, 1994 to December 31, 2003, were included in the study.
A selected dog was replaced if the owner had moved from the address listed in the NKC registry and could not be traced through the Norwegian telephone register or if a transfer of ownership had occurred and the new owner could not be traced. The next dog on the birth list from the NKC replaced the excluded dog.
This sample was presumed to be representative of the total Norwegian population of NSDTRs with regard to gender, reproductive status, health status, and other factors of possible interest.
Telephone interview and questionnaire
The owners of the dogs were interviewed by telephone using a standardized questionnaire concerning the clinical signs that can be associated with AM, if a diagnosis of AM had been established, and about treatment. An experienced neurologist conducted interviewer training, including instructions regarding the study design and principles of interviewing. All interviewers were qualified veterinarians.
Throughout questioning, the interviewers’ veterinary outlook was set aside, aiming at perceiving the disease from the owners’ point of view. The language was kept as ordinary as possible, and the questioning was adapted to the owner’s level of understanding; the interviews were conducted by 3 interviewers (KPF, FJHL, TRH).
First, it was established whether the dog was alive or not. If the dog had died, the owner was questioned about year and cause of death besides the questions regarding AM.
The owners were presented with 4 introductory questions that were posed to detect neck pain (as neck pain is a cardinal sign of AM). The owners were asked if the dog had experienced a course of disease where it 1) actively expressed signs of pain related to the neck area, for example, crying out in pain when moving the head, when being touched/manipulated in the neck region, or when wearing a collar and being walked on a leash; 2) was unwilling to reach for its food bowl; 3) was unwilling to turn its head; and 4) preferred to walk with a lowered head at a particular time.
Owners were then presented with a list of 6 additional clinical signs that are also often associated with AM. It was recorded whether the owners had observed fever, a stiff gait, depression, unwillingness to go for walks or play, unwillingness to open the mouth, and excessive panting (as a sign of pain) during the time of disease. Also, the owners were asked if a practising veterinarian had diagnosed their dog with AM, and if a medical treatment had been instituted. If the owner answered yes to this question, the name, address, and telephone number of the practising veterinarian was collected.
The practising veterinarians of all dogs considered preliminary positive for signs of AM after the interview were contacted by the investigators. Only in cases where the practising veterinarian could confirm that a dog had been clinically diagnosed with AM, and where potential differential diagnosis also associated with neck pain had been ruled out, the dog continued in the study.
Finally, the owners were asked whether they had knowledge of any AM positive dogs with familial relation to their own dog.
To further investigate any familial predisposition, the breeders of dogs diagnosed with AM by their practising veterinarian were contacted and asked whether they had any knowledge of other dogs diagnosed with AM that were related to any of the dogs with clinical signs of AM in this study. Pedigrees of these dogs were retrieved from the NCK. As most of the Norwegian dogs go back to Swedish imports, further pedigree information was obtained from the Swedish Kennel Club registry. The pedigrees of a control group of 14 randomly selected AM negative dogs were studied in order to investigate any consanguinity with AM suspect animals.
Each dog should fulfil all of the following criteria to finally be included in the study as a clinically positive AM case: 1) express more than 4 of the clinical signs characteristic of AM defined in the study; 2) the signs expressed by the dog lead to a clinical diagnosis of AM established by the practising veterinarian; 3) response to corticosteroid treatment should occur.
Being a retrospective population study, the design excluded the possibility of including cerebrospinal fluid (CSF) examination as a standard procedure.
Statistical analysis
A level of significance of 0.05 was used. The 95% confidence interval (CI) for the true prevalence of AM in the Norwegian population of NSDTRs was calculated as I ± 2 Sx̄ = I ± 2√where Sx̄ = standard error, P = prevalence estimate, q = 1 – P, and n = sample size). To determine if a gender predisposition could be found in this study, a chi-square test was performed.
Results
Study design
The study population consisted of 172 female and 190 male dogs, ranging from 1 to 11 years of age at the time of the survey. Of the 362 dogs initially sampled, 42 (11.6%) had to be replaced because their location could not be traced.
Prevalence
Nine dogs fulfilled the criteria to be included as clinically positive AM cases. This resulted in a prevalence of AM of 2.5% (95% CI 0.9% to 4.1%). Four females (4/172 = 2.3%) and 5 males (5/190 = 2.6%) were affected. The age of the 9 dogs at the time of onset of clinical signs consistent with AM ranged from 4 to 19 mo.
Clinical signs
Table I shows the clinical signs of the 9 affected dogs. Three dogs experienced all of the 10 clinical signs defined by the investigators as being associated with AM, while 3 dogs showed 9 and the remaining 3 dogs showed 6 to 7 of these signs (Table I). All dogs experienced fever during the episodes reported.
Table I.
Clinical signs in the 9 Nova Scotia duck tolling retrievers expressing signs of aseptic meningitis
| Dog number | 1 | 2 | 3 | 4a | 5 | 6 | 7 | 8 | 9a |
|---|---|---|---|---|---|---|---|---|---|
| Clinical signs of neck pain | |||||||||
| Actively expressing neck pain | + | + | + | + | + | + | − | + | + |
| Lowered head while walking | + | − | + | + | + | + | + | + | − |
| Reluctance to turn the head | + | − | + | + | + | + | + | + | + |
| Difficulty in lowering head to food bowl | + | − | + | + | + | + | + | + | − |
| Clinical signs also associated with AM | |||||||||
| Fever | + | + | + | + | + | + | + | + | + |
| Stiff gait | + | + | + | + | − | + | + | + | + |
| Unwillingness to go for walks or play | + | + | + | + | + | + | − | + | + |
| Depression | + | + | + | + | + | + | + | + | + |
| Unwillingness to open the mouth, e.g. carrying a stick/ball | + | + | + | + | + | + | − | + | + |
| Excessive panting | + | − | + | − | + | + | + | − | − |
In dogs number 4 and 9 the owner reported signs of hyperesthesia during the AM episode.
+ = The dog expressed the clinical sign.
−= The dog did not express the clinical sign.
In all dogs, the practising veterinarian established a diagnosis of AM based on the clinical signs and by ruling out other diseases presenting with neck pain, such as disc herniation and cervical facet joint arthritis. None of the 9 dogs had CSF examination.
All dogs showed improvement after corticosteroid treatment. Two dogs recovered during the 1st wk of corticosteroid treatment, and 3 dogs recovered within 3 wk. One dog was still on medication, occasionally showing signs after more than 6 mo of medication, and 1 dog that had relapsed several times was eventually euthanized. Another dog had 2 recurrences. Dog number 9, received a relatively low dose of corticosteroids and achieved a reduction in clinical signs but still suffered from mild signs of neck pain for several months. The recurrence frequency after withdrawal of treatment in the 9 dogs was 33.3% (3/9).
Familial predisposition
The owners of 2 dogs reported that dogs with familial relation to their own dog had been diagnosed with AM. One dog had 1 sister and 2 half-siblings with AM, and 1 dog had 1 half-sibling with the disease. This information, in addition to the interviews with the breeders, yielded the identities of an additional 7 dogs that had expressed multiple clinical signs associated with AM in this study. Due to the study design these dogs could not be included in calculating the prevalence.
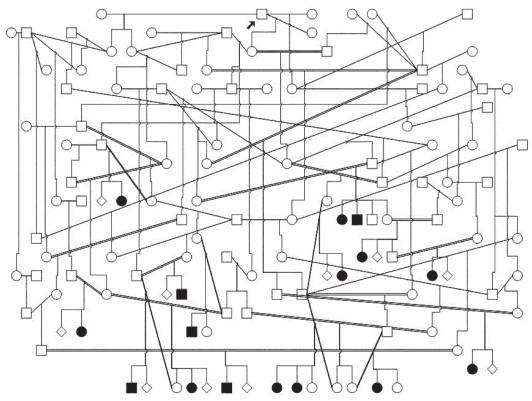
The pedigrees for all 16 affected dogs were drawn to see if there was any support for a simple Mendelian inheritance of the disease. For all the affected dogs, it was possible to trace the pedigree of both parents back to a specific founder dog that was born in Canada (Figure 1). A study of a control group of 14 randomly selected negative dogs showed that these dogs were also related through both parents to the same founder dog as were the affected dogs. Investigating the affected litters identified in the study showed that the percentage of affected dogs is < 17.4%.
Figure 1.
Pedigree of 16 Norwegian Nova Scotia duck tolling retrievers suffering from aseptic meningitis. Both parents of all affected dogs are related to a common ancestor (arrow). Affected litters have a total of 16 affected and 76 healthy siblings (⋄) (17.4% affected) (□ = males, ○ = females, ▪ = affected males, ● = affected females).
Gender predisposition (χ2 = 0.034, P = 0.8) was not found, as 5 affected dogs were males (2.6% of all male dogs) and 4 were females (2.3% of all female dogs).
Discussion
The NSDTR have been suspected of having an increased risk of AM. The history of AM was studied to reveal if this might be true in the Norwegian population of NSDTR. The study showed clear evidence that clinical signs associated with AM existed in young individuals in the population from 1994 to 2003.
This study estimated a prevalence of 2.5%, representing the risk an individual NSDTR in Norway has of developing AM some time during its life. No epidemiological studies have investigated the prevalence for AM in dogs in general or in specific breeds, and therefore our results cannot be compared to previous investigations. However, a prevalence estimate of 2.5% seems to be high, as AM is not a common disease.
When calculating the sample size, it was estimated that the true prevalence would be around 5%; however, our study revealed a prevalence of 2.5%. According to sample size theory, variability is at maximum at a prevalence of 50%, and hence, the closer the expected prevalence is to 50% the larger the sample size is required to estimate a prevalence with a given precision. A precision of 2% was used for calculating the sample size, but because the prevalence was only 2.5% instead of 5%, our 95% confidence interval was more precise than ± 2: 0.9% to 4.1%.
Both living and dead dogs born from 1994 to 2003 were included in this study. Excluding dead dogs would pose a risk of excluding a group of dogs with a higher incidence of AM than the rest of the population, thus creating selection bias. Regarding recall bias, it was judged that owners would recall the rather serious signs of AM even if they had occurred 10 years earlier.
As shown in the present study, the disease accumulated within specific families in which the risk of being affected with AM was considerable higher than 2.5%. As it appears from figure 1, half of the siblings in several litters had expressed clinical signs consistent with AM. This finding, and the fact that AM in many cases is a chronic and severe disease that may need lifelong therapy or lead to euthanasia, contributes to the seriousness of the present disease status in the Norwegian NSDTR population.
Dogs were initially classified as preliminary positive or negative with respect to AM based upon whether the owners had observed clinical expressions of neck pain and other, more unspecific signs associated with AM. Regardless of the answers to these questions, positive responders were not included in the present study unless a veterinarian had diagnosed the dog clinically as being affected with this disease. However, some of the dogs not included fulfilled many of the criteria of AM suspect positive dogs, and therefore the prevalence of AM reported in the present study is most likely an underestimate. Seven dogs that were identified by the breeders as being suspected of having AM were not included in calculating the prevalence because of the design of the study. Had these dogs been included, the actual incidence risk of AM would have been remarkably higher. The Canadian 2002 NSDTR Health Survey estimates a lower frequency of AM in the breed, 0.68/100 (28). This, however, may be an underestimate since it is not based upon statistical methods and thus may be biased by the fact that cases are self-reported and that breeders may want to conceal information. On the other hand, AM may very well represent a minor problem in the Canadian NSDTR population given the larger pool of founder animals.
The results of the present study confirm that AM is a disease that strikes the young animal. None of the affected dogs were older than 19 mo when the first signs of the disease appeared. This is in accordance with previously published data (13–15). The relationship between AM and young age may be connected with immune-mediated mechanisms, as the immune system is more easily activated in youth as opposed to the less active immune response seen in old age.
In this study, none of the 9 dogs suspected of being AM positive had a CSF examination. We acknowledge that identification of the cellular changes of the CSF that are often associated with especially the acute form of AM, would have supported the presumptive diagnosis of AM in these dogs, and thus would have strengthened the conclusions of this study (8,17,21). However, because of the need to search for historical cases, it was not possible to include CSF examination as a standard criterion. The practising veterinarians established the diagnosis of AM based on the clinical signs of neck pain, fever, the young age of the dogs, and by ruling out other diseases that present with neck pain — the main potential differential diagnoses being disc herniation and polyarthritis of the cervical facet joints. Going back in time, CSF collection and examination were not standard procedures at most clinics in Norway, and referrals to a neurologist are even today often hindered due to difficult geographical conditions.
As a rule, AM patients respond well to steroid therapy, as did the dogs in this study. This may support the suspicion of AM, although we acknowledge that other diseases that present with similar signs as AM do also respond to such therapy.
The main objective of the study was to investigate if clinical signs of AM existed in the population between 1994 and 2003, and to estimate the prevalence of AM in the Norwegian population. For this purpose, the clinical data with all 9 dogs being younger than 19 mo old, expressing multiple clinical signs associated with AM, and having a clinical diagnosis of AM established by a veterinarian, are strong enough to support the belief that these dogs probably did suffer from AM. It is, however, important to stress that the reported prevalence of this study is indeed an estimate.
For all the affected dogs, it was possible to trace the pedigree of both parents back to a specific founder dog. The pedigree studies indicate that genetic factors are involved in the etiology of the disease. However, it is not possible to prove a simple Mendelian inheritance in the present study, even if this cannot be excluded. Studies of pedigrees in small dog populations are easily confounded by the fact that small subpopulations of breeds of a relatively recent origin are often based on extensive line-breeding of a relatively small number of founder animals. Both dogs affected by specific diseases as well as unaffected controls will often be related to these ancestors. Special attention should, therefore, be taken to avoid making conclusions about simple inheritance based on pedigree information in such populations. A closer look into the affected litters identified in this study shows that the percentage of affected dogs is < 17.4%. For diseases with a simple recessive mode of inheritance, a frequency > 25% would be expected in population studies. This is due to the fact that litters without affected dogs, born from 2 carrier parents, are not included in such frequency estimates. If we assume that the population study picked up all affected dogs closely related to the probands, the number of affected (17.4%) is significantly lower than would have been expected in a population study with segregation of a simple recessive disease with complete penetrance.
The fact that the incidence of AM is high in this breed and accumulates within families, strongly suggests that the etiology of this disease is influenced by genetic factors. The existence of a specific ancestor for both parents of affected dogs could support a simple Mendelian recessive inheritance even if the frequency is lower than expected in a population study. However, this might be explained under a model with reduced penetrance, if potential environmental factors such as vaccination, infection, or parasites are involved in triggering the disease.
It would be interesting to pursue the hypothesis of AM having an autosomal recessive mode of inheritance in the NSDTR. This could be initiated by pedigree analysis and linkage analysis leading to gene mapping and identification of mutations. In this context, it would be of specific interest to study previous and potential new litters of carrier dogs. Collecting information of early cases of AM in the NSDTRs native country of Canada would also be important when trying to unravel the genetic mechanisms contributing to the spread of the disease within the breed.
In conclusion, we have a strong suspicion that AM represents at true problem in the NSDTR. Based upon the findings of this study, we suggest that AM affected animals should not be bred.
Acknowledgment
Support from the Norwegian Kennel Club is gratefully acknowledged.
References
- 1.Kelly DF, Grunsell CSG, Kenyon CJ. Polyarteritis in the dog: A case report. Vet Rec. 1973;92:363–366. doi: 10.1136/vr.92.14.363. [DOI] [PubMed] [Google Scholar]
- 2.Brooks PN. Necrotizing vasculitis in a group of Beagles. Lab Anim. 1984;18:285–290. [Google Scholar]
- 3.Albassam MA, Houston BJ, Greaves P, Barsoum N. Polyarteritis in a Beagle. J Am Vet Med Assoc. 1989;194:1595–1597. [PubMed] [Google Scholar]
- 4.Scott-Moncrieff JCR, Snyder PW, Glickman LT, Davis EL, Felsburg PJ. Systemic necrotizing vasculitis in nine young Beagles. J Am Vet Med Assoc. 1992;201:1553–1558. [PubMed] [Google Scholar]
- 5.Harcourt RA. Polyarteritis in a colony of beagles. Vet Rec. 1978;102:519–522. doi: 10.1136/vr.102.24.519. [DOI] [PubMed] [Google Scholar]
- 6.Kemi M, Usui T, Namara I, Takahashi R. Histopathology of spontaneous panarteritis in Beagle dogs. Jap J Vet Sci. 1990;52:55–61. doi: 10.1292/jvms1939.52.55. [DOI] [PubMed] [Google Scholar]
- 7.Meric SM, Child G, Higgins RJ. Necrotizing vasculitis of the spinal pachyleptomeningeal arteries in three Bernese Mountain dog littermates. J Am Anim Hosp Assoc. 1986;22:459–465. [Google Scholar]
- 8.Tipold A, Jaggy A. Steroid responsive meningitis-arteritis in dogs: Long-term study of 32 cases. J Small Anim Pract. 1994;35:311–316. [Google Scholar]
- 9.Tipold A, Vandevelde M, Zurbriggen A. Neuroimmunological studies in steroid-responsive meningitis-arteritis in dogs. Res Vet Sci. 1995;58:103–108. doi: 10.1016/0034-5288(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 10.Snyder PW, Kazacos EA, Scott-Moncrieff JC, et al. Pathologic features of naturally occurring juvenile polyarteritis in beagle dogs. Vet Pathol. 1995;32:337–345. doi: 10.1177/030098589503200401. [DOI] [PubMed] [Google Scholar]
- 11.Hoff EJ, Vandevelde M. Necrotizing vasculitis in the central nervous systems of two dogs: Case report. Vet Pathol. 1981;18:219–223. doi: 10.1177/030098588101800209. [DOI] [PubMed] [Google Scholar]
- 12.Tipold A, Moore P, Zurbriggen A, Vandevelde M. Lymphocyte subset distribution in steroid responsive meningitis-arteritis in comparison to different canine encephalitides. Zentralbl Veterinarmed A. 1999;46:75–86. doi: 10.1046/j.1439-0442.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 13.LeCouteur RA, Grandy JL. Diseases of the spinal chord. In: Ettinger SJ, Feldman EL, editors. Textbook of Veterinary Internal Medicine. 6. St. Louis: Elsevier Saunders; 2005. p. 856. [Google Scholar]
- 14.Meric S, Perman V, Hardy R. Corticosteroid-responsive meningitis in ten dogs. J Am Anim Hosp Assoc. 1985;21:677–684. [Google Scholar]
- 15.Irving G, Chrisman C. Long-term outcome of five cases of corticosteroid-responsive meningomyelitis. J Am Anim Hosp Assoc. 1990;26:324–328. [Google Scholar]
- 16.Presthus J. Aseptic suppurative meningitis in Bernese Mountain dogs. Euro J Comp Anim Pract. 1991;1:24–28. [Google Scholar]
- 17.Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: A retrospective study. J Vet Intern Med. 1995;9:304–314. doi: 10.1111/j.1939-1676.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 18.Poncelet L, Balligand M. Steroid responsive meningitis in three boxer dogs. Vet Rec. 1993;132:361–362. doi: 10.1136/vr.132.14.361. [DOI] [PubMed] [Google Scholar]
- 19.Hayes TJ, Roberts GK, Halliwell WH. An idiopathic febrile necrotizing arteritis syndrome in the dog: Beagle pain syndrome. Toxicol Pathol. 1989;17:129–137. doi: 10.1177/019262338901700109. [DOI] [PubMed] [Google Scholar]
- 20.Spencer A, Greaves P. Periarteritis in a Beagle colony. J Comp Pathol. 1987;97:121–128. doi: 10.1016/0021-9975(87)90031-4. [DOI] [PubMed] [Google Scholar]
- 21.Gandini G, Brini E, Bellotti D, Cipone M. Clinical and clinico-pathologic findings in three dogs with steroid responsive meningitis arteritis (SRMA) Vet Res Commun. 2003;27:763–765. doi: 10.1023/b:verc.0000014266.47886.8e. [DOI] [PubMed] [Google Scholar]
- 22.Tipold A, Somberg R, Felsburg P. Involvement of a superantigen in sterile purulent meningitis and arteritis of dogs. Tierarzt1 Prax. 1996;24:514–518. [PubMed] [Google Scholar]
- 23.Burgener I, Van Ham L, Jaggy A, Vandevelde M, Tipold A. Chemotactic activity and IL-8 levels in the cerebrospinal fluid in canine steroid responsive meningitis-arteritis. J Neuroimmunol. 1998;89:182–190. doi: 10.1016/s0165-5728(98)00134-9. [DOI] [PubMed] [Google Scholar]
- 24.Cizinauskas S, Jaggy A, Tipold A. Long-term treatment of dogs with steroid-responsive meningitis-arteritis: Clinical, laboratory and therapeutic results. J Small Anim Pract. 2000;41:295–301. doi: 10.1111/j.1748-5827.2000.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 25.Dougherty SA, Center Sa, Shaw EE, Erb HA. Juvenile-onset polyarthritis syndrome in Akitas. J Am Vet Med Assoc. 1991;198:849–856. [PubMed] [Google Scholar]
- 26.Meric SM. Canine meningitis — a changing emphasis. J Vet Intern Med. 1988;2:26–35. doi: 10.1111/j.1939-1676.1988.tb01974.x. [DOI] [PubMed] [Google Scholar]
- 27.Berendt M, Gredal H, Pedersen LG, Alban L, Alving J. A Cross-sectional study of epilepsy in Danish Labrador Retrievers: Prevalence and selected risk factors. J Vet Int Med. 2002;16:262–268. doi: 10.1892/0891-6640(2002)016<0262:acsoei>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 28.NSDTR Club of Canada. [Last accessed 20 April 2008]; [homepage on the Internet]. 2002 Nova Scotia Duck Tolling Retriever Health Survey. Available from www.toller.ca.