Abstract
One significant clinical symptom of familial hypobetalipoproteinemia [FHBL] due to defects in apolipoprotein B (apoB) is steatohepatosis. However, the increased hepatic fat content in apoB-related FHBL subjects was not associated with glucose intolerance, in contrast with what is the case in the metabolic syndrome. Meanwhile, in human subjects with similar apoB truncations, degree of obesity and insulin sensitivity, their liver triglyceride (TG) contents may vary considerably, suggesting that, in addition to defective apoB, other genes may affect the magnitude of hepatic TG accumulation. We hypothesized that genetic background affects the severity of hepatic steatosis and the expression of insulin sensitivity. To test the hypotheses, mouse apoB38.9-bearing congenies were bred under high, medium and low liver triglyceride (TG) backgrounds using “speed congenics” approach. These mice were fed on regular diet for 12 weeks. Their insulin sensitivity, serum and liver lipids were assessed. The highest liver fat strain [BALB/cByJ] accumulated significantly higher TG in the liver under apoB38.9 heterozygous condition, while the lowest liver fat strain [SWR/J] had the smallest liver TG change, suggesting that the genetic backgrounds affected the hepatic TG responses to the presence of the apoB38.9 mutation. Interestingly, only the low liver fat strain [SWR/J-apoB38.9] showed significant upward shifts of both glucose tolerance test (GTT) and insulin tolerance test (ITT) curves. Neither the glucose nor the insulin tolerance curves were altered in the two cognate congenics with higher liver fat content [BALB/cByJ and C57BL/6J]. Thus, hepatic TG contents and measures of glucose metabolism were dissociated from each other. It is tempting to conclude that hepatic TG per se may not be responsible for the insulin resistance seen in fatty liver. The genetic/molecular bases for the differences between SWR/J and the other two strains with respect to their glucose metabolic responses to increases in hepatic TG contents remain to be elucidated.
Introduction
Familial hypobetalipoproteinemia [FHBL] in its heterozygous form is a relatively mild condition characterized by low plasma concentrations of total- and LDL-cholesterol that segregates in most families as a Mendelian codominant. Its underlying genetic basis is unknown in most families, but several genes and susceptibility loci have been identified.1 One of the genes identified with FHBL specifies the production of apolipoprotein B100 [apoB100], a protein which in its mature form contains 4536 amino acid residues. Lipids stored in liver, combine with apoB100 in the endoplasmic reticulum [ER] and Golgi apparatus to form VLDL particles that are secreted into plasma. Thus, apoB100 is an integral, structural part of VLDL.2 Another form of apoB, apoB48, is secreted from intestine as part of chylomicrons. The assembly of chylomicrons in enterocytes resembles the assembly of VLDL in hepatocytes.3
As VLDL is converted to LDL in plasma, apoB100 remains with the metabolizing particles. Thus, apoB100 is the major protein associated with LDL.
Steatohepatosis in FHBL due to ApoB Mutations
C-terminal truncation-inducing mutations of apoB100 yield smaller than normal VLDL particles, with reduced capacities to bind TG.1 The small particles ferry suboptimal amounts of triglycerides [TG] from liver. In heterozygous individuals harboring truncation-producing mutations apoB, the product of the normal allele apoB100 is also produced at reduced rates.4 The two physiologic perturbations of the VLDL export system, i.e. small particles and reduced numbers of normal particles, combine to produce the retention of TG in hepatocytes. Another set of mutations, missense mutations of apoB in the 400–500 amino acid residue region, interferes with the productive association of lipid with the mutated apoB100 protein. As a result, reduced numbers of VLDL particles are formed and secreted.5
Domains near amino residue position 3500 of apoB100 serve as recognition sites for the LDL receptor, which is responsible for clearing LDL from plasma. Missense mutations in the LDL receptor recognition domain are associated with delayed clearance of LDL from plasma, and hypercholesterolemia.6 We shall limit the discussion here to FHBL due to mutations of apoB.
Hypotheses Based on Human Studies
Our human subjects with FHBL due to genetically defective forms of apoB accumulate more hepatic TG than controls, despite the similar mean levels of body mass index[BMI], similar masses of abdominal subcutaneous and intra-peritoneal adipose tissue, and similar glucose and insulin curves during oral glucose tolerance tests in the two groups.7, 8 This suggests that our FHBL subjects are especially susceptible to developing fatty liver. Moreover, the increased hepatic fat content in apoB-related FHBL subjects was not associated with glucose intolerance, in contrast with what is the case in the metabolic syndrome.7
Meanwhile, hepatic fat contents in humans with similar apoB truncations, and similar measures of obesity and glucose tolerance, may vary considerably, suggesting that, in addition to defective apoB, other genes may affect the magnitude of hepatic TG accumulation.9, 10 It is also likely that genetic background affects the expression of insulin sensitivity.
Mouse Studies
Hypotheses on the effects of genetic background on the extent of fatty liver and glucose tolerance are impossible to test formally in freely out-breeding human populations. Therefore, we used congenic mouse strains bearing the identical apoB truncation-producing mutation, apoB38.9, in differing genetic backgrounds.
Three steps were required to obtain congenic strains:
A mouse model of apoB38.9-FHBL was produced by site-directed mutagenesis, followed by homologous recombination in mouse stem cells using the lox-P-Cre system.11 This yielded a strain of apoB38.9-bearing mice in a mixed genetic background [∼50% C57BL/6J and 50% 129X1/SvJ], with low plasma cholesterol and high hepatic TG levels, resembling human FHBL.
To identify strains with varying basal hepatic TG contents, we surveyed ten strains and found that BALB/cByJ, C57BL6/J, and SWR/J, had high, intermediate, and low hepatic TG levels, respectively, demonstrating that genetic factors were important in setting hepatic TG contents.10
Finally, we bred the apoB38.9 mutation into each of the above three strains by backcrosses using genetic marker assisted selection, and tested the offspring for homozygosity at the markers known to differentiate among the strains [yielding “speed congenics”]. Since homozygosity at the apoB38.9 locus tends to be lethal in utero in mice, the data presented were derived from mice heterozygous for apoB38.9.
Results
Table 1 contains data on hepatic and serum lipids in both genders of our three congenic strains. The wildtype strains, BALB-apoB+/+, C57BL-apoB+/+, and SWR-apoB+/+ differed significantly with respect to hepatic TG levels [123, 79, and 46 mg/gm liver protein, respectively]. Analogous results were seen in females. The inter-strain differences in hepatic TG were likely due to differences in hepatic TG synthesis.10
TABLE 1.
| Strain | N | Liver TG mg/gm | Liver CHOL mg/gm | Serum TG mg/dL | Serum CHOL mg/dL |
|---|---|---|---|---|---|
| Female | |||||
| BALB-apoB+/+ | 38 | 123 (44)bC | 13.1 (1.7)bC | 86 (27)A | 61 (15)aA |
| BALB-apoB+/38.9 | 55 | 243 (57)aA | 16.4 (2.7)aA | 88 (33)A | 42 (14)bC |
| C57-apoB+/+ | 39 | 79 (26)bD | 14.4 (2.3)bBC | 56 (20)B | 64 (12)aA |
| C57-apoB+/38.9 | 49 | 157 (29)aB | 16.3 (2.4)aA | 61 (28)B | 50 (10)bBC |
| SWR-apoB+/+ | 12 | 46 (23)bE | 14.3 (1.5)bBC | 97 (29)A | 56 (10)aAB |
| SWR-apoB+/38.9 | 21 | 101 (29)aCD | 15.7 (2.2)aAB | 90 (29)A | 32 (14)bD |
| Male | |||||
| BALB-apoB+/+ | 23 | 132 (41)bBC | 13.2 (1.5)BC | 106 (30)A | 85 (21)aA |
| BALB-apoB+/38.9 | 49 | 287 (130)aA | 14.7 (2.6)AB | 93 (38)AB | 66 (23)bB |
| C57-apoB+/+ | 41 | 74 (28)bCD | 12.6 (2.0)C | 74 (23)B | 74 (15)AB |
| C57-apoB+/38.9 | 24 | 151 (44)aB | 13.9 (2.1)ABC | 76 (20)B | 71 (9)AB |
| SWR-apoB+/+ | 10 | 36 (14)bD | 14.2 (1.7)ABC | 84 (18)AB | 68 (21)AB |
| SWR-apoB+/38.9 | 12 | 62 (27)aD | 15.2 (1.3)A | 89 (19)AB | 60 (8)B |
1 Plasma and hepatic lipids were assessed using commercial methods described in 10. Liver triglyceride and cholesterol were expressed as mg/g liver protein. Results are means (SD).
2 The low-case superscript in each column (a and b) indicated significant difference (α = 0.01) between apoB genotype (apoB+/+ vs apoB+/38.9) within each genetic background (BALB, C57, or SWR). The upper-case superscript in each column (A, B etc) indicated the results of multiple comparisons among means of all congenics (Duncan's multiple range test, α = 0.01).
Hepatic TG levels were increased in each of the respective congenic strain [e.g. BALB-apoB+/+ vs. BALB-apoB+/38.9, etc.]. Differences in hepatic TG due to apoB38.9 were due to reduced hepatic TG secretion, compared with cognate wildtypes.4, 12 The delta increases in hepatic TG were significantly different among the strains [p < 0.001], and there were statistically significant interactions by strain and by apoB mutation [p < 0.001 for each gender], suggesting that the genetic backgrounds affected the hepatic TG responses to the presence of the apoB38.9 mutation. Similar to human apoB-related FHBL, the serum cholesterol levels were lower in congenics than in their cognate wildtypes [Table 1].
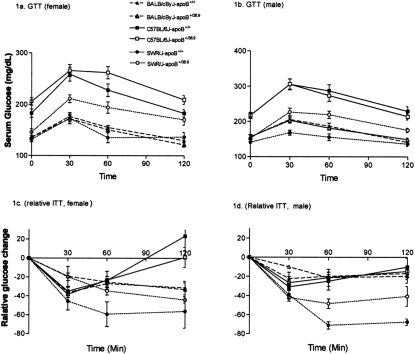
The glucose and insulin tolerance curves are shown in Figure 1. Body weight changes and areas under the intra-peritoneal glucose tolerance curves [AUC] are provided in Table 2. Within each strain, apoB38.9 truncation did not cause significant increase of body weight or body fat composition measured by a quantitative NMR system. Liver weight increased in female apoB+/38.9 mice, probably due to high liver fat content in these mice. The C57BL-apoB+/+ wildtypes had the most abnormal curves, although the BALB-apoB+/+ had nearly twice the hepatic TG contents. Neither the glucose nor the insulin tolerance curves were altered in the two cognate congenics. SWR-apoB+/+ had the lowest glucose levels among the three wildtype strains, but the presence of apoB38.9 in SWR-apoB+/38.9 resulted in significant upward shifts of both tolerance curves. Thus, hepatic TG contents and measures of glucose metabolism were dissociated from each other. Compatible findings, i.e. no changes in insulin sensitivity, were reported in mice over-expressing DGAT, the enzyme catalyzing hepatic TG synthesis, despite the resultant seventeen-fold increases in hepatic TG contents.13, 14
Fig. 1.
Congenic strains showed different insulin sensitivity by Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT). Procedures of GTT and ITT were described in 10 (1a) and (1b) showed 2-hours glucose curves for female and male congenic mice. (1c) and (1d) were relative plasma glucose changes over time (against baseline) for female (1c) and male (1d) after mice were administrated insulin intra-peritoneally at a rate of 0.75U/kg body weight.
TABLE 2.
Physiological alternations of apoB38.9-bearing congenic strains
| Strain | N | Body weight (g) | Liver weight (g) | Body Fat Content (%) | AUC-GTT | 30 min Insulin (ng/mL) |
|---|---|---|---|---|---|---|
| Female | ||||||
| BALB-apoB+/+ | 31 | 19.7 (5.1)A | 0.92 (0.22)aAB | 15.48 (1.68) | 31.9 (29.1)B | 0.8 (0.3)A |
| BALB-apoB+/38.9 | 33 | 20.7 (3.9)A | 1.08 (0.18)bA | 16.61 (2.95) | 21.4 (40.4)B | 0.6 (0.3)AB |
| C57-apoB+/+ | 24 | 19.2 (1.7)A | 0.83 (0.19)BC | 16.12 (3.69) | 72.0 (64.5)A | 0.5 (0.3)AB |
| C57-apoB+/38.9 | 40 | 20.7 (3.8)A | 0.92 (0.20)AB | 15.60 (1.49) | 74.0 (76.9)A | 0.6 (0.3)AB |
| SWR-apoB+/+ | 14 | 16.9 (0.8)B | 0.76 (0.07)aC | 15.51 (1.97) | 25.9 (44.2)Bb | 0.3 (0.1)B |
| SWR-apoB+/38.9 | 22 | 17.6 (1.7)B | 0.85 (0.09)bB | 15.61 (1.07) | 81.5 (49.5)Aa | 0.4 (0.3)AB |
| Male | ||||||
| BALB-apoB+/+ | 16 | 24.9 (6.7)A | 1.28 (0.44)A | 18.41 (1.59)Bb | 40.9 (39.5)B | 1.6 (0.9)A |
| BALB-apoB+/38.9 | 39 | 25.3 (6.1)A | 1.30 (0.37)A | 21.44 (1.66)Aa | 38.1 (36.9)B | 1.3 (0.6)AB |
| C57-apoB+/+ | 23 | 23.9 (2.2)A | 1.06 (0.13)B | 17.35 (0.74)BC | 101.9 (77.0)A | 1.0 (1.4)AB |
| C57-apoB+/38.9 | 34 | 23.8 (3.3)A | 1.06 (0.10)B | 14.73 (1.89)D | 85.6 (73.6)AB | 0.9 (0.3)AB |
| SWR-apoB+/+ | 16 | 19.9 (0.9)B | 1.02 (0.10)B | 15.53 (1.33)D | 22.1 (57.8)Bb | 0.5 (0.3)B |
| SWR-apoB+/38.9 | 23 | 20.1 (1.8)B | 1.04 (0.11)B | 16.41 (0.17)CD | 97.0 (69.9)Aa | 0.5 (0.1)B |
1 The number is expressed as Mean(SD).
2 Multiple comparisons were conducted at two levels: different small letters indicated significant differences (α = 0.01) between apoB wild-type and heterozygous within each strain; different capital letter indicated significant differences among genotypes within each gender (Duncan's multiple test, α =
It is tempting to conclude that hepatic TG per se may not be responsible for the insulin resistance seen in fatty liver. The genetic/molecular bases for the differences between SWR and the other two strains with respect to their glucose metabolic responses to increases in hepatic TG contents remain to be elucidated.
DISCUSSION
Chan, Houston: Gus, this was a really wonderful study from humans to mice and really tells a lot about the pathogenesis of the disorder. I was wondering whether you looked at the intracellular accumulation of fat, whether it is cytosolic or is in some of the organelles and things like. That, of course, I think that might also play a role in terms of the insulin resistance. The second question is what about the role of MTP in all these?
Schonfeld, St. Louis: Well, we've not done electron microscopic studies on these livers, at least not very systematically, but the few studies that we have done indicate that it's mostly cytosolic fat. It's not in the endoplasmic reticulum or in the mitochondria or anything like that. As to the role of MTP, we've done some micro arrays; the MTP mRNA doesn't seem to be altered by much.
Chan, Houston: MTP is, as you know, rated mostly at a post-translational level. So, actually, you might look at the protein level, as this could play a role.
Schonfeld, St. Louis: Yes, thank you.
Boxer, Ann Arbor: The same sort of phenomenon that you described has been observed with expression of von Willebrand's disease, both in mice and in humans with the ABO blood group. Do you have any plans to identify them, modifier genes, and if so, how do you plan to approach it?
Schonfeld, St. Louis: Well there are years of work to be done.
Boxer, Ann Arbor: Well, your next grant.
Schonfeld, St. Louis: There are a couple of approaches. One is to do crosses to identify what the modifier genes are. There are these F2-type crosses that you can do, and they give you an idea. It's sort of like a linkage study. The other is based on some of these physiologic studies on triglyceride synthesis and secretion. One can look at candidate genes in those pathways and see what's effective. The way we've begun it is by doing the micro-arrays, and there are a number of significant changes, differences by strain and also by the presence of the mutation.
Abboud, Iowa City: Can you shed some light on the separation of fatty liver from insulin resistance in these models without mechanistic reasons for that?
Schonfeld, St. Louis: Well, insulin resistance, as you know, is sort of a big Pandora's box. It may be related to faulty intracellular signaling, which in turn, may be related to phosphorylation of proteins in the signaling cascades, at specific sites; signaling is also related to the accumulation/depletion of certain lipids, such as fatty acylCoAs, and phospholipids. Ceramide may be one of them. So there are a number of different molecules one could examine, in a number of different pathways. That is certainly one of the areas that should be the next step in the research. In fact, that was the main reason to try to develop a mouse where you could disassociate mean fatty liver from insulin resistance.
Abboud, Iowa City: What about the skeletal muscle fat in these models? Have you looked at that?
Schonfeld, St. Louis: We've not looked at muscle in the mice. We've looked in humans. You can do MR spectroscopy in the human muscle, and there doesn't seem to be any more intra-myocellular fat in most humans. It's not like the metabolic syndrome.
Thorner, Charlottesville: Gus, that was a beautiful presentation. I was going to ask the question that Frank asked, but I guess I would like to take it one step further. What happens with visceral fat?
Schonfeld, St. Louis: In these mice?
Thorner, Charlottesville: In the mice or in the humans?
Schonfeld, St. Louis: Well, in humans, as I showed a slide where we did visceral fat in the humans by MRI, quantifying fat in cross sections of the abdomen, there was no difference in abdominal fat in those groups. We've not done the mice. The only thing we've done in the mice is to look at total body weight and total body fat, and the total body fat is no different.
Thorner, Charlottesville: So do you think this helps? I mean in the metabolic syndrome, people argue that visceral fat is a very important component of the development of insulin resistance, and I've always wondered whether it is an epiphenomenon or whether it is actually pathophysiologically related.
Schonfeld, St. Louis: This is not my area of expertise. Maybe Larry Chan wants to talk about this. My understanding of the literature is that adipose tissue secretes a lot of adipokines and cytokines and inflammatory molecules, and those molecules have direct effects on insulin resistance. So it's probably not an epiphenomenon. I wanted to look at this question of fatty liver and insulin resistance in a situation where you could dissociate the amount of triglyceride in the liver from insulin resistance, and I think we've accomplished that in these models. So now we have to assess any differences at the molecular level. That is the next stage of research.
Crowley, Boston: That is very interesting. I think your last point is critical in the sense of disassociating this from insulin resistance. There is undoubtedly a very important lesson there. Two suggestions, just as to follow up on the approaches: Number one, in terms of crossbreeding these mice, you could go chromosome by chromosome in one crossbreed. It seems like an awfully quick way to get from where you are into the gene locations, at least by chromosome with the crossbreed. Have you considered that? The second thing is that in terms of your expression studies, you do have complete knockout mice, albeit at a low level of fertility, but they would seem to be the best place to go for deriving cells lines to do your gene expression studies and to look for the specific pathways. Have you considered that?
Schonfeld, St. Louis: We've considered that and we do have two or three mice. We've been sort of hoarding them like diamonds. You are right. This is what we have to do. The problem is that you have to breed several hundred mice just to get a couple of homozygotes.
Crowley, Boston: Yeah, but then you've got cell lines from them. I mean, once you have one or two of them, then you can start to look tissue by tissue with the gene arrays if you regenerate the cell lines. And then the speed congenics is the issue that gets you to the chromosomes really quickly rather than a series or more elaborate crossbreeds. I think you are outlining a more elaborate crossbreeding, but that is a little tedious now. With these single chromosomes, you can land on a couple of them pretty quickly.
REFERENCES
- 1.Schonfeld G, Lin X, Yue P. Familial hypobetalipoproteinemia: genetics and metabolism. Cell Mol Life Sci. 2005;62(12):1372–8. doi: 10.1007/s00018-005-4473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277(20):17377–80. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 3.Levy E, Stan S, Delvin E, et al. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J Biol Chem. 2002;277(19):16470–7. doi: 10.1074/jbc.M102385200. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Fitzgerald RL, Li G, Davidson NO, Schonfeld G. Hepatic secretion of apoB-100 is impaired in hypobetalipoproteinemic mice with an apoB-38.9-specifying allele. J Lipid Res. 2004;45(1):155–63. doi: 10.1194/jlr.M300275-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Burnett JR, Shan J, Miskie BA, et al. A novel nontruncating APOB gene mutation, R463W, causes familial hypobetalipoproteinemia. J Biol Chem. 2003;278(15):13442–52. doi: 10.1074/jbc.M300235200. [DOI] [PubMed] [Google Scholar]
- 6.Boren J, Ekstrom U, Agren B, Nilsson-Ehle P, Innerarity TL. The molecular mechanism for the genetic disorder familial defective apolipoprotein B100. J Biol Chem. 2001;276(12):9214–8. doi: 10.1074/jbc.M008890200. [DOI] [PubMed] [Google Scholar]
- 7.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45(5):941–7. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Schonfeld G, Patterson BW, Yablonskiy DA, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003;44(3):470–8. doi: 10.1194/jlr.M200342-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Yue P, Tanoli T, Wilhelm O, Patterson B, Yablonskiy D, Schonfeld G. Absence of fatty liver in familial hypobetalipoproteinemia linked to chromosome 3p21. Metabolism. 2005;54(5):682–8. doi: 10.1016/j.metabol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Yue P, Chen Z, Schonfeld G. Hepatic triglyceride contents are genetically determined in mice: results of a strain survey. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1179–89. doi: 10.1152/ajpgi.00411.2004. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Fitzgerald RL, Averna MR, Schonfeld G. A targeted apolipoprotein B-38.9-producing mutation causes fatty livers in mice due to the reduced ability of apolipoprotein B-38.9 to transport triglycerides. J Biol Chem. 2000;275(42):32807–15. doi: 10.1074/jbc.M004913200. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Schonfeld G, Yue P, Chen Z. Hepatic fatty acid synthesis is suppressed in mice with fatty livers due to targeted apolipoprotein B38.9 mutation. Arterioscler Thromb Vasc Biol. 2002;22(3):476–82. doi: 10.1161/hq0302.105271. [DOI] [PubMed] [Google Scholar]
- 13.Monetti M, Levin MC, Watt MJ, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6(1):69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Choi CS, Savage DB, Kulkarni A, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282(31):22678–88. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]