Bioassay-guided fractionation of the EtOH extracts obtained from a plant identified as Didymochlaena truncatula led to the isolation of two cytotoxic alkaloids, camptothecin and 9-methoxycamptothecin. A second plant collection yielded three lignan derivatives, didymochlaenone A (1), didymochlaenone B (2) and (−)-wikstromol, one stilbene, (E)-3-methoxy-5-hydroxystilbene, and two stigmasterol derivatives, stigmast-4-en-3β-ol and stigmast-4-en-3-one, but no camptothecins, and it is probable that a coding error led to a mistaken identification of the original extract. The structures of the new compounds 1 and 2 were established on the basis of extensive interpretation of one and two dimensional NMR spectroscopic data.
In our continuing search for bioactive molecules from the Madagascar rainforests as part of an International Cooperative Biodiversity Group (ICBG) program,2 we obtained a cytotoxic extract (MG 1736A) from the roots of a plant collected in forest adjacent to the Zahamena National Park and identified as Didymochlaena truncatula (Sw.) J. Sm (Dryopteridaceae) collected in Madagascar. This extract, with an IC50 value of 3.9 µg/mL, was selected for bioassay-guided fractionation based on its cytotoxicity against the A2780 human ovarian cancer cell line, and also on the absence of any previous detailed phytochemical studies on the genus. The crude extract furnished the two known active alkaloids camptothecin3 and 9-methoxycamptothecin3d as the active constituents. A large recollection (MG 1736B) of D. truncatula was then obtained in an attempt to isolate other cytotoxic alkaloids analogous to camptothecin and 9-methoxycamptothecin, but surprisingly this collection did not contain detectable levels of camptothecins. Instead, four aromatic compounds and two stigmasterol derivatives were isolated as the weakly active constituents of this collection.
The second collected sample (MG 1736B) of D. truncatula was subjected to liquid-liquid partitioning to give a moderately active CH2Cl2 fraction with an IC50 value of 16 µg/mL in the A2780 assay. Activity-guided purification of this fraction by passage over a C18 open column and subjection of active fractions to further purification using C18 HPLC and PTLC led to the isolation of the two new compounds 1 and 2 and the four known compounds (−)-wikstromol,4 (E)-3-methoxy-5-hydroxystilbene,5 and the two stigmasterol derivatives, stigmast-4-en-3β-ol6 and stigmast-4-en-3-one.7 Here we report the structures of the two new components, didymochlaenone A (1), didymochlaenone B (2), as well as the activity of the isolates.
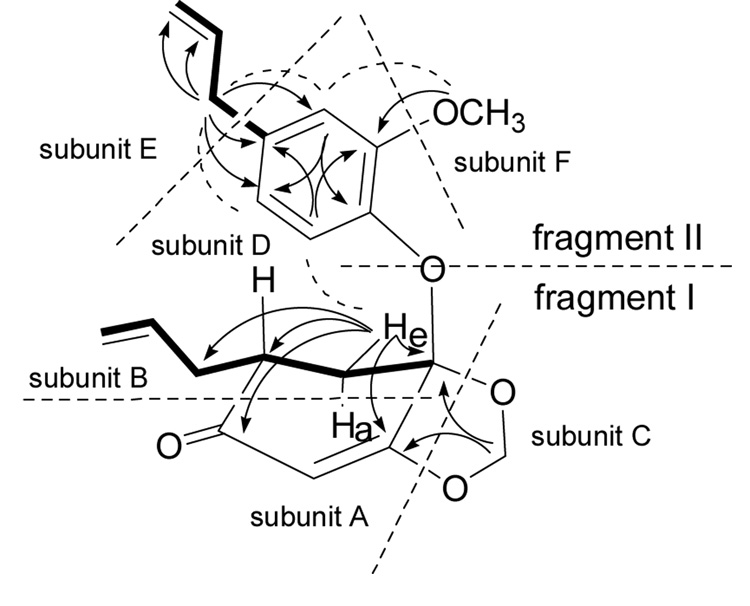
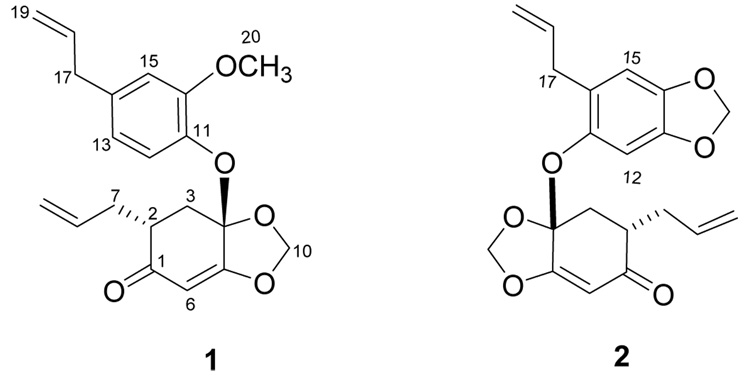
Didymochlaenone A (1) was obtained as a colorless oil. Its positive HRFABMS revealed a pseudo-molecular ion [(M+H)+] consistent with the molecular formula C20H22O5, requiring ten double-bond equivalents. The IR spectrum for 1 displayed a characteristic absorbance for an α,β-unsaturated ketone moiety (1653 cm−1),8 while the UV spectrum exhibited absorbances compatible with an oxygenated benzene ring and/or an α,β-unsaturated ketone moiety (283 and 246 nm).8 1H and 13C NMR spectra (Table 1) and DEPT data indicated the presence of three aromatic protons, four methines (three olefinic and one aliphatic), six methylenes (two olefinic, one di-oxygenated, and three aliphatic), one methoxy, and six quaternary carbons. The homonuclear 2D NMR COSY (Fig. 1) and TOCSY data for 1 identified connectivity sequences indicative of three coupling systems: subunit B: H2-3 through H-2, H2-7, and H-8 to H2-9 (CH2=CH-CH2-CH-CH2); subunit D: H-12 through H-13 to H-15 (a 1,2,4-trisubstituted benzene ring); subunit E: H2-17 through H-18 to H2-19 (an allyl group). Heteronuclear 2D NMR correlations [HSQCED and HMBC (Fig. 1)] permitted connection of subunits A-C (H-3e to C-1, C-5, C-7; H-6 to C-2, C-4; H2-10 to C-4, C-5), and subunits D-F (H-12 to C-14, C-16; H2-17 to C-13, C-14, C-15; H3-20 to C-16). Using this, two fragments, I and II, could be proposed for didymochlaenone A (1). Based on its molecular formula, it was concluded that fragments I and II were connected via an oxygen bridge between C-4 and C-11, which was confirmed by a ROESY correlation between H-12 and H-3e.
Table 1.
1H and 13C NMR Data (δ) for Compounds 1 and 2 in CDCl3
| no |
1 |
2 |
||
|---|---|---|---|---|
| 1H | 13C | 1H | 13C | |
| 1 | 199.7 | 199.4 | ||
| 2 | 2.47 m | 43.4 | 2.52 m | 43.2 |
| 3 | 3.05 br d (14.4) | 29.7 | 3.05 br d (14.7) | 30.2 |
| 2.17 dd (14.4, 8.0) | 2.20 dd (14.7, 8.5) | |||
| 4 | 103.5 | 103.1 | ||
| 5 | 168.7 | 169.0 | ||
| 6 | 5.57 br s | 101.0 | 5.60 br s | 100.7 |
| 7 | 2.60 m | 35.6 | 2.60 m | 36.2 |
| 2.40 m | 2.28 m | |||
| 8 | 5.54 m | 136.6 | 5.63 m | 136.2 |
| 9 | 4.82 br d (10.1) | 116.9 | 4.92 br d (10.5) | 117.6 |
| 4.46 br d (17.0) | 4.56 br d (16.3) | |||
| 10 | 5.56 br s | 98.5 | 5.52 br s | 98.8 |
| 5.49 br s | 5.22 br s | |||
| 11 | 140.9 | 143.2 | ||
| 12 | 7.30 d (8.1) | 119.5 | 6.86 br s | 100.7 |
| 13 | 6.70 dd (8.1, 2.2) | 120.4 | 146.0 | |
| 14 | 136.1 | 144.8 | ||
| 15 | 6.74 d (2.2) | 112.9 | 6.68 br s | 109.8 |
| 16 | 150.8 | 123.6 | ||
| 17 | 3.35 d (6.4) | 39.9 | 3.38 dd (15.2, 5.3) | 34.3 |
| 3.17 dd (15.2, 6.0) | ||||
| 18 | 5.94 m | 137.3 | 5.84 m | 136.4 |
| 19 | 5.09 br s | 115.9 | 5.04 br s | 116.1 |
| 5.07 br s | 5.02 br s | |||
| 20 | 3.77 s | 55.6 | 5.93 br s | 101.3 |
| 5.91 br s | ||||
Figure 1.
COSY (bold), key HMBC (arrows) and ROESY (dashed arc) correlations of 1
The α,β-unsaturated cyclohexanone ring had the same conformation as that of (2R)-illicinone F,9 a hemichair. This was evidenced by the fact that H-3e appeared as a broad doublet (J3e,3a = 14.4 Hz), indicating a small dihedral angle, while the coupling constant between H-2 and H-3a (J2,3a = 8 Hz) was consistent with a large dihedral angle, and hence H-2 was axial. Molecular modeling using the MM2 subprogram in Chem3D showed the dihedral angles H(2)-C(2)-C(3)-H(3a) and H(2)-C(2)-C(3)-H(3e) to be −177° and −60° respectively, consistent with the observed J2,3a of 8 Hz. The only possible alternate structure, with a syn orientation of the C-2 and C-4 substituents, was calculated to have dihedral angles of −45° and 69°, respectively; these values would require a much smaller J2,3a coupling constant. In addition, both H-2 and H-12 showed ROESY correlations to H-3e, but not H-3a, confirming the anti orientation of the C-2 and C-4—O—C-11 substituents. The CD spectrum of 1 showed similar positive Cotton effects at 240.9 and 314.3 nm, similar to those at 236 and 308 nm in (2R)-illicinone F,10 confirming that the absolute stereochemistry of 1 is (2R,4S).
Didymochlaenone B (2) was also obtained as a colorless oil, and its molecular formula was determined as C20H20O6 by HRFABMS and 13C NMR spectroscopy. Its 1H NMR spectrum was very similar to that of 1, but it contained resonances of an additional methylenedioxy instead of a methoxy group and a 1,2,4,5-tetrasubstotuted benzene ring instead of the 1,2,4-trisubstituted aromatic moiety in 1. The dioxymethylene group in fragment II of 2 was fused to the benzene ring at C-13/C-14, as H2-20 exhibited HMBC correlations with C-13 and C-14. The allyl group in fragment II of 2 was located at the 16-position since H2-17 showed a ROESY correlation with H-15. ROESY crosspeaks between H-3e and H-12, and between H-3e and H-2 were also observed, demonstrating the anti orientation of the C-2 and C-4—O—C-11 substituents. Hence, the structure of 2 was determined as shown.
The optical rotation and circular dichroism spectrum of 2 were approximately the opposite of those of 1, indicating that 2 most likely has the opposite stereochemistry to that of 1 at the 2 and 4 positions.10 Although it is unusual for a plant to produce two similar compounds with opposite stereochemistry, it is not unprecedented; thus, various Pinus sp. produce both (+) and (−)-α-pinene, and (+) and (−)-limonene both occur in peppermint.11
Camptothecin and 9-methoxycamptothecin isolated from the first collection (MG 1736A) were strongly cytotoxic, with IC50 values of 0.07 and 0.02 µg/mL, respectively, against the A2780 ovarian cancer cell line. All the isolated compounds from the second collection (MG 1736B) were evaluated for their cytotoxicity against the A2780 human ovarian cancer cell line. It was found that only (E)-3-methoxy-5-hydroxystilbene was active, with an IC50 value of 10 µg/mL.
As noted in the introduction, the bioactive compounds isolated from the first collection (MG 1736A) were not present in the second plant collection (MG 1736B). In the light of this surprising result, the two plant collections and the resulting two extracts were carefully compared. The herbarium specimens of each collection were compared and were confirmed to be of the same species. The two extracts did however show some major differences; not only were the camptothecins found in the first extract absent from the second extract, but also the major compounds found in the second extract were absent from the first extract. We thus concluded that a possible coding error may have occurred during the extraction process, and that the first extract could in fact be from another plant. The records of the extraction laboratory were used to identify plants which had been extracted at the same time as the original collection, and six extracts were identified as possible candidates for a coding error. These six extracts were all compared by HPLC analysis with the second and authentic D. truncatula extract, and extract MG 1726 (Coffea sp.) was found to correspond most closely with this extract. It is thus probable that the original extract that was coded as D. truncatula was in actuality a Coffea sp., and that this was the source of the camptothecins isolated. Unfortunately our collection work in the Zahamena region had ceased when this discovery was made, and it was not feasible to recollect the Coffea sp. to confirm this conclusion. It can be noted, however, that camptothecins have recently been shown to be produced by a fungus designated RJMEF001 isolated from Nothapodytes foetida,12 and it is thus very possible that the true source of these alkaloids is a fungal one.
Experimental Section
General Experimental Procedures
Optical rotations were recorded on a Perkin-Elmer 241 polarimeter. IR and UV spectra were measured on MIDAC M-series FTIR and Shimadzu UV-1201 spectrophotometers, respectively. CD analysis was performed on a JASCO-720 spectropolarimeter. NMR spectra were obtained on a JEOL Eclipse 500 and an Inova 400 spectrometer in CDCl3. The chemical shifts are given in δ (ppm), and coupling constants are reported in Hz. Mass spectra were obtained on a JEOL JMS-HX-110 instrument, in the positive ion mode. HPLC was performed on a Shimadzu LC-10AT instrument with a semi-preparative C18 and phenyl Varian Dynamax column (5 µm, 250 × 10 mm) and a preparative C18 Varian Dynamax column (8 µm, 250 × 21.4 mm). Finnigan LTQ LC/MS with a C18 Hypersil column (5 µm , 100 × 2.1 mm) was also used for crude sample analysis.
Cytotoxicity Bioassays
Cytotoxicity measurements were performed at Virginia Polytechnic Institute and State University against the A2780 ovarian cancer cell line as previously described.13,14 The A2780 cell line is a drug - sensitive ovarian cancer cell line.13
Plant Material
Roots of Didymochlaena truncatula (Sw.) J. Sm (Dryopteridaceae) were collected by Stephan Rakotonandrasana et al. 665 on December 2002, and Andrianjafy Mamisoa et al. 414 on 24 April 2004, 1 km north west of the village of Antenina, forest adjacent to the Zahamena National Park, in Toamasina Province, Madagascar (17. 30 25S; 48. 46. 03E, elevation ca. 860 m). Duplicate voucher specimens were deposited at Centre National d'Application des Recherches Pharmaceutiques (CNARP) and Direction des Recherches Forestieres et Piscicoles Herbarium in Antananarivo, Madagascar (TEF), Missouri Botanical Garden, St. Louis, Missouri (MO), and Museum National d'Histoire Naturelle in Paris, France (P).
Extraction and Isolation
Field-dried plant was ground in a hammer mill, then extracted by overnight percolation at room temperature with EtOH to give the crude extracts MG 1736A (first collection) and MG 1736B (second collection). MG 1736A (1 mg) was injected into a C18 Varian Dynamax column (5 µm; 250 ×10 mm; 2 mL/min 70% H2O to 100% MeCN for 30 min, then 100% MeCN for 10 min), and forty fractions were collected into a 96-well block. The block was evaporated (GenVac EZ-2), and the 40 dried fractions were tested in the A2780 assay. Only fractions 24 and 26 showed activity. Then 100 mg crude extract was loaded on a C18 Varian Dynamax column (8 µm, 250 × 21.4 mm, 10 mL/min 70% H2O to 100% MeCN for 30 min, then 100% MeCN for 10 min), and five fractions were collected. Fractions II and IV, corresponding to fractions 24 and 26 of the initial column respectively, were further purified using phenyl HPLC (65% MeOH) to furnish camptothecin (0.4 mg, tR 20 min) and 9-methoxycamptothecin (0.3 mg, tR 27 min). The structures of the camptothecins were confirmed by comparison of their spectroscopic data (NMR and MS) with those of the same compounds previously isolated by this group.3d
MG 1736B (23 g) was suspended in aqueous MeOH (MeOH-H2O, 9:1, 1000 mL) and extracted with hexane (3 × 1000 mL portions). The aqueous layer was then diluted to 50% MeOH (v/v) with H2O and extracted with CH2Cl2 (3 × 1000 mL portions). Both the hexane and the CH2Cl2 extracts were evaporated in vacuo to leave 86 mg and 1 g of residues (IC50: 12 and 16 µg/mL, respectively). The aqueous MeOH extract was inactive. The CH2Cl2 extract was selected due to its relatively greater quantity than the hexane extract, and this was fractionated by flash chromatography over 40 g of C18 reverse phase column using H2O-MeOH [(50 – 100%, in 10% increments, then 90% MeOH/CH2Cl2, 200 mL × 7)] to furnish seven fractions (I, II, III, IV, V, VI and VII with IC50 values of 19, 17, 13, 14, 15, 1000, 1000 µg/mL, respectively). Fraction V yielded an inseparable mixture of 1 and 2 (tR 15.5 min) over C18 HPLC using 80% MeOH, which could not be separated using phenyl HPLC with 75% MeCN (tR 9.3 min) either. But the mixture from fraction V could be separated by preparative TLC over Si gel developed with CH2Cl2 to afford 1 (1 mg, Rf 0.4) and 2 (1 mg, Rf 0.3). Further purification of fractions I and III was carried out by C18 HPLC with 50% and 70% MeOH as eluents to yield (−)-wikstromol (5 mg, tR 13.5 min) and (E)-3-methoxy-5-hydroxystilbene (3 mg, tR 23 min). Fraction II and the hexane extract were combined and chromatographed on a Si gel column eluted with hexane-CH2Cl2-MeOH (100:0:0; 100:1:0; 100:2:0; 100:3:0; 100:4:0; 100:5:0; 100:10:0; 0:100:0; 0:100:10). Fraction 9 of this separation yielded stigmast-4-en-3β-ol (2 mg, Rf 0.2) and stigmast-4-en-3-one (8 mg, Rf 0.3) after separation by preparative TLC developed with CH2Cl2.
Didymochlaenone A (1)
colorless oil; [α]D22 +112.5° (c 0.08, EtOH); IR (film) νmax 2917, 1653, 1507, 1350, 1264, 1185, 1126, 1037, 1023, 918; UV (EtOH) λmax (log ε) 246 (3.97), 283 (3.46) nm; CD (EtOH, c 0.04) [θ]225.0 −21.0, [θ]240.9 +43.3, [θ]314.3 +9.9; 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) see Table 1; HRFABMS m/z 343.1557 (calcd for C20H23O5, 343.1546).
Didymochlaenone B (2)
colorless oil; [α]D22 −27.5° (c 0.08, EtOH); IR (film) νmax 2921, 1653, 1502, 1482, 1345, 1259, 1185, 1037, 918; UV (EtOH) λmax (log ε) 243 (4.08), 298 (3.59) nm; CD (EtOH, c 0.04) [θ]236.4 +38.2, [θ]247.4 −12.3, [θ]316.2 −2.3; 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) see Table 1; HRFABMS m/z 357.1356 (calcd for C20H21O6, 357.1338).
Supplementary Material
Acknowledgment
This project was supported by the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313 with the International Cooperative Biodiversity Groups, and this support is gratefully acknowledged. We also thank Mr. Bill Bebout and Mr. Tom Glass for obtaining the HRFABMS and NMR spectra, respectively.
Footnotes
Supporting Information Available: 1H NMR spectra for compounds 1–2 and the structures of all isolated compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 18. For Part 17, seeChaturvedula VSP, Norris A, Miller JS, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2006;69 doi: 10.1021/np050376w. in press (np050376w)
- 2.(a) Adou E, Williams R, Schilling JK, Malone S, Meyer J, Wisse JH, Frederik D, Koese D, Werkhoven MCM, Snipes C, Werk TL, Kingston DGI. Bioorg. Med. Chem. 2005;13:6009–6014. doi: 10.1016/j.bmc.2005.07.026. [DOI] [PubMed] [Google Scholar]; (b) Murphy BT, Cao S, Norris A, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2005;68:417–419. doi: 10.1021/np049639x. [DOI] [PubMed] [Google Scholar]; (c) Cao S, Guza RC, Wisse JH, Evans R, van der Werff H, Miller JS, Kingston DGI. J. Nat. Prod. 2005;68:487–492. doi: 10.1021/np049629w. [DOI] [PubMed] [Google Scholar]; (d) Chaturvedula VSP, Farooq A, Schilling JK, Malone S, Derveld I, Werkhoven MCM, Wisse JH, Ratsimbason M, Kingston DGI. J. Nat. Prod. 2004;67:2053–2057. doi: 10.1021/np049696q. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. J. Am. Chem. Soc. 1966;88:3888–3890. [Google Scholar]; (b) Hsiang YH, Hertzberg R, Hecht S, Liu LF. J. Biol. Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]; (c) Hsiang YH, Liu LF. Cancer Res. 1988;48:1722–1726. [PubMed] [Google Scholar]; (d) Zhou BN, Hoch JM, Johnson RK, Mattern MR, Eng WK, Ma J, Hecht SM, Newman DJ, Kingston DGI. J. Nat. Prod. 2000;63:1273–1276. doi: 10.1021/np000058r. [DOI] [PubMed] [Google Scholar]
- 4.Sefkow M. J. Org. Chem. 2001;66:2343–2349. doi: 10.1021/jo001547z. [DOI] [PubMed] [Google Scholar]
- 5.Ngo KS, Brown GD. Phytochemistry. 1998;47:1117–1123. [Google Scholar]
- 6.(a) Marker RE, Lawson EJ, Wittle EL, Oakwood TS. J. Am. Chem. Soc. 1937;59:2714–2715. [Google Scholar]; (b) Gupta S, Ali M, Alam S, Niwa M, Sakai T. Phytochemistry. 1992;31:2558–2560. [Google Scholar]
- 7.(a) Lavie D, Kaye IA. J. Chem. Soc. 1963:5001–5002. [Google Scholar]; (b) Gaspar EMM, das Neves HJC. Phytochemistry. 1993;34:523–527. [Google Scholar]
- 8.Pretsch E, Bühlmann P, Affolter C. Structure Determination of Organic Compounds-Tables of Spectral Data. 3rd English Edition. New York: Springer; 2000. p. 288.p. 393.p. 395. [Google Scholar]
- 9.(a) Fukuyama Y, Shida N, Hata Y, Kodama M. Phytochemistry. 1994;36:1497–1503. [Google Scholar]; (b) Diaz DPP, Yoshida M, Gottlieb OR. Phytochemistry. 1980;19:285–288. [Google Scholar]
- 10.Berova N, Nakanishi K, Woody RW, editors. Circular Dichroism – Principles and Application. New York: Wiley-VCH; 2000. pp. 1–877. [Google Scholar]
- 11.Dewick PM. Medicinal Natural Products. A Biosynthetic Approach. Chichester: John Wiley and Sons, Ltd.; 2001. pp. 177–178. [Google Scholar]
- 12.Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. J. Nat. Prod. ASAP Article 10.1021/np0502802 S0163-3864(05)00280-6. [Google Scholar]
- 13.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]
- 14.Sai Prakash CV, Hoch JM, Kingston DGI. J. Nat. Prod. 2002;65:100–107. doi: 10.1021/np010405c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.