Abstract
Social defeat stress is a salient stressor that induces neuroadaptive changes in the mesocorticolimbic dopaminergic system. Substantial evidence indicates that μ-opioid receptors (MOR) modulate dopamine transmission in the ventral tegmental area (VTA). FosB/ΔFosB protein accumulation in dopaminergic projections during repeated treatments is thought to be involved in long-term neuroplasticity. In this study we characterize the magnitude and time-course of MOR mRNA expression and FosB/ΔFosB immunoreactivity in mesocorticolimbic regions following repeated social defeat stress. Effects of brief repeated social defeat stress or control handling procedures were studied in rats either 2 h after the last exposure, or 3, 7, 14, 21, 28 days later. We found that MOR mRNA expression in the VTA doubled after the last stress compared to handling, and remained 30–70% higher until day 21. The number of FosB/ΔFosB-labeled neurons in regions of the frontal cortex, nucleus accumbens (NAc) shell and core, and in the medial, central and basolateral amygdala increased significantly immediately after the last stress episode, and remained enhanced for 21 days. Another group of rats received bilateral intra-VTA infusion of the MOR agonist, DAMGO, seven days after the last stress. Prior social defeat stress augmented DAMGO-induced Fos expression in the NAc shell, suggesting that Fos expression in this region might be the direct result of MOR activity in the VTA. Social defeat stress leads to an increased capacity for MOR activation in the VTA, which may be relevant to enduring FosB/ΔFosB expression in mesocorticolimbic areas and to the behaviorally sensitized response to psychostimulant drugs.
Keywords: Stress, ventral tegmental area, n. accumbens, prefrontal cortex, DAMGO
Introduction
Species-specific defeat stress can be an unpredictable, uncontrollable stressor that is most salient for animals living in a social environment. Previously we showed that repeated social defeat stress up-regulates μ-opioid receptor (MOR) mRNA in the ventral tegmental area (VTA) of rats, and that effect lasts at least one week (Nikulina et al., 2005). Stimulation of MORs located in the VTA produces a dose-dependent enhancement of locomotor activity (Broekkamp et al., 1979; Kalivas et a.l, 1983). Furthermore, application of a selective MOR agonist into the VTA augments locomotor activity in previously stressed rats (Nikulina et al., 2005). Stress-sensitized rats exhibit increased dopamine metabolism in cortical and limbic terminal fields following intra-VTA MOR stimulation (Kalivas & Abhold, 1987), which induces dose-dependent Fos protein expression in the ventral striatum (Bontempi & Sharp, 1997), suggesting a prominent role of MORs in the regulation of mesolimbic dopaminergic projections (Di Chiara & Imperato, 1988; Spanagel et al., 1992).
Various stressors, including social defeat stress, are known to activate the mesocorticolimbic dopamine system (Abercrombie et al., 1989; Tidey & Miczek, 1996). Acute stress induces rapid and brief c-Fos expression in many regions of rat brain, but FosB immunoreactivity remains elevated for days after repeated daily stress (Stamp & Herbert, 1999). Among Fos family proteins, at least four major members have been identified: Fos, FosB, Fra-1 and Fra-2, which differ in their induction time and half-life (Cohen & Curran, 1988). ΔFosB, the truncated form of FosB, is of special interest because it gradually accumulates in response to chronic stimuli, such as repeated drug treatment, and can persist for long periods of time because of its high stability (Hope et al., 1994; Doucet et a.l, 1996; Nestler, 2001). Chronic restraint stress induces expression of ΔFosB in numerous brain regions (Perrotti et al., 2004). Repeated social defeat stress induces expression of Fos family proteins in mesocorticolimbic areas, which persists for at least one week after stress (Nikulina et al., 2004). It is not known at present whether repeated social defeat could produce long-term neuronal changes in mesocorticolimbic regions. Brief intermittent social stress induces long-lasting locomotor sensitization to psychostimulants (Nikulina et al., 2004; Covington et al., 2005). Dopamine and glutamate in mesocorticolimbic neurons regulate psychomotor sensitization (Cador et al., 1995; Wolf et al., 1995). Brain opioid peptides modulate the mesocorticolimbic dopamine system (Johnson & North, 1992; Mathon et al., 2005), and might exert a critical role in regulating the long-term consequences of social stress.
The objectives of the present study were to characterize the time-course and magnitude of MOR mRNA expression in the VTA, and FosB/ΔFosB immunoreactivity in the mesocorticolimbic terminals as a result of repeated exposure to brief social defeat stress. To determine whether a stress-induced increase in MOR was functionally relevant, we tested the consequence of stimulating MOR by intra-VTA infusion of a selective MOR agonist. If repeated social stress alters VTA dopaminergic function (tone) via MOR induction, then an altered functional response to MOR stimulation should be present in brain regions innervated by the affected VTA dopamine neurons.
Material and methods
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 270–320 g at the beginning of experiment, were allowed to acclimate to laboratory conditions for one week. They were then housed individually under a reverse, 12-h light-dark cycle (lights off at 0900 h) and maintained on food (Purina Rodent Chow) and water ad libitum in standard plastic cages. Male Long-Evans rats (weighing 550–700 g), termed “residents,” were used to induce social defeat stress in experimental animals. Residents were pair-housed together with a female in large cages (37 × 50 × 20 cm), and tested repeatedly for reliable display of aggressive attacks toward an intruder rat. All experimental procedures were approved by the Tufts-New England Medical Center Institutional Animal Care and Use Committee, and were conducted in accord with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2003). In addition, all efforts were made to minimize distress and to use the minimal number of subjects.
Experiment 1
Experiment 1 was designed to study the temporal effects of social defeat stress on MOR expression in the VTA and on FosB/ΔFosB expression in projection areas. Rats were randomly assigned to either an experimental “social defeat stress” group or a non-stressed, handled control group. The repeated handling procedure consisted of picking up each rat and returning it to its home cage, which represents a manipulation similar to that experienced by the experimental rat but without any aggressive interaction. Exposure to social defeat stress was conducted once daily for 5 days between 1000 and 1300 h, as described below; control rats were handled during the same period, but were not exposed to residents. After termination of stress, the rats were returned to their home cages. Six time-points after social defeat stress were studied (n=6 per group per time-point): 2 hr after the termination of social stress (day 0), or 3, 7, 14, 21, or 28 days later. Brain tissue was later collected at the same time of day.
Behavioral procedures
Social defeat stress consisted of a brief aggressive confrontation between experimental intruder rats and aggressive resident rats (Miczek, 1979). Females were removed from the resident cage prior to the social stress procedure. The experimental rat was placed with the resident until defeat behavior was demonstrated, consisting of the display of supine (submissive) posture by the intruder for at least four seconds, which usually occurred within the first 2–3 min of the encounter. As soon as the intruder rat displayed submissive behavior, a protective wire mesh cage was placed over the intruder, which was left in the resident’s cage for an additional 20 min.
Tissue preparation
At the appropriate time after the last defeat stress episode, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and then perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer for 15 min. Brains were removed, postfixed for 2 h in the same fixative at 4°C, and placed in graded concentrations of sucrose (12.5% and 25%) at 4°C for 3–4 days. Sections from same perfused brains were used to examine MOR mRNA by in situ hybridization histochemistry and FosB/ΔFosB labeling by immunohistochemistry.
Using a sliding microtome, coronal sections (20 μm) were collected from the level of frontal cortex (bregma +3.2 – 2.2; Paxinos & Watson, 1986), n. accumbens (NAc, bregma 1.0 – 1.2), amygdala (bregma −2.1 – −2.6), VTA (bregma −4.8 – −5.6) in chilled 0.1 M phosphate buffer (pH 7.4). Sections were mounted onto slides and stored at −80°C prior to histochemical procedures.
In situ hybridization histochemistry (ISHH)
A cRNA probe of 450 bases corresponding to transmembrane coding regions III and IV of the rat MOR (generously provided by Dr. H. Akil) was utilized. The radiolabeled probe was synthesized by standard in vitro transcription using 0.2 μg linearized plasmid cDNA, 25 μM [33P] UTP, excess GTP, CTP and ATP (500 μM each), 2 mM spermidine, 10 mM DTT, 4 U/μl RNAsin, and 5 U RNA polymerase (T7, Promega, Madison, WI, USA), incubated for 2 h at 37°C. 4.0 μl of DNAase (0.4 μl, Promega kit) was added to each reaction tube, and incubated for 15 min at 37°C, after which 2.0 μl of 0.2 M EDTA was added. The radiolabeled cRNA was purified by phenol/chloroform/isoamyl alcohol extraction and using a Quick Spin column (Boehringer Mannheim, Roche Diagnostic Corporation, Indianapolis, IN, USA). The probe was diluted to reach a final concentration of 2.0 pmol/ml in hybridization buffer (50% deionized formamide, 10% dextran sulfate, 4 X SSC, 1 X Denhardt solution, 100 mM DTT). Hybridization was accomplished at 55°C for 16 hours in a humidified chamber. Afterwards, sections were treated with RNAse A (30 min at 37°C), followed by a series of high stringency washes (15 min at room temperature in 1 X SSC; twice for 30 min at 67°C in 0.1 X SSC, followed by 10 min at room temperature in 0.1 X SSC). Sections were then rapidly dehydrated in graded alcohols and air-dried. The slides were exposed first to X-ray film (Biomax MR, Kodak, Rochester, NY, USA) for 5 days, then dipped in liquid photographic emulsion (Kodak, Rochester, NY, USA), exposed for 21 days at −35°C, developed, dried and coverslipped.
Image analysis
Regional X-ray film autoradiographs were assessed in defined VTA regions of both hemispheres by digitization using a CCD camera connected to a Macintosh computer. Quantification of regional optical density was performed using public domain image analysis software ImageJ (created by Wayne Rasband and available on the Internet at http://rsb.info.nih.gov/nih-image/) using calibrated radiostandards (ARC-146; American Radiolabeled Chemicals, St. Louis, MO, USA) to generate a calibration curve in cpm/mg. For microscopic analysis of grains, the number of labeled neurons was determined within 280 μm2 viewing fields from both sides of the VTA and the substantia nigra. Quantification of silver grains in labeled neurons was accomplished using ImageJ grain-counting software. Labeling was also measured in adjacent cell-sized regions that did not contain Nissl staining, which represented background labeling. Neurons were considered to be labeled if the number of autoradiographic grains was at least 3 times higher than background. The density of labeling was normalized as a labeling ratio that was calculated by dividing the number of grains per cell by the number of background grains (Hammer & Cooke, 1996). The labeling ratio was determined in 8–10 neurons in each of at least three adjacent sections from each region of interest in each rat. These data were averaged to determine mean values for each animal.
FosB/ΔFosB immunohistochemistry
Brain sections were washed three times in 0.05 M potassium phosphate buffered saline (KPBS, pH 7.4) and incubated in 5% normal goat serum/0.05M KPBS/0.4% Triton X for 60 min at room temperature. Primary antibody raised against an N-terminal region of FosB and ΔFosB (SC-48; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:2000 in 5% normal goat serum/0.05M KPBS/0.4% Triton X. This antibody recognizes a 32–37 kD protein corresponding to the molecular weight of ΔFosB-like proteins and full length FosB (Andersson et al., 2001; Perrotti et al., 2004). Following incubation for 48 hours at 4°C, sections were processed using the avidin-biotin-peroxidase (Vectastain ABC Elite kit, Vector Laboratories; Burlingame, CA, USA), then developed with a DAB peroxidase kit (SK-4100; Vector Laboratories, Burlingame, CA, USA) for 5 min at room temperature. Control procedures included preadsorption of the primary antibody using FosB peptide, and conducting these procedures in the absence of the primary antibody. There was no detectable labeling after either procedure (data not shown). Sections from stressed and handled brains were processed simultaneously throughout all stages of the immunohistochemical procedure.
Tissue sections were examined for the presence of a blue-black reaction product indicating immobilized antigen. For each rat in each treatment group, data were obtained from 3–4 sections through each brain region, and the mean regional value was calculated. Counts of immunostained profiles were determined using Image Pro Plus (version 4.1.0.0, Media Cybernetics, Silver Spring, MD, USA). Selected areas (20,576 μm2) were captured and digitized using a Cool Snap Pro videocamera interfaced to an Olympus BX2 microscope with a 20X objective. The mean density of non-specific background labeling was measured in cell-free areas of each experimental region, and digitally subtracted so as to eliminate any background staining. A specific threshold was set to detect only those labeled objects of appropriate size and staining intensity. The number of labeled profiles was determined in prelimbic and infralimbic regions of the frontal cortex, the NAc core and shell, medial, central and basolateral amygdaloid nuclei. These data were transformed to express the number of labeled nuclear profiles per mm2.
Experiment 2
Bilateral guide cannulae directed at the VTA were implanted under general anesthesia using isoflurane. The 23 ga guide cannulae (Plastics One; Roanoke, VA, USA), positioned 2 mm above their final destination, were directed towards the stereotaxic coordinates: AP=−5.0 mm; ML= ±0.23 mm from midline; DV= −8.2mm from the skull surface, angle 10° (Paxinos & Watson, 1986). The cannulae were permanently fixed to the skull with microscrews and dental cement. Removable stainless steel dummy cannulae of the same length were inserted into the guide cannulae to prevent clogging.
After 5–7 days of recovery, rats were subjected to repeated defeat stress (n=14) or handling procedures (n=14) as was described above (five daily social defeat stress exposures). Stressed and handled rats were randomly assigned to groups, which received either DAMGO ([D-Ala2,N-MePhe4,Gly-ol5]enkephalin) or vehicle by intra-VTA infusion (n=7 for each group). Infusions were performed one week after the last stress or handling exposure. Rats were adapted to head-mount manipulation for at least 3 days prior to the infusion procedure. DAMGO (Multiple Peptide Systems, San Diego, CA, USA) was infused (1.0 μg in 0.5 μl of 0.9% sterile saline) over 3 min using an infusion pump in awake, unrestrained rats. The dose of DAMGO was based on our previous observation that the same dose had a locomotor-stimulating effect after an intra-VTA infusion (Nikulina et al., 2005). The infusion cannulae remained in place for an additional 2 min to prevent back flow. The rats were anesthetized 2 hours later with sodium pentobarbital, and then were perfused transcardially with cold saline solution followed by 4% buffered paraformaldehyde. Tissue was prepared as described above in Experiment 1.
Fos immunohistochemistry
The effect of intra-VTA DAMGO infusion was assessed using Fos immunohistochemistry in sections from the level of the prefrontal cortex, NAc, and amygdala. Rabbit polyclonal antisera directed against amino acid residues 4–17 of human c-Fos (AB-5, Oncogene Research Products, San Diego, CA, USA) was utilized to recognize all Fos-like proteins. This antibody was applied for 48 hours at 4°C at 1:7,500 dilution in 5% normal goat serum/0.05M KPBS/0.4% Triton X. Additional procedures and image analysis were the same as those described for Experiment 1.
Histology
Sections from the VTA level were collected to confirm the location of each cannula track and tip. Coronal 20 μm sections of the VTA region were mounted on slides and stained with thionin, and examined under a light microscope. The cannula placements were located as in our previous experiment (Nikulina et al., 2005). Rats with cannula placements outside the VTA were excluded from immunohistochemical analysis.
Statistics
The data obtained from the time-course experiment were examined by two-way ANOVA comparing groups (handled v.s stressed) and time after stress termination (time points). After determining significant main effects and interactions, the appropriate post hoc comparison (Tukey test) was conducted to isolate the significant treatment effects. Immunohistochemical Fos induction data were analyzed using two-way ANOVA with conditions (handled, stressed) and drug (saline, DAMGO). The Bonferroni t-test was used for post hoc comparison of different treatment groups.
Results
Time-course of μ-opioid receptor mRNA expression in the VTA after stress
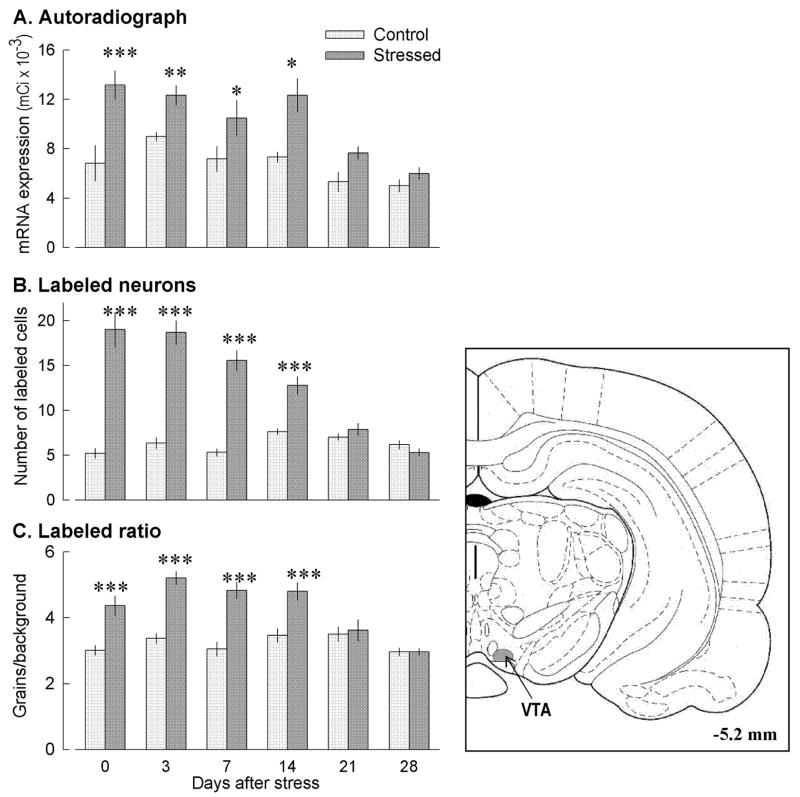
MOR mRNA expression levels in the VTA was of moderate intensity and did not vary significantly in handled control rats at different time points after handling (Figs. 1A and 2). By contrast, in previously defeated experimental rats, MOR mRNA expression in the VTA doubled 2 h after the last social defeat stress relative to handled control rats, and remained elevated by 30–70% for 3 weeks after the last defeat. Two-way ANOVA revealed significant effects of treatment group (stress or handling; F 1,60 = 47.32; p<0.01) and time after stress (F 5,60 = 11.04; p<0.001), with a nearly significant (F 5,60 = 2.28; p=0.057) treatment group x time interaction. Thus, repeated social stress induced a long-term increase in MOR mRNA expression in the area containing mesocorticolimbic cell bodies.
Fig 1.
Repeated defeat stress significantly increased the expression of MOR mRNA in the VTA 2 h after the last stress episode, and this level of mRNA expression remained high for at least 21 days. (A) optical density in film autoradiographs expressed as μCi/g of calibrated radiostandard (two-way ANOVA F 1,60 = 47.32; p<0.01) (B) number of labeled neurons in the VTA (F 1,60 = 176.82; p<0.001); (C) labeling ratio (number of grains per cell relative to background labeling) at different time-points after repeated defeat stress (F 1,60 = 70.15; p<0.001). *p<0.05; **p<0.01; *** p<0.001 compared with appropriate control group.
Fig 2.
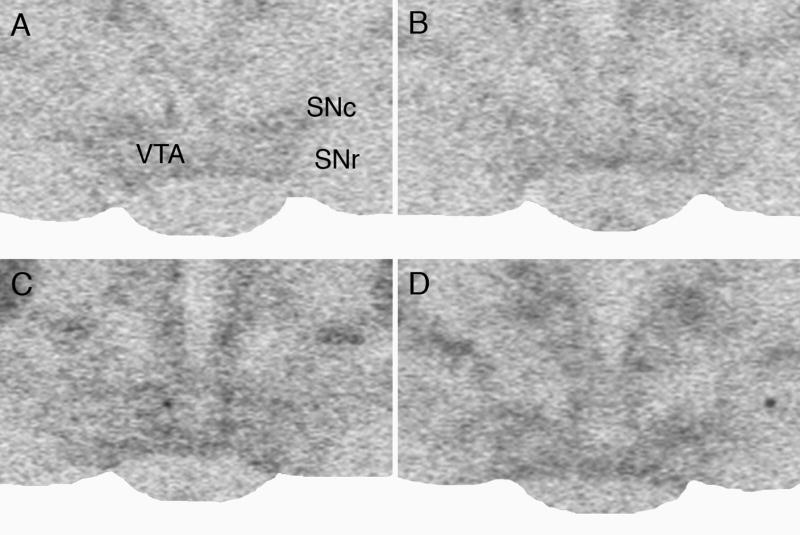
Representative film autoradiographs showing the effect of repeated social stress on MOR mRNA expression in the VTA of handled control and stressed rats 3 and 14 days after the last exposure. Handled control rat (A) 3 and (B) 14 days after handling; stressed rat (C) 3 and (D) 14 days after the last stress exposure. Note increased MOR mRNA expression after social defeat stress. SNc – substantia nigra, pars compacta; SNr - substantia nigra, pars reticulata.
A detailed analysis of the MOR-labeled neurons in the VTA was performed in sections processed for liquid emulsion autoradiography. The number of MOR mRNA-labeled neurons in Nissl-stained VTA sections showed clear differences between handled and stressed rats immediately after termination of repeated social stress, and for 3 more weeks (Fig. 1B). Silver particles indicating the presence of MOR mRNA were distributed more densely in labeled neurons of previously stressed rats relative to handled rats. Quantitative analysis of the number of cells expressing MOR mRNA indicated a three-fold increase in the number of labeled cells 2h after stress termination in comparison with handled animals, and at three and seven days after repeated social defeat exposures. Two-way ANOVA for the number of labeled neurons revealed significant effects of treatment group (F 1,60 = 176.82; p<0.001) and time after stress (F 5,60 = 17.36; p<0.001). There was a significant interaction between treatment and time (F 5,60 = 23.14; P<0.001). Similarly, the labeling ratios, which indicate the number of grains per cell relative to background labeling, increased significantly in stressed compared to handled rats until day 21 after stress (Fig 1C). Two-way ANOVA for labeling ratios revealed significant effects of treatment group (F 1,60 = 70.15; p<0.001) and time after stress (F 5,60 = 9.23; p<0.001). There was also a significant interaction between treatment and time (F 5,60 = 6.75; p<0.001).
FosB/ΔFosB in mesocorticolimbic areas after stress
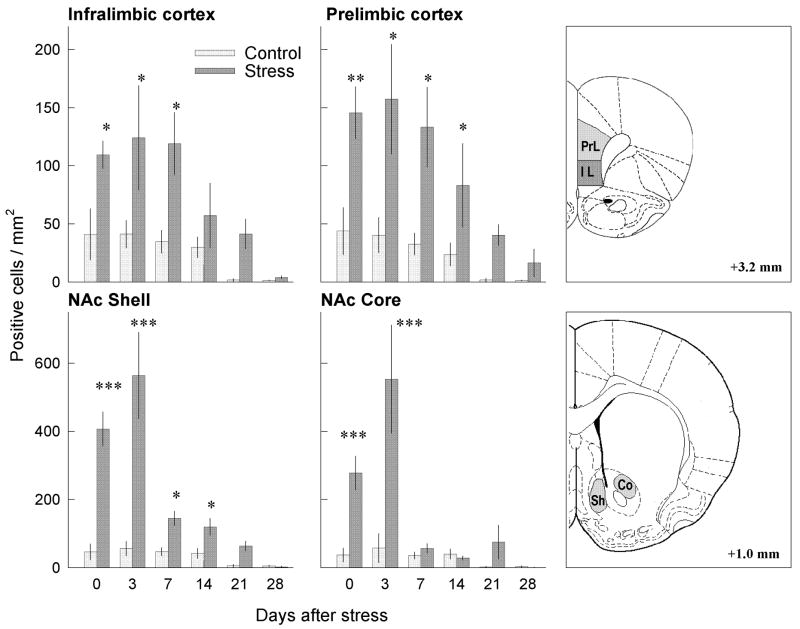
After termination of repeated social defeat stress, FosB/ΔFosB labeling increased in neurons of the frontal cortex, in the NAc shell and core, in all regions of the amygdala, and in the striatum in comparison to handled animals, and remained significantly elevated from days to weeks later. Repeated social defeat stress significantly increased FosB/ΔFosB labeling in both prelimbic and infralimbic regions of the frontal cortex (Figs. 3, 4). ANOVA indicated a main effect of treatment group for prelimbic (F 1,60 = 32.63; p<0.001) and infralimbic (F 1,60 = 20.82; p<0.001) regions, and also a significant effect of time after stress in the prelimbic (F 5,60 = 5.64; p<0.001) and infralimbic (F 5,60 = 5.91; p<0.001) cortex. There was no group x time interaction for prelimbic (p>0.05) or infralimbic (p>0.05) cortex.
Fig. 3.
Effects of repeated social defeat stress on the number of FosB/ΔFosB neurons in the medial prefrontal cortex and NAc shell and core at different time-points after stress. Repeated social defeat stress significantly increased the number of FosB/ΔFosB-positive neurons in the prelimbic (PrL; F1,60 = 32.63; p<0.001) and infralimbic cortical regions (IL; F1,60 = 20.82; p<0.001); NAc shell (Sh; F1,60 = 76.184; p<0.001) and NAc core (Co; F1,60 = 29.61; p<0.001). *p<0.05; **p<0.01; ***p<0.001 compared with appropriate control group.
Fig. 4.
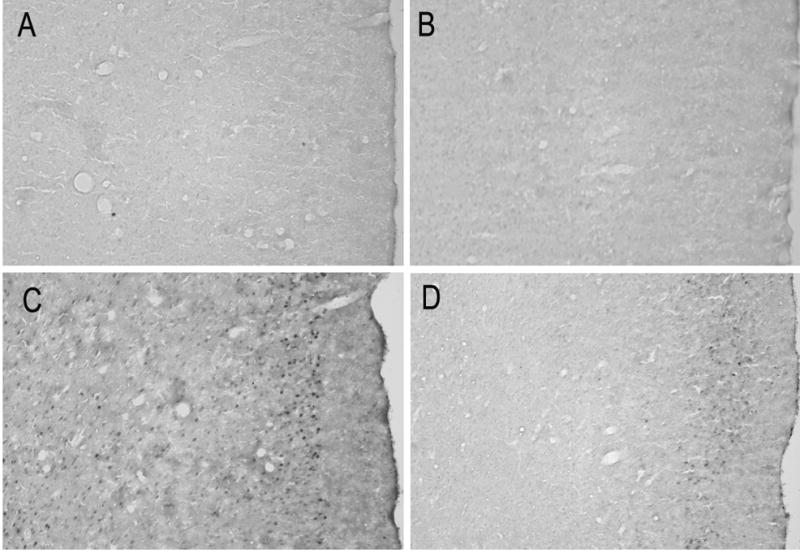
Photomicrographs showing FosB/ΔFosB labeling in prelimbic cortex from representative handled and stressed rats 3 or 14 days after the last exposure. Handled control rat (A) 3 and 14 (B) 14 days after handling; stressed rat (C) 3 and (D) 14 days after the last stress exposure. The number of FosB/ΔFosB profiles in the prelimbic cortical region significantly increased after exposure to repeated social defeat stress.
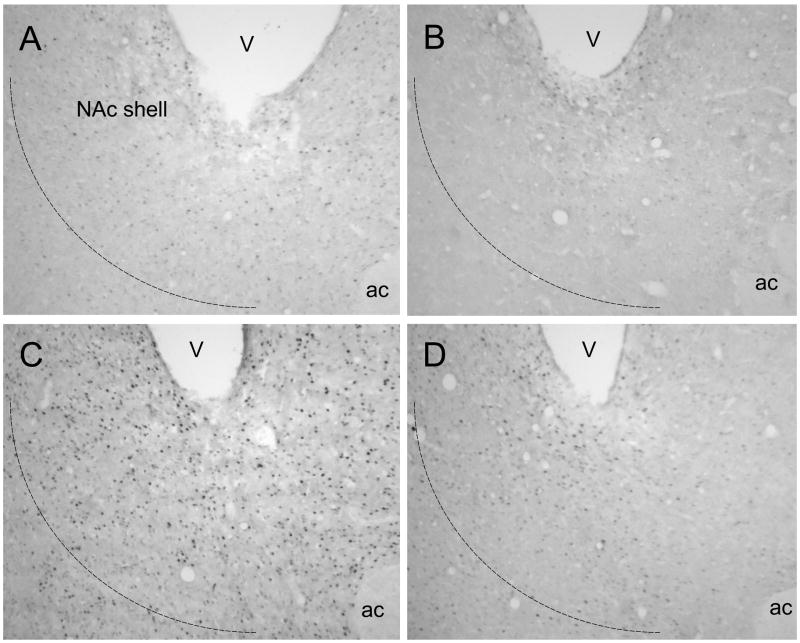
FosB/ΔFosB labeling in the NAc shell was highest 3 days after defeat, when the number of FosB/ΔFosB-positive neurons was 8-fold greater than after handling (Fig. 3, 5). At longer periods after stress termination (7–21 days), levels of FosB/ΔFosB labeling were reduced overall when compared to the level at the 3-day time-point, but remained elevated in comparison with the handled control group. A two-way ANOVA in the NAc shell revealed a significant effect of treatment group (F 1,60 = 76.84; p<0.001) and time after stress (F 5,60 = 21.48; p<0.001), as well as a significant treatment x time interaction (F 5,60 = 15.69; p<0.001). ANOVA for the NAc core also showed significant effects of treatment group (F 1,60 = 29.61; p<0.001) and time after stress (F 5,60 = 12.45; p<0.001), as well as significant treatment x time interaction (F 5,60 = 9.36; p<0.001).
Fig. 5.
Photomicrographs showing FosB/ΔFosB labeling in NAc shell from representative handled control and repeated social defeat stress rats 3 and 14 days after the last exposure. (A) – Handled control rat (A) 3 and 14 (B) 14 days after handling;stressed rat (C) 3 and (D) 14 days after the last stress exposure. The number of FosB/ΔFosB profiles in the NAc shell significantly increased after exposure to repeated social defeat stress. V – lateral ventricle; ac – anterior commissure.
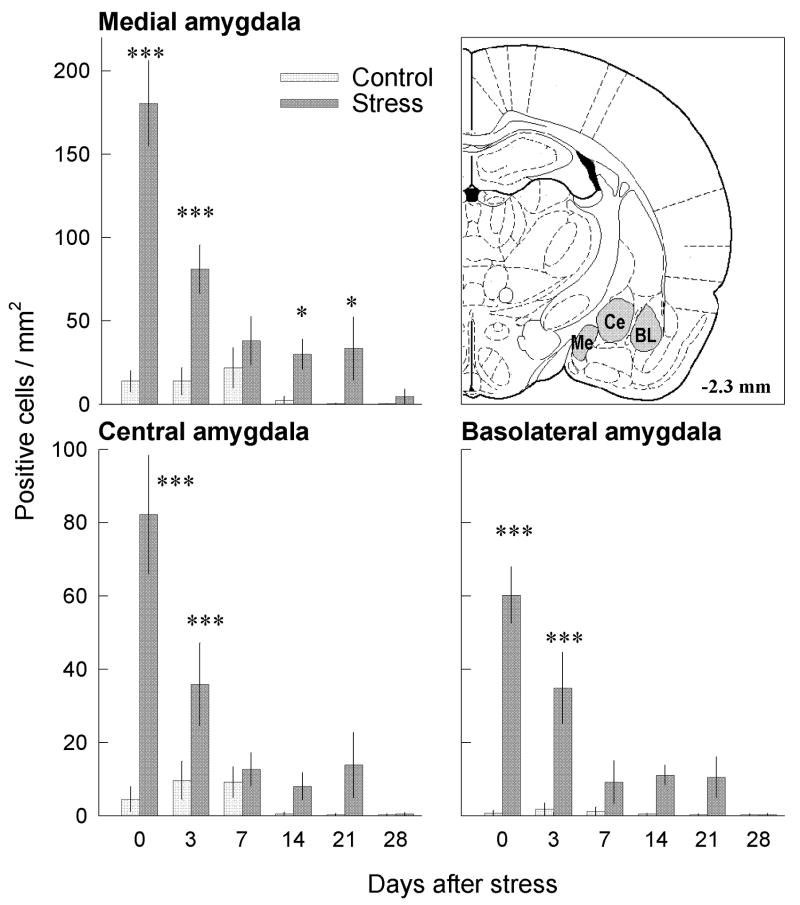
In the amygdala, two-way ANOVA showed significant effects of treatment and time after stress for FosB/ΔFosB labeling (Fig. 6). In the medial amygdala, there was a significant effect of treatment group (F 1,60 = 92.8; p<0.001) and time after stress (F 5,60 = 15.83; p<0.001), as well as significant treatment x time interaction (F 5,60 = 10.24; p<0.001). The central amygdala showed a significant effect of treatment group (F 1,60 = 76.184; p<0.001) and time after stress (F 5,60 = 17.77; p<0.001), as well as significant treatment x time interaction (F 5,60 = 15.07; p<0.001). In the basolateral amygdala, there was a significant effect of treatment group (F 1,60 = 44.48; p<0.001) and time after stress (F 5,60 = 7.85; p<0.001), as well as a significant treatment x time interaction (F 5,60 = 7.86; p<0.001). Post-hoc comparison for the medial amygdala indicated significant differences between the stressed and handled groups from days 0 to 21 (p<0.05); for central and basolateral amygdala, significant differences between stressed and handled groups were found only on days 0 and 3 after stress termination.
Fig. 6.
Time–course of repeated social defeat stress effects on FosB/ΔFosB labeling in medial (Me), central (Ce) and basolateral (BL) regions of amygdala. Repeated social defeat stress significantly increased the number of FosB/ΔFosB-positive neurons in Me (F1,60 = 92.8; p<0.001) Ce (F1,60 = 76.18; p<0.001) and BL regions (F1,60 = 44.48; p<0.001). *p<0.05; ***p<0.001 compared with appropriate control group.
A two-way ANOVA for FosB/ΔFosB labeling in the dorsomedial striatum also detected a significant effect of treatment group (F 1,60 = 28.78; p<0.001) and time after stress (F 5,60 = 15.17; p<0.001), as well as significant treatment x time interaction (F 5,60 = 13.07; p<0.001). However, post-hoc comparison indicated significant differences between stressed and handled groups only on days 0 and 3 after stress termination (p<0.05).
Fos in mesocorticolimbic areas after intra-VTA DAMGO infusion
Functional activation of mesocorticolimbic structures due to MOR up-regulation in the VTA after repeated social stress was studied after agonist stimulation of MORs in the VTA. Bilateral infusions of DAMGO were made 7 days after the last defeat stress or handling procedure. To investigate the cellular activation after DAMGO infusion, we used a pan-Fos antibody that recognized all Fos family proteins, including c-Fos, which is rapidly expressed after stimulation, and Fras (Fos-related antigens) proteins, which exhibit prolonged expression. Repeated social defeat stress itself significantly increased Fos labeling in the NAc shell (Fig. 7), prelimbic and infralimbic cortex (Fig. 8), and in the medial, central and basolateral amygdala (Fig. 8). A two-way ANOVA revealed a significant effect of group (handled and stressed) in the NAc shell (F 1,24 = 13.98; p<0.001). A significant main effect of DAMGO treatment was observed in NAc shell (F 1,24 = 7.37; p=0.012). However, there is no significant interaction between group x drug (p>0.05). In the NAc core there was a significant effect of group (handled, stressed; F 1,24 = 5.64; p<0.05), but no significant effect of treatment with DAMGO or saline (p>0.05). ANOVA revealed a main effect of group (handled, stressed) in prelimbic (F 1,24 = 11.21; p<0.005) and infralimbic cortex (F 1,24 = 4.35; p<0.05). There was no significant effect of DAMGO treatment in either prelimbic or infralimbic cortex (p>0.05), and no statistically significant interaction between group and DAMGO treatment. The number of Fos-positive neurons within regions of the amygdala significantly increased following prior repeated social defeat stress. The main effect of group (handled, stressed) was significant in the medial amygdala (F 1,24 = 7.53; p<0.02), central amygdala (F 1,24 = 7.10; p<0.02), and basolateral amygdala (F 1,24 = 6.69; p<0.02). There was no significant effect of DAMGO treatment in any amygdalar region (p>0.05), as well as no statistically significant interaction between group and DAMGO treatment. Thus, the NAc shell was the only region in handled and stressed rats that responded significantly to intra-VTA DAMGO infusion by increased Fos labeling. However, rats with previous repeated social defeat stress had higher Fos response in comparison to the handled control (t=2.7; df=12; p<0.05; Fig. 7).
Fig. 7.
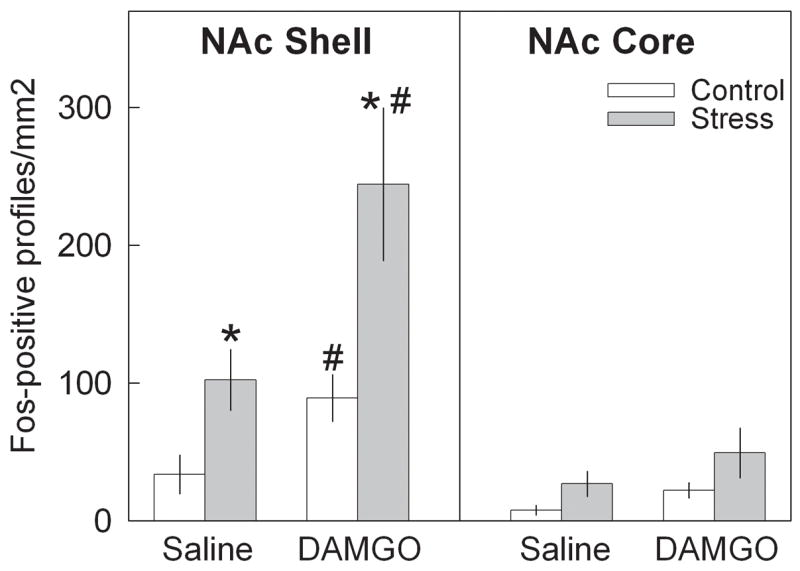
Effects of intra-VTA saline and DAMGO infusion on number of Fos-positive cells in NAc shell and core regions 7 days after repeated social defeat stress. * − p<0.05 control vs stress: # − p< saline vs DAMGO.
Fig. 8.
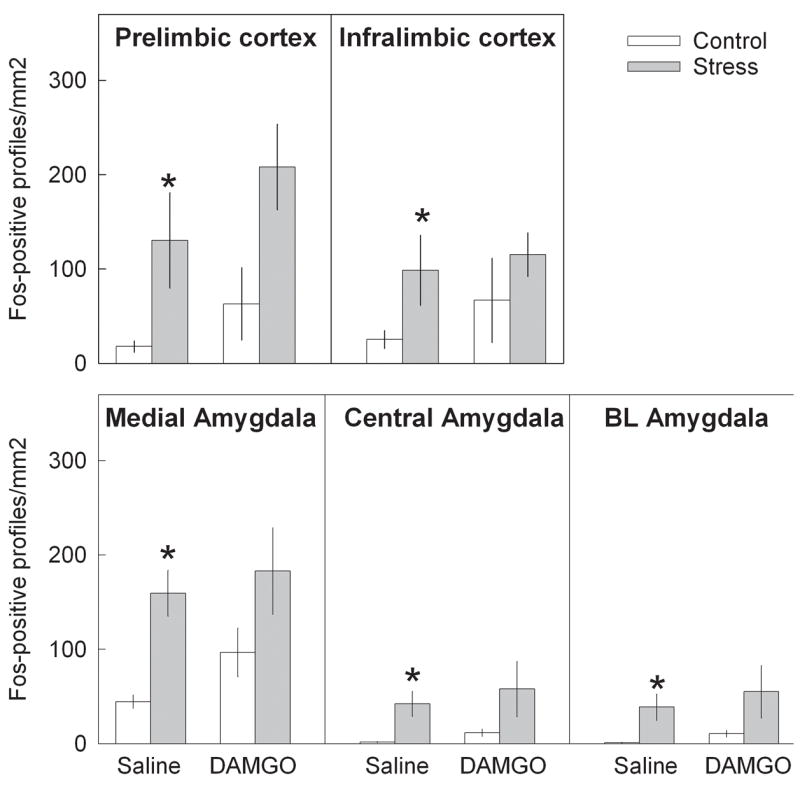
Effects of intra-VTA saline and DAMGO infusion on number of Fos-positive cells in prelimbic and infralimbic regions of frontal cortex; medial, central and basolateral regions of amygdala 7 days after repeated social defeat stress. * − p<0.05 control vs stress.
Discussion
In the present work, we have shown that repeated social defeat stress induced prolonged expression of MOR mRNA in the VTA, which accompanied a long-lasting neural expression of FosB/ΔFosB in mesocorticolimbic projections areas such as the NAc, frontal cortex, and amygdala. Intra-VTA MOR stimulation by DAMGO induced Fos labeling in the NAc shell in handled and stressed rats, although this increase appeared to be higher in stressed animals. Repeated social defeat stress initiated specific long-lasting changes in MOR modulation of the mesocorticolimbic dopamine system, which is an important element in the process of sensitization to drugs of abuse.
MOR activation in the VTA after social defeat stress
From an ethological point of view, social defeat is a highly salient stressor, which can induce long-term changes in behavioral parameters such as exploratory activity and anxiety (Koolhaas et al., 1997). Even a single exposure to social defeat stress may have long-term consequences for endocrine function, body temperature, food intake, and behavioral reactivity ranging from hours to days or weeks (Meerlo et al., 1996; Koolhaas et al., 1997). After defeat in a social confrontation, mice become analgesic (Miczek et al., 1982). This state is reversible using an antagonist, which acts on opioid receptors located in brain regions associated with pain processes (Rodgers & Randall, 1985; Miczek et al., 1985). Acute social defeat stress in rats induces rapid region-specific up-regulation of MOR mRNA expression in the VTA and periaqueductal grey matter, but not in substantia nigra (Nikulina et al., 1999; Miczek et al., 2004).
In our present experiments, repeated brief daily social defeat for five days induced prolonged MOR mRNA expression in the VTA, which is an integral site of the neurobiological circuit involved in regulation of the stress response and the reward system. There is a high level of correspondence between MOR mRNA expression and protein binding level (Mansour et al., 1994). Our previous work showed that elevated expression of MOR mRNA after social defeat stress is translated into enhanced functional MOR receptor protein in the VTA, which was illustrated by local MOR stimulation causing higher locomotor activity after social defeat stress than after handling (Nikulina et al., 2005). VTA MORs are located on GABAergic cells and their activation inhibits these interneurons, resulting in a decrease of tonic inhibition by GABA on dopamine neurons (Johnson & North, 1992). MOR activation thus indirectly stimulates dopamine VTA neurons, which send projections to mesocorticolimbic regions. The morphological substrate for such opioid-dopamine interaction is probably direct synaptic contact between opioid peptide-containing terminals and non-dopamine (i.e., GABA) inhibitory neurons in the VTA (Sesack & Pickel, 1992).
Adaptive changes in VTA neurons and their mesocorticolimbic projections following repeated social defeat stress may underlie altered behavior associated with psychostimulants and opiates. The impact of various stressors and drug administration on opioid activity has been shown by various studies. Foot-shock stress alters the level of the endogenous MOR agonist, met-enkephalin, in the VTA and activates dopamine projections to the prefrontal cortex and NAc (Kalivas & Abhold, 1987), and restraint stress increases MOR mRNA level in midbrain homogenate (Yamamoto et al., 2003). Cocaine treatment increases MOR binding in the NAc, ventral pallidum (Hammer, 1989), as well as MOR mRNA expression in the frontal cortex, the NAc, and the amygdala of rats (Yuferov et al., 1999). A powerful effect of different kinds of stress was shown on reinstatement of drug-seeking behavior, which particularly involved corticotrophin-releasing factor receptors in the VTA (Shaham et al., 2000; Wang et al., 2005; 2007). VTA dopamine neurons express glucocorticoid and corticotrophin-releasing factor receptors (Harfstrand et al, 1986; Van Pett et al., 2000), which their stimulation might enhance opioid-induced dopamine release because pretreatment with antagonists has been shown to reduce morphine-induced locomotion and stress-induced dopamine increase in the NAc and the VTA (Marinelli et al., 1998; Wang et al., 2005; 2007). Repeated intermittent social defeat stress resulted in robust long-lasting cross-sensitization to amphetamine, cocaine, and morphine (Covington & Miczek, 2001; Nikulina et al., 2004; Covington et al., 2005), which lasted up to 60 days and was accompanied by increased Fos expression in the VTA and amygdala.
Pretreatment with the opioid antagonist naloxone into the VTA significantly reduced dopamine concentration in the NAc after amphetamine administration (Schad et al., 2002). Similarly, increased cortical dopamine release after chronic stress can be reversed by naloxone pretreatment (Cuadra et al., 1999). Thus, MOR activity in the VTA might be critical for the process of stress-induced behavioral sensitization.
Up-regulation of MOR in the VTA appears to be involved in long-term drug sensitization (Magendzo & Bustos, 2003). Both δ- and MOR mRNA levels were elevated in the VTA of rats expressing amphetamine-induced sensitization when examined after a short drug-free interval. However, whereas δ-opioid receptor mRNA returned to the control level quite rapidly, MOR mRNA level remained elevated in the VTA of animals expressing behavioral sensitization for up to 14 days during the drug-free period. The expression of behavioral sensitization tested after 14 days of withdrawal was completely blocked by the opioid antagonist naloxone (Magendzo & Bustos, 2003).
The increased MOR mRNA expression in the VTA is consistent with the functional responsiveness of NAc shell neurons to DAMGO-induced stimulation of MORs in the VTA. Fos expression after DAMGO infusion was studied with a pan-Fos antibody, which recognizes all known Fos family proteins. Intra-VTA infusion of saline increased Fos expression in regions of the amygdala, prelimbic and infralimbic cortex, as well as in the NAc shell of previously stressed rats in comparison with handled rats. This enhanced labeling likely represents FosB/ΔFosB as a response to repeated social defeat stress exposure 7 days earlier. Two hours after DAMGO infusion into the VTA, we found that enhanced Fos labeling was observed in the NAc shell in both handled and stressed rats, which may represent a rapid induction of c-fos in response to DAMGO infusion. Similarly, morphine infusion into the VTA induced Fos expression in striatal and NAc neurons of rats (Bontempri & Sharp, 1997). However, it is worth noting that the site of DAMGO infusion in our study was localized predominantly in the rostral part of the VTA. The VTA consists of rostral and caudal subregions, which constitute two functionally and anatomically distinct areas (Ikemoto et al., 1997; Olson & Nestler, 2007; Zangen et al., 2002). A greater number of GABAergic neurons, which contain MORs (Johnson & North, 1992), are localized in the rostral VTA (Olson & Nestler, 2007). Therefore, targeting the rostral VTA was intended to produce maximal effect on VTA function and on regions to which it projects.
In the present experiment, the NAc shell, but not the core, was the only site where the number of Fos-positive profiles increased after DAMGO infusion. The NAc shell is considered to be the limbic part of the striatal complex, which is involved in the control of motivation and reward; in contrast, the NAc core is more closely related to the motor striatum (Heimer at al., 1991; Zahm & Brog, 1992). Indeed, heterogenous functional organization of the NAc in response to stress or injection of morphine or cocaine has been reported (Barrot et al., 1999; Kalivas & Duffy, 1995). Mild footshock presentation increases extracellular dopamine level in the NAc shell without any alteration of dopamine level in the NAc core (Kalivas & Duffy, 1995). Similarly, the NAc shell exhibited the largest increase in dopamine level and Fos expression after injection stress or administration of morphine or cocaine (Barrot et al., 1999). Previous episodes of repeated social stress induced an additional increase in Fos expression in the NAc shell after intra-VTA DAMGO in comparison to handled rats, which could suggest an augmentation of MOR-induced functional activation of projection neurons to the NAc shell. This pattern reinforces the idea that opioid modulation of VTA dopamine neurons contributes to the sensitized response to psychostimulants after social defeat stress.
Long-lasting FosB/ΔFosB expression in mesocorticolimbic areas after social defeat stress
Increased MOR mRNA expression in the VTA could underlie the long-term augmentation of FosB/ΔFosB expression in dopaminergic projection regions. The results of our time-course study suggest that repeated social defeat stress induced FosB/ΔFosB protein in mesocorticolimbic projection areas for extended periods of time (3 weeks) after termination of stress exposure. The transcription factor ΔFosB is a protein product of the immediate-early gene fosB, which accumulates after chronic treatment due to its stability (Chen et al., 1997). ΔFosB can bind to all Jun family proteins and can induce or suppress AP-1-mediated transcription (Chen et al., 1997; Nakabeppu & Nathans, 1991; Nye et al., 1995). Unlike FosB, which is rapidly induced and degraded within days, ΔFosB is induced after repeated stimulation and its expression persists for weeks (McClung et al., 2004). FosB/ΔFosB labeling in this study was assessed using an antiserum that recognizes both ΔFosB and full-length FosB (Andersson et al., 2001; Perrotti et al., 2004). Studies that use a different type of stressor have demonstrated that repeated restraint stress episodes over 10 days increase ΔFosB expression in the frontal cortex, NAc, and basolateral amygdala with little or no effect on FosB expression (Perrotti et al., 2004). The time pattern of expression observed herein suggests that FosB protein was expressed right away after repeated stress termination (0 day and 3 days). At later time points (7 days or later), the observed protein expression was most likely ΔFosB.
Various environmental stressors such as foot shock, food restriction, restraint or tail pinch induce cross-sensitization to psychostimulants via activation of mesocorticolimbic dopaminergic transmission (Kalivas & Stewart, 1991; Sorg, 1992). Following presentation of repeated social defeat stress, elevated dopamine release is observed in the NAc and prefrontal cortex, but this lasts only a short time (Tidey & Miczek, 1996). However, stress-induced excitation of dopamine neurons due to the up-regulation of MOR in the VTA is pronounced and prolonged, which could contribute to the development of behavioral sensitization to psychostimulants. Furthermore, it is known that ΔFosB accumulates in the NAc after chronic exposure to almost every drug of abuse involving dopaminergic neurotransmission (Hope et al., 1994; Nye & Nestler, 1996; Pich et al., 1997), and accumulation of ΔFosB could be a mechanism for the prolonged sensitization to drug exposure (Nestler, 2001).
The involvement of MOR in the expression of ΔFosB was demonstrated by repeated morphine treatment, which increased the level of chronic Fras and ΔFosB in the striatum and NAc. Furthermore, the induction of these proteins could be prevented by concomitant administration of the opioid receptor antagonist naltrexone (Nye et al., 1995). The absence of ΔFosB expression in fosB knockout mice prevents behavioral sensitization after repeated cocaine administration (Hiroi et al., 1997), and FosB/ΔFosB is expressed after social defeat stress in those sites that are critical for drug-induced behavioral sensitization (Cador et al., 1995). Our data support the suggestion that long-term FosB/ΔFosB expression in mesocorticolimbic projections might be a cause of stress-induced cross-sensitization to psychostimulant drugs (Nikulina et al., 2004).
In summary, the present study demonstrated that repeated social defeat stress induces MOR up-regulation in the VTA, which is associated with neuroadaptive changes in mesocorticolimbic areas. VTA MOR stimulation affected only the NAc shell, wherein Fos expression was significantly higher in defeated rats. Thus, MOR-induced activation of the VTA-NAc pathway, together with prolonged FosB/ΔFosB expression in mesocorticolimbic regions, could be a substrate for cross-sensitization to psychostimulant drugs following repeated social defeat stress exposure.
Acknowledgments
This research was supported by USPHS awards DA14327 (EMN), DA02632 (KAM), and MH066954 (RPH). We thank Mr. J. Thomas Sopko for assistance in the statistical analyses of the data and preparation of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- MOR
μ-opioid receptor
- NAc
nucleus accumbens
- VTA
ventral tegmental area
References
- Andersson M, Westin JE, Cenci MA. Time course of striatal ΔFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur J Neurosci. 2003;17:661–666. doi: 10.1046/j.1460-9568.2003.02469.x. [DOI] [PubMed] [Google Scholar]
- Abercrombie E, Keefe K, DiFrishia D, Zigmond M. Differential effects of stress in vivo dopamine release in striatum, nucleus accumbens and medial prefrontal cortex. J Neurochem. 1989;51:1657–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci. 1999;11:1155–1166. doi: 10.1046/j.1460-9568.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Sharp FR. Systemic morphine-induced Fos protein in the rat striatum and nucleus accumbens is regulated by μ-opioid receptors in the substantia nigra and ventral tegmental area. J Neurosci. 1997;17:8596–8612. doi: 10.1523/JNEUROSCI.17-21-08596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Phillips AG, Cools AR. Stimulant effects of enkephalin microinjection into the dopaminergic A10 area. Nature. 1979;278:560–562. doi: 10.1038/278560a0. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience. 1995;65:385–395. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DR, Curran T. Fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988;8:2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Repeated social-defeat stress, cocaine or morphine: Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Cuadra G, Zurita A, Lacerra C, Molina V. Chronic stress sensitizes frontal cortex dopamine release in response to a subsequent novel stressor: reversal by naloxone. Brain Res Bull. 1999;48:303–308. doi: 10.1016/s0361-9230(98)00179-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Cooke ES. Sensitization of neuronal response to cocaine during withdrawal following chronic treatment. NeuroReport. 1996;7:2041–2045. doi: 10.1097/00001756-199608120-00038. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Widerlov E, Stanley D, Breese G, Prange AJ., Jr Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther. 1983;227:229–237. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Abhold R. Enkephalin release into the ventral tegmental area in response to stress: modulation of mesocorticolimbic dopamine. Brain Res. 1987;414:339–348. doi: 10.1016/0006-8993(87)90015-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-induced and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Magendzo K, Bustos G. Expression of amphetamine-induced behavioral sensitization after short- and long-term withdrawal periods: participation of mu- and delta-opioid receptors. Neuropsychopharmacology. 2003;28:468–477. doi: 10.1038/sj.npp.1300063. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 1994;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci U S A. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathon DS, Ramakers GM, Pintar JE, Marinelli M. Decreased firing frequency of midbrain dopamine neurons in mice lacking mu opioid receptors. Eur J Neurosci. 2005;21:2883–2886. doi: 10.1111/j.1460-9568.2005.04123.x. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJ, Daan S, Van Den Hoofdakker RH, Koolhaas JM. Changes in behaviour and body weight following a single or double social defeat in rats. Stress. 1996;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology. 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, Nikulina EM, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Miczek KA, Kream RM. Social defeat stress increases expression of μ-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP. Prolonged effects of repeated social defeat stress on mRNA expression and function of mu-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, USA: 1986. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Randall JI. Social conflict analgesia: studies on naloxone antagonism and morphine cross-tolerance in male DBA/2 mice. Pharmacol Biochem Behav. 1985;23:883–887. doi: 10.1016/0091-3057(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Schad CA, Justice JB, Holtzman SG. Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther. 2002;300:932–938. doi: 10.1124/jpet.300.3.932. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Dual ultrastructural localization of enkephalin and tyrosine hydroxylase immunoreactivity in the rat ventral tegmental area: multiple substrates for opiate-dopamine interactions. J Neurosci. 1992;12:1335–1350. doi: 10.1523/JNEUROSCI.12-04-01335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sorg B. Mesocorticolimbic dopamine system: cross-sensitization between stress and cocaine. Ann NY Acad Sci. 1992;654:136–144. doi: 10.1111/j.1749-6632.1992.tb25962.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Tidey J, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Komori T, Matsumoto T, Zhang K, Miyahara S, Shizuya K, Okazaki Y. Effects of single and repeated prolonged stress on mu-opioid receptor mRNA expression in rat gross hypothalamic and midbrain homogenates. Brain Res. 2003;980:191–196. doi: 10.1016/s0006-8993(03)02969-x. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Zhou Y, Spangler R, Maggos CE, Ho A, Kreek MJ. Acute “binge” cocaine increases mu-opioid receptor mRNA levels in areas of the rat mesolimbic mesocortical dopamine system. Brain Res Bull. 1999;48:109–112. doi: 10.1016/s0361-9230(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]