Abstract
Orthostatic tolerance is reduced in the heat-stressed human. This study tested the following hypotheses: 1) whole body heat stress reduces cerebral blood velocity (CBV) and increases cerebral vascular resistance (CVR); and 2) reductions in CBV and increases in CVR in response to an orthostatic challenge will be greater while subjects are heat stressed. Fifteen subjects were instrumented for measurements of CBV (transcranial ultrasonography), mean arterial blood pressure (MAP), heart rate, and internal temperature. Whole body heating increased both internal temperature (36.4 ± 0.1 to 37.3 ± 0.1° C) and heart rate (59 ± 3 to 90 ± 3 beats/min); P < 0.001. Whole body heating also reduced CBV (62 ± 3 to 53 ± 2 cm/s) primarily via an elevation in CVR (1.35 ± 0.06 to 1.63 ± 0.07 mmHg · cm-1 · s); P < 0.001. A subset of subjects (n = 8) were exposed to lower-body negative pressure (LBNP 10, 20, 30, 40 mmHg) in both normothermic and heat-stressed conditions. During normothermia, LBNP of 30 mmHg (highest level of LBNP achieved by the majority of subjects in both thermal conditions) did not significantly alter CBV, CVR, or MAP. During whole body heating, this LBNP decreased MAP (81 ± 2 to 75 ± 3 mmHg), decreased CBV (50 ± 4 to 39 ± 1 cm/s), and increased CVR (1.67 ± 0.17 to 1.92 ± 0.12 mmHg · cm-1 · s); P < 0.05. These data indicate that heat stress decreases CBV, and the reduction in CBV for a given orthostatic challenge is greater during heat stress. These outcomes reduce the reserve to buffer further decreases in cerebral perfusion before presyncope. Increases in CVR during whole body heating, coupled with even greater increases in CVR during orthostasis and heat stress, likely contribute to orthostatic intolerance.
Keywords: hyperthermia, syncope, transcranial Doppler, cerebral vascular resistance
IN HUMANS, ORTHOSTATIC TOLERANCE is reduced during heat stress compared with normothermic conditions (1, 2, 19, 22, 37). In a recent study, 44% of participants could not complete a 10-min 60° head-up tilt after a mean increase in internal temperature of 0.8°C via whole body heating (42). Although mechanisms reducing orthostatic tolerance during heat stress are unclear, they are likely associated with factors that directly or indirectly affect cerebral perfusion pressure, cerebral blood flow, and thus oxygenation (24, 26, 41).
The effects of whole body heating, and subsequent orthostasis, on cerebral vascular regulation remain unclear. Previous research suggests that both mild (8) and moderate (42) passive-heat stresses decrease cerebral blood velocity (CBV) with little or no change in cerebral vascular resistance (CVR; 42). However, in the study in which CVR was calculated (42), there was a tendency for heat stress to increase cerebral vascular resistance, evidenced by a significant reduction in CBV coupled with the absence of a significant change in MAP. Thus the absence of an effect of whole body heating on cerebral vascular resistance in that study may be due to the relatively small number of subjects assessed resulting in a type II statistical error.
Accordingly, the first objective of this project was to test the hypothesis that whole body heat stress increases CVR, which contributes to the previously observed reduction in CBV (7, 42) in this thermal condition. Given that orthostatic intolerance is ultimately due to inadequate cerebral perfusion, it may be that the reduction in cerebral perfusion for a given orthostatic challenge is greater while subjects are heat stressed. Thus the second objective of this project was to test the hypothesis that during LBNP the decrease in CBV would be greater while subjects were heat stressed.
METHODS
Subjects
Fifteen subjects (7 men, 8 women) participated in this study; physical characteristics of male subjects were mean age of 34 ± 2 years, height of 178 ± 2 cm, and weight of 73 ± 5 kg; while female subjects had a mean age of 31 ± 2 years, height of 167 ± 2 cm, and weight of 60 ± 2 kg. Written informed consent was obtained from all participants before participating in this study. The protocol and informed consent were approved by the University of Texas Southwestern Medical Center and the Presbyterian Hospital of Dallas Human Institutional Review Boards.
Protocol 1
To determine the effect of heat stress on cerebral vascular variables, 15 subjects performed a total of 25 heat stresses. Eight subjects performed one heat stress, and to test the reproducibility of the responses, 7 subjects performed two heat stresses, and 3 of these 7 subjects each performed a third heat stress. During normothermia, thermoneutral water (35°C) was perfused through a high-density tube-lined suit (Carleton Technologies, Tampa Bay, FL). Whole body heating was performed until internal temperature increased ∼0.6-1.0°C. This was accomplished by perfusing 46°C water through the tube-lined suit. Water temperature perfusing the suit was slightly reduced (to 44-45°C) for 10 to 15 min before data collection to decrease the rate of rise of internal temperature during the ensuring data collection period. Hemodynamic and thermal data were averaged during both normothermic and heat stress periods while the subjects rested quietly in the supine position.
Protocol 2
To determine the effect of combined orthostatic and heat stresses on cerebral vascular variables, eight of the above participants (4 men, 4 women) were exposed to lower-body negative pressure (LBNP) while subjects were normothermic and at the end of the heat stress. Two-piece, water-perfused suits (upper and lower halves) were used for these subjects, thereby improving the seal of the LBNP chamber to the subject and reducing air leaking and associated skin cooling during LBNP. In both thermal conditions, after baseline measures, LBNP was applied for 3 min at 10, 20, 30, and 40 mmHg. LBNP was discontinued if signs or symptoms of syncope were observed (e.g., nausea, pallor, sudden decrease in heart rate and/or blood pressure, or a sustained decrease in systolic blood pressure <80 mmHg). A cumulative stress index was calculated by summing the product of duration and negative pressure at each gradation of LBNP tolerated by the subject (21).
Measurements
Heart rate was measured continuously from an electrocardiogram (SpaceLabs, Redmond, WA) interfaced to a cardiotachometer (CWE, Ardmore, PA). Arterial blood pressure was measured from the upper arm via electrosphygmomanometry (Sun-Tech, Raleigh, NC) and at the finger via the Penaz method (Finapres Ohmeda, Englewood, CO). Mean arterial blood pressure (MAP) was calculated as 1/3 pulse pressure plus diastolic blood pressure. Transthoracic impedance [an index of central blood volume (4, 9)] was measured to determine whether a greater fluid shift occurred per level of LBNP between normothermia and heat stress in protocol 2. This measure was accomplished by passing a 50-kHz 400-μA current between electrodes placed at the lateral neck at the approximate level of C7 and midaxillary line at the level of the xiphoid process (Biopac, Santa Barbara, CA).
CBV was measured from the middle cerebral artery via transcranial Doppler ultrasonography. A 2-MHz Doppler probe (DWL Elektronische Systeme, Sipplingen, Germany) was adjusted over the temporal window until an optimal signal was identified. The probe was then fixed using a mold constructed of polyvinylsiloxane impression medium and held in place with a headband strap to prevent subtle movement of the Doppler probe. CVR was calculated by MAP CBV-1. End-tidal CO2 (PetCO2) and respiratory rate were measured via nasal cannula (Criticare Systems, Waukesha, WI).
Forearm skin blood flow was measured via laser-Doppler flowmetry using an integrating flow probe (Perimed, North Rayalton, OH). Cutaneous vascular conductance was indexed by dividing laser-Doppler flux by MAP and expressed as a percentage of baseline, with baseline being set as 100% of the normothermic value. Forearm sweat rate was measured via capacitance hygrometry (Viasala, Woburn, MA). Internal temperature was obtained at 10-s intervals via an ingestible pill telemetry system (HTI Technologies, Palmetto, FL). Mean skin temperature was measured via the weighted average of six thermocouples attached to the skin (38).
Data analysis
Data were acquired at a minimum of 50 Hz throughout experimental procedures by a data acquisition system (Biopac, Santa Barbara, CA). In protocol 1, paired t-tests were used to compare thermal and hemodynamic responses between normothermic and heat-stressed conditions. Subjects who underwent repeated heat stress trials were analyzed for repeatability via intraclass correlation coefficient and coefficient of variation techniques. In protocol 2, the last minute of baseline and each LBNP stage were analyzed during both thermal conditions. It was expected that not all subjects would complete all LBNPs in the heat stress condition. To address this challenge, data were statistically analyzed from the LBNP that the majority of subjects were able to complete. Using this approach, we reported data that are more conservative given that subjects who became presyncopal at lower LBNPs would have exhibited even more pronounced responses relative to subjects who were able to complete all LBNPs. These data were analyzed via two-way repeated-measures ANOVA. If a significant main effect or interaction was identified, Student-Newman-Keuls post hoc analysis was performed. The α-level for all statistical analyses was set at 0.05. All values are reported as means ± SE.
RESULTS
Protocol 1
Whole body heating increased skin temperature (34.1 ± 0.2 to 38.3 ± 0.2°C; P < 0.001), which led to increases in internal temperature (36.4 ± 0.1 to 37.3 ± 0.1°C; P < 0.001). MAP was unchanged as a result of the heat stress (84 ± 1 to 84 ± 1 mmHg; P > 0.50), whereas heart rate was elevated (59 ± 3 to 90 ± 3 beats/min; P < 0.001). PetCO2 was reduced (42 ± 1 to 40 ± 1 mmHg; P < 0.001), and respiratory rate was slightly elevated (16 ± 1 to 18 ± 1 breaths/min; P < 0.001), during whole body heating. The heat stress significantly reduced CBV and increased CVR (Fig. 1). When data were analyzed from subjects who performed repeated trials, the reduced CBV response during whole body heating was similar between trials (P > 0.50), had a low coefficient of variation of the method error (9%), and had a high intraclass correlation coefficient (r = 0.87). These data indicate that whole body heating of this magnitude provides both stable and reproducible reductions in CBV.
Fig. 1.
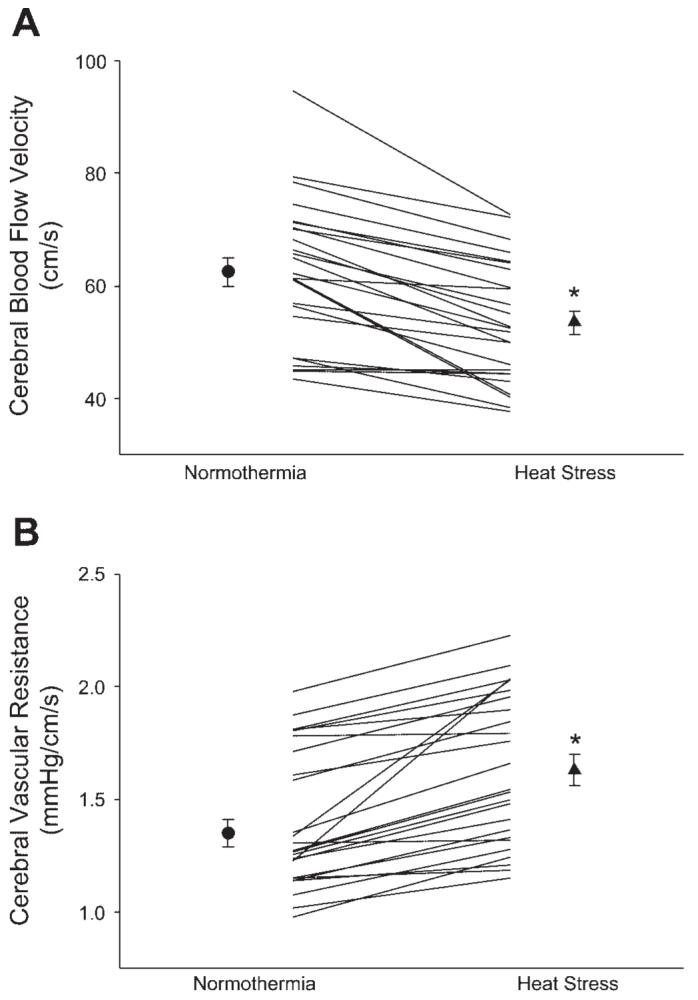
Effects of whole body heating on cerebral blood velocity from the middle cerebral artery (A) and cerebral vascular resistance (B). The symbols with error bars denote mean responses, while lines denote individual responses. *P < 0.001.
Protocol 2
All subjects were able to complete the LBNP protocol during normothermia. However, only one of eight was able to complete the LBNP protocol during whole body heating without exhibiting presyncopal signs and/or symptoms. During combined LBNP and whole body heating, the number of subjects completing stages without presyncopal symptoms was as follows: 10 mmHg, 8 subjects; 20 mmHg, 8 subjects; 30 mmHg, 5 subjects; 40 mmHg, 1 subject. This resulted in the cumulative stress index significantly decreasing from 300 mmHg/min during normothermia to 188 ± 21 mmHg/min during whole body heating. Table 1 provides data for the five subjects (3 men, 2 women) who completed 30 mmHg LBNP without presyncopal symptoms in the heated condition. It is important to emphasize that if responses from all 8 subjects were included in Table 1, reported changes due to LBNP while heated would be even more pronounced. However, interpretation of such data would be challenging given the differing number of subjects at each LBNP during heat stress (e.g., 8 subjects at 10 and 20 mmHg but only 1 subject at 40 mmHg) relative to the number of subjects included in the analysis during normothermia (e.g., eight subjects for each LBNP). Before LBNP, there was a tendency for differences in baseline CBV, CVR, and PetCO2 in these five subjects due to the heat stress, likely because of lower statistical power when compared with 15 subjects described above.
Table 1. Effect of LBNP during normothermia and heat stress on cardiovascular and ventilatory parameters for the five subjects who completed 30 mmHg LBNP during whole body heating.
| LBNP |
|||||
|---|---|---|---|---|---|
| Variable | Condition | Baseline | 10 mmHg | 20 mmHg | 30 mmHg |
| CBFV, cm/sec | Normothermia | 55±5 | 55±5 | 54±5 | 55±6 |
| Heat stress | 50±4 | 52±4 | 46±2*†‡ | 39±1*†‡ | |
| CVR, mmHg · cm-1 · s | Normothermia | 1.55±0.16 | 1.52±0.15 | 1.50±0.13 | 1.50±0.18 |
| Heat stress | 1.67±0.17 | 1.62±0.14 | 1.76±0.10*†‡ | 1.92±0.12*†‡ | |
| HR, beats/min | Normothermia | 61±8 | 61±8 | 65±9 | 66±10 |
| Heat stress | 91±8‡ | 96±9‡ | 107±11*†‡ | 117±12*†‡ | |
| MAP, mmHg | Normothermia | 83±2 | 80±3 | 79±2 | 79±1 |
| Heat stress | 81±3 | 81±2 | 80±3 | 75±3* | |
| Zo, %change | Normothermia | — | 0.9±0.5 | 1.9±0.9* | 2.3±1.1* |
| Heat stress | — | 0.8±0.4 | 1.7±0.6* | 2.4±0.8* | |
| Bf, breaths/min | Normothermia | 15±1 | 15±1 | 12±1* | 12±1* |
| Heat stress | 18±2‡ | 16±1*†‡ | 14±1*† | 13±2* | |
| PetCO2, Torr | Normothermia | 41±1 | 41±1 | 40±1 | 41±2 |
| Heat stress | 39±1 | 39±2 | 37±2*†‡ | 35±2*†‡ | |
Values are listed as means ± SE. HR, heart rate; MAP, mean arterial blood pressure; Zo, thoracic impedance; Bf, breathing frequency; PetCO2, end-tidal carbon dioxide; LBNP, lower body negative pressure.
Significant difference (P < 0.05) from baseline.
Significant difference (P < 0.05) from the preceding time point.
Significant difference (P <0.05) between normothermia and heat stress.
Heart rate significantly increased during LBNP; however, in normothermia the increase in heart rate occurred only at 40 mmHg LBNP, compared with significant increases in heart rate at 20 mmHg LBNP during whole body heating (Table 1). Similarly, MAP and PetCO2 decreased at 40 mmHg LBNP during normothermia, while these variables significantly decreased at a lower LBNP during whole body heating (e.g., 20 mmHg and 30 mmHg, respectively; see Table 1). LBNP increased thoracic impedance (indicating a decrease in central blood volume), but the magnitude of this increase was unaffected by the thermal condition.
LBNP significantly decreased CBV during both normothermia and heat stress (Table 1). However, significant reductions in CBV occurred at an earlier LBNP during heat stress relative to normothermia (Fig. 2). During normothermia, LBNP did not alter CVR regardless of the level of negative pressure, while LBNP during heat stress increased cerebral vascular resistance (Table 1). The combination of heat stress and LBNP resulted in greater increases in CVR compared with normothermia (Fig. 3). Together, these data clearly indicate that there were greater decreases in CBV and increases in CVR per gradation LBNP (i.e., 20 and 30 mmHg) during heat stress compared with normothermia.
Fig. 2.
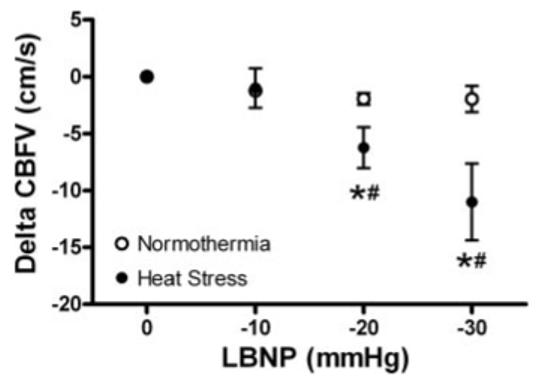
Effects of normothermia and heat stress on the change in cerebral blood velocity (CBFV) during lower body negative pressure (LBNP). Baseline represents 0 mmHg LBNP. *Significant difference from baseline. #Significant difference from the previous LBNP. n = 5 given that only five subjects tolerated 30 mmHg LBNP while heat stressed. The three subjects who did not tolerate 30 mmHg LBNP were excluded from this figure to appropriately compare the paired and interactive effects of whole body heating and LBNP.
Fig. 3.
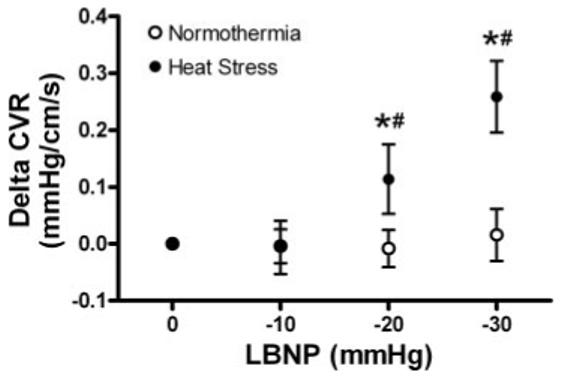
Effects of normothermia and heat stress on the change of cerebral vascular resistance (CVR) during LBNP. Baseline represents 0 mmHg LBNP. *Significant difference from baseline. #Significant difference from the previous LBNP. n = 5 given that only five subjects tolerated 30 mmHg LBNP. The three subjects who did not tolerate 30 mmHg LBNP were excluded from this figure to appropriately compare the paired and interactive effects of whole body heating and LBNP.
DISCUSSION
The major findings of this study are that 1) whole body heating increases CVR leading to reductions in CBV, 2) combined heat and orthostatic stress causes further increases in CVR and accompanying greater reductions in CBV per gradation of LBNP relative to orthostatic stress in normothermia, and 3) whole body heating causes considerable decreases in orthostatic tolerance during LBNP. These results suggest that changes in the cerebral vasculature during heat stress likely contribute to reduced orthostatic tolerance.
During heat stress, CBV is reduced and CVR is elevated before orthostatic stress compared with normothermia. This observation suggests that heat stress reduces the reserve by which further reductions in CBV can occur before the onset of syncopal symptoms. The observed increase in CVR is in contrast to our previous findings in which we observed only a tendency for an increase in this variable during heat stress (42). Differences in these findings between studies are likely due to a lower subject number, and thus statistical power, in the prior study compared with the present study. Compounding these lower baseline (i.e., pre-LBNP) CBV values during heat stress are greater decreases in CBV per gradation of LBNP (see Fig. 3), together leading to greater reductions in orthostatic tolerance. The mechanism by which the same LBNP caused greater reductions in CBV during heat stress was likely due to a combination of greater reductions in cerebral perfusion pressure coupled with greater increases in CVR per gradation of LBNP relative to these responses during LBNP in normothermia (see Table 1).
It is widely recognized that autoregulation is important in controlling CBF during perturbations that change perfusion pressure (31). It is interesting to note that increased CVR during LBNP, while subjects were heat stressed, is in contrast to what would be expected for cerebrovascular autoregulation. That is, given that perfusion pressure was reduced by LBNP in heat-stressed subjects, cerebrovascular autoregulation would dictate reductions in CVR to maintain flow, which is in contrast to the observed increases in this variable. This observation suggests that heat stress impairs cerebrovascular autoregulatory capabilities. In contrast to this hypothesis, Doering et al. (7) reported an increase in the autoregulation index after a hypotensive challenge during heat stress compared with responses during both normothermia and cold stress. Two important methodological differences exist between the present study and that of Doering et al. (7). First, Doering et al. (7) used a single brief hypotensive challenge, vs. a sustained and graded orthostatic stress used in this study. Second, the current study used a greater thermal stress, which resulted in pronounced increases in internal temperature, mean skin temperature, heart rate, cutaneous vascular conductance, and sweat rate. In the cited study (7), cerebral autoregulation was assessed after internal temperature was elevated only ∼0.4°C and there were no reports of mean skin temperature, heart rate, or thermoregulatory effector responses (e.g., skin blood flow and sweat rate). Therefore, it is difficult to directly compare these studies. Nevertheless, it is possible that slight increases in internal temperature may not affect or may improve cerebrovascular autoregulation, while a greater heat stress impairs this response and contributes to heat-related syncope.
Heat stress increases sympathetic nerve activity to kidneys, muscle, and skin (34). Orthostatic stress is also a potent activator of the sympathetic nervous system (20). Sympathetic stimulation during a hypotensive episode has previously been identified to cause vasoconstriction of cerebral blood vessels (10, 23). Thus a component of the increase in CVR during heat stress alone, and combined with LBNP, could be due to sympathetically mediated cerebral vasoconstriction. Compounding identified changes in CBV and CVR during heat stress, is that increases in sympathetic activity may cause a rightward shift of the cerebrovascular autoregulation curve (31). A rightward shift would move the operating point to a location closer to the steep descending portion of the curve, resulting in greater reductions in cerebral blood flow per change in perfusion pressure. The possibility that these sympathetically mediated events alter cerebrovascular control in heat stress subjects warrants further investigation.
MAP was not significantly different between normothermia and heat stress trials and was maintained during low levels of LBNP in both conditions. Recent evidence suggests that despite similar MAP, central blood volume can affect CBV during orthostasis (40). To account for the potential of greater decreases in central blood volume during whole body heating, we measured thoracic impedance as an index of central blood volume (4, 9). There were no significant differences in the change in thoracic impedance during LBNP between normothermia and heat stress. A second cardiovascular parameter that can also alter CBV is cardiac output (30). Although we did not measure cardiac output in this study, previous results from our laboratory have identified increases in cardiac output during heat stress and similar decreases in cardiac output during head-up tilt between normothermia and heat stress (42). Thus it is doubtful that differences in central blood volume or cardiac output contributed appreciably to differences in CBV responses observed in this study.
PetCO2 decreased during whole body heating and continued to decrease during subsequent LBNP. These decreases in PetCO2 paralleled increases in CVR and likely contribute to the reduction in cerebral perfusion, given the observed strong relationship between decreases in PetCO2 and decreases in CBV (16, 36). It has been proposed that in resting humans every 1 Torr change in PaCO2 changes cerebral blood flow ∼3% in the same direction (33). Using this calculation with the present data, we found that a significant component, but not all, of the elevation in CVR can be accounted for by the decrease in PaCO2. Nybo and Nielsen (29) determined that during an exercise-heat stress, the decrease in PetCO2 accounted for approximately one-half of the reduction in CBV, but the other half was likely due to other mechanisms. Followup studies have identified increases in CO2 reactivity (32) and strong relations between PaCO2 and global cerebral blood flow (28) during exercise in the heat. However, responses during an exercise-heat stress paradigm may be very different relative to passive heating because during exercise, variables such as ventilation rate, cardiac output (heart rate and stroke volume), and systolic blood pressure are elevated to a greater extent relative to during passive heating (6, 17, 29, 39). Given these data, it is difficult to directly compare exercise-heat and passive-heat stress conditions with respect to the impact of changes in PaCO2 on the control of CVR and cerebral perfusion.
Comparative observations also have identified decreases in brain and spinal cord blood flow (assessed via microspheres) during heat stress in panting, but not nonpanting, species (12-14). This indicates that high respiratory frequencies that result in decreases in PaCO2 can be influential in the decrease in brain blood flow during heating. Our data identify significant increases in respiration frequency and decreases in PetCO2 during heat stress. However, some caution must be made given the small increase in respiratory rate observed in the current subjects relative to panting species, such as the canine, which can respire over 300 times per minute (15). Nevertheless, it is clear that PetCO2 and PaCO2 play an important, but not solitary causatory role, in orthostatic intolerance in humans (18, 35) and that increasing inspiratory CO2 can improve orthostatic tolerance in a number of conditions (3, 27).
Limitations
Whole body heating reduces central venous pressure 3-4 mmHg (5, 25). In the present experiment, central venous pressure was not measured, although changes in this variable are expected to follow those previously observed. Given that perfusion pressure is the pressure gradient between arterial and venous circulatory regions, a reduction in venous pressure during heat stress results in a slight increase in perfusion pressure despite a lack of change in arterial pressure. However, if actual perfusion pressure (i.e., arterial-venous pressure) had been used to calculate CVR, then even greater increases in vascular resistance would have been identified during heat stress. Thus the limitation of not accounting for venous pressures in the calculation of vascular resistance does not affect the interpretation of the heat stress results.
In the current study, middle cerebral artery velocity was used as an index of cerebral blood flow. We recognize that velocity is representative of flow only if the diameter of the vessel remains unchanged. In support of this concept, investigators directly measured the middle cerebral artery diameter in humans and found that the diameter of this large vessel was either not changed or was only minimally affected by changes in MAP and PetCO2 (11, 36). Thus it is likely that changes in CBV reported in the present investigation reflect changes in cerebral blood flow.
In summary, heat stress dramatically reduces orthostatic tolerance in humans. Whole body heating reduces CBV and increases CVR during nonorthostatic and orthostatic conditions. Additionally, heat stress resulted in greater decreases in CBV and increases in CVR during LBNP compared with normothermia. These cerebral vascular changes occurred in combination with decreases in PetCO2 and presumably increases in cerebral sympathetic activity, indicating potential mechanisms for the observed cerebral vasoconstriction under these conditions. Collectively, compromised cerebral perfusion contributes to the reduction in orthostatic tolerance during heat stress.
ACKNOWLEDGMENTS
The authors would like to express their appreciation to Scott L. Davis, Sylvan Durand, and Kimberly Williams for their technical assistance, and to the subjects for their willing participation in this project.
GRANTS
This research project was funded in part by grants from the National Heart, Lung, and Blood Institute (HL-61388, HL-67422, and HL-10488) and an American Heart Association Post-Doctoral Fellowship Award and Beginning Grant-in-Aid.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +G z acceleration. J Appl Physiol. 1972;33:418–420. doi: 10.1152/jappl.1972.33.4.418. [DOI] [PubMed] [Google Scholar]
- 2.Bean WB, Eichna LW. Performance in relation to environmental temperature. Fed Proc. 1943;2:144–158. [Google Scholar]
- 3.Blaber AP, Bondar RL, Moradshahi P, Serrador JM, Hughson RL. Inspiratory CO2 increases orthostatic tolerance during repeated tilt. Aviat Space Environ Med. 2001;72:985–991. [PubMed] [Google Scholar]
- 4.Cai Y, Holm S, Jenstrup M, Stromstad M, Eigtved A, Warberg J, Hojgaard L, Friberg L, Secher NH. Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol. 2000;89:1569–1576. doi: 10.1152/jappl.2000.89.4.1569. [DOI] [PubMed] [Google Scholar]
- 5.Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol. 1999;86:605–610. doi: 10.1152/jappl.1999.86.2.605. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard MK, Ogoh S, Dawson EA, Yoshiga CC, Quistorff B, Secher NH. Cerebral carbohydrate cost of physical exertion in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R534–R540. doi: 10.1152/ajpregu.00256.2004. [DOI] [PubMed] [Google Scholar]
- 7.Doering TJ, Aaslid R, Steuernagel B, Brix J, Niederstadt C, Breull A, Schneider B, Fischer GC. Cerebral autoregulation during whole body hypothermia and hyperthermia stimulus. Am J Phys Med Rehab. 1999;78:33–38. doi: 10.1097/00002060-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Doering TJ, Brix J, Schneider B, Rimpler M. Cerebral hemodynamics and cerebral metabolism during cold and warm stress. Am J Phys Med Rehabil. 1996;75:408–415. doi: 10.1097/00002060-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- 10.Fitch W, MacKenzie ET, Harper AM. Effects of decreasing arterial blood pressure on cerebral blood flow in the baboon. Influence of the sympathetic nervous system. Circ Res. 1975;37:550–557. doi: 10.1161/01.res.37.5.550. [DOI] [PubMed] [Google Scholar]
- 11.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- 12.Hales JR, Rowell LB, King RB. Regional distribution of blood flow in awake heat-stressed baboons. Am J Physiol Heart Circ Physiol. 1979;237:H705–H712. doi: 10.1152/ajpheart.1979.237.6.H705. [DOI] [PubMed] [Google Scholar]
- 13.Hales JRS. Effects of exposure to hot environments on total and regional blood flow in the brain and spinal cord of the sheep. Pflügers Arch. 1973;344:327–337. doi: 10.1007/BF00592785. [DOI] [PubMed] [Google Scholar]
- 14.Hales JRS. The redistribution of cardiac output in the dog during heat stress. J Therm Biol. 1975;1:29–34. [Google Scholar]
- 15.Hemingway A. The panting response of normal unanesthetized dogs to measured dosages of diathermy heat. Am J Physiol. 1938;121:747–754. [Google Scholar]
- 16.Ide K, Eliasziw M, Poulin MJ. The relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 2003;95:129–137. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- 17.Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand. 1998;162:13–20. doi: 10.1046/j.1365-201X.1998.0280f.x. [DOI] [PubMed] [Google Scholar]
- 18.Immink RV, Secher NH, Roos CM, Pott F, Madsen PL, Lieshout JJ. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol. 2006;96:609–614. doi: 10.1007/s00421-006-0136-6. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35:798–803. doi: 10.1152/jappl.1973.35.6.798. [DOI] [PubMed] [Google Scholar]
- 20.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation. 1994;90:298–306. doi: 10.1161/01.cir.90.1.298. [DOI] [PubMed] [Google Scholar]
- 21.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- 22.Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- 23.Linder J. Effects of cervical sympathetic stimulation on cerebral and ocular blood flows during hemorrhagic hypotension and moderate hypoxia. Acta Physiol Scand. 1982;114:379–386. doi: 10.1111/j.1748-1716.1982.tb06998.x. [DOI] [PubMed] [Google Scholar]
- 24.Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58:541–560. doi: 10.1016/s0301-0082(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 25.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- 26.Njemanze PC. Critical limits of pressure-flow relation in the human brain. Stroke. 1992;23:1743–1747. doi: 10.1161/01.str.23.12.1743. [DOI] [PubMed] [Google Scholar]
- 27.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29:1876–1881. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 28.Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- 29.Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol. 2001;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. AOY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 32.Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol. 2006;96:299–304. doi: 10.1007/s00421-005-0079-3. [DOI] [PubMed] [Google Scholar]
- 33.Ringelestien EB, Otis SM. Physiological testing of vasomotor reserve. In: Newell DW, Aaslid R, editors. Transcrianial Doppler. Raven Press; New York: 1992. pp. 83–99. [Google Scholar]
- 34.Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15:505–507. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- 35.Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1087–R1093. doi: 10.1152/ajpregu.00446.2005. [DOI] [PubMed] [Google Scholar]
- 36.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 37.Shvartz E, Strydom NB, Kotze H. Orthostatism and heat acclimation. J Appl Physiol. 1975;39:590–595. doi: 10.1152/jappl.1975.39.4.590. [DOI] [PubMed] [Google Scholar]
- 38.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- 39.Thomas SN, Schroeder T, Secher NH, Mitchell JH. Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol. 1989;67:744–748. doi: 10.1152/jappl.1989.67.2.744. [DOI] [PubMed] [Google Scholar]
- 40.van Lieshout JJ, Pott F, Madsen PL, van Goudoever J, Secher NH. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. 2001;32:1546–1551. doi: 10.1161/01.str.32.7.1546. [DOI] [PubMed] [Google Scholar]
- 41.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–848. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- 42.Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]


