Abstract
The PI3K/Akt/mTOR pathway is a prototypic survival pathway that is constitutively activated in many types of cancer. Mechanisms for pathway activation include loss of tumor suppressor PTEN function, amplification or mutation of PI3K, amplification or mutation of Akt, activation of growth factor receptors, and exposure to carcinogens. Once activated, signaling through Akt can be propagated to a diverse array of substrates, including mTOR, a key regulator of protein translation. This pathway is an attractive therapeutic target in cancer because it serves as a convergence point for many growth stimuli, and through its downstream substrates, controls cellular processes that contribute to the initiation and maintenance of cancer. Moreover, activation of the Akt/mTOR pathway confers resistance to many types of cancer therapy, and is a poor prognostic factor for many types of cancers. This review will provide an update on the clinical progress of various agents that target the pathway, such as the Akt inhibitors perifosine and PX-866 and mTOR inhibitors (rapamycin, CCI-779, RAD-001) and discuss strategies to combine these pathway inhibitors with conventional chemotherapy, radiotherapy, as well as newer targeted agents. We will also discuss how the complex regulation of the PI3K/Akt/mTOR pathway poses practical issues concerning the design of clinical trials, potential toxicities and criteria for patient selection.
Keywords: PI3 kinase, Akt, combination, chemotherapy, cancer, wortmannin, PX-866, perfosine, mTOR inhibitors, rapamycin, RAD-001, PI-103, triciribine (API-2)
1. Introduction
1.1. Activation of the PI3K/Akt/mTOR pathway
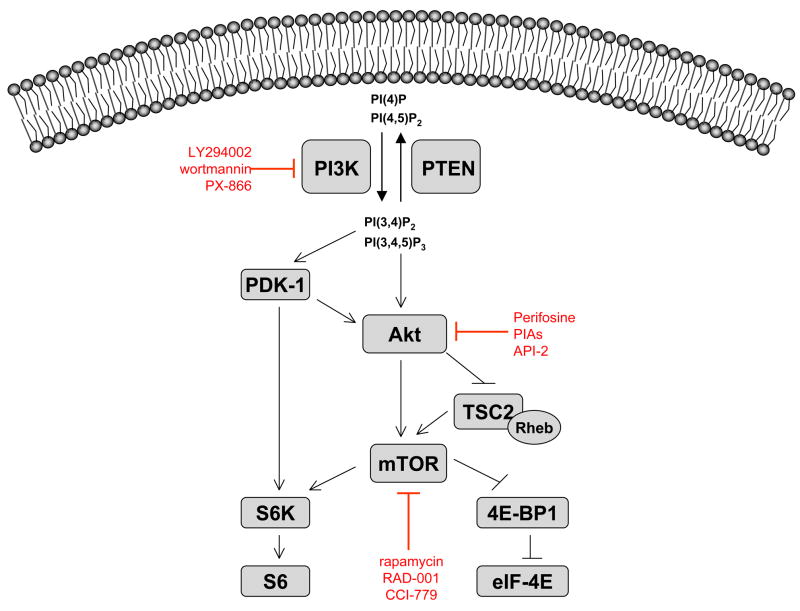
Signaling through the PI3K/Akt/mTOR pathway can be initiated by several mechanisms, all of which increase activation of the pathway in cancer cells. Once activated, the PI3K/Akt/mTOR pathway can be propagated to various substrates, including mTOR, a master regulator of protein translation. Initial activation of the pathway occurs at the cell membrane, where the signal for pathway activation is propagated through class IA PI3K (Figure 1). Activation of PI3K can occur through tyrosine kinase growth factor receptors such as epidermal growth factor receptor (EGFR) and insulin-like growth factor-1 receptor (IGF-1R), cell adhesion molecules such as integrins, G-protein-coupled receptors (GPCRS), and oncogenes such as Ras. PI3K catalyzes phosphorylation of the D3 position on phosphoinositides to generate the biologically active moieties phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3) and phosphatidylinositol-3,4-bisphosphate (PI(3,4)P2). Upon generation, PI(3,4,5)P3 binds to the pleckstrin homology (PH) domains of PDK-1 (3′phosphoinositide-dependent kinase 1) and the serine/threonine kinase Akt, causing both proteins to be translocated to the cell membrane where they are subsequently activated. The tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) antagonizes PI3K by dephosphorylating PI(3,4,5)P3 and (PI(3,4)P2), thereby preventing activation of Akt and PDK-1.
Figure 1. Pharmacological inhibition of the PI3K/Akt/mTOR pathway.
Receptor tyrosine kinases (RTK) such as IGF-IR and EGFR, integrins, and G-protein coupled receptors (GPCR) can all stimulate PI3K. PI3K phosphorylates PI(4)P and PI(4,5)P2 at the 3′-position to generate PI(3,4)P2 and PI(3,4,5)P3, respectively. PTEN opposes the function of PI3K by removing 3′-phosphate groups. LY294002 is a reversible small molecule inhibitor of PI3K, while wortmannin and its analogue, PX-866, are irreversible inhibitors. Generation of 3′-phosphoinositides activates both Akt and PDK-1, which phosphorylates Akt at T308. Akt propagates its signal to affect transcription, apoptosis, and cell cycle progression. Perifosine and phosphatidylinositol ether lipid analogues (PIAs) are lipid-based Akt inhibitors that interact with the PH domain of Akt and prevent its translocation to the membrane, while API-2, also known as triciribine, is a small molecule that inhibits the kinase activity of Akt. Akt can activate mTOR directly by phosphorylation at S2448 or indirectly, by phosphorylation and inactivation of TSC2. When TSC2 is inactivated, the GTPase Rheb is maintained in its GTP-bound state, allowing for increased activation of mTOR. mTOR (TORC1) activates S6 kinase 1, which activates ribosomal protein S6 and leads to increased protein translation. TORC1 also phosphorylates 4EBP-1, causing it to dissociate from eIF4E, and freeing eIF4E to participate in formation of the translation initiation complex. Inhibitors of mTOR include rapamycin and its analogues, RAD-001 and CCI-779, all of which bind to the FK506-binding protein, FKBP-12, which then binds and inhibits mTOR.
Akt exists as three structurally similar isoforms, Akt1, Akt2 and Akt3, which are expressed in most tissues (Zinda et al., 2001). Activation of Akt1 occurs through two crucial phosphorylation events, the first of which occurs at T308 in the catalytic domain by PDK-1 (Andjelkovic et al., 1997; Walker et al., 1998). Full activation requires a subsequent phosphorylation at S473 in the hydrophobic motif, which can be mediated by several kinases such as PDK-1 (Balendran et al., 1999), integrin-linked kinase (ILK) (Delcommenne et al., 1998; Lynch et al., 1999), Akt itself (Toker and Newton, 2000), DNA-dependent protein kinase (Feng et al., 2004; Hill et al., 2002), or mTOR (when bound to Rictor in so called TORC2 complexes (Santos et al., 2001)). Phosphorylation of homologous residues in Akt2 and Akt3 occurs by the same mechanism. Phosphorylation of Akt at S473 is also controlled by a recently described phosphatase, PHLPP (PH domain leucine-rich repeat protein phosphatase), that has two isoforms that preferentially decrease activation of specific Akt isoforms (Brognard et al., 2007). In 3 addition, amplification of Akt1 has been described in human gastric adenocarcinoma (Staal, 1987), and amplification of Akt2 has been described in ovarian, breast, and pancreatic carcinoma (Bellacosa et al., 1995; Cheng et al., 1996). Although mutation of Akt itself is rare, Carpten et al. recently described somatic mutations occurring in the PH domain of Akt1 in a small percentage of human breast, ovarian, and colorectal cancers (Carpten et al., 2007).
1.2. Downstream substrates of activated Akt
Akt recognizes and phosphorylates the consensus sequence RXRXX(S/T) when surrounded by hydrophobic residues. Because this sequence is present in many proteins, numerous Akt substrates have been identified and validated (Obenauer et al., 2003). These substrates control key cellular processes such as apoptosis, cell cycle progression, transcription, and translation. For instance, Akt phosphorylates the FoxO subfamily of forkhead family transcription factors, which inhibits transcription of several pro-apoptotic genes, e.g., Fas-L, IGFBP1 and Bim (Datta et al., 1997; Nicholson and Anderson, 2002). Additionally, Akt can directly regulate apoptosis by phosphorylating and inactivating pro-apoptotic proteins such as BAD, which controls release of cytochrome c from mitochondria, and ASK1 (apoptosis signal-regulating kinase-1), a mitogen-activated protein kinase kinase involved in stress-and cytokine-induced cell death (Datta et al., 1997; del Peso et al., 1997; Zha et al., 1996). In contrast, Akt can phosphorylate IKK, which indirectly increases the activity of nuclear factor kappa B (NF-kB) and stimulates the transcription of pro-survival genes (Ozes et al., 1999; Romashkova and Makarov, 1999; Verdu et al., 1999). Cell cycle progression can also be effected by Akt through its inhibitory phosphorylation of the cyclin-dependent kinase inhibitors, p21WAF1/CIP1 and p27KIP1 (Liang et al., 2002; Shin et al., 2002; Zhou et al., 2001), and inhibition of GSK3β by Akt stimulates cell cycle progression by stabilizing cyclin D1 expression (Diehl et al., 1998). Recently, a novel pro-survival Akt substrate, PRAS40 (proline-rich Akt substrate of 40kDa), has been described (Vander Haar et al., 2007), whereby phosphorylation of PRAS40 by Akt attenuates its ability to inhibit mTORC1 kinase activity. It has been suggested that PRAS40 may be a specific substrate of Akt3 (Madhunapantula et al., 2007). Thus, Akt inhibition might have pleiotropic effects on cancer cells that could contribute to an anti-tumor response.
The best-studied downstream substrate of Akt is the serine/threonine kinase mTOR (mammalian target of rapamycin). Akt can directly phosphorylate and activate mTOR, as well as cause indirect activation of mTOR by phosphorylating and inactivating TSC2 (tuberous sclerosis complex 2, also called tuberin), which normally inhibits mTOR through the GTP-binding protein Rheb (Ras homolog enriched in brain). When TSC2 is inactivated by phosphorylation, the GTPase Rheb is maintained in its GTP-bound state, allowing for increased activation of mTOR. mTOR exists in two complexes: the TORC1 complex, in which mTOR is bound to Raptor, and the TORC2 complex, in which mTOR is bound to Rictor. In the TORC1 complex, mTOR signals to its downstream effectors S6 kinase/ribosomal protein S6 and 4EBP-1/eIF-4E to control protein translation. Although mTOR is generally considered a downstream substrate of Akt, mTOR can also phosphorylate Akt when bound to Rictor in TORC2 complexes, perhaps providing a level of positive feedback on the pathway (Sarbassov et al., 2005). Finally, the downstream mTOR effector S6 kinase-1 (S6K1) can also regulate the pathway by catalyzing an inhibitory phosphorylation on insulin receptor substrate (IRS) proteins. This prevents IRS proteins from activating PI3K, thereby inhibiting activation of Akt (Harrington et al., 2004; Shah et al., 2004).
1.3. Rationale for targeting the PI3K/Akt/mTOR pathway
In addition to preclinical studies, many clinical observations support targeting the PI3K/Akt/mTOR pathway in human cancer. First, immunohistochemical studies using antibodies that recognize Akt when phosphorylated at S473 have shown that activated Akt is detectable in cancers such as multiple myeloma, lung cancer, head and neck cancer, breast cancer, brain cancer, gastric cancer, acute myelogenous leukemia, endometrial cancer, melanoma, renal cell carcinoma, colon cancer, ovarian cancer, and prostate cancer (Alkan and Izban, 2002; Choe et al., 2003; Dai et al., 2005; Ermoian et al., 2002; Gupta et al., 2002; Horiguchi et al., 2003; Hsu et al., 2001; Kanamori et al., 2001; Kreisberg et al., 2004; Kurose et al., 2001; Malik et al., 2002; Min et al., 2004; Nakayama et al., 2001; Nam et al., 2003; Perez-Tenorio and Stal, 2002; Roy et al., 2002; Schlieman et al., 2003; Sun et al., 2001; Terakawa et al., 2003; Yuan et al., 2000). Immunohistochemical analysis has also been used to demonstrate prognostic significance of Akt activation. Phosphorylation of Akt at S473 has been associated with poor prognosis in cancers of the skin (Dai et al., 2005), pancreas (Schlieman et al., 2003; Yamamoto et al., 2004), liver (Nakanishi et al., 2005), prostate (Kreisberg et al., 2004), breast (Perez-Tenorio and Stal, 2002), endometrium (Terakawa et al., 2003), stomach (Nam et al., 2003), brain (Ermoian et al., 2002), and blood (Min et al., 2004). Tsurutani et al. recently extended these studies by using antibodies against two sites of Akt phosphorylation, S473 and T308, to show that Akt activation is selective for NSCLC tumors versus normal tissue and is a better predictor of poor prognosis in NSCLC tumors than S473 alone (Tsurutani et al., 2006). In addition, amplification of Akt isoforms has been observed in some cancers, albeit at a lower frequency (Bellacosa et al., 1995; Cheng et al., 1996; Ruggeri et al., 1998; Staal, 1987).
Another frequent genetic event that occurs in human cancer is loss of tumor suppressor PTEN function. PTEN normally suppresses activation of the PI3K/Akt/mTOR pathway by functioning as a lipid phosphatase. Loss of PTEN function in cancer can occur through mutation, deletion, or epigenetic silencing. Multiple studies have demonstrated a high frequency of PTEN mutations or deletions in a variety of human cancers, including brain, bladder, breast, prostate, and endometrial cancers (Ali et al., 1999; Aveyard et al., 1999; Dahia, 2000; Dreher et al., 2004; Li et al., 1997; Rasheed et al., 1997), making PTEN the second most frequently mutated tumor suppressor gene (Stokoe, 2001). In tumor types where PTEN mutations are rare, such as lung cancer, epigenetic silencing may occur (Forgacs et al., 1998; Kohno et al., 1998; Yokomizo et al., 1998). Several studies have also demonstrated the prognostic significance of PTEN loss in multiple human cancers, where mutation, deletion, or epigenetic silencing of PTEN correlates with poor prognosis and reduced survival (Bertram et al., 2006; Perez-Tenorio et al., 2007; Saal et al., 2007; Smith et al., 2001; Yoshimoto et al., 2007). Collectively, these studies have established that the loss of PTEN is a common mechanism for activation of the PI3K/Akt/mTOR pathway and poor prognostic factor in human cancer.
Finally, activation of PI3K has been described in human cancers. It can result from amplification, overexpression or from mutations in the p110 catalytic or p85 regulatory subunits. Amplification of the 3q26 chromosomal region, which contains the gene PIK3CA that encodes the p110α catalytic subunit of PI3K, occurs in 40% of ovarian (Shayesteh et al., 1999) and 50% of cervical carcinomas (Ma et al., 2000). Somatic mutations of this gene have also been detected in several cancer types and result in increased kinase activity of the mutant PI3K relative to wild-type PI3K. Mutations in the regulatory p85 subunit have also been detected (Jimenez et al., 1998; Philp et al., 2001). Because any of these alterations in individual components would result in activation of the pathway, these studies suggest that pathway activation is one of the most frequent molecular alterations in cancer.
1.4. PI3K/Akt/mTOR pathway and chemotherapeutic resistance
The rationale for targeting the PI3K/Akt/mTOR pathway in combination therapy comes from data describing constitutive or residual pathway activation in cells that have developed resistance to conventional chemotherapy and radiation (West et al., 2002), as well as to other targeted therapies such as EGFR antagonism. In these cases, combining chemotherapy or radiation with a pathway inhibitor can overcome acquired resistance to EGFR tyrosine kinase inhibitors (TKI). Some standard chemotherapeutic agents appear to directly inhibit Akt in vitro, and the cytotoxicity may be a direct consequence of inhibition of Akt signaling (Asselin et al., 2001; Hayakawa et al., 2002). Because Akt is integrally involved in cellular survival, many groups have investigated the effects of combining chemotherapy with pathway inhibitors. Preclinical studies that have investigated this concept will be discussed below.
2. Preclinical combination data
2.1. Combining pathway inhibitors with conventional chemotherapy and radiation
2.1.1. PI3 kinase inhibitors: LY294002 and wortmannin
Targeting PI3 kinase, the most proximal pathway component, has advantages over targeting more distal components such as Akt and mTOR. Inhibitors of PI3K diminish signaling to Rac as well as Akt, providing a broader inhibition of downstream signaling than distal inhibition. The pharmacologic agents LY294002 and wortmannin both target the p110 catalytic subunit of PI3K. Although these commercially available inhibitors effectively inhibit PI3K, poor solubility and high toxicity have limited their clinical application. However, these compounds provide powerful preclinical tools to study the cellular consequences of pathway inhibition. Both of these inhibitors of PI3K sensitize cancer cells to various types of conventional chemotherapy. LY294002 increases cytotoxicity induced by antimicrotubule agents such as taxanes and vinca alkaloids in glioma, ovarian cancer, esophageal cancer, and lung cancer cells in vitro and in vivo (Hu et al., 2002; Mabuchi et al., 2002; Nguyen et al., 2004; Shingu et al., 2003). Wortmannin has also been shown to enhance apoptosis of several cell lines when used in combination with paclitaxel (Hu et al., 2002), cisplatin (Asselin et al., 2001), gemcitabine (Ng et al., 2000), or 5-fluorouracil (Wang et al., 2002), where potentiation of apoptosis caused by wortmannin was associated with inhibition of Akt activation. In another study, wortmannin enhanced cytoxicity of etoposide in eight tumorigenic cell lines, predominantly through inhibition of PI3K-dependent phosphorylation of protein kinase C zeta (PKCζ) (Reis et al., 2005; Skladanowski et al., 2007). Wortmannin can also increase the efficacy of chemotherapeutic agents in vivo. For example, gemcitabine-induced apoptosis of orthotopic pancreatic cancer in xenografts was potentiated by treatment with wortmannin and was associated with decreased Akt phosphorylation (Ng et al., 2001). In addition, the treatment of human ovarian cancer xenografts with wortmannin plus paclitaxel increased apoptosis and decreased tumor burden compared to either agent alone (Hu et al., 2002). Wortmannin combined with cisplatin increased the efficacy of cisplatin in an ovarian cancer model where cancer cells were injected into the peritoneum of nude mice (Ohta et al., 2006). In this study, wortmannin increased cisplatin-induced apoptosis and inhibition of intra-abdominal dissemination of cancer cells. Additionally, several studies have identified PI3K inhibitors as radiosensitizers and augmentation of radiation-induced cytotoxicity has been observed with nanomolar doses of wortmannin (Edwards et al., 2002; Gupta et al., 2003; Sarkaria et al., 1998). Although wortmannin and LY294002 are not clinically useful, newer inhibitors of PI3K such as PX-866 are being developed, but none of these have been combined with traditional chemotherapies.
2.1.2. Akt inhibitors
Perifosine
Due to feedback activation of Akt that results from mTOR inhibition, inhibiting Akt directly may have advantages over targeting more distal components of the pathway. To date, the most developed inhibitor of Akt is perifosine, a lipid-based inhibitor. In vitro, perifosine inhibits translocation of Akt to the cell membrane, and inhibits the growth of melanoma, lung, prostate, colon, and breast cancer cells in association with inhibition of Akt activity (Crul et al., 2002; Kondapaka et al., 2003). Additional in vitro data demonstrates synergistic effects of perifosine and traditional chemotherapeutic agents such as etoposide in leukemia cells (Nyakern et al., 2006), doxorubicin in multiple myeloma cells (Hideshima et al., 2006), and temozolomide in glioma cells (Momota et al., 2005). In the latter study, the combination of perifosine and temozolomide was more effective than temozolomide alone in inhibiting growth of glioma xenografts. Perifosine has also been found to sensitize cancer cells to apoptosis and cell cycle arrest induced by radiation in vitro and in vivo (Caron et al., 2005; Ruiter et al., 1999; Vink et al., 2006a).
PIAs
A relatively new group of lipid-based Akt inhibitors are the phosphatidylinositol ether lipid analogues (PIAs). PIAs were designed to interact with the PH domain of Akt and are structurally similar to the products of PI3 kinase. Although less clinically developed, PIAs are well-characterized in vitro. In addition to Akt inhibition, Gills et al recently identified several molecular targets that contribute to the cytotoxicity of PIAs, including activation of the stress kinase p38α, and PIA-induced cytotoxicity correlates with inhibition of Akt phosphorylation in the NCI60 cell line panel. (Gills et al., 2007; Gills et al., 2006). PIAs mitigate drug resistance to several traditional chemotherapies and ionizing radiation in vitro (Martelli et al., 2003; Tabellini et al., 2004; Van Meter et al., 2006). PIAs also enhance the apoptosis induced by other agents such as tumor necrosis factor-related ligand (TRAIL), all-trans-retinoic acid (ATRA) and motexafin gadolinium (Martelli et al., 2003; Puduvalli et al., 2005; Ramos et al., 2006).
Triciribine (API-2)
API-2, also known as triciribine phosphate, was identified as an Akt inhibitor after screening the National Cancer Institute (NCI)’s structural diversity set. Triciribine inhibits Akt2 phosporylation at both sites (T309 and S474) and inhibits EGF-induced phosphorylation of all three isoforms of Akt in vitro. In vivo, treatment with low doses of triciribine stimulated apoptosis in xenografts with constitutively activated Akt or PTEN mutations, but not in tumors with low Akt activity (Yang et al., 2004). Triciribine has not been preclinically combined with standard chemotherapies or radiation.
2.1.3. mTOR inhibitors: rapamycin and its analogues
The PI3K/Akt/mTOR pathway inhibitors that are most clinically developed, target a more distal pathway component, mTOR. Rapamycin, the prototypic mTOR inhibitor, was discovered in 1975 as a potent anti-fungicide, and is produced naturally by Steptomyces hygroscopicus (Sehgal et al., 1975; Vezina et al., 1975). The ability of rapamycin to inhibit the proliferation of cancer cell lines was shown over 20 years ago (Douros and Suffness, 1981; Eng et al., 1984; Houchens et al., 1983). More recently, rapamycin analogues such as CCI-779 and RAD-001 have been explicitly designed for development as anticancer drugs. These inhibitors of mTOR bind to the FK506-binding protein, FKBP-12, which then binds and inhibits mTOR. Inhibition of mTOR decreases phosphorylation of two downstream targets, 4E-BP1 and S6K, resulting in inhibition of protein synthesis.
Rapamycin and its analogues have been studied in combination with standard chemotherapies. For example, treatment of orthotopic neuroblastoma-bearing mice with rapamycin and vinblastine resulted in inhibition of tumor growth and angiogenesis, with an increase in survival compared to either drug alone (Marimpietri et al., 2007; Marimpietri et al., 2005). Similar results were observed in hepatocellular carcinoma (Ribatti et al., 2007). In vitro, synergy has been observed with rapamycin and paclitaxel, carboplatin, or vinorelbine. In lymphoma models, RAD-001 demonstrates in vitro synergy with rituximab, doxorubicin, and vincristine (Haritunians et al., 2007; Wanner et al., 2006), predominantly through induction of cell cycle arrest. Combinations of RAD-001 and anti-estrogen agents tamoxifen and letrozole also demonstrated enhanced levels of apoptosis than with either drug alone (Boulay et al., 2005; Treeck et al., 2006). Interestingly, RAD-001 sensitizes tumor cells to cisplatin-induced apoptosis in a p53-dependent manner via inhibition of mTOR function, resulting in reduced p21 translation (Beuvink et al., 2005). CCI-779, another rapamycin analogue, has been successfully combined with cisplatin, gemcitabine, and camptothecin in vitro and in vivo (Geoerger et al., 2001; Ito et al., 2006; Thallinger et al., 2007a; Thallinger et al., 2007b; Wu et al., 2005).
Rapamycin and RAD-001 are also potent radiosensitizers through mTOR-dependent enhancement of radiation-induced autophagy (Albert et al., 2006; Kim et al., 2006; Paglin et al., 2005; Moretti et al., 2007). In a recent study, RAD-001 sensitized PTEN wild-type and PTEN null cancer cells to ionizing radiation, but induced more cytotoxicity in PTEN null cells (Cao et al., 2006). RAD-001 also enhances radiation-induced damage of tumor vasculature in vivo through induction of apoptosis of vasculature endothelial cells (Shinohara et al., 2005). Taken together, these data indicate that combining mTOR inhibition with chemotherapy or radiation could be a potentially effective approach in cancer treatment.
2.2. Combining pathway inhibitors with other targeted therapies
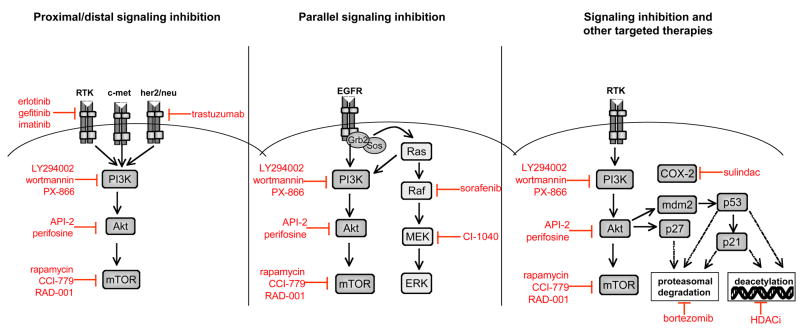
Because signaling of multiple receptor tyrosine kinases (RTKs) is propagated through Akt, simultaneous inhibition of RTKs such as IGF-IR or erbB family members with pathway components such as Akt or mTOR may circumvent feedback activation seen with either approach alone. Such an approach can be considered proximal and distal signaling inhibition (Figure 2, left panel). Based on the observed feedback activation of Akt by mTOR inhibitors, it is possible that they might be most effective when combined with proximal pathway inhibitors. For example, synergistic effects between rapamycin and LY294002, an upstream inhibitor of PI3K, are commonly observed in vitro (Breslin et al., 2005; Sun et al., 2005; Takeuchi et al., 2005). Most recently, Fan et al showed that a dual inhibitor of PI3Kα and mTOR, PI-103, was able to inhibit Akt activity as well as proliferation in glioma cells, regardless of PTEN or EGFR status (Fan et al., 2006). PI-103 was effective in inhibiting the growth of glioma xenografts in the absence of toxicity, most likely through a cytostatic mechanism.
Figure 2. Combinatorial approaches with inhibitors of the PI3K/Akt/mTOR pathway.
Several approaches can be employed when combining pathway inhibitors with other targeted therapies. Inhibition of proximal pathway components, such as receptor tyrosine kinases (RTKs) and oncogenes, combined with distal inhibition of Akt or mTOR may be an effective approach to circumvent feedback activation that could occur with distal inhibition alone (left panel). Alternatively, dual inhibition of parallel signaling pathways prevents compensatory activation of redundant pro-survival pathways (middle panel). Finally, pathway inhibition can be combined with several other types of targeted therapies, including inhibition of histone deacetylase complexes (HDAC), the proteasome and cyclooxygenase-2 (COX-2) (right panel).
Another possible approach is to combine inhibition of the PI3K/Akt/mTOR pathway with inhibition of a parallel pro-survival signaling pathway such as the MEK/ERK pathway (Tortora et al., 2007) (Figure 2, middle panel). This approach abrogates compensatory activation of other pro-survival pathways when the PI3K/Akt/mTOR pathway is inhibited. For example, combining an inhibitor of PI3K with an inhibitor of MEK causes a synergistic increase in apoptosis in both PTEN mutant and wild-type cells (She et al., 2005). Both approaches will be discussed below.
2.2.1. Pathway inhibitors and inhibitors of receptor tyrosine kinases (RTKs)
i) Combinations with EGFR antagonists
Perhaps the most extensive data on proximal and distal signaling inhibition exists for combining PI3K/Akt/mTOR pathway inhibitors with EGFR antagonists. The epidermal growth factor receptor (EGFR/erbB1) is overexpressed or amplified in a variety of tumor types and is a major target in cancer therapy. Patients who respond to EGFR TKIs eventually develop resistance and progressive disease. Elucidated mechanisms of resistance in NSCLC include a somatic T790M mutation in the kinase domain of EGFR (Engelman et al., 2006), epithelial to mesenchymal transition (EMT)(Irie et al., 2005; Thomson et al., 2005; Yauch et al., 2005), amplification of the Met oncogene (Engelman et al., 2005; Engelman et al., 2007) and downregulation of BIM activity (Cragg et al., 2007; Gong et al., 2007). All of these mechanisms of resistance are associated with maintenance and continued activation of the PI3K/Akt/mTOR pathway.
Cancer cell lines with mutant PTEN, which have high levels of Akt are resistant to EGFR antagonists such as gefitinib. PTEN reconstitution can restore sensitivity to EGFR inhibition. She et al. showed that this approach inhibited growth of breast cancer xenografts, which was not seen with either EGFR inhibition or PTEN induction alone (She et al., 2005). Similar results have been observed in NSCLC, prostate, and leukemia cell lines, thereby linking PTEN status and Akt activity with sensitivity to EGFR inhibition (Festuccia et al., 2005; Janmaat et al., 2003; Janmaat et al., 2006; Kokubo et al., 2005; Li et al., 2006). In PTEN-null gefitinib-resistant cells, reintroduction of PTEN function or treatment with LY294002 restores gefitinib sensitivity (She et al., 2003).
Many different PI3K inhibitors can restore sensitivity to EGFR inhibitors (Bianco et al., 2003; She et al., 2003). Sordella et al. found that NSCLC cells transfected with gefitinib-sensitizing EGFR mutations had increased levels of activated Akt, and these cells were more sensitive than their wild-type counterparts not only to gefitinib, but also to LY294002 (Sordella et al., 2004). In another study with PX-866, a PI3K inhibitor selective for p110α, PX-866 was able to abolish gefitinib resistance in NSCLC xenografts. Toxicities associated with PX-866 administration were decreased glucose tolerance and hyperglycemia, both of which were reversed upon discontinuation of drug treatment (Ihle et al., 2005).
Synergistic effects of rapamycin and EGFR TKIs have been observed in several in vitro systems, including glioblastoma multiforme (Hjelmeland et al., 2007; Rao et al., 2005; Wang et al., 2006), prostate cancer (Buck et al., 2006; Masiello et al., 2007), pancreatic cancer (Buck et al., 2006), squamous cell carcinoma (Jimeno et al., 2007), renal cell carcinoma (Costa et al., 2007; Gemmill et al., 2005), leukemia (Mohi et al., 2004), cervical carcinoma (Birle and Hedley, 2006), and non-small cell lung cancer (Buck et al., 2006). Several of these studies extended the efficacy of these combinations to xenograft experiments (Birle and Hedley, 2006; Buck et al., 2006; Jimeno et al., 2007). Buck et al noted re-sensitization and synergistic growth inhibition with the combination of rapamycin and erlotinib in cells lines that were previously resistant to erlotinib (Buck et al., 2006). Li et al noted significant regression of lung tumors in transgenic mice that possessed the secondary resistance mutation T790M when treated with the combination of rapamycin and the irreversible EGFR TKI, HKI-272 (Li et al., 2007). In human glioma cell lines with mutant PTEN, addition of the dual PI3K/mTOR inhibitor PI-103 to erlotinib was necessary to induce growth arrest, suggesting that activation of the PI3K/Akt/mTOR pathway by EGFR-independent mechanisms confers resistance to EGFR inhibitors, which can nonetheless be overcome by the addition of pathway inhibitors. Collectively, these data suggest that the use of EGFR antagonists with pathway inhibitors may be especially beneficial in patients whose tumors harbor mutations in EGFR and/or PTEN, as well as patients who have developed resistance to EGFR TKIs.
ii) Combinations with erbB2 antagonists
Another potentially useful combination is proximal inhibition of erbB2, also known as her-2/neu, with distal inhibition of Akt or mTOR. Inhibition of Akt phosphorylation is a requirement for the anti-proliferative effects of the her-2/neu antagonist, trastuzumab, and trastuzumab-resistant cells exhibit sustained activation of the PI3K/Akt/mTOR pathway (Chan et al., 2005a; Nagata et al., 2004; Yakes et al., 2002). A preclinical study was recently reported combining triciribine with trastuzumab in an effort to circumvent trastuzumab resistance due to loss of PTEN (Lu et al., 2007). In breast cancer cell lines and xenografts, triciribine restored sensitivity to trastuzumab, concomitant with induction of apoptosis and inhibition of tumor growth. In the same study, RAD-001 was also able to re-sensitize trastuzumab resistant cells to apoptosis in vitro and in vivo. Similar results have been observed with rapamycin (Liu et al., 2005; Wang et al., 2007), and classical PI3K inhibitors have also been successfully combined with trastuzumab in vitro (Chan et al., 2005a; Nagata et al., 2004).
iii) Combinations with IGF-IR antagonists
Monoclonal antibodies directed against the insulin-like growth factor 1 receptor (IGF-IR), a transmembrane RTK, have been used extensively in preclinical studies. When bound by IGF-I or IGF-II, IGF-IR is autophosphorylated and activates PI3K. Additionally, feedback activation of Akt induced by mTOR inhibition is partially mediated via upregulation of insulin receptor substrate 1 (IRS1), and subsequent signaling through IGF-IR, suggesting that dual inhibition of IGF-IR and mTOR may be more effective than mTOR inhibition alone. For example, combining rapamycin with a small molecule inhibitor of IGF-IR abrogated feedback activation of Akt and enhanced cytotoxicity of rapamycin in glioma cells (Steinbach et al., 2004). Similarly, combination of a neutralizing antibody directed against IGF-IR with RAD-001 reversed Akt phosphorylation induced by RAD-001, and resulted in additive anti-proliferative effects in leukemic cells (Tamburini et al., 2007). These data demonstrate that proximal inhibition of IGF-IR combined with inhibition of distal pathway components, such as Akt and mTOR, may abrogate feedback activation that results from mTOR inhibition alone.
2.2.2. Pathway inhibitors and other targeted agents
In addition to combining PI3K/Akt/mTOR inhibitors with agents that inhibit either the same or parallel pro-survival signaling pathways, PI3K/Akt/mTOR inhibitors have also been combined with targeted agents that defy easy categorization such as imatinib and those that do not directly affecting signaling pathways, e.g., histone deacetylase (HDAC) inhibitors and proteasome inhibitors (Landis-Piwowar et al., 2006) (Figure 2, right panel). Although the mechanisms behind the efficacy of these combinations are not completely understood, they represent potentially useful combinations for patients whose tumors do not respond to more conventional therapy regimens.
i) PI3 kinase and Akt inhibitors
PI3K inhibitors have been successfully combined with imatinib in leukemic cells (Klejman et al., 2002), as well as sulindac, a non-steroidal anti-inflammatory drug that inhibits COX-2 (Yip-Schneider et al., 2003). LY294002 and wortmannin sensitize cancer cells to histone deacetylase (HDAC) inhibitor-induced apoptosis in vitro and in vivo (Denlinger et al., 2005; Rahmani et al., 2003) (Wang et al., 2002). Rahmani et al (Rahmani et al., 2005) found that treatment with LY294002 inhibited ERK phosphorylation and p21 induction, both of which normally protect leukemic cells from HDAC inhibitor-induced apoptosis. They concluded that the latter mechanisms, rather than inhibition of Akt signaling led to increased cell death. In contrast, sensitization of A549 NSCLC xenografts by LY294002 to HDAC inhibitor-induced apoptosis resulted from Akt-dependent regulation of nuclear factor kappa B transcription (Denlinger et al., 2005). Combined treatment with an HDAC inhibitor and LY294002 inhibited tumor growth concurrently with inhibition of Akt in vivo. In addition to PI3K inhibitors, the Akt inhibitor perifosine has been combined with a handful of other targeted therapies in vitro. Perifosine treatment of PTEN-deficient breast and prostate cancer cells enhanced growth inhibition induced by cetuximab (Li et al., 2006), as well as apoptosis induced by HDAC inhibitors in leukemic cells (Rahmani et al., 2005).
ii) mTOR inhibitors
mTOR inhibitors have also been successfully combined pre-clinically with other targeted therapies. In chronic myelogenous leukemia cells with moderate resistance to imatinib, treatment with imatinib and rapamycin or its analogue, RAD-001, resulted in synergistic inhibition of leukemic cell growth. Rapamycin has also been effectively combined in breast cancer models with targeted agents such as herceptin (Wang et al., 2007), cotylenin A (Kasukabe et al., 2005), and luteolin (Chiang et al., 2007). In multiple myeloma (MM), rapamycin sensitizes MM cells to apoptosis induced by hsp90 inhibitors (Francis et al., 2006), dexamethasone, and thalidomide analogs (Raje et al., 2004; Stromberg et al., 2004). In addition, rapamycin acts cooperatively with small molecule inhibitors of c-met and VEGF, where in the latter study, combination therapy inhibited primary and metastatic growth of orthotopic pancreatic cancer tumors, as well as liver metastasis (Ma et al., 2005; Stephan et al., 2004). mTOR inhibition can be combined with other types of therapeutic approaches. For example, rapamycin and RAD-001 enhance the efficacy of oncolytic viruses that target tumor cells in medulloblastoma and colon cancer xenografts (Lun et al., 2007; Homicsko et al., 2005). Recently, it was shown that infection with a herpes simplex viral vector activates Akt in cancer cells, and that concurrent treatment with viral particles and LY294002 enhanced the efficacy of the virus (Liu et al., 2007). This may be a common feature of pathway inhibition, whereby oncolytic viral infection activates the PI3K/Akt/mTOR pathway, and this activation is amenable to pharmacologic inhibition.
3. Clinical trials with PI3K/Akt/mTOR pathway inhibitors as single agents and in combination with other therapies
Although combinations of pathway inhibitors with various types of chemotherapy have been investigated extensively in preclinical studies, only a few clinical trials with Akt inhibitors and mTOR inhibitors have been reported up to now, while no clinical trials using PI3K inhibitors have been published. These data will be discussed below (Table 1).
Table 1.
Clinical trials combining PI3K/Akt/mTOR pathway inhibitors with other anti-cancer agents
| Pathway Inhibitor | Combination Agent | Trial phase/tumor type | Toxicity (CTC grade 3–5) ≥10% of patients | Response Rates | Comments | Reference |
|---|---|---|---|---|---|---|
| Perifosine | Radiation | Phase I Inoperable solid tumors | Nausea/ vomiting (10%), dysphagia (10%) | 6/21 (29%) CR
5/21 (24%) PR |
Perifosine as radiosensitizer is safe and tolerable | Vink et al, 2006 |
| Rapamycin | Radiation/cisplatin | Phase I Stage III NSCLC | Dysphagia (14%) MTD not defined | Not reported | Rapamycin as radiosensitizer is safe and tolerable | Sarkaria et al, 2007 |
| Gefitinib | Phase I Recurrent malignant glioma | Mucositis (68%), diarrhea (42%), fatigue (37%), anemia (32%), leukopenia (32%), infection (26%), hypercholesterolemia (21%), hypertriglyceridemia (21%), thrombocytopenia (21%), AST/ALT (16%), nausea/vomiting (16%). | 2/34 (6%) PR
13/34 (38%) SD |
Increased grade 3–4 toxicity due to high doses in attempt to cross blood-brain barrier
Combination of EGFR TKI and mTOR inhibitor in GBM currently in phase II |
Reardon et al, 2006 | |
| CCI-779 | 5-FU/leucovorin | Phase I Advanced solid tumors | Asthenia (19%), mucositis (19%), hyperglycemia (15%), diarrhea (15%), anemia (15%), nausea/vomiting (11%) | 3/26 (12%) PR
11/26 (42%) SD |
Discontinued due to two treatment- related deaths. Combination of agents at this dosing schedule not recommended | Punt et al, 2003 |
| IFN-alfa | Phase III Advanced RCC | Asthenia (28%), anemia (38%), dyspnea (10%), infection (11%), neutropenia (15%) | 8.1% CR/PR
28.1% SD |
No difference OS or PFS for combination vs. IFN-a alone
Single agent CCI-779 superior OS and PFS to IFN-a and combination Larger sample size (~210 patients per group) |
Hudes et al, 2007 | |
| RAD-001 | Gefitinib | Phase I Advanced NSCLC | Hypotension (11%), acidosis (11%), azotemia (11%), lymphopenia (11%), stomatitis (11%) | 2/8 (25%) PR | 2/2 responses seen in former smokers
Currently in phase II trials |
Riely et al, 2007 |
| Bevacizumab | Phase I Solid tumors | Pain (71%), mucositis (64%), anorexia (57%), bleeding (50%), rash(50%), hyperlipidemia (43%), fatigue (43%) | 2/16 (13%) PR
8/16 (50%) SD |
Presented only in abstract form
Currently in phase II trials |
Zafar et al, 2006 | |
| Imatinib | Phase I/II GI stromal tumors | Fatigue, diarrhea, vomiting, nausea, anemia, edema, headache, and rash | 2/31 (6%) PR
8/31 (26%) SD |
Presented only in abstract form | Van Oosterom et al, 2005 |
CR = complete response, PR = partial response, SD = stable disease, MTD = maximally tolerated dose, OS = overall survival, PFS = progression-free survival, TKI = tyrosine kinase inhibitor
3.1. Akt inhibitors
3.1.1. Perifosine
A number of phase I and II clinical trials investigating perifosine monotherapy in a variety of tumor types have been completed. In initial phase I trials employing high daily doses of perifosine, gastrointestinal toxicity led to frequent treatment discontinuations (Crul et al., 2002). More recent phase I trials utilized a loading dose followed by smaller daily maintenance doses of perifosine, allowing for rapid achievement of steady state plasma concentrations, and reduced gastrointestinal toxicity during the maintenance phase (Van Ummersen et al., 2004). The loading dose followed by maintenance perifosine has been used in Phase II clinical trials in breast cancer, pancreatic cancer, prostate cancer, head and neck cancer, melanoma, and sarcoma (Argiris et al., 2006; Bailey et al., 2006; Ernst et al., 2005; Knowling et al., 2006; Leighl et al., 2007; Marsh Rde et al., 2007; Posadas et al., 2005). In these trials, the activity of single agent perifosine in solid tumors has been disappointing, with few objective responses noted. Gastrointestinal and constitutional toxicities were problematic, particularly in a trial in advanced pancreatic cancer (Marsh Rde et al., 2007). A phase II trial of perifosine in twenty three patients with soft tissue sarcomas yielded a partial response of nine months duration in one patient with chondrosarcoma, as well as stabilization of disease in five patients at eight weeks. Despite not meeting their objectives for progression free survival (≥ 40% at 6 months) in this trial, the investigators plan to conduct further studies of perifosine in patients with selected soft tissue sarcoma histologies, perhaps enriching their patient population with patients whose tumors bear high expression of p-Akt, and attempting to achieve high steady state plasma levels of perifosine (>6μg/ml) that appears to correlate with clinical benefit (Bailey et al., 2006).
Given the limited efficacy of perifosine monotherapy in a variety of solid malignancies in phase II trials, an alternative strategy would be to combine perifosine with chemotherapy, radiation therapy and targeted agents in an attempt to enhance cytotoxicity and overcome chemotherapeutic resistance through inhibition of the Akt pathway. A phase I trial combining perifosine and radiotherapy in advanced solid tumors demonstrated that perifosine could be safely utilized as a radiation sensitizer, and phase II trials with this strategy are in development (Vink et al., 2006b). In addition, preliminary reports from a number of phase I trials investigating the combination of perifosine with traditional cytotoxic chemotherapeutic agents such as taxanes and gemcitabine indicate that these combinations can be safely administered (Cervera et al., 2006; Ebrahimi et al., 2006; Goggins et al., 2006; Weiss et al., 2006).
Multiple myeloma (MM) is a rational target for perifosine combination therapy based upon in-vitro data in which perifosine induces cytotoxicity in MM cell lines and patient MM cells resistant to conventional therapy (Hideshima et al., 2006). Perifosine also shows antitumor activity in a human plasmacytoma mouse model (Catley et al., 2007). Preliminary results from a phase II study of perifosine alone or in combination with dexamethasone for patients with relapsed or refractory multiple myeloma revealed that single agent perifosine induced stabilization of disease in 6 of 25 evaluable patients. The addition of dexamethasone to perifosine in patients who progressed on perifosine monotherapy conferred a minor response in 3 of 9 evaluable patients and stabilization of disease in 2 of 9 patients (Richardson et al., 2006). Based upon the promising activity of perifosine as a single agent and in combination with dexamethasone, further studies of perifosine in multiple myeloma utilizing different dosing schedules as well as in combination with the proteosome inhibitor bortezomib are planned.
3.1.2. Triciribine (API-2)
In the 1980’s and 1990’s, a number of phase I and II clinical trials were performed utilizing triciribine as a cytotoxic agent in various advanced malignancies at different dosing schedules (Feun et al., 1993; Feun et al., 1984; Hoffman et al., 1996; Mittelman et al., 1983; O’Connell et al., 1987; Schilcher et al., 1986). Minimal efficacy was observed with few objective responses, and triciribine at high doses caused a number of serious toxicities, including hepatotoxicity, hyperglycemia and hypertriglyceridemia. Whether the hyperglycemia and hypertriglyeridemia were related to inhibition of Akt 2 is unknown. In these trials, pharmacokinetic analysis revealed erratic drug levels of triciribine, particularly with a 5-day continuous infusion dosing schedule. With the recent discovery of triciribine as a bona fide Akt inhibitor, phase I clinical trials are currently underway utilizing lower doses of triciribine-phosphate by weekly IV infusions in patients with metastatic solid tumors whose tumors bear high expression of phospho-AKT as well as in patients with myeloid malignancies. In addition, trials combining triciribine-phosphate with tyrosine kinase inhibitors such as erlotinib and lapatinib to overcome primary and secondary resistance mechanisms to ErbB family inhibitors are currently in development.
3.2. mTOR inhibitors: rapamycin, RAD-001, and CCI-779
3.2.1. Clinical trials of mTOR inhibitors as single agents
The mTOR inhibitors, CCI-779 and RAD-001 have been tested as single agents in Phase II trials in a variety of tumor types, and objective responses and stabilization of disease have been noted in breast cancer (Chan et al., 2005b), glioblastoma (Chang et al., 2005; Galanis et al., 2005), neuroendocrine carcinoma (Duran et al., 2006), renal cell carcinoma (Atkins et al., 2004), mantle cell lymphoma (Witzig et al., 2005), and myeloid malignancies (Yee et al., 2006). A recent phase III trial of CCI-779 in poor risk metastatic renal cell carcinoma randomized patients to CCI-779, interferon-alpha (IFN-α) or a combination of the two. Single agent CCI-779 showed statistically significant improvement in progression free survival (5.5 vs 3.1 months) and overall survival (10.9 vs 7.3 months) as compared to IFN-α, while the combination of CCI-779 and IFN-α showed no statistically significant differences in progression free survival (4.7 months) or overall survival (8.4 months) (Hudes et al., 2007). This trial resulted in regulatory approval of single agent CCI-779 as front-line therapy in advanced renal cell carcinoma.
3.2.2. Clinical trials combining mTOR inhibitors with conventional chemotherapy and radiation
Although responses have been observed with single agent rapamycin analogues in a variety of tumor types, in most cases activity is modest and of short duration. This may not be unexpected, as pre-clinical data have shown not only that rapamycin and its analogues are predominantly cytostatic in vitro, but also that feedback activation of Akt after mTOR inhibition may limit the efficacy of mTOR inhibitors as single agents. Therefore, a number of clinical trials are utilizing mTOR inhibitors in combination with chemotherapy and radiation to overcome resistance mechanisms and enhance response. A phase I trial added rapamycin to concomitant radiation and cisplatin for patients with unresectable stage III non-small cell lung cancer (Sarkaria et al., 2007). Unfortunately, this trial was terminated prematurely due to lack of further funding. Despite not reaching the maximal tolerated dose of rapamycin with combination chemo-radiation, the feasibility of utilizing mTOR inhibitors as radiosensitizing agents was established.
Other trials that combined mTOR inhibitors with conventional cytotoxic chemotherapy have revealed some unexpected toxicities. For example, a phase I trial combining CCI-779 with 5-FU and leucovorin in patients with advanced solid tumors was discontinued due to two treatment-related deaths associated with bowel perforation. Based on the overlapping mucocutaneous toxicities of CCI-779 with 5-FU, the combination of these agents at this schedule was not recommended for further development (Punt et al., 2003). Preliminary results of a phase I trial in advanced cancers with weekly gemcitabine at 600mg/m2 (a dose lower than usually administered) and weekly RAD-001 revealed that the combination was not tolerated in a majority of patients due to myelosuppression (Pacey et al., 2004). Pharmacokinetic analysis of these trials did not suggest an interaction between the mTOR inhibitor and the cytotoxic agent. Clearly, based on the unexpected toxicities observed in these trials, investigators should be attentive to potential overlapping toxicities between mTOR inhibitors and conventional chemotherapy.
3.2.3. Clinical trials combining mTOR inhibitors with EGFR antagonists
Because preclinical studies showed that PI3K/Akt/mTOR inhibitors can augment the efficacy and overcome resistance to EGFR TKIs, phase I and II clinical trials are underway testing the combination of EGFR TKI and mTOR inhibitors. A phase I trial in patients with malignant glioma combining gefitinib with rapamycin revealed that daily administration of these agents is feasible, and that rapamycin does not significantly affect gefitinib drug levels. Out of 34 pretreated patients with refractory disease, 2 attained a partial radiographic response and 13 achieved stable disease (Reardon et al., 2006). Based on these results, a number of phase II trials utilizing various combinations of EGFR TKIs and mTOR inhibitors in malignant glioma are underway. A phase I trial combining gefitinib and RAD-001 in patients with advanced NSCLC patients who had not previously been treated with an EGFR TKI yielded partial responses in 2 out of 8 evaluable patients (Milton et al., 2007). The investigators have initiated a phase II clinical trial to further assess the efficacy of this combination.
mTOR inhibitors are also being studied for their ability to overcome secondary resistance to EGFR TKI therapy in NSCLC. In NSCLC patients who progressed after initially responding to EGFR TKI therapy and were continued on the EGFR TKI with subsequent addition of RAD-001, no objective responses were seen three weeks after the addition of RAD-001 (Riely et al., 2007). In spite of these negative preliminary findings, the addition of mTOR inhibitors to EGFR inhibitors as a means of overcoming mechanisms of secondary resistance is not associated with undue toxicity and could be further investigated in clinical trials.
3.2.4. Clinical trials combining mTOR inhibitors with other targeted therapies
A number of clinical trials are investigating the combination of mTOR inhibitors with multi-targeted tyrosine kinase inhibitors other than EGFR TKIs, such as imatinib, sunitinib and sorafenib in a variety of malignancies. Preliminary data from a phase I/II clinical trial combining RAD-001 with imatinib in 31 patients with GI stromal tumors refractory to imatinib resulted in stabilization of disease for greater than four months in eight patients. Two patients subsequently achieved partial responses, suggesting that mTOR inhibition may re-sensitize tumors to imatinib (van Oosterom et al., 2005).
Given that mTOR inhibitors have direct anti-angiogenic effects through regulation of HIF-1α, dual angiogenic inhibition may be a rational approach. Encouraging efficacy data have been reported from a phase I trial, which combined RAD-001 with the anti-VEGF monoclonal antibody bevacizumab in various solid tumors. In a preliminary analysis, the investigators reported partial responses in 2 out of 16 evaluable patients, with an additional 8 out of 16 patients attaining minor responses or stability of disease. The combination appeared well tolerated with minimal overlapping toxicities and no dose limiting toxicities (Zafar et al., 2006).
Based on strong preclinical in vivo data, a number of phase II and III randomized, controlled clinical trials are underway to determine the efficacy and safety of aromatase inhibitors and mTOR inhibitors in hormone receptor positive breast cancer. Despite promising preliminary phase II data from a randomized trial of CCI-779 in combination with letrozole in postmenopausal women with hormone receptor metastatic breast cancer (Carpenter et al., 2005), a phase III trial investigating this combination in the same patient population was terminated after an interim analysis determined that the combination yielded no benefit over letrozole alone (Leary and Dowsett, 2006). Despite this negative trial, the combination of mTOR inhibitors with other molecularly targeted agents remains a promising approach to enhance cytotoxicity, overcome resistance and limit toxicity.
3.3. Prediction of response to PI3K/Akt inhibitors and pathway modulation
3.3.1. Phospho-specific antibodies in IHC and immunoblotting
The clinical use of PI3K/Akt/mTOR pathway inhibitors will be optimized by identifying biomarkers to predict response to therapy and to assess target inhibition in vivo. Traditional methods of assessing pathway activation include immunohistochemistry (IHC) and immunoblotting using phospho-specific antibodies that recognize pathway components when phosphorylated at specific residues. Phosphorylation at these specific sites is indicative of activation. The advantage of IHC is the ability to localize pathway proteins intracellularly, including the plasma membrane, cytoplasm and nucleus. A potential disadvantage is that IHC is not easy to quantify objectively. Several clinical trials have measured pathway components by IHC before and after drug treatment. For example, in a study of CCI-779 in neuroendocrine carcinomas, paired tumor biopsies were obtained at baseline and two weeks following treatment. The only pre-treatment marker evaluated (including PTEN, p53, phospho-S6, phospho-mTOR, phospho-Akt) that correlated with improved tumor response was an elevated baseline level of phospho-mTOR. After 2 weeks of therapy, CCI-779 effectively decreased levels of phospho-S6, validating that the drug inhibited its intended target. Increased levels of p-AKT expression and decreased levels of p-mTOR expression after 2 weeks of treatment were associated with a statistically significant delayed time to progression (Duran et al., 2006). In another phase II study with CCI-779 in recurrent glioblastoma multiforme, increased levels of phospho-p70S6 kinase in baseline tumor specimens were shown to correlate with radiographic response (Galanis et al., 2005). From these small trials, measurement of pathway components such as phospho-Akt, phospho-mTOR and its downstream substrates may serve as predictive biomarkers for patients most likely to respond to PI3K/Akt/mTOR inhibitors, either as monotherapy or in combination with other agents. Future trials should make every effort to incorporate analysis of pathway activation and target modulation in pre- and post-treatment tumor tissue.
Depending on the tumor site, this may, however, require several invasive procedures, which are either not feasible or not safe. Therefore, immunoblotting can be used to assess biomarkers in readily accessible surrogate tissues, such as peripheral blood mononuclear cells (PBMCs). Several trials with mTOR inhibitors have incorporated analysis of downstream substrates of mTOR in PBMCs as a correlate for clinical response. Yee et al. analyzed PBMCs in patients treated with RAD-001 for relapsed or refractory hematologic malignancies. In 6 out of 9 samples studied, RAD-001 decreased phosphorylation of mTOR substrates, including in 3 patients who demonstrated evidence of a clinical response. Interestingly, treatment with RAD-001 led to inhibition of p-AKT in up to two thirds of specimens analyzed, including in all samples where inhibition of mTOR was noted, suggesting that feedback activation of Akt may not be clinically relevant in hematologic malignancies (Yee et al., 2006). Although less invasive than serial tumor biopsies, it is not known whether PBMCs are a valid surrogate tissue in which to measure target inhibition in non-hematologic malignancies.
3.3.2. Imaging modalities
i) Molecular imaging
Ideally, the least invasive and most sensitive way to measure inhibition of pathway components would be to quantify kinase activity of tumor tissue in situ. This has been achieved pre-clinically, where a reporter system was developed to quantitatively measure Akt kinase activity via bioluminescence of an Akt reporter molecule, whereby an increase in luminescence was indicative of inhibition of Akt (Zhang et al., 2007). They showed a dose- and time-dependent inhibition of Akt in several human xenografts following administration of perifosine and API-2 (triciribine) to nude mice. Use of this technology in humans would require stable integration of the reporter construct into tumor cells, which is not currently feasible. However, monitoring Akt activity within live tumor cells provides a dynamic preclinical tool with which to assess target modulation in vivo.
ii) FDG-PET
The use of [18F] Fluorodeoxyglucose (FDG)-positron emission tomography (PET) as a non-invasive means to assess early response to signal transduction inhibitors has been established for gastrointestinal stromal tumor patients treated with imatinib (Gayed et al., 2004). Studying FDG-PET as a pharmacodynamic marker of biochemical modulation by PI3K/Akt/mTOR inhibitors is based on evidence that activation of the pathway controls hexokinase activity and glycolysis (Majewski et al., 2004) and that FDG accumulation within tumors depends on hexokinase activity. Xenografts of (VHL null) renal cell carcinoma tumors showed a two-fold increase in FDG-PET uptake relative to parental tumor cells. This FDG-PET uptake was reduced to baseline levels 24 hours after administration of CCI-779 (Thomas et al., 2006). These data raise the possibility that FDG-PET can be used to detect modulation of the mTOR pathway in patients treated with rapamycin analogues. In a prospective assessment of the addition of RAD-001 to gefitinib or erlotinib in NSCLC, Reily et al. showed that 3 weeks following the addition of the mTOR inhibitor, 5 of 10 patients had a decrease in Standard Uptake Value (SUV) of >15%, with a median reduction in SUV of 18% (Riely et al., 2007). These results need to be confirmed in additional clinical trials, where FDG-PET could be further investigated as a surrogate marker for inhibition of the PI3K/Akt/mTOR pathway.
4. Some clinical considerations for the combination of pathway inhibitors with other chemotherapies
There is substantial preclinical evidence that PI3K/Akt/mTOR pathway inhibitors can be effectively combined with chemotherapy, radiotherapy and targeted agents to enhance efficacy and overcome mechanisms of resistance. The early clinical trials suggest that pathway inhibitors may be beneficial when added to other anti-cancer therapies, particularly other targeted therapies such as EGFR TKIs, imatinib, and bevacizumab, although there remains a paucity of phase II and phase III data to corroborate these findings. Two major issues that will determine whether pathway inhibitors can be successfully used in combination with other anticancer agents are toxicity considerations and patient selection, which will be discussed below in more detail.
4.1. Toxicity concerns
The toxicity of pathway inhibitors will vary with the particular drug as well as with the class of inhibitor. For example, within the class of Akt inhibitors, lipid based compounds such as perifosine or the PIAs may induce more gastrointestinal toxicity than a nucleoside analogue such as triciribine. Certain toxicities, however, may be class specific to all pathway inhibitors, such as de-regulation of glucose and lipid metabolism, which have been clinically observed with Akt inhibitors such as triciribine (dose-limiting hyperglycemia and hypertriglyceridemia in phase I and II trials) as well as with mTOR inhibitors such as rapamycin and its analogues. Whether a therapeutic index can be achieved with pathway inhibitors is presently unknown, as normal cells also rely on the activation of the PI3K/AKT/mTOR pathway. However, cancer cells may have greater reliance on pathway activation for survival than normal cells because they are selectively exposed to stressors such as hypoxia or aneuploidy, which increase activation of PI3k/Akt/mTOR. Thus, pathway inhibition may result in selective cytotoxicity of cancer cells. It has yet to be determined whether the development of severe hyperglycemia and/or hyper-cholesterolemia would correlate with patient response, in a manner analogous to rash in patients responding to EGFR inhibitors. In support of this possibility in a study of CCI-779 in glioblastoma, development of grade 2 or greater hyperlipidemia was associated with a higher rate of radiographic response (Galanis et al., 2005).
A significant concern with mTOR inhibitors is immune suppression, given that rapamycin is FDA approved for the prevention of allograft rejection and blocks IL-2 induced T cell proliferation. However, there is little evidence in the literature to suggest that the mTOR inhibitors cause significant immune compromise when used as single agents. Hidalgo et al., in a phase I trial of CCI-779 in advanced malignancy, found no changes in lymphocyte cell surface phenotypic markers and lymphocyte subsets. Furthermore, there was no significant change in lymphocyte proliferation assays nor was there clinical evidence of immune compromise (Hidalgo et al., 2006). In a different study, Yee et al noted a high frequency of infectious episodes in patients with hematologic malignancies treated with RAD-001, but no opportunistic infections were observed. The investigators noted that this increased frequency could be due to underlying immune-compromised states associated with hematologic malignancies (Yee et al., 2006).
In trials combining mTOR inhibitors with conventional chemotherapy, unexpected toxicities in two trials lead to early discontinuation of the studies (Punt et al., 2003; Pacey et al., 2004). However, overlapping toxicities were not observed in preliminary data from trials combining perifosine with conventional chemotherapy (Cervera et al., 2006; Ebrahimi et al., 2006; Goggins et al., 2006; Weiss et al., 2006). Nevertheless, combining pathway inhibitors with conventional cytotoxic chemotherapy could result in more toxicity than when combining inhibitors with molecularly targeted agents. If overlapping toxicities with combination agents are a concern, phase I trials should be designed utilizing doses lower than established single agent doses, even if it resulted in slower achievement of biologically effective pathway inhibition in vivo.
4.2. Patient selection
In designing clinical trials for pathway inhibitors in combination with other agents, particularly phase II trials, investigators should stratify patients by relative strength of pathway activation, or alternatively exclude patients whose tumors do not demonstrate pathway activation. If the PI3K/Akt/mTOR pathway is not activated in tumor cells, then pathway inhibitors would not be expected to have efficacy, assuming that these agents’ clinical activities will not be due to off-target effects. Of the pathway inhibitors discussed in this review, rapamycin is exquisitely specific for mTOR, and has no described off-target effects. One could argue that patients whose tumors did not exhibit mTOR activation would not be expected to benefit from an mTOR inhibitor. Certainly, any changes in design of early phase clinical trials that results in exclusion of patients based on molecular criteria must be accompanied by the development of validated assays that can reliably measure activation of pathway components.
In addition to utilizing activation state specific antibodies in IHC or immunoblotting, other methods for measuring pathway activation are in development. Recently, Saal et al developed a gene expression signature for PTEN loss which correlated with adverse outcomes in breast, prostate, and bladder cancer (Saal et al., 2007). Future trials could prospectively assess cancer cell gene expression signatures of key components of the pathway. Comparisons of pathway component gene expression at baseline and after therapy may be a means by which to determine if an inhibitor is altering gene expression of pathway components and to evaluate if a given gene signature is predictive of response to a pathway inhibitor. An alternative strategy to validate target modulation by pathway inhibitors early in their clinical development would be to test these agents in a patient population with uniform activation of the PI3K/Akt/mTOR pathway and accessible tissues. Such populations might include patients with PTEN hamartomatous tumor syndromes (PHTS) such as Cowden Syndrome. These are rare syndromes in which patients possess germline mutations of PTEN, leading to constitutive activation of the PI3K/Akt/mTOR pathway in benign and malignant tumors. Patients with this syndrome are at increased risk for developing certain malignancies, including thyroid, breast and endometrial cancer. Agents that effectively modulate the pathway in tissues such as PBMCs, gastrointestinal hamartomas, and skin trichilemmomas might have promise as anticancer therapeutics. Those agents that demonstrated modulation of the pathway in patients with PHTS could subsequently be tested in the general population of cancer patients whose tumors bear pathway activation.
In conclusion, the proper selection of patients for clinical trials and reliable demonstration of target inhibition in vivo will be critical to the development of PI3K/Akt pathway inhibitors as anticancer therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5:1183–1189. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- Ali UI, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- Alkan S, Izban KF. Immunohistochemical localization of phosphorylated AKT in multiple myeloma. Blood. 2002;99:2278–2279. doi: 10.1182/blood-2001-01-0317. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Argiris A, Cohen E, Karrison T, et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- Aveyard JS, Skilleter A, Habuchi T, Knowles MA. Somatic mutation of PTEN in bladder carcinoma. Br J Cancer. 1999;80:904–908. doi: 10.1038/sj.bjc.6690439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HH, Mahoney MR, Ettinger DS, et al. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107:2462–2467. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- Balendran A, Casamayor A, Deak M, et al. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- Bertram J, Peacock JW, Fazli L, et al. Loss of PTEN is associated with progression to androgen independence. Prostate. 2006;66:895–902. doi: 10.1002/pros.20411. [DOI] [PubMed] [Google Scholar]
- Beuvink I, Boulay A, Fumagalli S, Zilbermann F, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- Birle DC, Hedley DW. Signaling interactions of rapamycin combined with erlotinib in cervical carcinoma xenografts. Mol Cancer Ther. 2006;5:2494–2502. doi: 10.1158/1535-7163.MCT-05-0504. [DOI] [PubMed] [Google Scholar]
- Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- Breslin EM, White PC, Shore AM, Clement M, Brennan P. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. Br J Pharmacol. 2005;144:791–800. doi: 10.1038/sj.bjp.0706061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Buck E, Eyzaguirre A, Brown E, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- Caron RW, Yacoub A, Li M, et al. Activated forms of H-RAS and K-RAS differentially regulate membrane association of PI3K, PDK-1, and AKT and the effect of therapeutic kinase inhibitors on cell survival. Mol Cancer Ther. 2005;4:257–270. [PubMed] [Google Scholar]
- Carpenter JT, Campone JT, Colomer M, et al. Randomized 3-arm, phase 2 study of temsirolimus (CCI-779) in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol ASCO Ann Meeting Proc. 2005;23(16S Suppl):564. [Google Scholar]
- Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Catley L, Hideshima T, Chauhan D, et al. Alkyl phospholipid perifosine induces myeloid hyperplasia in a murine myeloma model. Exp Hematol. 2007;35:1038–1046. doi: 10.1016/j.exphem.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Cervera A, Nemunaitis A, Ebrahimi JJ, et al. Online Collaborative Oncology Group, Perifosine (P) can be combined with docetaxel (T) without dose reduction of either drug. J Clin Oncol ASCO Ann Meeting Proc Part I. 2006 June 20;24(Suppl):13066. [Google Scholar]
- Chan CT, Metz MZ, Kane SE. Differential sensitivities of trastuzumab (Herceptin)-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005a;91:187–201. doi: 10.1007/s10549-004-7715-1. [DOI] [PubMed] [Google Scholar]
- Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005b;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci (USA) 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CT, Way TD, Lin JK. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21(WAF1/CIP1) expression with rapamycin. Mol Cancer Ther. 2007;6:2127–2138. doi: 10.1158/1535-7163.MCT-07-0107. [DOI] [PubMed] [Google Scholar]
- Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- Costa LJ, Gemmill RM, Drabkin HA. Upstream signaling inhibition enhances rapamycin effect on growth of kidney cancer cells. Urology. 2007;69:596–602. doi: 10.1016/j.urology.2007.01.053. [DOI] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PloS Med. 2007;4:e316. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crul M, Rosing H, de Klerk GJ, et al. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Dahia PL. PTEN, a unique tumor suppressor genex. Eur J Cancer. 2000;7:115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci (USA) 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger CE, Rundall BK, Jones DR. Inhibition of phosphatidylinositol 3-kinase/Akt and histone deacetylase activity induces apoptosis in non-small cell lung cancer in vitro and in vivo. J Thorac Cardiovasc Surg. 2005;130:1422–1429. doi: 10.1016/j.jtcvs.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douros J, Suffness M. New antitumor substances of natural origin. Cancer Treat Rev. 1981;8:63–87. doi: 10.1016/s0305-7372(81)80006-0. [DOI] [PubMed] [Google Scholar]
- Dreher T, Zentgraf H, Abel U, et al. Reduction of PTEN and p27kip1 expression correlates with tumor grade in prostate cancer. Analysis in radical prostatectomy specimens and needle biopsies. Virchows Arch. 2004;444:509–517. doi: 10.1007/s00428-004-1004-6. [DOI] [PubMed] [Google Scholar]
- Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi B, Shiffman B, Birch R, et al. Online Collaborative Oncology Group, A phase 1 study of daily oral perifosine with weekly paclitaxel. J Clin Oncol ASCO Ann Meeting Proc (Part I) 2006;24(Suppl):13117. [Google Scholar]
- Edwards E, Geng L, Tan J, et al. Phosphatidylinositol 3-kinase/Akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002;62:4671–4677. [PubMed] [Google Scholar]
- Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo) 1984;37:1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Janne PA, Mermel C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci (USA) 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Ermoian RP, Furniss CS, Lamborn KR, et al. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–1106. [PubMed] [Google Scholar]
- Ernst DS, Eisenhauer E, Wainman N, et al. Phase II study of perifosine in previously untreated patients with metastatic melanoma. Invest New Drugs. 2005;23:569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- Festuccia C, Muzi P, Millimaggi D, et al. Molecular aspects of gefitinib antiproliferative and pro-apoptotic effects in PTEN-positive and PTEN-negative prostate cancer cell lines. Endocr Relat Cancer. 2005;12:983–998. doi: 10.1677/erc.1.00986. [DOI] [PubMed] [Google Scholar]
- Feun LG, Blessing JA, Barrett RJ, Hanjani P. A phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Am J Clin Oncol. 1993;16:506–508. doi: 10.1097/00000421-199312000-00010. [DOI] [PubMed] [Google Scholar]
- Feun LG, Savaraj N, Bodey GP, et al. Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res. 1984;44:3608–3612. [PubMed] [Google Scholar]
- Forgacs E, Biesterveld EJ, Sekido Y, et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17:1557–1565. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- Francis LK, Alsayed Y, Leleu X, et al. Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res. 2006;12:6826–6835. doi: 10.1158/1078-0432.CCR-06-1331. [DOI] [PubMed] [Google Scholar]
- Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- Gayed I, Vu T, Iyer R, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- Gemmill RM, Zhou M, Costa L, Korch C, Bukowski RM, Drabkin HA. Synergistic growth inhibition by Iressa and Rapamycin is modulated by VHL mutations in renal cell carcinoma. Br J Cancer. 2005;92:2266–2277. doi: 10.1038/sj.bjc.6602646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoerger B, Kerr K, Tang CB, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–1532. [PubMed] [Google Scholar]
- Gills JJ, Castillo SS, Zhang C, et al. Phosphatidylinositol ether lipid analogues that inhibit AKT also independently activate the stress kinase, p38alpha, through MKK3/6-independent and -dependent mechanisms. J Biol Chem. 2007;282:27020–27029. doi: 10.1074/jbc.M701108200. [DOI] [PubMed] [Google Scholar]
- Gills JJ, Holbeck S, Hollingshead M, Hewitt SM, Kozikowski AP, Dennis PA. Spectrum of activity and molecular correlates of response to phosphatidylinositol ether lipid analogues, novel lipid-based inhibitors of Akt. Mol Cancer Ther. 2006;5:713–722. doi: 10.1158/1535-7163.MCT-05-0484. [DOI] [PubMed] [Google Scholar]
- Goggins TF, Shiffman TF, Birch R, et al. Online Collaborative Oncology Group, A phase 1 study of daily oral perifosine with every 3-week paclitaxel. J Clin Oncol ASCO Ann Meeting Proc (Part I) 2006;24 (Supplement):13134. [Google Scholar]
- Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PloS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Cerniglia GJ, Mick R, et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56:846–853. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
- Gupta AK, McKenna WG, Weber CN, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- Haritunians T, Mori A, O’Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, et al. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Tasaka K, et al. Regulation of the PRL promoter by Akt through cAMP response element binding protein. Endocrinology. 2002;143:13–22. doi: 10.1210/endo.143.1.8586. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Feng J, Hemmings B. Identification of a plasma membrane raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12:1251. doi: 10.1016/s0960-9822(02)00973-9. [DOI] [PubMed] [Google Scholar]
- Hjelmeland AB, Lattimore KP, Fee BE, et al. The combination of novel low molecular weight inhibitors of RAF (LBT613) and target of rapamycin (RAD001) decreases glioma proliferation and invasion. Mol Cancer Ther. 2007;6:2449–2457. doi: 10.1158/1535-7163.MCT-07-0155. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Holmes FA, Fraschini G, et al. Phase I-II study: triciribine (tricyclic nucleoside phosphate) for metastatic breast cancer. Cancer Chemother Pharmacol. 1996;37:254–258. doi: 10.1007/BF00688325. [DOI] [PubMed] [Google Scholar]
- Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65:6882–6890. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- Horiguchi A, Oya M, Uchida A, Marumo K, Murai M. Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol. 2003;169:710–713. doi: 10.1097/01.ju.0000038952.59355.b2. [DOI] [PubMed] [Google Scholar]
- Houchens DP, Ovejera AA, Riblet SM, Slagel DE. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur J Cancer Clin Oncol. 1983;19:799–805. doi: 10.1016/0277-5379(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Hsu J, Shi Y, Krajewski S, et al. The AKT kinase is activated in multiple myeloma tumor cells. Blood. 2001;98:2853–2855. doi: 10.1182/blood.v98.9.2853. [DOI] [PubMed] [Google Scholar]
- Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomcat P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- Ihle NT, Paine-Murrieta G, Berggren MI, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Fujimoto K, Mori T, et al. In vivo antitumor effect of the mTOR inhibitor CCI-779 and gemcitabine in xenograft models of human pancreatic cancer. Int J Cancer. 2006;118:2337–2343. doi: 10.1002/ijc.21532. [DOI] [PubMed] [Google Scholar]
- Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer. 2006;118:209–214. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Jones DR, Rodriguez-Viciana P, et al. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Kulesza P, Wheelhouse J, et al. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br J Cancer. 2007;96:952–959. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001;7:892–895. [PubMed] [Google Scholar]
- Kasukabe T, Okabe-Kado J, Kato N, Sassa T, Honma Y. Effects of combined treatment with rapamycin and cotylenin A, a novel differentiation-inducing agent, on human breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res. 2005;7:R1097–1110. doi: 10.1186/bcr1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Mutter RW, Cao C, et al. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling, J. Biol Chem. 2006;281:36883–36890. doi: 10.1074/jbc.M607094200. [DOI] [PubMed] [Google Scholar]
- Klejman A, Rushen L, Morrione A, Slupianek A, Skorski T. Phosphatidylinositol-3 kinase inhibitors enhance the anti-leukemia effect of STI571. Oncogene. 2002;21:5868–5876. doi: 10.1038/sj.onc.1205724. [DOI] [PubMed] [Google Scholar]
- Knowling M, Blackstein M, Tozer R, et al. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- Kohno T, Takahashi M, Manda R, Yokota J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer. 1998;22:152–156. doi: 10.1002/(sici)1098-2264(199806)22:2<152::aid-gcc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kokubo Y, Gemma A, Noro R, et al. Reduction of PTEN protein and loss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA. Br J Cancer. 2005;92:1711–1719. doi: 10.1038/sj.bjc.6602559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- Kreisberg JI, Malik SN, Prihoda TJ, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097–2106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Milacic V, Chen V, et al. The proteasome as a potential target for novel anticancer drugs and chemosensitizers. Drug Resist Updates. 2006;9:263–273. doi: 10.1016/j.drup.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Leary A, Dowsett M. Combination therapy with aromatase inhibitors: the next era of breast cancer treatment? Br J Cancer. 2006;95:661–666. doi: 10.1038/sj.bjc.6603316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl NB, Dent S, Clemons M, et al. A Phase 2 study of perifosine in advanced or metastatic breast cancer. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9584-x. in press. [DOI] [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li X, Luwor R, Lu Y, Liang K, Fan Z. Enhancement of antitumor activity of the anti-EGF receptor monoclonal antibody cetuximab/C225 by perifosine in PTEN-deficient cancer cells. Oncogene. 2006;25:525–535. doi: 10.1038/sj.onc.1209075. [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Liu M, Howes A, Lesperance J, et al. Antitumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res. 2005;65:5325–5336. doi: 10.1158/0008-5472.CAN-04-4589. [DOI] [PubMed] [Google Scholar]
- Liu TC, Wakimoto H, Martuza RL, Rabkin SD. Herpes Simplex virus Us3(−) mutant as oncolytic strategy and synergizes with phosphatidylinositol 3-kinase-Akt-targeting molecular therapeutics. Clin Cancer Res. 2007;13:5897–5902. doi: 10.1158/1078-0432.CCR-07-1013. [DOI] [PubMed] [Google Scholar]
- Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming Trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- Lun XQ, Zhou H, Alain T, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DK, Ellis CA, Edwards PA, Hiles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18:8024–8032. doi: 10.1038/sj.onc.1203258. [DOI] [PubMed] [Google Scholar]
- Ma PC, Schaefer E, Christensen JG, Salgia R. A selective small molecule c-MET Inhibitor, PHA665752, cooperates with rapamycin. Clin Cancer Res. 2005;11:2312–2319. doi: 10.1158/1078-0432.CCR-04-1708. [DOI] [PubMed] [Google Scholar]
- Ma YY, Wei SJ, Lin YC, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19:2739–2744. doi: 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- Mabuchi S, Ohmichi M, Kimura A, et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002;277:33490–33500. doi: 10.1074/jbc.M204042200. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–3636. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Malik SN, Brattain M, Ghosh PM, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- Marimpietri D, Brignole C, Nico B, et al. Combined therapeutic effects of vinblastine and rapamycin on human neuroblastoma growth, apoptosis and angiogenesis. Clin Cancer Res. 2007;13:3977–3988. doi: 10.1158/1078-0432.CCR-06-2757. [DOI] [PubMed] [Google Scholar]
- Marimpietri D, Nico B, Vacca A, et al. Synergistic inhibition of human neuroblastoma-related angiogenesis by vinblastine and rapamycin. Oncogene. 2005;24:6785–6795. doi: 10.1038/sj.onc.1208829. [DOI] [PubMed] [Google Scholar]
- Marsh Rde W, Rocha Lima CM, Levy DE, et al. A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2007;30:26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Tazzari PL, Tabellini G, et al. A new selective AKT pharmacological inhibitor reduces resistance to chemotherapeutic drugs, TRAIL, all-trans-retinoic acid, and ionizing radiation of human leukemia cells. Leukemia. 2003;17:1794–1805. doi: 10.1038/sj.leu.2403044. [DOI] [PubMed] [Google Scholar]
- Masiello D, Mohi MG, McKnight NC, et al. Combining an mTOR antagonist and receptor tyrosine kinase inhibitors for the treatment of prostate cancer. Cancer Biol Ther. 2007;6:195–201. doi: 10.4161/cbt.6.2.3588. [DOI] [PubMed] [Google Scholar]
- Milton DT, Riely GJ, Azzoli CG, et al. Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer. 2007;110:599–605. doi: 10.1002/cncr.22816. [DOI] [PubMed] [Google Scholar]
- Min YH, Cheong JW, Kim JY, et al. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004;64:5225–5231. doi: 10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- Mittelman A, Casper ES, Godwin TA, Cassidy C, Young CW. Phase I study of tricyclic nucleoside phosphate. Cancer Treat Rep. 1983;67:159–162. [PubMed] [Google Scholar]
- Mohi MG, Boulton C, Gu TL, et al. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc Natl Acad Sci (USA) 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist Updates. 2007;10:135–143. doi: 10.1016/j.drup.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer. 2005;103:307–312. doi: 10.1002/cncr.20774. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037–3044. doi: 10.1002/1097-0142(20011215)92:12<3037::aid-cncr10171>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nam SY, Lee HS, Jung GA, et al. Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. Apmis. 2003;111:1105–1113. doi: 10.1111/j.1600-0463.2003.apm1111205.x. [DOI] [PubMed] [Google Scholar]
- Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7:3269–3275. [PubMed] [Google Scholar]
- Ng SS, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine- induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5455. [PubMed] [Google Scholar]
- Nguyen DM, Chen GA, Reddy R, Tsai W, Schrump WD, Cole G, Jr, Schrump DS. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J Thorac Cardiovasc Surg. 2004;127:365–375. doi: 10.1016/j.jtcvs.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Nyakern M, Cappellini A, Mantovani I, Martelli AM. Synergistic induction of apoptosis in human leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Mol Cancer Ther. 2006;5:1559–1570. doi: 10.1158/1535-7163.MCT-06-0076. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Rubin J, Hahn RG, Kvols LK, Moertel CG. Phase II clinical trial of tricyclic nucleoside phosphate for advanced colorectal cancer. Cancer Treatm Rep. 1987;71:333–334. [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Ohmichi M, Hayasaka T, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147:1761–1769. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine- threonine kinase [see comments] Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pacey S, Steven N, Brock C, et al. Results of a phase 1 clinical trial investigating a combination of the oral mTOR-inhibitor everolimus (E, RAD001) and gemcitabine (GEM) in patients (pts) with advanced cancers. J Clin Oncol ASCO Ann Meeting Proc. 2004;22 (Suppl):3120. [Google Scholar]
- Paglin S, Lee NY, Nakar C, et al. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- Perez-Tenorio G, Alkhori L, Olsson B, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp AJ, Campbell IG, Leet C, et al. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- Posadas EM, Gulley J, Arlen PM, et al. A Phase II study of Perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- Puduvalli VK, Sampath D, Bruner JM, Nangia J, Xu R, Kyritsis AP. TRAIL-induced apoptosis in gliomas is enhanced by Akt-inhibition and is independent of JNK activation. Apoptosis. 2005;10:233–243. doi: 10.1007/s10495-005-6078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt CJ, Boni J, Bruntsch U, Peters M, Thielert C. Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol. 2003;14:931–937. doi: 10.1093/annonc/mdg248. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Reese E, Dai Y, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Yu C, Reese E, Ahmed W, Hirsch K, Dent P, Grant S. Inhibition of PI-3 kinase sensitizes human leukemic cells to histone deacetylase inhibitor-mediated apoptosis through p44/42 MAP kinase inactivation and abrogation of p21(CIP1/WAF1) induction rather than AKT inhibition. Oncogene. 2003;22:6231–6242. doi: 10.1038/sj.onc.1206646. [DOI] [PubMed] [Google Scholar]
- Raje N, Kumar S, Hideshima T, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104:4188–4193. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- Ramos J, Sirisawad M, Miller R, Naumovski L. Motexafin gadolinium modulates levels of phosphorylated Akt and synergizes with inhibitors of Akt phosphorylation. Mol Cancer Ther. 2006;5:1176–1182. doi: 10.1158/1535-7163.MCT-05-0280. [DOI] [PubMed] [Google Scholar]
- Rao RD, Mladek AC, Lamont JD, et al. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia. 2005;7:921–929. doi: 10.1593/neo.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed BK, Stenzel TT, McLendon RE, et al. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res. 1997;57:4187–4190. [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh JJ, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- Reis C, Giocanti N, Hennequin C, et al. A role for PKCzeta in potentiation of the topoisomerase II activity and etoposide cytotoxicity by wortmannin. Mol Cancer Ther. 2005;4:1457–1464. doi: 10.1158/1535-7163.MCT-05-0156. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Mangieri D, et al. In vivo inhibition of human hepatocellular carcinoma related angiogenesis by vinblastine and rapamycin. Histol Histopathol. 2007;22:285–289. doi: 10.14670/HH-22.285. [DOI] [PubMed] [Google Scholar]
- Richardson P, Lonial S, Jakubowiak A, et al. A multicenter phase II study of Perifosine (KRX-0401) alone and in combination with dexamethasone (Dex) for patients with relapsed or relapsed/refractory multiple myeloma (MM) ASH Ann Meeting Abstracts. 2006:108. [Google Scholar]
- Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling [see comments] Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Roy HK, Olusola BF, Clemens DL, et al. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. [PubMed] [Google Scholar]
- Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyl-lysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res. 1999;59:2457–2463. [PubMed] [Google Scholar]
- Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci (USA) 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SC, Lacronique V, Bouchaert I, et al. Constitutively active STAT5 variants induce growth and survival of hematopoietic cells through a PI 3-kinase/Akt dependent pathway. Oncogene. 2001;20:2080–2090. doi: 10.1038/sj.onc.1204308. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Schwingler P, Schild SE, et al. Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J Thorac Oncol. 2007;2:751–757. doi: 10.1097/JTO.0b013e3180cc2587. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- Schilcher RB, Haas CD, Samson MK, Young JD, Baker LH. Phase I evaluation and clinical pharmacology of tricyclic nucleoside 5′-phosphate using a weekly intravenous regimen. Cancer Res. 1986;46:3147–3151. [PubMed] [Google Scholar]
- Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, et al. PIK3CA is implicated as an oncogene in ovarian cancer [see comments] Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- Shingu T, Yamada K, Hara N, et al. Synergistic augmentation of antimicrotubule agent-induced cytotoxicity by a phosphoinositide 3-kinase inhibitor in human malignant glioma cells. Cancer Res. 2003;63:4044–4047. [PubMed] [Google Scholar]
- Shinohara ET, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24:5414–5422. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]
- Skladanowski A, Bozko P, Sabisz M, Larsen AK. Dual inhibition of PI3K/Akt signaling and the DNA damage checkpoint in p53-deficient cells with strong survival signaling: implications for cancer therapy. Cell Cycle. 2007;6:2268–2275. doi: 10.4161/cc.6.18.4705. [DOI] [PubMed] [Google Scholar]
- Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci (USA) 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach JP, Eisenmann C, Klumpp A, Weller M. Co-inhibition of epidermal growth factor receptor and type 1 insulin-like growth factor receptor synergistically sensitizes human malignant glioma cells to CD95L-induced apoptosis. Biochem Biophys Res Commun. 2004;321:524–530. doi: 10.1016/j.bbrc.2004.06.175. [DOI] [PubMed] [Google Scholar]
- Stephan S, Datta K, Wang E, et al. Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clin Cancer Res. 2004;10:6993–7000. doi: 10.1158/1078-0432.CCR-04-0808. [DOI] [PubMed] [Google Scholar]
- Stokoe D. PTEN Curr Biol. 2001;11:R502. doi: 10.1016/s0960-9822(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Stromberg T, Dimberg A, Hammarberg A, et al. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004;103:3138–3147. doi: 10.1182/blood-2003-05-1543. [DOI] [PubMed] [Google Scholar]
- Sun M, Wang G, Paciga JE, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- Tabellini G, Tazzari PL, Bortul R, et al. Novel 2′-substituted, 3′-deoxy-phosphatidyl-myo-inositol analogues reduce drug resistance in human leukaemia cell lines with an activated phosphoinositide 3-kinase/Akt pathway. Br J Haematol. 2004;126:574–582. doi: 10.1111/j.1365-2141.2004.05073.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- Tamburini J, Chapuis N, Bardet V, et al. mTORC1 inhibition activates PI3K/Akt by up-regulating IGF-1R signaling in acute myeloid leukemia: rational for therapeutic inhibition of both pathways. Blood. 2007 doi: 10.1182/blood-2007-03-080796. in press. [DOI] [PubMed] [Google Scholar]
- Terakawa N, Kanamori Y, Yoshida S. Loss of PTEN expression followed by Akt phosphorylation is a poor prognostic factor for patients with endometrial cancer. Endocr Relat Cancer. 2003;10:203–208. doi: 10.1677/erc.0.0100203. [DOI] [PubMed] [Google Scholar]
- Thallinger C, Poeppl W, Pratscher B, et al. CCI-779 plus cisplatin is highly effective against human melanoma in a SCID mouse xenotranplantation model. Pharmacology. 2007a;79:207–213. doi: 10.1159/000101008. [DOI] [PubMed] [Google Scholar]
- Thallinger C, Werzowa J, Poeppl W, et al. Comparison of a treatment strategy combining CCI-779 plus DTIC versus DTIC monotreatment in human melanoma in SCID mice. J Invest Dermatol. 2007b;127:2411–2417. doi: 10.1038/sj.jid.5700872. [DOI] [PubMed] [Google Scholar]
- Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- Tortora G, Bianco R, Daniele G, et al. Overcoming resistance to molecularly targeted anticancer therapies: rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug Resist Updates. 2007;10:81–100. doi: 10.1016/j.drup.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck O, Wackwitz B, Haus U, Ortmann O. Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol. 2006;102:292–299. doi: 10.1016/j.ygyno.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- Van Meter TE, Broaddus WC, Cash D, Fillmore H. Cotreatment with a novel phosphoinositide analogue inhibitor and carmustine enhances chemotherapeutic efficacy by attenuating AKT activity in gliomas. Cancer. 2006;107:2446–2454. doi: 10.1002/cncr.22248. [DOI] [PubMed] [Google Scholar]
- Van Oosterom A, Reichardt P, Blay J, et al. A phase I/II trial of the oral mTOR-inhibitor eEverolimus and imatinib mesylate in patients with gastrointestinal stromal tumor (GIST) refractory to im: Study update. ASCO Ann Meeting Abstract # 9033 2005 [Google Scholar]
- Van Ummersen L, Binger K, Volkman J, et al. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Vink SR, Lagerwerf S, Mesman E, et al. Radiosensitization of squamous cell carcinoma by the alkylphospholipid perifosine in cell culture and xenografts. Clin Cancer Res. 2006a;12:1615–1622. doi: 10.1158/1078-0432.CCR-05-2033. [DOI] [PubMed] [Google Scholar]
- Vink SR, Schellens JH, Beijnen JH, et al. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother Oncol. 2006b;80:207–213. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Chan JL, Li W. Rapamycin together with herceptin significantly increased anti-tumor efficacy compared to either alone in ErbB2 over expressing breast cancer cells. Int J Cancer. 2007;121:157–164. doi: 10.1002/ijc.22606. [DOI] [PubMed] [Google Scholar]
- Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li N, Wang X, Kim MM, Evers BM. Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3′-kinase inhibition in the KM20 human colon cancer cell line. Clin Cancer Res. 2002;8:1940–1947. [PubMed] [Google Scholar]
- Wanner K, Hipp S, Oelsner M, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol. 2006;134:475–484. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- Weiss S, Diaz-Lacayo M, Birch R, et al. Online Collaborative Oncology Group, A phase 1 study of daily oral perifosine and weekly gemcitabine. J Clin Oncol ASCO Ann Meeting Proc Vol. 2006;24 (Suppl):13084. [Google Scholar]
- West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updates. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- Wu C, Wangpaichitr M, Feun L, Kuo MT, Robles C, Lampidis T, Savaraj N. Overcoming cisplatin resistance by mTOR inhibitor in lung cancer. Mol Cancer. 2005;4:25. doi: 10.1186/1476-4598-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- Yamamoto S, Tomita Y, Hoshida Y, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Januario T, Eberhard DA, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- Yip-Schneider MT, Wiesenauer CA, Schmidt CM. Inhibition of the phosphatidylinositol 3′-kinase signaling pathway increases the responsiveness of pancreatic carcinoma cells to sulindac. J Gastrointest Surg. 2003;7:354–363. doi: 10.1016/s1091-255x(02)00156-7. [DOI] [PubMed] [Google Scholar]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene. 1998;17:475–479. doi: 10.1038/sj.onc.1201956. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Cunha IW, Coudry RA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97:678–685. doi: 10.1038/sj.bjc.6603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZQ, Sun M, Feldman RI, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- Zafar Y, Lager J, Yu D, et al. Preliminary results of a phase I study of bevacizumab in combination with everolimus in patients with advanced solid tumors. J Clin Oncol ASCO Ann Meeting Proc. 2006;24 (Suppl):3097. [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-LL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lee KC, Bhojani MS, et al. Molecular imaging of Akt kinase activity. Nat Med. 2007;13:1114–1119. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- Zinda MJ, Johnson MA, Paul JD, et al. AKT-1, -2, and -3 are expressed in both normal and tumor tissues of the lung, breast, prostate and colon. Clin Cancer Res. 2001;7:2475–2479. [PubMed] [Google Scholar]