Abstract
Karenia mikimotoi is one of the most common red-tide dinoflagellates proliferating in the eastern North Atlantic and around Japan. Kills of marine fauna are associated with its blooms. In mixed water columns it migrates vertically, while in stratified water columns, the population remains confined within pycnocline layers. Wind events, increasing mixing and agitation initiate declines in its populations. This paper is focused on the formulation of mortality rate relative to shear rate. Autotoxicity is demonstrated by the use of a synthetic toxin. Bioconvection observed in cultures allows the establishment of a trade-off between phototropism, which leads to the local accumulation of cells, and their autotoxicity, which would prevent cell concentration. The combination of these processes allows diffusion of the toxin into the underlying water, where it subsequently degrades. Confinement of the population in the pycnocline layer results also from another trade-off between growth conditions and shear-rate-modulated mortality. A simplified encounter kernel was introduced into the population dynamics equation to account for a mortality factor. Under realistic forcing conditions with a small number of parameters, this model reproduced the confinement of the population in the pycnocline layer, the proper timing and the duration of the recurrent K. mikimotoi bloom on the Ushant front (France).
Keywords: autotoxicity, allelopathy, ichthyotoxicity, bioconvection, encounter rate, trade-offs
1. Introduction
Known in the literature successively under different names (Gyrodinium aureolum, Gymnodinium cf. aureolum, Gymnodinium nagasakiense, Gymnodinium mikimotoi), Karenia mikimotoi is one of the most common red-tide dinoflagellate proliferating in the eastern North Atlantic regions and around Japan. Blooms of this species are commonly associated with kills of marine fauna. The vegetative niche of this species has been outlined by Gentien (1998).
In the case of mixed or slightly stratified water columns, K. mikimotoi is observed to vertically migrate daily with a range of up to 15 m (Koizumi et al. 1996). When stratification is greater, the population exhibits a non-migrating maximum in the pycnocline layer (Bjoernsen & Nielsen 1991; Arzul et al. 1993), relying mainly on nitrogen remineralization (Le Corre et al. 1993). It is not possible to estimate from the available data the lowest density gradient through which migration still persists. The reasons for this shift in behaviour have not yet been elucidated, but they are of great importance when modelling is done with a view to prediction.
Sharp pycnocline layers are associated with high shear between water masses and, if phototropism modulated by cell quota were the only driving force behind population movement, cells escaping from the transition zone would flush out; observations of high concentrations forming layers could be partly the result of this selection. Even if fine layering can result from purely physical processes (Franks 1995), persistence of populations in narrow layers at a small scale suggests that other factors, such as chemotropism or higher survival rates in layers of low turbulent energy, may be involved in the maintenance of high-concentration populations within these layers. Maintenance and growth of the population is possible, considering the temperature and light regimes at these depths. These boundary layers may also exhibit limited residual movement, allowing the population to develop with limited dispersion. In this paper, we address the possibility of higher survival rates in pycnocline layers.
Increased stability of the water column due to stratification and calm weather is generally favourable to red tides, while storm events terminate them (see Iizuka et al. 1989). Wind strength tends to be inversely related to bloom maintenance (Yamamoto & Seike 2003). Small scale physical–biological interactions may have different effects on dinoflagellates, including lowering the growth rate (Pollingher & Zemel 1981; Juhl & Latz 2002; Sullivan et al. 2003), associated sometimes with increased mortality and changes in morphology (Berdalet 1992). However, the thresholds for the appearance of such negative effects are species specific (Sullivan et al. 2003). Although the above-cited works involved thecate dinoflagellates, similar effects may also apply to K. mikimotoi, which is athecate. Even if commonly observed and reported, the major processes underlying these effects have never been formulated in population models.
In the case of K. mikimotoi, the effect does not seem to be a repression in growth rate due to the arrest of the cell cycle. Agitation in cultures reduces the cell concentration to the point that specific care in manipulating cultures has to be taken. This species produces exotoxins with a haemolytic effect due to non-specific inhibition by 18 : 5n3 fatty acid of membrane ATPases (Fossat et al. 1999). By preventing osmoregulation, it could be that the same toxin kills K. mikimotoi while inhibiting competitors (Gentien & Arzul 1990), killing fishes and other organisms (Sola et al. 1999) and deforming bivalve shells (Erard-Le Denn et al. 1990). We report here the role of this toxin in the sensitivity of K. mikimotoi to agitation.
Here, we report on the autotoxicity demonstrated using synthetic toxin. From detailed studies conducted in still cultures, we examine the trade-off between cell concentration induced by phototropism and autotoxicity. A simplified formulation of the collision kernel was applied to a one-dimensional model with realistic forcing in order to test the importance of this control process on population dynamics.
2. Material and methods
(a) Cell cultures and sampling
Karenia mikimotoi cells were sampled during a toxic bloom from the Rade de Brest, France. The cells isolated were batch cultured without agitation in a sterile Guillard's f/2 medium at 18±1°C under a 12 h light : 12 h dark cycle at 60 μE m2 s−1. Since the species is very sensitive to agitation, special care was taken in homogenizing the cultures, prior to sampling; the same person always did the mixing, very gently, before sampling, thus ensuring the best reproducibility. Repartition into aliquots was done at least 12 h prior to experimentation in order to limit the numbers of non-viable cells. Samples were taken either by syringe or by siphoning into tubes previously filled with the required amount of Lugol's fixative. Maximum growth rate was determined at each degree Celsius between 12 and 20°C, after acclimation for at least two months in the culture cabinet (two to three cultures). Since no growth was observed at 12°C, cultures were acclimated at 13°C prior to the growth rate estimation at 12°C. Maximum growth rates at each temperature were estimated using a nonlinear regression procedure (Nlreg software by P. H. Sherrod; Dennis et al. 1981).
(b) Viability test
Fluorescein diacetate (FDA) is non-fluorescent and apolar. It was added to cell suspensions to allow the viable cells to be counted. After entering the cell, it may be hydrolysed into fluorescein by non-specific esterases. If the cell membrane is intact, fluorescence, which is polar, accumulates in the cell. It is therefore a marker of esterase activity and membrane integrity, and therefore is an index of cell viability. FDA dissolved in acetone (1 mg ml−1) is added to the cell suspension (2 μl ml−1), which is incubated in the dark for 10 min. The proportion of viable cells (number of green cells per total number) is estimated under epifluorescence in at least 200 cells.
(c) Synthesis of the all-cis-octadecapentaenoic acid
The lability of the all-cis-octadecapentaenoic acid identified previously as one of the major agents toxic to K. mikimotoi (Parrish et al. 1993) prevents any direct estimation of the dose–effect relationship on phytoplankton cells. This fatty acid was synthesized in sufficient amounts (approx. 100 mg), according to the method described by Kuvlev et al. (1992), a method involving a γ-iodolactonization of 22 : 6n3. This method has previously been used to identify the mode of action of the K. mikimotoi toxic principle (Fossat et al. 1999; Sola et al. 1999). The structure of the synthesized fatty acid was confirmed by gas chromatography—and fast atom bombardment—mass spectrometry, ionising radiation and 1H-nuclear magnetic resonance and by comparison with a sample isolated from cultures. The standard fatty acid mixture contained 82% 18 : 5n3, the major impurities being 20 : 5n3 (5.4%), 18 : 4n3 (2%) and 22 : 6n3 (1%) fatty acids. The fatty acid was stored immediately after synthesis in vacuum-sealed ampoules in aliquots of approximately 100 mg at −20°C in the dark. The content of each ampoule was dissolved in 1 ml methanol and the exact fatty acid weight determined by weighing. Possible degradation of the fatty acid into aldehydes and oxidation products with a shorter retention time was checked by GC prior to any toxicity testing. Experiments were conducted with a fatty acid standard above 90% purity.
(d) Oxygen radical production measurements
Degradation of the fatty acid was followed indirectly by trapping the hydroxyl radicals produced onto the oxygen radical trap HPPA (hydroxyphenylpropionic acid), following the method described by Palenik & Morel (1988). HPPA is oxidized into a fluorescent dimer (excitation 320 nm; emission 410 nm). An increase in fluorescence provides an integral measurement of the oxygen radicals produced. Estimation of the half-life of the fatty acid was measured in the dark at ambient temperature (18°C).
(e) Toxicity of the all-cis-octadecapentaenoic acid
The autotoxic effect of the 18 : 5n3 was tested in 50 ml aliquots of K. mikimotoi cultures. The fatty acid standard was dissolved in 1 ml methanol. The maximum volume added to test vials was 70 μl. Blanks were performed with 70 μl pure methanol in 50 ml cell suspensions. All measurements were done in triplicate. The concentration of viable cells was determined as described above.
(f) Cell behaviour measured by laser sheet trajectography
Cell behaviour was observed by laser sheet trajectography. An argon laser source was tuned at 488 nm, conditioned through a polarizer and a half-wavelength slide and through an optoacoustic deflector (AA-DTS-X-250). After adjustment of the conditioning optics to maximize intensity of the first-order diffraction and minimize intensity for the zeroth order (18° incidence), a cylindrical lens was used to obtain a light sheet. An intensified NanoCam camera equipped with a 50 mm Nikon lens mounted back-to-front allowed a 22 enlargement factor. Sharp cell images could be obtained with a shutter speed of 1 ms. The camera was mounted on a motorized stage allowing controlled displacements. Synchronization and generation of pulses for the optoelectronic deflector was performed by Pascal software through an IEEE-488 bus, a multifunction and impulse generator. The motorized stage was driven through an RS-232. After calibration of the depth of field, it was then possible to measure cell concentration and, by superposition of successive frames, to measure cell speed. In the descending plumes, cell concentration was so high that only movements of the fronts of the clouds of cells could be used to assess velocity measurements.
(g) Migration experiments
Aliquots of cultures at least 10 days old were transferred to square-sectioned cells (5×5×25 cm) at least 12 h prior to the experiment. Three millilitres of distilled water were added carefully at the water surface. Under light, the cells tended to concentrate in a surface layer from which descending plumes developed. An apparent steady state, as judged from the length of the descending plumes, developed in approximately 2–3 h. Before the establishment of descending plumes, underlying water was gently siphoned out. The elevated cell concentration remaining was counted every 30 min for 1.5 h. Each experiment was conducted in triplicate. After incubation periods, an FDA viability test was performed and each vial was counted for live cells after a 10 min incubation period in the dark.
(h) One-dimensional physical modelling
The hydrodynamic model is a one-dimensional dynamic and numerical model forced by wind and tide. In order to simulate tidal effects, free surface elevation gradients are considered. The model has five state variables, namely temperature, salinity, velocities (u and v) and turbulent kinetic energy. The turbulence closure is achieved by an algebraic formulation of the mixing length.
The two components of the velocity were
| (2.1) |
where t is the time, z is the vertical coordinate (positive upward), u is the E–W velocity (m s−1), v is the N–S velocity (m s−1), g is the gravitational acceleration (9.81 m s−2), f is the Coriolis parameter (10−4 s−1), nz is the vertical eddy viscosity (m2 s−1) and (∂ξ/∂x), (∂ξ/∂y) is the free surface elevation gradient.
The surface condition was
the surface wind stress components where ρ is the density of seawater (kg m−3).
The bottom condition was
where Cd is the drag coefficient (2.5×10−3) and ub, vb the velocities in the bottom layer.
For tidal forcing, we applied the linear theory of tide which indicates that the horizontal gradient induced by a tidal wave propagating in one direction can be expressed as the following horizontal gradient
where T is the M2 tidal period (44 712 s) and U0 is the maximum tidal current reached during a tidal cycle.
The turbulence closure model was based on the turbulent kinetic energy (TKE) state equation and an algebraic formulation of the mixing length (Luyten et al. 1996)
where k is the TKE (m2 s−2); ϵ is the dissipation rate of TKE (m−2 s−3); production of TKE by vertical velocity gradient, Ps=nz((∂u/∂v)2+(∂v/∂z)2); reduction of TKE by vertical density gradient, G=−gkz(1/ρ)(∂ρ/∂z); and kz is the vertical eddy diffusivity (m2 s−1). In the chosen turbulence closure scheme, ε is given by a function of TKE and the mixing length l according to the equation
where ϵ0=0.166 and lz=κz(1−z/H)1/2, with Karman constant κ=0.4 and H the depth of the water column.
Finally, turbulent eddy viscosity and eddy diffusivity are given by nz=Suk2/ϵ and kz=Sbk2/ε, where Su and Sb are the stability functions, the expressions of which can be found in Luyten et al. (1996).
Though similar to that of Westgard (1989), our model differs from it in two ways. In our model, (i) tidal current is taken into account (which was not the case in Westgard 1989) and (ii) ε is estimated as a function of the mixing length (we have a one-equation k closure scheme and not a two-equation k-ε closure scheme as in Westgard 1989). Luyten et al. (1996) compared different turbulence closure schemes for shelf stratified waters and concluded that there was no difference in the results between the two schemes, the k closure scheme being less computer intensive. This model can be applied to situations with homogeneous or stratified vertical profiles of temperature and salinity; in particular, it can accommodate any type of gradient in turbulent eddy diffusivity due to complex haloclines on the shelf under the influence of river plumes. The model can also estimate the steady state vertical distribution as well as time-dependent distributions.
3. Results and discussion
We investigated the behaviour of K. mikimotoi in still cultures, and then applied the results in a simplified one-dimensional model in order to test the importance of crowding on population dynamics as a control process.
(a) In vitro cultures of Karenia mikimotoi
When cultured in batch, phytoplankton species follow a growth described by the logistic equation that takes into account an asymptotically stable census limit. In the case of our strain of K. mikimotoi, the maximum cell concentration reached in batch cultures never exceeded 4×107 cell l−1. Assuming a Poisson distribution (Rothschild 1992), the mean nearest neighbour distance at this cell concentration, C, is d=0.55C−1/3=175 μm. Each cell requires on average a vital volume that corresponds to a travel time of roughly 2 s, as measured from laser sheet trajectography of individual cells. It should be noted that during these experiments in still cultures, cell collisions were never observed.
Different bioactive agents have been reported to be excreted by K. mikimotoi, namely the all-cis-3,6,9,12,15-octadecapentaenoic acid (in short 18 : 5n3) and its glycerides (Parrish et al. 1993), as well as three volatile sesquiterpenoids (Kajiwara et al. 1992). The volatile sesquiterpenoids appear to be rather stable in culture conditions, but unfortunately their production has not been studied in detail. Parrish et al. (1994) showed that the concentrations of 18 : 5n3 vary greatly with environmental (temperature and light) culture conditions. It can reach 34% of total fatty acids at 18°C and 35 μE m−2 s−1. This fatty acid inhibits Na-, K- and Mg-ATPase activities in a non-specific way (Fossat et al. 1999; Sola et al. 1999), and it could therefore act on different biological targets. Furthermore, these authors showed that toxicity from oxygen-free radicals produced by the degradation of the fatty acid was not involved in the process.
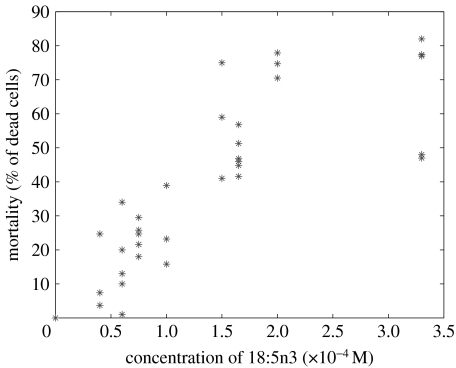
The lability of the octadecapentaenoic acid precluded the testing of extracts of K. mikimotoi culture medium on itself. The fatty acid was therefore synthesized from 22 : 6n3 using a γ-iodolactonization step, as described in §2. After checking the stereochemistry and purity, the lethal concentration 50(LC50) for K. mikimotoi was found to be 1.5×10−4 M (figure 1). Controls consisting of 70 μl pure methanol gave results under 3% mortality. It clearly shows that K. mikimotoi is sensitive to its own toxin but to a lesser degree than potential competitors, as 1 μM 18 : 5n3 totally inhibits Chaetoceros gracile growth (Gentien 1998). These concentrations should not be extrapolated to nature as the fatty acid adsorbs on the wall, and on the air–water interface. In nature, the toxic agent is distributed around the producing cells and not dissolved in the aqueous phase; this renders the extrapolation even more difficult. However, this test demonstrates a different sensitivity of the two biological targets.
Figure 1.
Dose–effect titration of all-cis-3,6,9,12,15-octadecapentaenoic acid on Karenia mikimotoi.
Allelopathic properties in K. mikimotoi have been demonstrated (Gentien & Arzul 1990; Arzul et al. 1993); they provide this species with a competitive advantage over the other phytoplankton species. However, the sensitivity of K. mikimotoi to its own toxin could counteract this advantage. In order to estimate the potential effect of this compound released at the cell membrane, it is essential to define how it is distributed around the cell.
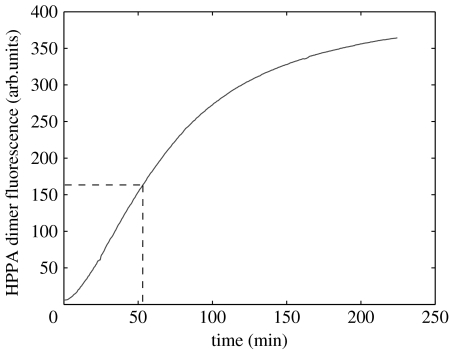
To this effect, its half-life was measured indirectly by trapping the oxygen radicals produced by its degradation with HPPA. The increase in fluorescence due to HPPA dimer formation gives an integrated measure of oxygen radicals produced during the decay of the 18 : 5n3 (figure 2). The half-life of this molecule in seawater and in the dark at 18°C is approximately 50 min. This half-life should be regarded as a maximum, since decay of degradation products can produce extra radicals. The average vital distance between cells could result from a balance between the flux from the cell and the molecular diffusion on the one hand and the toxin degradation and the LC50 on the other. Owing to the rapid decay of the toxin excreted by the cells, the action distance is very short and the estimated distance of 175 μm seems to be the upper limit. When transported in the viscous range, cells that continuously produce the toxin move with their own cloud of toxin.
Figure 2.
Integrated production of oxygen radicals during degradation of the 18 : 5n3 fatty acid (as estimated from the formation of the fluorescent dimer of hydroxyphenylpropionic acid).
Even if the time required for the toxin to act irreversibly is unknown, any increase in cell concentration would have a negative effect on cell viability. In culture, during the dark phase, cells tend to be evenly distributed throughout the culture volume, but during the light phase, cells crowd at the air–water interface in very thin layers (2–5 mm in thickness). Thus, cell concentration can locally be much higher than the average limit concentration in a culture. Subsampling in the surface layer showed local cell concentration over 3×108 cell l−1 (average neighbour distance of approx. 90 μm). The apparent contradiction with the observations reported above can be solved by a careful analysis of the processes. While the surface layer concentrates, descending plumes from the surface layer are observed. These descending plumes are so concentrated in cells that it was impossible to identify individual cells with the visualization system. Once the maximum vertical extent of the plume was reached, cells separated from their plume and progressively reached the surface layer at instantaneous speeds of 90–100 μm s−1 on average. This speed is similar to that observed for individual cells in the dark phase, but under illumination, the azimuths of the trajectories (data not shown) are oriented towards the surface, resulting in a continuous cell exchange between surface layer and underlying water. This phenomenon, called ‘bioconvection’, has been described for many flagellates (Hopkins & Fauci 2002). As reported by Harashima et al. (1988), the essence of this phenomenon is that gravity acts on the concentrated layer, not on the water or micro-organisms separately but on their mixture. The energy source of bioconvection is the active transport of buoyancy given internally by the upward swimming of micro-organisms. These patterns increase the vertical diffusion of dissolved compounds released by cells, including possible exotoxins. Continuity requires an inflow of underlying water to compensate for the flux driven by descending plumes. If the residence time in the underlying water is sufficient, then the toxin would decay and the compensating flux to the surface layer would be free of toxin.
The speed of the descending plume front was observed to be approximately 200 μm s−1. The upper part of the plumes can be approximated as cylinders of 4–6 mm (measured with the laser sheet system equipped with the optoelectronic deflector). These plumes induce a downward flux from the surface layer of 15.7 mm3 s−1 per plume. Three to five plumes were observed in the 5×5 cm section containers. The total downward flux for an average of three plumes is in the range 30–68 mm3 s−1. The renewal time of water in the surface layer is between 1.2 and 7 min. Since no adverse effects on cell concentration were observed, this time is not sufficient at a distance of 90 μm to promote an irreversible effect on cells. The renewal time of underlying water is of the order of a few hours, allowing the toxin to decay and the compensation water entering the surface layer to be free of toxin. This mechanism explains why cells do not suffer from crowding in the surface layer.
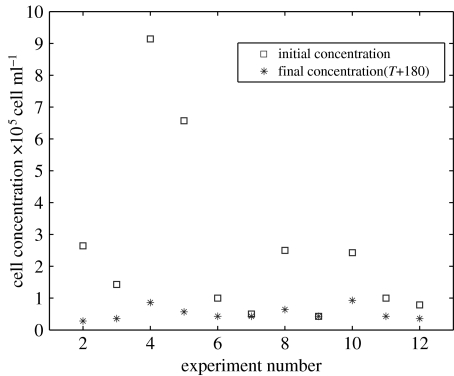
To confirm the hypothesis that a high residence time is necessary for the toxin to decay, underlying water was carefully withdrawn while the cell concentration was building up in the top layer. The results are illustrated in figure 3. The initial cell concentration in the concentrate is highly variable since it depends on various experimental conditions. The number of live cells was estimated (FDA measurements) after 180 min in the concentrate. On average, the final cell concentration observed in 12 experiments was 4×107 cell l−1, with over 90% of the remaining cells still viable. This limit cell concentration is the one found in batch cultures.
Figure 3.
Evolution of cell concentration in 11 batches after withdrawal of the underlying dilution volume.
The importance of bioconvection in maintaining the crowded layer has been further confirmed by the following experiment. Bottom illumination of a culture flask blackened on the sides caused cell crowding at the bottom. Bioconvection could not occur in these conditions. After a light period of 4 h, all the cells had died; after return of the culture to normal illumination condition, the culture failed to grow again owing to the absence of a viable inoculum.
In summary, K. mikimotoi produces a short-lived toxin that acts at a short range. By the inhibition of competitors at low cell concentrations (104 cell l−1), this toxin provides a competitive advantage (Arzul et al. 1993) but controls, at the same time, its own maximum cell yield. In quiet conditions, the phototropism-driven cell behaviour leads to local accumulation of cells without negative effects on cell concentration owing to the exploitation of physical instabilities.
The range of action of the toxin is the result of a balance between the production flux at the cell membrane, the molecular diffusion and the toxin decay rate. Local saturation of the medium does not occur since the toxin is unstable and the toxic effect acts only at a short distance. This finding is in accordance with the report by Uchida et al. (1999) that the inhibitory effect of K. mikimotoi on Heterocapsa circularisquama in bialgal cultures occurred mainly by direct cell contact. Therefore, allelopathic and autotoxic processes have an action at a short range (less than 175 μm). Hereafter, K. mikimotoi are considered as virtual particles with a maximum diameter of 175 μm.
We further examined the implications of this intrinsic property of K. mikimotoi on its own population development under realistic conditions.
(b) In situ population development
A common feature in field observations of K. mikimotoi is that it often occurs in or near the pycnocline layer during some stage of its population development. There are cases where populations develop in weakly stratified water bodies. However, along the Atlantic coast of Europe, blooms occur mainly on the stratified side of hydrographic fronts (Partensky & Sournia 1986). Blooms in stratified water columns remain confined mainly within the pycnocline layers (Birrien et al. 1991). Bjoernsen & Nielsen (1991) studied the distribution of G. aureolum in a pycnocline layer in the Kattegat with a high-resolution sampler. They observed a strong heterogeneity of the dinoflagellate population in the decimetre scale and concluded that G. aureolum at that time formed a more or less coherent ‘magic carpet’ in the pycnocline layer. These authors supposed that the inhibition of potential predators could be an important factor in the maintenance of a high phytoplankton biomass in the pycnocline layer.
Karenia mikimotoi has been shown to produce exotoxins that are detrimental to the growth of other algae (Gentien & Arzul 1990). On the Ushant front, K. mikimotoi's maximum concentration corresponded to a minimum for diatoms (Arzul et al. 1993), and the minimum cell concentration for a reduction in the diatom growth rate was approximately 104 cell l−1 (Gentien 1998). Therefore, allelopathy exerted by K. mikimotoi may have been playing an effective role at the onset of the population development. We reported above that this adaptive advantage may be countered by autotoxicity above a limit in cell concentration; it could be that the population thus benefits from this confinement in a layer while, at the same time, being limited by it.
In §4, we test the hypothesis that exotoxin production is an essential control factor in the population dynamics. Hereafter, we consider that two cells have encountered each other when the vital volumes of these two cells intersect. Under quiet conditions in vitro, motile cells have the possibility of avoiding each other. In situ, cells are transported and they may enter into ‘contact’; at a given cell concentration, turbulence would increase the frequency at which individual cells are within a certain distance of another. We treated this increase in frequency as the encounter of virtual particles with a diameter range between 25 μm (the cell diameter) and 175 μm (the vital volume around each cell).
Encounter rate is a function of the sizes of colliding particles, their concentrations and environmental parameters. The encounter rate of particles is given by βcC2, where C is the concentration of particles and βc is the coagulation kernel (product of the encounter kernel (β) and the efficiency kernel (α)). The encounter kernel represents the average percentage of particle pairs that will encounter per unit time and unit volume. It is the sum of the terms describing the different processes that bring particles into contact. Three major processes can generate encounter: Brownian motion, differential sedimentation and shear (Pruppacher & Klett 1978, adapted by Jackson 1990).
Owing to their own motility, cells present a cell diffusivity that could be treated in the same way as Brownian motion if the cells were colliding in still conditions. Measurements of cell distance show that cells always maintain a minimum distance between them; collisions or cell doublets have never been observed under quiet conditions. Even if the cell diffusivity is quite high, it can be supposed that in turbulent conditions, it does not have an important contribution to the encounter kernel.
Differential sedimentation means that each large settling particle generates a wake with a downward-induced motion. Such a large descending particle is expected to accumulate a cluster of smaller ones in its wake. The observations of cells in contact show a release of intracellular material due to lysis. In the first approximation, we suppose that released matter would be in the form of colloids, with a zero sedimentation speed. This process was therefore neglected.
The third encounter mechanism is due to shear: differences in fluid velocity cause two particles to approach each other. Considering only the latter mechanism and the fact that only the same-sized particles are concerned, the encounter kernel formulation for particles of the same size reduces to β=βSh=10.4γr3 according to Pruppacher & Klett (1978), where γ is the shear rate (s−1) and r (μm) is the cell active diameter, which can be larger than the cell diameter. The term expressing mortality will therefore be formulated as −KγC2, where γ is the shear rate (s−1) , where ϵ is the energy dissipation rate and v is the kinematic viscosity; Moum & Lueck 1985); C is the cell concentration (m−3); and K is a scaling parameter that takes into account the effective cross-section diameter and a scaling factor (K=0.1 for cell concentration expressed in dm−3).
The effect of the mortality process due to autotoxicity was tested under real conditions occurring on the Ushant front. The Iroise Sea, off West Brittany (France), in the eastern North Atlantic, shows a well-developed tidal and seasonal frontal system, the northern part of which is termed the Ushant front, its physical, chemical and biological properties having been well described (Pingree et al. 1975, 1977). This area was selected as communities of dinoflagellates, including K. mikimotoi, recur frequently in the pycnocline layers on the stratified side of this tidal front (Holligan & Harbour 1977). Realistic forcing has been applied to a one-dimensional model with realistic tides. Wind data have been obtained from the Ushant meteorological station.
Growth formulation was kept as simple as possible to test the effect of mortality induced by cell encounter. The maximum growth rate was observed to be 0.6 d−1 (Gentien 1998). The growth rate in relation to temperature was obtained by a polynomial fit to in vitro measurements: (μ=2.5×10−3T3−0.15T2 +2.8775 T−17.25). This equation reproduces the zero growth rate observed at 12°C for this strain. The relative stability of maximum growth rate (more than 0.5 d−1) between 14 and 18°C probably reflects the acclimation time allowed before growth rate measurements at different temperatures.
The pigment composition and bio-optical characteristics of K. mikimotoi, being very plastic with respect to adaptation to the growth light regime, allow it to benefit from both low and high levels of light (Johnsen & Sakshaug 1993). A light regime such as those reported from the pycnocline layers (1–5% incident light) during summer can support net growth of the population, albeit at non-saturated rates (Richardson & Kullenberg 1987). In the first approach, light limitation was not considered at all, even if this 1% limit may be encountered around 25–30 m depth in summer.
In stratified water columns, cells do not migrate and remain in the pycnocline layer. This has been taken as fact and a model of vertical migration was not implemented. The explanation for this shift in behaviour should be the subject of further work.
The time evolution of the tracer K. mikimotoi is therefore
This model was run under realistic forcing for the years 1996–1998. K was kept constant at 5×10−5 (for a cell concentration expressed in cell l−1), even though it is probable that K varies according to the cell physiological status. Mucopolysaccharide excretion would tend to lower γ at a given energy dissipation rate (ε) by local changes in the kinematic viscosity (ν). However, this process was not considered in this first approach model.
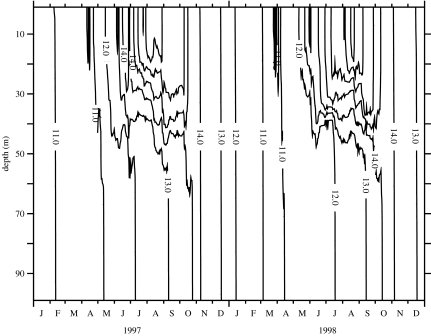
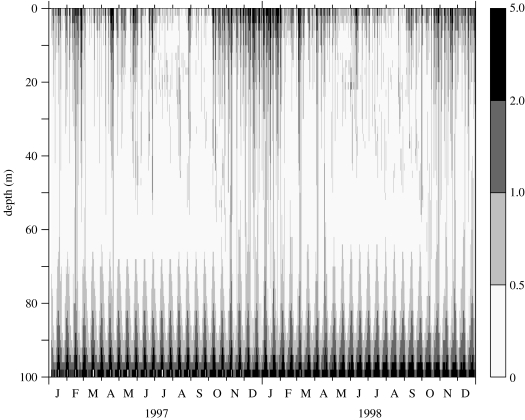
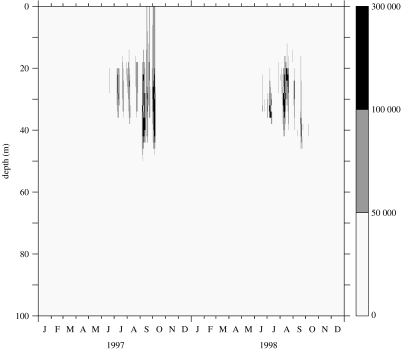
Initial conditions were set at the level of 1 cell l−1 distributed in the water column, with no reset during wintertime. Figures 4–6 show results from the model run with 50 evenly distributed layers in the 50 m-depth water column. It was started on 1 January 1996, but the stabilization period of the model is omitted from the figure and the results are presented for 1997–1998. Figure 4 represents the time–depth evolution of the isotherms; the gross features of the stratification offshore of the front are correctly reproduced in terms of temperature range and timing of stratification. Figure 5 represents the evolution of the calculated shear rate resulting from the influence of wind events and the modulation of tidal friction on the bottom. Two low-shear-rate (less than 0.5 s−1) periods occur at mid-depth in the summer months. During these periods, the K. mikimotoi-like tracer (figure 6) appears to develop in the pycnocline layer. The resulting pattern is similar to that reported by Holligan & Harbour (1977). Figure 6 shows time discontinuities in cell densities closely associated with bursts of agitation induced by wind.
Figure 4.
Modelling results using meteorological forcing time-series (1996–1998) from Ushant meteorological station: temperature evolution (Julian days starting from 1 January 1997).
Figure 5.
Modelling results using meteorological forcing time-series (1996–1998) from Ushant meteorological station: evolution of shear rate (s−1).
Figure 6.
Modelling results using meteorological forcing time-series (1996–1998) from Ushant meteorological station: time and depth distribution of the K. mikimotoi-like tracer (isolines spacing: 5×104 cell l−1).
4. Conclusions
The ichthyotoxicity of K. mikimotoi is due to the production of a fatty acid (all-cis-octadecapentaenoic acid) or its glycoglycerolipids (Parrish et al. 1998). This fatty acid is labile, and therefore acts at local scales on algal competitors and other biological targets. Each cell of K. mikimotoi is surrounded by a cloud of toxin that does not exceed a 175 μm diameter. This toxin provides a competitive ecological advantage to K. mikimotoi over the other species encountered. However, K. mikimotoi cells are sensitive to their own toxins. Autotoxicity is a well-known process in terrestrial plants and has profound implications in agroecosystems (Singh et al. 1999). This is one of the few documented cases of autotoxicity in the marine environment (Pratt & Fong 1940; Imada et al. 1992). In culture, possible autotoxicity due to cell crowding resulting from concentration of motile cells by phototropism was shown to be countered by the exploitation of physical instabilities.
The dependency on turbulence was tested as a major possible control in population dynamics. The modelling exercise should be considered as an experiment to evaluate the importance of this control process. To this effect, growth formulation has been kept as simple as possible, with a growth rate depending on temperature and mortality expressed by an encounter kernel depending solely on shear rate. This growth equation is similar in structure to the logistic equation used in still batch cultures with one major difference: the mortality factor in C2 depends on external forcing factors (tide and wind). Quite surprisingly, this simple formulation reproduces the gross features of the development of K. mikimotoi on the Ushant front. Population confinement in the pycnocline layer (Birrien et al. 1991; Gentien 1998) is not mainly driven by diurnal migration capabilities, as observed vertical distributions can be reproduced without using diurnal migration complex formulations. Hence, one can conclude that the confinement in the pycnocline layer is probably not due to active behaviour but to an increased survival rate.
This approach differs from the general ‘ecological’ models derived from models of phytoplanktonic biomass, in that it does not consider competition for nutritive substrate as the determinant of the competition outcome between the various phytoplanktonic species, but rather relies on intrinsic properties of a given species.
Ranking of control factors allows reduction in the number of parameters needed from several tens to six, four of them (the coefficients of μ=f(T)) being experimentally measurable, and therefore allows improvement of the robustness of the model. The higher the cell concentration, the higher will be the mortality rate. The population dependency on shear rate is likely to control the termination of the bloom more effectively than the possible depletion in nutrients. This result should be further investigated for these hydrodynamic conditions, using better shear rate estimates and a better understanding of the processes leading to collision between cells. This simple scheme may need some adaptation for shallow weakly stratified seas, where biological control factors may be more important than the physical ones. Nonetheless, the rate of cell mortality due to encounters should be considered as one of the major control factors of population growth for this species.
Acknowledgments
This work was supported by the Ifremer program ALTOX and partly by the EC-INTERREG programme NEMEDA. Fruitful discussions with Prof. T. Osborn and Dr I. R. Jenkinson are gratefully acknowledged.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Arzul G, Erard-Le Denn E, Videau C, Jegou A.M, Gentien P. Diatom growth-repressing factors during an offshore bloom of Gyrodinium cf. aureolum. In: Smayda T.J, Shimizu Y, editors. Toxic phytoplankton blooms in the sea. Elsevier Science; Amsterdam, The Netherlands: 1993. pp. 719–724. [Google Scholar]
- Berdalet E. Effects of turbulence on the marine dinoflagellate Gymnodinium nelsonii. J. Phycol. 1992;28:267–272. doi:10.1111/j.0022-3646.1992.00267.x [Google Scholar]
- Birrien J.L, Wafar M.V, Le Corre P, Riso R. Nutrients and primary production in a shallow stratified ecosystem in the Iroise Sea. J. Plankton Res. 1991;13:721–742. doi:10.1093/plankt/13.4.721 [Google Scholar]
- Bjoernsen P.K, Nielsen T.G. Decimeter scale heterogeneity in the plankton during a pycnocline bloom of Gyrodinium aureolum. Mar. Ecol. Prog. Ser. 1991;73:263–267. [Google Scholar]
- Dennis J.E, Gay D.M, Welsch R.E. An adaptive non-linear least-squares algorithm. ACM Trans. Math. Software. 1981;7:348–369. doi:10.1145/355958.355965 [Google Scholar]
- Erard-Le Denn E, Morlaix M, Dao J.C. Effects of Gyrodinium cf. aureolum on Pecten maximus (post larvae, juveniles and adults) In: Granéli E, Sundström B, Edler L, Anderson D.M, editors. Toxic marine phytoplankton. Elsevier Science; Amsterdam, The Netherlands: 1990. pp. 132–136. [Google Scholar]
- Fossat B, Porthe-Nibelle J, Sola P, Masoni A, Gentien P, Bodennec G. Toxicity of fatty acid 18 : 5n3 from Gymnodinium cf. mikimotoi: II. Intracellular pH and KC uptake in isolated trout hepatocytes. J. Appl. Toxicol. 1999;19:275–278. doi: 10.1002/(sici)1099-1263(199907/08)19:4<275::aid-jat578>3.0.co;2-b. doi:10.1002/(SICI)1099-1263(199907/08)19:4<275::AID-JAT578>3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- Franks P.J.S. Thin layers of phytoplankton: a model of formation by near-inertial waves shear. Deep-Sea Res. I. 1995;42:75–91. [Google Scholar]
- Gentien P. Bloom dynamics and ecophysiology of the Gymnodinium mikimotoi species complex. In: Anderson D.M, Cembella A.D, Hallegraeff G.M, editors. Physiological ecology of harmful algal blooms. NATO ASI Series. vol. G41. Springer; Berlin, Germany: 1998. pp. 155–173. [Google Scholar]
- Gentien P, Arzul G. Exotoxin production by Gyrodinium cf. aureolum (Dinophyceae) J. Mar. Biol. Assoc. UK. 1990;70:571–581. [Google Scholar]
- Harashima A, Watanabe M, Fujishiro I. Evolution of bioconvection patterns in a culture of motile flagellates. Phys. Fluids. 1988;31:764–775. doi:10.1063/1.866812 [Google Scholar]
- Holligan P.M, Harbour D.S. The vertical distribution and succession of phytoplankton in the western English Channel in 1975 and 1976. J. Mar. Biol. Assoc. UK. 1977;57:1075–1093. [Google Scholar]
- Hopkins M.M, Fauci L.J. A computational model of the collective fluid dynamics of motile micro-organisms. J. Fluid Mech. 2002;455:149–174. doi:10.1017/S0022112001007339 [Google Scholar]
- Iizuka S, Sugiyama H, Hirayama K. Population growth of Gymnodinium nagasakiense red tide in Omura Bay. In: Okaichi T, Anderson D.M, Nemoto T, editors. Red tides: biology, environmental science and toxicology. Elsevier; New York, NY: 1989. pp. 269–272. [Google Scholar]
- Imada N, Kobayashi K, Isomura K, Saito H, Kimura S, Tahara K, Oshima Y. Isolation and identification of an autoinhibitor produced by Skeletonema costatum. Nippon Suisan Gakkaishi. 1992;58:1687–1692. [Google Scholar]
- Jackson G.A. A model of the formation of marine algal flocs by physical coagulation processes. Deep-Sea Res. 1990;37:1197–1211. doi:10.1016/0198-0149(90)90038-W [Google Scholar]
- Johnsen G, Sakshaug E. Bio-optical characteristics and photoadaptive responses in the toxic and bloom-forming dinoflagellates Gymnodinium aureolum, Gymnodinium galatheanum and two strains of Prorocentrum minimum. J. Phycol. 1993;29:627–642. doi:10.1111/j.0022-3646.1993.00627.x [Google Scholar]
- Juhl A.R, Latz M.I. Mechanisms of fluid shear-induced inhibition of population growth in a red-tide dinoflagellate. J. Phycol. 2002;38:683–694. doi:10.1046/j.1529-8817.2002.00165.x [Google Scholar]
- Kajiwara T, Ochi S, Kodama K, Matsui K, Hatakana A, Fujimura T, Ikeda T. Cell-destroying sesquiterpenoids from red tide of Gymnodinium nagasakiense. Phytochemistry. 1992;31:783–785. [Google Scholar]
- Koizumi Y, Uchida T, Honjo T. Diurnal vertical migration of Gymnodinium mikimotoi during a red tide in Hoketsu Bay, Japan. J. Plankton Res. 1996;18:289–294. doi:10.1093/plankt/18.2.289 [Google Scholar]
- Kuvlev D.V, Aizdaicher N.A, Imbs A.B, Bezuglov V.V, Latishev N.A. All-cis-3,6,9,12,15-octadecapentaenoic acid from the unicellular alga Gymnodinium kowalevskii. Phytochemistry. 1992;31:2401–2403. doi:10.1016/0031-9422(92)83286-8 [Google Scholar]
- Le Corre P, L'Helguen S, Wafar M. Nitrogen source for uptake by Gyrodinium cf. aureolum in a tidal front. Limnol. Oceanogr. 1993;38:446–451. [Google Scholar]
- Luyten P, Deleersnijder E, Ozer J, Ruddick K. Presentation of a family of turbulence closure models for stratified shallow water flows and preliminary application to the Rhine outflow region. Cont. Shelf Res. 1996;16:101–130. doi:10.1016/0278-4343(95)93591-V [Google Scholar]
- Moum J.N, Lueck R.G. Causes and implications of noise in oceanic dissipation measurements. Deep-Sea Res. 1985;32:379–392. doi:10.1016/0198-0149(85)90086-X [Google Scholar]
- Palenik B, Morel F.M.M. Dark production of H2O2 in the Sargasso Sea. Limnol. Oceanogr. 1988;33:1606–1611. [Google Scholar]
- Parrish C.C, Bodennec G, Sebedio J.-L, Gentien P. Intra- and extra-cellular lipids in cultures of the toxic dinoflagellate, Gyrodinium aureolum. Phytochemistry. 1993;32:291–295. doi:10.1016/S0031-9422(00)94983-5 [Google Scholar]
- Parrish C.C, Bodennec G, Gentien P. Time courses of intracellular and extracellular lipid classes in batch cultures of the toxic dinoflagellate, Gymnodinium cf. nagasakiense. Mar. Chem. 1994;48:71–82. doi:10.1016/0304-4203(94)90063-9 [Google Scholar]
- Parrish C.C, Bodennec G, Gentien P. Haemolytic glycoglycerolipids from Gymnodinium species. Phytochemistry. 1998;47:783–787. doi: 10.1016/s0031-9422(97)00661-4. doi:10.1016/S0031-9422(97)00661-4 [DOI] [PubMed] [Google Scholar]
- Partensky F, Sournia A. Le dinoflagellé Gyrodinium cf. aureolum dans le plankton de l'Atlantique Nord: identification, écologie, toxicité. Crypt. Algol. 1986;7:251–275. [Google Scholar]
- Pingree R.D, Pugh P.R, Holligan P.M, Forster G.R. Summer phytoplankton blooms and red tides along tidal fronts in the approaches to the English Channel. Nature. 1975;258:672–677. doi:10.1038/258672a0 [Google Scholar]
- Pingree R.D, Holligan P.M, Head R.N. Survival of dinoflagellate blooms in the western English Channel. Nature. 1977;265:266–269. doi:10.1038/265266a0 [Google Scholar]
- Pollingher U, Zemel E. In-situ and experimental evidence of the influence of turbulence on cell division processes of Peridinium cinctum forma Westii (Lemm.) Lefevre. Br. Phycol. J. 1981;16:281–287. [Google Scholar]
- Pratt R, Fong J. Studies on Chlorella vulgaris. II. Further evidence that Chlorella cells form a growth-inhibiting substance. Am. J. Bot. 1940;27:431–436. doi:10.2307/2436459 [Google Scholar]
- Pruppacher H.R, Klett J.D. The microphysics of clouds and precipitation. Reidel; Boston, MA: 1978. p. 714. [Google Scholar]
- Richardson K, Kullenberg G. Physical and biological interactions leading to plankton blooms: a review of a bloom of Gyrodinium aureolum blooms in Scandinavian waters. Rapp. P.-V. Reun. Cons. Int. Explor. Mer. 1987;187:19–26. [Google Scholar]
- Rothschild B.J. Application of stochastic geometry to problems in plankton ecology. Phil. Trans. R. Soc. B. 1992;336:225–237. doi:10.1098/rstb.1992.0058 [Google Scholar]
- Singh H.P, Batish D.R, Kohli R.K. Autotoxicity: concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999;18:757–772. doi:10.1080/07352689991309478 [Google Scholar]
- Sola P, Masoni A, Fossat B, Porthe-Nibelle J, Gentien P, Bodennec G. Toxicity of fatty acid 18 : 5n3 from Gymnodinium cf. mikimotoi. I. Morphological and biochemical aspects on Dicentrarchus labrax gills and intestine. J. Appl. Toxicol. 1999;19:279–284. doi: 10.1002/(sici)1099-1263(199907/08)19:4<279::aid-jat579>3.0.co;2-x. doi:10.1002/(SICI)1099-1263(199907/08)19:4<279::AID-JAT579>3.0.CO;2-X [DOI] [PubMed] [Google Scholar]
- Sullivan J.M, Swift E, Donaghay P.L, Rines J.E.B. Small-scale turbulence affects the division rate and morphology of two red-tide dinoflagellates. Harmful Algae. 2003;2:183–199. doi:10.1016/S1568-9883(03)00039-8 [Google Scholar]
- Uchida T, Toda S, Matsuyama Y, Yamaguchi M, Kotani Y, Honjo T. Interactions between the red tide dinoflagellates Heterocapsa circularisquama and Gymnodinium mikimotoi in laboratory cultures. J. Exp. Mar. Biol. Ecol. 1999;241:285–299. doi:10.1016/S0022-0981(99)00088-X [Google Scholar]
- Westgard T. Two models for the vertical distribution of pelagic fish eggs in the turbulent upper layer of the ocean. Rapp. P.-V. Réun. Const. Int. Explor. Mer. 1989;191:195–200. [Google Scholar]
- Yamamoto T, Seike T. Modelling the population dynamics of the toxic dinoflagellate Alexandrium tamarense in Hiroshima Bay Japan. II. Sensitivity to physical and biological parameters. J. Plankton Res. 2003;25:63–81. doi:10.1093/plankt/25.1.63 [Google Scholar]