Abstract
Fishes suspended in water are subject to the complex nature of three-dimensional flows. Often, these flows are the result of abiotic and biotic sources that alter otherwise uniform flows, which then have the potential to perturb the swimming motions of fishes. The goal of this review is to highlight key studies that have contributed to a mechanistic and behavioural understanding of how perturbing flows affect fish. Most of our understanding of fish behaviour in turbulence comes from observations of natural conditions in the field and laboratory studies employing controlled perturbations, such as vortices generated in the wake behind simple geometric objects. Laboratory studies have employed motion analysis, flow visualization, electromyography, respirometry and sensory deprecation techniques to evaluate the mechanisms and physiological costs of swimming in altered flows. Studies show that flows which display chaotic and wide fluctuations in velocity can repel fishes, while flows that have a component of predictability can attract fishes. The ability to maintain stability in three-dimensional flows, either actively with powered movements or passively using the posture and intrinsic compliance of the body and fins, plays a large role in whether fish seek out or avoid turbulence. Fish in schools or current-swept habitats can benefit from altered flows using two distinct though not mutually exclusive mechanisms: flow refuging (exploiting regions of reduced flow relative to the earth frame of reference) and vortex capture (harnessing the energy of environmental vortices). Integrating how the physical environment affects organismal biomechanics with the more complex issue of behavioural choice requires consideration beyond simple body motions or metabolic costs. A fundamental link between these two ways of thinking about animal behaviour is how organisms sense and process information from the environment, which determines when locomotor behaviour is initiated and modulated. New data are presented here which show that behaviour changes in altered flows when either the lateral line or vision is blocked, showing that fish rely on multi-modal sensory inputs to negotiate complex flow environments. Integrating biomechanics and sensory biology to understand how fish swim in turbulent flow at the organismal level is necessary to better address population-level questions in the fields of fisheries management and ecology.
Keywords: turbulence, vortices, kinematics, lateral line, muscle activity, flow visualization
1. Introduction
Most studies of fish locomotion have sought to simplify the hydrodynamic environment to isolate and thus better understand the complex movements of swimming. Pioneering studies have provided fundamental insights into the kinematics, physiology and hydrodynamics of fish locomotion. For example, by swimming fish through a tank of still water, Gray (1933) revealed that the speed of the body wave travelling towards the tail is greater than the speed of a fish moving forward, providing a quantitative framework to evaluate the mechanism of undulatory locomotion. Blazka et al. (1960) and Brett (1964) championed the use of the swim tunnel respirometer, which measured oxygen consumption as an indicator of physiological effort during swimming in controlled uniform flow conditions. This provided a crucial link between swimming velocity and its associated metabolic cost. Employing a tank of still water with milk layered on the bottom, Rosen (1959) was the first to visualize the regular pattern of vortices shed from the undulating body of a fish, catalysing our understanding of the hydrodynamics of locomotion. Since then, great technological and analytical advancements have been made in each of these subfields, enabling more precise measurements and new insight into the movements, costs and hydrodynamics of fish swimming in uniform flows (Webb 1975; Müller et al. 1997; Shadwick et al. 1999; Lauder & Drucker 2002). Our current understanding of fish locomotion is biased by the historical predominance of studies conducted in still water or steady flow. Much less is known about the effect of hydrodynamics that deviate from these conditions. This is unfortunate since the majority of fishes commonly experience altered flows given the prevalence of these conditions in nature. As such, understanding how fish swim in complex flows is a topic of considerable interest for a broad scientific audience (Fausch 1993; Shuler et al. 1994; Anderson et al. 1998; McLaughlin & Noakes 1998; Pavlov et al. 2000; Heggenes 2002; Odeh et al. 2002; Triantafyllou et al. 2002; Enders et al. 2003; Liao et al. 2003a; Smith & Brannon 2005). No synthesis has been attempted to bring together results from such disparate fields as fisheries management, fluid dynamics, physiology, ecology and sensory biology. Thus, a broad review of the literature is timely. The goal of this paper is to review key studies that have informed us about the behaviour of fish swimming in altered flows and to begin to define the mechanisms by which fish can exploit them.
(a) Definitions
Hydrodynamic terms in the literature, when considered across disciplines, are often ambiguous and used interchangeably. This makes it challenging to categorize the types of fluid phenomena experienced by fishes across studies, especially when behaviour is not quantified. It is necessary from the outset to define terms with which to encapsulate the sheer diversity of hydrodynamic regimes that fish experience. This review is mainly concerned with altered or complex flows (i.e. velocity varies spatially or temporally relative to the earth frame of reference) that have a perturbing effect on fish. Flows that perturb fish are defined broadly as flows that generate an angular or linear translation of the body (i.e. displacement relative to the earth frame of reference), or flows that cause deformations of the body (i.e. alteration of swimming motions relative to the fish frame of reference). Altered flows will be used as an umbrella term that encompasses, but is not limited to, more traditional terms such as turbulence and unsteady flow that have been used in literature, which implicitly use the environment as the frame of reference. Note that steady flow can also have a perturbing effect under this definition, such as when a uniform current linearly or angularly displaces the body (translation and rotation, respectively). Although it eludes simple description, for the purpose of this review turbulence is defined by chaotic vortical flows of multiple strengths and sizes superimposed onto a mean flow velocity (Kirkbride 1993; Warhaft 2002). Studies have also found it useful to describe turbulence using a metric called turbulence intensity, which is a measure of the magnitude of flow fluctuation about the average flow. More specifically, it is the standard deviation of the flow velocity divided by the mean flow velocity (Odeh et al. 2002; Enders et al. 2003). The term unsteady flow applies to situations when fluid velocity varies with respect to time for a given point in space. Additionally, viscous flows contain velocity gradients which may or may not lead to the formation of discrete vortices. Whether environmental vortices will affect fish behaviour depends largely on the spatial scale of vortical flows relative to the fish size. It can also be useful to define perturbing flows as forces relative to the fish frame of reference (Goodwin 2004). Depending on the direction of force (e.g. perpendicular or parallel to the body corresponding to normal or shear strain, respectively), flow may cause shape deformations or rotational movements.
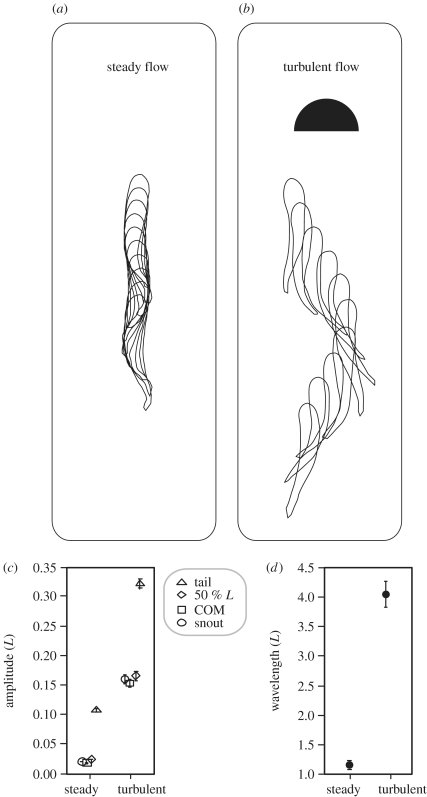
It is equally important to define the different effects of altered flows on fish behaviour. The term kinematics will be used here to describe the changes in body shape (i.e. motions or rates of deformation) relative to the fish frame of reference. For example, a fish encountering faster flow velocities will, with respect to its own frame of reference, alter its swimming kinematics (e.g. increase lateral tail-beat amplitude) to swim faster (Webb et al. 1984). Alternatively, steady flows can perturb a fish without substantially changing its kinematics by displacing the location of the body relative to the environment. In this example, the body of a swimming fish will drift downstream relative to the earth frame of reference if its forward velocity is less than the current it is swimming against. Finally, unsteady flows can change both the kinematics and the absolute position of the fish, as when lateral body amplitude and body wavelength increase when vortices buffet fish in turbulent conditions (figure 1a,b). Thus, it is important to recognize that a fish can be described as perturbed if displaced in three-dimensional space either with or without a change in kinematics.
Figure 1.
Turbulent flow can drastically alter swimming motions by deforming as well as translating the body (see text for discussion). Silhouette outlines for a 10 cm trout (standard length, L) swimming in: (a) a 45 cm s−1 steady current versus (b) turbulent flow created behind a 5 cm diameter cylinder. Ten body outlines for each treatment are shown for approximately one tail-beat cycle, illustrating the differences in body position relative to the earth frame of reference as well as body postures relative to the fish frame of reference. Although the lateral amplitude of selected points along the body is similar in pattern between swimming in steady and turbulent flow (c), the absolute amplitude values relative to the earth frame of reference are larger for fish in turbulence due to the lateral translation of the body by vortices (Liao et al. 2003b). In addition to displacing the body, turbulence can also generate body deformations (i.e. influence body kinematics), as evidenced by longer body wavelengths (d). Standard error bars are shown.
(b) The effect of turbulent flows on fish
There has been conflicting evidence concerning the effect of turbulence on fish swimming. The source of this difficulty can be described simply: depending on the hydrodynamic conditions specific to a study fish may be attracted to, or repelled by, turbulence. As a constraint, turbulence has been well documented to increase the cost of locomotion (Pavlov et al. 1982, 2000; Hinch & Rand 1998; Webb 1998; Enders et al. 2003); at extremely high levels, such as near hydroelectric dam turbines, shear stresses can even cause bodily damage to fishes (Odeh et al. 2002). On the other hand, altered flows that remain steady or maintain an aspect of predictability can be exploited by swimming fishes. Numerous field and laboratory studies have also shown that fish can reduce locomotory costs by exploiting turbulence (figure 2) generated by water moving past physical structures or by the propulsive movements of other fishes (Breder 1965; Weihs 1973; Sutterlin & Waddy 1975; McMahon & Gordon 1989; Fausch 1993; Shuler et al. 1994; Gerstner 1998; Herskin & Steffensen 1998; Webb 1998; Hinch & Rand 2000; Liao et al. 2003b; Montgomery et al. 2003; Smith 2003). Under these circumstances, turbulence may be considered a feature of the hydrodynamic environment that is a benefit rather than a constraint.
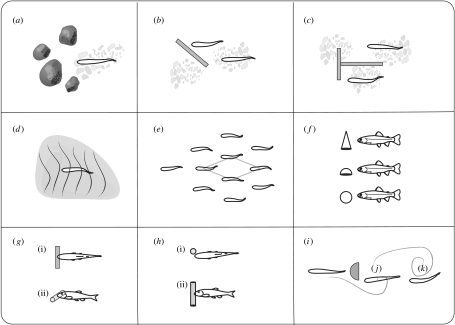
Figure 2.
Schematic summary of key field, laboratory and theoretical studies which have investigated the effects of biotically and abiotically generated wakes on fish swimming kinematics and behaviour. Stream observations of salmonids taking advantage of reduced flows behind aggregations of (a) boulders (Shuler et al. 1994), (b) log baffles (McMahon & Gordon 1989) and (c) Plexiglas T-structures (Fausch 1993). (d) Laboratory manipulations of substratum ripple height and spacing on cod (Gerstner 1998). (e) A schooling fish swimming in the vortex wake of preceding members can gain a hydrodynamic advantage if a diamond formation is adopted (Breder 1965; Weihs 1973). (f) Lateral view showing that brook trout choose to swim behind cones, half-spheres and spheres less when their ability to detect flow is blocked (Sutterlin & Waddy 1975). (g(i)) Dorsal and (g(ii)) lateral view of fish entraining on relatively small diameter (less than 30% of standard body length) horizontal cylinders (Webb 1998). (h(i)) Dorsal and (h(ii)) lateral view of brook trout, river chub and smallmouth bass entraining on relatively small diameter vertical cylinders (Sutterlin & Waddy 1975; Webb 1998; Montgomery et al. 2003). Rainbow trout (i) riding in the bow wake, (j) entraining and (k) exploiting vortices behind a relatively large (50% standard body length) D-section cylinder (Liao et al. 2003a,b; Liao 2006). See text for details.
Whenever water flows past a structure, such as a rock or the moving tail of a fish, velocity gradients are created which form vortices that range in intensity and size, interact with each other and decay over different time courses (Vogel 1994; Zdravkovich 1997). These conditions can alter body motions (figure 1) both relative to the earth frame of reference (displacement) and the fish frame of reference (kinematics; Liao et al. 2003b). Turbulence can also have broader effects, influencing daily behavioural routine (Webb 2002) and habitat choice (Fausch 1993; Webb 1993; McLaughlin & Noakes 1998; Heggenes 2002; Enders et al. 2003; Smith 2003). Swimming performance can be drastically influenced by flows altered by abiotic as well as biotic sources (such as schooling; Weihs 1973; Abrahams & Colgan 1987; Gerstner 1998; Herskin & Steffensen 1998; Webb 1998; Fish 1999; Liao et al. 2003a). This review will discuss examples from these categories, with emphasis placed on laboratory studies employing relatively well-characterized wakes from simple geometric objects. The mechanisms of flow usage in fish schools will be discussed as an example of biotically generated flows. Exploiting self-generated flows, while in certain situations an important mechanism to enhance locomotory performance (Streitlien & Triantafyllou 1996; Dickinson et al. 1999), will not be covered in this review.
As early as the 1960s, Breder (1965) identified the potential benefits of swimming in turbulent flows. He suggested that fish swimming among vortices may be ‘taking advantage of the lessened flow downstream of the side of the vortex which is moving countercurrent…taking advantage of (the vortex) may be the only means by which such fishes are able to negotiate flows as strong as they can be observed to negotiate’. Since then, many studies have shown that vortical flows provide an energy-rich environment which can be exploited to enhance swimming performance (Breder 1965; Weihs 1973; Streitlien & Triantafyllou 1996; Coutant & Whitney 2000; Hinch & Rand 2000; Liao et al. 2003a). There are at least two mechanisms by which fish can exploit unsteady flows generated by abiotic or biotic sources to increase swimming performance. First, fish can take advantage of regions of reduced flow velocity behind bluff bodies. In this case, the average velocity in the turbulent wake is reduced relative to both its steady-state condition prior to interaction with the bluff body and to the earth frame of reference. When fish seek refuge from the main current, they can be described as ‘flow refuging’ (i.e. exploiting regions of reduced flow velocity; Webb 1998). This behaviour usually occurs near the interface between a fluid and a solid, and can present an opportunity for ‘station holding’, which is the ability to maintain position in a current relative to the earth frame of reference without actively swimming (Gerstner 1998). This is the most commonly implicated mechanism of flow exploitation in the fisheries literature (Puckett & Dill 1984; McMahon & Gordon 1989; Shuler et al. 1994; Heggenes 2002). The second mechanism involves capturing the energy of discrete, environmentally generated vortices, and this depends on an appropriate ratio of vortex diameter to fish length as well as low levels of background turbulence (Triantafyllou et al. 2002; Liao et al. 2003a). These mechanisms are useful categories by which to think about how fish use altered flows, but are to some extent arbitrary since both are ultimately taking advantage of vortical flows.
2. The role of stability in negotiating turbulence
Fish subjected to complex flows require an ability to maintain stability. Stability can be achieved passively by self-correcting mechanisms or actively with powered movements (Webb & Weihs 1994; Jindrich & Full 2002; Liao 2002; Webb 2004). In general, fish are attracted to turbulent flows if their mechanisms of stability are sufficient for a given hydrodynamic environment, usually if the flow environment has a predictable spatiotemporal component. Fish tend to avoid flows that have unpredictable, wide fluctuations in velocity (Enders et al. 2003; Smith 2003), or otherwise have flow features at a spatial and temporal scale that interfere with swimming trajectories. Since fish are not equally sensitive to disturbances in all planes, perturbation direction relative to the body plays a pivotal role in determining the nature of the response (Webb 2004). For example, fish swimming quickly through regions of high turbulence are unstable and show irregular pitching motions (Pavlov et al. 2000). This sensitivity to vertically oriented perturbations suggests that correction for pitching, which requires the production of vertical forces either anterior or posterior to the centre of mass (COM), is more difficult than for disturbances from other directions. Webb (2004) found that flow surges would displace fish similarly from their holding stations near cylinders regardless of their body morphology, and that rolling perturbations induced by jets of water were more quickly corrected than perturbations that produced slip, yaw or pitch motions (figure 3). Since a rolling moment grows slowly at the beginning of a roll, stabilization may be enhanced by unilateral thrust forces generated by powered fin movements early on. Quicker correction times for rolling perturbations may also reflect contributions from passive mechanisms. Webb & Weihs (1994) found this to be the case for sunfish, which possess a hydrostatic rolling equilibrium because the gas bladder and COM are positioned at the same height. In the absence of powered motions, simply the posture of the fins may also facilitate self-righting behaviour when water moves relative to a fish (Webb 1998; Liao 2002). The shape of the body and fins and their intrinsic compliance can also contribute to passive mechanisms of stability. In response to sudden postural disturbances caused by jets of water, fusiform-shaped fish can self-correct more quickly than laterally compressed fish (Webb 2004). The passive return of the body to its resting state due to the intrinsic compliance of the musculoskeletal system has been termed a ‘preflex’ for terrestrial animals (Jindrich & Full 2002). Passive self-correction to a perturbation is independent of neural processes and has been documented to facilitate the mechanisms of stability in terrestrial animals. This area holds great promise for future studies of stability in aquatic animals.
Figure 3.
To investigate the ability of species with different body morphologies to stabilize their body in response to a hydrodynamic disturbance, sudden flow perturbations were generated (Webb 2004). (a) A strut oriented perpendicular to the flow direction (i) is quickly flipped 90° (ii). The resulting surge displaces fish similarly relative to the earth frame of reference regardless of body morphology. (b) A jet of water was directed at a specific location on the body to assess the recovery response time, revealing that fusiform-shaped fish can self-correct more quickly than laterally compressed fish.
3. Facilitating group dynamics: biotically generated flows during schooling
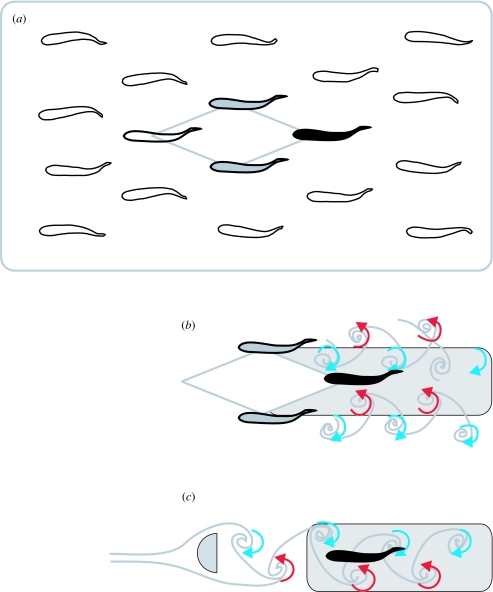
Of the studies investigating the beneficial effects of biotically generated flows, fish schooling has by far received the most attention. In addition to hydrodynamic reasons, schooling formations are established as a result of many social and sensory factors (Parker 1973; Weihs 1973; Pitcher et al. 1976; Partridge & Pitcher 1980; Partridge et al. 1983; Krause 1993; DeBlois & Rose 1996; Bumann et al. 1997). Individuals in a school swim for a longer duration, with lower tail-beat frequencies and respiratory rates, compared with fish swimming alone (Belyayev & Zuyev 1969; Abrahams & Colgan 1987; Fitzsimmons & Warburton 1992; Ross & Backman 1992; Herskin & Steffensen 1998; Svendsen et al. 2003). However, it is a physical fact that a swimming fish generates vortices in its wake, so it follows that individuals in a school swimming behind each other will encounter vortices from the propulsive wakes of preceding members (Breder 1965). Experimental and theoretical studies have invoked flow refuging, vortex capture, wall effects and tip vortices to explain the hydrodynamic benefit and energetic savings associated with schooling. For example, if fish in a school adopt a diamond formation (Weihs 1973), an individual located behind and in between two preceding members can take advantage of the average reduced velocity associated with the thrust wakes of the two preceding members (figure 4a). Owing to the rotation of the vortices in a reverse Kármán street (i.e. thrust wake), downstream flow outside the wake will be slower than the jet of water through the centreline of the wake (relative to the earth frame of reference). A fivefold energy saving is predicted for the Weihs' model, given the condition of a two-dimensional school constrained to a single horizontal plane (i.e. one ‘layer’ of fish, with no members above or below this layer). The lateral proximity of neighbours in a school, if less than one body length apart, can increase an individual's swimming performance through wall or channelling effects (Weihs 1973; Webb 1993; Fish 1999). Similarly, the proximity of the school to the substrate can potentially lead to ground effects (Blake 1983). These mechanisms can theoretically increase the thrust of an individual by tens of percentages without additional energy expenditure (Weihs 1973). Three-dimensional schools in nature may have additional mechanisms to enhance individual swimming performance, such as tip vortices shed by pectoral fins (Lissman & Schollenberger 1970; Weihs 1973; Fish 1999).
Figure 4.
(a) Schematic illustrating the hydrodynamic benefit gained by an individual fish swimming in a two-dimensional school if a diamond formation is adopted. A reverse von Kármán street (thrust wake) is generated by the propulsive motions of two individuals (grey fill), which can be exploited by a downstream individual (black fill) if positioned between the two preceding individuals. Side-by-side thrust wakes generated by two individuals in a school (b) create a Kármán street (drag wake, highlighted in the grey box) analogous to that seen behind a bluff body in the flow such as a D-section cylinder (c; Weihs 1973). Fish can exploit the energy of discrete vortices as well as the average reduced velocity in the Kármán street. Additional mechanisms are discussed in the text. Blue represents clockwise vorticity and red represents counter-clockwise vorticity.
Conducting experimental work on schooling fish is challenging and empirical evidence supporting the maintenance of a diamond formation has been conflicting (Weihs 1973; Breder 1976; Partridge & Pitcher 1979). Respirometry measurements remain difficult owing to the mutually exclusive requirements of having a large working area to minimize wall effects and behavioural abnormalities, and having a small water volume necessary for accurate oxygen measurements. With these precautions in mind, the metabolic cost of an individual swimming in a school has been estimated to be less than for an individual swimming in solitude based on reduced tail-beat frequencies (Herskin & Steffensen 1998). However, care needs to be exercised when extrapolating data to group situations (Tang & Boisclair 1993; Anras et al. 1997). Reduced tail-beat frequency is thought to correspond to reduced energy expenditure based on the assumption that individuals benefit from flow refuging, wall effects, vortex capture or some combination of these mechanisms. Despite the occurrence of decreased tail-beat frequency for schooling fishes, additional data are needed before we can distinguish between these hydrodynamic mechanisms.
Observations of individual positions in a school have provided useful information, but still almost no hydrodynamic or physiological data exist to evaluate the hypothesis that fish can increase swimming performance by taking advantage of the wake of other members. Fortunately, the hydrodynamic environment behind two members in a school can be approximated by the drag wake of a bluff body in flow. This is because a drag wake is created between two parallel thrust wakes (figure 4b,c). Studies strongly suggest that fish can benefit energetically from swimming in drag wakes by capturing the energy of discrete vortices (Liao et al. 2003a; Liao 2004), so by extension it is reasonable to predict that individuals in a school can also benefit from vortices. Future investigations to visualize flow between two tail-sized flapping foils would lend strong support to the hypothesis that fish can benefit from vortices in a three-dimensional schooling environment.
One useful experiment to conduct would be to quantify kinematics and muscle activity from an individual swimming in a school. The results would help support or refute the contributions of flow refuging, wall effects or vortex capture during schooling. Flow refuging or wall effects might be revealed as lower amplitude lateral body waves and reduced tail-beat frequency along with decreased muscle activity concentrated caudally, corresponding to what is expected for fish swimming at lower velocities or in a constricted channel. Vortex capture might translate into more dramatic kinematic differences or novel patterns of muscle activation, as has been shown previously (Liao 2004).
4. Understanding mechanisms of behaviour in altered flows
(a) Field observations and manipulations
Fisheries biologists have long observed that fishes living in current-swept habitats will associate with certain types and sizes of structure, such as boulders and woody debris, to take advantage of velocity refuges (Hartman 1965; Bustard & Narver 1975; Shirvell & Dungey 1983; deGraaf & Bain 1986). Field observations and experimental manipulations have revealed valuable information about behaviour simply by observing preferences for structures and average flow velocities. Unfortunately, limitations in technology can lead to qualitative hydrodynamic and behavioural results, which detract from the ability to inform decisions on habitat management and modelling distribution patterns at the population level (Fausch 1984; Hughes & Dill 1990; Hill & Grossman 1993; Rincon & Lobon-Cervia 1993; Nislow et al. 1999). While experiments placing geometric objects in the field have revealed that current velocity is the major variable determining microhabitat selection in stream-dwelling salmonids (McMahon & Gordon 1989; Fausch 1993), there is a need to measure actual kinematics of fish suspended in natural flows before we can better understand the mechanistic effects of turbulence on stability and locomotion. Swimming kinematics are rarely recorded from the field in part because they require a dorsal (i.e. imaging through surface waves) or ventral (i.e. underwater) camera perspective which is difficult to obtain. Most field video recordings adopt a lateral view which precludes analysis of informative kinematic variables, such as tail-beat frequency, amplitude and body wavelength. In one exception, McLaughlin & Noakes (1998) measured swimming kinematics for wild brook trout (Salvelinus fontinalis) in natural streams and reported a difference from that of fishes in laboratory flumes. Tail-beat frequencies and amplitudes varied in response to unsteady hydrodynamic phenomena such as the boundary layer above the substrate and the inherent ebb and surge of current velocity in natural streams. Tail-beat frequency in particular was dramatically influenced by temporal and spatial flow heterogeneities and was higher than that found for fish in the laboratory for a given swimming speed. Without these detailed descriptions of kinematics, it is difficult to determine if fish in natural habitats are simply swimming in reduced flow behind structures or if they are additionally interacting with discrete vortices in the wake of structures.
(b) Measuring the response to fluid perturbations: a case for laboratory studies
A detailed description of how fishes interact with the immediate spatial and temporal features of flow is necessary if we are to understand behaviour across different hydrodynamic environments. Laboratory experiments have proven invaluable in accelerating a quantitative understanding of swimming mechanics in turbulent flows at the organismal level. This is because, at present, only in the laboratory can flow velocity and turbulence levels be controlled and techniques such as flow visualization (Lauder et al. 1996; Müller et al. 1997; Anderson et al. 2001), electromyography (EMG; Jayne & Lauder 1994; Altringham & Ellerby 1999) and high-speed video (Jayne & Lauder 1995; Gillis 1996) be employed to their full potential. Care must be taken since the results of this controlled approach are applicable to natural conditions only if physical structures responsible for altering flows approximate those from the field. For example, by systematically subjecting cod (Gadus morhua) to substratum ripples of different natural heights and spacings in a laboratory flume, Gerstner (1998) and Webb (1998) were able to identify a specific range that elicited station-holding behaviour. Fish would be displaced if flow velocity exceeded the upper limit of this range, and avoid substratum ripples when velocity was slower than the lower limit of this range. Similarly, Webb (1998) found that fishes only preferred to swim behind horizontally oriented cylinders approximating submerged tree branches at intermediate flow speeds (figure 5). During periods of fast flow, fish were displaced from the cylinder and during slow flow they avoided cylinders altogether. A preference to exploit a specific range of turbulence reveals that fish can be quite sensitive to turbulent cues. Indeed, Smith (2003) found that, for juvenile rainbow trout (Oncorhynchus mykiss) swimming at a constant average flow velocity, differences in turbulence level can provide sufficient information to discriminate between habitats.
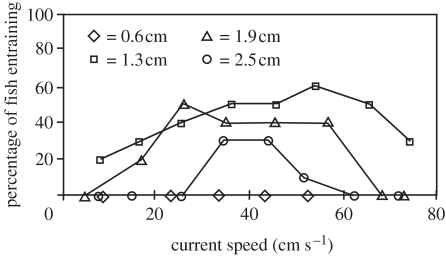
Figure 5.
Percentage of fish entraining on horizontally oriented cylinders of various diameters with increasing flow speeds. Cylinder diameters (cm) are represented from smallest to largest as geometric symbols: diamonds, squares, triangles and circles, respectively. Fish have a preferred range of turbulence, shown by the fact that intermediate diameter cylinders (triangles and squares) cause the most fish to entrain across the widest span of current speeds (Webb 1998).
(c) Exploiting steady flows around a cylinder: the bow wake and entraining
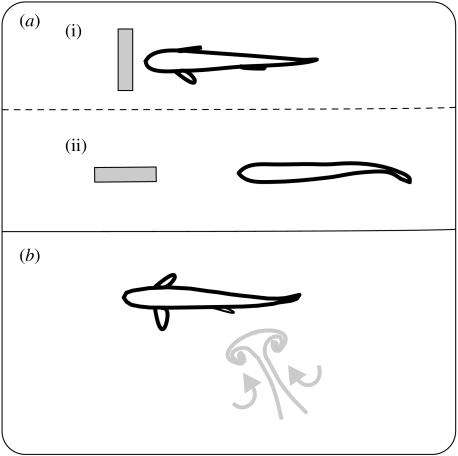
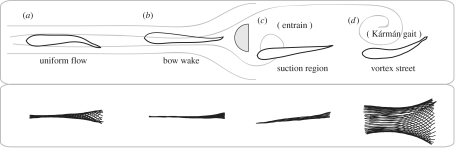
Steady flow becomes altered when it encounters a bluff body such as cylinder. A high-pressure region (i.e. stagnation point where fluid velocity is zero) is established at the upstream side of the cylinder, and a stable low-pressure suction region is established (i.e. attached eddy) at the downstream side of the cylinder (Vogel 1994; Zdravkovich 1997), both of which fish can exploit. For example, compared to fish swimming in steady flow (figure 6a), fish holding station in the reduced-flow region of the bow wake (figure 6b) exhibit very little body undulation (Liao et al. 2003b). Similarly, when fish entrain near the low-pressure suction region of the cylinder (figure 6c) they make no regular axial swimming motions and yet can still balance thrust and drag forces to hold station relative to the earth frame of reference. When fish entrain they position their heads close to, but not touching, the cylinder and their fins are continuously in motion without any discernable pattern (Webb 1998; Liao 2004). This is one example of how altered flows can still be considered ‘steady’. Presumably, fish are taking advantage of the steady aspects of the attached eddy and making fine-scale corrections with their fins. Instantaneous velocity measurements in the region where fish entrain are needed to provide support for this hypothesis. Although no axial muscle activity or oxygen consumption measurements have been made, the drastically reduced body motions while in the bow wake or entraining strongly suggest that these positions are energetically favourable. At times, swimming in the bow wake or entraining (figure 6b,c) is preferred over swimming in uniform flow or in the vortex street behind a cylinder (figure 6a,d; Webb 1998; Liao et al. 2003b). Video analyses reveal that fish often spend the entire duration of the experiment in the bow wake or entraining, and if displaced they respond by swimming through the cylinder wake to resume position (Liao et al. 2003b; Liao 2006). Only under laboratory conditions can detailed observations of fin/body kinematics and position be made across different hydrodynamic conditions with such resolution and consistency.
Figure 6.
Summary schematic showing the anatomy of the flow around a D-cylinder and the positions and associated fish swimming midlines from approximately one tail-beat cycle. (a) A trout swimming in uniform flow is shown at the far left for comparison. Trout in the (b) bow wake (Liao et al. 2003b), (c) entraining in the suction region (Sutterlin & Waddy 1975; Webb 1998; Montgomery et al. 2003; Liao 2006) and (d) Kármán gaiting in the vortex street. In all cases except a, trout are holding station, defined as maintaining position in a current relative to the earth frame of reference without actively swimming (see text for discussion on Kármán gait kinematics and neuromuscular activity). By convention, fish are flow refuging whenever they maintain position (relative to the cylinder) in regions where the average flow velocity is reduced (b, d and potentially c). Flow refuging and vortex capture may therefore not be mutually exclusive, as exemplified in Kármán gaiting. A hydrodynamic reason for why the body of an entraining trout (c) is angled into the wake is currently unclear (Liao et al. 2003b; Liao 2006).
(d) Unsteady flows behind a cylinder: kinematics in a Kármán street
Fish will seek and maintain position a few body lengths downstream of a cylinder (Liao et al. 2003b) within a range of Reynolds numbers, which is a dimensionless number that describes the ratio of inertial to viscous forces (Vogel 1994). For Reynolds numbers between 300 and 150 000, flow past a stationary cylinder generates a wake known as a Kármán vortex street (Blevins 1990). It is possible to systematically alter the frequency at which vortices are shed, as well as the downstream spacing between successive vortices (i.e. wake wavelength), by changing the flow velocity and the cylinder diameter. This provides an ability to generate consistent, repeatable and controlled hydrodynamic perturbations. Depending on their body length, rainbow trout will adopt a novel gait when stationed behind D-section cylinders (the sharp trailing edge promotes a more consistent vortex shedding frequency), such that they synchronize their body motions to the vortex shedding frequency across different combinations of cylinder and flow treatments. This unique pattern of motion, termed the Kármán gait, is characterized by a lower tail-beat frequency and larger lateral body amplitudes and curvatures than for swimming in uniform flow of comparable velocity (Liao et al. 2003b). Are these motions due to the lateral translation of the body by vortices or do kinematics actually change relative to the fish frame of reference? Several lines of evidence demonstrate that Kármán-gaiting trout are not just employing steady-swimming kinematics while being buffeted from side to side by vortices (Liao 2004, 2006). Body kinematics in the vortex street differ from those in the bow wake or entraining, the latter of which shows no correlation to the expected vortex shedding frequencies when cylinder size and flow speed are varied (Webb 1998; Liao et al. 2003b). The Kármán gait is an example of how a novel mode of locomotion can be revealed when we move beyond studying animals in simple environments. While characterization of complex habitats is relatively tractable in terrestrial systems, this challenge is made more difficult in a three-dimensional fluid environment.
5. Visualizing flow at the organismal level
The application of quantitative flow visualization techniques to biological studies has accelerated our understanding of aquatic locomotion by making it feasible to quantify water flow in the wake of freely swimming fishes. These studies have provided new information on how swimming speed and species membership can sculpt the features of the propulsive wake, how flow behaves at the interface of the body and fins and what is the contribution of forces along the body that enables stable swimming (Willert & Gharib 1991; Lauder et al. 1996; Müller et al. 1997; Drucker & Lauder 1999; Anderson et al. 2001; Bartol et al. 2002). This approach has been applied with great success to understand the fundamental aspects of locomotion under simple hydrodynamic conditions.
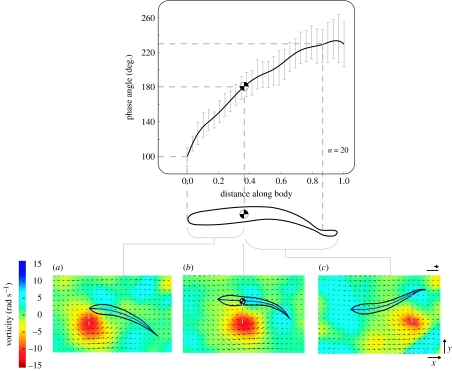
Flow visualization also presents a unique opportunity to quantify directly the effect of complex flows on fish behaviour. Simultaneous visualization of a cylinder wake and Kármán gait kinematics revealed that fish move with the lateral component of the sinusoidal flow in a vortex street, slaloming between vortices rather than swimming through each vortex centre (Liao et al. 2003a). Lateral body displacement (y-axis, figure 7) can be described using an arbitrary phase relationship relative to the position of the drifting vortices. For example, when a vortex drifts down to the (x-position) region of the head (figure 7a), the head and the vortex have a phase relationship of approximately 100°, indicating that the head is moving laterally away from the vortex. When a vortex drifts down to the body's COM (chequered circle, figure 7b), the COM has reached its lateral-most excursion from that vortex (i.e. phase relationship is 180°, or anti-phase) and is starting to move back towards the midline of the wake. At all body points posterior to the COM, the phase relationship is greater than 180°. Thus, when a vortex drifts to the tail (figure 7c), the phase relationship between vortex and body is approximately 230°, so the tail is moving laterally towards the vortex. The phase relationships between different points along the body and vortices are consistent with the observation that the body is being translated by the lateral component of the flow established by the preceding vortex.
Figure 7.
(a–c) Time-series of a trout Kármán gaiting in a vortex street generated from a 5 cm cylinder to the left (not shown) illustrating the phase relationship between points on the body and the position of a drifting vortex. Fish outlines (black) and midlines (blue) are superimposed onto vorticity and velocity vector plots of the cylinder wake. Black arrows indicate flow direction (arrow orientation) and speed (scale arrow=56 cm s−1 or 4.5 body lengths per second; scale bar, 1 cm). Vorticity (radians per second) is colour-coded, where red represents counter-clockwise vorticity, green represents zero vorticity and blue represents clockwise vorticity. An arbitrary, external reference system was established to quantify the spatial relationship between the centre of the vortex and the position of a specific body point at a given time in the Kármán gait cycle (n=20 tail-beat cycles). (a) When a counter-clockwise vortex reaches the location of the head, the head is moving away from the vortex (phase relationship less than 180°; in this case, it is approx. 100°) due to the largely passive movement of the body with the lateral component of the flow induced by the previous clockwise vortex (not shown). (b) When the vortex drifts down to the point of the body where the COM is located, the COM is at its lateral-most excursion away from the vortex (phase relationship of 180°) and is about to start moving back towards the midline (dashed white line) of the wake. (c) When the vortex drifts down to the caudal peduncle, the caudal peduncle is moving towards the vortex (phase relationship greater than 180°; in this case, it is approx. 230°). Put another way, a phase relationship of 180° represents slaloming in between vortices, and 0 or 360° (not shown) represents vortex interception. The COM is shown as a chequered circle. Standard error bars are in light grey. Images are approximately 40 ms apart (Liao et al. 2003a).
The ability to Kármán gait relies predominantly on encountering vortical flows of the proper strength and size, which is determined by the flow velocity and ratio of cylinder diameter to fish length. Altering this ratio can substantially affect the preference of fish to associate with cylinders (Liao et al. 2003b). From a hydrodynamic viewpoint, it stands to reason that fish in natural environments will distribute themselves accordingly among different size structures, where structural preference has been correlated to body length in the field (Shirvell & Dungey 1983).
6. The cost of swimming in altered flows
How does one best evaluate the cost of swimming in turbulent flows? Traditional metrics gathered from swimming kinematics, such as tail-beat frequency, slip and Strouhal number, have been used to assess the performance of propulsive, thrust-based locomotion in uniform flows, but are inappropriate in unsteady flows (Liao et al. 2003b). As a first approximation, simply observing if fish prefer or avoid turbulent habitats may indicate if an energetic benefit exists. However, care must be exercised since cover complexity, which is intimately linked to turbulence in current-swept environments, offers other advantages such as visual isolation from conspecifics and predators in addition to hydrodynamic benefits (Smith 2003).
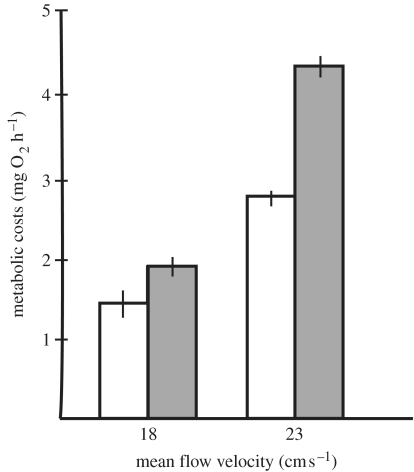
Oxygen consumption is the most direct measure of metabolic cost required during fish locomotion (Blazka et al. 1960), yet few studies have assessed the energetic expenditure of fishes swimming in turbulent flow. One exception showed that juvenile Atlantic salmon (Salmo salar) swimming at a constant average flow velocity increase their energy expenditure when exposed to turbulence (figure 8), generated as wide fluctuations of flow velocity around a mean flow value (Enders et al. 2003). This increase in cost with turbulence was especially dramatic for trials conducted at higher mean flow velocities. Migratory fishes commonly encounter high levels of turbulence and show difficulty maintaining proper orientation. These fish exhibit increased respiratory rates and a decreased ability to perform escape behaviours after experimental turbulence trials (Odeh et al. 2002). Thus, a picture has emerged where fish choose habitats not only based on average flow velocity but also on the degree of variation in flow velocity (Pavlov et al. 2000; Smith & Brannon 2005). These studies make it clear that models need to take velocity fluctuations into consideration when estimating the costs of locomotion in natural flow environments. The nature of the data makes it difficult to determine exactly what flow features are responsible for the increase in swimming costs. Oxygen consumption is a time-averaged measurement and thus cannot provide the temporal resolution necessary to determine the proximate hydrodynamic mechanisms that affect physiology. If combined with quantitative flow visualization, the opportunity to correlate metabolic swimming costs to specific turbulent features (i.e. vortex size, strength and frequency) will be possible for the first time. Complementary data, such as kinematics and muscle activity patterns will contribute substantially to our ability to decipher the hydrodynamic mechanisms of turbulence-induced swimming costs.
Figure 8.
Cost of turbulence as measured by oxygen consumption for juvenile Atlantic salmon. Turbulence was experimentally generated by varying flow velocities around two different mean velocity values. Oxygen consumption for salmon increases in turbulence (grey) compared to swimming in steady flows (white) for a given mean flow velocity. This trend is more dramatic at higher mean flow velocity. Vertical lines represent 95% confidence intervals (Enders et al. 2003).
7. Turning a constraint into a benefit: reduced muscle activity in vortical flows
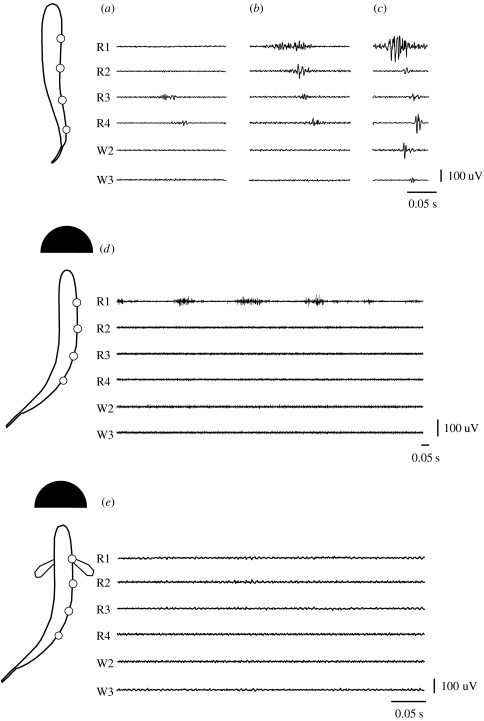
We currently have little understanding of how hydrodynamic perturbations affect the neuromuscular control of locomotion, in contrast to the extensive literature available for studies of steady swimming (Grillner & Kashin 1976; Gillis 1998b; Hammond et al. 1998; Jayne & Lauder 1994; Wardle et al. 1995; Altringham & Ellerby 1999). Observations have shown that fishes in current-swept environments position themselves in the wake behind objects to take advantage of the reduced velocity and presumably reduce muscle activity (Heggenes 1988; McMahon & Gordon 1989; Fausch 1993; Shuler et al. 1994). Yet, kinematics alone cannot conclusively reveal mechanisms of flow exploitation or the extent of energy expenditure involved under these circumstances. When muscle recordings and flow visualization techniques are employed in the laboratory (Liao et al. 2003a), it is clear that trout holding station behind a cylinder are not just seeking refuge in reduced flow or using a rostrocaudal sequence of muscle activity (figure 9a–c), but using the energy of the vortices (figure 9d,e). This is because although the body participates in a large-amplitude mechanical wave, electromyograms (EMGs) reveal that only the anterior, axial red muscles are activated in a stereotypical manner when exploiting vortices (figure 9d; Liao 2004). At times, fish actively oscillating their pectoral fins do not exhibit any red or white axial muscle activity (figure 9e). This demonstrates that trout holding position in the vortex street (figure 10) can generate enough thrust to balance drag just using their fins (not shown) and/or the intrinsic compliance of their musculoskeletal system (Liao et al. 2003a,b).
Figure 9.
Red and white axial muscle EMG traces of a trout swimming in uniform flow at: (a–c) different speeds, and in a turbulent vortex street behind a cylinder (d) without and (e) with pectoral fin activity. Red muscle electromyograms (R1–R4) are approximately aligned with the electrode positions along the body outlines to the left, with the W2 and W3 white muscle electromyograms corresponding to the same longitudinal placement as R2 and R3 insertion sites (Liao 2004). (a) When swimming in uniform flow at 1.8 L s−1, red muscle activity propagates down to the posterior end of the body. (b) At 3.5 L s−1, a wave of red muscle activity travels the entire length of the body. (c) At 5.0 L s−1, white muscle is recruited. (d) Vortex street 3.5 L s−1. The same fish Kármán gaiting behind a cylinder placed in a 3.5 L s−1 flow activates only its anterior-most red muscles (R1). Several swimming cycles are shown to illustrate the rhythmicity and variation of activity. (e) Vortex street with pectoral fin activity 3.5 L s−1. At times during the Kármán gait when the pectoral fins are active, there is no appreciable axial muscle activity along the entire body. Black scale bars are given for time (s) and muscle activity (uV).
Figure 10.
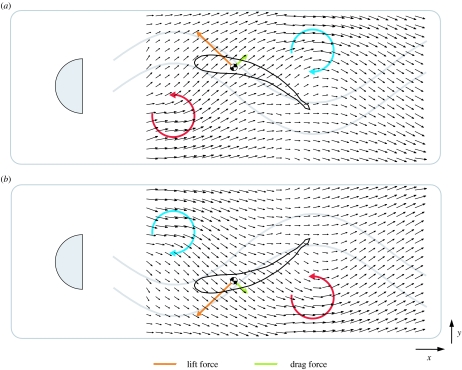
Hypothesized mechanism of vortex capture for thrust production of a fish in a vortex street based on theoretical and experimental work with oscillating foils, live and euthanized fish (see text for details). The direction and length of the black arrows represent flow velocity. (a) A low-pressure, counter-clockwise vortex (red circle) is shed from the cylinder and approaches the head of the swimming trout, causing the incident flow to be directed at an angle to the body. The relatively large angle of attack of the body produces a lift force (orange arrow) normal to the path of the incident flow and a drag force (green arrow) parallel to the flow. The region of average reduced flow behind the cylinder is approximated by the grey sinusoidal lines. (b) After a certain period of time, the sinusoidal flow of the Kármán street translates the fish back towards the midline of the wake such that the fish will then encounter a clockwise vortex (blue circle). Force vectors in (b) are the same as in (a), only flipped in the y-direction. Owing to vorticity decay, the upstream vortex has a lower pressure than the downstream vortex, which may facilitate holding position in a certain region along the x-axis of the wake. The ability to use the energy of vortices provides an additional explanation (to accompany flow refuging) as to why fish swim in the wake behind bluff bodies (Liao et al. 2003b).
The ability to station hold in fast turbulent flow while exhibiting relatively little axial muscle activity is possible because the high-amplitude, whole-body undulatory motions seen during Kármán gait are largely generated by vortices (Liao et al. 2003a). Put another way, Kármán-gaiting trout are not exclusively using a travelling wave of muscle activity to generate propulsive movements, as during swimming in uniform flow, but rather capturing the energy of cylinder vortices to generate thrust. Anterior red muscle activity during the Kármán gait has a significantly lower intensity and longer duration when compared with swimming in the absence of a cylinder (Liao 2004). This suggests that muscle activity is playing a role in controlling body posture and compliance rather than generating propulsive body movements. Rhythmic anterior red muscle activity may be positioning the head favourably in the wake to use vortices, either by resisting or exacerbating head movement caused by vortices. An analogy to the nautical mechanism of ‘tacking’ was proposed to explain the Kármán gait, whereby the alternating camber and angle of the body, facilitated by the lateral flow component of the oscillating wake (y-axis, figure 10), generates thrust passively (Liao et al. 2003b). Decreased axial muscle activity in response to vortices (Liao 2004) provides a physiological mechanism to explain the preference of fish to associate with turbulent flow rather than swim in uniform flow.
Sections 1–6 have shown that under certain conditions fish can move against a current without activating much axial musculature. Thus, at first glance flow features which might initially be thought to be destabilizing or impose a constraint to swimming performance may, upon closer inspection, actually turn out to be a benefit to swimming fish. This finding forces us to reconsider the definition of an environmental constraint and cautions against overestimating the cost of locomotion in naturally turbulent flows. For example, for a given swimming speed, tail-beat frequency is higher in the field than in the laboratory (McLaughlin & Noakes 1998). Swimming in natural flows can be interpreted as less efficient only if the energetic cost of oscillating the tail is the same in both the field and laboratory. This cannot be assumed since it is possible that less muscle activity is required to beat the tail in the field, given what we know about vortex-assisted swimming. This underscores the importance of a physiological measurement for swimming cost to accurately assess the relationship between flow conditions and locomotion.
The extent of axial muscle activity reduction in response to other unsteady flow regimes remains unexplored. As flow velocity increases from rest past a cylinder, swimming costs should be low as the vortex street develops and fish begin to Kármán gait. When trout are unable to use vortices in a vortex street, such as when encountering extreme wake wavelengths or high levels of background turbulence, axial muscle activity is predicted to increase as propulsive movements are introduced. Above a certain flow speed (and thus vortex shedding frequency), muscle activity and hence swimming cost may be expected to rise as fish become unstable, resulting ultimately in abandoning the cylinder wake. Similarly, the size of the vortices will influence the ability of fish to exploit them. If vortex size is more than an order of magnitude smaller than the body length fish may swim steadily through them. Likewise, if they are much larger than the body (e.g. an oceanic gyre) they may linearly displace the body but not affect swimming kinematics.
8. Passive thrust production in a vortex street
As we have seen, the benefit of swimming behind a bluff body is not limited to using the region of reduced flow relative to the earth frame of reference. Instead, the energy of individual, discrete vortices can be exploited. Vorticity control is a relatively new paradigm in fish locomotion (Anderson 1996; Streitlien & Triantafyllou 1996; Triantafyllou et al. 2000, 2002) and may help explain how migratory fishes can ascend highly turbulent rivers for extended periods. Harnessing the energy inherent in turbulent environments can be substantial; it has been experimentally and theoretically demonstrated that thrust can be generated entirely passively by a foil when placed in an oscillating flow (Wu & Chwang 1975; Bose & Lien 1990; Anderson 1996; Beal 2003). The ability to extract energy from vortices can greatly enhance propulsive efficiency (Golpalkrishnan et al. 1994; Anderson 1996; Streitlien & Triantafyllou 1996). This is largely because as the lateral component of the flow heaves a foil from side to side, the foil's chord maintains a favourable angle of attack with respect to the incident flow and facilitates both lift and thrust production (Wu & Chwang 1975; Bose & Lien 1990). Analogous to a passive foil exploiting vortices (Wu & Chwang 1975), live trout can Kármán gait temporarily without any axial muscle activity (Liao 2004). Consequently, dead trout towed behind a cylinder can generate thrust passively and move upstream on a slack line when the natural resonance of their flexible body allows for synchronization with vortices (Liao 2004; Beal et al. 2006). Interestingly, only freshly killed trout could generate thrust and move upstream. Towed trout at various stages of rigor mortis, frozen and then thawed trout, and rubber models of varying stiffness did not show similar upstream movement but rather rolled unpredictably against the taut tow line. This reveals both the importance and subtlety of proper body compliance in facilitating vortex capture in fishes. The oscillating motions of dead, towed trout were remarkably similar to live Kármán-gaiting trout. In both cases, head angle was the same, lateral body oscillations were similar in amplitude (relative to the fish frame of reference), frequency approached the vortex shedding frequency of the cylinder and body wavelength was longer than the wake wavelength (Liao 2004). Statistically identical head angles measured relative to the midline swimming trajectory for dead and live trout suggest that vortices and not anterior muscle or pectoral fin activity control head orientation (Liao 2004). This assumes that fish are positioned at an appropriate distance downstream from the cylinder. Paradoxically, these data reveal that, given proper musculoskeletal compliance and body position in the vortex street, at times no axial muscle activity is needed to maintain station or even move upstream in turbulent flows.
Differences in behaviour are obviously expected between live Kármán-gaiting fish and dead, towed fish and these differences can be informative. Compared to live fish Kármán gaiting, dead fish towed behind a cylinder pitched, rolled and were more frequently drawn upstream into the suction region of the cylinder. This suggests that in order to Kármán gait continuously live fish must be able to produce drag selectively to remain in the appropriate downstream region of the vortex street. If fish hold station too close to the cylinder, they will be abruptly drawn forward by the low-pressure suction region associated with the attached eddies. If fish hold station too far downstream from the cylinder, the energy dissipation of the vortices will render Kármán gaiting less effective and fish will need to recruit more muscle and swim propulsively. Taken together, differences and similarities in behaviour and kinematics between towed and live trout indicate that selective drag production by the fins may play a critical role in negotiating turbulent flows.
9. Fin activity in altered flows
Fishes move multiple fins to stabilize their body when suspended in turbulent flow. The range of stability conferred by the fins may cause body shape and habitat specialization to play less of a role in swimming in turbulence than swimming in steady flow (Bioly & Magnan 2002). For example, fin activity is probably responsible for the fact that fish can exploit vortices shed behind a cylinder despite large differences in body morphology, phylogenetic membership and ecological niche (Liao et al. 2003a). Species as different as lentic, laterally compressed bluegill sunfish (Lepomis macrochirus) and lotic, fusiform rainbow trout both spend a large proportion of time holding station in a vortex street behind a cylinder. Similarity of behaviour despite obvious differences in body morphology is also seen for fish manoeuvring through slits (Webb et al. 1996). This behavioural convergence suggests that a premium is placed on the ability to stabilize the body during swimming in turbulent flows. Perhaps, this is because in nature exploitable turbulent flows are a valuable and limited resource and are often associated with resting positions and foraging opportunities. It is probable that species with extreme body morphologies, especially those with lateral profiles that cannot be substantially modified by median fin activity (e.g. eels), will encounter more difficulty stabilizing in, and thus avoid, turbulent flows. Fine control of the median fins by muscle groups (Winterbottom 1974; Jayne et al. 1996) can alter lateral body profiles and enhance yaw and roll stability (Drucker & Lauder 2001; Liao 2002; Standen & Lauder 2005), as well as dampen perturbations by increasing resistance to translational and rotational moments (Webb 2004).
Species with similar body and fin morphologies can also display different abilities to swim in turbulent flows. Webb (1998) found that fusiform-shaped chub (Nocomis micropogon) entrained behind cylinders for longer durations and across a wider range of flow speeds than similarly shaped smallmouth bass (Micropterus dolomieu). Chub used their fins mostly for passive self-correcting control (i.e. the fin is held out from the body and not actively oscillated relative to the body) whereas bass used pronounced powered motions (i.e. the fin actively moves relative to the body) to abduct the fin and generate thrust. Trimming forces take advantage of passive lift-generating mechanisms established by the orientation and shape of the abducted fins, a common behaviour for fishes swimming in both turbulent and steady flows (Webb 1998; Pavlov et al. 2000; Bartol et al. 2002; Liao 2002). In some fishes, the posture and position of the paired pelvic fins allow them to act as negative dihedrals, and thus affect rolling stability (Wilga & Lauder 1999). Powered propulsive motions, such as the rowing movements of pectoral fin swimmers (Drucker & Jensen 1997; Walker & Westneat 1997), were not observed for fishes swimming in the vortex street behind a D-section cylinder.
Selective ablations (Pavlov et al. 2000) and detailed observations (McLaughlin & Noakes 1998; Webb 1998; Liao et al. 2003b) suggest that pectoral fins play a very active role in maintaining stability in complex flows. As control surfaces located anterior to the COM, pectoral fins can potentially exert large influence in orienting and stabilizing the body. The movements of the pectoral fins can vary widely during swimming in turbulent flows, ranging from slow oscillating bilateral motions to sustained unilateral abductions. For example, the increased drag produced by the ipsilateral abduction of a pectoral fin can cause the body to yaw to the same side, thereby changing the angle of the head to the oncoming current (Liao et al. 2003b). Faced with turbulence in a vortex street, trout stabilize their bodies using their pectoral fins for both self-correcting control and powered movements (Webb 1998; Liao et al. 2003b). Observations in the field support this finding: in natural flows, the pectoral fins of brook trout are often continually active (McLaughlin & Noakes 1998). These observations suggest that paired fins may play a larger role in perturbing versus steady flows, at least for non-labriform swimmers (Gibb et al. 1994; Walker & Westneat 1997; Drucker & Lauder 2003). Unfortunately, no kinematic fin descriptions yet exist for fish swimming in turbulence. This is an area which would benefit from the application of flow visualization, since without a way to evaluate flow perturbations fin kinematics become difficult to interpret.
10. Sensory feedback in altered flows: the role of vision and the lateral line in kinematics
Expanding the scope of locomotion studies to include more complex environments leads to novel insights into the mechanisms of movement (Roberts et al. 1997; Gillis 1998a; Biewener & Gillis 1999; Jindrich & Full 2002). The key to successfully manoeuvring through unpredictable and non-uniform habitats for fishes lies in the ability to sense, process and react appropriately to environmental changes. Thus, one of the most challenging and yet crucial aspects of studying animal locomotion is to understand the role of sensory feedback in modulating the mechanisms of movement as well as mediating higher-order behaviours like avoidance or preference. The remainder of the paper will be devoted to discussing the role of sensory perception and how it affects aspects of fish swimming such as kinematics and habitat preference.
For the majority of fishes, the two most important sensory modalities for swimming are vision and the lateral line sense. The lateral line system detects flow velocity and acceleration via a series of mechanosensory hair cells that are distributed on or just under the skin along the head and body (Coombs et al. 1989). Studies have shown that fishes use both hydrodynamic cues (Dijkgraaf 1963; Montgomery et al. 1997; Engelmann et al. 2000; Coombs et al. 2001) and high-contrast visual cues (Ingle 1971) to swim steadily in uniform flow or in still water. There is a degree of functional redundancy between these two modalities such that blocking the lateral line does not alter the ability to swim in uniform flow if vision is kept intact (Dijkgraaf 1963). In a more complex hydrodynamic environment devoid of visual cues, blocking the lateral line interferes with the ability to swim in steady and unsteady flows. Specifically, fish swimming in the dark without a functional lateral line (i.e. lacking both superficial and canal neuromasts) entrain behind a cylinder less than fish with an intact lateral line (Montgomery et al. 2003). Without sufficient visual cues, the ability of trout to entrain behind obstacles was reduced by bilateral denervation of the posterior lateral line system (Sutterlin & Waddy 1975). This led to the hypothesis that the suction region behind a bluff body represents a discontinuity with the downstream flow, and that the posterior lateral line (consisting mostly of canal neuromasts) was needed to detect this pressure difference along the body. All evidence suggests that both superficial and canal neuromasts are required for obstacle entrainment (Sutterlin & Waddy 1975; Montgomery et al. 2003). This is plausible since the wakes of objects contain both flow velocity and acceleration components, which can be detected by superficial and canal neuromasts, respectively (Coombs et al. 1989; Montgomery et al. 1997). Clear divisions of function between these two neuromast types remain undefined for fish swimming in turbulent flows, unlike for other behaviours such as rheotaxis (Montgomery et al. 1997) and prey detection (Coombs et al. 2001).
The sinusoidal flow of a vortex street represents a considerably different environment than the relatively stable low-pressure suction region directly behind a cylinder. Blocking the lateral line with a cobalt chloride treatment (Karlsen & Sand 1987) alters certain kinematics associated with maintaining position (relative to the earth frame of reference) in a vortex street. Compared to control animals, trout with a blocked lateral line have a faster body wave speed, a lower maximum body curvature, exhibit lower amplitudes, and adopt a longer and more variable body wavelength (Liao 2006). When lateral line functionality is held constant, the presence or absence of visual ability does not change kinematics in a vortex street. In other words, irregardless of whether fish have an intact or blocked lateral line in a vortex street, they display the same motions in both the light and dark. This illustrates that hydrodynamic feedback has a larger effect on Kármán gait kinematics than visual feedback. The duration of time that trout preferred to Kármán gait in the vortex street varied depending on the sensory cues available to them. Trout with a blocked lateral line will only stay in a vortex street for prolonged periods of time if they have visual cues available to orient them to the cylinder (Liao 2006).
Interestingly, trout without hydrodynamic and visual cues can still hold position in the vortex street, albeit in brief bouts. How can fish maintain a spatial coordinate in the absence of vision and flow information? There are two probable explanations for this behaviour. The first explanation has been described earlier as a mechanism of passive thrust generation in an oscillating wake. Apparently, no sensory feedback is necessary to Kármán gait for short periods of time, as evidenced by the ability of dead, towed fish to Kármán gait. The second explanation is that other sensory inputs that were not controlled during the experiments may have contributed to the ability to Kármán gait in the cylinder wake. For example, sound cues generated by shed vortices or flow-induced accelerations of the body can be detected by the acoustic and vestibular ear systems, respectively, and used to facilitate station holding in the appropriate region of the vortex street (Atema et al. 1988).
11. The effect of sensory feedback on the preference to associate with vortical flows
It is important to make the distinction between the ability to Kármán gait in a vortex street and the preference to maintain this behaviour. While most fishes can stabilize their bodies when exposed to flow perturbations, they may not choose to stay in those habitats for extended periods of time. For example, fish may prefer to Kármán gait for a large proportion of time if presented with certain sensory stimuli, but choose to entrain near the suction region when presented with other stimuli.
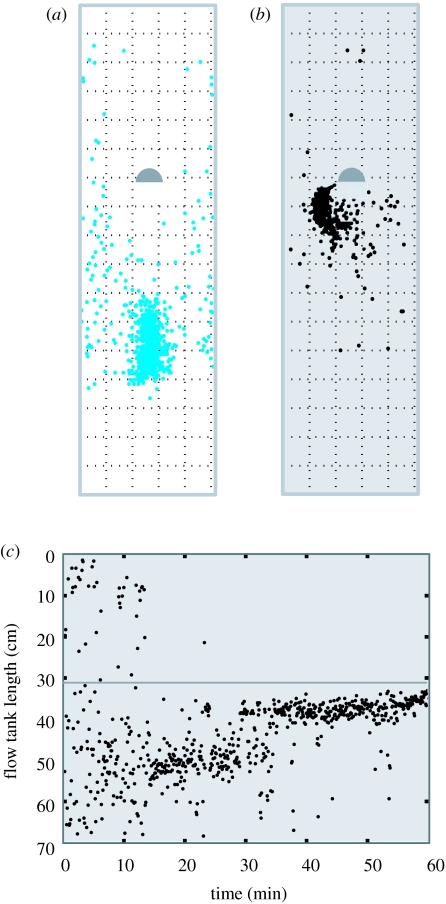
In contrast to body kinematics, the preference to Kármán gait depends more on visual cues than hydrodynamic cues. For example, trout in the light choose to spend most of their time holding station in a turbulent vortex street even without a functional lateral line (figure 11a). This suggests that trout are using vision to maintain position relative to the cylinder to ensure that they occupy the appropriate region of the vortex street. Indeed, early on Dijkgraaf (1963) observed that ‘the visual system appears to be the most likely sensory channel to provide fish with a reference point as an indicator of body displacement’. Observations support this hypothesis: fish are more likely to associate with turbulent flows at higher light levels (Pavlov et al. 2000) and brown trout move from turbulent regions during the day to low velocity, low-turbulence regions at night (Hubert et al. 1994). It may be the case that fish that choose to maintain station in vortical flows in the dark require other sensory cues in addition to the lateral line. In the dark, fish generally avoided Kármán gaiting in the vortex street, preferring to entrain just downstream and to one side of the cylinder regardless of whether they had a functional lateral line (figure 11b). This implies that trout swimming in the dark cannot maintain position in the turbulent vortex street using only hydrodynamic cues. However, fish are evidently using their lateral line to search for favourable flow regimes around the cylinder (figure 11c). Fish in the dark use their lateral line to explore the vortex street, but ultimately prefer to entrain on the cylinder, perhaps because flow characteristics are less variable.
Figure 11.
Time budget illustrating the importance of vision over the lateral line in the preference to hold station in a vortex street. (a,b) Plots for a representative fish (12 cm standard length, L), where each dot reveals the position of the head every 5 s for 1 h relative to the position of a 5 cm D-cylinder. Flow (3.5 L s−1) is from top to bottom. Dashed grid shows 5×5 cm partitions superimposed over the entire area of the flow tank. When the lateral line is blocked (blue dots) and the experiment is conducted in the light (a), fish can visually locate the cylinder and prefer to spend most of their time in the vortex street. The same fish with a functional lateral line (prior to lateral line blocking, black dots) in the dark (denoted by grey fill) does not spend much time in the vortex street (b), but instead prefers to hold station just downstream and to the side of the cylinder near the suction region (‘entraining’, see text for details). (c) Time course of positional preference reveals that in the dark, fish with a functional lateral line require time to search out favourable hydrodynamic positions around the cylinder before holding station. The downstream edge of the D-section cylinder is represented by a grey line located approximately 30 cm downstream from the top edge of the flow tank. Note that by halfway through the experiment (30 min) fish take position approximately 5 cm downstream from the edge of the cylinder, which illustrates that they are entraining (as in b) rather than Kármán gaiting (i.e. holding station further downstream from the cylinder, as in a).
The position that entraining trout adopt next to a cylinder in the dark (figure 6c) is analogous to the position described for two other stream species of distant phylogenetic relationship (Webb 1998). This illustrates that the exploitation of the low-pressure suction region directly downstream of a bluff body may be a common microhabitat preference for fishes living in moving water. The kinematics, and presumably swimming costs, associated with these positions can differ significantly from each other (Liao et al. 2003b). Entraining trout with an intact lateral line choose to alternate between right and left sides of the cylinder (flow past the cylinder was symmetrical), reflecting a tendency to explore their hydrodynamic surroundings. In contrast, fish with a blocked lateral line show fidelity to one side of the cylinder and did not adopt other positions for extended periods (Liao 2006).
12. Beyond hydrodynamics
There is a robust correlation between fish size and the scale of turbulence with which they prefer to associate (Shirvell & Dungey 1983; Webb 1998; Liao et al. 2003b). However, it has also been shown that fish inhabit turbulent flows for reasons other than hydrodynamic benefits. Size-dependant sorting of fishes may also be determined by intraspecific competition, a factor which has been documented to play an important role in group behaviour for salmonids (Heggenes 2002). Complex physical structures, such as submerged tree branches, provide three-dimensional cover, shade and visual isolation from other fish, which reduce territorial needs (Fausch & White 1981; Doloff 1986; McMahon & Gordon 1989; Fausch 1993; Imre et al. 2002; Smith 2003). These factors may be more important than hydrodynamic-related energy savings, since it allows individuals to avoid antagonistic intra- and interspecific interactions. Increasing the structural complexity in natural streams leads to an increase in population density (Moore & Gregory 1988), with dominant fish establishing territories that presumably contain the most favourable combination of these variables (Puckett & Dill 1984).
Visual isolation from other fish is just one of the several potential factors influencing why fish choose to position themselves in habitats where turbulent flows are common. Physiological state, such as hunger, may motivate the choice to hold station in turbulent flows. Fish are attracted to microhabitats where steep flow velocity gradients exist. This is because fish can swim in the slower flow to minimize energy expenditure while maximizing food intake by foraging on disoriented prey in the faster current nearby (Jenkins 1969; Everest & Chapman 1972; Fausch & White 1981; Fausch 1984; Puckett & Dill 1984; Hayes & Jowett 1994; McLaughlin & Noakes 1998; Heggenes 2002). High-turbulence levels can increase the number of predator–prey encounters and thus increase foraging efficiency (MacKenzie & Kiorboe 1995; Lewis & Pedley 2001). This may explain why starved fish prefer more turbulent currents and fish increasingly seek out turbulent flows as they become hungry (Pavlov et al. 2000). At higher levels of turbulence, a trade-off may develop whereby more frequent prey encounters are offset by greater difficulty in capturing prey. The upper limit of turbulence, then, could be set by the destabilization ‘threshold’ of a foraging fish. This limit can be affected by other physical aspects of the environment, such as illumination levels discussed earlier. Wild fishes preferentially forage in turbulence zones under high illumination (Pavlov et al. 2000). Laboratory results confirm these observations: in the light fish hold position in a vortex street and will leave temporarily to feed, while in the dark fish choose to hold station in less complex flows and make no feeding attempts (Liao et al. 2003b; Liao 2006).
13. Conclusions and future directions
The study of how environmental perturbations influence animal behaviour has proven to be a productive line of inquiry that has resulted in previously unknown mechanisms of movement, control and energy economy. A mechanistic understanding of fish behaviour in turbulent flows at the individual level is still in its early stages, but continued work promises to shed insight into fundamental principles of stability and propulsion in complex fluid environments. The cross-disciplinary nature of studies included in this review illustrates the broad interest and strong potential for integrated and applied work essential to understand the relationship between structure, flow and fish behaviour.
This review summarizes a growing body of work showing that complex flows can have dramatic effects on swimming performance. What is clear is that swimming costs in the field can no longer be estimated from variables measured under steady flow conditions. The effects of complex flows can be beneficial or detrimental, depending on the specific features of the flow environment. The most conclusive data to elucidate the relationship between locomotion and flow conditions have come from analysis of kinematics and physiology in laboratory settings. This trend will probably continue with an emphasis on more closely imitating the actual flow conditions that fish experience in natural environments. Furthermore, there is currently a discrepancy between the spatial and temporal resolution of flows at which hydraulics are modelled and at which fish respond at the level of the individual. The ability to measure and accurately model vorticity and velocity gradients at the organismal level will lead to a stronger understanding of population-level behaviour in both natural or man-made turbulence (Kondolf et al. 2000; Crowder & Diplas 2002; Odeh et al. 2002; Lai et al. 2003; Smith 2003; Roy et al. 2004; Goodwin et al. 2006). Qualitative descriptions (e.g. ‘eddies’ or ‘riffles’) and variables, such as average current velocity, depth and substrate complexity, do not describe the dynamic flow features that fish encounter in nature, and make meaningful comparisons across studies difficult. While advances in technology will undoubtedly provide a more sophisticated evaluation of hydrodynamic features at the individual level, equally comprehensive physiology measurements must be made to understand the biological influence of unsteady flows on behaviour.
Laboratory and field studies will continue to inform and inspire one another, especially in light of advancing technologies that allow remote, real-time measurements of the physiological costs of swimming. Locomotor and cardiac muscle activity have been used to estimate swimming costs previously in the field. EMG transmitters can be inserted into fish and calibrated to known tail-beat frequencies and swimming speeds and then released into natural habitats (reviewed in Cooke et al. 2004), with proper precautions (Webb 1991; McLaughlin & Noakes 1998). EMG telemetry has already been shown to be effective in monitoring other behaviours, such as heartbeat, jaw motions associated with feeding and spawning events. Calibration of EMGs to oxygen consumption has been conducted in the laboratory for steady swimming and a good correlation seems to exist between oxygen consumption and muscle activity (Cooke et al. 2004). Coupled with underwater video verification of swimming kinematics near structures, field recordings of EMGs may reveal the extent to which fish encounter or prefer to associate with turbulence. The main drawback is that the relationship between timing of EMGs and force generation is not clear, and signal variation can be substantial based on electrode placement and temperature effects (Loeb & Gans 1986). Another alternative is to measure directly pressure changes along the body of the fish to determine power production. Sensors implanted at the caudal peduncle of cod have been used to measure tail-beat pressure, which has the advantage of revealing lateral amplitude in addition to tail-beat frequency (Webber et al. 2001). At low swimming speeds, frequency can vary widely for a given speed (Webb 1971). Thus, pressure sensors potentially represent a more accurate method to measure swimming velocity in freely swimming fish. This technique also has limitations. In natural flows, velocity gradients lead to inherent pressure differences independent of body and fin motion influences, and thus may obscure the interpretation of these data. Although currently out of reach for field studies, simultaneous measurements of kinematics, flow and physiological costs are the most comprehensive way to evaluate the effect of turbulent flows on fish behaviour.
Until we have these technological advancements in hand, controlled laboratory studies continue to be one of the strongest approaches to determine the fundamental mechanisms by which fish can exploit unsteady flow. Future experiments include combining flow visualization and respirometry experiments to quantify the metabolic costs of locomotion in response to well-characterized hydrodynamic perturbations. For studies involving cylinder wakes, a systematic exploration of the lower and upper limit of vortex size at which fish can no longer entrain or Kármán gait is needed. The existing data indicate that the ratio of cylinder diameter to body length needs to fall between 1 : 12 and 1 : 2 for fish to be attracted to the wake, but this range is by no means the result of an exhaustive search (Sutterlin & Waddy 1975; Webb 1998; Liao et al. 2003b; Montgomery et al. 2003). Similarly, the behaviour of fish in the wake behind different orientations and aggregations of simple geometric objects representing natural structures (e.g. multiple cylinders as tree branches or half-spheres on the substrate approximating boulders) should be explored. There is an extensive body of work in the hydrodynamics literature that establish the conditions which generate specific flow phenomena (Gerrard 1966; Grass et al. 1991; Zdravkovich 1997), and this information can be used to initiate the next generation of investigations.
The applications of understanding how fish associate with natural and man-made turbulence are especially timely and important, given the state of fisheries worldwide. River rehabilitation efforts try to increase fish diversity and population density by introducing structures to increase physical heterogeneity. However, these attempts are based on a limited understanding of the underlying causal mechanisms of how fish interact with turbulent flows (Moore & Gregory 1988; McMahon & Gordon 1989), and have met with varied success (Pretty et al. 2003). Similar arguments could be made for modelling population dynamics and design of fish passageways to mitigate the effect of dams on fish migrations. Strong steps are already being taken in this direction (Odeh et al. 2002; Smith 2003) with work starting to incorporate sensory aspects involved in negotiating or avoiding turbulent flows (Goodwin et al. 2006). The hope is that this work will inform vital decisions that could enhance the sustainability of important fisheries in the years to come.
Acknowledgments
I would like to extend my thanks to Paolo Domenici for inviting me to participate in this symposium. I would also like to thank Paul Webb and an anonymous reviewer for suggesting several references and for comments that substantially improved the manuscript. Funding was provided by the NIH NRSA postdoctoral fellowship NS051009-01 to J.C.L.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Abrahams M.V, Colgan P.W. Fish schools and their hydrodynamic function: a reanalysis. Environ. Biol. Fishes. 1987;20:79–80. doi:10.1007/BF00002028 [Google Scholar]
- Altringham J, Ellerby D.J. Fish swimming: patterns in muscle function. J. Exp. Biol. 1999;202:3397–3403. doi: 10.1242/jeb.202.23.3397. [DOI] [PubMed] [Google Scholar]
- Anderson J.M. Joint program in oceanography/applied ocean science engineering. Massachusetts Institute of Technology/Woods Hole Oceanographic Institution; Cambridge, MA: 1996. Vorticity control for efficient propulsion; pp. 1–193. [Google Scholar]
- Anderson J.M, Streitlien K, Barrett D.S, Triantafyllou M.S. Oscillating foils of high propulsive efficiency. J. Fluid Mech. 1998;360:41–72. doi:10.1017/S0022112097008392 [Google Scholar]
- Anderson E.J, McGillis W.R, Grosenbaugh M.A. The boundary layer of swimming fish. J. Exp. Biol. 2001;204:81–102. doi: 10.1242/jeb.204.1.81. [DOI] [PubMed] [Google Scholar]
- Anras M.B, Lagardere J.P, Lafaye J. Diel activity rhythm of seabass tracked in a natural environment: group effects on swimming patterns and amplitudes. Can. J. Fish. Aquat. Sci. 1997;54:162–168. doi:10.1139/cjfas-54-1-162 [Google Scholar]
- Atema J, Fay R.R, Popper A, Tavolga W, editors. Sensory biology of aquatic animals. Springer; New York, NY: 1988. [Google Scholar]
- Bartol I.K, Gharib M, Weihs D, Webb P.W, Hove J.R, Gordon M.S. Hydrodynamic stability of swimming in ostraciid fishes: role of the carapace in the smooth trunkfish Lactophrys triqueter (Teleostei: Ostraciidae) J. Exp. Biol. 2002;206:725–744. doi: 10.1242/jeb.00137. doi:10.1242/jeb.00137 [DOI] [PubMed] [Google Scholar]
- Beal, D. N. 2003 Propulsion through wake synchronization using a flapping foil. Ph.D. thesis, Massachusetts Institute of Technology, Cambridge, MA.
- Beal D.N, Hover F.S, Triantafyllou M.S, Liao J.C, Lauder G.V. Passive propulsion in vortex wakes. J. Fluid Mech. 2006;549:385–402. doi:10.1017/S0022112005007925 [Google Scholar]
- Belyayev V.V, Zuyev G.V. Hydrodynamic hypothesis of school formation in fishes. Probl. Ichthyol. 1969;9:578–584. [Google Scholar]
- Biewener A.A, Gillis G.B. Dynamics of muscle function during locomotion: accommodating variable conditions. J. Exp. Biol. 1999;202:3387–3396. doi: 10.1242/jeb.202.23.3387. [DOI] [PubMed] [Google Scholar]
- Bioly P, Magnan P. Relationship between individual variation in morphological characters and swimming costs in brook char (Salvelinus fontinalis) and yellow perch (Perca flavescens) J. Exp. Biol. 2002;205:1031–1036. doi: 10.1242/jeb.205.7.1031. [DOI] [PubMed] [Google Scholar]
- Blake R.W. Cambridge University Press; Cambridge, MA: 1983. Fish locomotion. [Google Scholar]
- Blazka P, Volf M, Cepela M. A new type of respirometer for the determination of the metabolism of fish in an active state. Physiol. Bohemoslov. 1960;9:553–558. [Google Scholar]
- Blevins R.D. Krieger Publishing Company; Malabar, FL: 1990. Flow induced vibration. [Google Scholar]
- Bose N, Lien J. Energy absorption from ocean waves: a free ride for cetaceans. Proc. R. Soc. B. 1990;240:591–605. doi: 10.1098/rspb.1990.0054. doi:10.1098/rspb.1990.0054 [DOI] [PubMed] [Google Scholar]
- Breder C.M. Vortices and fish schools. Zoologica. 1965;50:97–114. [Google Scholar]
- Breder C.M. Fish schools as operational structures. Fish. Bull. 1976;74:471–502. [Google Scholar]
- Brett J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964;21:1183–1226. [Google Scholar]
- Bumann D, Krause J, Rubenstein D. Mortality risk of spatial positions in animal groups: the danger of being in front. Behaviour. 1997;134:1063–1076. [Google Scholar]
- Bustard D.R, Narver D.W. Aspects of the winter ecology of juvenile coho salmon (Oncorhynchus kisutch) and steelhead trout (Salmo gairdneri) J. Fish. Res. Board Can. 1975;32:667–680. [Google Scholar]
- Cooke S.J, Thorstad E.B, Hinch S.G. Activity and energetics of free-swimming fish; insights from electromyogram telemetry. Fish Fish. 2004;5:21–52. [Google Scholar]
- Coombs S, Gorner P, Munz H, editors. The mechanosensory lateral line: neurobiology and evolution. Springer; New York, NY: 1989. [Google Scholar]
- Coombs S, Braun C.B, Donovan B. The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. J. Exp. Biol. 2001;204:337–348. doi: 10.1242/jeb.204.2.337. [DOI] [PubMed] [Google Scholar]
- Coutant C.C, Whitney R.R. Fish behavior in relation to passage through hydropower turbines: a review. Trans. Am. Fish. Soc. 2000;129:351–380. doi:10.1577/1548-8659(2000)129<0351:FBIRTP>2.0.CO;2 [Google Scholar]
- Crowder D.W, Diplas P. Vorticity and circulation: spatial metrics for evaluating flow complexity in stream habitats. Can. J. Fish. Aquat. Sci. 2002;59:633–645. doi:10.1139/f02-037 [Google Scholar]
- DeBlois E, Rose G. Cross-shoal variability in the feeding habits of migrating Atlantic cod (Gadus morhua) Oecologia. 1996;108:192–196. doi: 10.1007/BF00333231. doi:10.1007/BF00333231 [DOI] [PubMed] [Google Scholar]
- deGraaf D.A, Bain L.H. Habitat use by and preferences of juvenile Atlantic salmon in two Newfoundland rivers. Trans. Am. Fish. Soc. 1986;115:671–681. doi:10.1577/1548-8659(1986)115<671:HUBAPO>2.0.CO;2 [Google Scholar]
- Dickinson M.H, Lehmann F, Sane S.P. Wing rotation and the aerodynamic basis of insect flight. Science. 1999;284:1954–1960. doi: 10.1126/science.284.5422.1954. doi:10.1126/science.284.5422.1954 [DOI] [PubMed] [Google Scholar]
- Dijkgraaf S. The functioning and significance of the lateral-line organs. Biol. Rev. Cambridge Philos. Soc. 1963;38:51–105. doi: 10.1111/j.1469-185x.1963.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Doloff C.A. Effects of stream cleaning on juvenile coho salmon and Dolly Varden in southeast Alaska. Trans. Am. Fish. Soc. 1986;115:743–755. doi:10.1577/1548-8659(1986)115<743:EOSCOJ>2.0.CO;2 [Google Scholar]
- Drucker E.G, Jensen J.S. Kinematic and electromyographic analysis of steady pectoral fin swimming in surfperches. J. Exp. Biol. 1997;200:1709–1723. doi: 10.1242/jeb.200.12.1709. [DOI] [PubMed] [Google Scholar]
- Drucker E.G, Lauder G.V. Locomotor forces on a swimming fish: three-dimensional vortex wake dynamics quantified using digital particle image velocimetry. J. Exp. Biol. 1999;202:2393–2412. doi: 10.1242/jeb.202.18.2393. [DOI] [PubMed] [Google Scholar]
- Drucker E.G, Lauder G.V. Locomotor function of the dorsal fin in teleost fishes: an experimental analysis of wake forces in sunfish. J. Exp. Biol. 2001;204:2943–2958. doi: 10.1242/jeb.204.17.2943. [DOI] [PubMed] [Google Scholar]
- Drucker E.G, Lauder G.V. Function of the pectoral fins in rainbow trout: behavioral repertoire and hydrodynamic forces. J. Exp. Biol. 2003;206:813–826. doi: 10.1242/jeb.00139. doi:10.1242/jeb.00139 [DOI] [PubMed] [Google Scholar]
- Enders E.C, Boisclair D, Roy A.G. The effect of turbulence on the cost of swimming for juvenile Atlantic salmon (Salmo salar) Can. J. Fish. Aquat. Sci. 2003;60:1149–1160. doi:10.1139/f03-101 [Google Scholar]
- Engelmann J, Hanke W, Mogdans J, Bleckmann H. Hydrodynamic stimuli and the fish lateral line. Nature. 2000;408:51–52. doi: 10.1038/35040706. doi:10.1038/35040706 [DOI] [PubMed] [Google Scholar]
- Everest F.H, Chapman D.W. Habitat selection and spatial interaction by juvenile chinook salmon and steelhead trout in two Idaho streams. J. Fish. Res. Board Can. 1972;29:91–100. [Google Scholar]
- Fausch K.D. Profitable stream positions for salmonids: relating specific growth rate to net energy gain. Can. J. Fish. Aquat. Sci. 1984;62:441–451. [Google Scholar]
- Fausch K.D. Experimental analysis of microhabitat selection by juvenile steelhead (Oncorhynchus mykiss) and coho salmon (O. kisutch) in a British Columbia stream. Can. J. Fish. Aquat. Sci. 1993;50:1198–1207. [Google Scholar]
- Fausch K.D, White R.J. Competition between brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) for positions in a Michigan stream. Can. J. Fish. Aquat. Sci. 1981;38:1220–1227. [Google Scholar]
- Fish F.E. Energetics of swimming and flying in formation. Comm. Theor. Biol. 1999;5:283–304. [Google Scholar]
- Fitzsimmons S.D, Warburton K. Fish movement behaviour: variability within and between groups. Behav. Process. 1992;26:211–216. doi: 10.1016/0376-6357(92)90015-6. doi:10.1016/0376-6357(92)90015-6 [DOI] [PubMed] [Google Scholar]
- Gerrard J.H. Formation region of vortices behind bluff bodies. J. Fluid Mech. 1966;25:401–413. doi:10.1017/S0022112066001721 [Google Scholar]
- Gerstner C.L. Use of substratum ripples for flow refuging by Atlantic cod, Gadus morhua. Environ. Biol. Fishes. 1998;51:455–460. doi:10.1023/A:1007449630601 [Google Scholar]
- Gibb A, Jayne B.C, Lauder G.V. Kinematics of pectoral fin locomotion in the bluegill sunfish Lepomis macrochirus. J. Exp. Biol. 1994;189:133–161. doi: 10.1242/jeb.189.1.133. [DOI] [PubMed] [Google Scholar]
- Gillis G.B. Undulatory locomotion in elongate aquatic vertebrates: anguilliform swimming since Sir James Gray. Am. Zool. 1996;36:656–665. [Google Scholar]
- Gillis G.B. Environmental effects on undulatory locomotion in the American eel Anguilla rostrata: kinematics in water and on land. J. Exp. Biol. 1998a;201:949–961. [Google Scholar]
- Gillis G.B. Neuromuscular control of anguilliform locomotion: patterns of red and white muscle activity during swimming in the American eel Anguillla rostrata. J. Exp. Biol. 1998b;201:3245–3256. doi: 10.1242/jeb.201.23.3245. [DOI] [PubMed] [Google Scholar]
- Golpalkrishnan R, Triantafyllou M.S, Triantafyllou G.S, Barrett D.S. Active vorticity control in a shear flow using a flapping foil. J. Fluid Mech. 1994;274:1–21. doi:10.1017/S0022112094002016 [Google Scholar]
- Goodwin A.R. Civil and environmental engineering. Cornell University; Ithaca, NY: 2004. Hydrodynamics and juvenile salmon movement behavior at Lower Granite Dam: decoding the relationship using 3-D space–time (CEL Agent IBM) simulation. [Google Scholar]
- Goodwin A.R, Nestler J.M, Anderson J.J, Weber L.J, Loucks D.P. Forecasting 3-D fish movement behavior using a Eulerian–Lagrangian-agent method (ELAM) Ecol. Model. 2006;192:197–223. doi:10.1016/j.ecolmodel.2005.08.004 [Google Scholar]
- Grass A.J, Stuart R.J, Mansou-Tehrani M. Vortical structures and coherent motion in turbulent flow over smooth and rough boundaries. Phil. Trans. R. Soc. A. 1991;336:35–65. doi:10.1098/rsta.1991.0065 [Google Scholar]
- Gray J. Studies in animal locomotion. I. The movement of fish with special reference to the eel. J. Exp. Biol. 1933;10:88–104. [Google Scholar]
- Grillner S, Kashin S. On the generation and performance of swimming in fish. In: Herman R.M, Grillner S, Stein P.S.G, Stuart D.G, editors. Neural control of locomotion. Plenum; New York, NY: 1976. pp. 181–202. [Google Scholar]
- Hammond L, Altringham J, Wardle C. Myotomal slow muscle function of rainbow trout Oncorhynchus mykiss during steady swimming. J. Exp. Biol. 1998;201:1659–1671. doi: 10.1242/jeb.201.10.1659. [DOI] [PubMed] [Google Scholar]
- Hartman G.F. The role of behaviour in the ecology and interactions of underyearling coho salmon (Oncorhynchus kisutch) and steelhead trout (Salmo gairdneri) J. Fish. Res. Board Can. 1965;22:1035–1081. [Google Scholar]
- Hayes J.W, Jowett I.G. Microhabitat models of large drift-feeding brown trout in three New Zealand rivers. N. Am. J. Fish. Manage. 1994;14:710–725. doi:10.1577/1548-8675(1994)014<0710:MMOLDF>2.3.CO;2 [Google Scholar]
- Heggenes J. Effects of short-term flow fluctuations on displacement of, and habitat use by, brown trout in a small stream. Trans. Am. Fish. Soc. 1988;117:336–344. doi:10.1577/1548-8659(1988)117<0336:EOSFFO>2.3.CO;2 [Google Scholar]
- Heggenes J. Flexible summer habitat selection by wild, allopatric brown trout in lotic environments. Trans. Am. Fish. Soc. 2002;131:287–298. doi:10.1577/1548-8659(2002)131<0287:FSHSBW>2.0.CO;2 [Google Scholar]
- Herskin J, Steffensen J.F. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J. Fish Biol. 1998;53:366–376. doi:10.1111/j.1095-8649.1998.tb00986.x [Google Scholar]
- Hill J, Grossman G.D. An energetic model of microhabitat use for rainbow trout and rosyside dace. Ecology. 1993;74:685–698. doi:10.2307/1940796 [Google Scholar]
- Hinch S.G, Rand P.S. Swim speeds and energy use of up-river migrating sockeye salmon (Oncorhynchus nerka): role of the local environment and fish characteristics. Can. J. Fish. Aquat. Sci. 1998;55:1821–1831. doi:10.1139/cjfas-55-8-1821 [Google Scholar]
- Hinch S.G, Rand P.S. Optimal swimming speeds and forward-assisted propulsion: energy-conserving behaviours of upriver-migrating adult salmon. Can. J. Fish. Aquat. Sci. 2000;57:2470–2478. doi:10.1139/cjfas-57-12-2470 [Google Scholar]
- Hubert W.A, Harris D.D, Wesche T.A. Diurnal shifts in the use of summer habitat by age-0 brown trout in a regulated mountain stream. Hydrobiologia. 1994;284:147–156. doi:10.1007/BF00006886 [Google Scholar]
- Hughes N.F, Dill L.M. Position choice by drift-feeding salmonids: model and test for Arctic grayling (Thymallis arcticus) in subarctic streams, interior Alaska. Can. J. Fish. Aquat. Sci. 1990;47:2039–2048. [Google Scholar]
- Imre I, Grant J.W.A, Keeley E.R. The effect of visual isolation on territory size and population density of juvenile rainbow trout (Oncorhynchus mykiss) Can. J. Fish. Aquat. Sci. 2002;59:303–309. doi:10.1139/f02-010 [Google Scholar]
- Ingle D, editor. Vision: the experimental analysis of visual behavior. Fish physiology. Sensory systems and electric organs. Academic Press; New York, NY: 1971. [Google Scholar]
- Jayne B.C, Lauder G.V. How swimming fish use slow and fast muscle fibers: implications for models of vertebrate muscle recruitment. J. Comp. Physiol. A. 1994;175:123–131. doi: 10.1007/BF00217443. doi:10.1007/BF00217443 [DOI] [PubMed] [Google Scholar]
- Jayne B.C, Lauder G.V. Speed effects on midline kinematics during steady undulatory swimming of largemouth bass, Micropterus salmoides. J. Exp. Biol. 1995;198:585–602. doi: 10.1242/jeb.198.2.585. [DOI] [PubMed] [Google Scholar]
- Jayne B.C, Lozada A, Lauder G.V. Function of the dorsal fin in bluegill sunfish: motor patterns during four locomotor behaviors. J. Morphol. 1996;228:307–326. doi: 10.1002/(SICI)1097-4687(199606)228:3<307::AID-JMOR3>3.0.CO;2-Z. doi:10.1002/(SICI)1097-4687(199606)228:3<307::AID-JMOR3>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Jenkins T.M. Social structure, position choice and micro-distribution of two trout species (Salmo trutta and Salmo gairdneri) resident in mountain streams. Anim. Behav. Monogr. 1969;2:56–123. [Google Scholar]
- Jindrich D.L, Full R.B. Dynamic stabilization of rapid hexapedal locomotion. J. Exp. Biol. 2002;205:2803–2823. doi: 10.1242/jeb.205.18.2803. [DOI] [PubMed] [Google Scholar]
- Karlsen H.E, Sand O. Selective and reversible blocking of the lateral line in freshwater fish. J. Exp. Biol. 1987;133:249–262. [Google Scholar]
- Kirkbride A.D. Turbulence: perspectives on flow and sediment transport. Wiley; Chichester, UK: 1993. Observations of the influence of bed roughness on turbulence structure in depth limited flows over gravel beds. [Google Scholar]
- Kondolf G.M, Larsen E.W, Williams J.G. Measuring and modeling the hydraulic environment for assessing instream flows. N. Am. J. Fish. Manage. 2000;20:1016–1028. doi:10.1577/1548-8675(2000)020<1016:MAMTHE>2.0.CO;2 [Google Scholar]
- Krause J. The relationship between foraging and shoal position in a mixed shoal of roach (Rutilus rutilus) and chub (Leuciscus cephlus): a field study. Oecologia. 1993;93:356–359. doi: 10.1007/BF00317878. doi:10.1007/BF00317878 [DOI] [PubMed] [Google Scholar]
- Lai Y.G, Weber L.J, Patel V.C. Nonhydrostatic three-dimensional model for hydraulic flow simulation. II: validation and application. J. Hydraul. Eng. 2003;129:206–214. doi:10.1061/(ASCE)0733-9429(2003)129:3(206) [Google Scholar]
- Lauder G.V, Drucker E.G. Forces, fishes, and fluids: hydrodynamic mechanisms of aquatic locomotion. News Physiol. Sci. 2002;17:235–240. doi: 10.1152/nips.01398.2002. [DOI] [PubMed] [Google Scholar]
- Lauder G.V, Connon C, Dunn-Rankin D. Visualization of flow behind the tail of swimming fish: new data using DPIV techniques. Am. Zool. 1996;36:7A. [Google Scholar]
- Lewis D.M, Pedley T.J. The influence of turbulence on plankton predation strategies. J. Theor. Biol. 2001;210:347–365. doi: 10.1006/jtbi.2001.2310. doi:10.1006/jtbi.2001.2310 [DOI] [PubMed] [Google Scholar]
- Liao J.C. Swimming in needlefish (Belonidae): anguilliform locomotion with fins. J. Exp. Biol. 2002;205:2875–2884. doi: 10.1242/jeb.205.18.2875. [DOI] [PubMed] [Google Scholar]
- Liao J.C. Neuromuscular control of trout swimming in a vortex street: implications for energy economy during the Karman gait. J. Exp. Biol. 2004;207:3495–3506. doi: 10.1242/jeb.01125. doi:10.1242/jeb.01125 [DOI] [PubMed] [Google Scholar]
- Liao J.C. The role of the lateral line and vision on body kinematics and hydrodynamic preference of rainbow trout in turbulent flow. J. Exp. Biol. 2006;209:4077–4090. doi: 10.1242/jeb.02487. doi:10.1242/jeb.02487 [DOI] [PubMed] [Google Scholar]
- Liao J.C, Beal D.N, Lauder G.V, Triantafyllou M.S. Fish exploiting vortices decrease muscle activity. Science. 2003a;302:1566–1569. doi: 10.1126/science.1088295. doi:10.1126/science.1088295 [DOI] [PubMed] [Google Scholar]
- Liao J.C, Beal D.N, Lauder G.V, Triantafyllou M.S. The Kármán gait: novel kinematics of rainbow trout swimming in a vortex street. J. Exp. Biol. 2003b;206:1059–1073. doi: 10.1242/jeb.00209. doi:10.1242/jeb.00209 [DOI] [PubMed] [Google Scholar]
- Lissman P.S.S, Schollenberger C.A. Formation flight of birds. Science. 1970;168:1003–1005. doi: 10.1126/science.168.3934.1003. doi:10.1126/science.168.3934.1003 [DOI] [PubMed] [Google Scholar]
- Loeb G.E, Gans C. University of Chicago Press; Chicago, IL: 1986. Electromyography for experimentalists. [Google Scholar]
- MacKenzie B.R, Kiorboe T. Encounter rates and swimming behavior of pause-travel and cruise larval fish predators in calm and turbulent laboratory environments. Limnol. Oceanogr. 1995;40:1278–1289. [Google Scholar]
- McLaughlin R.L, Noakes D.L.G. Going against the flow: an examination of the propulsive movements made by young brook trout in streams. Can. J. Fish. Aquat. Sci. 1998;55:853–860. doi:10.1139/cjfas-55-4-853 [Google Scholar]
- McMahon T.E, Gordon F.H. Influence of cover complexity and current velocity on winter habitat use by juvenile coho salmon (Oncorhynchus kisutch) Can. J. Fish. Aquat. Sci. 1989;46:1551–1557. [Google Scholar]
- Montgomery J, Baker C, Carton A. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. doi:10.1038/40135 [Google Scholar]
- Montgomery J.C, McDonald F, Baker C.F, Carton A.G, Ling N. Sensory integration in the hydrodynamic world of rainbow trout. Proc. R. Soc. B. 2003;270:S195–S197. doi: 10.1098/rsbl.2003.0052. doi:10.1098/rsbl.2003.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.M.S, Gregory S.V. Response of young-of-the-year cutthroat trout to manipulation of habitat structure in a small stream. Trans. Am. Fish. Soc. 1988;117:162–170. doi:10.1577/1548-8659(1988)117<0162:ROYOTY>2.3.CO;2 [Google Scholar]
- Müller U.K, Van den Heuvel B, Stamhuis E.J, Videler J.J. Fish footprints: morphology and energetics of the wake behind a continuously swimming mullet (Chelon labrosus Risso) J. Exp. Biol. 1997;200:2893–2906. doi: 10.1242/jeb.200.22.2893. [DOI] [PubMed] [Google Scholar]
- Nislow K.H, Folt C.L, Parrish D.L. Favorable foraging locations for young Atlantic salmon: application to habitat and population restoration. Ecol. Appl. 1999;9:1085–1099. doi:10.2307/2641353 [Google Scholar]
- Odeh, M., Noreika, J. F., Haro, A., Maynard, A., Castro-Santos, T. & Cada, G. F. 2002 Evaluation of the effects of turbulence on the behavior of migratory fish. In Final Report 2002, Report to Bonneville Power Administration, Contract No. 00000022, pp. 1–55.
- Parker F.R.J. Reduced metabolic rates in fishes as a result of induced schooling. Trans. Am. Fish. Soc. 1973;102:125–131. doi:10.1577/1548-8659(1973)102<125:RMRIFA>2.0.CO;2 [Google Scholar]
- Partridge B.L, Pitcher T.J. Evidence against a hydrodynamic function for fish schools. Nature. 1979;279:418–419. doi: 10.1038/279418a0. doi:10.1038/279418a0 [DOI] [PubMed] [Google Scholar]
- Partridge B.L, Pitcher T.J. The sensory basis of fish schools: relative roles of the lateral line and vision. J. Comp. Physiol. A. 1980;135:315–325. doi:10.1007/BF00657647 [Google Scholar]
- Partridge B.L, Johansson J, Kalish J. The structure of schools of giant bluefin tuna in Cape Cod Bay. Environ. Biol. Fishes. 1983;9:253–262. doi:10.1007/BF00692374 [Google Scholar]
- Pavlov D.S, Skorobogatov M.A, Shtaf L.G. The critical current velocity of fish and the degree of flow turbulence. Rep. USSR Acad. Sci. 1982;267:1019–1021. [Google Scholar]
- Pavlov D.S, Lupandin A.I, Skorobogatov M.A. The effects of flow turbulence on the behavior and distribution of fish. J. Ichthyol. 2000;40:S232–S261. [Google Scholar]
- Pitcher T.J, Partridge B.L, Wardle C.S. A blind fish can school. Science. 1976;194:963–965. doi: 10.1126/science.982056. doi:10.1126/science.982056 [DOI] [PubMed] [Google Scholar]
- Pretty J.L, Harrison S.S.C, Shepherd D.J, Smith C, Hildrew A.G, Hey R.D. River rehabilitation and fish populations: assessing the benefit of instream structures. J. Appl. Ecol. 2003;40:251–265. [Google Scholar]
- Puckett K.J, Dill L.M. The energetics of feeding territoriality in juvenile coho salmon (Oncorhynchus kisutch) Behaviour. 1984;92:97–110. [Google Scholar]
- Rincon P.A, Lobon-Cervia J. Microhabitat use by stream-resident brown trout: bioenergetic consequences. Trans. Am. Fish. Soc. 1993;122:575–587. doi:10.1577/1548-8659(1993)122<0575:MUBSRB>2.3.CO;2 [Google Scholar]
- Roberts T.J, March R.L, Weyand P, Taylor C.R. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. doi:10.1126/science.275.5303.1113 [DOI] [PubMed] [Google Scholar]
- Rosen M.W. Water flow about a swimming fish. Nav. Ordinance Test Station Tech. Paper. 1959;2298:1–96. [Google Scholar]
- Ross R.M, Backman T.W.H. Group–size-mediated metabolic rate reduction in American shad. Trans. Am. Fish. Soc. 1992;121:385–390. doi:10.1577/1548-8659(1992)121<0385:NGMRRI>2.3.CO;2 [Google Scholar]
- Roy A.G, Buffin-Belanger T, Lamarre H, Kirkbride A.D. Size, shape and dynamics of large-scale turbulent flow structures in a gravel-bed river. J. Fluid Mech. 2004;500:1–27. doi:10.1017/S0022112003006396 [Google Scholar]
- Shadwick R.E, Katz S.L, Korsmeyer K.E, Knower T, Covell J.W. Muscle dynamics in skipjack tuna: timing of red muscle shortening in relation to activation and body curvature during steady swimming. J. Exp. Biol. 1999;202:2139–2150. doi: 10.1242/jeb.202.16.2139. [DOI] [PubMed] [Google Scholar]
- Shirvell C.S, Dungey R.G. Microhabitats chosen by brown trout for feeding and spawning in rivers. Trans. Am. Fish. Soc. 1983;112:355–367. doi:10.1577/1548-8659(1983)112<355:MCBBTF>2.0.CO;2 [Google Scholar]
- Shuler S.W, Nehring R.B, Fausch K.D. Diel habitat selection by brown trout in the Rio Grande river, Colorado, after placement of boulder structures. N. Am. J. Fish. Manag. 1994;14:99–111. doi:10.1577/1548-8675(1994)014<0099:DHSBBT>2.3.CO;2 [Google Scholar]
- Smith, D. L. 2003 The shear flow environment of juvenile salmonids. PhD thesis, University of Idaho.
- Smith D.L, Brannon E.L. Response of juvenile rainbow trout to turbulence produced by prismatoidal shapes. Trans. Am. Fish. Soc. 2005;134:741–753. doi:10.1577/T04-069.1 [Google Scholar]
- Standen E.M, Lauder G.V. Dorsal and anal fin function in bluegill sunfish Lepomis macrochirus: three-dimensional kinematics during propulsion and maneuvering. J. Exp. Biol. 2005;208:2753–2763. doi: 10.1242/jeb.01706. doi:10.1242/jeb.01706 [DOI] [PubMed] [Google Scholar]
- Streitlien K, Triantafyllou G.S. Efficient foil propulsion through vortex control. AIAA J. 1996;34:2315–2319. [Google Scholar]
- Sutterlin A.M, Waddy S. Possible role of the posterior lateral line in obstacle entrainment by brook trout (Salvelinus fontinalis) J. Fish. Res. Board Can. 1975;32:2441–2446. [Google Scholar]
- Svendsen J.C, Skov J, Bildsoe M, Steffensen J.F. Intra-school positional preference and reduced tailbeat frequency on trailing positions in schooling roach under experimental conditions. J. Fish Biol. 2003;62:834–846. doi:10.1046/j.1095-8649.2003.00068.x [Google Scholar]
- Tang M, Boisclair D. Influence of the size of enclosures on the swimming characteristics of juvenile brook trout (Salvelinus fontinalis) Can. J. Fish. Aquat. Sci. 1993;50:1786–1793. [Google Scholar]
- Triantafyllou M.S, Triantafyllou G.S, Yue D.K.P. Hydrodynamics of fishlike swimming. Annu. Rev. Fluid Mech. 2000;32:33–53. doi:10.1146/annurev.fluid.32.1.33 [Google Scholar]
- Triantafyllou G.S, Techet A.H, Zhu Q, Beal D.N, Hover F.S, Yue D.K.P. Vorticity control in fish-like propulsion and maneuvering. Integr. Comp. Biol. 2002;42:1026–1031. doi: 10.1093/icb/42.5.1026. doi:10.1093/icb/42.5.1026 [DOI] [PubMed] [Google Scholar]
- Vogel S. Princeton University Press; Princeton, NJ: 1994. Life in moving fluids: the physical biology of flow. [Google Scholar]
- Walker J.A, Westneat M.W. Labriform propulsion in fishes: kinematics of flapping aquatic flight in the bird wrasse Gomphosus varius (Labridae) J. Exp. Biol. 1997;200:1549–1569. doi: 10.1242/jeb.200.11.1549. [DOI] [PubMed] [Google Scholar]
- Wardle C.S, Videler J.J, Altringham J.D. Tuning in to fish swimming waves: body form, swimming mode and muscle function. J. Exp. Biol. 1995;198:1629–1636. doi: 10.1242/jeb.198.8.1629. [DOI] [PubMed] [Google Scholar]
- Warhaft Z. Turbulence in nature and in the laboratory. Proc. Natl Acad. Sci. USA. 2002;99:2481–2486. doi: 10.1073/pnas.012580299. doi:10.1073/pnas.012580299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P.W. The swimming energetics of trout. I. Thrust and power output at cruising speeds. J. Exp. Biol. 1971;55:489–520. doi: 10.1242/jeb.55.2.489. [DOI] [PubMed] [Google Scholar]
- Webb P.W. Hydrodynamics and energetics of fish propulsion. Bull. Fish. Res. Board Can. 1975;190:1–159. [Google Scholar]
- Webb P.W. Composition and mechanics of routine swimming of rainbow trout Oncorhynchus mykiss. Can. J. Fish. Aquat. Sci. 1991;48:583–590. [Google Scholar]
- Webb P.W. The effect of solid and porous channel walls on steady swimming of steelhead trout Oncorhynchus mykiss. J. Exp. Biol. 1993;178:97–108. [Google Scholar]
- Webb P.W. Entrainment by river chub Nocomis micropogon and smallmouth bass Micropterus dolomieu on cylinders. J. Exp. Biol. 1998;201:2403–2412. doi: 10.1242/jeb.201.16.2403. [DOI] [PubMed] [Google Scholar]
- Webb P.W. Control of posture, depth, and swimming trajectories of fishes. Integr. Comp. Biol. 2002;42:94–101. doi: 10.1093/icb/42.1.94. doi:10.1093/icb/42.1.94 [DOI] [PubMed] [Google Scholar]
- Webb P.W. Response latencies to postural differences in three species of teleostean fishes. J. Exp. Biol. 2004;207:955–961. doi: 10.1242/jeb.00854. doi:10.1242/jeb.00854 [DOI] [PubMed] [Google Scholar]
- Webb P.W, Weihs D. Hydrostatic stability of fish with swim bladders: not all fish are unstable. Can. J. Zool. 1994;72:1149–1154. [Google Scholar]
- Webb P.W, Kostecki P.T, Stevens E.D. The effect of size and swimming speed on the locomotor kinematics of rainbow trout. J. Exp. Biol. 1984;109:77–95. [Google Scholar]
- Webb P.W, LaLiberte G.D, Schrank A.J. Does body and fin form affect the maneuverability of fish traversing vertical and horizontal slits? Environ. Biol. Fishes. 1996;46:7–14. doi:10.1007/BF00001692 [Google Scholar]
- Webber D.M, Boutilier R.G, Kerr S.R, Smale M.J. Caudal differential pressure as a predictor of swimming speed of cod (Gadus morhua) J. Exp. Biol. 2001;204:3561–3570. doi: 10.1242/jeb.204.20.3561. [DOI] [PubMed] [Google Scholar]
- Weihs D. Hydromechanics of fish schooling. Nature. 1973;241:290–291. doi:10.1038/241290a0 [Google Scholar]
- Wilga C.D, Lauder G.V. Locomotion in sturgeon: function of the pectoral fins. J. Exp. Biol. 1999;202:2413–2432. doi: 10.1242/jeb.202.18.2413. [DOI] [PubMed] [Google Scholar]
- Willert C.E, Gharib M. Digital particle image velocimetry. Exp. Fluids. 1991;10:181–193. doi:10.1007/BF00190388 [Google Scholar]
- Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc. Acad. Nat. Sci. Philadelphia. 1974;125:225–317. [Google Scholar]
- Wu T.Y, Chwang A.T. Extraction of flow energy by fish and birds in a wavy stream. Swimm. Fly. Nat. 1975;2:687–702. [Google Scholar]
- Zdravkovich M.M. Oxford Science Publications, Oxford University Press; Oxford, UK: 1997. Flow around circular cylinders: a comprehensive guide through flow phenomena, experiments, applications, mathematical models, and computer simulations. [Google Scholar]