Abstract
Investigating the biological mechanisms linking environmental variability to fish production systems requires the disentangling of the interactions between habitat, environmental adaptation and fitness. Since the number of environmental variables and regulatory processes is large, straightening out the environmental influences on fish performance is intractable unless the mechanistic analysis of the ‘fish-milieu’ system is preceded by an understanding of the properties of that system. While revisiting the key points in our currently poorly integrated understanding of fish ecophysiology, we have highlighted the explanatory potential contained within Fry's (Fry 1947 Univ. Toronto Stud. Biol. Ser. 55, 1–62) concept of metabolic scope and categorization of environmental factors. These two notions constitute a pair of powerful tools for conducting an external (at the emerging property level) analysis of the environmental influences on fish, as well as an internal (mechanistic) examination of the behavioural, morphological and physiological processes involved. Using examples from our own and others work, we have tried to demonstrate that Fry's framework represents a valuable conceptual basis leading to a broad range of testable ecophysiological hypotheses.
Keywords: scope for metabolic activity, environmental conditions, environmental adaptation, fish
1. Introduction
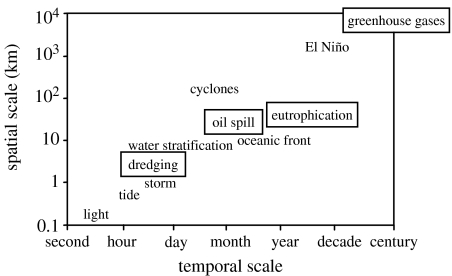
Marine ecosystems throughout the world are increasingly affected by the development of human activities, but the scales at which these biological systems are impacted range widely in both temporal and spatial terms (figure 1). Accidental spills of contaminants, for instance, are generally short lived and impinge on relatively restricted areas (e.g. ‘The Erika oil spill’, special issue of Aquat. Living Resour. 17, 2004). On the other hand, climatic changes resulting from the over-production of greenhouse gases are likely to have long-term influences on rather large portions of our planet (McGowan 1990; Brander 1996; O'Brien et al. 2000; Stebbing et al. 2002). Obviously, these man-made influences add together with habitat-specific natural constraints, some of which fluctuate on time-bases ranging from a few seconds (light intensity) to decades (El-Niño). In such a complex and changeable context, one question that has been central to generations of fish physiologists, ecologists and evolutionary biologists revolves around the disentangling of the interactions between habitat, environmental adaptation and fitness. What is at stake in analysing these interactions is essentially the understanding of the biological mechanisms relating environmental variability and ecosystem properties, and living organisms being viewed as one element of that ecosystem.
Figure 1.
Time–space scales of forcing factors in a marine system (man-made in boxes). Modified from Clark & Frid (2001).
The quantitative estimate of fish production is a key ingredient to effective fisheries and ecosystem management. However, despite the large number of studies that have investigated the effects of environmental variability upon fish activities and performance, our current ability to predict the influence of environmental contingencies upon fish production is limited (Neill et al. 1994; van-der-Veer et al. 2000). The past 20–30 years have largely contributed to demonstrate the economical and societal costs resulting from this deficiency, e.g. the collapse of the northwest Atlantic cod stocks 15 years ago. Three major reasons explain this consequential situation. The first one follows on from the fact that fish are exposed to a multidimensional environment, the complexity and dynamics of which are very difficult to replicate experimentally, or indeed mathematically. The second reason results from the fact that predicting animal movements in a heterogeneous environment requires addressing a number of questions about potential fitness gain, individual movement ability and decision-making process (Kramer et al. 1997). The last one relates to the difficulty of transferring our understanding of environmental adaptation from the organismal to the population level (Huey 1991; Neill et al. 1994; Miller 1997). In the following, we will examine our current understanding of these points and how Fry's notion of ‘metabolic scope for activity’ has been instructive in linking autecological levels of analysis with the synecological levels of organization (see also Kerr 1990).
2. Environmental constraints and the regulatory repertoire of fish
Before we begin, we shall define the notion of performance as used in the current manuscript. The term performance designates a volume of capacities, where that volume is determined by the environmental conditions and interactions among the systems which contribute to those capacities (Bennett 1989). The term performance will apply to various levels of biological organization, from simple physiological functions to complex organismal traits.
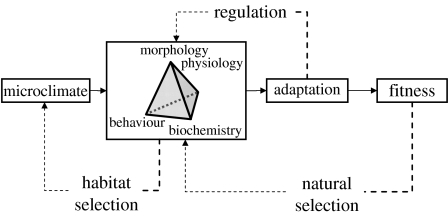
One simplistic and admittedly naive way of formulating the issue at stake is that the environment presents a problem and the organism must provide a solution to the problem posed by the environment. An ecological niche is a multidimensional system where each dimension corresponds to one environmental variable. Environmental factors interact with each other and combine to give rise to constraints with far-reaching influences upon the physiological performance of living organisms, ultimately affecting their ability to grow, survive and reproduce. How environmental conditions influence organisms' activities and performance is graphically summarized in figure 2. The microclimate that characterizes a selected habitat largely determines the triptych physiology–biochemistry–morphology, which, in turn, constitutes the operational framework for behaviour. The three feedback loops represented in figure 2 are meant to illustrate that fish are not helpless when facing environmental problems. The first loop summarizes regulatory physiological mechanisms, the second one implies a behavioural mitigation of the environmental contingencies and the last loop involves long-term evolutionary changes potentially affecting all the organismal components. Imbedded in this representation is the understanding that these solutions are largely dictated by the need to adjust to environmental heterogeneity and dynamics under the broad assumption that improved fitness lies beneath physiological regulation, habitat selection or evolutionary changes in performance (Beitinger & Fitzpatrick 1979; Huey 1991; Davenport & Sayer 1993; Huntingford 1993).
Figure 2.
Influence of habitat conditions on the activities, performance and fitness of an organism. Dotted lines indicate feedback loops. See text for details. Modified from Huey (1991).
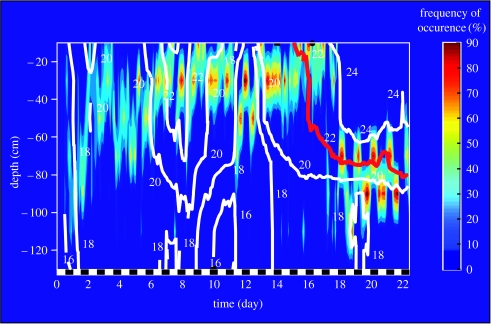
A demonstrative example of the accuracy of small-scale, microclimate adjustments in fish is given in figure 3. In this experiment, the influence of the thermal structure of the water column on the vertical distribution of telemetered sea bass was investigated. Note that the experimental mesocosm was only 1.3 m deep and fish body depth was of the order of 12 cm. When the water column was thermally homogenous (days 1–6) sea bass vertical positioning followed a daily cycle, which more or less covered the entire depth range. In a thermally stratified water column, on the contrary, profound changes in fish distribution pattern were observed. Two cases were distinguished. During the first period (days 7–17), the progressive warming of the surface, and cooling at the bottom, rapidly constrained the amplitude of sea bass' daily ‘migratory’ pattern, fish essentially occurring between −20 and −40 cm. During the second period (days 18–22), surface temperature went beyond the species optimal temperature (22°C; Claireaux & Lagardère 1999) and fish responded by following the 22°C isotherm as it moved down the water column.
Figure 3.
Frequency of occurrence of sea bass (0.8–1.1 kg) in a thermally heterogeneous and variable water column. White solid lines represent the isotherms, the red isotherm corresponding to the species optimal temperature (22°C; Claireaux & Lagardère 1999). Fish vertical position (accuracy±10 cm) was obtained using acoustic telemetry tags and receiver (Vemco, Shad-Bay, Canada). On the x-axis, the succession of black and white boxes indicates the photoperiod. A full description of the mesocosm can be found in Schurmann et al. (1998).
Since the number of environmental variables and adaptive processes is large, the problem of disentangling the environmental influences on fish performance and fitness is intractable unless the internal (i.e. mechanistic) analysis is preceded by the observation and theory at the external (i.e. emergence) level of analysis (Kerr 1976; Rose 1999; Underwood et al. 2000). Along this route, an absolute first step is the definition of an appropriate currency of fitness. Somatic and gonadic growths have been classically viewed as interim measures of fitness. Since body weight or length, unlike fertility, are readily measured in the field, numerous authors have investigated the environmental influences on fish distribution and activities under the premise that they are essentially driven by the need to maximize growth rate or food intake. Werner et al. (1983), combining optimal foraging theory, laboratory estimates of foraging cost and field observations, have shown that the habitat use of bluegill sunfish (Lepomis macrochirus) could be predicted on the basis of a maximizing feeding rate hypothesis. Brandt et al. (1992) also demonstrated that under optimal thermal conditions, the distribution of striped bass (Morone saxatilis) in the Chesapeake Bay matched calculated maps of growth rate potential. However, this study also revealed that under suboptimal environmental conditions, the distribution pattern of stripped bass in the Bay ceased to be determined by this growth rate potential. This mismatch most probably derived from the fact that, under challenging conditions, fish performance breadth was reduced and prioritization of activities occurred. Generally, prioritization of internal energy flow happens at the expense of activities which are not directly involved with short-term survival, typically growth or reproduction (Priede 1985). It is our contention that Fry's (1947) notion of metabolic scope for activity is a more universally applicable gauge for an external analysis of the energetics of habitat selection than growth or reproductive performance. While the scope for metabolic activity is a measure of the instantaneous rate of metabolic energy expenditure available in a given environmental and physiological context, growth or reproductive performance is a measure of the cumulated energy surplus earned and stored over a relatively extended period of time (Ware 1982). Moreover, the temporal resolution of the regulation of metabolic scope is compatible with that of behavioural or physiological regulatory responses (minutes to hours) but is less congruent with the temporal context of the maximization of growth or fertility (week to month). Readers will find a more detailed discussion of the time-scale issues in bioenergetics in Priede (1985).
3. The concept of metabolic scope for activity
The essentials of the Fry paradigm have been explored at length (e.g. Kerr 1976, 1990; Priede 1977, 1985; Evans 1990; Hochachka 1990; Kelsch & Neill 1990; Neill et al. 1994, 2004; Miller 1997) and it is not our intention to reiterate. In the context of the current dissertation, the most relevant points of the Fry paradigm worth mentioning here are that the environmental influences on animals' activity are mediated through metabolism and environmental factors can be classified on the basis of their metabolic consequences. Fry's paradigm discriminates five types of factors. Briefly, controlling factors (e.g. temperature) govern the kinematics of biophysical and biochemical reactions involved in metabolism. These factors set both active and standard metabolic rates. Limiting factors (e.g. oxygen, ammonia) interfere with oxygen supply and constrain active metabolic rate. Masking factors (e.g. salinity) increase the maintenance metabolic demand owing to the supplementary energetic costs associated with internal homeostasis. Lethal factors (e.g. pollutants) block metabolic processes and lead to animal death. Finally, directive factors (e.g. photoperiod) funnel the animal towards habitats or physiological states it is potentially more ‘fitted to’.
Environmental factors shape the adaptive responses of living organisms, and one of Fry's major scientific contributions was to propose an external level of analysis of that reaction norm using the metabolic scope for activity as a metric of an organisms' ability to cope with environmental demands. In operational terms, the metabolic scope for activity measures, in units of metabolic energy dissipation, the difference between the active (or maximum) and the standard (or maintenance) metabolic rates. The metabolic scope therefore gauges the metabolic confines within which aerobic activities must be undertaken. According to Fry's definition, activities include all energy-requiring work, which not only means mechanical work but also growth, physiological regulation of the internal environment or fighting diseases and other stresses. All together, Fry's concept of metabolic scope and categorization of environmental factors provide a set of functional linkages which allow an external (at the emerging property level) analysis of the environmental influences on fish, as well as an internal (mechanistic) examination of the behavioural, morphological and physiological processes involved.
4. Linking scope for activity and fitness
(a) External level of analysis: growth performance
We generally recognize that biological systems exhibit hierarchical organization. The organizational spectrum organism–populations–communities–ecosystem is one such example of hierarchy (Kerr 1976). From the notion of hierarchy follows the idea of emergent properties. As we move up the organizational scale from, say molecular or cellular levels into increasingly complex integration plans, new structural and functional properties are readily observed, their number and interaction increasing exponentially as we proceed. Since biological systems are hierarchically structured, it has been argued that their analysis should begin with the examination of their emergent behaviours before making recourse to an internal description of the mechanisms involved (Kerr 1976). Fitness is an emergent property occurring at the organismal level, although the proximal causes, ultimately energy flow and allocation, lie at a lower plane of organization. As previously discussed, fitness being difficult to assess, growth is generally considered as an acceptable and workable correlate to one organism's lifetime ability to transfer its genes to the next generations. Even though situations resulting in a reduction in metabolic scope are likely to be tied in with reduced growth performance (Priede 1985; Evans 1990), very few studies have actually examined the shape of the relationship between scope for metabolic activity and growth. We can offer two examples in support of this contention.
Many fish species feed discontinuously, periods of starvation alternating with periods of intense feeding. One such species is the Atlantic cod (Gadus morhua) and it has been shown that the post-prandial oxygen demand of self-feeding cod could represent up to 90% of their scope for aerobic activity (Soofiani & Hawkins 1982; Claireaux et al. 2000). Because so much energy is derived towards food processing and digestion, the ability of ‘bout-feeders’ to grow is believed to be tightly linked to their ability to maximize their scope for activity. There are two reasons in favour of this assertion. The most obvious one is that the larger the scope for activity, the more food is potentially processed per unit of time and, therefore, the sooner the next meal. The second reason derives from the fact that, although excursions of metabolic rate at or near the limits of metabolic scope are possible, they are at the expense of mandatory activities, for instance, the repayment of an oxygen debt incurred during evasion from an unexpected predator. On that basis, Priede (1977) argued that during these excursions into metabolic ‘highs’, fish are confronted with a reduced metabolic security margin and, consequently, face higher probability of mortality. Resolving this trade-off between growth and survival is fundamental and increased scope for activity is an evident component of the solution.
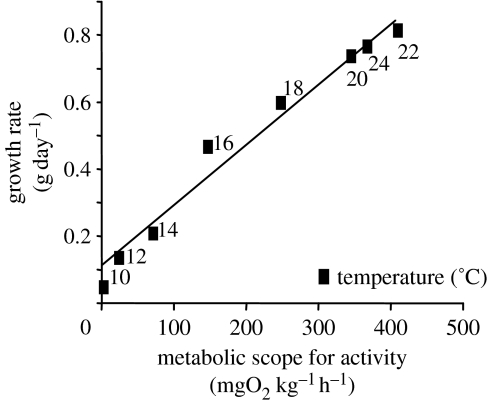
In Fry's categorization of the environment, ambient oxygenation is a limiting factor, meaning that it is a determinant of internal energy flow, impinging on active metabolic rate. Chabot & Dutil (1999) have shown that hypoxia-exposed Atlantic cod displayed significantly reduced growth in 60–70% air-saturated water and a 45% reduction in weight gain was measured in individuals reared in 40% saturated water. These authors attributed this result to reduced food intake and not to impaired food conversion efficiency. Reinterpreting the Chabot and Dutil dataset, Claireaux et al. (2000) argued that the reported reduction in ingestion rate could be ascribed to a behaviourally mediated adaptive response to the dwindling scope for activity, fish adjusting meal size according to their food processing ability. We illustrated this contention by revealing the linear relationship linking cod scope for activity and growth performance. More recently, similar relationships have been reported in various environmental circumstances in sea bass (Dicentrarchus labrax; figure 4) and turbot (Scophthalmus maximus; Mallekh & Lagardère 2002).
Figure 4.
Influence of water temperature on metabolic scope for activity and daily growth rate in the European sea bass. Growth data are from Lefebvre et al. (2001).
(b) External level of analysis: habitat selection
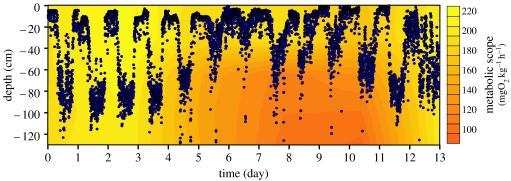
When studying habitat selection, we are generally asking questions about the capacities of an individual organism to assess changes in its environment and its abilities to respond to these changes. In order to sense the biotic and abiotic resources available, and then customize the most appropriate regulatory strategy, fish have at their own disposal banks of externally oriented sensors, which monitor the value and rate of change of a range of environmental factors (Burleson & Smatresk 1990; McKenzie et al. 1991). Moreover, internally oriented sensors also allow fish to sense their own metabolism relative to its maximum level by cues such as blood gas tension, ventilation rate, etc. (Randall & Smith 1967; Burleson & Milsom 1993). Studies on water temperature and oxygenation have produced classical examples of the sharpness of fish behavioural adjustments when environmental variables depart from optimal (Steffel et al. 1976; Claireaux et al. 1995a,b; Schurmann & Steffensen 1997; Schurmann et al. 1998; Shingles et al. 2005). At the basis of all these studies is the widespread acceptance that in a spatio-temporally heterogeneous environment, habitat selection by fish is mostly driven by the need to optimize metabolic scope (Evans 1990; Neill & Bryan 1991; Neill et al. 1994). Yet, very few studies have actually established this point (Kelsch & Neill 1990). The experiments summarized in figure 5 illustrate one such attempt. In this experiment, the vertical distribution of telemetered sea bass is analysed in varying conditions of oxygenation (6–3 mgO2 l−1) and temperature (11–22°C). When fish movements are analysed with regard to their influence on scope for activity (colour-coded background), a causal relationship cannot be ascertained. What is certain, however, is that day-to-day variability in the amplitude of the vertical distribution pattern contributes to the preserving of bass' metabolic performance breadth. This experiment also clarifies the adaptive significance of habitat selection decisions and the constraints which impede those decisions (Kramer et al. 1997).
Figure 5.
Linking sea bass distribution pattern to its associated scope for activity (recalculated from fig. 5 in Schurmann et al. (1998)). The spatially explicit colour-coded background represents sea bass metabolic scope calculated by entering the prevailing temperature and oxygenation conditions in the model of Claireaux & Lagardère (1999). In this experiment, temperature ranged between 11 and 22°C and water oxygenation between 3 and 6 mg l−1. Fish vertical position (accuracy±10 cm) was obtained using acoustic telemetry tags and receiver (Vemco, Shad-Bay, Canada).
(c) Internal level of analysis: energy acquisition and allocation
The proximal effects of environment on fish activity are mediated through metabolism. Unravelling the physiological mechanisms involved in environmental adaptation is of fundamental importance to understanding individual performance as well as population demography, dynamics and evolution (Neill et al. 1994). The level of fitness of an organism is the product of a dynamic and multidimensional equilibrium between that individual and its habitat. According to Fry, the number of options available to an organism in establishing that equilibrium is a direct function of its scope for activity in that environment. As previously discussed, any environmental situation resulting in a reduction of scope for activity is prone to generating energy budgeting conflicts between competing demands or functions. Prioritization in internal energy allocation then occurs, generally at the expense of somatic or gonadic growth.
In metabolically challenging situations, a general redistribution of blood flow may occur in accordance with priorities of internal energy allocation. Investigations of the energetic burden imposed by digestion and its interactions with fish ability to swim (Blaikie & Kerr 1996) or to tolerate lowered oxygen availability (Claireaux et al. 2000) have provided compelling examples of this mechanism.
In unfed fish, blood flow to the gastrointestinal tract accounts for 20–30% of the total cardiac output measured under resting conditions (Axelsson et al. 1989, 2000; Axelsson & Fritsche 1991; Thorarensen et al. 1994; Farrell et al. 2001). Within hours post-feeding, however, blood flow to the gut increases in the order of 60–70% to facilitate absorption of food and shorten digestion time (Axelsson et al. 1989, 2000; Axelsson & Fritsche 1991). Axelsson et al. (2002) have shown in sea bass that hypoxic conditions, and the associated reduction in scope for metabolic activity, were tied to parallel decreases in cardiac output and gut blood flow. This response was interpreted as a sign of reduction in total energy flow while upholding priorities with regard to relative energy allocation. Conversely, when fed sea bass were challenged with a standardized exercise protocol in a swim-tunnel, the increased metabolic demand by the working muscle mass was afforded via a sharp increase in cardiac output, associated with a decrease in the blood flow to the gastrointestinal tract (Altimiras et al. submitted). Contrary to sea bass, Atlantic cod (G. morhua) fed to satiation were observed to empty their stomach when exposed to hypoxia (30% saturation), indicating that systems other than the gastrointestinal tract were prioritized (Claireaux et al. 2000).
5. Integrating the past
Up to now, we have argued that Fry's scope for metabolic activity reflected the integrated aerobic metabolic potential of the whole animal in a given environment. We then followed Priede's (1985) reasoning that using the concept of scope for activity to investigate the environmental influences on fish performance implied that an appropriate time base be chosen, probably of the order of minutes to hours, depending on the adaptive process under study and on the temporal resolution of the experimental set-up used. In the following paragraph, we will add a ‘historical’ dimension to the notion of scope for activity.
As they develop, grow and age, individual fish follow ‘lifelines’ that are inevitably associated with phenotypic variation in physiological regulations and functions. We argue that some of the inter-individual diversity in scope for activity is the result of this difference in lifelines. Time-integrating sources of variations in individual lifelines are many and strong interactions between these sources exist. Nutritional diet is an example of such a source of phenotypic diversity.
Even though the study of fish swimming has a long history, we still know very little about the variation of performance among individual fish and the sources of that variation (reviewed in Kolok 1999; Plaut 2001; Nelson et al. 2002; Nelson & Claireaux 2005). Previous studies have revealed that diet, and particularly dietary fatty acids, can have profound influences on fish swimming performance. McKenzie et al. (1998) and Wagner et al. (2004) have shown that the fatty acid composition of the diet had a marked impact on the range and repeatability of locomotor ability in Atlantic salmon (Salmo salar). In eel (Anguilla anguilla) and Adriatic sturgeon (Acipenser naccarii), diet composition was found to be a determinant of maintenance metabolic cost as well as an influential factor in cardiovascular performance under reduced oxygenation conditions (McKenzie et al. 1995, 1999, 2000; Agnisola et al. 1996). A recent study by Chatelier et al. (2006) has brought a novel perception of the possible ecological repercussions of fish nutritional diet. In their study, Chatelier and co-workers started by showing that within three to four months, tissue fatty acid profiles of relatively large sea bass (200 g) reflected the fatty acid composition of their diet. Their second major observation was that these changes in tissue fatty acid composition were correlated with parallel changes in fish scope for activity and swimming performance. Taken together, these results indicate that although dietary fatty acids exert their effects at the cellular level, these effects translate across the levels of organismal organization to influence the physiology of the whole animal, and ultimately its fitness. This raises interesting questions about how food quality might influence the energetic strategy and the ecological performance of fish in their natural environment. In marine fish, essential fatty acids such as the n-3HUFA are obtained exclusively through diet (Sargent & Whittle 1981; Sargent et al. 1999) and juvenile fish, which feed near the base of the food web, naturally experience variations in the availability of these essential molecules (Volkmann et al. 1989; Galois et al. 1996). The links between fatty acid availability and tissue fatty acid profile in wild fish populations are unknown. Yet, the time-scale at which tissue impregnation occurs, together with the extent of the associated changes in fitness-related performance, make it tempting to assume that qualitative changes in feeding conditions are potential sources of year-to-year variability in fish recruitment.
6. Ecosystem management
(a) Fisheries
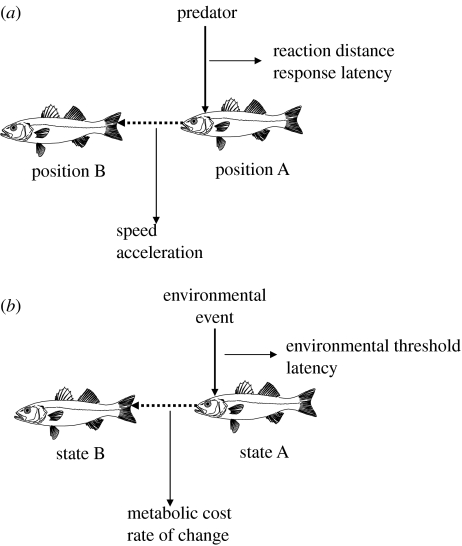
The cause of inter-annual variability in recruitment is the most disputed issue in fisheries sciences (Sinclair 1988; Hilborn & Walters 1992) and the question of its tractability is still at the centre of controversies. Miller (1997) summarized the issue at stake: ‘For instance, we all know temperature affects growth. But linking temperature to recruitment is a matter of linking an effect at the metabolic level to a response at the individual level (growth); then the individual level effect to a subpopulation level response (production); and finally, the subpopulation effect to a population level response (recruitment)’. In an attempt to provide a framework to link environmental variability to recruitment variability, Neill et al. (1994) extended Fry's construct of metabolic scope and factor types to higher levels of organization (subpopulation and population). At the basis of their reasoning is the analogy that environment operates on individuals through metabolism, on populations through recruitment and on communities through abiotic and biotic diversification. In this context, scope for population increase, for instance, is the difference between maximum and maintenance recruitment into the spawning stock. Revisiting Neill et al. and Miller's papers is beyond the scope of the current essay but we certainly encourage readers to examine these pivotal contributions to the alleviation of confusion around the origin of recruitment variability. The conceptual scheme provided by Neill and co-workers, like the original one drafted by Fry 60 years ago, fills an important gap by providing an array of testable hypotheses concerning the links between ecophysiological performance and fish life-history strategies and population dynamics. In this regard, a fertile parallel can be drawn between the methodologies followed to investigate environmental influences on fish performance and those implemented to examine the kinematics of escape response in relation to predator–prey interactions (figure 6). Investigators who study escape response in fish classically measure the reaction distance of the prey to the predator and the response latency to the startle stimulus. These studies are also interested in estimating the maximal escape swimming speed (m s−1) as the fish moves from point A (its initial position) to point B (supposedly out of reach of the predator), as well as the acceleration (m s−2) to that speed. By analogy, environmental contingencies can be viewed as the predator and survival is then linked to the time lag to completion of an appropriate response. Taking our analogy one step further, we can argue that ecophysiologists are also interested in determining environmental thresholds associated with the triggering of adaptive regulatory responses as well as how individual fish proceed from ‘adaptive state’ A (where it is energetically at risk) to B (where energy budgeting conflict are minimized). They also want to measure how much power (J s−1) is required in the process and the rate at which that power is being mobilized (J s−2). The above analogy may be a bit far-fetched but it brings to light a set of testable questions.
What are the determinants of the distribution of reactivity-related traits within a population?
To what extent do past or current environmental conditions influence that distribution?
What are the links between diversity in these traits and populations' resilience?
Figure 6.
Analogy between investigating the influence of environmental contingencies on fish performance and investigating escape response. (a) Experimental approach and quantification of escape response. (b) Extension to studying fishes' adaptive responses. See text for further details.
(b) Aquaculture
Ensuring the well being of domesticated fish requires that the status of the equilibrium between their adaptive capacity and the prevailing environmental constraints be monitored appropriately. Since it reflects the balance between fish power generating potential and environmentally driven metabolic demands, the extent of scope for aerobic activity has been proposed as a proximal indicator of welfare in aquaculture (Neill & Bryan 1991). Claireaux et al. (2005) have provided evidence that farmed rainbow trout fingerlings with poor swimming abilities had an impaired ability to raise their metabolic rate and perform aerobic work when tested as adults. They were able to demonstrate that poorly performing individuals actually suffered from abnormal cardiac morphologies and reduced myocardium working abilities. In 2002, the Fisheries Society of the British Isles defined the ‘five freedoms’ to secure good welfare in farmed fish. Among these was the freedom from injury, disease and functional impairment. On that basis, the decreased scope for aerobic work observed in a fraction of Claireaux et al.'s experimental rainbow trout population proved to be an operational indicator of detrimental cardiovascular morpho-functional characteristics which were incompatible with the need to guarantee the ability of fish, not only to operate under routine conditions, but also to mobilize metabolic power in response to environmental challenges.
7. Perspectives
Capacity for adaptive responses to environmental conditions has long been considered a property of living organisms. However, its significance for the process of evolution in fish has not been extensively explored. In §5, we argued that nutrient availability is one of the factors of the reaction norm that describes the environmental influence on organisms' adaptive ability. Sorting out the elements involved in environmental adaptation is critical to the determination of synecological properties such as trait heritability and links to fecundity. To our knowledge, published data documenting the heritability or between-individual variability in the scope for activity are unavailable. Likewise, the metabolic cost of the reaction norm has never been assessed nor analysed from an evolutionary perspective (Kerr 1990).
In terms of energy, the most important problem facing an animal trying to survive is to attain the power output required by its selected niche. It has been proposed that power budgeting could be more important in determining fitness than energetic efficiency per se (Priede 1985). The same author also suggested that natural selection should favour phenotypes having relatively large metabolic scope and/or reduced energetic cost of activity. These two potential evolutionary trends are not mutually exclusive and the actual balance between ‘maximizing performance breadth’ and ‘operating at the lowest cost’ may denote species-specific adaptive strategy. Clearly, this question is still open and awaits in-depth scrutiny.
Ware (1982) proposed that power budget, instead of energy budget, should be used for testing adaptive strategy. Ware's contention is that optimal foraging theory and optimal life-history theory are concerned with metabolic power acquisition and allocation while natural selection operates to increase surplus power. In Ware's view, surplus power corresponds to the power available after maintenance and routine activities have been provided for. Depending on the ontogeny stage, surplus power is allocated differently. During larval and juvenile development, surplus power is almost exclusively allocated to somatic growth. On the other hand, during the adult phase, the fraction of energy allocated to somatic development is reduced in favour of gonadic growth. Although the Ware and Fry concepts follow parallel lines of reasoning, one difference must be noted. Whereas, energy budget (joule) does not contain a time dimension, power (joule per unit of time) budgeting implies the selection of a time-scale. This is not trivial for the final result. For instance, fluctuation in heart rate or swimming activity occur at a time-scale of a few seconds while growth must be integrated over days and most likely weeks. Such discrepancies in the time-scale at which metabolic events take place are very difficult to reconcile within the framework of a power budget.
We have argued that fast growth necessarily implied maximized metabolic scope or surplus power, hence should be positively correlated with fitness. It is important to note, however, that fitness does not increase endlessly with the build-up of energy reserves and trade-offs are responsible for the levelling-off of that relationship. One of these trade-offs results from interaction between energy storage, body shape and swimming performance. In many fish species, swimming performance is influenced by morphological characters affecting manoeuvrability, acceleration or cost of sustained transport (Videler 1993; Domenici & Blake 1997). Boily & Magnan (2002) have shown that in yellow perch (Perca flavescens) accumulation of reserves can lead to stout body shape which is associated with higher net cost of transport. These authors suggest a link between the individual variations in swimming cost and morphological traits affecting drag and thrust forces. However, the same study failed to identify a trade-off between energy build-up and swimming ability in brook charr (Salvelinus fontinalis).
It is generally presumed that inter-individual differences in internal energy flow are subject to natural selection. However, the causal relationships between energetic strategies (acquisition and allocation) and fitness largely remain to be established (Ware 1982; Rudstam & Magnuson 1985; Dill 1987; Huey 1991; Nisbet et al. 2001). A possible first step along that path is to adequately relate metabolic scope for activity to surplus power (Ware 1982) or probability of survival (Priede 1977). Although claimed by various authors (Priede 1985; Evans 1990; Neill & Bryan 1991; Miller 1997; Claireaux & Lagardère 1999; Claireaux et al. 2000; Lefrançois & Claireaux 2003), the existence of such a relationship still awaits experimental corroboration.
We previously discussed the possibility that aerobic metabolic demand may temporarily reach the limit of metabolic scope. We also mentioned, after Priede (1977), that such occurrences are believed to result in increased probability of mortality due to a reduced metabolic safety margin. Under specific conditions, total metabolic demand of fish can actually exceed the aerobic scope for activity. In these conditions, supplementary energy needs are provided for by anaerobic glycolysis, the depleting of carbohydrate reserves and the accumulation of organic acids in the tissues, essentially lactic acid. Tissues may operate anaerobically for a time but the build-up of intra-cellular lactate levels represents an oxygen debt that must be cleared before acidosis reaches a level which interfere with normal cellular functions. At first glance, the contribution of anaerobic metabolic pathways to lifetime energy need may be viewed as minor because they are so time constrained. However, anaerobically fuelled metabolic processes play crucial, life-preserving roles, for instance, during burst-type attack or escape responses, as well as during episodes of reduced oxygen availability. To our knowledge, the link between anaerobic performance and fitness has never been formally established except in the case of burst, anaerobic swimming in relation to survival in larvae (Fuiman et al. 2006). Hochachka (1990) examined the ability of organisms to suppress their metabolism below basal metabolic rate and suggested that it was a conceptual mirror-image of Fry's scope for activity. However, the existence of a possible trade-off between aerobic and anaerobic performance, and the evaluation of its adaptive value, remain unclear and represent promising avenues for future research.
8. Concluding remarks
The objective of this essay was to revisit key points in our currently poorly integrated understanding of the environmental influences on fish production systems. We have shown that following Fry's (1947) original monograph, numerous authors have highlighted the explanatory potential contained within the notions of metabolic scope and categorization of the environment. We also reckoned that the contribution of Fry's paradigm to fisheries and environmental sciences, as well as to evolutionary biology, remains relatively limited despite its utility and power. This reality is surprising if one considers the number of testable hypotheses contained within this conceptual scheme. In the introductory section, we claimed that our current inability to relate environmental contingencies to fish production derives from a difficulty in grasping the interplay between the environmental matrix and the fish regulatory arsenal. We also suggested that taking into account the hierarchical organization of biological systems, and identifying the emerging property relevant at the organizational level considered, are essential in this process. With some examples extracted from our own and others work, we have illustrated how the unravelling of the environmental influences on phenotype performance and adaptive flexibility opens new fields of research, particularly in linking ecophysiology and evolutionary biology. Recent works have considered phenotypic accommodation and responsiveness to environmental constraints as the first step in the process of Darwinian adaptive evolution. Since environmental factors can affect a whole population, they are believed to be more effective initiators of selectable evolutionary novelties than mutations, which initially only affect one individual (West-Eberhard 2003, 2005). It is our firm conviction that, all together, Fry's original concept and later expansions delineate a fantastic playground in which to test these exciting ideas.
Acknowledgments
The authors would like to thank D. J. McKenzie, J. A. Nelson and P. Domenici for sharing their ideas with us as well as I. G. Priede for his comments on the manuscript. Financial support by the European Union, Directorate Fisheries, through contract QLRS-2002-00799, Project ETHOFISH, is also acknowledged.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator-prey interactions in aquatic organisms’.
References
- Agnisola C, McKenzie D.J, Taylor E.W, Bolis C.L, Tota B. Cardiac performance in relation to oxygen supply varies with dietary lipid composition in sturgeon. Am. J. Phys. 1996;271:417–425. doi: 10.1152/ajpregu.1996.271.2.R417. [DOI] [PubMed] [Google Scholar]
- Altimiras, J., Claireaux, G., Sandblom, E., Farrell, A. P., McKenzie, D. J. & Axelsson, M. Submitted. Gastrointestinal blood flow and postprandial metabolism in swimming sea bass (Dicentrarchus labrax). J. Exp. Biol [DOI] [PubMed]
- Axelsson M, Fritsche R. Effects of exercise, hypoxia and feeding on the gastrointestinal blood flow in the Atlantic cod, Gadus morhua. J. Exp. Biol. 1991;158:181–198. doi: 10.1242/jeb.158.1.181. [DOI] [PubMed] [Google Scholar]
- Axelsson M, Driedzic W.R, Farrell A.P, Nilsson S. Regulation of cardiac output and gut flow in the sea raven, Hemitripterus americanus. Fish Physiol. Biochem. 1989;6:315–326. doi: 10.1007/BF01881686. doi:10.1007/BF01881686 [DOI] [PubMed] [Google Scholar]
- Axelsson M, Thorarensen M, Nilsson S, Farrell A.P. Gastrointestinal blood flow in the red Irish lord, Hemilepidotus hemilepidotus: long-term effects of feeding and adrenergic control. J. Comp. Physiol. B. 2000;170:145–152. doi: 10.1007/s003600050269. doi:10.1007/s003600050269 [DOI] [PubMed] [Google Scholar]
- Axelsson M, Altimiras J, Claireaux G. Post-prandial blood flow to the gastrointestinal tract is not compromised during hypoxia in the sea bass Dicentrarchus labrax. J. Exp. Biol. 2002;205:2891–2896. doi: 10.1242/jeb.205.18.2891. [DOI] [PubMed] [Google Scholar]
- Beitinger T.L, Fitzpatrick L.C. Physiological and ecological correlates of preferred temperature in fish. Am. Zool. 1979;19:319–329. [Google Scholar]
- Bennett A.F. Integrated studies of locomotor performance. In: Wake D.B, Roth G, editors. Complex organismal functions: integration and evolution in vertebrates. Wiley; Chichester, UK: 1989. pp. 191–202. [Google Scholar]
- Blaikie H.B, Kerr S.R. Effect of activity level on apparent heat increment in Atlantic cod, Gadus morhua. Can. J. Fish. Aquat. Sci. 1996;53:2093–2099. doi:10.1139/cjfas-53-9-2093 [Google Scholar]
- Boily P, Magnan P. Relationship between individual variation in morphological characters and swimming costs in brook charr (Salvelinus fontinalis) and yellow perch (Perca falvescens) J. Exp. Biol. 2002;205:1031–1036. doi: 10.1242/jeb.205.7.1031. [DOI] [PubMed] [Google Scholar]
- Brander K. Effects of climate change on cod (Gadus morhua) stocks. In: Wood C.M, McDonald D.G, editors. Global warming: implications for freshwater and marine fish. S.E.B. seminar series. Cambridge University Press; Cambridge, UK: 1996. pp. 255–278. [Google Scholar]
- Brandt S.B, Mason D.M, Patrick V. Spatially explicit models of fish growth rate. Fisheries. 1992;17:23–33. doi:10.1577/1548-8446(1992)017<0023:SMOFGR>2.0.CO;2 [Google Scholar]
- Burleson M.L, Milsom W.K. Sensory receptors in the first gill arch of rainbow trout. Resp. Physiol. 1993;93:97–110. doi: 10.1016/0034-5687(93)90071-h. doi:10.1016/0034-5687(93)90071-H [DOI] [PubMed] [Google Scholar]
- Burleson M.L, Smatresk N.J. Evidence for two oxygen-sensitive chemoreceptor loci in channel catfish, Ictalurus punctatus. Physiol. Zool. 1990;63:208–221. [Google Scholar]
- Chabot D, Dutil J.-D. Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J. Fish Biol. 1999;55:472–491. doi:10.1111/j.1095-8649.1999.tb00693.x [Google Scholar]
- Chatelier A, McKenzie D.M, Prinet A, Galois R, Zambonino J, Claireaux G. Linking tissue fatty acid composition with major physiological traits of performance and metabolism in the sea bass (Dicentrarchus labrax) J. Exp. Biol. 2006;209:3429–3439. doi: 10.1242/jeb.02347. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Lagardère J.-P. Influence of temperature, oxygen and salinity on the metabolism of European sea bass. J. Sea Res. 1999;42:157–168. doi:10.1016/S1385-1101(99)00019-2 [Google Scholar]
- Claireaux G, Webber D.M, Kerr S.R, Boutilier R.G. Physiology and behaviour of free swimming Atlantic cod, Gadus morhua, facing fluctuating temperature conditions. J. Exp. Biol. 1995a;198:49–60. doi: 10.1242/jeb.198.1.49. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Webber D.M, Kerr S.R, Boutilier R.G. Physiology and behaviour of free swimming Atlantic cod, Gadus morhua, facing fluctuating salinity and oxygenation conditions. J. Exp. Biol. 1995b;198:61–69. doi: 10.1242/jeb.198.1.61. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Webber D.M, Lagardère J.-P, Kerr S.R. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua) J. Sea Res. 2000;44:257–265. doi:10.1016/S1385-1101(00)00053-8 [Google Scholar]
- Claireaux G, McKenzie D.J, Genge A.G, Chatelier A, Aubin J, Farrell A.P. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 2005;208:1775–1784. doi: 10.1242/jeb.01587. doi:10.1242/jeb.01587 [DOI] [PubMed] [Google Scholar]
- Clark R.A, Frid C.L.J. A review of long term changes in the North Sea ecosystem. Environ. Rev. 2001;9:131–187. doi:10.1139/er-9-3-131 [Google Scholar]
- Davenport J, Sayer M.D.J. Physiological determinant of distribution in fish. J. Fish Biol. 1993;43:121–145. [Google Scholar]
- Dill L. Animal decision-making and its ecological consequences: the future of aquatic ecology and behaviour. Can. J. Zool. 1987;65:803–811. [Google Scholar]
- Domenici P, Blake R.W. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 1997;200:1165–1178. doi: 10.1242/jeb.200.8.1165. [DOI] [PubMed] [Google Scholar]
- Evans D.O. Metabolic thermal compensation by rainbow trout: effect on standard metabolic rate and potential usable power. Trans. Am. Fish. Soc. 1990;119:585–600. doi:10.1577/1548-8659(1990)119<0585:MTCBRT>2.3.CO;2 [Google Scholar]
- Farrell A.P, Thorarensen H, Axelsson M, Crocker C.E, Gamperl A.K, Cech J.J. Gut blood flow in fish during exercise and severe hypercapnia. Comp. Biochem. Physiol. 2001;128:551–563. doi: 10.1016/s1095-6433(00)00335-4. [DOI] [PubMed] [Google Scholar]
- Fry F.E.J. Effect of the environment on animal activity. Univ. Toronto Stud. Biol. Ser. 1947;55:1–62. [Google Scholar]
- Fuiman L.A, Rose K.A, Cowan J.H, Smith E.P. Survival skills required for predator evasion by fish larvae and their relationship to laboratory measures of performance. Anim. Behav. 2006;71:1389–1399. doi:10.1016/j.anbehav.2005.11.013 [Google Scholar]
- Galois R, Richard P, Fricourt B. Seasonal variations in suspended particulate matter in the Marennes-Oléron Bay, France, using lipids as biomarkers. Estuar. Coast Shelf Sci. 1996;43:335–357. doi:10.1006/ecss.1996.0074 [Google Scholar]
- Hilborn R, Walters C.J. Chapman and Hall; New York, NY: 1992. Quantitative fisheries stock assessment: choice, dynamics and uncertainty; pp. 1–570. [Google Scholar]
- Hochachka P. Scope for survival: a conceptual ‘mirror’ of Fry's scope for activity. Trans. Am. Fish. Soc. 1990;119:622–628. doi:10.1577/1548-8659(1990)119<0622:SFSACT>2.3.CO;2 [Google Scholar]
- Huey R.B. Physiological consequences of habitat selection. Am. Nat. 1991;137:91–115. doi:10.1086/285141 [Google Scholar]
- Huntingford F.A. Can cost-benefit explain fish distribution pattern? J. Fish Biol. 1993;43:289–308. [Google Scholar]
- Kelsch S.W, Neill W.H. Temperature preference versus acclimation in fishes: selection for changing metabolic optima. Trans. Am. Fish. Soc. 1990;119:601–610. doi:10.1577/1548-8659(1990)119<0601:TPVAIF>2.3.CO;2 [Google Scholar]
- Kerr S.R. Ecological analysis and the Fry paradigm. J. Fish. Res. Board Can. 1976;33:329–335. [Google Scholar]
- Kerr S.R. The Fry paradigm: its significance for contemporary ecology. Trans. Am. Fish. Soc. 1990;119:779–785. doi:10.1577/1548-8659(1990)119<0779:TFPISF>2.3.CO;2 [Google Scholar]
- Kolok A.S. Inter-individual variation in the prolonged locomotor performance of ectothermic vertebrates: a comparison of fish and herpetofaunal methodologies and a brief review of the recent fish literature. Can. J. Fish. Aquat. Sci. 1999;56:700–710. doi:10.1139/cjfas-56-4-700 [Google Scholar]
- Kramer D.L, Rangeley R.W, Chapman L.J. Habitat selection: patterns of spatial distribution from behavioural decisions. In: Gaudin J.-G, editor. Behavioural ecology of teleost fishes. Oxford University Press; Oxford, UK: 1997. pp. 37–79. [Google Scholar]
- Lefebvre S, Bacher C, Meuret A, Hussenot J. Modelling nitrogen cycling in a mariculture ecosystem as a tool to evaluate its outflow. Estuar. Coast. Shelf Sci. 2001;52:305–325. doi:10.1006/ecss.2000.0707 [Google Scholar]
- Lefrançois C, Claireaux G. Influence of ambient oxygenation and temperature on metabolic scope and scope for heart rate in the common sole Solea solea. Mar. Ecol. Prog. Ser. 2003;259:273–284. [Google Scholar]
- Mallekh R, Lagardère J.P. Effect of temperature and dissolved oxygen concentration on the metabolic rate of the turbot and the relationship between metabolic scope and feeding demand. J. Fish Biol. 2002;60:1105–1115. doi:10.1111/j.1095-8649.2002.tb01707.x [Google Scholar]
- McGowan J.A. Climate and change in oceanic ecosystems: the value of time series data. Trends Ecol. Evol. 1990;5:293–300. doi: 10.1016/0169-5347(90)90084-Q. doi:10.1016/0169-5347(90)90084-Q [DOI] [PubMed] [Google Scholar]
- McKenzie D.J, Burleson M.L, Randall D.J. The effects of branchial denervation and pseudobranch ablation on cardio-ventilatory control in an air-breathing fish. J. Exp. Biol. 1991;161:347–365. [Google Scholar]
- McKenzie D.J, Piraccini G, Steffensen J.F, Bolis C.L, Bronzi P, Taylor E.W. Effect of diet on spontaneous locomotor activity and oxygen consumption in Adriatic sturgeon (Acipenser nascarii) Fish Physiol. Biochem. 1995;14:341–355. doi: 10.1007/BF00003373. doi:10.1007/BF00003373 [DOI] [PubMed] [Google Scholar]
- McKenzie D.J, Higgs D.A, Dosanjh B.S, Deacon G, Randall D.J. Dietary fatty acid composition influences swimming performance in Atlantic salmon (Salmo salar) in seawater. Fish Physiol. Biochem. 1998;19:111–122. doi:10.1023/A:1007779619087 [Google Scholar]
- McKenzie D.J, Piraccini G, Agnisola C, Steffensen J.F, Bronzi P, Bolis C.L. The influence of dietary fatty acid composition on the respiratory and cardiovascular physiology of Adriatic sturgeon (Acipenser naccarii): a review. J. Appl. Ichthyol. 1999;15:265–269. [Google Scholar]
- McKenzie D.J, Piraccini G, Piccolella M, Steffensen J.F, Bolis C.L, Taylor E.W. Effects of dietary fatty acid composition on metabolic rate and responses to hypoxia in the European eel, Anguilla anguilla. Fish Physiol. Biochem. 2000;22:281–296. doi:10.1023/A:1007865327923 [Google Scholar]
- Miller J.M. Opening address of the third flatfish symposium. J. Sea Res. 1997;37:183–186. doi:10.1016/S1385-1101(97)00028-2 [Google Scholar]
- Neill W.H, Bryan J.D. Responses of fish to temperature and oxygen, and response integration through metabolic scope. In: Brune D.E, Tomasso J.R, editors. Aquaculture and water quality, advances in world aquaculture. The World Aquaculture Society; Baton Rouge, LA: 1991. pp. 30–57. [Google Scholar]
- Neill W.H, Miller J.M, Van Der Veer H.K, Winemiller K.O. Ecophysiology of marine fish recruitment: a conceptual framework for understanding interannual variability. Neth. J. Sea Res. 1994;32:135–152. doi:10.1016/0077-7579(94)90037-X [Google Scholar]
- Neill W.H, et al. Ecophys. Fish: a simulation model of fish growth in time-varying environmental regime. Rev. Fish. Sci. 2004;12:233–288. doi:10.1080/10641260490479818 [Google Scholar]
- Nelson J.A, Claireaux G. Sprint swimming performance of juvenile European sea bass. Trans. Am. Fish. Soc. 2005;134:1274–1284. doi:10.1577/T04-087.1 [Google Scholar]
- Nelson J.A, Gotwalt P.S, Reidy S.P, Webber D.M. Beyond Ucrit: matching swimming performance tests to the physiological ecology of the animal, including a new fish ‘drag strip’. Comp. Biochem. Physiol. 2002;133:289–302. doi: 10.1016/s1095-6433(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Nisbet R.M, Muller E.B, Lika K, Kooijman S.A.L.M. From molecules to ecosystems through dynamic energy budget models. J. Anim. Ecol. 2001;69:913–926. doi:10.1046/j.1365-2656.2000.00448.x [Google Scholar]
- O'Brien C.M, Fox C.J, Planque B, Casey J. Climate variability and North Sea cod. Nature. 2000;404:142. doi: 10.1038/35004654. doi:10.1038/35004654 [DOI] [PubMed] [Google Scholar]
- Plaut I. Critical swimming speed: its ecological relevance. Comp. Biochem. Physiol. 2001;131:41–50. doi: 10.1016/s1095-6433(01)00462-7. doi:10.1016/S1095-6433(01)00462-7 [DOI] [PubMed] [Google Scholar]
- Priede I.G. Natural selection for energetic efficiency and relationship between activity level and mortality. Nature. 1977;267:610–612. doi: 10.1038/267610a0. doi:10.1038/267610a0 [DOI] [PubMed] [Google Scholar]
- Priede I.G. Metabolic scope in fish. In: Tyler P, Calow P, editors. Fish energetics: new perspectives. Croom Helm; London, UK: 1985. pp. 33–64. [Google Scholar]
- Randall D.J, Smith J.C. The regulation of cardiac activity in fish in a hypoxic environment. J. Exp. Biol. 1967;40:104–113. [Google Scholar]
- Rose S. Précis of lifelines: biology freedom, determinism. Behav. Brain Sci. 1999;22:871–921. doi: 10.1017/s0140525x99002204. doi:10.1017/S0140525X99002204 [DOI] [PubMed] [Google Scholar]
- Rudstam L.G, Magnuson J.J. Predicting the vertical distribution of fish populations: analysis of cisco, Coregonus artedii, and yellow perch, Perca flavescens. Can. J. Fish. Aquat. Sci. 1985;42:1178–1188. [Google Scholar]
- Sargent J.R, Whittle K.J. Lipids and hydrocarbons in the marine food web. In: Longhurst A.R, editor. Analysis of marine ecosystems. Academic Press; London, UK: 1981. pp. 491–533. [Google Scholar]
- Sargent J, Bell G, McEvoy L, Tocher D, Estevez A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture. 1999;177:191–199. doi:10.1016/S0044-8486(99)00083-6 [Google Scholar]
- Schurmann H, Steffensen J.F. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J. Fish Biol. 1997;50:1166–1180. [Google Scholar]
- Schurmann H, Claireaux G, Chartois H. Advances in invertebrates and fish telemetry. In: Lagardère J.P, Bégout-Anras M.L, Claireaux G, editors. Developments in hydrobiology series. Kluwer Academic Publisher; Dordrecht, The Netherlands; Boston, MA; London, UK: 1998. pp. 207–213. [Google Scholar]
- Shingles A, McKenzie D.J, Claireaux G, Domenici P. Reflex cardioventilatory responses to hypoxia in the flathead gray mullet (Mugil cephalus) and their behavioral modulation by perceived threat of predation and water turbidity. Physiol. Biochem. Zool. 2005;78:744–755. doi: 10.1086/432143. doi:10.1086/432143 [DOI] [PubMed] [Google Scholar]
- Sinclair M. University of Washington Press; Seattle, WA: 1988. Marine populations. [Google Scholar]
- Soofiani N.M, Hawkins A.D. Energetic costs at different levels of feeding in the juvenile cod, Gadus morhua. J. Fish Biol. 1982;21:577–592. doi:10.1111/j.1095-8649.1982.tb02861.x [Google Scholar]
- Stebbing A.R.D, Turk S.M.T, Wheeler A, Clarke K.R. Immigration of southern fish species to south-west England linked to warming of the North Atlantic (1960–2001) J. Mar. Biol. Ass. UK. 2002;82:177–180. doi:10.1017/S0025315402005325 [Google Scholar]
- Steffel S, Dizon A.E, Magnuson J.J, Neill W.H. Temperature discrimination by captive free-swimming tuna, Euthynnus affinis. Trans. Am. Fish. Soc. 1976;105:588–591. doi:10.1577/1548-8659(1976)105<588:TDBCFT>2.0.CO;2 [Google Scholar]
- Thorarensen H, Gallaugher P.E, Kiessling A.K, Farrell A.P. Intestinal blood flow in swimming chinook salmon Oncorhynchus tshawytscha and the effects of haematocrit on blood flow distribution. J. Exp. Biol. 1994;179:115–129. doi:10.1016/0022-0981(94)90020-5 [Google Scholar]
- Underwood A.J, Chapman M.G, Connell S.D. Observations in ecology: you can't make progress on processes without understanding the patterns. J. Exp. Mar. Biol. Ecol. 2000;250:97–115. doi: 10.1016/s0022-0981(00)00181-7. doi:10.1016/S0022-0981(00)00181-7 [DOI] [PubMed] [Google Scholar]
- van-der-Veer H.W, Berghahn R, Miller J.M, Rijnsdorp A.D. Recruitment in flatfish, with special emphasis on North Atlantic species: progress made by the Flatfish Symposia. ICES J. Mar. Sci. 2000;57:202–215. doi:10.1006/jmsc.1999.0523 [Google Scholar]
- Videler J.J. Fish swimming. Chapman and Hall; London, UK: 1993. p. 260. [Google Scholar]
- Volkmann J.K, Jeffrey S.W, Nichols P.D. Fatty acids and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989;128:219–240. doi:10.1016/0022-0981(89)90029-4 [Google Scholar]
- Wagner G.N, Balfry S.K, Higgs D.A, Lall S.P, Farrell A.P. Dietary fatty acid composition affects the repeat swimming performance of Atlantic salmon in seawater. Comp. Biochem. Physiol. 2004;137:567–576. doi: 10.1016/j.cbpb.2003.11.005. doi:10.1016/j.cbpb.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Ware D.M. Power and evolutionary fitness of teleosts. Can. J. Fish. Aquat. Sci. 1982;39:3–13. [Google Scholar]
- Werner E.E, Mittelbach G.G, Hall D.H, Gilliam J.F. Experimental tests of optimal habitat use in fish: the role of relative habitat profitability. Ecology. 1983;64:1525–1539. doi:10.2307/1937507 [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]
- West-Eberhard M.J. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. 2005;304:1–9. doi: 10.1002/jez.b.21071. [DOI] [PubMed] [Google Scholar]