Abstract
Complex physiological traits, such as routine aerobic metabolic rate or exercise performance, are indicators of the functional integrity of fish that can reveal sub-lethal toxicological effects of aquatic pollutants. These traits have proved valuable in laboratory investigations of the sub-lethal effects of heavy metals, ammonia and various xenobiotics. It is not known, however, whether they can also function as biomarkers of the complex potential range of effects upon overall functional integrity caused by exposure to mixtures of chemicals in polluted natural environments. The current study used portable swimming respirometers to compare exercise performance and respiratory metabolism of fish exposed in cages for three weeks to either clean or polluted sites on three urban European river systems: the river Lambro, Milan, Italy; the rivers Blythe, Cole and Tame, Birmingham, UK; and the river Amstel, Amsterdam, The Netherlands. The UK and Italian rivers were variously polluted with high levels of both bioavailable heavy metals and organics, and the Amstel by mixtures of bioavailable organics at high concentrations. In both the UK and Italy, indigenous chub (Leuciscus cephalus) exposed to clean or polluted sites swam equally well in an initial performance test, but the chub from polluted sites could not repeat this performance after a brief recovery interval. These animals were unable to raise the metabolic rate and allocate oxygen towards exercise in the second trial, an effect confirmed in successive campaigns in Italy. Swimming performance was therefore a biomarker indicator of pollutant exposure in chub exposed at these sites. Exposure to polluted sites on the river Amstel did not affect the repeat swimming performance of cultured cloned carp (Cyprinus carpio), indicating either a species-specific tolerance or relative absence of heavy metals. However, measurements of oxygen uptake during swimming revealed increased rates of routine aerobic metabolism in both chub and carp at polluted sites in all of the rivers studied, indicating a sub-lethal metabolic loading effect. Therefore, the physiological traits of exercise performance and metabolic rate have potential as biomarkers of the overall sub-lethal toxic effects of exposure to complex mixtures of pollutants in rivers, and may also provide insight into why fish do not colonize some polluted environments.
Keywords: biomarker, exercise performance, metabolic rate, sub-lethal, river, pollution
1. Introduction
It is commonly accepted that physiological adaptation by fishes to their environment will influence the success with which they can colonize particular habitats (Fry 1947, 1971; Prosser 1950). This has led to many proposals that complex physiological traits, in particular, of functional integrity and energetics, can be used as indicators of the sub-lethal toxicological effects of aquatic pollution (Brett 1958; Cairns 1966; Sprague 1971).
One aspect of functional integrity that has received much attention is the ability to perform prolonged exercise. Fish species that pursue an active lifestyle perform prolonged exercise to forage, migrate and maintain position against currents, and this activity requires the coordinated activity of systems at various levels of organismal organization (Brett 1958; Randall 1982; Moyes & West 1995). A single integrative trait known as critical swimming speed (Ucrit; as conceived by Brett 1964) has proven to be sensitive to many environmental stressors (Randall & Brauner 1991), including pollutants such as low pH (Ye & Randall 1991; Butler et al. 1992), dissolved metals (e.g. Waiwood & Beamish 1978; Wilson et al. 1994; Beaumont et al. 1995a,b, 2003), ammonia (e.g. Shingles et al. 2001; Wicks et al. 2002; McKenzie et al. 2003a) and various other toxic chemicals such as organophosphate pesticides (Petersen 1974), organochlorine fungicides (MacKinnon & Farrell 1992; Nikl & Farrell 1993; Wood et al. 1996) and bleached kraft pulp-mill effluents (Howard 1975; McGeer et al. 1999).
A complex energetic trait that has been proposed as a potentially valuable indicator of sub-lethal pollution is routine aerobic metabolic rate, measured as rates of oxygen consumption (Fry 1971; Sprague 1971; Rice 1990). Metabolic rate can be considered the unifying currency of adaptation to the environment (Wikelski & Ricklefs 2001) and can reveal increased energetic costs associated with occupying polluted habitats (Rice 1990). There is evidence to indicate that increased metabolic rate is a general indicator of stress in fish (Schreck 1990; Wendelaar Bonga 1997) and studies have shown it to be raised by exposure to various pollutants such as organochlorine pesticides (Holmberg & Saunders 1975; Farrell et al. 1998), methylmercury (Rodgers & Beamish 1981) and some specific herbicides (Johansen & Geen 1990; Janz et al. 1991).
The literature investigating the effects of pollutants upon fish exercise performance and metabolic rates is, however, almost exclusively laboratory based and, with few exceptions (Howard 1975; McLeay & Brown 1979; Hopkins et al. 2003), comprises the exposure of salmonid species to single toxicants. Very little is known about the potential physiological effects of exposure to polluted natural environments, as revealed in situ (Farrell et al. 2004). Indeed, many aquatic habitats are continuously loaded with mixtures of chemicals released by human communities and industries, and many of these pollutants have been shown to exert adverse impacts upon the resident biota. The last few decades have, therefore, seen an increasing interest in the use of ‘biomarkers’ to establish early warning signals of exposure and toxic effects of specific pollutants or pollutant classes (reviewed by Van der Oost et al. 2003). In a broad sense, a biomarker can be defined as any biological response by an organism to exposure to environmental chemicals, or to their toxic effects (Peakall 1994). The term is most commonly used, however, to refer to measurements in body fluids, cells or tissues, which are indicative of bioaccumulation of toxic chemicals, biochemical and cellular modifications provoked by specific toxicants, or secondary responses of host tissues to these toxicants (Van der Oost et al. 2003). The assumption is that such modifications at these lower orders of biological organization are indicative of, or directly linked to, modifications in systemic and organismal function which, in turn, lead to changes in the populations and communities that comprise the ecosystem. These assumptions and, in particular, the link between expression of such biomarkers and the functional integrity of the whole organism remain, however, to be demonstrated (Van der Oost et al. 2003).
Both swimming performance and metabolic rate may be valuable in this sense because they directly reflect the functional integrity of fish and are also of immediate relevance to their ecology (Sprague 1971; Rice 1990; MacKinnon & Farrell 1992). Nothing is known, however, about whether such traits can be used as physiological biomarkers of sub-lethal toxic effects of the complex mixtures of pollutants that can prevail in natural environments. These complex traits that are reliable measures of the responses of many physiological systems, together with analyses of other more-specific biomarkers, may contribute towards an understanding of whether and how sub-lethal toxicological impacts might underlie changes in fish populations and community composition in polluted environments.
The current study used custom-built portable swim-tunnel respirometers to compare exercise performance (as Ucrit) and associated aerobic metabolism of fish exposed in cages for three to four weeks at either clean or polluted sites on three urban European river systems, across different seasons (spring, summer and winter). The rivers studied were the Lambro (Milan, Italy), the Blythe, Cole and Tame (Birmingham, UK) and the Amstel (Amsterdam, The Netherlands). Two species of cyprinid were chosen as models, the chub (Leuciscus cephalus) was studied in Italy and the UK, whereas the carp (Cyprinus carpio) was studied in The Netherlands. Swimming performance was assessed with a ‘repeat-exercise’ protocol, which measures the ability of fish to perform two sequential Ucrit tests with a brief intervening recovery interval, because Jain et al. (1998) have demonstrated that this protocol provides significantly more sensitive information about fish health and water quality than a single exercise test alone. Measurements were made of routine rates of oxygen uptake under standard conditions of sustained low-intensity exercise, to investigate whether this trait was influenced by the differences in water toxicant load at the various sites. Other metabolic traits, such as the maximum oxygen uptake during prolonged exercise and aerobic metabolic scope, were also derived during the swim tests, to gain insight into proximate physiological mechanisms that underlie any impairment to exercise performance (Beamish 1978; Wilson et al. 1994; McKenzie et al. 2003a; Pane et al. 2004). In the chub, the effects on performance of seasonal variations in temperature were studied under controlled laboratory conditions to enable this variable to be discriminated from the pollutant effects during seasonal caging campaigns.
2. Material and methods
(a) River sites
River sites in each country were selected based upon prior knowledge of their pollution status (UK and The Netherlands), or following visual inspection of potential sites (Italy). Table 1 summarizes some general water physicochemical characteristics at all of the sites, as measured with a multiparametric probe (Ocean Seven 316, Idronaut, Brugherio (MI), Italy) throughout the caging experiments, and also the concentrations of major nutrients, as measured on repeated spot samples (E. Garofalo & S. Ceradini 2003, unpublished data). Overall pollution status at each of the sites was assessed using concurrent deployment of diffusive gradient in thin film (DGT) and semipermeable membrane device (SPMD) passive samplers. Table 2 shows bioavailable heavy metals at the sites, as assessed with DGTs, which sample metals in solution that are not bound with complex organic macromolecules or adsorbed onto particulate matter (Garofalo et al. 2004). Table 3 shows bioavailable organics, assessed with SPMDs which sample fractions of these pollutants in the aqueous phase and can mimic bioconcentration processes in fishes (Verweij et al. 2004).
Table 1.
General physicochemical variables and concentrations of major nutrients in the river sites studied in Italy, the UK and The Netherlands. (For the physicochemical variables, values are means of hourly measurements taken with a multiparametric probe throughout the caging campaign for the Italian and Dutch river sites. For the UK sites, data are taken from Winter et al. (2005). For the nutrients, values are means of at least three spot samples taken on separate days. Standard deviations are not given for the sake of clarity. These were never greater than 10% of the mean values, except for water O2 levels at the Vrouwenakker and Amsterdam sites in The Netherlands, where there was a wide diurnal fluctuation. Temp., temperature; DO, dissolved oxygen; % sat., percentage air saturation; Cond., conductivity; NH4+, ammonium ions; , nitrate ions, , phosphate ions; I, Italy; NL, The Netherlands.)
| country, river, site | month | temp. (°C) | DO (% sat.) | pH | cond. (μS cm−1) | (mg l−1) | (mg l−1) | (mg l−1) |
|---|---|---|---|---|---|---|---|---|
| I, Lambro, Merone (unpolluted) | May | 14.8 | 98 | 7.2 | 4.6 | <0.02 | 10.2 | 1.04 |
| September | 19.6 | 86 | 6.9 | 4.2 | 0.17 | 8.1 | <0.02 | |
| February | 8.1 | 101 | 8.1 | 4.1 | <0.02 | 10.8 | 0.11 | |
| I, Lambro, Brugherio (polluted) | May | 14.9 | 107 | 7.3 | 5.7 | <0.02 | 15.3 | 1.55 |
| September | 19.4 | 86 | 6.9 | 4.2 | 0.18 | 11.8 | 0.67 | |
| February | 8.0 | 99 | 8.2 | 4.2 | <0.02 | 19.1 | 0.36 | |
| UK, Blythe (unpolluted) | June/July | 13.7 | 67 | 8.1 | 5.5 | 0.05 | 41.1 | 3.01 |
| February | 7.5 | 75 | 7.7 | 2.9 | <0.02 | 35.3 | 1.41 | |
| UK, Cole (polluted) | June/July | 15.8 | 70 | 7.9 | 5.2 | 0.08 | 8.6 | 0.46 |
| February | 8.3 | 79 | 8.3 | 3.5 | 0.03 | 19.5 | 0.20 | |
| UK, Tame (heavily polluted) | June/July | 18.4 | 61 | 7.5 | 8.3 | 0.39 | 55.4 | 5.31 |
| February | 9.6 | 66 | 7.7 | 5.7 | 0.51 | 40.4 | 4.98 | |
| NL, Amstel, Vrouwenakker (unpolluted) | September | 18.7 | 146 | 7.9 | 0.4 | 0.2 | 2.3 | 0.64 |
| NL, Amstel, Amsterdam (polluted) | September | 18.8 | 44 | 6.7 | 1.2 | 0.4 | 10.0 | 1.80 |
| NL, Volgermeerpolder (heavily polluted) | September | 17.8 | 66 | 7.1 | 0.9 | 0.3 | 0.2 | 0.07 |
Table 2.
Concentrations of bioavailable metal ions sampled by diffusive gradient in thin film devices (DGTs) in the river sites studied in Italy, the UK and The Netherlands. (All data are taken from Garofalo et al. 2004. I, Italy; NL, The Netherlands.)
| country, river, site | Zn (μg l−1) | Ni (μg l−1) | Cu (μg l−1) | Pb (μg l−1) | Cd (μg l−1) | Mn (μg l−1) |
|---|---|---|---|---|---|---|
| I, Lambro, Merone | 0.65 | 0.61 | 0.33 | 0.06 | 0.001 | 2.50 |
| I, Lambro, Brugherio | 3.98 | 3.43 | 2.74 | 0.33 | 0.006 | 5.07 |
| UK, Blythe | 1.44 | 0.31 | 0.31 | 0.08 | 0.005 | 3.89 |
| UK, Cole | 8.42 | 1.08 | 0.77 | 0.27 | 0.016 | 4.16 |
| UK, Tame | 23.84 | 4.22 | 0.77 | 0.10 | 0.011 | 10.16 |
| NL, Amstel, Vrouwenakker | 2.93 | 0.00 | 0.24 | 0.01 | 0.003 | 12.00 |
| NL, Amstel, Amsterdam | 1.34 | 0.42 | 0.13 | 0.00 | 0.001 | 6.04 |
| NL, Volgermeerpolder | 1.73 | 0.21 | 0.17 | 0.00 | 0.002 | 9.77 |
Table 3.
Major classes of bioavailable organic pollutants sampled by semipermeable membrane devices (SPMDs) in the river sites studied in Italy, the UK and The Netherlands. (Values are averages from at least two sampling campaigns, reported as the total nanograms collected during a three-week exposure period (Garofalo & Ceradini 2003, unpublished data). Values for the UK rivers have been reported previously in Winter et al. 2005. Values for the NL Volgermeerpolder site are derived from Verweij et al. 2004. PAHs, polycyclic aromatic hydrocarbons; PCB, polychlorinated biphenyls; OCPs, organochlorine pesticides. I, Italy; NL, The Netherlands.)
| country, river, site | ΣPAHs (ng) | ΣPCBs (ng) | ΣOCPs (ng) |
|---|---|---|---|
| I, Lambro, Merone | 607 | 2 | 10 |
| I, Lambro, Brugherio | 2723 | 9 | 13 |
| UK, Blythe | 1493 | 14 | 137 |
| UK, Cole | 6218 | 22 | 277 |
| UK, Tame | 5825 | 70 | 443 |
| NL, Amstel, Vrouwenakker | 5374 | 64 | 16 |
| NL, Amstel, Amsterdam | 6857 | 139 | 12 |
| NL, Volgermeerpolder | 12 379 | 522 | 7810 |
Italy. The river Lambro flows through the Lombardy region, rises in the foothills of the Alps in Como province and flows in a southerly direction through the provinces and cities of Monza and Milan, eventually becoming a tributary of the river Po. The Monza and Milan provinces are major centres of population and industry, and the river Lambro has historically been a recipient of both domestic and industrial wastes. Two sites were studied, one (Merone) in Como province and the other (Brugherio San Rocco) in Monza province, just north of the city of Milan. Physicochemical conditions and nutrient concentrations did not differ markedly between these two sites. Overall water quality, however, was higher at the upstream site, Merone, compared with the downstream Brugherio site. As shown in table 2, the Brugherio site was polluted by bioavailable zinc, nickel and copper, metals that were only found at very low levels at the Merone site (Garofalo et al. 2004). As shown in table 3, the Brugherio site also exhibited higher pollution by bioavailable organics, such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs), than the Merone site (Garofalo & Ceradini 2003, unpublished data).
UK. The confluent Blythe, Cole and Tame lie within the Birmingham conurbation in the West Midlands. This is a major centre of industry and population, with a consequent legacy of pollution that is reflected in reduced water quality in many of the surrounding watercourses (e.g. Harkness 1982). The river Tame, which flows through an area of dense urban development and industrialization (Beavan et al. 2001), receives large amounts of industrial and urban inputs (Webster et al. 2001). The Cole has a predominantly urban course and, although it has relatively few industrial inputs, it receives deleterious inputs from urban run-off (Williams 2000). In contrast, the river Blythe has been designated a Site of Special Scientific Interest (Box & Walker 1994), has an extensively rural course and receives relatively little detrimental chemical input.
Physicochemical conditions and nutrient concentrations did not differ markedly between these three sites (table 1). As shown in table 2, however, the Tame was polluted with a complex mixture of metals, largely zinc, nickel and copper; the Cole was also polluted with these metals but to a lesser extent, whereas the Blythe exhibited only a low level of bioavailable zinc (Garofalo et al. 2004). As shown in table 3, sampling of bioavailable organics with SPMDs revealed that the Tame had four- to fivefold higher levels of PAHs, PCBs and OCPs than the Blythe, which had low levels of all these contaminants. The Cole exhibited similar levels of PAHs to the Tame, but levels of PCBs and OCPs intermediate between Tame and Blythe (Winter et al. 2005).
The Netherlands. The Amstel River is 40 km long, running south to north from a rural area into and through the centre of Amsterdam. A number of sites on the river have been used previously for studies upon biomarker responses in caged fishes (Van der Oost et al. 1998; Verweij et al. 2004). Among these sites, three were identified for the current investigation; two of these were on the river proper, whereas the third was an associated series of drainage trenches (polders). The clean site, Vrouwenakker, is in the southern part of the river, which is relatively unpolluted despite some agricultural run-off (site A1 in Verweij et al. 2004). This was compared with a site in downtown Amsterdam, where the river is polluted by industrial and domestic wastes, particularly as a result of run-off from an industrial accident several years ago (site A3 in Verweij et al. 2004). The third site, Volgermeerpolder, is heavily polluted because it is surrounded by a sanitary landfill (site VM in Verweij et al. 2004). These sites presented very little bioavailable heavy metal pollution (table 2; Garofalo et al. 2004) but quite severe pollution by organics, particularly at the Volgermeerpolder site, where there were high levels of PAHs and OCPs (Verweij et al. 2004) in comparison with both the Italian and UK sites (table 3).
(b) Experimental animals
Chub, L. cephalus, are common in relatively fast-flowing rivers throughout Europe and are indigenous to the rivers studied in Italy and the UK. They are omnivorous, eating invertebrates and plant material when young but becoming active piscivores as they mature. Chub are, however, rare in the slow-flowing lower reaches of the Amstel (F. Verweij & R. van der Oost 2004, unpublished observations). Therefore, the common carp, C. carpio, was studied on this river. Common carp inhabit ponds, lakes and slow-flowing rivers; native to Eastern Europe and Asia, they have been introduced in many other countries, including The Netherlands. Also being omnivorous, they feed on bottom-dwelling invertebrates and plant material.
Italy. Wild chub were captured by electrofishing in the river Lambro, at the clean Merone site. Fish were transported to the La Casella Fluvial Hydrobiology Station (via Argine del Ballottino, 29010 Sarmato (PC), Italy), where they were stocked in 4 m2 fibreglass tanks provided with a flow of water within a recirculating biofiltered system (volume 90 m3). The animals were fed daily with pelleted feed and maintained for at least two weeks at prevailing seasonal ambient temperatures and photoperiods prior to use in either caging or laboratory experiments, as described later.
UK. Farm-reared chub were obtained from the Environment Agency fish farm (Calverton, Nottinghamshire) and transported to the animal holding facilities at the School of Biosciences, University of Birmingham. Fish were held at prevailing seasonal temperatures and photoperiods in laboratory aquaria in biofiltered dechlorinated Birmingham tap water and fed daily with pelleted feed for at least two weeks prior to use in either caging or laboratory experiments (Winter et al. 2004).
The Netherlands. Caging experiments were performed with genetically identical male carp from a cultured F1 hybrid fish line produced and maintained at the Agricultural University of Wageningen (Van der Oost et al. 1998; Verweij et al. 2004). This experimental group offered the clear advantage of reduced variability between individuals in the toxicity tests (Van der Oost et al. 2003).
(c) Caging exposures
Italy. Exposures were performed in May 2001 (spring), September 2001 (late summer) and February 2002 (winter). The cages were constructed of 25 mm mesh Galvaplax plastic-coated steel fencing, bent and hooked together to form a cage (120×100×45 cm, volume 540 l). Two of these were positioned completely submerged on the riverbed at each site, in areas of gentle flow (less than 20 cm s−1) and anchored to riverside trees and a large submerged stone. Ten chub were placed in each cage and exposed to the prevailing conditions for at least three weeks.
UK. Caging experiments were performed in June/July 2002 (summer) and in February 2003 (winter), with the materials and methods described by Winter et al. (2005). Briefly, two submerged cages (66×87×69 cm, volume 396 l) were positioned at each site and 10 chub were exposed in each cage for at least three weeks.
The Netherlands. Caging exposures were performed in September 2002, following the practices described by Van der Oost et al. (1998). Briefly, submerged cages (volume approx. 1000 l) were anchored in the water column at each site, 20 carp were placed in each cage and exposed for at least three weeks.
No attempt was made to feed the fish during the caging exposures. In both Italy and the UK, the caged fish had access to both the water column and the riverbed and fish were observed visually to feed upon naturally available feed items that entered the cages, and post-mortem analysis of gut contents showed evidence of feeding (Winter et al. 2005).
(d) Exercise respirometry
Exercise performance and metabolism were measured using custom-built portable swimming respirometers, designed to exercise individual fish in a non-turbulent water flow with a uniform velocity profile (Steffensen et al. 1984). One respirometer constructed of PVC, with a cross-sectional area of 225 cm2 to the swimming chamber and a respirometric volume of 49.0 l, has been described in detail previously (McKenzie et al. 2001). The second was of similar design but had a cross-sectional area of 100 cm2 to the swim chamber, a respirometric volume of 13.4 l and was constructed of Plexiglas. In each respirometer, water flow was generated by a moulded thermoplastic propeller attached to a variable speed DC permanent magnet motor. Motor speed was controlled by a PC and Labview software (National Instruments, Inc.), calibrated to deliver water velocities in centimetres per second and, hence, swimming speeds corrected for the solid blocking effect of the fish (Bell & Terhune 1970). The respirometer chambers were thermostatted by immersion in larger outer tanks that received a constant flow of the appropriate source of exposure water.
The swimming respirometry for the caging campaigns was performed in a mobile laboratory created in a large transit van. This was parked adjacent to the study sites, on wooden blocks to ensure that the respirometers were completely level. A large submersible pump delivered a constant flow of river water to a 200 l plastic reservoir, whence a 24 V submersible pump provided a constant flow to the respirometers positioned in the van. Equipment was powered by a 220 V diesel-driven generator with a voltage stabilizer. Fish were transferred to the respirometer without exposure to air, by coaxing them gently into a submerged plastic bag in their cage and then out of the water-filled bag and into the swimming chamber of the respirometer. They were then permitted 4 h recovery from the handling stress while swimming gently at a current speed of 20 cm s−1, prior to testing their repeat swimming performance (Jain et al. 1998).
All fish were exercised by progressive increments in swimming speed of 10 cm s−1 every 30 min until fatigue. Fatigue was unambiguous in both the chub and the carp, they swam vigorously until they collapsed against the back screen and would not resume in response to sudden increases in current velocity or gentle manual encouragement. The fish were then allowed 40 min recovery from the first swim test (T1) after which they were exposed to exactly the same protocol a second time (T2). Critical swimming speed for both T1 and T2 was calculated in centimetres per second (cm s−1), as described by Brett (1964). The repeat performance ratio was calculated as T2/T1 (Jain et al. 1998). Instantaneous O2 uptake MO2 was measured at each swimming speed by intermittent flow-through respirometry (Steffensen 1989) controlled by a PC and Labview software as described in detail by McKenzie et al. (2001). For both T1 and T2, active (maximum) metabolic rate (AMR) was estimated as the MO2 measured at the highest swimming speed immediately prior to fatigue (Fry 1971; Beamish 1978). For both T1 and T2, an estimate of functional aerobic scope for activity was also calculated by subtracting rates of O2 uptake measured at the lowest swimming speed (20 cm s−1, defined as routine metabolic rate, RMR) from the measured AMR.
(e) Laboratory studies
In Italy and the UK, exercise performance and associated respiratory metabolism were also measured on chub under controlled laboratory conditions to allow comparison with the field data. Experiments were performed on Italian chub acclimatized to prevailing seasonal temperatures of 7°C (February), 15°C (May) and 24°C (September), and on UK chub acclimatized to prevailing temperatures of 8°C (February) and 14°C (June/July).
The studies were performed with the same swimming respirometers and exercise protocol as for the field campaigns. The fish were, however, allowed to acclimate to the respirometer for approximately 16 h (overnight) while swimming gently at a current speed of 20 cm s−1, with their repeat exercise performance measured the following day. Least squares exponential regression was applied to the relationship between swimming speed and MO2 for the data obtained in T1. Extrapolation back to the y-intercept was used to derive the notional metabolic rate of the immobile fish (IMR; McKenzie et al. 2003b); this correction for the metabolic costs of locomotor activity is considered to provide a valid estimate of the minimum metabolic rate required for maintenance (Brett 1964; Fry 1971). This then allowed an estimate of absolute aerobic scope for T1, as AMR minus IMR (Fry 1971). Calculation of IMR for T2 was, by definition, not valid because the fish exhibited an elevated post-exercise increase in MO2 (EPOC) subsequent to T1 (Farrell et al. 1998). By the same logic, no attempt was made to derive IMR in the field campaigns because this would have been confounded by the short period of acclimation to the swim tunnel.
(f) Statistics
Each single measurement campaign in the field was analysed separately, as it was considered that direct statistical comparisons between campaigns in different seasons or countries would be subject to spurious confounding factors. Thus, for each campaign, the objective was simply to reveal any statistical differences in the physiological variables between the sites that differed in their pollution status. To analyse the effect of the repeated exercise protocol upon measured variables, these were compared between groups by two-way analysis of variance (ANOVA) for repeated samples, where the interacting factors were the group (i.e. caging sites) and the repetition of the exercise protocol. To compare the repeat performance ratio between groups, the t-test was used to compare the two Italian sites for each separate campaign, a one-way ANOVA to compare the three sites in the UK and the same test to compare the three sites in The Netherlands. For the laboratory studies on the effects of seasonal temperature acclimatization, variables were compared between temperatures (seasons) by two-way ANOVA for repeated samples, where the interacting factors were the season and the repetition of the exercise protocol. A one-way ANOVA was used to compare the repeat performance ratio at the three temperatures. Holm–Sidak post hoc tests were used to identify the differences among individual group means. In all cases, p<0.05 was taken as the fiducial level for statistical significance.
3. Results
The meristic characters of the fishes studied for their swimming performance are listed in table 4. In Italy, there were no significant differences in meristics among any of the groups, whether caged at river sites or studied in the laboratory. In the UK, the chub studied in winter were from the same population as those studied in summer, so they had grown significantly larger. In The Netherlands, there were no meristic differences among the groups of cloned carp caged at each river site.
Table 4.
Biometrics of the chub (Leuciscus cephalus) and carp (Cyprinus carpio) used for the swimming experiments in Italy, the UK and The Netherlands. (Values are means±s.d. Condition factor is calculated as (mass length−3)×100. I, Italy; NL, The Netherlands. In Italy, ‘Laboratory’ refers to the La Casella Fluvial Hydrobiology Station; in UK, ‘Laboratory’ refers to the School of Biosciences, University of Birmingham, Birmingham.)
| species | country, river, site | month (temp, °C) | n | mass (g) | fork length (cm) | condition factor |
|---|---|---|---|---|---|---|
| L. cephalus | I, Lambro, Merone | May | 6 | 183±53 | 23.7±3.1 | 1.38±0.33 |
| September | 6 | 196±52 | 24.1±2.0 | 1.38±0.15 | ||
| February | 6 | 178±58 | 23.8±2.3 | 1.30±0.14 | ||
| I, Lambro, Brugherio | May | 6 | 194±48 | 24.3±4.7 | 1.36±0.41 | |
| September | 6 | 209±44 | 25.1±1.8 | 1.31±0.05 | ||
| February | 6 | 211±77 | 25.0±3.2 | 1.31±0.12 | ||
| I, Laboratory | May | 7 | 200±69 | 24.3±2.9 | 1.36±0.20 | |
| September | 7 | 198±61 | 23.7±4.2 | 1.35±0.19 | ||
| February | 7 | 188±58 | 24.1±2.2 | 1.30±0.22 | ||
| L. cephalus | UK, Blythe | June/July | 5 | 57±6 | 17.9±0.3 | 1.19±0.10 |
| February | 6 | 108±12 | 20.3±0.7 | 1.28±0.87 | ||
| UK, Cole | June/July | 6 | 62±7 | 17.7±0.9 | 1.11±0.03 | |
| February | 6 | 112±24 | 20.0±0.9 | 1.38±0.18 | ||
| UK, Tame | June/July | 5 | 69±7 | 16.9±0.6 | 1.20±0.11 | |
| February | 6 | 112±13 | 20.9±0.7 | 1.22±0.57 | ||
| UK, Laboratory | June/July | 6 | 67±15 | 17.7±1.1 | 1.21±0.10 | |
| February | 6 | 115±24 | 19.9±1.0 | 1.44±0.12 | ||
| Cyprinus carpio | NL, Amstel, Vrouwenakker | September | 6 | 178±37 | 19.3±2.3 | 2.55±0.79 |
| NL, Amstel, Amsterdam | September | 6 | 169±24 | 19.5±0.5 | 2.28±0.19 | |
| NL, Volgermeerpolder | September | 6 | 196±40 | 20.2±1.7 | 2.36±0.30 |
(a) Caging studies
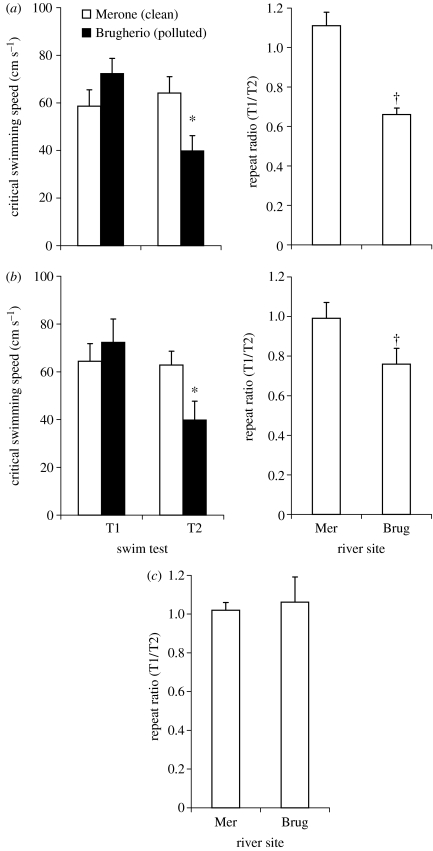
Italy. Figure 1a shows the swimming performance of chub in the clean site (Merone) and the polluted site (Brugherio), as measured in May 2001 at a water temperature of 17°C. At both sites, the fish swam equally well in T1. The fish from the clean site at Merone were able to repeat this performance in T2, but those from the polluted site at Brugherio were not, and exhibited a significant decline in their Ucrit. As a result, the fish from the clean site had a repeat ratio that was not significantly different from 1, whereas the fish from the polluted site had a significantly lower ratio of around 0.7 (figure 1a). Figure 1b shows the swimming performance of another set of chub, exposed at the same two sites in September 2001 at a water temperature of 20°C. The results obtained were very similar to those obtained in May. Thus, at both sites, the fish swam equally well in T1, but, while the fish from the clean site (Merone) were able to repeat this performance in T2, those from the polluted site (Brugherio) exhibited a significant decline in their Ucrit. As a result, the fish from the clean site had a repeat ratio that was not significantly different from 1, whereas the fish from the polluted site had a significantly lower ratio of around 0.7 (figure 1b). As shown in figure 1c, however, there was no significant difference in the ability of the animals from either site to repeat their exercise performance in February 2002, at a water temperature of 8°C.
Figure 1.
Swimming performance of chub (Leuciscus cephalus) following exposure in submerged cages at two sites on the river Lambro, Italy: a relatively clean site (Merone) or a polluted site (Brugherio). Results from (a) June 2001, (b) September 2001 and (c) January 2002. The graphs show mean (±s.e.m.) critical swimming speed (Ucrit) as measured twice, with the second swim test (T2) measured 40 min following fatigue in the first swim test (T1), and the corresponding repeat performance ratio (Ucrit T2/Ucrit T1). In all cases, n=6. Asterisk, significant difference between T1 and T2 for that river site in that campaign; dagger, significant difference between the two sites for the relevant variable in that campaign; Mer, Merone; Brug, Brugherio.
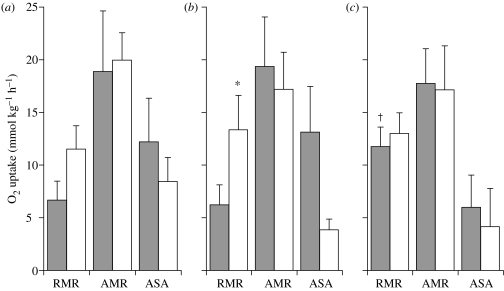
Figure 2a shows the respirometric measures taken during the swim tests in May 2001. Prior to T1, the RMR of chub from the polluted site (Brugherio) was significantly elevated relative to fish from the clean site (Merone). In both groups, T1 caused a significant increase in metabolic rate, with AMR and functional aerobic scope for activity being similar in both groups. The fish from the clean site exhibited a significant contribution of EPOC to their RMR prior to T2, but, nonetheless, they showed a similar AMR to that in T1 and, therefore, a functional aerobic scope in T2, which was similar to that measured in T1 (figure 2a). In the chub from the polluted site, there was no visible contribution of EPOC to their elevated RMR following T1, but they exhibited a significant reduction in AMR during T2, and thus in consequent functional aerobic scope for activity in T2 when compared with T1 (figure 2a). As shown in figure 2b, the respirometric measures collected in September 2001 were generally similar to those obtained in May, with the exception that RMR was not significantly different between fish at the clean site (Merone) and the polluted site (Brugherio); fish at the clean site showed no significant EPOC following T1, whereas those at the polluted site did. Nonetheless, the metabolic responses to T2 were similar to those seen in May, whereby the fish from the clean site were able to maintain AMR and functional aerobic scope unchanged from T1, but the fish from the polluted site showed a severe and significant decline in these variables (figure 2b). Unfortunately, respirometric analyses could not be collected in the campaign at low seasonal temperatures in February 2002, due to technical difficulties.
Figure 2.
Swimming respirometry of chub (Leuciscus cephalus) following exposure in submerged cages to two sites on the river Lambro, Italy: a relatively clean site (Merone) or a polluted site (Brugherio). Results from (a) June 2001 and (b) September 2001. The graphs show mean (±s.e.m.) RMR measured prior to the exercise challenge on fish swimming steadily at 20 cm s−1, the AMR achieved during the exercise challenge and the functional aerobic scope for activity (ASA) calculated as AMR–RMR. Each variable was measured twice, with the second swim test (T2, open columns) measured 40 min following fatigue in the first swim test (T1, filled columns). In all cases, n=6. Asterisk, significant difference between T1 and T2 for that river site in that campaign; dagger, significant difference between the two sites for the relevant variable in that campaign.
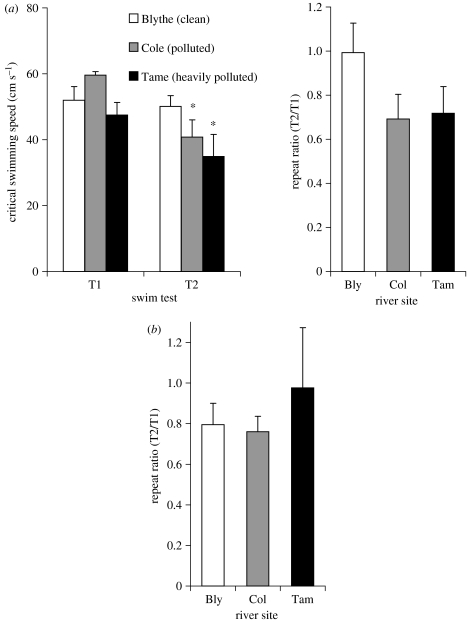
UK. Figure 3a shows the swimming performance of chub caged in the Blythe (clean), Cole (polluted) and Tame (heavily polluted), as measured in June/July 2002 in water at temperatures between 13 and 15°C. At all sites, the fish swam equally well in T1. The fish from the clean Blythe site were able to repeat this performance in T2, while those from the polluted Cole and heavily polluted Tame sites were not, and exhibited a significant decline in their Ucrit relative to T1. The fish from the Tame also had a significantly lower Ucrit in T2 than that measured in fish from the Blythe (figure 3a). As a result, the fish from the clean Blythe had a repeat ratio that was not significantly different from 1, whereas the fish from the polluted Cole and heavily polluted Tame had a significantly lower ratio of around 0.7 (figure 3a). Figure 3b also shows the repeat performance ratio of chub caged in the clean Blythe, polluted Cole and heavily polluted Tame, in February 2003, at water temperatures between 7 and 10°C. In this campaign, none of the groups exhibited a repeat ratio that differed significantly from unity.
Figure 3.
Swimming performance of chub (Leuciscus cephalus) following exposure in submerged cages in three confluent rivers in the UK: the Blythe (clean); the Cole (polluted); and the Tame (heavily polluted). Results from (a) June 2002 and (b) January 2003. The graphs show mean (±s.e.m.) critical swimming speed (Ucrit) as measured twice, with the second swim test (T2) measured 40 min following fatigue in the first swim test (T1), and the corresponding repeat performance ratio (Ucrit T2/Ucrit T1). In all cases, n=6. Asterisk, significant difference between T1 and T2 for that river site; Bly, Blythe; Col, Cole; Tam, Tame.
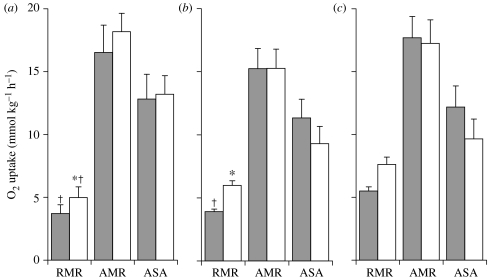
Figure 4 shows the respirometric measures taken during the campaign in the UK in June/July 2002. Prior to T1, the fish from the heavily polluted Tame exhibited elevated RMR relative to those from the clean Blythe. All fish, however, showed a statistically similar AMR and functional aerobic scope in T1 (although the mean value appears visibly lower in the Tame River, where there was much variability in exercise metabolism among the fish). Fish from the clean Blythe and polluted Cole exhibited a significant contribution of EPOC to their RMR prior to T2, but no contribution from EPOC was visible against the elevated RMR of fish from the heavily polluted Tame. In T2, chub from the Blythe achieved similar AMRs and functional aerobic scopes to those measured in T1. The fish from the Cole, however, showed significant declines in their functional scope in T2 relative to T1 (figure 4). In the campaign at low seasonal water temperatures in February 2003, all groups were able to maintain aerobic scope for activity unchanged between T1 and T2 (data not shown).
Figure 4.
Swimming respirometry of chub (Leuciscus cephalus) following exposure in submerged cages in three confluent rivers in the UK: (a) the Blythe (clean), (b) the Cole (polluted) and (c) the Tame (heavily polluted), in June 2002. The graphs show mean (±s.e.m.) RMR measured prior to the exercise challenge on fish swimming steadily at 20 cm s−1, the AMR achieved during the exercise challenge and the functional ASA calculated as AMR-RMR. Each variable was measured twice, with the second swim test (T2, open columns) measured 40 min following fatigue in the first swim test (T1, filled columns). In all cases, n=6. Asterisk, significant difference between T1 and T2 for that river site; dagger, significant difference between the Tame and the other two sites for the relevant variable.
The Netherlands. Figure 5 shows the exercise performance of carp caged at the clean Vrouwenakker site, polluted Amsterdam site and heavily polluted Volgermeerpolder sites in September 2002. There were no differences in performance between the carp caged in the sites with different pollution status; all animals exhibited a repeat performance ratio that was statistically identical to unity. Despite the absence of any differences in swimming performance between the groups, there were differences in their respiratory metabolism (figure 6). Prior to T1, the groups differed in their RMR, which was lower at the clean Vrouwenakker and polluted Amsterdam sites when compared with the severely polluted Volgermeerpolder site. All groups showed a contribution of EPOC to RMR prior to T2, but the carp at the Vrouwenakker site still had significantly lower RMR than those at the Volgermeerpolder site. Despite the apparent metabolic loading in the animals in the heavily polluted Volgermeerpolder site, there were no differences in AMR or functional aerobic scope for activity at any site, in either T1 or T2 (figure 6).
Figure 5.
Swimming performance of carp (Cyprinus carpio) following exposure in submerged cages to three sites on the river Amstel in The Netherlands: Vrouwenakker (relatively clean), Amsterdam (polluted) and Volgermeerpolder (heavily polluted), in September 2002. The graphs show mean (±s.e.m.) critical swimming speed (Ucrit) as measured twice, with the second swim test (T2) measured 40 min following fatigue in the first swim test (T1) and the corresponding repeat performance ratio (Ucrit T2/Ucrit T1). In all cases, n=6. Vro, Vrouwenakker; Ams, Amsterdam; Vol, Volgermeerpolder.
Figure 6.
Swimming respirometry of carp (Cyprinus carpio) following exposure in submerged cages to three sites on the river Amstel in The Netherlands: (a) Vrouwenakker (relatively clean), (b) Amsterdam (polluted) and (c) Volgermeerpolder (heavily polluted), in September 2002. The graphs show mean (±s.e.m.) RMR measured prior to the exercise challenge on fish swimming steadily at 20 cm s−1, the AMR achieved during the exercise challenge and the functional ASA calculated as AMR–RMR. Each variable was measured twice, with the second swim test (T2, filled columns) measured 40 min following fatigue in the first swim test (T1, open columns). In all cases, n=6. Asterisk, significant difference between T1 and T2 for that river site; dagger, significant difference from the Volgermeerpolder site for the relevant variable; Vro, Vrouwenakker; Ams, Amsterdam; Vol, Volgermeerpolder.
(b) Laboratory studies
Table 5 shows the repeat swimming performance of Italian chub acclimatized to different seasonal temperatures. Temperature had no significant effect upon Ucrit for either T1 or T2; the chub were able to repeat their swimming performance well with repeat performance ratios that did not differ significantly from unity (table 5).
Table 5.
Selected performance and metabolic variables in chub (Leuciscus cephalus) seasonally acclimatized to three different temperatures and maintained in clean biofiltered water, when exposed to a repeated swimming respirometry protocol (in all cases, n=7). (Please see text for further details. The presence of a common superscript for a given variable indicates no difference between temperatures. Asterisk, difference between swim test 1 and swim test 2 for that variable.)
| seasonal water temperature (°C) | |||
|---|---|---|---|
| 7 | 15 | 21 | |
| IMR (mmol O2 kg−1 h−1) | 1.32±0.24a | 2.37±0.26b | 3.40±0.19c |
| swim test 1 | |||
| Ucrit (cm s−1) | 70.6±7.9 | 62.4±3.8 | 59.8±5.0 |
| RMR (mmol O2 kg−1 h−1) | 2.20±0.32a | 4.06±0.45b | 5.74±0.32c |
| AMR (mmol O2 kg−1 h−1) | 7.33±0.56a | 10.76±1.69a,b | 14.73±1.19b |
| ASA (AMR-RMR) | 5.14±0.61 | 6.70±1.59 | 8.99±1.29 |
| swim test 2 | |||
| Ucrit (cm s−1) | 69.9±7.5 | 62.1±5.7 | 63.7±6.4 |
| repeat ratio (Ucrit T2/Ucrit T1) | 1.00±0.24 | 1.00±0.08 | 1.02±0.03 |
| RMR (mmol O2 kg−1 h−1) | 4.18±0.75a* | 5.13±0.36b | 7.68±1.13c* |
| AMR (mmol O2 kg−1 h−1) | 9.06±1.21a | 12.21±1.33a,b | 14.87±0.95b |
| ASA (AMR–RMR) | 4.88±0.91 | 7.08±1.25 | 7.19±1.21 |
The respirometric data derived from these exercise studies is also shown in table 5. IMR showed a significant effect of temperature, being lowest at 7°C, intermediate at 15°C and highest at 22°C. A similar trend was observed for RMR before both T1 and T2. The AMR measured in T1 was significantly lower at 7°C than at 22°C, with an intermediate value at 15°C (table 5). There was a significant increase in RMR prior to T2 at all temperatures, which is evidence of a contribution from EPOC. In all groups, however, AMR and functional aerobic scope in T2 were unchanged relative to T1. As seen in T1, the AMR measured in T2 was significantly lower at 7°C than at 22°C, with an intermediate value at 15°C (table 5). There was no effect of temperature upon absolute aerobic scope, factorial aerobic scope or functional aerobic scope for activity, which were statistically similar in all groups (table 5).
Laboratory studies on UK chub acclimatized to a summer temperature of 14°C revealed that fish had mean (±s.e.m.) Ucrits in T1 and T2 of 56±7 and 50±5 cm s−1, respectively, which were similar to those observed in fish caged in the clean Blythe sites (figure 3). The mean (±s.e.m.) repeat performance ratio was 0.92±0.06 (n=6), which was not statistically different from 1. The fish exhibited no change in functional aerobic scope in T2 relative to T1 (data not shown). Interestingly, comparison of their respirometry with that obtained in the UK summer caging campaign revealed that they had a mean RMR prior to T1 of 4.09±0.56 mmol O2 kg−1 h−1 (n=6), which was similar to that measured in fish caged in the unpolluted Blythe, but significantly lower than the RMR of fish caged in the heavily polluted Tame (figure 4). UK chub in winter showed the same performance pattern in the laboratory as had been observed in the conspecifics exposed in cages at the three river sites. In other words, the Ucrit values in T1 and T2 were statistically the same as those observed in the caged chub (data not shown) and the mean (±s.e.m.) repeat performance ratio was 0.85±0.14 (n=6). Although apparently rather low, this ratio was not statistically different from 1 and was not statistically different from the repeat ratios observed in the chub caged at the river sites. As observed in the caged animals, there were no statistical differences in the metabolic variables between T1 and T2 (data not shown).
4. Discussion
The caging studies demonstrated that measured values of the exercise performance and metabolic rate of fish vary with the recorded conditions in their sites of exposure, so that these indices of physiological performance can be used to demonstrate the sub-lethal effects of the complex mixtures of chemicals which prevail in polluted urban rivers. In both Italy and the UK, chub exposed to sites polluted with bioavailable heavy metals (primarily Zn, Ni and Cu) and organics (PAHs, PCBs and OCPs) exhibited impairments to their exercise physiology, revealed as a reduced ability to repeat their swimming performance in a standard Ucrit test. Such impairments were not observed in the carp exposed in The Netherlands to sites that were heavily polluted with bioavailable organics but not with heavy metals. In both chub and carp, however, there was evidence of metabolic disruption following exposure to polluted sites, with fish exhibiting elevated RMR. These results indicate that traits of performance such as Ucrit and the ability to repeat a swim test, and traits of metabolism such as RMR during sustained low-intensity aerobic exercise, can be employed as physiological biomarkers of sub-lethal aquatic pollution. The seasonal studies on chub revealed that the sub-lethal physiological effects of aquatic pollution were most obvious in fish acclimatized to warm spring/summer temperatures.
The swimming performance studies upon the chub provide strong support for the proposal of Jain et al. (1998) that a protocol of repeated exercise performance can provide more sensitive information about fish health and water quality than a standard single Ucrit test. Indeed, there were no differences in swimming performance and exercise metabolism for T1 at any of the sites. An impact of the bioavailable metals and organics at the polluted sites in Italy and the UK was only revealed by T2, where chub seasonally acclimatized to warm spring/summer temperatures showed an approximately 30% reduction in performance relative to their T1, leading to a repeat ratio of approximately 0.7. The fact that similar results were obtained over two successive campaigns in Italy, and that chub at the polluted sites in the UK showed qualitatively similar responses, is good circumstantial evidence to indicate that impaired performance was indeed due to the bioavailable pollution evident at the sampling sites. This supposition gains further support from the fact that, in each country, chub at the clean sites exhibited very similar Ucrits in T1 and T2 to those measured in ‘control’ fish acclimatized to similar seasonal temperatures, but maintained in clean biofiltered water in the laboratory. The absence of any effect of water quality upon exercise performance or exercise metabolism of chub during the winter campaigns may have been because the sub-lethal toxic effects of the pollutants at each site were less pronounced when physicochemical activities and fish metabolism were depressed by the low water temperatures (Butler et al. 1992; Beaumont et al. 1995b). Under these circumstances, chub at all sites exhibited similar performance to that observed in their respective control laboratory population. The absence of any effect of water quality upon exercise performance in the carp exposed to the sites on the river Amstel may reflect species differences in tolerance of sub-lethal pollution, or differences in the relative concentrations of particular pollutants between the Italian, UK and Dutch sites, as discussed later.
The respirometry data collected during the swim tests allow some general statements to be made about the proximate mechanisms underlying the impaired repeat performance in chub exposed to polluted river sites. In teleost fish, increased metabolic rate during sustained aerobic exercise is largely due to increased work, and consequent oxygen demand, by oxidative (red) skeletal muscle (Jones & Randall 1978; Taylor et al. 1996). This increased demand is met by increased oxygen uptake at the gills and increased cardiac work and oxygen convection in the blood (Jones & Randall 1978; Taylor et al. 1996). The effectiveness of these processes, muscle work and the allocation of oxygen to the muscles, will be reflected in the measurements of functional aerobic scope during the swim tests. The poor performance in T2 by chub at polluted sites was consistently linked to reduced aerobic scope which, in turn, was due to a combination of increased RMR prior to T2 and reduced AMR during exercise. Increased RMR prior to T2 was due to EPOC following T1, an impaired ability to recover from the metabolic costs incurred during the first swim test which would have compromised the allocation of further oxygen to the muscle to perform for T2. Reduced AMR in T2 could reflect compromised oxygen uptake at the gills. If, however, gas transfer at the gills were significantly compromised by the sub-lethal effects of pollution, this would have caused measurable reductions in AMR, aerobic scope and swimming performance in the first swim test (e.g. Nikl & Farrell 1993; Pane et al. 2004). The reduced AMR during T2 could, therefore, reflect impaired red muscle function and/or an impaired ability to deliver oxygen, perhaps due to impaired cardiac function.
Extensive laboratory tests would be required to identify which toxicant, or combination of toxicants, may ultimately have been responsible for the decline in aerobic scope in T2 and the consequent impaired repeat swimming performance in the chub. Ammonia causes significant impairments to Ucrit in salmonids (Shingles et al. 2001; Wicks et al. 2002; McKenzie et al. 2003a), but ammonia levels were generally low at all sites. A number of metals have been shown to impair the Ucrit of salmonids, including copper (Waiwood & Beamish 1978; Beaumont et al. 1995a, 2003), zinc (Alsop et al. 1999) and nickel (Pane et al. 2004), which all exhibited elevated bioavailable levels at the polluted Italian and UK sites. In brown trout, Salmo trutta, copper impairs performance by interfering with ammonia excretion, causing plasma ammonia accumulation and a consequent depolarization of white muscle (Beaumont et al. 2000a,b), which compromises white muscle recruitment and so swimming performance at the highest speeds (Shingles 2002; Beaumont et al. 2003). Zinc may have a similar mode of action (Alsop et al. 1999). Nickel, on the other hand, appears to act by damaging the gills (Pane et al. 2004) and, although this did lead to a reduced AMR during a standard Ucrit test in rainbow trout, Oncorhynchus mykiss (Pane et al. 2004), any such gill damage in the chub in the present study should have impaired exercise performance in T1, which was not the case.
Among the bioavailable organic chemicals found at the polluted sites, various PCBs had no impact upon the Ucrit of rainbow trout (Brauner et al. 1994) and, although pentachlorophenol, an OCP, caused increased metabolic rate in sockeye salmon, Oncorhynchus nerka, this did not affect their capacity to repeat Ucrit with a repeat ratio of unity (Farrell et al. 1998). The potential effects of PAHs upon performance have not been assessed, but it seems unlikely that they would affect performance except at acutely toxic concentrations (Farrell et al. 2004). Thus, the impaired repeat performance of the chub at the polluted sites may have been a result of the combined action of bioavailable heavy metals. The absence of any impairment to the exercise performance of carp exposed to the polluted sites on the river Amstel may therefore have been due to the absence of significant bioavailable metals. It is also possible, however, that the impaired performance in the chub was a result of a generalized stress response to all of the combined pollutants in their environment (Schreck 1990; Wendelaar Bonga 1997), and that the carp were simply more tolerant.
An impact of pollution was however visible in both species, as an increase in metabolic rate prior to T1, during low-intensity sustained aerobic exercise at a water speed of 20 cm s−1 (defined as RMR for the purposes of the current study). The advantage of comparing the groups when they were exercising at a common sustained level, is that it avoids confounding effects that can derive from variability in spontaneous activity, and consequent metabolic rate, among individuals (Schreck 1990). Therefore, the differences in RMR can be ascribed to an impact of the environment upon the respiratory metabolism of the fish. The elevated RMR that was measured in the chub and carp exposed to polluted sites is unlikely to have derived from higher metabolic costs for the low-level sustained exercise per se, because any such increased costs of locomotion should have caused the fish to reach their maximum capacity for oxygen uptake (AMR) at a lower swimming speed, and so have been visible as a decline in swimming performance in a single Ucrit test. It might also be argued that, in Italy, fish were taken from an environment to which they had previously adapted (the clean site) and were exposed to a novel environment (the polluted site) and this in itself might have led to increased metabolic rate (Nelson et al. 1996). However, the chub and carp from the UK and The Netherlands, respectively, were derived from captive, cultured, populations and they also exhibited increased metabolic rate when exposed to polluted sites. Thus, it seems reasonable to suggest that the increased RMR was in fact a metabolic load imposed upon fish exposed to polluted sites.
Controlled laboratory exposures of salmonids to sub-lethal concentrations of Zn, Cu or Ni have not reported any effects upon metabolic rate under ‘routine’ conditions (McGeer et al. 1999; Beaumont et al. 2003; Pane et al. 2004). The most elevated RMR was observed in carp in The Netherlands, where there were very low levels of bioavailable metal pollution. Exposure of rainbow trout to various PCBs had no effect on their RMR (Yang & Randall 1997). Organochlorine pesticides such as pentachlorophenol are metabolic poisons that cause mitochondrial uncoupling. Exposure to pentachlorophenol leads to profound increases in routine oxygen consumption in the American eel, Anguilla rostrata (Holmberg & Saunders 1975), and in sockeye salmon (Farrell et al. 1998). All of the polluted sites at which fish exhibited elevated RMR also had significant bioavailable OCP levels, particularly the Volgermeerpolder site in The Netherlands (Verweij et al. 2004; Winter et al. 2005; E. Garofalo & S. Ceradini 2003, unpublished observations). There is also a body of evidence to suggest that elevated metabolic rates in fish can be an indicator of chronic non-specific stress (Schreck 1990; Wendelaar Bonga 1997).
Whatever the mechanisms by which exposure to polluted river sites caused a reduced ability to repeat strenuous exercise and/or elevated RMRs, these traits do seem to offer some potential as physiological biomarkers of sub-lethal toxic stress. Stegeman et al. (2002) and Van der Oost et al. (2003) have proposed a set of six criteria by which to evaluate the strengths or weaknesses of a biomarker:
The assay to quantify the biomarker should be reliable (with quality assurance), relatively cheap and easy to perform. Both exercise performance and related respiratory metabolism can be measured reliably, and laboratory studies can provide expected values for specific traits, for quality assurance. In particular, the repeat performance ratio is expected to be unity in healthy unstressed fish. Modern swim-tunnel respirometers, such as those used in the present study, are relatively easy to operate by suitably trained personnel and standardized models are now available commercially. One important aspect of quality assurance would be training in the handling of live animals for in vivo experiments. Specific protocols (such as those described in this paper) should be established to ensure that animals are stressed as little as possible by the experimenter. After the initial outlay in equipment and training, the main running cost will be the personnel to perform the protocol. This may differ from biochemical and molecular markers, where the costs of consumable materials for the analyses must also be considered.
The biomarker response should be sensitive to pollution exposure and/or effects in order to serve as an early warning parameter. Laboratory studies have provided extensive evidence that traits such as swimming ability and metabolic rate can be sensitive to specific pollutants and also to environmental stressors (see citations in this article). There is evidence that wild fish populations can recover from sub-lethal toxicological impacts upon performance and metabolism (e.g. Taylor et al. 2004), and hence these biomarkers can serve as an early warning for more severe toxicological impacts. The ability to repeat exercise performance may be a particularly sensitive sub-lethal marker.
Baseline data for the biomarker should be well defined in order to distinguish natural variability (noise) and contaminant-induced stress (signal). These requirements can be met by appropriate studies of annual variation in any of the traits (e.g. as related to breeding cycles), and comparison of field data with parallel laboratory studies upon seasonally acclimated animals in clean water. These studies may be rather time consuming because handling of live animals prior to experimentation can also introduce ‘noise’, and so relatively extended recovery periods (24 h) may be required prior to experimentation on any single fish. These caveats may however also hold for many biochemical and molecular markers.
The impact of confounding factors on the biomarker response should be well established. Once again, this criterion can be met by appropriate laboratory experiments in parallel to field studies. The advantage of the repeat performance ratio as a biomarker is that it provides a single number which should be independent of major confounding factors such as fish size or water temperature.
The underlying mechanism for the relationship between pollutant exposure (dosage, time) and biomarker response should be established. This criterion is more difficult to meet for complex physiological traits, in particular, because some effects may be engendered by an overall state of non-specific stress (Schreck 1990; Wendelaar Bonga 1997).
The toxicological significance of the biomarker, e.g. the relationships between its response and the (long-term) impact on the organism, should be established. This criterion may be the most difficult to meet for all biomarkers. Physiological traits have the distinct advantage, however, that they are more direct indicators of higher-order consequences than biochemical markers (Van der Oost et al. 2003), and can provide insights into why fish may fail to colonize specific areas. For example, a reduced ability to repeat exercise performance may have a particular impact during river spates, or for species that undergo upstream spawning migrations. Increased metabolic costs associated with occupying a polluted habitat can be expected to compromise allocation of energy towards growth and reproduction (Rice 1990).
Thus, the physiological traits appear to meet criteria (i)–(iv) as satisfactorily as molecular and biochemical biomarkers (Van der Oost et al. 2003). With regard to criterion (v), physiological traits are perhaps weaker than some molecular/biochemical biomarkers, because the physiological markers may potentially be engendered by chronic non-specific stress rather than by a defined pollutant. Nonetheless, the most well established of the biomarkers for organic pollutants, the so-called ‘phase 1 enzymes’, mixed-function oxidases that metabolize hydrophobic organics, have been shown to be modulated by members of all the major organic classes: PAHs, PCBs and OCPs (Van der Oost et al. 2003). Exercise performance is therefore not any less specific, as it is known to be unaffected by PCBs (Brauner et al. 1994) but influenced by metals and some specific xenobiotics (see previous citations in the text). Similar arguments can be made for metabolic rate. With regard to criterion (vi), however, the physiological traits are more valuable than suborganismal biomarkers. In other words, even for the most well established of the suborganismal biomarkers, the exact significance of the response for the functional integrity of the organism still requires validation (Van der Oost et al. 2003) and, therefore, these do not yet provide any insight into why fish might fail to colonize polluted areas.
The same (or similar) caging experiments at the UK and Dutch river sites were also used to provide samples for measurements of body burdens of pollutants, and for the analysis of some tissue and body fluid biomarkers. In both countries, contamination with bioavailable PAHs, PCBs and OCPs was statistically linked to increased levels of these contaminants in fish tissues and increased bile metabolites of PAHs (Verweij et al. 2004; Winter et al. 2005). Interestingly, it has been shown that uptake of lipophilic organic toxicants is directly related to rates of oxygen uptake in fishes, because these latter determine the volume of pollutant-laden water that is presented to the gill epithelium (Brauner et al. 1994; Randall et al. 1998; Yang et al. 2000). Thus, the increased rates of routine metabolism in the fish at polluted sites can be expected to exacerbate body burdens of organic pollutants. The accumulation of organics in the tissues of fish caged in the UK and The Netherlands was linked to increased hepatic CYP1A activity (as ethoxyresorufin-O-deethylase, or EROD activity; Winter et al. 2005; Verweij & van der Oost 2004, unpublished observations). In UK rivers, there was also evidence of genotoxic impacts at the polluted Cole and Tame sites, measured as DNA strand breaks and adducts, presumably as a consequence of the organic pollution (Winter et al. 2004). The site with the highest bioavailable metal levels, among the three countries, was the Tame river in the UK (Garofalo et al. 2004), but this did not coincide with a significant elevation of metallothionein in the gills or liver of chub from the Tame site when compared to those from the moderately polluted Cole or clean Blythe sites (Hayes et al. 2004). Thus, the physiological biomarkers appear to be as reliable as the ‘classical’ biomarkers of organic pollution (EROD and bile PAH metabolites) and genotoxic indicators (DNA damage), and more reliable than metallothionein, at least with respect to those fish analysed from the UK sampling sites.
Work on these cyprinid fishes is of particular relevance when studying urban rivers as they are typically lowland species characterizing the ‘cyprinid reach’, where water flows are relatively slow and temperatures and oxygen levels are often variable, even in the absence of pollution. Both species performed sustained exercise well, and exhibited similar large increases in metabolic rate as they swam to fatigue. The current study is the first report of repeated exercise performance in cyprinids and any fish other than salmonids. It is interesting that their relative critical swimming speeds and their capacity to repeat the Ucrit test were similar to those of migratory salmonids (Farrell et al. 1998; Jain et al. 1998), which are considered to represent excellent fish ‘athletes’ (Brett 1964; Beamish 1978; Farrell et al. 1998). The absence of any effects of seasonal temperature acclimation upon exercise performance in the chub is similar to the results obtained in the brown trout (Butler et al. 1992; Day & Butler 2005), but differs from many species such as the sockeye salmon (Brett & Glass 1973), the rainbow trout (Taylor et al. 1996) and the smallmouth buffalo, Ictiobus bubalus (Adams & Parson 1998), where clear seasonal thermal optima exist. The absence of any effect of seasonal temperature on repeat performance in the chub also differs from the results obtained in rainbow trout, where a seasonal increase in temperature, from 8 to 15°C, caused a significant increase in Ucrit 1 but a significant decline in the ability to repeat performance in Ucrit 2 (Jain & Farrell 2003). Taken together, these results indicate that the chub is a good model species for the investigation of the physiological biomarkers of aquatic pollution. Unfortunately, the chub is not common in all aquatic habitats in Europe, being rare in the slow-flowing lower reaches of the river Amstel (F. Verweij & R. van der Oost 2004, unpublished observations).
In conclusion, the results indicate that physiological traits of performance and metabolism offer potential as physiological biomarkers of sub-lethal aquatic pollution. Traits such as the ability to repeat Ucrit swim tests, and RMR, meet many of the criteria required of biomarkers (Stegeman et al. 2002; Van der Oost et al. 2003). While they may be less specific than biochemical or molecular biomarkers in terms of revealing particular types of pollution, they are reliable integrated measures of the responses of many physiological systems, and so can provide insight into why fish fail to colonize some polluted habitats. The physiological biomarkers may therefore be particularly useful within programmes of ecological risk assessment which also comprise analyses of a suite of other biochemical and molecular markers. Further studies are required to investigate potential model species for different aquatic habitat types and confirm whether an absence of any impairment to swimming performance in carp exposed to polluted sites on the river Amstel was due to an absence of bioavailable heavy metals, or to a species-specific tolerance of abiotic stresses.
Acknowledgments
We are particularly indebted to the late Ted Bentham-Evans, a member of the mechanical workshop team in Birmingham and to his team leader, the late Chris Hardeman, who together designed and built the swimming respirometers to our specifications. Our work, using this equipment, is dedicated to their memories and to that of our friend and colleague, the late Frank Verweij. We are grateful to Bassiano Stoppelli for his expertise in organizing the field studies and to Alan Gardner and Peter Hall for animal care at the University of Birmingham. This research was funded by the European Union (CITYFISH project EVK1-CT-1999-00009).
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Adams S.R, Parsons G.R. Laboratory-based measurements of swimming performance and related metabolic rates of field-sampled smallmouth buffalo (Ictiobus bubalus): a study of seasonal changes. Physiol. Zool. 1998;71:350–358. doi: 10.1086/515419. [DOI] [PubMed] [Google Scholar]
- Alsop D.H, McGeer J.C, McDonald D.G, Wood C.M. Costs of chronic waterborne zinc exposure and the consequences of zinc acclimation on the gill/zinc interactions of rainbow trout in hard and soft water. Environ. Toxicol. Chem. 1999;18:1014–1025. doi:10.1897/1551-5028(1999)018<1014:COCWZE>2.3.CO;2 [Google Scholar]
- Beamish F.W.H. Swimming capacity. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. VII. Academic Press; New York, NY: 1978. pp. 101–187. [Google Scholar]
- Beaumont M.W, Butler P.J, Taylor E.W. Exposure of brown trout, Salmo trutta, to sub-lethal copper concentrations in soft acidic water and its effects upon sustained swimming performance. Aquat. Toxicol. 1995a;33:45–63. doi: 10.1016/s0166-445x(00)00109-0. doi:10.1016/0166-445X(95)00007-Q [DOI] [PubMed] [Google Scholar]
- Beaumont M.W, Butler P.J, Taylor E.W. Plasma ammonia concentration in brown trout (Salmo trutta) exposed to acidic water and sub-lethal copper concentrations and its relationship to decreased swimming performance. J. Exp. Biol. 1995b;198:2213–2220. doi: 10.1242/jeb.198.10.2213. [DOI] [PubMed] [Google Scholar]
- Beaumont, M. W., Butler, P. J. & Taylor, E. W. 2000a Tissue ammonia levels and swimming performance of brown trout exposed to copper in soft, acidic water. In Fish physiology, fish toxicology and fisheries management (ed. R. V. Thurston), pp. 51–68. Athens, GA: United States Environmental Protection Agency.
- Beaumont M.W, Butler P.J, Taylor E.W. Exposure of brown trout, Salmo trutta, to a sub-lethal concentration of copper in soft acidic water: effects upon muscle metabolism and membrane potential. Aquat. Toxicol. 2000b;51:259–272. doi: 10.1016/s0166-445x(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Beaumont M.W, Butler P.J, Taylor E.W. Exposure of brown trout, Salmo trutta, to a sub-lethal concentration of copper in soft acidic water: effects upon gas exchange and ammonia accumulation. J. Exp. Biol. 2003;206:153–162. doi: 10.1242/jeb.00060. doi:10.1242/jeb.00060 [DOI] [PubMed] [Google Scholar]
- Beavan L, Sadler J, Pinder C. The invertebrate fauna of a physically modified urban river. Hydrobiologia. 2001;445:97–108. doi:10.1023/A:1017584105641 [Google Scholar]
- Bell W.H, Terhune L.D.B. Water tunnel design for fisheries research. Fish. Res. Board Can. Tech. Rep. 1970;195:1–169. [Google Scholar]
- Box J.D, Walker G.J. Conservation of the river Blythe, a high quality river in a major urban area in England. Aquat. Conserv. 1994;4:75–85. doi:10.1002/aqc.3270040107 [Google Scholar]
- Brauner C.J, Randall D.J, Neuman J.F, Thurston R.V. The effect of exposure to 1,2,4,5 tetrachlorobenzene and the relationship between toxicant and oxygen uptake in rainbow trout (Oncorhynchus mykiss) and cutthroat trout (Oncorhynchus clarkii) during exercise. Environ. Toxicol. Chem. 1994;13:1813–1820. [Google Scholar]
- Brett J.R. Implications and assessments of environmental stress. In: Larkin P.A, editor. The investigation of fish-power problems. Institute of Fisheries; University of BC, Canada: 1958. pp. 69–93. [Google Scholar]
- Brett J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964;21:1183–1226. [Google Scholar]
- Brett J.R, Glass N.R. Metabolic rates and critical swimming speeds of sockeye salmon (Oncorhynchus nerka) in relation to size and temperature. J. Fish. Res. Board Can. 1973;30:379–387. [Google Scholar]
- Butler P.J, Day N, Namba K. Interactive effects of seasonal temperature and low pH on resting oxygen uptake and swimming performance of adult brown trout Salmo trutta. J. Exp. Biol. 1992;165:195–212. [Google Scholar]
- Cairns J. Don't be half-safe—the current revolution in bioassay techniques. Eng. Bull. Purdue Univ. Proc. 1966;21:559–567. [Google Scholar]
- Day N, Butler P.J. The effects of acclimation to reverse seasonal temperatures on the swimming performance of adult brown trout Salmo trutta. J. Exp. Biol. 2005;208:2683–2692. doi: 10.1242/jeb.01669. doi:10.1242/jeb.01669 [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Gamperl A.K, Birtwell I.K. Prolonged swimming, recovery and repeat swimming performance of mature sockeye salmon Oncorhynchus nerka exposed to moderate hypoxia and pentachlorophenol. J. Exp. Biol. 1998;201:2183–2193. doi: 10.1242/jeb.201.14.2183. [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Kennedy C.J, Kolok A. Effects of wastewater from oil refining operations on survival, haematology, gill histology and swimming performance of fathead minnows. Can. J. Zool. 2004;82:1519–1527. doi:10.1139/z04-128 [Google Scholar]
- Fry F.E.J. The effects of the environment on animal activity. Univ. Toronto Stud. Biol. Ser. 1947;55:1–62. [Google Scholar]
- Fry F.E.J. The effect of environmental factors on the physiology of fish. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. VI. Academic Press; New York, NY: 1971. pp. 1–98. [Google Scholar]
- Garofalo E, Ceradini S, Winter M.J. The use of diffusive gradients in thin-film (DGT) passive samplers for the measurement of bioavailable metals in river water. Ann. Chim. 2004;94:515–520. doi: 10.1002/adic.200490065. doi:10.1002/adic.200490065 [DOI] [PubMed] [Google Scholar]
- Harkness N. The river Tame—a short history of water pollution and control within an industrial river basin. Water Sci. Technol. 1982;14:153–165. [Google Scholar]
- Hayes R.A, Regondi S, Winter M.J, Butler P.J, Agradi E, Taylor E.W, Chipman J.K. Cloning of a chub metallothionein gene and development of competitive RT-PCR of chub metallothionein mRNA as a potential biomarker of heavy metal exposure. Mar. Environ. Res. 2004;58:665–669. doi: 10.1016/j.marenvres.2004.03.059. doi:10.1016/j.marenvres.2004.03.059 [DOI] [PubMed] [Google Scholar]
- Holmberg B, Saunders R.L. The effects of pentachlorophenol on swimming performance and oxygen consumption in the American eel (Anguilla rostrata) Rapp. P.-v. Reun. Cons. Int. Explor. Mer. 1975;174:144–149. [Google Scholar]
- Hopkins W.A, Snodgrass J.W, Staub B.P, Jackson B.P, Congdon J.D. Altered swimming performance of a benthic fish (Erimyzon sucetta) exposed to contaminated sediments. Arch. Environ. Contam. Toxicol. 2003;44:383–389. doi: 10.1007/s00244-002-2030-5. doi:10.1007/s00244-002-2030-5 [DOI] [PubMed] [Google Scholar]
- Howard T.E. Swimming performance of juvenile coho salmon (Oncorhynchus kisutch) exposed to bleached kraft pulpmill effluent. J. Fish. Res. Board Can. 1975;32:789–793. [Google Scholar]
- Jain K.E, Farrell A.P. Influence of seasonal temperatures on the repeat swimming performance of rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 2003;206:3569–3579. doi: 10.1242/jeb.00588. doi:10.1242/jeb.00588 [DOI] [PubMed] [Google Scholar]
- Jain K.E, Birtwell I.K, Farrell A.P. Repeat swimming performance of mature sockeye salmon following a brief recovery period: a proposed measure of fish health and water quality. Can. J. Zool. 1998;76:1488–1496. doi:10.1139/cjz-76-8-1488 [Google Scholar]
- Janz D.M, Farrell A.P, Morgan J.D, Vigers G.A. Acute physiological stress responses of juvenile coho salmon (Oncorhynchus kisutch) to sub-lethal concentrations of Garlon-4, Garlon-3A and Vision herbicides. Environ. Toxicol. Chem. 1991;10:81–90. [Google Scholar]
- Johansen J.A, Geen G.H. Sublethal and acute toxicity of the ethylene glycol butyl ether ester formulation of triclopyr to juvenile coho salmon (Oncorhynchus kisutch) Arch. Environ. Contam. Toxicol. 1990;19:610–616. doi: 10.1007/BF01059083. doi:10.1007/BF01059083 [DOI] [PubMed] [Google Scholar]
- Jones D.R, Randall D.J. The respiratory and circulatory systems during exercise. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. VII. Academic Press; New York, NY: 1978. pp. 425–501. [Google Scholar]
- MacKinnon D.L, Farrell A.P. The effects of 2(thiocyanomethylthio)benzothiazole on juvenile coho salmon (Oncorhynchus kisutch)—sublethal toxicity testing. Environ. Toxicol. Chem. 1992;11:1541–1548. [Google Scholar]
- McGeer J.C, Szebedinszky C, McDonald D.G, Wood C.M. Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout. I. Iono-regulatory disturbance and metabolic costs. Aquat. Toxicol. 1999;50:231–243. doi: 10.1016/s0166-445x(99)00105-8. doi:10.1016/S0166-445X(99)00105-8 [DOI] [PubMed] [Google Scholar]
- McKenzie D.J, Cataldi E, Owen S, Taylor E.W, Bronzi P. Effects of acclimation to brackish water on the growth, respiratory metabolism and exercise performance of Adriatic sturgeon (Acipenser naccarii) Can. J. Fish. Aquat. Sci. 2001;58:1104–1112. doi:10.1139/cjfas-58-6-1104 [Google Scholar]
- McKenzie D.J, Shingles A, Taylor E.W. Sub-lethal plasma ammonia accumulation and the swimming performance of salmonids. Comp. Biochem. Physiol. 2003a;135:515–526. doi: 10.1016/s1095-6433(03)00116-8. doi:10.1016/S1095-6433(03)00116-8 [DOI] [PubMed] [Google Scholar]
- McKenzie D.J, Martinez R, Morales A, Acosta J, Morales R, Taylor E.W, Steffensen J.F, Estrada M.P. Effects of growth hormone transgenesis on metabolic rate, exercise performance and hypoxia tolerance in tilapia hybrids. J. Fish Biol. 2003b;63:398–409. doi:10.1046/j.1095-8649.2003.00162.x [Google Scholar]
- McLeay D.J, Brown D.A. Stress and chronic effects of untreated and treated bleached kraft pulpmill effluent on the biochemistry and stamina of juvenile coho salmon (Oncorhynchus kisutch) J. Fish. Res. Board Can. 1979;36:1049–1059. [Google Scholar]
- Moyes C.D, West T.G. Exercise metabolism of fish. In: Hochachka P.W, Mommsen T.P, editors. Biochemistry and molecular biology of fishes. vol. 4. Elsevier Science; Amsterdam, The Netherlands: 1995. pp. 367–392. [Google Scholar]
- Nelson J.A, Tang Y, Boutilier R.G. The effects of salinity change on the exercise performance of two Atlantic cod (Gadus morhua) populations inhabiting different environments. J. Exp. Biol. 1996;199:1295–1309. doi: 10.1242/jeb.199.6.1295. [DOI] [PubMed] [Google Scholar]
- Nikl D.L, Farrell A.P. Reduced swimming performance and gill structural changes in juvenile salmonids exposed to 2-(thiocyanomethylthio)benzothiazole. Aquat. Toxicol. 1993;27:245–263. doi:10.1016/0166-445X(93)90057-8 [Google Scholar]
- Pane E.F, Haque A, Goss G.G, Wood C.M. The physiological consequences of exposure to chronic, sub-lethal waterborne nickel in rainbow trout (Oncorhynchus mykiss): exercise vs resting physiology. J. Exp. Biol. 2004;207:1249–1261. doi: 10.1242/jeb.00871. doi:10.1242/jeb.00871 [DOI] [PubMed] [Google Scholar]
- Peakall D.W. Biomarkers: the way forward in environmental assessment. Toxicol. Ecotoxicol. News. 1994;1:55–60. [Google Scholar]
- Petersen R.H. Influence of fenitrothion on swimming velocities of brook trout (Salvelinus fontinalis) J. Fish. Res. Board Can. 1974;31:1757–1762. [Google Scholar]
- Prosser C.L. 1st edn. W. B. Saunders; Philadelphia, PA; London, UK: 1950. Comparative animal physiology. [Google Scholar]
- Randall D.J. The control of respiration and circulation in fish during exercise and hypoxia. J. Exp. Biol. 1982;100:175–188. [Google Scholar]
- Randall D.J, Brauner C. Effects of environmental factors on exercise in fish. J. Exp. Biol. 1991;160:113–126. [Google Scholar]
- Randall D.J, Connell D.W, Yang R, Wu S.S. Concentrations of persistent lipophilic compounds in fish are determined by exchange across the gills, not through the food chain. Chemosphere. 1998;37:1263–1270. doi: 10.1016/s0045-6535(98)00124-6. doi:10.1016/S0045-6535(98)00124-6 [DOI] [PubMed] [Google Scholar]
- Rice J.A. Bioenergetics modelling approaches to evaluation of stress in fishes. Am. Fish. Soc. Symp. 1990;8:90–92. [Google Scholar]
- Rodgers D.W, Beamish F.W.H. Uptake of waterborne methylmercury by rainbow trout (Salmo gairdneri) in relation to oxygen consumption and methylmercury concentration. Can. J. Fish. Aquat. Sci. 1981;38:1309–1315. [Google Scholar]
- Schreck C.B. Physiological, behavioural and performance indicators of stress. Am. Fish. Soc. Symp. 1990;8:29–37. [Google Scholar]
- Shingles, A. 2002 The effects of some environmental pollutants on ammonia excretion and swimming performance of trout. Ph.D. thesis, University of Birmingham, Birmingham, UK.
- Shingles A, McKenzie D.J, Taylor E.W, Moretti A, Butler P.J, Ceradini S. Effects of sub-lethal ammonia exposure on swimming performance in rainbow trout (Oncorhynchus mykiss) J. Exp. Biol. 2001;204:2699–2707. doi: 10.1242/jeb.204.15.2691. [DOI] [PubMed] [Google Scholar]
- Sprague J.B. Measurement of pollutant toxicity to fish. III. Sublethal effects and ‘safe’ concentrations. Water Res. 1971;5:245–266. doi:10.1016/0043-1354(71)90171-0 [Google Scholar]
- Steffensen J.F. Some errors in the respirometry of water breathers: how to avoid and correct for them. Fish Physiol. Biochem. 1989;6:49–59. doi: 10.1007/BF02995809. [DOI] [PubMed] [Google Scholar]
- Steffensen J.F, Johansen K, Bushnell P.G. An automated swimming respirometer. Comp. Biochem. Physiol. A. 1984;79:437–440. doi:10.1016/0300-9629(84)90541-3 [Google Scholar]
- Stegeman J.J, Rento K.W, Woodin B.R, Zhang Y.-S, Addison R.F. Molecular responses to environmental contamination: enzyme and protein systems as indicators of chemical exposure and effect. In: Hugget R.J, Kimerly R.A, Mehrle M, Bergman H.L, editors. Biomarkers: biochemical, physiological and histological markers of anthropogenic stress. Lewis Publishers; Chelsea, MI: 2002. pp. 235–265. [Google Scholar]
- Taylor S.E, Egginton S, Taylor E.W. Seasonal temperature acclimatisation of rainbow trout: cardiovascular and morphometric influences on maximum sustainable exercise level. J. Exp. Biol. 1996;199:835–845. doi: 10.1242/jeb.199.4.835. [DOI] [PubMed] [Google Scholar]
- Taylor L.N, McFarlane W.J, Pyle G.G, Couture P, McDonald D.G. Use of performance indicators in evaluating chronic metal exposure in wild yellow perch (Perca flavescens) Aquat. Toxicol. 2004;67:371–385. doi: 10.1016/j.aquatox.2004.01.018. doi:10.1016/j.aquatox.2004.01.018 [DOI] [PubMed] [Google Scholar]
- Van der Oost R, Lopes S.S.C, Komen H, Satumalay K, van den Bos R, Heida H, Vermeulen N.P.E. Assessment of environmental quality and inland water pollution using biomarker responses in caged carp (Cyprinus carpio): use of a bioactivation:detoxican ratio as a biotransformation index (BTI) Mar. Environ. Res. 1998;46:315–319. doi:10.1016/S0141-1136(97)00096-2 [Google Scholar]
- Van der Oost R, Beyer J, Vermeulen N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. doi:10.1016/S1382-6689(02)00126-6 [DOI] [PubMed] [Google Scholar]
- Verweij F, Booij K, Satumalay K, van der Molen N, van der Oost R. Assessment of bioavailable PAH, PCB and OCP concentrations in water, using semipermeable membrane devices (SPMDs), sediments and caged carp. Chemosphere. 2004;54:1675–1689. doi: 10.1016/j.chemosphere.2003.10.002. doi:10.1016/j.chemosphere.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Waiwood K.G, Beamish F.W.H. Effects of copper, pH and hardness on the critical swimming performance of rainbow trout (Salmo gairdneri Richardson) Water Res. 1978;12:611–619. doi:10.1016/0043-1354(78)90141-0 [Google Scholar]
- Webster P, West J.R, Gurnell A.M, Petts G.E, Sadler J.P, Forster C.F. Development, flood risk and the urban environment: experiences from the river Tame. J. Chart. Inst. Water. Environ. Manage. 2001;15:167–173. [Google Scholar]
- Wendelaar Bonga S.E. The stress response in fish. Physiol. Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- Wicks B.J, Joensen R, Tang Q, Randall D.J. Swimming and ammonia toxicity in salmonids: the effect of sub-lethal exposure on the swimming performance of coho salmon and the acute toxicity of ammonia in swimming and resting rainbow trout. Aquat. Toxicol. 2002;59:55–69. doi: 10.1016/s0166-445x(01)00236-3. doi:10.1016/S0166-445X(01)00236-3 [DOI] [PubMed] [Google Scholar]
- Wikelski M, Ricklefs R.E. The physiology of life histories. Trends Ecol. Evol. 2001;16:479–481. doi:10.1016/S0169-5347(01)02279-0 [Google Scholar]
- Williams P. The Environment Agency; Bristol, UK: 2000. River rehabilitation, practical aspects from 16 case studies—River Cole, project Kingfisher; p. 8. [Google Scholar]
- Wilson R.W, Bergman H.L, Wood C.M. Metabolic costs and physiological consequences of acclimation to aluminium in juvenile rainbow trout (Oncorhynchus mykiss). 2. Gill morphology, swimming performance, and aerobic scope. Can. J. Fish. Aquat. Sci. 1994;51:527–535. [Google Scholar]
- Winter M.J, Day N, Hayes R.A, Butler P.J, Taylor E.W, Chipman J.K. DNA strand breaks and adducts determined in feral and caged chub (Leuciscus cephalus) exposed to rivers exhibiting variable water quality around Birmingham, UK. Mutat. Res. 2004;552:163–175. doi: 10.1016/j.mrfmmm.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Winter M.J, et al. Tissue levels and biomarkers of organic contaminants in feral and caged chub (Leuciscus cephalus) from rivers in the West Midlands, UK. Aquat. Toxicol. 2005;73:394–405. doi: 10.1016/j.aquatox.2005.05.001. doi:10.1016/j.aquatox.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Wood A.W, Johnston B.D, Farrell A.P, Kennedy C.J. Effects of didecyldimethylammonium chloride (DDAC) on the swimming performance, gill morphology, disease resistance, and biochemistry of rainbow trout (Oncorhynchus mykiss) Can. J. Aquat. Sci. 1996;53:2424–2432. doi:10.1139/cjfas-53-11-2424 [Google Scholar]
- Yang R, Randall D.J. Oxygen consumption of juvenile rainbow trout (Oncorhynchus mykiss) exposed to sublethal concentrations of 1,2,3,5-tetrachlorobenzene and tetrachloroguaiacol. Bull. Environ. Contam. Toxicol. 1997;59:479–485. doi: 10.1007/s001289900503. doi:10.1007/s001289900503 [DOI] [PubMed] [Google Scholar]
- Yang R, Brauner C, Thurston V, Neuman J, Randall D.J. Relationship between toxicant transfer kinetic processes and fish oxygen consumption. Aquat. Toxicol. 2000;48:95–108. doi: 10.1016/s0166-445x(99)00050-8. doi:10.1016/S0166-445X(99)00050-8 [DOI] [PubMed] [Google Scholar]
- Ye X, Randall D.J. The effect of water pH on swimming performance in rainbow trout (Salmo gairdneri R.) Fish Physiol. Biochem. 1991;9:15–21. doi: 10.1007/BF01987607. doi:10.1007/BF01987607 [DOI] [PubMed] [Google Scholar]