Abstract
The rates of metabolism in animals vary tremendously throughout the biosphere. The origins of this variation are a matter of active debate with some scientists highlighting the importance of anatomical or environmental constraints, while others emphasize the diversity of ecological roles that organisms play and the associated energy demands. Here, we analyse metabolic rates in diverse marine taxa, with special emphasis on patterns of metabolic rate across a depth gradient, in an effort to understand the extent and underlying causes of variation. The conclusion from this analysis is that low rates of metabolism, in the deep sea and elsewhere, do not result from resource (e.g. food or oxygen) limitation or from temperature or pressure constraint. While metabolic rates do decline strongly with depth in several important animal groups, for others metabolism in abyssal species proceeds as fast as in ecologically similar shallow-water species at equivalent temperatures. Rather, high metabolic demand follows strong selection for locomotory capacity among visual predators inhabiting well-lit oceanic waters. Relaxation of this selection where visual predation is limited provides an opportunity for reduced energy expenditure. Large-scale metabolic variation in the ocean results from interspecific differences in ecological energy demand.
Keywords: metabolism, deep sea, scaling, oxygen consumption, locomotion, marine
1. Introduction
The rates of metabolic processes in animals vary tremendously throughout the biosphere (e.g. Bennett 1991; Seibel 2007). The origins and scope of this variation are a matter of active debate. Some emphasize geometric and environmental constraints or resource limitation as its source (e.g. Gillooly et al. 2001; Brown et al. 2004), while others emphasize the diversity of ecological roles that organisms play and the associated energy demands (Childress 1995; Suarez 1996; Reinhold 1999; Clarke 2004; Seibel 2007) as a primary driver of metabolic variation. With this in mind, the present paper addresses three seemingly simple questions: (i) what is the extent of variation in the rate of metabolism across diverse taxa and environments, (ii) what environmental and ecological demands act on organisms to drive selection for high metabolic capacity, and (iii) under what environmental circumstances might such selection be relaxed or capacity be constrained?
These questions have been pursued vigorously for decades but the clarity of the debate has been diminished by the subtlety of differences in capacity between the taxa most thoroughly studied, that often provide too small a signal to be clearly detected against the background noise inherent in animal physiology and its measurement. For example, Taylor and colleagues went to great lengths to successfully demonstrate important differences in metabolic rate between athletic and more sedentary mammalian species (Taylor et al. 1981; Weibel & Hoppeler 2005). However, both groups fall within the scatter of data found in the larger ‘mouse-to-elephant’ curve that describes the relationship between basal metabolism and body mass in mammals (Kleiber 1932; White & Seymour 2003). Here, we increase the ‘signal-to-noise’ ratio by comparing animals of dramatically different abilities (i.e. extreme animals) living in extremes of environmental conditions. More specifically, we review the metabolic capacity of diverse marine species across a depth gradient. However, we believe that the analysis provides generalities about the evolution of metabolic rates that may be broadly applicable.
Recent interest in the metabolism of deep-sea organisms is based on a very real need to understand global carbon flux and the consequences of ocean-based global-warming mitigation strategies (Angel 1989; Longhurst et al. 1990; Childress & Thuesen 1995; Seibel & Walsh 2001, 2003; del Giorgio & Duarte 2002; Hernandez-Leon & Ikeda 2005; Thistle et al. 2005). However, an intense inherent interest stems from an anthropocentric view of the deep ocean as a hostile environment. Deep-sea organisms face a number of seemingly insurmountable challenges, including high hydrostatic pressure, cold temperatures, low food availability, constant darkness and hypoxia (see below). Not surprisingly, metabolism in the deep sea is often viewed as universally low and environmentally constrained. In particular, food limitation is widely entertained as an important determinant of deep-sea organismal metabolism (e.g. Collins et al. 1999; Poulson 2001; Dalhoff 2004). At the opposite end of the spectrum, some have claimed that all organisms tend towards a common mass- and temperature-corrected metabolic rate (Gillooly et al. 2001). If true, the oft-cited decline in metabolism with depth must be artefactual. A third view claims that patterns of metabolism across a depth gradient reflect demand for energy for predator–prey interactions and that such interactions are dependent, primarily, on vision and light (Childress 1995; Seibel et al. 1997, 2000). Thus, some groups (e.g. those with image-forming eyes, living in environments with limited refuge from predators) show marked declines in metabolism with depth while others do not.
The state of our knowledge remains technology and resource limited, but steady advances (Childress et al. 1978; Childress 1985; Robison 2000; Koyama et al. 2002; Drazen et al. 2005) have allowed the live capture, surface maintenance and measurement of a surprisingly large number of deep-sea animals. A necessarily smaller number of oxygen consumption measurements have been made in situ on the deep-sea floor but techniques for these types of investigations have also seen many advances in recent years (Smith 1978; Smith & Baldwin 1997; Priede & Bagley 2000; Bailey et al. 2002). Oxygen consumption rates have now been measured for deep- and shallow-living representatives of many major phyla, both benthic and pelagic, and in several regions. Biochemical proxies of metabolism have provided estimates of metabolic capacity in many additional species (Torres & Somero 1988; Childress & Somero 1990; Seibel et al. 1998, 2000; Drazen 2002a; Seibel & Walsh 2003; Treberg et al. 2003; Dalhoff 2004; Thuesen et al. 2005b), while submersibles, landers and new tagging techniques have provided detailed studies of locomotion and behaviour in the deep sea (Priede et al. 1990; Marshall & Diebel 1995; Villanueva et al. 1997; Hunt & Seibel 2000; Bailey et al. 2003, 2005; Drazen & Robison 2004; Robison 2004; Seibel et al. 2005). More than 10 years have passed since the last specific reviews of deep-sea metabolic rates (Childress 1995; Mahaut et al. 1995). The present review consolidates the available data on metabolic rates of marine organisms from a diversity of phyla, habitats, depths and regions. We analyse the variation for discernable patterns and test various hypotheses that have been put forward to explain these patterns. We conclude that the primary determinant of routine metabolic rate for marine organisms is energy demand for locomotion and that the observed variation in locomotory capacity stems from the extent that species participate in visually mediated predator–prey interactions. In outlining the case for this ‘visual interactions hypothesis’ (Childress & Mickel 1985) below, we will explain why ‘limitation’ or ‘constraint’ hypotheses are inconsistent with what is known about the evolution of metabolic capacity and we will review literature supporting a link between the locomotory activity and the rates of metabolism in resting and active states.
2. Approach
The rate of oxygen consumption has been measured on a variety of deep-living species. These data have been augmented by measurements of the activities of key enzymes of intermediary metabolism, which usually correlate well with oxygen consumption rates (Childress & Somero 1979; Dalhoff 2004; Moyes & LeMoine 2005; Weibel & Hoppeler 2005; Seibel 2007). We have summarized (routine) metabolic rates and enzymatic data from our own studies and those in the literature. For each group of animals examined (or for which sufficient data are available), all oxygen consumption values were measured at, or adjusted to, 5°C and enzymatic activities were measured at, or normalized to, 10°C (fish) or 20°C (cephalopods). Published temperature coefficients were used where available or we assumed a Q10 of 2. Use of other available models for temperature correction (e.g. Gillooly et al. 2001) would have resulted in subtle differences in the elevations and slopes in some of the relationships observed but would not have altered our conclusions. For comparison of species across a depth gradient, species rates were adjusted to a common body size using published scaling coefficients where available, using scaling coefficients derived in the present study, or assuming a scaling coefficient of −0.25 for mass-specific metabolism. Quarter-power scaling is by no means universal (Glazier 2005; Seibel 2007) but is the safest assumption in the absence of empirical data (Schmidt-Nielsen 1984). Normalized rates were analysed both as a function of body mass and minimum habitat depth (minimum depth of occurrence (MDO), the depth below which 90% of the individuals of a given species were captured).
For inclusion in the present analysis, published studies of oxygen consumption must have satisfied a few basic criteria, although, given the general paucity of deep-sea data, we were not able to be highly selective. In all studies, spontaneous activity and the effects of feeding during measurement were minimized or specifically controlled. All measurements were made over at least 4 h and most experiments lasted 12–48 h with at least a 12 h period of food deprivation prior to measurement. Microbial respiration was controlled and animal incubation chambers were kept in the dark. Where activity was monitored, a rate corresponding to a minimum activity was used. Most measurements are of ‘routine’ metabolism that makes some allowance for spontaneous activity in the absence of obvious external stimuli.
3. Results
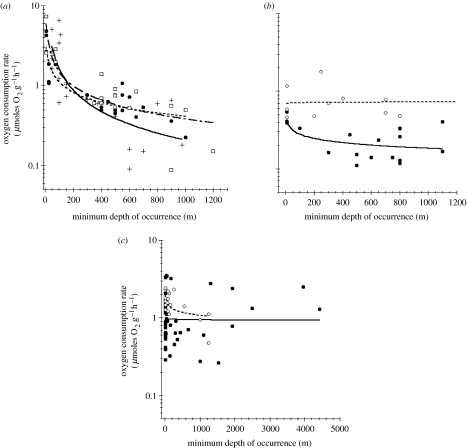
Figure 1 shows temperature- and size-normalized metabolic rates of individual species plotted as a function of MDO, the depth below which 90% of the individuals of a given species were captured. Thus, each data point represents the metabolic rate of an individual species of a common size at a common temperature. Visually orienting pelagic species (figure 1a) show strong declines in metabolism with depth. Bathypelagic species of fish, crustaceans and cephalopods have metabolic rates as much as 200-fold lower than shallow-living relatives and similar to many gelatinous zooplankton species found in both deep and shallow environments (figure 1b). Benthic (figure 1c), as well as non-visual pelagic (figure 1b), species have similar metabolic rates regardless of habitat depth.
Figure 1.
Metabolic rates of diverse marine species as a function of minimum habitat depth (see text). (a) Pelagic groups with image-forming eyes, including fish (closed circles, 8.08 MDO−0.43), cephalopods (plus signs, 105.16 MDO−0.90) and crustaceans (open squares, 23.02 MDO−0.59) show significant (p<0.05) declines in oxygen consumption rates with increasing depth. (b) Pelagic taxa lacking image-forming eyes, including chaetognaths (open circles, n.s.) and medusae (closed circles, 0.64 MDO−0.18; p<0.05), show only subtle declines with depth where significant. (c) Benthic carridean decapod crustaceans (open circles, 2.6 MDO−0.13) show a modest decline with depth, while most benthic crustaceans (closed circles, n.s.), including crabs, non-carridean decapods and aphipods, do not. Benthic fish and cephalopods exhibit similarly modest declines, if any (data not shown, but see figure 3). Sources of all data can be found in tables 1–3 the electronic supplementary material.
The similar metabolic rates of deep- and shallow-living benthic species are clearly seen in the scaling curves presented in figure 2a–d. Interspecific metabolic variation is low within benthic fish, crustaceans, cephalopods and echinoderms, once mass and temperature influences are taken into account, regardless of habitat depth. Benthopelagic species (those typically found near, but not on, the bottom) and pelagic species, in contrast, show large variation independent of temperature and body mass (figure 2a,e). Scaling curves are effective at showing the large-scale variations between highly diverse taxa as well. Again, we find that the variation within and between benthic groups is much less than that found in pelagic taxa. While variability found between sighted pelagic groups (figure 3a) is high and largely depth-related, the variation among gelatinous zooplankton and benthic taxa is lower and independent of habitat depth. Metabolic variability in unsighted pelagic groups (figure 3b) appears to reflect different feeding strategies ranging from ‘float-and-wait’ predators to the more active tactile foraging found in some gymnosome molluscs. Benthic taxa tend towards slightly more robust bodies and higher metabolic rates relative to gelatinous zooplankton.
Figure 2.
Metabolic rates (MO2) of diverse marine taxa from deep (closed symbols) and shallow (open symbols) benthic habitats as a function of body mass (M). Relationships are in the form MO2=aMb, where a is a normalization constant independent of size and temperature and b is a scaling coefficient representing the slope of the size scaling relationships. (a) Benthic (circles) and benthopelagic (squares) fish. Deep and shallow benthic fish are not significantly different and do not scale significantly with body mass over the limited size-range available. Shallow-living benthopelagic fish, primarily cods, (4.9 M−0.20±0.06) have significantly higher rates (ANCOVA, p<0.05) than deep-living benthopelagic fish (0.88 M−0.26±0.06) including macrourids. The arrow indicates a single rate for the deep-living benthic eel Synaphobranchus kaupii that most probably represents an active rate, whereas others are standard or routine rates (see text). (b) No significant difference between deep- and shallow-living benthic crustaceans, including carideans, is observed. When combined, the metabolic rates of all benthic crustaceans are significantly correlated with body mass (1.9 M−0.28±0.03). (c) Echinoderm metabolism declines significantly with body mass (0.62 M−0.39). No differences are observed between deep- and shallow-living species. (d) Benthic cephalopod metabolism declines significantly with body mass (3.4 M−0.27) but the limited data reveal no differences between deep- and shallow-living species. (e) Pelagic cephalopod metabolism declines significantly with body mass for individual families, including Loliginidae (8.2 M−0.84±0.01; triangles), Gonatidae (open squares, n.s.), Histioteuthidae (1.36 M−0.24±0.83; closed circles), Cranchidae (0.53 M−0.19±0.09; open circles), Bolitaenidae (0.27 M−0.25±0.072; plus signs) and Vampyroteuthis infernalis (0.14 M−0.23±0.115; closed squares). The normalization constants are highly correlated with minimum habitat depth (Seibel 2007). n.s., not significant. Sources of all data are available in tables 1–3 in the electronic supplementary material.
Figure 3.
Summarized metabolic scaling relationships comparing diverse marine taxa. (a) The diversity of metabolic rates found among cephalopods is greater than that found between the fastest and the slowest fish. (b) More than 100-fold variation is demonstrated between different groups of gelata, ranging from active gymnosomatous pteropod molluscs, Clione antarctica (Seibel & Dierssen 2003) to the inactive polychaete, Peoebius meseres (Thuesen & Childress 1993b) and a lobate ctenophore, Mnemiopsis leidyi (H. Chang 2007, University Rhode Island, unpublished data). (c) Benthic taxa scaling relationships drawn from combined deep- and shallow-species data in figure 2. Sources of all data are available in tables 1–3 in the electronic supplementary material.
The patterns noted here for oxygen consumption rates are generally mirrored by enzymatic indicators of aerobic and anaerobic metabolism. Mean citrate synthase and lactate or octopine dehydrogenase activities in locomotory muscles decline strongly with depth for pelagic species of fish and cephalopods (figure 4), independent of mass and temperature.
Figure 4.
Aerobic (citrate synthase, CS; closed symbols) and anaerobic (lactate or octopine dehydrogenase, LDH or ODH; open symbols) enzymatic activities in marine animals as a function of minimum depth of occurrence (MDO). (a) Pelagic fish show strong declines in both CS (10.4 MDO−0.56; Childress & Thuesen 1995) and LDH (734.0 MDO−0.69; Childress & Somero 1979) with habitat depth. (b) Pelagic cephalopods show very strong declines in both CS (1398 MDO−1.23) and ODH (37 893 MDO−1.54) with increasing depth (Seibel et al. 2000).
4. Rates of metabolism in relation to environmental variables
(a) Metabolic rate is correlated with habitat depth only in visually orienting pelagic animals
Previous studies, confirmed by our analysis here, have shown that deep-living pelagic (and benthopelagic) fish (Meek & Childress 1973; Gordon et al. 1976; Torres et al. 1979; Smith & Laver 1981; Torres & Somero 1988; Koslow 1996), crustaceans (Childress 1971, 1975; Ikeda 1988; Cowles et al. 1991; Yamada & Ikeda 2003) and cephalopods (Seibel et al. 1997, 2000; Seibel & Carlini 2001) have 10- to 200-fold lower metabolic rates than shallow-living pelagic relatives, a trend that cannot be explained by temperature or size differences (figures 1 and 2e). The distinction between benthic and pelagic is important as similar declines are not evident, or are not as pronounced, in benthic species (figure 2a). These declines are mirrored by patterns of enzymatic activity (Childress & Somero 1979; Sullivan & Somero 1980; Gibbs & Somero 1990; Seibel et al. 1998, 2000; Janssens et al. 2000; Seibel 2007; figure 4), buffering capacity (Castelini & Somero 1981; Dickson & Somero 1987; Seibel et al. 1997) and protein content (Childress & Nygaard 1974; Bailey & Robison 1986; Childress et al. 1990b; Drazen 2002b; Seibel et al. 2004) in locomotory muscles.
Much less work has been published on the metabolism of deep-sea benthic animals in part because collecting specimens in good health is more complicated (Childress et al. 1990a). Benthic species are better represented by measurements of metabolic enzyme activities that serve as proxies for metabolic capacity. The majority of oxygen consumption and aerobic metabolic enzyme measurements in deep-sea benthic species, including echinoderms (Smith 1983), meiobenthic animals (Shirayama 1992), crabs and shrimp (Childress & Mickel 1985; Henry et al. 1990; Walsh & Henry 1990; Childress et al. 1990a; Bailey et al. 2005), amphipods (Smith & Baldwin 1982; Treude et al. 2002), sponges (Witte & Graff 1996) and octopods (Seibel & Childress 2000) were at most one- to fivefold lower than shallow-living species after temperature correction and many of these groups show no decline at all (e.g. figures 1c, 2, 3c and 4). For comparison, epipelagic fish and squids have rates that are 10- to 200-fold higher than most bathy- or deep benthopelagic species (figures 3a,c and 4). Among the benthic groups, the more active caridean shrimps exhibit a significant decline with depth as was originally shown with data from California species alone (figure 1c; Childress et al. 1990a). The slope reported here, with the inclusion of data from Mediterranean species (Company & Sarda 1998), is approximately 50% less than initially reported.
Literature values seem to indicate a significant decline in anaerobic metabolic enzymes, lactate and octopine dehydrogenase, with depth in both pelagic and benthic taxa (figure 4; Childress & Somero 1979; Somero & Childress 1980; Sullivan & Somero 1980; Torres & Somero 1988; Seibel et al. 2000; Seibel & Childress 2000). However, patterns of anaerobic metabolic enzymes in fish should be interpreted cautiously as they are typically measured only in white muscle even though the proportions of white and red muscle probably change with depth. Scaling patterns of anaerobic metabolic enzymes appear to reflect predator–prey interactions and are thus much more variable making accurate normalization difficult (Siebenaller et al. 1982; Childress & Somero 1990; Seibel et al. 1998, 2000; Thuesen et al. 2005b). Depth-related patterns of metabolic enzyme activities in benthic and benthopelagic fish will be reviewed in detail elsewhere (Drazen & Seibel submitted).
Hypotheses investigated as potential explanations of the above patterns have included pressure-induced metabolic limitation (Somero & Siebenaller 1979), oxygen and food limitation (Childress 1971; Smith & Hessler 1974; Yang et al. 1992; Collins et al. 1999). Each of these hypotheses focuses on a perceived limitation of metabolism in the deep sea. However, as stated above, many groups show no decline in metabolism with depth, while the shallow ocean is dominated by low metabolic rate species as well (e.g. gelata; Haddock 2004). Therefore, an approach may be to explain why some environments, such as the epipelagic open ocean, select for exceptionally high rates among visual predators and their sighted prey. To our knowledge, only one hypothesis put forward to date explains this dichotomy and the patterns of metabolic rate with depth. The visual interactions hypothesis suggests that high light levels and limited refuge in the epipelagic realm has resulted in strong selection for high locomotory capacity for predator–prey interactions among visually orienting taxa and that high metabolic rates follow. Relaxation of this selection in darkened bathypelagic waters, or among visually limited taxa, allows less expensive lifestyles and low metabolic rates (Thuesen & Childress 1993a,b, 1994; Childress 1995; Seibel et al. 1997, 2000).
(b) Temperature alone does not explain the patterns of metabolism with depth
The influence of temperature on biochemical reactions is well known and includes a doubling or tripling of rate with a temperature increase of 10°C (equivalent to a Q10 of 2–3). Although not fully understood, the effect of temperature on whole-organism metabolism usually mirrors that on chemical reaction rates (Hochachka & Somero 2002; Clarke 2004). With the exception of a few notable regions, temperature decreases with increasing depth to approximately 1–5°C throughout most of the deep (more than 1000 m) ocean. Thus, the difference in oxygen consumption between an organism living near the surface in warm tropical waters (30°C) and one living at 4000 m on the bottom of the ocean or in polar surface waters (−2 to 1°C) may be as much as 27-fold, all else being equal. A large body of literature reveals little or no evidence of elevated resting metabolic rates (i.e. metabolic cold adaptation) in polar animals relative to the rates expected by extrapolation from warm-water measurements (see reviews by Clarke 1983; Clarke & Johnston 1999) and no elevation in response to cold would be expected in the deep sea, as selection for elevated rates appears generally absent (see below).
To account for temperature-related metabolic variation and its influence on patterns of metabolism with depth, three approaches have been taken. Firstly, metabolic rates of some groups were measured at a temperature experienced by all species in the analysis. For example, the pelagic cephalopods examined by Seibel et al. (1997) off California all experience 5°C at some point during their diel or ontogenetic vertical migrations and this temperature was chosen as a common measurement temperature (figure 1a). A second approach is to normalize metabolic rates to a common temperature using measured or assumed temperature coefficients. A third approach takes advantage of the isothermal water column, found in regions such as the Southern Ocean and Mediterranean Sea, to separate the influences of depth and temperature. All rates in the present study were measured at, or adjusted to, a common temperature of 5°C (see §2), effectively eliminating temperature as a potential influence on our conclusions. Certainly, lower temperatures in the deep sea cause a reduction in metabolism in nature, but the metabolic measurements at a single temperature presented here and elsewhere still demonstrate large declines in inherent metabolic rate with depth in some groups (Childress & Somero 1979; Sullivan & Somero 1980; Childress 1995; Seibel et al. 1997). Furthermore, studies of animals inhabiting the isothermal Antarctic water column have also shown depth-related declines in metabolism (Ikeda 1988; Torres & Somero 1988).
(c) Pressure does not restrict metabolism in the deep sea
Pressure increases linearly with depth and selects for enzymes that are resistant to volume changes during catalysis, an adaptation that reduces catalytic efficiency as well (Somero & Siebenaller 1979; Somero 1992). Pressure may further select for cellular constituents that counteract pressure effects on macromolecules (Yancey 2005; Samerotte et al. 2007). However, capacity adaptations allow an organism to overcome pressure-induced inefficiencies in enzymes to maintain a required level of performance (Childress & Somero 1979; Sullivan & Somero 1980; Siebenaller et al. 1982). In addition, several investigations of oxygen consumption rates under varying pressure indicate that there is little or no effect in some fish (Meek & Childress 1973; Belman & Gordon 1979), crustaceans (Childress 1977), cephalopods (Belman 1978) and polychaetes (Thuesen & Childress 1993c). A small elevation of metabolism measured in situ at mesopelagic depths, relative to shipboard measurements, was reported for some gelatinous zooplankton (Bailey et al. 1994) and was explained as a reduction in motor activity due to decompression upon recovery to the surface. However, metabolism in these groups is not known to decline with depth (Thuesen & Childress 1994). The absence of metabolic declines with depth, and hence pressure, in gelatinous zooplankton as well as most benthic taxa, argues strongly that decompression during shipboard measurements does not explain the declines in metabolism with depth observed in some other groups.
(d) Hypoxia does not drive the decline in metabolism with depth
Low metabolic rates in deep-sea animals are clearly advantageous in the low oxygen concentrations found in the oxygen minimum layer off the coast of California, where most of the metabolic rates have been measured. However, as explained below, a low metabolic rate is insufficient for survival in the extreme hypoxia found at depth in some regions.
With increasing habitat depth, photosynthetically available light and atmospheric mixing is diminished, while organismal biomass decreases. Thus, both provision and consumption of oxygen decrease with depth, but at different rates. Near the surface, consumption is high but supply is generally sufficient to maintain oxygen saturation. At intermediate depths, consumption outpaces oxygen supply and a layer of hypoxic water (i.e. oxygen minimum layer, OML) is formed. At deeper depths, consumption is further diminished and oxygen levels are elevated above that in the minimum layer. The spatial extent and intensity of OMLs depend on the productivity in overlying waters and on the elapsed time since a deep water mass was last in contact with the atmosphere (i.e. the ‘age’ of the water). Thus, the most intense OMLs are in the eastern tropical Pacific and Arabian Seas, while midwaters of the North Atlantic are generally not oxygen depleted. Oxygen minimum layers have a profound influence on the distribution, abundance and physiology of marine animals (Wishner et al. 1990; Childress & Seibel 1998; Helly & Levin 2004). The regional variation in oxygen content has allowed the separation of this confounding variable from other depth-related influences on metabolism (Childress & Seibel 1998).
A variety of circulatory and morphological adaptations to the OML have been identified. Permanent residents of the OML have elevated gill surface areas, high ventilation volumes and respiratory proteins with a high affinity for oxygen (Childress & Seibel 1998; Seibel et al. 1999). However, low rates of oxygen consumption do not appear to be an adaptation specifically for life in low oxygen waters (Childress & Seibel 1998). Related species living at comparable depths in regions with higher oxygen levels have similarly low metabolic rates but lesser capacity for oxygen extraction and transport (Childress & Seibel 1998; Seibel et al. 1999). Thus, a low metabolic rate may be necessary, but it is not sufficient for aerobic survival in the OML. Furthermore, regional oxygen content is not correlated with metabolic rate, reflecting the fact that some animal groups show declines in metabolism with depth regardless of oxygen content.
(e) Food availability does not constrain metabolic rates in the deep sea
An additional and more obvious consequence of reduced biomass in the deep sea is a reduction in food availability for deep-sea organisms. With the exception of spatially confined habitats such as hydrothermal vents, primary production is dependent on sunlight and is thus restricted to near-surface waters. Zooplankton and benthic faunal biomass also decline nearly exponentially with depth resulting in an order of magnitude difference between the surface and 1000 m (Angel & Baker 1982). Earlier studies explained the low metabolic rates of deep-sea animals as an adaptation to lower food availability at depth (Childress 1971; Smith & Hessler 1974), a hypothesis that has not withstood experimental testing but persists nonetheless. As explained in detail below, low food supply in the deep sea does not specifically select for reduced metabolic demand nor does it preclude high rates of metabolism.
Since biomass at depth depends on the upper water column productivity (Thurston et al. 1994, 1998), food availability in deep eutrophic waters can be comparable to that near the surface in oligotrophic ones. Thus, if organisms living at 1000 m depth off California have low metabolic rates because they are food limited, organisms living in surface waters off Hawaii should also have low metabolic rates because biomass levels are similarly low. Clearly, this is not the case (Cowles et al. 1991; Seibel et al. 1997). One would also expect animals living at depth in an oligotrophic area to have lower rates than those living in a more eutrophic environment at the same depth. However, this also does not appear to be the case (Cowles et al. 1991; Seibel et al. 1997). Thus, there appears to be no correlation between food supply and metabolic rates in the ocean.
Although fish inhabiting more productive regions have higher lipid contents, reflecting access to food supplies, they do not have significantly different protein contents, which are indicative of locomotory activity and correlate with metabolic rates (Bailey & Robison 1986; Childress et al. 1990b). As explained below, many animal groups, with a wide range of metabolic rates, show no depth-related trends, a fact also that argues against food limitation in the deep sea.
Very high metabolic rates could be precluded from food-poor environments, all else being equal. However, the finding that highly active animals occupy oligotrophic surface waters, where food availability is apparently less than in the deep sea of more productive regions, suggests that food supply in the deep sea does not preclude even the highest rates of metabolism. Lastly, in those groups that show declines in metabolism with depth, no additional decline is observed below approximately 800 m even though food availability (biomass) continues to decline to the deepest depths.
Poulson (2001) argues that the asymptote of metabolic rate decline near 800 m may reflect a limit of reduction even though food supply continues to decline with depth. In other words, he argues that metabolic rates lower than that of bathypelagic fish and crustaceans cannot be attained. He similarly argues that the absence of a decline in unsighted animals such as chaetognaths and jellyfishes may also be explained by their already very low rates. This reasoning implies that rates of metabolism that are already food limited at 800 m are somehow provided for at greater depths with even less food. Furthermore, fish and crustaceans have not reached the limits of reduction as some other animal groups have much lower metabolic rates than even deep-sea representatives of these groups (figure 3). Mass- and temperature-corrected rates of some gelatinous animals are nearly 100-fold lower than those of bathypelagic fish. Poulson's reasoning further does not explain the absence of a strong decline with depth in most benthic organisms, which typically have higher metabolic rates than bathypelagic fishes. Lastly, any energy saved via reduced metabolism does not represent total lifetime economy as many deep-living pelagic fishes have high rates of growth (Childress et al. 1980).
Of prime importance to the above analysis is an understanding that metabolism represents a cost to the organism (cf. Clarke & Johnston 1999). In other words, an elevated metabolic rate is not, itself, a benefit to an organism and selection will not act to elevate metabolism in the absence of energy demand. Thus, organisms with lower rates are apparently taking advantage of opportunities for energy savings (i.e. of decreased energy demand; cf. Clarke 1983; Childress & Somero 1990). In this context, food limitation or other constraints on metabolism make little sense. While food could theoretically (but apparently does not) limit the evolved metabolic rates of otherwise metabolically active species, the organisms that are found in food-poor environments cannot be food limited.
Food limitation implies that organisms in the deep sea would evolve a higher capacity for locomotion and metabolism, and hence consume more energy, only if they had access to more food. But to what end? Unless swimming faster and burning more energy provide a selective advantage, high metabolic rates should not be found. One could also argue that selection acts to achieve greater efficiency where resources are in short supply thus lowering metabolic expenditure in the food-poor deep sea. However, one must also explain why such efficiency is not advantageous and under strong selection in all environments. Only if such efficiency includes a trade-off with metabolic or locomotory capacity might efficiency be selected against in organisms with demanding lifestyles. Such a trade-off would only be acceptable in food-poor environments if metabolic capacity were not under strong selection itself.
For example, Seibel et al. (2000) argued that part of the reduction with depth in metabolism of pelagic cephalopods results from increased efficiency of locomotion in deep- versus shallow-living species. As discussed above, shallow squids swim by jet propulsion at low Froude efficiencies. Deep-living species, in contrast, swim primarily using fins at lower speeds but greater efficiency. This transition from jet propulsion to fin swimming could be selected for by the low food availability demanding greater efficiency. However, the transition is only possible because high-speed locomotion is not under strong selection in the deep sea. Conversely, the only reason that such inefficiency of propulsion persists in shallow-living squids is because high speed confers tremendous selective advantage to visual predators and prey in well-lit epipelagic waters.
(f) Light-mediated relaxation of selection for metabolic capacity
Arguably, the leading hypothesis explaining the observed declines in metabolism with depth, the visual interactions hypothesis (Childress & Mickel 1985), suggests that in the absence of light, the distances over which predators and prey interact are reduced (Lythgoe 1988) resulting in relaxed selection pressure for rapid locomotory capacity for pursuit and evasion (Childress 1995). Owing to the absorption of light by water and its narrowing spectrum, the intensity of light available for vision falls rapidly with depth (Warrant 2004). Light intensity declines by approximately 2.6 log units in the first 100 m but less rapidly below that (1.5 orders of magnitude every 100 m) due to increased clarity of the water with depth. Light at 600 m depth is equivalent to starlight and no visible light remains below approximately 1000 m (Warrant 2004; Warrant & Lockett 2004). The influence of light on metabolism is not immediately obvious but, as we will see, light influences predator–prey interactions that in turn dictate locomotory and metabolic requirements.
In support of the visual interactions hypothesis, only sighted pelagic taxa exhibit strong depth-related metabolic declines (figure 1a). Metabolism declines to the limits of visible solar light (approx. 800–1000 m; Lythgoe 1988; Warrant & Locket 2004), but is fairly constant below this depth (Childress 1995). In contrast, the metabolic rates and enzyme activities of non-visual pelagic taxa such as chaetognaths, copepods, medusa, pteropod molluscs and polychaetes do not show any trend with habitat depth (figure 1b; Smith & Teal 1973; Thuesen & Childress 1993a,b, 1994; Thuesen et al. 1998).
Metabolic rates of taxa living in benthic environments are much less variable than those of pelagic groups and strong declines with depth are not typically observed, reflecting the increased opportunities for crypsis and refuge near the bottom at all depths (figures 1c, 2 and 3; Childress et al. 1990a; Hamner 1995; Seibel & Childress 2000). Childress et al. (1990a) hypothesized that differences in metabolism between groups of benthic crustaceans can be explained by mode of locomotion (sedentary versus active swimmers), but that the slopes of the relationships with depth vary with exposure to predators on and off the bottom (Childress et al. 1990a; Childress & Thuesen 1995). In the open pelagic environment, animals may hide only by being transparent or small (Hamner 1995; Johnsen 2001). In the benthic realm, the substrate affords many hiding places and the opportunity for camouflage of even large organisms. Therefore, some species are very sedentary regardless of the depth of habitat and light levels, while others are much more mobile.
The visual interactions hypothesis explains the patterns observed and the taxonomic and lifestyle variations in them. It further explains the absence of declines in benthic and non-visual taxa and the absence of additional metabolic decline below the limit of downwelling light in visually orienting pelagic groups. It explains striking convergence between some deep-sea and cave animals (reviewed in Poulson 2001). Perhaps most importantly, it is consistent with current understanding of how metabolic capacity evolves and the overriding influence of locomotory activity. However, there are some assumptions upon which the hypothesis is based that require additional support, which is provided below.
5. Predator–prey interactions, locomotion and metabolism
(a) The link between resting and active metabolic rates
Implicit in the visual interactions hypothesis is a link between locomotory activity, required for visual predator–prey interactions, and the routine or resting metabolic rates that have been measured in diverse taxa. A relationship between locomotory speed and oxygen consumption has been demonstrated (Taylor et al. 1981; O'Dor & Webber 1986) and is easily explained by the increase in ATP hydrolysis supporting muscle contraction (Suarez 1996). Not so easily explained is the relationship between active or maximum sustained metabolic rate and resting, basal or maintenance metabolic rate (Bennett 1991; Reinhold 1999). Certainly, the machinery required to support locomotory activity must confer higher maintenance costs. For example, muscle will consume oxygen at a higher rate than the same mass of gelatinous tissue, even at rest. Similarly, greater mitochondrial volumes presumably require higher protein synthesis rates and will contribute more to basal metabolism via proton leak all else being equal (Porter & Brand 1993).
Stated in evolutionary terms, a trade-off exists between a low resting metabolic rate and adaptations of metabolism for activity (Reinhold 1999). This suggests that selection to reduce resting metabolism is less intense in active species than in species where resting metabolism constitutes a large proportion of total metabolic costs. Those animals that spend more energy on activity should therefore have a higher resting metabolic rate than animals that spend less energy on activity (Reinhold 1999). As argued below, where selection for activity levels is lessened, selection for locomotory efficiency and neutral buoyancy is strengthened and low metabolic rates follow.
(b) Locomotory activity and depth
An additional assumption of the visual interactions hypothesis is that locomotory activity in visually orienting predators and prey declines with depth and light levels. As a corollary, diverse feeding strategies that do not involve lengthy predatory pursuit (e.g. bioluminescent prey attraction) will emerge or become more prominent. However, only a handful of studies have directly assessed locomotion or behaviour in deep-sea animals. Several submersible-, or autonomous platform-, based locomotory observations (Cowles 1994; Roper & Vecchione 1997; Villanueva et al. 1997; Priede & Bagley 2000; Robison 2004) exist for diverse pelagic species, but only a few are quantitative (e.g. Bailey et al. 2003, 2005; Zeidberg 2004). Laboratory ‘swim tunnel’ measurements have been made for a variety of shallow-living fishes (e.g. Dewar & Graham 1994; Nelson & Claireaux 2005) and squids (e.g. O'Dor 1982; Bartol et al. 2001), but only for a few mesopelagic crustaceans (Cowles & Childress 1988; Cowles 2001). New tagging methods are revealing swimming capacity and movement patterns of a variety of both deep- and shallow-living species (Priede et al. 1990; Collins et al. 1999; Block et al. 2002; O'Dor 2002; Markaida et al. 2005).
An important point to keep in mind is that the vast majority of marine animals, at least those that are not permanently fixed to the bottom, can swim. The expectation of poor swimming ability in benthic, gelatinous or globular species has led researchers to describe observed agility and speed in numerous deep-sea taxa as ‘surprising’. However, even Vampyroteuthis infernalis, a gelatinous animal with the lowest metabolic rate of any cephalopod, can achieve speeds of nearly 0.5 m s−1 (two body lengths) for short distances (Hunt 1996; Seibel et al. 1998), similar to routine swimming speeds in many epipelagic fishes and squids (e.g. Zeidberg 2004). Furthermore, energetic cost increases with velocity cubed such that small errors in speed determination can result in very large errors in metabolic capacity estimation. Thus, accurate measures of activity duration, speed, body size and metabolic state of species under observation are required for meaningful comparisons of metabolic capacity derived from locomotory observations. These requirements have been only partially met in only a few studies. Nevertheless, observations of locomotory performance are in their infancy and show great promise for further testing hypotheses of capacity in deep-sea organisms. Much more data, over a greater depth and taxonomic range, will undoubtedly provide new perspective in deep-sea biology.
Although many species appear to be classic ‘sit-and-wait’ predators (Robison 2004; Youngbluth & Bamstedt 2001), behavioural observations reveal a diversity of predatory strategies within pelagic taxa, suggesting that this label may be an oversimplification (Matsumoto & Harbison 1993; Hunt & Lindsay 1998; Robison 2004). For example, the narcomedusae, Solmissus sp., is a gelatinous predator that swims with tentacles extended forward to attack prey on approach (Raskoff 2002). Similarly, the vampire squid forages by deploying a single long sensory filament and swimming an arc around it (Hunt 1996). A variety of mesopelagic species have adopted a strategy of protective mimicry, rather than locomotion, as a first response to predators (Arkihpkin & Bizikov 1996; Robison 1999). Others use complex bioluminescent displays that minimize the requirements for locomotion during predator–prey interactions (Herring 1977; Young 1983; Hunt 1996; Widder 2002; Robison et al. 2003; Robison 2004; Haddock et al. 2005) and still others use larger animals as cover from predators (Drazen & Robison 2004).
Midwater animals have developed diverse locomotory strategies, but during routine swimming they tend towards efficiency at the expense of speed. For example, while most deep-sea cephalopods are capable of jet propulsion to achieve at least modest escape speeds, biochemical measurements (Seibel et al. 1998, 2000) and behavioural observations (Hunt 1996; Roper & Vecchione 1997; Villanueva et al. 1997) reveal a reliance on more efficient (but sluggish) swimming using the fins or arms and webbing for propulsion.
In one of the earliest reports of midwater animal behaviour in situ, Barham (1971) described the orientation and activity levels of several species of vertically migrating midwater fishes in the California Current. At their deeper daytime depths, most individuals were ‘passively drifting’ while at shallower night-time depths, they were ‘actively swimming’. Barham (1971) attributed this daytime ‘lethargy’ to the low oxygen levels in the deep sea. Similar in situ behaviour was reported for the vertically migrating mesopelagic squid, Gonatus onyx. Hunt & Seibel (2000) observed that shallower individuals were quick to dart away when approached by the submersible, while deeper individuals held their positions and escaped only after persistent harassment by the investigators. Cowles (1994) reports equal activity levels during both day and night off California for the vertically migrating shrimp, Sergestes similis, but the shrimp were always swimming downward in response to the lights of the submersible. His laboratory analysis, in contrast, revealed a reduction in oxygen consumption and swimming speed with declining oxygen concentrations. The limited data available are consistent with at least partial metabolic suppression by vertical migrators during deeper diurnal forays into oxygen-depleted waters (Childress & Seibel 1998). Vertical migrators appear to possess metabolic and locomotory capacity more similar to their shallower, non-migrating relatives than to permanently deep-living species, but operate on apparent diel cycles of activity.
An examination of an abyssal scavenging shrimp in the Mediterranean found swimming speeds similar to shallow-living crustaceans at similar temperatures but a lack of burst locomotory performance (Bailey et al. 2005), consistent with limited enzymatic activity data reported for other taxa (e.g. Seibel & Childress 2000; Treberg et al. 2003). Routine swimming speeds in deep-living rattail fishes are very low (approx. 0.1 m s−1; Priede et al. 1990; Priede & Bagley 2000), while the blue hake, Antimora rostrata, and a deep-sea eel, Synaphobranchus kaupii, reportedly have high routine swimming rates and burst swimming abilities similar to shallow-living pelagic species at equivalent temperatures (Bailey et al. 2003, 2005). However, as indicated above, swimming speeds, especially those measured remotely following attraction to bait, may be a poor indicator of capacity (Childress et al. 1990a). Whether the reported speeds represent a routine or maximum sustainable speed, or possibly even depend to some extent on burst swimming using anaerobic metabolism, is impossible to know using these methods but is crucial for accurate comparison with shallow-living species. The in situ measurement of oxygen consumption for S. kaupii (1.74 μmol g−1h−1, 5°C; figure 2a) approaches standard rates for shallow pelagic fish and thus is probably not a standard or even routine oxygen consumption rate. Rather, this measurement may represent an active, well-fed animal of an otherwise sluggish species. While A. rostrata and S. kaupii are clearly capable of greater swimming performance than rattails and bathypelagic anglerfishes, we suspect the reported swimming performances of these species are elevated in response to the bait.
Another recent comparative study found that routine swimming rates of a scavenging morid fish, A. rostrata, inhabiting the continental slope (approx. 2500 m), were only approximately twice that of Coryphaenoides armatus, a species with a deeper, but overlapping, depth range (Collins et al. 1999). The authors concluded that the differences between these two unrelated species were adaptations to food availability in the main part of their respective habitats. Priede et al. (2003) similarly invoked food availability to explain the fact that Coryphaenoides spp., living at abyssal depths under eutrophic regions of the north Pacific, have higher swimming rates than individuals in oligotrophic regions. However, as argued above, rates and speeds should not be arbitrarily elevated simply because more food is available. In this context, the appropriate question is why do species from eutrophic regions require higher swimming speeds than those from oligotrophic ones? Selection for early arrival at food falls (as larger numbers of competitors are probably in eutrophic regions) is a reasonable hypothesis (Collins et al. 1999).
6. The evolution of metabolic variation
(a) Convergence and divergence of metabolic and locomotory capacity
There appears to be an arms race between visual predators and prey in their attack and evasion tactics that we believe has been instrumental in the evolution of metabolic rates, especially in the marine environment. In environments where opportunities for refuge and crypsis are rare, such as the epipelagic realm, this race has resulted in a variety of unique predatory behaviours and countermeasures such as schooling, inking, colour change and even aerial flight in marine animals. However, the more important component of predator–prey interactions for visual animals is thought to be pursuit or evasion via active aquatic locomotion (Domenici 2002). One consequence of this has been a striking convergence of adaptations among disparate epipelagic taxa that permits high-speed locomotion and high aerobic and anaerobic metabolic capacity. Such convergence has been thoroughly described between various groups of fish (Farrell 1991; Dickson 1995, 1996; Bernal et al. 2001; Block & Stevens 2001; Donley et al. 2004) as well as between fish and cephalopods (O'Dor & Webber 1986; Packard 1972; Seibel et al. 2000; Pörtner 2002; Webber et al. 2000). Although less data exist, a case could also be made for convergence towards high metabolic and locomotory capacity in marine mammals (Williams 1999), pelagic decapod crustaceans (Quetin et al. 1994; Childress 1995) and marine reptiles (Humphries & Ruxton 2002).
Convergence among pelagic fish and squids towards high speed and continuous swimming is evident in their torpedo-shaped bodies, large masses of locomotory muscle with high protein content (as percentage wet mass), high oxygen transport capacity, elevated concentrations of metabolic enzymes in both ‘red’ and ‘white’ muscle equivalents, increased muscle buffering capacity and enhanced gill diffusion capacities. Tunas, lamnid sharks and oceanic squids have become dependent on continuous swimming for hydrodynamic lift and for water flow across the gills. The convergence between pelagic cephalopods and fish is especially informative, as these groups have very different anatomies and operate under different constraints, thus high-lighting the features most critical for an active pelagic lifestyle independent of phylogeny.
Among the most important differences between these groups is the mode of locomotion. Cephalopods swim by jet propulsion, a highly inefficient means of transport relative to caudal fin undulation. While the broad caudal fin typical of pelagic fish allows a relatively slow acceleration of a larger mass of water, jet propulsion requires rapid acceleration of a small volume of water to achieve similar thrust. The result is low Froude propulsion efficiency. ‘Squids thus require twice as much oxygen to go half as fast as a similar sized fish’ (O'Dor & Webber 1986).
Convergence between squids and fish is all the more striking considering that oxygen transport is constrained in cephalopods relative to fish. Haemocyanin, the cephalopod respiratory protein, is a large extracellular molecule that, due to viscosity constraints, can be found only at low concentrations relative to haemoglobin in fish (O'Dor & Webber 1986; Pörtner 2002). Thus, oxygen-carrying capacity is low in squids, relatively to similarly active fish, while oxygen demand is high. Their haemocyanin has, like haemoglobin in most pelagic fish, a low oxygen affinity and high pH sensitivity of oxygen binding that promotes release of oxygen at the tissues (Bridges 1994). In fact, active squids consume all available oxygen from the blood on each pass through the body leaving no venous reserve and must acquire as much as 60% of their total oxygen demand across the skin (Pörtner 2002). The additional oxygen taken up across the skin, in combination with maximized circulatory performance (Shadwick et al. 1990; Pörtner 2002), allows squids to achieve athletic performance comparable to heterothermic fish.
The phylogenetic and anatomical constraints in epipelagic cephalopods, and the requirements for high-speed locomotion and continuous swimming, have led to the highest mass- and temperature-corrected metabolic rates in the animal kingdom (Seibel 2007; figure 3a). Loliginid and ommastrephid squids have higher standard metabolic rates than scombrid fish, or even mammals, at equivalent sizes and temperatures. This difference is especially pronounced at large sizes due to differences in scaling between these groups (Seibel 2007; figure 3a).
By comparison, swimming and metabolic capacity in visually limited, tactile predators and herbivores is truly underwhelming. The capacity adaptations that allow high performance in active squids and fish are clearly absent from these organisms. Elongate (eel-like) or globular body forms, poorly equipped for speed, replace the torpedo-shaped bodies predominant among visual predators. Muscle mass is substantially reduced and protein contents are lower by an order of magnitude or more (Childress et al. 1990b; Drazen 2002b, 2007; Seibel et al. 2004). Activities of aerobically and, in many cases, anaerobically poised enzymes and the capacity to buffer the acidic end products of anaerobic metabolism are also orders of magnitude lower (figure 4; Sullivan & Somero 1980; Castellini & Somero 1981; Dickson & Somero 1987; Torres & Somero 1988; Gibbs & Somero 1990; Seibel et al. 1998, 2000; Drazen 2002a; Seibel 2007). Oxygen-carrying capacity is reduced, as is gill-diffusion capacity (with the exception of species living permanently in extreme hypoxia; Childress & Seibel 1998; Seibel et al. 1999). Some benthopelagic scavenging fish retain some locomotory ability, perhaps to compete for limited food falls on the deep-sea floor as discussed above. Seamount-associated fish in the deep sea may also display considerable locomotory abilities that facilitate maintenance of position at seamounts against strong currents (Koslow 1996). Where such requirements for locomotion are absent, visually limited predators and prey have dramatically reduced metabolic rates.
The gelatinous zooplankton, or ‘gelata’, encompasses diverse phyla such as cnidarians, ctenophores, chaetognaths, annelids and molluscs, too fragile to sample with conventional oceanographic nets (Haddock 2004). The fragility reflects their planktonic lifestyle where locomotory capacity is not under strong selection, contact with solid surfaces is minimal and accumulation of metabolically inert tissue (i.e. gel) is a cost-effective buoyancy strategy. The role of ‘gel’ in the biology of gelata is poorly understood but is probably structural and may facilitate oxygen storage (Thuesen et al. 2005a). Layers of gelatinous tissues certainly increase size without excessive energetic investment. Regardless, gel is an obvious disadvantage where strong locomotion is required. Thus, its accumulation highlights a trade-off between ‘power density’ for locomotion and density reduction (i.e. buoyancy) for energy savings (Childress & Nygaard 1974; Webber et al. 2000; O'Dor 2002; Seibel et al. 2004). Large deposits of metabolically inert tissue provide lift but displace muscle tissue that could otherwise be used for propulsion. Only at slow speeds is such a strategy for buoyancy cost effective (Webber et al. 2000). Thus, neutral buoyancy is typically only found in environments (or species) that do not require strong swimming abilities. Only gas-filled swimbladders and intracellular chemical buoyancy aides (e.g. trimethylamine oxide; Sanders & Childress 1988; Withers et al. 1994; Seibel & Walsh 2002) can confer neutral buoyancy without substantial reductions in locomotory capacity. However, the most actively swimming pelagic fish, squids and crustaceans tend to be negatively buoyant.
(b) Light, vision and low habitat complexity in the evolution of high metabolic capacity
Recent findings from diverse fields are providing strong support for the idea that visual pursuit and evasion selects for high locomotory capacity, especially where low habitat complexity provides little refuge. High metabolic capacity follows and may play an important role in structuring pelagic ecosystems. For example, it is now well documented that the perceived light field (resulting from the interaction of prevailing light, animal visual systems and water turbidity) plays an important role in the vertical distribution of pelagic organisms hoping to avoid visual predators (Frank & Widder 1997). Prey visibility determines predation rates (Aksnes & Giske 1993), alters predator and prey behaviour (Domenici 2002), drives selection for adaptations that render animals less (e.g. transparency: Johnsen 2001) or more (e.g. bioluminescence: Widder 2002) conspicuous and can ultimately drive species composition and the structure of pelagic ecosystems (Eiane et al. 1999). Furthermore, de Queiroz (1999) found, looking only at shallow-living taxa, a strong association between image-forming eyes and activity levels.
The streamlined (i.e. torpedo shape) morphology of visual predators in the illuminated open ocean is maintained across a wide size and phylogenetic spectrum. However, ‘morphology becomes more diverse wherever the environment offers protection from visual predators, [as exemplified by] the myriad shapes of deep-sea fish’ (Verity et al. 2002). Similarly, deep-sea, non-visual and, to a lesser extent, benthic taxa are variably ‘freed’ from the need to actively swim from attacking predators and exhibit a much wider range of shapes than their visually foraging counterparts. Thus, escape from predators appears to be a major factor constraining the morphotype of epipelagic fish. Body form is constrained, in clear, well-lit oceanic waters, by the demand for streamlined bodies that consist largely of locomotory muscles used for high-speed locomotion during predator–prey interactions. Vision-limited predators rely more on tactile or sit-and-wait predation strategies and, in fact, turbid water masses favour tactile predators by diminishing the efficiency of visual predation (Eiane et al. 1999; Aksnes et al. 2004).
Exceptions to this general rule (i.e. visual predators with low locomotory capacity living in epipelagic waters) exist but typically involve a compensatory predator avoidance mechanism. For example, species may be unpalatable (Bullard & Hay 2002) or abduct a species that is (McClintock & Janssen 1990); have evolved startle responses and bioluminescent ‘burglar alarms’ (Widder 2002) or other behavioural (e.g. schooling or inking) or anatomical predator-avoidance strategies (spikes, stinging cells, etc); or may remain undetected through one of the few means of camouflage available in this refuge-free pelagic zone: countershading, reflection, extremely small size or transparency (Johnsen 2001). For example, cranchiid squids are sluggish swimmers with globular bodies and low metabolic rates (Seibel et al. 1997) but have good image-forming eyes and are often found in shallow oceanic water. However, they are highly transparent earning them the common name ‘glass squids’ and are thus able to minimize predation without strong locomotory abilities.
Obviously, unsighted pelagic taxa (e.g. gelata) do not engage in lengthy, high-speed predator evasion or pursue prey over long distances. The strong convergence between gelata and species with image-forming eyes living in the deep sea suggests that the latter also interact with predators and prey only over limited spatial scales and at speeds requiring minimal locomotory capacity. However, such species do retain functional eyes, often enhanced in size and sensitivity (Warrant 2004; Warrant & Lockett 2004), which begs the question of how eyes are used and to what extent predator–prey interactions require locomotion in the deep sea. At depths shallower than 800 m, where visible light is present, discrimination of visual targets depends on both the characteristics of the predator's eyes and the body size, shape and optical properties of the prey (Johnsen 2001). Interestingly, the ability to resolve an image is also highly dependent on the relative speed of the object being observed (Warrant 2004). This suggests that while a bathypelagic fish may be able to discriminate a motionless object in dim light, an object in motion (or one viewed by a rapidly swimming predator) is probably not readily discernable. Thus, high-resolution vision in dim light precludes high-speed locomotion (and vice versa). The large well-developed eyes in deep-sea organisms are most probably used to detect bioluminescence, a transient light-source that is also not likely to elicit lengthy, high-speed predator–prey interactions.
7. Conclusion
Metabolism and locomotion are complex traits representing the integration of numerous physiological, morphological and behavioural traits. Thus, adaptive changes in performance depend on how selection acts on multiple integrated traits such as growth and reproduction (Ghalambor et al. 2003). These considerations are especially important in the deep sea where longevity and reproductive investment may be very different than in shallow water (Calliet et al. 2001). What we have explained here are broad interspecific patterns of metabolism in a system with a high signal-to-noise ratio in metabolic measurements. More subtle changes with depth in benthic or non-visual groups where present, or differences between any two isolated species, cannot readily be predicted by any combination of environmental traits or by phylogenetic relationships and must be viewed in the context of life history, ecology and evolution of the species involved.
This review reveals remarkable interspecific variation in mass- and temperature-corrected metabolic rates throughout the world's oceans (figure 3). More than 300-fold variation exists between the fastest and slowest marine animals independent of body mass and temperature. This finding stands in stark contrast to recent claims (Gillooly et al. 2001; Brown et al. 2004) that the primary drivers of metabolic variation are body mass and temperature. Shallow-living, visually orienting pelagic predators (epipelagic squids, fish and, data not shown, crustaceans; figure 3a) spend at least some portion of their day in active pursuit of prey in near-surface waters and exhibit the highest energy consumption rates measured (figure 3a). Visually limited pelagic groups, including representatives of sighted taxa living in light-limited waters (more than 100 m) and most gelata (figure 3a,b), tend towards tactile, sit-and-wait predation strategies and have low, but variable, rates of energy consumption at all depths. Benthic organisms generally have intermediate energy consumption rates that are also independent of habitat depth. These patterns argue strongly that metabolic rates are not severely limited by environmental parameters in the deep sea. However, strong convergent evolution among sighted pelagic predators in shallow water, and between unsighted and sighted pelagic predators in deep water, is evidence of a common selective regime, mediated by the presence or absence of visual predator–prey interactions, operating at each depth (cf. Harvey & Pagel 1991). The present analysis suggests that the well-documented divergence of metabolic rates with depth in visually orienting pelagic animals must result, not from selection for low rates in the deep sea due to some environmental constraint, but from strong selection for high locomotory capacity among shallow-living species with demanding lifestyles.
Acknowledgments
This manuscript benefited tremendously from the presentations and many enlightening discussions emanating from the symposium ‘Environmental Constraints on Locomotion’ at the Society for Experimental Biology Conference in Barcelona, Spain (2005) and from the thoughtful contributions of anonymous reviewers. The ideas put forward in this review are founded on the pioneering efforts of, among others, Drs J. J. Childress and K. L. Smith.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
Supplementary Material
Table 1. Metabolic scaling relationships of marine animals; Table 2. Metabolic rates of benthic crustaceans, echinoderms and fishes; Table 3. Metabolic rates of cephalopods
References
- Angel M.V. Does mesopelagic biology affect the vertical flux? In: Berger W.H, Smetacek V.S, Wefer G, editors. Productivity of the ocean: present and past. Wiley; New York, NY: 1989. pp. 155–173. [Google Scholar]
- Angel M.V, Baker A.C. Vertical distribution of the standing crop of plankton and micronekton at three stations in the northeast Atlantic. Biol. Oceanogr. 1982;2:1–30. [Google Scholar]
- Aksnes D.L, Giske J. A theoretical model of aquatic visual feeding. Ecol. Model. 1993;67:233–250. doi:10.1016/0304-3800(93)90007-F [Google Scholar]
- Aksnes D.L, Nejstgaard J, Soedberg E, Sornes T. Optical control of fish and zooplankton populations. Limnol. Oceanogr. 2004;49:233–238. [Google Scholar]
- Arkhipkin A, Bizikov V. Possible imitation of jellyfish by the squid paralarvae of the family Gonatidae (Cephalopoda, Oegopsida) Polar Biol. 1996;16:531–534. [Google Scholar]
- Bailey T.G, Robison B.H. Food availability as a selective factor on the chemical compositions of midwater fishes in the eastern North Pacific. Mar. Biol. 1986;91:131–141. doi:10.1007/BF00397578 [Google Scholar]
- Bailey T.G, Torres J.J, Youngbluth M.J, Owen G.P. Effect of decompression on mesopelagic gelatinous zooplankton: a comparison of in situ and shipboard measurements of metabolism. Mar. Ecol. Prog. Ser. 1994;113:12–27. [Google Scholar]
- Bailey D.M, Jamieson A.J, Bagley P.M, Collins M.A, Priede I.G. Measurement of in situ oxygen consumption of deep-sea fish using an autonomous lander vehicle. Deep Sea Res. I. 2002;49:1519–1529. doi:10.1016/S0967-0637(02)00036-5 [Google Scholar]
- Bailey D.M, Bagley P.M, Jamieson A.J, Collins M.A, Priede I.G. In situ investigation of burst swimming and muscle performance in the deep-sea fish Antimora rostrata. J. Exp. Mar. Biol. Ecol. 2003;285–286:295–311. doi:10.1016/S0022-0981(02)00534-8 [Google Scholar]
- Bailey D.M, Bagley P.M, Jamieson A.J, Cromarty A, Collins M.A, Tselepidis A, Priede I.G. Life in a warm deep sea: routine activity and burst swimming performance of the shrimp Acanthephyra eximia in the abyssal Mediterranean. Mar. Biol. 2005;146:1199–1206. doi:10.1007/s00227-004-1525-1 [Google Scholar]
- Barham E.G. Deep-sea fishes: lethargy and vertical orientation. In: Farquhar G.B, editor. Proc. Int. Symp. Biological Sound Scattering in the Ocean. Superintendent of Documents; Washington, DC: 1971. pp. 100–118. [Google Scholar]
- Bartol I.K, Mann R, Patterson M.R. Aerobic respiratory costs of swimming in the negatively buoyant brief squid Lolliguncula brevis. J. Exp. Biol. 2001;204:3639–3653. doi: 10.1242/jeb.204.21.3639. [DOI] [PubMed] [Google Scholar]
- Belman B.W. Respiration and the effects of pressure on the mesopelagic vertically migrating squid Histioteuthis heteropsis. Limnol. Oceanogr. 1978;23:735–739. [Google Scholar]
- Belman B.W, Gordon M.S. Comparative studies on the metabolism of shallow-water and deep-sea marine fishes. 5. Effects of temperature and hydrostatic pressure on oxygen consumption in the mesopelagic Melanostigma pammelas. Mar. Biol. 1979;50:275–281. doi:10.1007/BF00394209 [Google Scholar]
- Bennett A.F. The evolution of activity capacity. J. Exp. Biol. 1991;160:1–23. doi: 10.1242/jeb.160.1.1. [DOI] [PubMed] [Google Scholar]
- Bernal D, Dickson K.A, Shadwick R.E, Graham J.B. Review: analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comp. Biochem. Physiol. A. 2001;129:695–726. doi: 10.1016/s1095-6433(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Block B.A, Stevens E.D. Academic Press; London, UK: 2001. Tuna physiology, ecology and evolution. [Google Scholar]
- Block B.A, Costa D.P, Boehlert G.W, Kochevar R.E. Revealing pelagic habitat use: the tagging of Pacific pelagics program. Oceanologica Acta. 2002;25:255–266. doi:10.1016/S0399-1784(02)01212-4 [Google Scholar]
- Bridges C.R. Bohr and Root effects in cephalopod haemocyanins—paradox or pressure in Sepia officinalis? In: Portner H.O, O'Dor R.K, MacMillan D.L, editors. Physiology of cephalopod molluscs: lifestyle and performance adaptations. Gordon and Breach; New York, NY: 1994. pp. 121–130. [Google Scholar]
- Brown J.H, Gillooly J.F, Allen A.P, Savage V.M, West G.B. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Bullard S.G, Hay M.E. Palatability of marine macro-holoplankton: nematocysts, nutritional quality, and chemistry as defense against consumers. Limnol. Oceanogr. 2002;47:1456–1467. [Google Scholar]
- Cailliet G.M, Andrews A.H, Burton E.J, Watters D.L, Kline D.E, Ferry-Graham L.A. Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 2001;36:739–764. doi: 10.1016/s0531-5565(00)00239-4. doi:10.1016/S0531-5565(00)00239-4 [DOI] [PubMed] [Google Scholar]
- Castelini M.A, Somero G.N. Buffering capacity of vertebrate muscle: correlations with potentials for anaerobic function. J. Comp. Physiol. B. 1981;143:191–198. [Google Scholar]
- Childress J.J. Respiratory rate and depth of occurrence of midwater animals. Limnol. Oceanogr. 1971;16:104–106. [Google Scholar]
- Childress J.J. The respiratory rates of midwater crustaceans as a function of depth of occurrence and relation to the oxygen minimum layer off southern California. Comp. Biochem. Physiol. A. 1975;50:787–799. doi: 10.1016/0300-9629(75)90146-2. doi:10.1016/0300-9629(75)90146-2 [DOI] [PubMed] [Google Scholar]
- Childress J.J. Effects of pressure, temperature and oxygen on the oxygen-consumption rate of the midwater copepod Gausia princeps. Mar. Biol. 1977;39:19–24. doi:10.1007/BF00395588 [Google Scholar]
- Childress J.J. Capture and live recovery of deep-sea crustaceans. Natl Geogr. Soc. Res. Rep. 1985;21:67–69. [Google Scholar]
- Childress J.J. Are there physiological and biochemical adaptations of metabolism in deep-sea animals? Trends Ecol. Evol. 1995;10:30–36. doi: 10.1016/s0169-5347(00)88957-0. doi:10.1016/S0169-5347(00)88957-0 [DOI] [PubMed] [Google Scholar]
- Childress J.J, Mickel T.J. Metabolic rates of animals from the hydrothermal vents and other deep-sea habitats. Biol. Soc. Wash. 1985;6:249–260. [Google Scholar]
- Childress J.J, Nygaard M.H. Chemical composition and buoyancy of midwater crustaceans as function of depth of occurrence off Southern California. Mar. Biol. 1974;27:225–238. doi:10.1007/BF00391948 [Google Scholar]
- Childress J.J, Seibel B.A. Life at stable low oxygen: adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 1998;201:1223–1232. doi: 10.1242/jeb.201.8.1223. [DOI] [PubMed] [Google Scholar]
- Childress J.J, Somero G.N. Depth-related enzymatic activities in muscle, brain, and heart of deep-living pelagic teleosts. Mar. Biol. 1979;52:273–283. doi:10.1007/BF00398141 [Google Scholar]
- Childress J.J, Somero G.N. Metabolic scaling: a new perspective based on scaling of glycolytic enzyme activities. Am. Zool. 1990;30:161–173. [Google Scholar]
- Childress J.J, Thuesen E.V. Metabolic potentials of deep-sea fishes: a comparative approach. In: Hochachka P.W, Mommsen T.P, editors. Biochemistry and molecular biology of fishes. Elsevier Science; Berlin, Germany: 1995. pp. 175–195. [Google Scholar]
- Childress J.J, Barnes A.T, Quetin L.B, Robison B.H. Thermally protecting cod ends for the recovery of living deep-sea animals. Deep Sea Res. 1978;25:419–422. doi:10.1016/0146-6291(78)90568-4 [Google Scholar]
- Childress J.J, Taylor S.M, Cailliet G.M, Price M.H. Patterns of growth, energy utilization and reproduction in some meso- and bathypelagic fishes off southern California. Mar. Biol. 1980;61:27–40. doi:10.1007/BF00410339 [Google Scholar]
- Childress J.J, Cowles D.L, Favuzzi J.A, Mickel T.J. Metabolic rates of benthic deep-sea decapod crustaceans decline with increasing depth primarily due to the decline in temperature. Deep Sea Res. 1990a;37:929–949. doi:10.1016/0198-0149(90)90104-4 [Google Scholar]
- Childress J.J, Price M.H, Favuzzi J, Cowles D. Chemical composition of midwater fishes as a function of depth of occurrence off the Hawaiian Islands: food availability as a selective factor? Mar. Biol. 1990b;105:235–246. doi:10.1007/BF01344292 [Google Scholar]
- Clarke A. Life in cold water: the physiological ecology of polar marine ectotherms. Oceanogr. Mar. Biol. 1983;21:341–453. [Google Scholar]
- Clarke A. Is there a universal temperature dependence of metabolism? Funct. Ecol. 2004;18:252–256. doi:10.1111/j.0269-8463.2004.00842.x [Google Scholar]
- Clarke A, Johnston N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999;68:893–905. doi:10.1046/j.1365-2656.1999.00337.x [Google Scholar]
- Collins M.A, Priede I.G, Bagley P.M. In situ comparison of activity in two deep-sea scavenging fishes occupying different depth zones. Proc. R. Soc. B. 1999;266:2011–2016. doi:10.1098/rspb.1999.0879 [Google Scholar]
- Company J.B, Sarda F. Metabolic rates and energy content of deep-sea benthic decapod crustaceans in the western Mediterranean Sea. Deep Sea Res. I. 1998;45:1861–1880. doi:10.1016/S0967-0637(98)00034-X [Google Scholar]
- Cowles D.L. Swimming dynamics of the mesopelagic vertically migrating penaeid shrimp Sergestes similis: modes and speeds of swimming. J. Crustacean Biol. 1994;14:247–257. doi:10.2307/1548905 [Google Scholar]
- Cowles D.L. Swimming speed and metabolic rate during routine swimming and simulated diel vertical migration of Sergestes similis in the laboratory. Pac. Sci. 2001;55:215–226. doi:10.1353/psc.2001.0021 [Google Scholar]
- Cowles D.L, Childress J.J. Swimming speed and oxygen consumption of the bathypelagic mysid Gnathophausia ingens. Biol. Bull. 1988;175:111–121. doi:10.2307/1541898 [Google Scholar]
- Cowles D.L, Childress J.J, Wells M.E. Metabolic rates of midwater crustaceans as a function of depth of occurrence off the Hawaiian Islands: food availability as a selective factor? Mar. Biol. 1991;110:75–83. doi:10.1007/BF01313094 [Google Scholar]
- Dalhoff E.P. Biochemical indicators of stress and metabolism: applications for marine ecological studies. Annu. Rev. Physiol. 2004;66:183–207. doi: 10.1146/annurev.physiol.66.032102.114509. doi:10.1146/annurev.physiol.66.032102.114509 [DOI] [PubMed] [Google Scholar]
- del Giorgio P.A, Duarte C.M. Respiration in the open ocean. Nature. 2002;420:379–384. doi: 10.1038/nature01165. doi:10.1038/nature01165 [DOI] [PubMed] [Google Scholar]
- de Queiroz A. Do image-forming eyes promote evolutionary diversification? Evolution. 1999;53:1654–1664. doi: 10.1111/j.1558-5646.1999.tb04551.x. doi:10.2307/2640429 [DOI] [PubMed] [Google Scholar]
- Dewar H, Graham J. Studies of tropical tuna swimming performance in a large water tunnel—kinematics. J. Exp. Biol. 1994;192:45–59. doi: 10.1242/jeb.192.1.45. [DOI] [PubMed] [Google Scholar]
- Dickson K.A. Unique adaptations of the metabolic biochemistry of tunas and billfishes for life in the pelagic environment. Environ. Biol. Fishes. 1995;42:65–97. doi:10.1007/BF00002352 [Google Scholar]
- Dickson K.A. Locomotor muscle of high-performance fishes: what do comparisons of tunas with ectothermic sister taxa reveal? Comp. Biochem. Physiol. A. 1996;113:39–49. doi:10.1016/0300-9629(95)02056-X [Google Scholar]
- Dickson K.A, Somero G.N. Partial characterization of the buffering components of the red and white myotomal muscle of marine teleosts, with special emphasis on scombrid fishes. Physiol. Zool. 1987;60:699–706. [Google Scholar]
- Domenici P. The visually mediated escape response in fish: predicting prey responsiveness and the locomotor behaviour of predators and prey. Mar. Freshw. Behav. Physiol. 2002;35:87–110. doi:10.1080/10236240290025635 [Google Scholar]
- Donley J, Sepulveda C.A, Konstantinidis P, Gemballa S, Shadwick R.E. Convergent evolution in mechanical design of lamnid sharks and tunas. Nature. 2004;429:61–65. doi: 10.1038/nature02435. doi:10.1038/nature02435 [DOI] [PubMed] [Google Scholar]
- Drazen J.C. Energy budgets and feeding rates of Coryphaenoides acrolepis and C. armatus. Mar. Biol. 2002a;140:677–686. doi:10.1007/s00227-001-0747-8 [Google Scholar]
- Drazen J.C. A seasonal analysis of the nutritional condition of deep-sea macrourid fishes in the north-east Pacific. J. Fish Biol. 2002b;60:1280–1295. doi:10.1111/j.1095-8649.2002.tb01720.x [Google Scholar]
- Drazen J.C. Depth related trends in proximate composition of demersal fishes in the eastern North Pacific. Deep Sea Res. I. 2007;54:203–219. doi:10.1016/j.dsr.2006.10.007 [Google Scholar]
- Drazen J.C, Robison B.H. Direct observations of the association between deep-sea fish and a giant scyphomedusa. Mar. Freshw. Behav. Physiol. 2004;37:209–214. doi:10.1080/10236240400006190 [Google Scholar]
- Drazen J.C, Seibel B.A. Depth-related trends in metabolism of benthic and benthopelagic fishes. Limnol. Oceanogr. Submitted [Google Scholar]
- Drazen J.C, Bird L.B, Barry J.P. Development of a hyperbaric trap-respirometer for the capture and maintenance of live deep-sea organisms. Limnol. Oceanogr. Methods. 2005;3:488–498. [Google Scholar]
- Eiane K, Aksnes D.L, Bagoien E, Kaartvedt S. Fish or jellies—a question of visibility? Limnol. Oceanogr. 1999;44:1352–1357. [Google Scholar]
- Farrell A.P. From hagfish to tuna: a perspective on cardiac function in fish. Physiol. Zool. 1991;64:1137–1164. [Google Scholar]
- Frank T, Widder E. The correlation of downwelling irradiance and staggered vertical migration patterns of zooplankton in Wilkinson Basin Gulf of Maine. J. Plankton Res. 1997;19:1975–1991. doi:10.1093/plankt/19.12.1975 [Google Scholar]
- Ghalambor C.K, Walker J.A, Reznick D.N. Multi-trait selection, adaptation, and constraints on the evolution of burst swimming performance. Integr. Comp. Biol. 2003;43:431–438. doi: 10.1093/icb/43.3.431. doi:10.1093/icb/43.3.431 [DOI] [PubMed] [Google Scholar]
- Gibbs A, Somero G.N. Na+–K+–adenosine triphosphatase activities in gills of marine teleost fishes: changes with depth, size and locomotory activity level. Mar. Biol. 1990;106:315–321. doi:10.1007/BF01344307 [Google Scholar]
- Gillooly J.F, Brown J.H, West G.B, Savage V.M, Charnov E.L. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. doi:10.1126/science.1061967 [DOI] [PubMed] [Google Scholar]
- Glazier D.S. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005;80:611–662. doi: 10.1017/S1464793105006834. doi:10.1017/S1464793105006834 [DOI] [PubMed] [Google Scholar]
- Gordon M.S, Belman B.W, Chow P.H. Comparative studies on the metabolism of shallow-water and deep-sea marine fishes. IV. Patterns of aerobic metabolism in the mesopelagic deep-sea fangtooth fish Anoplogaster cornuta. Mar. Biol. 1976;35:287–293. doi:10.1007/BF00396876 [Google Scholar]
- Haddock H.D. A golden age of gelata: past and future research on planktonic ctenophores and cnidarians. Hydrobiologia. 2004;530/531:549–556. doi:10.1007/s10750-004-2653-9 [Google Scholar]
- Haddock H.D, Dunn C.W, Pugh P.R, Schnitzler C.E. Bioluminescent and red fluorescent lures in a deep-sea siphonophore. Science. 2005;309:263. doi: 10.1126/science.1110441. doi:10.1126/science.1110441 [DOI] [PubMed] [Google Scholar]
- Hamner W.M. Predation, cover, and convergent evolution in epipelagic oceans. Mar. Freshw. Behav. Physiol. 1995;26:71–89. [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Helly J.J, Levin L.A. Global distribution of naturally occurring marine hypoxia on continental margins. Deep Sea Res. 2004;51:1159–1168. doi:10.1016/j.dsr.2004.03.009 [Google Scholar]
- Henry R.P, Handley H.L, Krarup A, Perry H.M. Respiratory and cardiovascular physiology of two species of deep-water crabs, Chaceon fenneri and C. quinquidens: in nomoxia and hypoxia. J. Crustacean Biol. 1990;10:413–422. doi:10.2307/1548331 [Google Scholar]
- Hernandez-Leon S, Ikeda T. A global assessment of mesozooplankton respiration in the ocean. J. Plankton Res. 2005;27:153–158. doi:10.1093/plankt/fbh166 [Google Scholar]
- Herring P.J. Bioluminescence of marine animals. Nature. 1977;267:788–793. doi:10.1038/267788a0 [Google Scholar]
- Hochachka P.W, Somero G.N. Oxford University Press; Oxford, UK: 2002. Biochemical adaptation: mechanism and process in physiological evolution. [Google Scholar]
- Humphriese S, Ruxton G.D. Why did some ichthyosaurs have such large eyes? J. Exp. Biol. 2002;205:439–441. doi: 10.1242/jeb.205.4.439. [DOI] [PubMed] [Google Scholar]
- Hunt, J. C. 1996 The behavior and ecology of midwater cephalopods from Monterey Bay. PhD dissertation, UMI Press, pp. 231.
- Hunt J.C, Lindsay D.J. Observations on the behavior of Atolla (Scyphozoa: Coronatae) and Nanomia (Hydrozoa: Physonectae): use of the hypertrophied tentacle in prey capture. Plankton Biol. Ecol. 1998;45:239–224. [Google Scholar]
- Hunt J.C, Seibel B.A. Life history of Gonatus onyx (Cephalopoda: Teuthoidea): ontogenetic changes in habitat, behavior and physiology. Mar. Biol. 2000;136:543–552. doi:10.1007/s002270050714 [Google Scholar]
- Ikeda T. Metabolism and chemical composition of crustaceans from the Antarctic mesopelagic zone. Deep Sea Res. I. 1988;35:1991–2002. doi:10.1016/0198-0149(88)90121-5 [Google Scholar]
- Janssens B, Childress J.J, Baguet F, Rees J. Reduced enzymatic antioxidative defense in deep-sea fish. J. Exp. Biol. 2000;203:3717–3725. doi: 10.1242/jeb.203.24.3717. [DOI] [PubMed] [Google Scholar]
- Johnsen S. Hidden in plain sight: the ecology and physiology of organismal transparency. Biol. Bull. 2001;201:301–318. doi: 10.2307/1543609. doi:10.2307/1543609 [DOI] [PubMed] [Google Scholar]
- Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–352. [Google Scholar]
- Koslow J.A. Energetic and life-history patterns of deep-sea benthic, benthopelagic and seamount-associated fish. J. Fish Biol. 1996;49A:54–74. doi:10.1111/j.1095-8649.1996.tb06067.x [Google Scholar]
- Koyama S, Miwa T, Horii M, Ishikawa Y, Horikoshi K, Aizawa M. Pressure-stat aquarium system designed for capturing and maintaining deep-sea organisms. Deep Sea Res. I. 2002;49:2095–2102. doi:10.1016/S0967-0637(02)00098-5 [Google Scholar]
- Longhurst A.R, Bedo A.W, Harrison W.G, Head E.J.H, Sameoto D.D. Vertical flux of respiratory carbon by oceanic diel migrant biota. Deep Sea Res. I. 1990;37:685–694. doi:10.1016/0198-0149(90)90098-G [Google Scholar]
- Lythgoe J.N. Light and vision in the aquatic environment. In: Atema J, Fay R.R, Popper A, Tavolga W.N, editors. Sensory biology of aquatic animals. Springer; New York, NY: 1988. pp. 57–87. [Google Scholar]
- Mahaut M.L, Sibuet M, Shirayama Y. Weight-dependent respiration rates in deep-sea organisms. Deep Sea Res. I. 1995;42:1575–1582. doi:10.1016/0967-0637(95)00070-M [Google Scholar]
- Markaida U, Rosenthal J.J.C, Gilly W.F. Tagging studies of the Humboldt squid Dosidicus gigas, in the Gulf of California, Mexcio. Fish. Bull. 2005;103:219–226. [Google Scholar]
- Marshall N.J, Diebel C. Deep-sea ‘spiders’ that walk through the water. J. Exp. Biol. 1995;198:1371–1379. doi: 10.1242/jeb.198.6.1371. [DOI] [PubMed] [Google Scholar]
- Matsumoto G.I, Harbison G.R. In situ observations of foraging, feeding, and escape behavior in three orders of oceanic ctenophores: Lobata, Cestida, and Beroida. Mar. Biol. 1993;117:279–287. doi:10.1007/BF00345673 [Google Scholar]
- McClintock J.B, Janssen J. Pteropod abduction as a chemical defense in a pelagic antarctic amphipod. Nature. 1990;346:462–464. doi:10.1038/346462a0 [Google Scholar]
- Meek R.P, Childress J.J. Respiration and the effect of pressure in the mesopelagic fish Anoplogaster cornuta (Beryciformes) Deep Sea Res. 1973;20:1111–1118. [Google Scholar]
- Moyes C.D, LeMoine C.M.R. Control of muscle bioenergetic gene expression: implications for allometric scaling relationships of glycolytic and oxidative enzymes. J. Exp. Biol. 2005;208:1601–1610. doi: 10.1242/jeb.01502. doi:10.1242/jeb.01502 [DOI] [PubMed] [Google Scholar]
- Nelson J.A, Claireaux G. Spring swimming performance of juvenile European Sea Bass. Trans. Am. Fish. Soc. 2005;134:1274–1284. doi:10.1577/T04-087.1 [Google Scholar]
- O'Dor R.K. Respiratory metabolism and swimming performance of the squid Loligo opalescens. Can. J. Fish. Aquat. Sci. 1982;39:580–587. [Google Scholar]
- O'Dor R.K. Telemetered cephalopod energetics: swimming, soaring and blimping. Integr. Comp. Biol. 2002;42:1065–1070. doi: 10.1093/icb/42.5.1065. doi:10.1093/icb/42.5.1065 [DOI] [PubMed] [Google Scholar]
- O'Dor R.K, Webber D.M. The constraints on cephalopods: why squid aren't fish. Can. J. Zool. 1986;64:1591–1605. [Google Scholar]
- Packard A. Cephalopods and fish: the limits of convergence. Biol. Rev. 1972;47:241–307. doi:10.1086/407285 [Google Scholar]
- Porter R.K, Brand M.D. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature. 1993;362:628–630. doi: 10.1038/362628a0. doi:10.1038/362628a0 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Environmental and functional limits to muscular exercise and body size in marine invertebrate athletes. Comp. Biochem. Physiol. A. 2002;133:303–321. doi: 10.1016/s1095-6433(02)00162-9. doi:10.1016/S1095-6433(02)00162-9 [DOI] [PubMed] [Google Scholar]
- Poulson T.L. Adaptations of cave fishes with some comparisons to deep-sea fishes. Environ. Biol. Fishes. 2001;62:345–364. doi:10.1023/A:1011893916855 [Google Scholar]
- Priede I.G, Bagley P.M. In situ studies on deep-sea demersal fishes using autonomous unmanned ladder platforms. Annu. Rev. Oceanogr. Mar. Biol. 2000;38:357–392. [Google Scholar]
- Priede I.G, Smith K.L, Jr, Armstrong J.D. Foraging behavior of abyssal grenadier fish: inferences from acoustic tagging and tracking in the North Pacific Ocean. Deep Sea Res. 1990;37:81–101. doi:10.1016/0198-0149(90)90030-Y [Google Scholar]
- Priede I.G, Deary A.R, Bailey D.M, Smith K.L. Low activity and seasonal change in population size structure of granadiers in the oligotrophic abyssal central North Pacific Ocean. J. Fish Biol. 2003;63:187–196. doi:10.1046/j.1095-8649.2003.00142.x [Google Scholar]
- Quetin L.B, Ross R.M, Clarke A. Krill energetics: seasonal and environmental aspects of the physiology of Euphausia superba. In: El-Sayed S.Z, editor. Southern ocean ecology: the BIOMASS perspective. Cambridge University Press; Cambridge, UK: 1994. pp. 165–184. [Google Scholar]
- Raskoff K.A. Foraging, prey capture, and gut contents of the mesopelagic narcomedusae, Solmissus spp. (Cnidaria: Hydrozoa) Mar. Biol. 2002;141:1099–1107. doi:10.1007/s00227-002-0912-8 [Google Scholar]
- Reinhold K. Energetically costly behavior and the evolution of resting metabolic rate in insects. Funct. Ecol. 1999;13:221–224. doi:10.1046/j.1365-2435.1999.00300.x [Google Scholar]
- Robison B.H. Shape-change behavior by mesopelagic animals. Mar. Freshw. Behav. Physiol. 1999;32:17–25. [Google Scholar]
- Robison B.H. The coevolution of undersea vehicles and deep-sea research. Mar. Tech. Soc. J. 2000;33:65–73. [Google Scholar]
- Robison B.H. Deep pelagic biology. J. Exp. Mar. Biol. Ecol. 2004;300:245–264. doi:10.1016/j.jembe.2004.01.012 [Google Scholar]
- Robison B.H, Reisenbichler K.R, Hunt J.C, Haddock S.H.D. Light production by the arm tips of the deep-sea cephalopod Vampyroteuthis infernalis. Biol. Bull. 2003;205:102–109. doi: 10.2307/1543231. doi:10.2307/1543231 [DOI] [PubMed] [Google Scholar]
- Roper C.F.E, Vecchione M. In situ observations test hypotheses of functional morphology in Mastigoteuthis (Cephalopoda, Oegopsida) Vie Milieu. 1997;47:87–94. [Google Scholar]
- Samerotte S.L, Drazen J.C, Brand G.L, Seibel B.A, Yancey P.H. Correlation of trimethylamine oxide and habitat depth within and among species of teleost fishes: an analysis of causation. Physiol. Biochem. Zool. 2007;80:197–208. doi: 10.1086/510566. doi:10.1086/510566 [DOI] [PubMed] [Google Scholar]
- Sanders N.K, Childress J.J. Ion replacement as a buoyancy mechanism in a pelagic deep-sea crustacean. J. Exp. Biol. 1988;138:333–343. [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, UK: 1984. Scaling: why is animal size so important? [Google Scholar]
- Seibel B.A. On the depth and scale of metabolic rate variation: scaling of oxygen consumption and enzymatic activity in the Class Cephalopoda (Mollusca) J. Exp. Biol. 2007;210:1–11. doi: 10.1242/jeb.02588. doi:10.1242/jeb.02588 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Carlini D.B. Metabolism of pelagic cephalopods as a function of habitat depth: a reanalysis using phylogenetically independent contrasts. Biol. Bull. 2001;201:1–5. doi: 10.2307/1543519. doi:10.2307/1543519 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Childress J.J. Metabolism of benthic octopods (Cephalopoda) as a function of habitat depth and oxygen concentration. Deep Sea Res. 2000;47:1247–1260. doi:10.1016/S0967-0637(99)00103-X [Google Scholar]
- Seibel B.A, Dierssen H.M. Tip of the iceberg: cascading trophic impacts of the iceberg, B-15, in the Ross Sea, Antarctica. Biol. Bull. 2003;205:93–99. doi: 10.2307/1543229. doi:10.2307/1543229 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Walsh P.J. Potential impacts of CO2 injection on deep-sea biota. Science. 2001;294:319–320. doi: 10.1126/science.1065301. doi:10.1126/science.1065301 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Walsh P.J. Trimethylamine oxide accumulation in marine animals: relationship to acylglycerol storage. J. Exp. Biol. 2002;205:297–306. doi: 10.1242/jeb.205.3.297. [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Walsh P.J. Biological impacts of deep-sea carbon dioxide injection inferred from indices of physiological performance. J. Exp. Biol. 2003;206:641–650. doi: 10.1242/jeb.00141. doi:10.1242/jeb.00141 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Thuesen E.V, Childress J.J, Gorodezky L.A. Decline in pelagic cephalopod metabolism with habitat depth reflects differences in locomotory efficiency. Biol. Bull. 1997;192:262–278. doi: 10.2307/1542720. doi:10.2307/1542720 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Thuesen E.V, Childress J.J. Flight of the vampire: ontogenetic gait-transition in Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha) J. Exp. Biol. 1998;201:2413–2424. doi: 10.1242/jeb.201.16.2413. [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Chausson F, Lallier F.H, Zal F, Childress J.J. Vampire blood: respiratory physiology of the vampire squid (Cephalopoda: Vampyromorpha) in relation to the oxygen minimum layer. Exp. Biol. Online. 1999;4:1–10. [Google Scholar]
- Seibel B.A, Thuesen E.V, Childress J.J. Light-limitation on predator–prey interactions: consequences for metabolism and locomotion of deep-sea cephalopods. Biol. Bull. 2000;198:284–298. doi: 10.2307/1542531. doi:10.2307/1542531 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Goffredi S.K, Thuesen E.V, Childress J.J, Robison B.H. Ammonium content and buoyancy in midwater cephalopods. J. Exp. Mar. Biol. Ecol. 2004;313:375–387. doi:10.1016/j.jembe.2004.08.015 [Google Scholar]
- Seibel B.A, Robison B.H, Haddock S.D.H. First observations of post-spawning egg-care in a squid. Nature. 2005;438:929. doi: 10.1038/438929a. doi:10.1038/438929a [DOI] [PubMed] [Google Scholar]
- Shadwick R.E, O'Dor R.K, Gosline J.M. Respiratory and cardiac function during exercise in squid. Can. J. Zool. 1990;68:792–798. [Google Scholar]
- Shirayama Y. Respiration rates of bathyal meiobenthos collected using a deep-sea submersible SHINKAI 2000. Deep Sea Res. 1992;39:781–788. doi:10.1016/0198-0149(92)90120-I [Google Scholar]
- Siebenaller J.F, Somero G.N, Haedrich R.L. Biochemical characteristics of macrourid fishes differing in their depths of distribution. Biol. Bull. 1982;163:240–249. doi:10.2307/1541512 [Google Scholar]
- Smith K.L., Jr Metabolism of the abyssopelagic rattail Coryphaenoides armatus measured in situ. Nature. 1978;274:362–364. doi:10.1038/274362a0 [Google Scholar]
- Smith K.L., Jr Metabolism of two dominant epibenthic echinoderms measured at bathyal depths in the Santa Catalina Basin. Mar. Biol. 1983;72:249–256. doi:10.1007/BF00396830 [Google Scholar]
- Smith K.L, Jr, Baldwin R.J. Laboratory and in situ methods for studying deep-sea fishes. In: Randall D.J, Farrell A.P, editors. Deep-sea fishes. Academic Press; San Diego, CA: 1997. pp. 351–378. [Google Scholar]
- Smith Jr K.L, Baldwin R.J. Scavenging deep-sea amphipods: effects of food odour on oxygen consumption and a proposed metabolic strategy. Mar. Biol. 1982;68:287–298. doi:10.1007/BF00409595 [Google Scholar]
- Smith K.L, Jr, Hessler R.R. Respiration of benthopelagic fishes: in situ measurements at 1230 meters. Science. 1974;184:72–73. doi: 10.1126/science.184.4132.72. doi:10.1126/science.184.4132.72 [DOI] [PubMed] [Google Scholar]
- Smith K.L, Jr, Laver M.B. Respiration of the bathypelagic fish Cyclothone acclinidens. Mar. Biol. 1981;61:261–266. doi:10.1007/BF00401564 [Google Scholar]
- Smith K.L, Jr, Teal J.M. Temperature and pressure effects on respiration of thecosomatous pteropods. Deep Sea Res. 1973;20:853–858. [Google Scholar]
- Somero G.N. Adaptations to high hydrostatic pressure. Annu. Rev. Physiol. 1992;54:557–577. doi: 10.1146/annurev.ph.54.030192.003013. doi:10.1146/annurev.ph.54.030192.003013 [DOI] [PubMed] [Google Scholar]
- Somero G.N, Childress J.J. A violation of the metabolism-size scaling paradigm: activities of glycolytic enzymes in muscle increase in larger-size fish. Physiol. Zool. 1980;53:322–337. [Google Scholar]
- Somero G.N, Siebenaller J.F. Inefficient lactate dehydrogenases of deep-sea fishes. Nature. 1979;282:100–102. doi: 10.1038/282100a0. doi:10.1038/282100a0 [DOI] [PubMed] [Google Scholar]
- Suarez R.K. Upper limits to mass-specific metabolic rates. Annu. Rev. Physiol. 1996;58:583–605. doi: 10.1146/annurev.ph.58.030196.003055. doi:10.1146/annurev.ph.58.030196.003055 [DOI] [PubMed] [Google Scholar]
- Sullivan K.M, Somero G.N. Enzyme activities of fish skeletal muscle and brain as influenced by depth of occurrence and habits of feeding and locomotion. Mar. Biol. 1980;60:91–99. doi:10.1007/BF00389152 [Google Scholar]
- Taylor C.R, Maloiy G.M, Weibel E.R, Langman V.A, Kamau J.M, Seeherman H.J, Heglund N.C. Design of the mammalian respiratory system. III. Scaling maximum aerobic capacity to body mass: wild and domestic mammals. Respir. Physiol. 1981;44:25–37. doi: 10.1016/0034-5687(81)90075-x. doi:10.1016/0034-5687(81)90075-X [DOI] [PubMed] [Google Scholar]
- Thistle D, Carman K.R, Sedlacek L, Brewer P.G, Fleeger J.W, Barry J.P. Deep-ocean, sediment-dwelling animals are sensitive to sequestered carbon dioxide. Mar. Ecol. Prog. Ser. 2005;289:1–4. [Google Scholar]
- Thuesen E.V, Childress J.J. Enzymatic activities and metabolic rates of pelagic chaetognaths: lack of depth-related declines. Limnol. Oceanogr. 1993a;38:935–948. [Google Scholar]
- Thuesen E.V, Childress J.J. Metabolic rates, enzyme activities and chemical compositions of some deep-sea pelagic worms, particularly Nectonemertes mirabilis (Nemertea; Hoplonemertinea) and Poeobius meseres (Annelida; Polychaeta) Deep Sea Res. I. 1993b;40:937–951. doi:10.1016/0967-0637(93)90082-E [Google Scholar]
- Thuesen E.V, Childress J.J. Effects of hydrostatic pressure on metabolic rates of six deep-sea gelatinous zooplankton. Limnol. Oceanogr. 1993c;38:665–670. [Google Scholar]
- Thuesen E.V, Childress J.J. Oxygen consumption rates and metabolic enzyme activities of oceanic California medusae in relation to body size and habitat depth. Biol. Bull. 1994;187:84–98. doi: 10.2307/1542168. doi:10.2307/1542168 [DOI] [PubMed] [Google Scholar]
- Thuesen E.V, Miller C.B, Childress J.J. Ecophysiological interpretation of oxygen consumption rates and enzymatic activities of deep-sea copepods. Mar. Ecol. Prog. Ser. 1998;168:95–107. [Google Scholar]
- Thuesen E.V, Rutherford L.D, Jr, Brommer P.L, Garrison K, Gutowska M.A, Towanda T. Intragel oxygen promotes hypoxia tolerance of scyphomedusae. J. Exp. Biol. 2005a;208:2475–2482. doi: 10.1242/jeb.01655. doi:10.1242/jeb.01655 [DOI] [PubMed] [Google Scholar]
- Thuesen E.V, McCullough K.D, Childress J.J. Metabolic enzyme activities in swimming muscle of medusae: is the scaling of glycolytic activity related to oxygen availability? J. Mar. Biol. Assoc. UK. 2005b;85:603–611. doi:10.1017/S0025315405011537 [Google Scholar]
- Thurston M.H, Bett B.J, Rice A.L, Jackson P.A.B. Variations in the invertebrate abyssal megafauna in the North Atlantic Ocean. Deep Sea Res. I. 1994;41:1321–1348. doi:10.1016/0967-0637(94)90100-7 [Google Scholar]
- Thurston M.H, Rice A.L, Bett B.J. Latitudinal variation in invertebrate megafaunal abundance and biomass in the North Atlantic Ocean Abyss. Deep Sea Res. II. 1998;45:1–3. doi:10.1016/S0967-0645(97)00077-5 [Google Scholar]
- Torres J.J, Somero G.N. Metabolism, enzymic activities and cold adaption in Antarctic mesopelagic fishes. Mar. Biol. 1988;98:169–180. doi:10.1007/BF00391192 [Google Scholar]
- Torres J.J, Belman B.W, Childress J.J. Oxygen consumption rates of midwater fishes as a function of depth of occurrence. Deep Sea Res. 1979;26:185–197. doi:10.1016/0198-0149(79)90075-X [Google Scholar]
- Treberg J.R, Martin R.A, Driedzic W.R. Muscle enzyme activities in a deep-sea squaloid shark, Centroscyllium fabricii, compared with its shallow-living relative, Squalus acanthias. J. Exp. Zool. A. 2003;300:133–139. doi: 10.1002/jez.a.10318. doi:10.1002/jez.a.10318 [DOI] [PubMed] [Google Scholar]
- Treude T, Janssen F, Queisser W, Witte U. Metabolism and decompression tolerance of scavenging lysianassoid deep-sea amphipods. Deep Sea Res. I. 2002;49:1281–1289. doi:10.1016/S0967-0637(02)00023-7 [Google Scholar]
- Verity P.G, Smetacek V, Smayda T.J. Status, trends and the future of the marine pelagic ecosystem. Environ. Conserv. 2002;29:207–237. doi:10.1017/S0376892902000139 [Google Scholar]
- Villanueva R, Segonzac M, Guerra A. Locomotion modes of deep-sea cirrate octopods (Cephalopoda) based on observations from video recordings on the Mid- Atlantic Ridge. Mar. Biol. 1997;129:113–122. doi:10.1007/s002270050152 [Google Scholar]
- Walsh P.J, Henry R.P. Activities of metabolic enzymes in the deep-water crabs Chaceon fenneri and C. quinquedens and the shallow-water crab Callinectes sapidus. Mar. Biol. 1990;106:343–346. doi:10.1007/BF01344310 [Google Scholar]
- Warrant E.J. Vision in the dimmest habitats on Earth. J. Comp. Physiol. A. 2004;190:765–789. doi: 10.1007/s00359-004-0546-z. doi:10.1007/s00359-004-0546-z [DOI] [PubMed] [Google Scholar]
- Warrant E.J, Locket N.A. Vision in the deep-sea. Biol. Rev. Camb. Phil. Soc. 2004;79:671–712. doi: 10.1017/s1464793103006420. doi:10.1017/S1464793103006420 [DOI] [PubMed] [Google Scholar]
- Webber D.M, Aitken J, O'Dor R.K. Costs of vertical locomotion and vertic dynamics of cephalopods and fish. Physiol. Biochem. Zool. 2000;73:651–662. doi: 10.1086/318100. doi:10.1086/318100 [DOI] [PubMed] [Google Scholar]
- Weibel E, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J. Exp. Biol. 2005;208:1635–1644. doi: 10.1242/jeb.01548. doi:10.1242/jeb.01548 [DOI] [PubMed] [Google Scholar]
- White C.R, Seymour R.S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl Acad. Sci. USA. 2003;100:4046–4049. doi: 10.1073/pnas.0436428100. doi:10.1073/pnas.0436428100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder E.A. Bioluminescence and the pelagic visual environment. Mar. Freshw. Behav. Physiol. 2002;35:1–26. doi:10.1080/10236240290025581 [Google Scholar]
- Williams T.M. The evolution of cost efficient swimming in marine mammals: limits to energetic optimization. Phil. Trans. R. Soc. B. 1999;354:193–201. doi:10.1098/rstb.1999.0371 [Google Scholar]
- Wishner K, Levin L.A, Gowing M, Mullineaux L. Involvement of the oxygen minimum in benthic zonation on a deep seamount. Nature. 1990;346:57–59. doi:10.1038/346057a0 [Google Scholar]
- Withers P, Hefter G, Pang T.S. The role of urea and methylamines in buoyancy of elasmobranchs. J. Exp. Biol. 1994;188:175–189. doi: 10.1242/jeb.188.1.175. [DOI] [PubMed] [Google Scholar]
- Witte U, Graf G. Metabolism of deep-sea sponges in the Greenland–Norwegian Sea. J. Exp. Mar. Biol. Ecol. 1996;198:223–235. doi:10.1016/0022-0981(96)00006-8 [Google Scholar]
- Yamada Y, Ikeda T. Metabolism and chemical composition of four pelagic amphipods in the Oyashio region, western subarctic Pacific Ocean. Mar. Ecol. Prog. Ser. 2003;253:233–241. [Google Scholar]
- Yancey P.H. Organic osmolytes as compatible, metabolic and counteracting croprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. doi:10.1242/jeb.01730 [DOI] [PubMed] [Google Scholar]
- Yang T.H, Lai N.C, Graham J.B, Somero G.N. Respiratory, blood, and heart enzymatic adaptations of Sebastolobus alascanus (Scorpaenidae; Teleostei) to the oxygen minimum zone: a comparative study. Biol. Bull. 1992;183:490–499. doi: 10.2307/1542026. doi:10.2307/1542026 [DOI] [PubMed] [Google Scholar]
- Young R.E. Oceanic bioluminescence: an overview of general functions. Bull. Mar. Sci. 1983;33:829–845. [Google Scholar]
- Youngbluth M.J, Båmstedt U. Distribution, abundance, behavior and metabolism of Periphylla periphylla, a mesopelagic coronate medusa in a Norwegian fjord. Hydrobiolgia. 2001;451:321–333. doi:10.1023/A:1011874828960 [Google Scholar]
- Zeidberg L.D. Allometry measurements from in situ video recordings can detemine the size and swimming speeds of juvenile and adult squid Loligo opalescens (Cephalopoda: Myopsida) J. Exp. Biol. 2004;207:4195–4203. doi: 10.1242/jeb.01276. doi:10.1242/jeb.01276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Metabolic scaling relationships of marine animals; Table 2. Metabolic rates of benthic crustaceans, echinoderms and fishes; Table 3. Metabolic rates of cephalopods