Abstract
While aquatic environments have long been thought to be more moderate environments than their terrestrial cousins, environmental data demonstrate that for some systems this is not so. Numerous important environmental parameters can fluctuate dramatically, notably dissolved oxygen, turbidity and temperature. The roles of dissolved oxygen and turbidity on predator–prey interactions have been discussed in detail elsewhere within this issue and will be considered only briefly here. Here, we will focus primarily on the role of temperature and its potential impact upon predator–prey interactions. Two key properties are of particular note. For temperate aquatic ecosystems, all piscine and invertebrate piscivores and their prey are ectothermic. They will therefore be subject to energetic demands that are significantly affected by environmental temperature. Furthermore, the physical properties of water, particularly its high thermal conductivity, mean that thermal microenvironments will not exist so that fine-scale habitat movements will not be an option for dealing with changing water temperature in lentic environments. Unfortunately, there has been little experimental analysis of the role of temperature on such predator–prey interactions, so we will instead focus on theoretical work, indicating that potential implications associated with thermal change are unlikely to be straightforward and may present a greater threat to predators than to their prey. Specifically, we demonstrate that changes in the thermal environment can result in a net benefit to cold-adapted species through the mechanism of predator–prey interactions.
Keywords: temperature, predator–prey, scaling, dissolved oxygen, turbidity
1. Introduction
The fundamental tenet underlying studies of animal design is evolution by natural selection. Simply stated, it argues that within the constraints imposed by phylogenetic history and genetic mechanisms, organisms will evolve phenotypes that are well adapted to their environment (Mayr 1983). While the level to which organisms can be adapted to their environment has been debated for some time (e.g. Gould & Lewontin 1979), it is undeniable that the environment which confronts organisms will be a major evolutionary force. From this perspective, a changing environment will then present organisms with a new range of challenges.
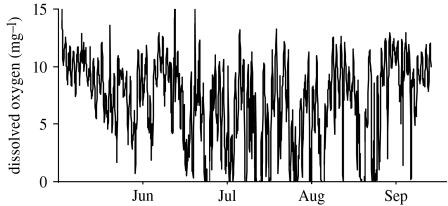
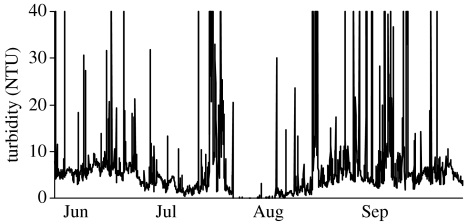
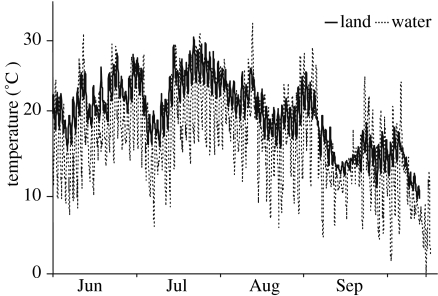
It is assumed that aquatic environments will be less affected by a changing environment, since the physical properties of water should buffer aquatic environments from extreme change. However, temperate aquatic environments can exhibit dramatic fluctuations in a number of key environmental parameters through time. Most notable among these are dissolved oxygen (DO), turbidity and temperature. Within central North America, placement of automated data loggers (YSI 6920 data sondes) within the shallow waters of Delta marsh (located at the southern tip of Lake Manitoba, 49.973081° N–98.291244° E) indicates that all these parameters undergo a wide range of variation through time. Dissolved oxygen levels through most of the summer months tend to remain below 5 mg l−1 and can become almost completely anoxic for considerable periods of time whereas the spring and autumn months are characterized by water that is well oxygenated (figure 1). Similarly, turbidity within this location is highly variable (figure 2). Typically, we have observed that turbidity in excess of 13 nephelometric turbidity units (NTUs) can significantly alter interactions between predators and their prey (Abrahams 1994; Abrahams & Kattenfeld 1997). The mechanism generating turbidity at this location is suspended inorganic particles and we believe that periods of high turbidity are then associated with anything that generates water turbulence (i.e. wind or movement of large fish). Owing to this mechanism, the pattern of turbidity within this environment is highly variable and much less predictable than changes in dissolved oxygen. Because of the shallow depth, this location also experiences substantial changes in temperature, with daytime levels approaching 30°C. Unlike many other aquatic environments, temperatures within the water do not appear to be greatly buffered when compared with those recorded on land (figure 3).
Figure 1.
Dissolved oxygen levels (mg l−1) measured 10 cm above the substrate in Delta marsh, located at the south end of Lake Manitoba.
Figure 2.
Turbidity (measured in NTUs) measured 10 cm above the substrate in Delta marsh, located at the south end of Lake Manitoba.
Figure 3.
Temperature measured 10 cm above the substrate in Delta marsh, located at the south end of Lake Manitoba.
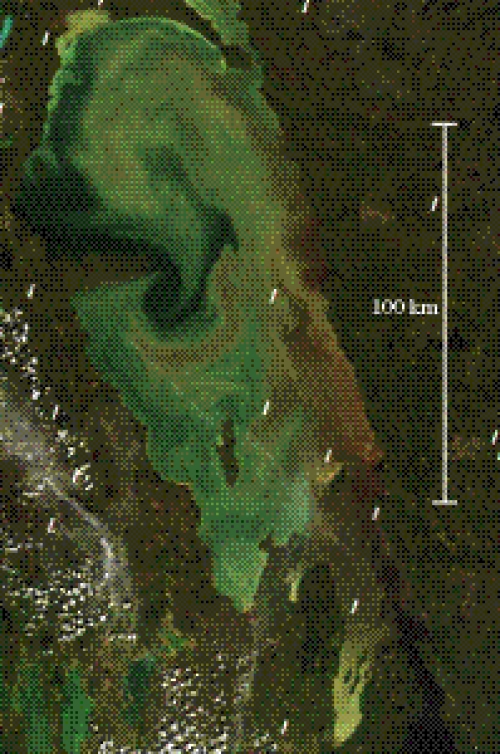
While this environment experiences significant changes through time, so too can these parameters vary through location. Satellite imagery of Lake Winnipeg, the world's tenth largest lake and located close to Delta marsh, exhibits large variation in turbidity. This is primarily due to the effect of different rivers flowing into this lake. Relatively clear water flowing in from the Canadian Shield mixes with turbid water running off the North American prairies to produce large gyres that vary in turbidity (figure 4). Such a physical parameter is readily visible from satellite imagery and it is not unreasonable to assume that rivers providing water with other different physical characteristics, as well as variation in local biological conditions can generate similar spatial variation in key environmental parameters.
Figure 4.
Satellite image (June 2005) of the north basin of Lake Winnipeg illustrating the pattern of turbid water throughout the basin.
With this background of the physical environment, we describe the impact that variation in some environmental parameters can have on predator–prey interactions within temperate aquatic ecosystems, and in particular, we seek to determine whether it is the predator or prey that is the beneficiary of such change.
In considering the impact of these environmental parameters, we are assuming that both predator and prey are fish species, and that the predator must be significantly larger than the prey. The actual size difference between the predators and prey has been developed elsewhere (Damsgård 1995; Persson et al. 1996; Nilsson & Brönmark 2000; Magnhagen & Heibo 2001) and is a well-known phenomenon within aquatic biology.
(a) Dissolved oxygen
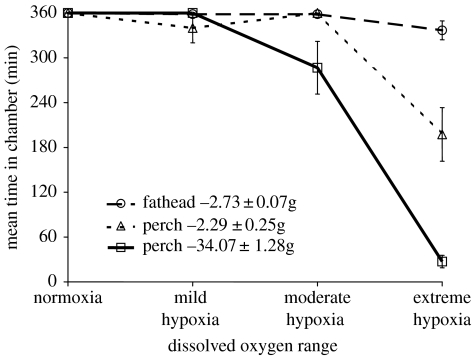
The species upon which most of the work on dissolved oxygen and turbidity we describe is based on the fathead minnow (Pimephales promelas) as prey and the yellow perch (Perca flavescens) as predator. Earlier work (Robb & Abrahams 2003) has demonstrated that fathead minnows are considerably more tolerant of hypoxic environments than their predator (figure 5). In these experiments, we measured the time until loss of equilibrium in four different concentrations of dissolved oxygen (measured in mg l−1): 7.2 (normoxic), 3.5 (mild hypoxic), 2.5 (hypoxic) and 1.8 (extreme hypoxia). Three different groups of individuals were used: adult fathead minnows, yellow perch of a size capable of consuming these minnows and juvenile yellow perch that were the same size as the minnows. Our data (figure 4) demonstrated that of all three groups, the fathead minnows were the most tolerant of the hypoxic environment. In most instances, they were capable of withstanding all hypoxic conditions for the duration of the 3 h trial. The only exceptions were some of the largest minnows within the extreme hypoxic condition. This was in stark contrast to the performance of the large yellow perch. Most could not tolerate the moderate hypoxic environment for the duration of the 3 h trial, and all trials within the extreme hypoxia treatment were terminated within 30 min. Using yellow perch that were the same size as the fathead minnows generated an intermediate response. These smaller yellow perch could tolerate more hypoxic environments but still were unable to achieve the performance of the fathead minnows. These data suggest that tolerance to hypoxic environments, at least among these species, is largely based upon allometric issues. Furthermore, we have found that prey are able to detect hypoxic stress within their predators at dissolved oxygen levels that can normally be tolerated by their predator. In a behavioural experiment, we measured the impact of predation risk on habitat quality using the technique of Abrahams & Dill (1989), under normoxic and mild hypoxic conditions (3.5 mg l−1), a level of hypoxia that generated no observable stress in either the predator or the prey. These experiments demonstrate that under mild hypoxic conditions prey are able to detect hypoxic stress within their predators at dissolved oxygen levels that can normally be tolerated by their predator. Even though these levels of hypoxia generate no measurable levels of stress, it apparently renders them ineffective as a predator (Robb & Abrahams 2003). The ecological implication of this result is that prey may intentionally seek hypoxic environments under the risk of predation as a refuge. To test this hypothesis, we created a three-chambered apparatus (see Abrahams & Sloan submitted for details) that allowed fathead minnows and a yellow perch predator to freely move between and choose among environments. Two of the barrels contained normoxic water, the third was hypoxic and all locations contained food for the prey. The barrels were arranged in a triangle and connected to each other by a 1.7 m pipe. Each fish (20 prey and 1 predator) contained a passive integrated transponder (PIT) tag and their movements were monitored by six PIT tag readers that recorded when fish moved in or out of any specific habitat. By the location of the readers and synchronizing their internal clocks, we were able to generate a dataset that allowed us to determine the location of all individuals continuously for a two-week period and this was done separately for five different groups.
Figure 5.
The time until loss of equilibrium for fathead minnows and yellow perch exposed to four different dissolved oxygen environments. Results illustrate the mean±1 s.e. The maximum time available within the apparatus is 360 min. From Robb & Abrahams (2003) and reproduced with permission from Blackwell Publishing.
These data demonstrated that prey initially preferred the hypoxic environment, and were able to effectively avoid the predator using this habitat. However, through time the refuge became increasingly less effective as the predator was able to make increased use of that habitat. Our interpretation of these data is that the predator is able to acclimate to the hypoxic environment and hence defeats this environment's capacity to function as a refuge.
We believe that differences in physiological tolerance that are within the range of physiological adaptation through acclimation will only be of ecological significance if they are ephemeral. Furthermore, hypoxic environments that can be occupied by some species are likely to be avoided under conditions of low risk since their use will probably incur some energetic cost (unless this energetic cost is compensated by benefits through competition). However, under threat of predation, they will probably become preferred habitats due to diminished risk. The ecological value of hypoxic environments may become even more pronounced when the role of aerial predators is considered. One option that piscivorous fish can exploit to use hypoxic environments is aquatic surface respiration (ASR). But in so doing, these fish then make themselves vulnerable to their aerial predators. If there is a range of dissolved oxygen levels that require ASR by the predators, and not their prey, then it is at this point that aerial predators may amplify the value of hypoxic environments to prey species (Kramer 1987).
There is evidence that hypoxic refuges may have occurred in African lakes that were subject to the introduction of the Nile perch (Lates niloticus). This is a species that grows to a very large size and is a voracious piscivore. It was introduced into lakes that contained primarily small species including the diverse haplochromine assemblage. The consequence of this introduction was the extinction of many species of haplochromine cichlids, including some species that were important herbivores. Within Lake Victoria, the removal of these herbivores shifted a lake that was mesotrophic dominated by diatoms to a eutrophic lake dominated by blue-green algae (Hecky 1993). Large amounts of decaying vegetation within the lake resulted in large regions that became hypoxic or anoxic, and it was within wetlands adjacent to the lake that several species of haplochromines that were thought to be extinct were discovered (Chapman et al.1996a,b, 1999). While such locations do provide the structural complexity important in evading predators, it was noted that such locations contained hypoxic waters that cannot be tolerated by the Nile perch.
From the perspective of who benefits under conditions of hypoxia, the answer will hinge critically on the physiological basis for hypoxia tolerance. In the yellow perch/fathead minnow system we have described, the benefits accrue primarily to smaller individuals suggesting that hypoxic refuges may exist for the prey. However, there is also evidence that some fish are capable of reducing aerobic demands and increasing anaerobic capacity by increasing levels of lactate and malate dehydrogenase within major organs as they become larger (Almeida-Val et al. 1995, 2000). Responses of individuals less tolerant of hypoxic conditions may be to concentrate them in locations of water higher in DO but in so doing may make them more vulnerable to predators.
(b) Turbidity
It has been recognized for some time that the risk of predation may exert at least as great, if not a greater impact upon an ecosystem than the actual act of predation. For predators to exert this influence, it is necessary that prey be able to detect the presence of predators and modify their behaviour in a way that will affect their probability of being killed (reviewed by Lima & Dill 1990). Many fish species have very well developed eyes and it is not unreasonable to assume that they are capable of detecting their predators visually (Blaxter & Fuiman 1990; Fuiman & Magurran 1994). However, varying turbidity levels, as experienced within our study location and also within many other aquatic ecosystems will significantly alter fishes' ability to detect their predators (Miner & Stein 1996). There are two non-mutually exclusive actions that can occur when this happens. Considering only visual information for identifying predators and the subsequent risk they impose, increasing turbidity will result in reducing the distance at which predators can be detected. Ultimately, predators may be only detected at the point where their location is so close that the outcome of a predator–prey interaction will be determined by who detects whom first. Under this condition, these interactions will very closely approximate the conditions that underlie the Lotka–Volterra predator–prey model and hence it is reasonable to assume that predator–prey interactions will not be affected by modified behaviours associated with the risk of predation. Evidence in support of this assertion is the experiment by Abrahams & Kattenfeld (1997) that demonstrated that size-dependent mortality became size-independent when the environment became turbid. This result may be due to size-dependent antipredator behaviours, such as burst swimming, becoming ineffective in a turbid environment.
Extending this scenario, detection rates will then be determined by the speed at which predators and prey move through their environment (Werner & Anholt 1993). Prey rates of movement will probably be optimized by the conflicting demands of increasing encounter rates of food while decreasing encounter rates with predators. Predators will only be selected to move rapidly to increase encounter rates with prey, subject to the constraints associated with the energetics of motion. Under these conditions, it seems reasonable that predators may benefit more than their prey.
This conclusion hinges critically on the mechanism that is used to detect predators and their prey. It is well known that fish within the superorder Ostariophysi rely also on chemical cues to assess the level of predation risk within their environment, specifically the presence of hypoxanthine-3-N-oxide (Pfeiffer et al. 1985; Smith 1992). There is also evidence that other species within the Salmonidae are also capable of detecting this chemical, even though they apparently do not release the chemical themselves (Brown & Smith 1998). Indeed, the conflicting demands of chemical versus visual information have been well demonstrated in comparative studies that show reduction in the size of the optic nerve and other components of the visual system with increasing water turbidity (Huber & Rylander 1992; van Staaden et al. 1994). In situations where both predator and prey are using multiple sources of information, the outcome of predator–prey interactions will be determined by their relative effectiveness (for more detail, see Abrahams 2005).
(c) Temperature
To our knowledge, all temperate freshwater fishes are ectotherms. Furthermore, the thermal conductivity of water is 24.5 times greater than air (Hammel 1955). This high thermal conductivity means that there is unlikely to be much fine-scale variation in temperature beyond that normally associated with vertical stratification or the confluence of waters whose origins differ in temperature. For example, unlike terrestrial environments, there should be little significant difference in temperature between locations that are locally shaded versus those exposed to direct sunlight. In addition, because both predator and prey are ectothermic, their energetic requirements will be tightly linked to environmental temperature.
Increasing temperature should have a significant impact upon predator–prey interactions. For both predator and prey, energetic demands will increase (Elliott 1976). Prey species are required to move through their environment to locate their prey (sensu Werner & Anholt 1993) and if they require more energy then they must spend more time feeding, or when feeding they must do so more actively. Yet, their predators' energetic demands are probably also increasing and therefore should be expected to forage vigorously. The implications for prey are that mortality rates are likely to be much higher with increasing temperature. Unfortunately, very little is known about changing temperature on predator–prey interactions. A few studies have examined or noted the effects of temperature on predator–prey interactions in aquatic ecosystems. Juvenile Atlantic salmon (Salmo salar) were observed to switch from diurnal to nocturnal feeding cycles with decreasing temperature (Fraser et al. 1993, 1995). At lower temperatures, the salmon have reduced swimming abilities while their endothermic terrestrial predators are not impaired. The nocturnal strategy therefore is a simple and effective strategy that reduces encounter rates with predators whose relative effectiveness increases with decreasing temperature (Greenwood & Metcalfe 1998).
While prey species become more passive with decreasing temperature, the reverse also appears to be true. With increasing temperature, guppies (Poecilia reticulata) switch from relatively passive antipredator behaviour to a much more active mode (Weetman et al. 1998). At 22°C, individuals are relatively inactive and have a much reduced feeding rate compared with that at 26°C. At 26°C, individuals also have greater swimming ability, tend to spend more time schooling and engage in predator inspection behaviour. Given that predators are likely to be more active at higher temperatures, these authors also suggest that water temperature may be used as a cue to assess the risk of predation within the environment.
To better understand the integration between the physical environment, an animal's physiology and behaviour and their impact upon predator–prey interactions in the much longer term, we have sought to integrate information about brown trout (Salmo trutta) foraging on Arctic charr (Salvelinus alpinus) within Scottish Lochs. We used this system since there is a relatively large amount of published material on the physiology and behaviour for these two temperate species that was necessary for the development of the model. But unlike previous information that we have presented, this model operates over a much longer time-scale allowing us to investigate its impact at the population level.
The model we used to study the impact of a changing thermal environment was originally developed elsewhere, and is described in detail in Mangel & Abrahams (2001). The published version of this model was developed to identify the role that ecological and biochemical adaptations play in the longevity of a rare morph of the brown trout, the ferox trout. In exploring such adaptations, we developed an individual-based model that contained both brown trout and charr that occupied a typical lake within Scotland. This model tracked individuals on a weekly basis, and confronted them with the thermal and photoperiod conditions associated with a typical Scottish lake at 60° N latitude.
Both trout and charr were subject to size-independent (e.g. disease and parasites) and size-dependent (predation) mortality. Weekly mortality for a fish of mass w is then described by
| (1.1) |
where m0 represents size-independent mortality and m1 is the parameter associated with size-dependent mortality. For the purposes of the simulations described here, we set m0=0.01 and m1=0.03. We also characterized lakes as containing two distinct volumes that correspond to the littoral zone (VL) and the benthic region (VB). These values were set to 500 and 1600 for VL and VB, respectively. This parameter combination allowed the model to routinely produce the ferox trout morph which is the major predator required for an exploration of the impact that the thermal environment has predator–prey dynamics (for details on other parameters and their values, see Mangel & Abrahams (2001)).
Temperature is a critical parameter in these simulations as it affects behaviour and a number of key physiological parameters. We quantified the impact of temperature by including a temperature-dependent assimilation parameter, ϕT that is distinct for trout and charr
| (1.2) |
In this equation, cn is a normalization constant that is selected so that the maximum value of ϕT is 1. The variables Tmin, T* and Tmax are species-specific parameters that characterize lethal minimum temperature, a temperature for the optimal value of food gathering and conversion ability, and lethal maximum temperature, respectively. This equation generates a peaked function for the food gathering and assimilation ability of the fish (Elliott 1994).
In the analysis of growth dynamics below, we also use the temporal average of ϕT, defined by
| (1.3) |
Growth rate of fish is described by the variable f0 and we assume that growth rate within the population has a lognormal distribution with a mean and a standard deviation σ0, which we assume is 30% of the mean (Jobling 1994).
Growth rate within this model is calculated every week and represents a balance of anabolic and catabolic factors as
| (1.4) |
where α is a measure of metabolic cost; W(t) is the current weight of the fish; and D(t) is the daylight hours on day t. Assuming that maximum size of a fish is Wmax, the right-hand side of the preceding equation should be W(t) when W(t)=Wmax. However, there is time dependence to the right-hand side. We approximate the value of α using the mean values for daylight hours (0.5), temperature and food gathering, and assimilation abilities. When W(t)=Wmax, the second two terms on the right-hand side of that equation sum to 0,
| (1.5) |
where Ta is the average temperature. We conclude that
| (1.6) |
The value for was determined by selecting the value that most closely approximated known growth rates as reported by Vøllestad et al. (1993) and Vøllestad & L'Abee-Lund (1994).
Of particular importance is the role of temperature on metabolic rates. The metabolic parameter in the preceding equations is αWe0.071T. Within our simulations, we determined the value for α by an iterative solution that resulted in fish that had achieved their maximum size being unable to grow further at the average environmental temperature. However, the key component and the one most relevant to this discussion is that metabolic rates will scale isometrically with body mass and nonlinearly with temperature. While the precise shape of this relation varies among species and is probably much more complicated than that used in this simulation, it does present a useful null model with which we base our interpretation.
Within our model, we make the assumption that trout are primarily adapted to feeding in the peripheral regions of the lake where they are capable of establishing feeding territories, and charr will occur and feed predominantly within the open water and benthic regions of the lake. Rates of competition among individuals will then depend on their relative competitive abilities within these two environments, as well as the density of both trout and charr in these locations.
Charr are known to become cannibalistic at a median size of 33.5 cm (Griffiths 1994). For this reason, we approximated a size-specific piscivore threshold of 30 cm for both trout and charr. Until these fish achieve a body length of 30 cm, we assume that their prey will primarily include zooplankton and other invertebrate prey. At this stage, their rate of energy acquisition is then determined by density-dependent competition within their environment. Once they exceed 30 cm, we assume they switch to becoming obligate piscivores. Their diet will consist of relatively small individuals (0+ trout and charr, and cyprinids) and larger individuals to a maximum size determined by a gape limitation model (Damsgård 1995). The effect is to provide piscivores with an energy-rich niche that they can exploit to achieve the very large size typified by the piscivorous ferox trout. The equations that provide the detailed energy benefits of piscivory are described in Mangel & Abrahams (2001).
With these parameters set, we could then address the question of the impact that a changing thermal regime has on predator–prey interactions. Our model operates by adapting animals to one thermal regime (as described previously) and then confronting them with another. In developing this approach, we took the approach of simply adding either 3 or 7°C to average weekly temperatures, a range predicted by the Arctic Climate Impact Assessment-designated models (ACIA 2005).
While the role of global warming is unlikely to operate this way (e.g. the frequency of intense weather events and the variability of climactic conditions between years were ignored), we felt that such an approach would allow us to precisely examine the role that temperature per se has on the interaction between these two species. Furthermore, we also ignored the potential impact that changing environmental conditions would have on other ecologically important species such as the availability and abundance of zooplankton and benthic invertebrates. Our focus is exclusively on the interaction between animal physiology, behaviour and their environment.
We ran our simulations for 100 years, but ignored the first 25 years of data due to transient effects associated with the simulation. We also used a pseudorandom number generator that generated the same series of random numbers between simulations. This was important since this is an individual-based population model; many events have probabilistic outcomes, particularly those associated with behaviour. By running the simulations in this manner, it was then possible to make direct comparisons when manipulating environmental variables. We then plot the results of our simulations using the annual population size without environmental manipulation as the x-coordinate, and the population size for that year with environmental manipulation as the corresponding y-coordinate. As a reference, we provide an x=y line on each plot that corresponds to the conditions in which no net change would be observed under the two environmental treatments.
2. Results and discussion
With the parameter values that we used, piscivores made up only approximately 5% of the total population, with the majority of these unable to consume many of the trout and charr within our populations. The occurrence of exceptionally large individuals (those capable of consuming all size classes of non-piscivorous trout and charr) was a rare event. However, the presence of these large individuals resulted in higher mortality rates for small individuals, effectively stunting both the population of trout and charr and generating two distinct modes in the size distribution.
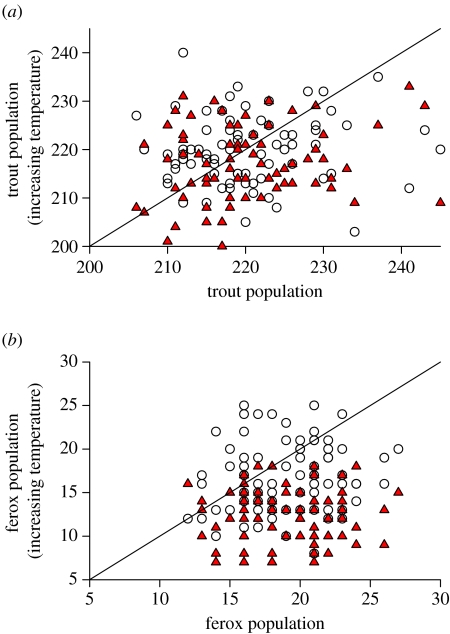
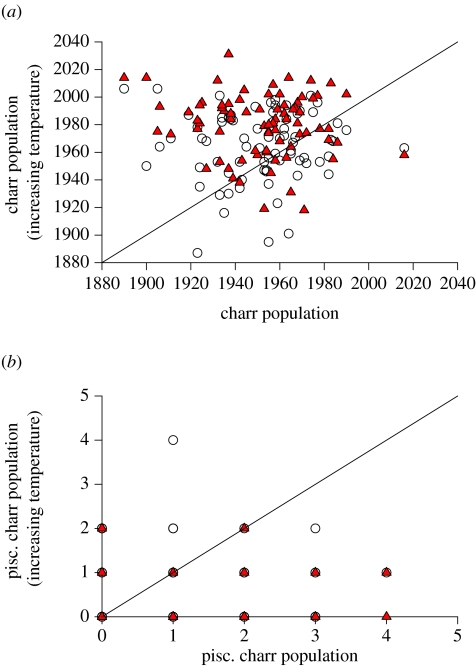
The impact of warming the environment by either 3 or 7°C is summarized in figures 6 and 7 and statistically analysed in table 1. These data demonstrate that the prevalence of very large piscivorous morphs within both the brown trout and charr population becomes much lower with increasing temperature (note that most data points for the piscivore populations occur below the diagonal line, figure 6b) with the effect most pronounced with a 7°C increase in ambient temperature (table 1). The impact on the non-piscivorous trout population is less dramatic. For both the 3 and 7°C changes in temperature, the trout population exhibits the smallest change in population size based upon an effect size index of all the fish types explored in this simulation (table 1).
Figure 6.
The effect of a warming environment on (a) the trout population and (b) the ferox trout population. The horizontal axis corresponds to the population size when there is no change in temperature and the vertical axis represents the population size with increased temperatures. The coordinates for this plot are the corresponding points for both conditions over a 75-year period (years 26–101). If the data lie above the diagonal (x=y), then increasing temperatures increase population size. If the data fall below this line, populations decline in response to global warming. Data that fall around the diagonal indicate no influence of global climate change. Open circle and filled triangle correspond to an increase of 3 and 7°C, respectively.
Figure 7.
The effect of a warming environment on (a) the charr population and (b) the piscivorous charr population. These plots and their interpretation are the same as for figure 6.
Table 1.
Quantitative summary of the response of trout and charr populations to a 3 and 7°C increases in their thermal environment (see text for details). Statistical analysis used a one-way ANOVA for three different thermal regimes with 222 d.f. in the error term. The reported magnitude of the effect was determined by calculating effect size (Cohen 1988)—the difference in the estimated means divided by a pooled estimate of the standard error. Positive values for effect size indicate increasing population size; negative values indicate a decreasing population size.
| trout | ferox trout | charr | piscivorous charr | |
|---|---|---|---|---|
| F | 4.45 | 71.76 | 26.71 | 18.07 |
| p | 0.013 | 9.15 × 10−25 | 4.02 × 10−11 | 5.37 × 10−8 |
| effect size 0–3°C increase | −0.30 | −0.96 | 0.82 | −0.47 |
| effect size 0–7°C increase | −0.45 | −1.95 | 1.28 | −1.06 |
The impact of this temperature change is similar for the charr that are large enough to be piscivorous, although they occur much less frequently in our model (figure 7b). The most surprising and counterintuitive result is the response of the charr population (figure 7a and table 1). Unlike every other population in this simulation, the population size of the charr increases with increasing temperature (table 1).
The probable mechanism responsible for this change hinges on the mortality imposed by the large piscivores in this population. Based upon the data we used, charr suffer higher mortality from the piscivorous fish simply because they tend to grow more slowly than the trout and hence are less likely to grow outside the susceptible prey range defined by our gape limitation function. The predators will be unable to maintain their growth rates, since their metabolic costs will be increasing solely due to higher temperatures. This will both impair their growth rates and increase their mortality rates simply due to starvation and their major prey, Arctic charr, will be the beneficiary.
The role of metabolic rates in influencing the growth of brown trout has attracted considerable attention within the literature, with a diverse range of views on this relation. Lahti et al. (2002) argue that metabolic rate correlates positively with dominance status and negatively with latitude. Interpretation of these data is complex but ultimately it may be determined by the pattern of food availability. In conditions where the food distribution is stable through time, becoming dominant can be economically advantageous since these individuals will be able to monopolize a much larger proportion of the resources. This is a disadvantageous position when the availability of food becomes uncertain, since the elevated metabolic rate associated with dominance also renders these individuals the first to succumb to starvation (Álvarez & Nicieza 2005). If metabolic rates are elevated simply as a consequence of elevated temperatures, starvation may become much more common for those individuals occupying niches that contain a less predictable food supply.
We hesitate to conclude with certainty this will be the consequence of a warming environment on predator–prey interactions, but we do believe that this process forces us to think of the energetic consequences of such change. Given that aquatic environments require that predators be significantly larger than their prey, we must understand the combined roles of scaling and temperature on metabolic rates. Endothermic and ectothermic animals alike show metabolic rate scaling with body mass with roughly a 3/4 power (Hemmingsen 1960; Kleiber 1961; Dodds et al. 2001). While the mechanisms and precise value of this scaling factor remain under debate (Chaui-Berlinck et al. 2005; Makarieva et al. 2005; West & Brown 2005), the result is that larger organisms have a lower specific metabolic rate and therefore expend resources at a slower rate per unit body mass. From the perspective of predator–prey interactions, this means that predators that are much larger than their prey should be more tolerant of the hypoxia associated with warmer environments owing to their lower specific metabolic rate. But prey are much smaller and have ultimately less biomass to support and should therefore require smaller quantities of oxygen from the environment to meet their metabolic demands suggesting that they may ultimately be the beneficiaries of a changing environment. Further, confounding the issue is the temperature-dependent solubility of oxygen in water. As temperature increases the solubility and therefore the concentration of oxygen in water decreases just as metabolic demands are increasing.
It is also important to note that changes in water turbidity can exacerbate these energetic consequences. In particular, if both predator and prey rely primarily upon vision to detect prey and avoid predators, their response to increasing turbidity may be to increase their rate of movement to acquire more energy (Werner & Anholt 1993). Under such a scenario, we believe that the ecosystem may be inherently unstable since increased temperature may increase energetic demands while increasing turbidity will make it more expensive to meet these demands. Ultimately, the combined effects of temperature and turbidity will leave no more behavioural options to predators to compensate for factors that reduce their net foraging rate. Once that point is crossed, the environment becomes uninhabitable for the predators.
Schultz & Conover (1999) examined the rate of energy reserve depletion in Altantic silversides held at 4 or 8°C and found that metabolic rates increased with temperature and that smaller individuals depleted their energy reserves relatively faster. While such a result would suggest that smaller individuals would be more vulnerable to starvation, they also noted that smaller fish reduced their rate of energy depletion more effectively than larger individuals and that survival probability was unrelated to body size. Hence, the impact of body size can be complicated by these responses.
The pressures to increase body size to escape gape-limited predators or accumulate sufficient energy stores to survive periods of starvation can also have dramatic effects on animal behaviours. Rainbow trout (Onchorhyncus mykiss) incur greater predation risks in low energy systems to achieve growth rates which allow to escape from gape-limited predators and accumulation of sufficient energy reserves for over winter survival (Biro et al. 2005). The accumulation of energy reserves for over winter survival is heavily documented and confirms the superiority of larger body size for persistence during times of starvation.
The opposition of specific metabolic rates and total supported body mass has two major results. First, owing to lower biomass and superior metabolic adaptability, smaller individuals will be able to use habitats where resources can be acquired from the environment in nearly continuous small doses which would provide an inadequate consumption rate for larger fishes. Second, large individuals are better adapted to surviving conditions when resources are wholly or nearly unavailable for protracted periods (Schultz & Conover 1999; Biro et al. 2004). These results can be confounded by relative abilities for metabolic acclimation (Schultz & Conover 1999) or adaptations which allow the acquisition of resources from alternate environments (i.e. surface respiration) and the ability to increase anaerobic capacity (Almeida-Val et al.1995, 2000).
3. General conclusion
It is generally conceded that the globe is currently undergoing a period of rapid climate change and the three parameters discussed in this paper; dissolved oxygen, turbidity and temperature, will change in a way that will challenge aquatic ecosystems. While knowing with certainty what the consequences of such change will be is impossible, it does seem reasonable that changes in these parameters may probably shift the balance in favour of prey, although a good knowledge of precise mechanisms will be required and there are certain to be many exceptions to the rule. We have also ignored the role of evolutionary response to change and this may also alter our conclusions (Jones & Ellner 2004). If this is the general trend, then it is probable that species that occupy higher trophic levels within such communities will be threatened and that the ultimate consequence will be a reduction in biodiversity.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Abrahams M.V. Risk of predation and its influence on the relative competitive abilities of two species of freshwater fishes. Can. J. Fish. Aquat. Sci. 1994;51:1629–1633. [Google Scholar]
- Abrahams M.V. The physiology of antipredator behaviour: what you do with what you've got. In: Sloman K.A, Wilson R.W, Balshine S, editors. Behaviour and physiology of fish; Vol. 24, Fish physiology. Academic Press; London, UK: 2005. pp. 79–108. [Google Scholar]
- Abrahams M.V, Dill L.M. A determination of the energetic equivalence of the risk of predation. Ecology. 1989;70:999–1007. doi:10.2307/1941368 [Google Scholar]
- Abrahams M.V, Kattenfeld M.G. The role of turbidity as a constraint on predator–prey interactions in aquatic environments. Behav. Ecol. Sociobiol. 1997;40:169–174. doi:10.1007/s002650050330 [Google Scholar]
- Abrahams, M. V. & Sloan, J. Submitted. Hypoxic environments and their impact upon habitat selection decisions involving the risk of predation.
- ACIA. Cambridge University Press; Cambridge, UK: 2005. Arctic climate impact assessment. p. 1042. [Google Scholar]
- Almeida-Val V.M.F, Farias I.P, Silva M.N.P, Duncan W.P, Val A.L. Biochemical adjustments to hypoxia by Amazon cichlids. Braz. J. Med. Biol. Res. 1995;28:1257–1263. [PubMed] [Google Scholar]
- Almeida-Val V.M.F, Val A.L, Duncan W.P, Souza F.C.A, Paula-Silva M.N, Land S. Scaling effects on hypoxia tolerance in the Amazon fish Astronotus ocellatus (Perciformes: Cichlidae): contribution of tissue enzyme levels. Comp. Biochem. Phys. B. 2000;125:219–226. doi: 10.1016/s0305-0491(99)00172-8. doi:10.1016/S0305-0491(99)00172-8 [DOI] [PubMed] [Google Scholar]
- Álvarez D, Nicieza A.G. Is metabolic rate a reliable predictor of growth and survival of brown trout (Salmo trutta) in the wild? Can. J. Fish. Aquat. Sci. 2005;62:643–649. doi:10.1139/f04-223 [Google Scholar]
- Biro P, Abrahams M.V, Post J.R, Thompson C. Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc. R. Soc. B. 2004;271:2232–2237. doi: 10.1098/rspb.2004.2861. doi:10.1098/rspb.2004.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P, Post J.R, Abrahams M.V. Ontogeny of energy allocation reveals selective pressure promoting risk-taking behaviour in young fish cohorts. Proc. R. Soc. B. 2005;272:1443–1448. doi: 10.1098/rspb.2005.3096. doi:10.1098/rspb.2005.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter J.H.S, Fuiman L.A. The role of sensory systems of herring larvae in evading predatory fishes. J. Mar. Biol. Assoc. UK. 1990;70:418–427. [Google Scholar]
- Brown G.E, Smith R.J.F. Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery reared fish to recognize chemical cues of predator. Can. J. Fish. Aquat. Sci. 1998;55:611–617. doi:10.1139/cjfas-55-3-611 [Google Scholar]
- Chapman L.J, Chapman C.A, Chandler M. Wetland ecotones as refugia for endangered fishes. Biol. Conserv. 1996a;78:263–270. doi:10.1016/S0006-3207(96)00030-4 [Google Scholar]
- Chapman L.J, Chapman C.A, Ogutu-Ohwayo R, Chandler M, Kaufman L, Keiter A.E. Refugia for endangered fishes from an introduced predator in Lake Nabugabo, Uganda. Conserv. Biol. 1996b;10:554–561. doi:10.1046/j.1523-1739.1996.10020554.x [Google Scholar]
- Chapman L.J, Chapman C.A, Brazeau D, McGlaughlin B, Jordan M. Papyrus swamps and faunal diversification: geographical variation among populations of the African cyprinid Barbus neumayeri. J. Fish. Biol. 1999;54:310–327. [Google Scholar]
- Chaui-Berlinck J.G, Navas C.A, Monteiro L.H.A, Bicudo J.E.P.W. Control of metabolic rate is a hidden variable in the allometric scaling of homeotherms. J. Exp. Biol. 2005;208:1709–1716. doi: 10.1242/jeb.01421. doi:10.1242/jeb.01421 [DOI] [PubMed] [Google Scholar]
- Cohen J. Lawrence Erlbaum Associates; New Jersey, NJ: 1988. Statistical power analysis for the behavioural sciences. [Google Scholar]
- Damsgård B. Arctic charr Salvelinus alpinus (L.), as prey for piscivorous fish: a model to predict prey vulnerabilities and prey size refuges. Nord. J. Freshw. Res. 1995;71:190–196. [Google Scholar]
- Dodds P.S, Rothman D.H, Weitz J.S. Re-examination of the 3/4-law of metabolism. J. Theor. Biol. 2001;209:9–27. doi: 10.1006/jtbi.2000.2238. doi:10.1006/jtbi.2000.2238 [DOI] [PubMed] [Google Scholar]
- Elliott J.M. The energetics of feeding, metabolism, and growth of brown trout (Salmo trutta) in relation to body weight, water temperature, and ration size. J. Anim. Ecol. 1976;45:923–948. doi:10.2307/3590 [Google Scholar]
- Elliott J.M. Oxford University Press; New York, NY: 1994. Quantitative ecology and the brown trout. [Google Scholar]
- Fraser N.H.C, Metcalfe N.B, Thorpe J.E. Temperature-dependent switch between diurnal and nocturnal foraging in salmon. Proc. R. Soc. B. 1993;252:135–139. doi:10.1098/rspb.1993.0057 [Google Scholar]
- Fraser N.H.C, Heggenes J, Metcalfe N.B, Thorpe J.E. Low summer temperatures cause juvenile Atlantic salmon to become nocturnal. Can. J. Zool. 1995;73:446–451. [Google Scholar]
- Fuiman L.A, Magurran A.E. Development of predator defences in fishes. Rev. Fish Biol. Fisheries. 1994;4:145–183. doi:10.1007/BF00044127 [Google Scholar]
- Gould S.J, Lewontin R.C. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. doi:10.1098/rspb.1979.0086 [DOI] [PubMed] [Google Scholar]
- Greenwood M.F.D, Metcalfe N.B. Minnows become nocturnal at low temperatures. J. Fish Biol. 1998;53:25–32. doi:10.1111/j.1095-8649.1998.tb00105.x [Google Scholar]
- Griffiths R.D. The size structure of lacustrine Arctic charr (Pisces: Salmonidae) populations. Biol. J. Linn. Soc. 1994;51:337–357. doi:10.1006/bijl.1994.1028 [Google Scholar]
- Hammel H.T. Thermal properties of fur. Am. J. Physiol. 1955;182:369–376. doi: 10.1152/ajplegacy.1955.182.2.369. [DOI] [PubMed] [Google Scholar]
- Hecky R.E. The eutrophical of Lake Victoria. Berhand. Int. Ver. Theor. Ang. Limnol. 1993;25:39–48. [Google Scholar]
- Hemmingsen A.M. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Mem. Hosp. (Copenhagen) 1960;9:1–110. [Google Scholar]
- Huber R, Rylander M.K. Quantitative histological study of the optic nerve in species of minnows (Cyprinidae, Teleostei) inhabiting clear and turbid water. Brain Behav. Evol. 1992;40:250–255. doi: 10.1159/000113916. [DOI] [PubMed] [Google Scholar]
- Jobling M. Chapman & Hall; London, UK: 1994. Fish bioenergetics. [Google Scholar]
- Jones L.E, Ellner S.P. Evolutionary tradeoff and equilibrium in an aquatic predator–prey system. Bull. Math. Biol. 2004;66:1547–1573. doi: 10.1016/j.bulm.2004.02.006. doi:10.1016/j.bulm.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Kleiber M. Wiley; New York, NY: 1961. The fire of life: an introduction to animal energetics. [Google Scholar]
- Kramer D.L. Dissolved oxygen and fish behaviour. Environ. Biol. Fishes. 1987;18:81–90. doi:10.1007/BF00002597 [Google Scholar]
- Lahti K, Huuskonen H, Laurila A, Piironen J. Metabolic rate and aggressiveness between brown trout populations. Funct. Ecol. 2002;16:167–174. doi:10.1046/j.1365-2435.2002.00618.x [Google Scholar]
- Lima S.L, Dill L.M. Behavioural decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Magnhagen C, Heibo E. Gape size allometry in pike reflects variation between lakes in prey availability and relative body depth. Funct. Ecol. 2001;15:754–762. doi:10.1046/j.0269-8463.2001.00576.x [Google Scholar]
- Makarieva A.M, Gorshokov V.G, Li B.-L. Biochemical universality of living matter and its metabolic implications. Funct. Ecol. 2005;19:547–557. doi:10.1111/j.1365-2435.2005.01005.x [Google Scholar]
- Mangel M, Abrahams M.V. Age and longevity in fish, with consideration of the ferox trout. Exp. Gerontol. 2001;36:765–790. doi: 10.1016/s0531-5565(00)00240-0. doi:10.1016/S0531-5565(00)00240-0 [DOI] [PubMed] [Google Scholar]
- Mayr E. How to carry out the adaptationist program? Am. Nat. 1983;121:324–334. doi:10.1086/284064 [Google Scholar]
- Miner J.G, Stein R.A. Predator detection and habitat choice by small bluegills: effects of turbidity. Trans. Am. Fish. Soc. 1996;125:97–103. doi:10.1577/1548-8659(1996)125<0097:DOPAHC>2.3.CO;2 [Google Scholar]
- Nilsson P.A, Brönmark C. Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos. 2000;88:539–546. doi:10.1034/j.1600-0706.2000.880310.x [Google Scholar]
- Persson L, Andersson J, Wahlström E, Eklov P. Size-specific interactions in lake systems: predator game limitation and prey growth rate and mortality. Ecology. 1996;77:900–911. doi:10.2307/2265510 [Google Scholar]
- Pfeiffer W, Riegelbauer G, Meier G, Scheibler B. Effect of hypoxanthine-3-N oxide and hypoxanthine-1-N-oxide on central nervous excitation of the black tetra Gymnocorymbus ternetzi (Characaidae, Ostariophysi, Pisces) indicated by dorsal light response. J. Chem. Ecol. 1985;11:507–523. doi: 10.1007/BF00989562. doi:10.1007/BF00989562 [DOI] [PubMed] [Google Scholar]
- Robb T, Abrahams M.V. Variation in tolerance to hypoxia in a predator and prey species: an ecological advantage to being small? J. Fish. Biol. 2003;62:1067–1081. doi:10.1046/j.1095-8649.2003.00097.x [Google Scholar]
- Schultz E.T, Conover D.O. The allometry of energy reserve depletion: test of a mechanism for size-dependent winter mortality. Oecologia. 1999;119:474–483. doi: 10.1007/s004420050810. doi:10.1007/s004420050810 [DOI] [PubMed] [Google Scholar]
- Smith R.J.F. Alarm signals in fishes. Rev. Fish. Biol. Fish. 1992;2:33–63. doi:10.1007/BF00042916 [Google Scholar]
- van Staaden M.J, Huber R, Kaufman L.S, Liem K.F. Brain evolution in cichlids of the African Great Lakes: brain and body size, general patterns, and evolutionary trends. Zoology. 1994;98:165–178. [Google Scholar]
- Vollestad L.A, L'Abee-Lund J.H. Evolution of the life history of Arctic char Salvelinus alpinus. Evol. Ecol. 1994;8:315–327. doi:10.1007/BF01238281 [Google Scholar]
- Vollestad L.A, L'Abee-Lund J.H, Saegrov H. Dimensionless numbers and life history in brown trout. Evol. Ecol. 1993;7:207–218. doi:10.1007/BF01239389 [Google Scholar]
- Weetman D, Atkinson D, Chubb J.C. Effects of temperature on anti-predator behaviour in the guppy, Poecilia reticulate. Anim. Behav. 1998;55:1361–1372. doi: 10.1006/anbe.1997.0666. doi:10.1006/anbe.1997.0666 [DOI] [PubMed] [Google Scholar]
- Werner E.E, Anholt B.R. Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am. Nat. 1993;142:242–272. doi: 10.1086/285537. doi:10.1086/285537 [DOI] [PubMed] [Google Scholar]
- West G.B, Brown J.H. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. doi:10.1242/jeb.01589 [DOI] [PubMed] [Google Scholar]