Abstract
Hypoxia is a phenomenon occurring in marine coastal areas with increasing frequency. While hypoxia has been documented to affect fish activity and metabolism, recent evidence shows that hypoxia can also have a detrimental effect on various antipredator behaviours. Here, we review such evidence with a focus on the effect of hypoxia on fish escape responses, its modulation by aquatic surface respiration (ASR) and schooling behaviour. The main effect of hypoxia on escape behaviour was found in responsiveness and directionality. Locomotor performance in escapes was expected to be relatively independent of hypoxia, since escape responses are fuelled anaerobically. However, hypoxia decreased locomotor performance in some species (Mugilidae) although only in the absence of ASR in severe hypoxia. ASR allows fish to show higher escape performance than fish staying in the water column where hypoxia occurs. This situation provides a trade-off whereby fish may perform ASR in order to avoid the detrimental effects of hypoxia, although they would be subjected to higher exposure to aerial predation. As a result of this trade-off, fishes appear to minimize surfacing behaviour in the presence of aerial predators and to surface near shelters, where possible.
For many fish species, schooling can be an effective antipredator behaviour. Severe hypoxia may lead to the disruption of the school unit. At moderate levels, hypoxia can increase school volume and can change the shuffling behaviour of individuals. By altering school structure and dynamics, hypoxia may affect the well functioning of schooling in terms of synchronization and execution of antipredator manoeuvres. School structure and volume appear to be the results of numerous trade-offs, where school shape may be dictated by the presence of predators, the need for energy saving via hydrodynamic advantages and oxygen level.
The effects of hypoxia on aquatic organisms can be taxon specific. While hypoxia may not necessarily increase the vulnerability of fish subject to predation by other fish (since feeding in fish also decreases in hypoxia), predators from other taxa such as birds, jellyfish or aquatic mammals may take advantage of the detrimental effects of hypoxia on fish escape ability. Therefore, the effect of hypoxia on fish antipredator behaviours may have major consequences for the composition of aquatic communities.
Keywords: hypoxia, antipredator behaviours, fish, escape, schooling, aquatic surface respiration
1. Introduction
Environmental fluctuations in coastal areas, whether they are man-made or natural occurrences, can provoke dramatic changes in the abundance and distribution of organisms. The impact of these disturbances on coastal ecosystems is modulated by the behaviour of the organisms (Kramer et al. 1997). In-depth knowledge of the behavioural processes that regulate the interactions between environmental variations and ecologically relevant parameters, such as growth and survival, can be fundamental for understanding and predicting the effects of such interactions. Hypoxia has become one of the main environmental constraints affecting coastal areas due to the increasing occurrence of eutrophication (Rydberg et al. 1990; Rabalais et al. 2002; Druon et al. 2004; Hagy et al. 2004). At severe levels, hypoxia is known to affect fish growth and survival (e.g. Plante et al. 1998; Dean & Richardson 1999; Smith & Able 2003). Above lethal oxygen levels, hypoxia can affect fish activity and distribution as a result of various effects on their behaviour and physiology. Hypoxia is known to affect various physiological functions in fish, such as metabolism (Duthie 1982; Van Den Thillart et al. 1994; Lefrançois & Claireaux 2003) and cardiovascular regulation (Fritsche & Nilsson 1989). These effects lead to limitations in metabolic scope and, consequently, reduction in feeding, growth (Chabot & Dutil 1999; Thetmeyer et al. 1999; Eby et al. 2005) as well as activity levels (Metcalfe & Butler 1984; Fisher et al. 1992; Bushnell et al. 1984; Steffensen & Farrell 1998).
Fish activity depends mainly on the ability of the cardiovascular system to provide tissues with the required amount of oxygen and nutrients. Some parameters have fundamental roles such as oxygen diffusion at the gills and the blood properties for gas transport. Heart rate and stroke volume also play a key role as they modify convection processes involved in oxygen transport towards tissues where aerobic processes take place (Gilmour 1997). During progressive hypoxia, some fish have adapted to cope with the oxygen rarefaction (i.e. regulators) mainly through cardiovascular and respiratory adjustments (Randall 1982). For instance, increase of the ventilation frequency, bradycardia and elevation of the stroke volume have been observed in hypoxia (Satchell 1971; Marvin & Burton 1973; Cech et al. 1977; Randall 1982; Fritsche & Nilsson 1989). This is likely to improve the gas exchange at the branchial surface by causing blood to remain in the gills for a longer time (Randall 1968; Satchell 1971). Recruitment of gill lamellue is also known to contribute to oxygen uptake at the gill. For instance, in crucian carp (Carassius carassius) exposed to long-term hypoxia (7 days), an increase by approximately 7.5-fold of the gill surface was accompanied by an increased capacity for oxygen uptake (Sollid et al. 2003). Such cardiovascular and respiratory adjustments temporarily counteract the reduced oxygen available in the environment and contribute to the preservation of fish aerobic metabolism. However, as oxygen rarefaction becomes more severe, these adaptive responses are no longer adequate, oxygen uptake shows a steep decline, oxygen delivery to various tissues falls and systemic tissue hypoxia occurs. This level was described as the limiting oxygen concentration (LOC, Beamish 1964; Fry 1971; Gehrke 1988; Neill & Bryan 1991; Lefrançois & Claireaux 2003). The particular LOC below which the standard metabolic rate (i.e. the oxygen consumption of a resting, fasted and non-maturing fish, Fry 1971) cannot be maintained is called the critical oxygen saturation (Scrit). Below Scrit, fish cannot sustain maintenance activities, such as ventilation or osmoregulation, and survival is uncertain. Dalla Via et al. (1998) investigated the response to hypoxia in the common sole, Solea solea. They found that swimming behaviour was markedly affected by hypoxia and notably observed a reduction in activity when oxygen decreased from 80 to 20% of oxygen saturation and a ‘panic state’ when approaching Scrit.
Behaviour was shown to be a relevant modulating factor in fish coping with hypoxia. It may contribute to reducing the limiting effect of severe hypoxic conditions by taking advantage of the oxygen heterogeneity of the environment or reducing the animal's oxygen requirements. For instance, decreased swimming activity was also observed in dogfish (Scyliorhinus canicula; Metcalfe & Butler 1984) and eelpout (Zoarces viviparus; Fisher et al. 1992), and has been considered a means of reducing oxygen needs in accordance with the hypoxia-related decrease in fish aerobic metabolic scope. In contrast with expectations based on physiological knowledge, swimming activity in certain species increases in hypoxia (Dizon 1977; Bejda et al. 1987; Domenici et al. 2000a). Increase in activity has been interpreted as a behavioural response to hypoxia, in that highly active fish may increase their chances of finding a better oxygenated environment. Similarly, hypoxia was shown to affect habitat selection in various species of fish (Congleton 1980; Matthews et al. 1985; Suthers & Gee 1986; Eby & Crowder 2002). For instance, some species showed changes in vertical distribution while performing aquatic surface respiration (ASR; Kramer et al. 1983; Kramer 1987). These travel to the surface of the water column to ventilate well-oxygenated water in contact with the air.
While hypoxia can have a profound effect on fish activity and distribution, recent work has shown that hypoxia may also affect predator–prey interactions in fish (e.g. Breitburg et al. 1999), and in particular, their antipredator behaviour (e.g. Lefrançois et al. 2005), as well as schooling (e.g. Domenici et al. 2002). This review focuses on the effect of hypoxia on antipredator behaviours and schooling, as schooling can be considered primarily as an antipredator adaptation (Pitcher & Parrish 1993), and many of the effects of hypoxia on schooling patterns trade-off with antipredator behaviours.
2. Hypoxia and predator–prey encounters
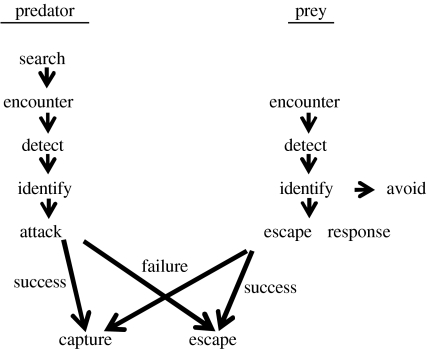
The events leading to a predator–prey encounter are summarized in figure 1. Once prey detect and identify a predator, they could ‘leave the game’ by moving out of sight of the predator, thus avoiding any further interaction (i.e. ‘predator avoidance’, Smith 1997). Once prey are attacked by a predator, their only option is escaping. Avoidance is not always possible as predators may detect the prey before the prey detects the predator. In addition, prey may take risks in situations in which they share a certain habitat (e.g. for foraging) with their predators (Hugie & Dill 1994). In addition, schooling may be involved in all the phases described in figure 1, such as in searching for prey and antipredator manoeuvres (Pitcher & Parrish 1993). Hypoxia may have an effect on the vulnerability of fish prey, as a result of various effects on the different phases outlined in figure 1. For example, hypoxia may lead to species-specific changes in fish activity (Schurmann & Steffensen 1994; Domenici et al. 2000a). It is possible that these changes in activity may affect the encounter rate between predators and prey. Hypoxia can have a dramatic effect on the vertical distribution of various fish species, particularly air-breathers and fish that perform ASR. This can lead to a higher encounter rate with predators, especially aerial ones. Following an attack, fish resort to escaping using a fast-start. Although fast-starts are fuelled anaerobically (Wakeling & Johnston 1998; Webb 1998), and their performance was hypothesized to be relatively independent of hypoxia (Beamish 1978), recent work shows that hypoxia can have a detrimental effect on fish escape responses (Lefrançois et al. 2005; Lefrançois & Domenici 2006) thereby increasing prey vulnerability to predation.
Figure 1.
The main phases of predator–prey encounters. Based on Batty & Domenici (2000).
3. The effect of hypoxia on fish escape responses
Many authors have suggested that oxygen fluctuations influence predator–prey interactions in fish and other aquatic organisms (Breitburg et al. 1994, 1999; Sandberg & Bonsdorff 1996; Robb & Abrahams 2002; Shoji et al. 2005; Shimps et al. 2005). These effects may be attributed to changes in the predator's feeding rates and/or in the prey vulnerability. Whether hypoxia shifts the balance in favour of the predator or the prey may depend on their relative tolerance to hypoxia. In isopod–amphipod interactions, as predator and prey, respectively, Sandberg & Bonsdorff (1996) suggested that hypoxia-related changes in the prey's behaviours may influence its vulnerability and therefore modify the predator's diet. Breitburg et al. (1994) showed that the attack rates on goby larvae by two species of fish predators, juvenile striped bass (Morone saxatilis) and adult naked goby (Gobiosoma bosc) decreased with oxygen level. On the other hand, when exposed to sea nettles (Chrysaora quinquecirrha) mortality by predation increased in the goby larvae (Breitburg et al. 1994). A similar oxygen-related change in vulnerability was observed in red sea bream larvae (Pagrus major), i.e. in hypoxia, predation by moon jellyfish (Aurelia aurita) increased while predation by Spanish mackerel (Scomberomorus niphonius) decreased (Shoji et al. 2005). Therefore, these studies show that predator–prey interactions involving fish as predator may decrease in hypoxia (as appetite and feeding are inhibited by hypoxia in fish, Chabot & Dutil 1999), while predation on fish may increase when predators belong to other taxa that are relatively tolerant of hypoxia. Breitburg et al. (1994) suggests that the high mortality rates observed in fish larvae attacked by sea nettles, a relatively hypoxia-resistant predator, may be due to the effect of hypoxia on prey escape ability. Escape responses are fuelled anaerobically (Wakeling & Johnston 1998; Webb 1998), hence, hypoxia might be expected to have no effect on the brief, anaerobic burst of activity associated with escape (Beamish 1978; Wolf & Kramer 1987). On the other hand, systemic hypoxia could impair brain and sensory functions, which are fundamental for the execution of escape responses. Therefore, low oxygen levels may increase the vulnerability of fish when attacked by predators, by impairing the escape performance variables other than locomotory ones (e.g. responsiveness).
Studies concerning the effect of hypoxic conditions on escape performance have been carried out recently on various species of coastal fishes from areas where hypoxic events occur chronically (golden grey mullet, Liza aurata, Lefrançois et al. 2005; European sea bass, Dicentrarchus labrax, Lefrançois & Domenici 2006; flathead grey mullet, Mugil cephalus, Shingles et al. in preparation). Escape responses are used by many fish as the main defence against predator attacks, and they have been studied extensively in terms of kinematics, performance, behaviour and physiology (see Domenici & Blake (1997) for a review). Escape responses consist of a brief sudden acceleration, usually in a direction away from the startling stimulus (Domenici & Blake 1993a). They are typically triggered by one of a pair of giant neurons, the Mauthner cells, although alternative pathways have been described (Eaton & Hackett 1984). These giant neurons allow for a quick response time, of the order of a few milliseconds, i.e. as short as 5–10 ms (Eaton & Hackett 1984). Following stimulation (i.e. by an artificial stimulus or a predator attack), fish usually bend into a C-shape as a result of the unilateral contraction of the axial musculature contralateral to the stimulus. This corresponds to stage 1, which may be followed by a second contraction (i.e. stage 2) of the opposite side of the body (Foreman & Eaton 1993; Domenici & Blake 1997). Beyond stage 2, locomotor behaviour is variable and can include steady swimming or coasting (Weihs 1973a). Certain species can also exhibit an S-shaped escape response (e.g. pike, Exos masquinongy), in which fish bend into an S-shape resulting from simultaneous contractions of both sides of the axial musculature during stage 1 (Hale 2002). The success of an escape response depends mainly on the prey's locomotor and sensory performance and can be influenced by factors such as size (Wardle 1975; Webb 1976; Domenici & Blake 1993b, Domenici 2001), ontogenetic stage (Fuiman et al. 1999; Hale 1999; Wakeling et al. 1999), stimulus intensity and/or orientation (Eaton & Hackett 1984; Domenici & Blake 1993a) and environmental factors such as temperature and oxygen (e.g. Webb 1978; Webb & Zhang 1994; Lefrançois et al. 2005).
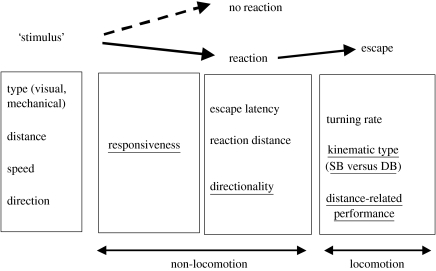
Figure 2 shows the series of events and relative variables that can be observed in a prey exposed to a startling stimulus. Laboratory stimulation may be visual or mechanical. Stimulus characteristics include its distance from the prey, speed and direction. After the onset of a startling stimulus, fishes may or may not respond. The responsiveness, i.e. the proportion of animals responding to the stimulus, is an indicator of the fish's acoustic or visual sensitivity and as well as its motivation to escape. If the prey reacts to the threatening stimulus, its response will show a certain response latency (i.e. the interval between stimulus onset to the first detectable movement leading to the escape of the animal) and reaction distance (the distance between the stimulus, or predator, and the prey at the onset of the response). Escaping fish usually exhibit a C-shape opposite to the side of the stimulation (Domenici & Blake 1993a). The proportion of these ‘away responses’ represents the directionality of the response, which is of the order of 80–90% in non-stress conditions (Domenici & Blake 1993a; Domenici & Batty 1997). Responsiveness, response latency, reaction distance and directionality are likely to be related to the fish's sensory performance and motivation and are defined as ‘non-locomotor’ variables in figure 2. Once the escape response is triggered, performance can be evaluated by a set of locomotor variables, such as turning rate, distance-related performance (e.g. speed and acceleration) and kinematic types. Work on predator–prey interactions suggests that both non-locomotor and locomotor variables affect prey vulnerability to predator attacks (Barber et al. 2004; Walker et al. 2005).
Figure 2.
The phases of an escape response, from stimulation to escape and relative variables. Variables are divided into non-locomotion and locomotion ones. Variables that are underlined indicate those on which hypoxia was shown to have an effect. SB and DB indicate single bend and double bend response, respectively (Lefrançois et al. 2005).
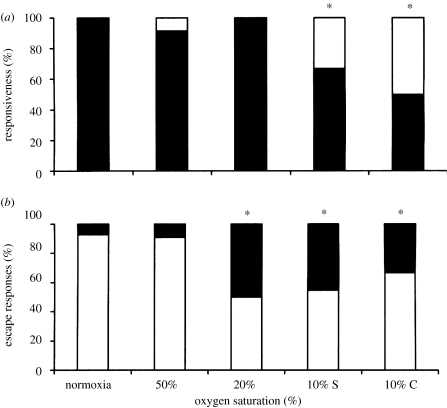
Hypoxia has been shown to mainly affect most non-locomotor variables studied (Lefrançois et al. 2005; Lefrançois & Domenici 2006; Shingles et al. in preparation). Responsiveness (figure 2) was affected in all the species investigated; in L. aurata (figure 3a) and D. labrax (startled using mechanical stimulus; Lefrançois et al. 2005; Lefrançois & Domenici 2006) as well as in M. cephalus (startled with visual stimulus; Shingles et al. in preparation). Levels at which hypoxia significantly affected responsiveness appeared to be species specific. In addition, the two species of Mugilidae perform ASR in hypoxia, while D. labrax do not. Therefore, the experimental design included a treatment in hypoxia (at 10% oxygen saturation) in which access to the surface was denied, by using a mesh suspended at the surface (Lefrançois et al. 2005). In L. aurata, 100% of the individuals responded to the threatening stimulus when exposed to normoxia, while only about 69% responded at 10% oxygen saturation and even less (about 40%) when access to the surface was denied (figure 3a). Similarly, the responsiveness of M. cephalus decreased significantly in hypoxia. Significant differences were found in hypoxia between fishes that performed ASR (92% of responders) versus fishes which did not (20% of responders; Shingles et al. in preparation.). Clearly, ASR allows the prey to retain a responsiveness similar to that of fish in normoxia, presumably because this behaviour prevents systemic hypoxia. However, responsiveness in golden grey mullets is impaired even when performing ASR (Lefrancois et al. 2005). Therefore, decrease in motivation (an important component of escape response, Webb 1986), possibly due to stress, may be a further factor contributing to the low responsiveness in hypoxia. Sea bass do not perform ASR and their responsiveness was as low as 37% at 10% oxygen saturation (Lefrançois et al. 2005), which is much lower than the responsiveness of golden grey mullet whether performing ASR or not.
Figure 3.
(a) The percentage of golden grey mullet (L. aurata) that responded to a mechanical stimulation in normoxia and various hypoxic conditions (black bars). Ten per cent C indicated no access to the surface at 10% oxygen saturation. Asterisks indicate significant difference from normoxia. (b) The percentage of fish that made an escape response in a direction away (white bars) or towards (black bars) the mechanical stimulus. Asterisks indicate different from random. From Lefrançois et al. (2005).
The absence of an escape response may have significant ecological relevance, since it leads to the capture of prey, unless the predator makes an error. The onset of escape responses depends on the sensory ability of the fish to detect a mechano-acoustic or visual signal (Eaton & Hackett 1984). Absence of escape response suggests that hypoxia may impair this ability. It can be hypothesized that, once systemic hypoxia occurs, decreased oxygen delivery to the sensorial cells/organs may affect responsiveness. Hypoxia has been shown to impair the function of the ciliated inner ear cells in bullfrog (Sitko & Honrubia 1986). Similarity between sensory cells of the teleosts' lateral line and the amphibians' ciliated inner ear cells (Shellart & Wubbels 1998) suggests that a similar, negative effect of hypoxia may also occur in fish. Interestingly, however, hypoxia had no effect on the timing of the escape response (i.e. the latency and the reaction distance; figure 2). It is therefore possible that low oxygen availability could alter the fish's sensory threshold involved in triggering an escape response, while the time course to initiate the response does not appear to be affected (Lefrançois et al. 2005).
A further, ‘non-locomotor’ variable that is likely to be related to the fish's sensory performance is directionality. Fish are able to turn away from startling stimuli by using the directional nature of particle motion, which determines which of the two Mauthner cells fires first (Canfield & Eaton 1990). A C-bend oriented towards the predator may induce a significant delay in the effort of the prey to escape away from the predator, and this may reduce the probability of escape success. The effect of hypoxia on directionality was studied in L. aurata and D. labrax (Lefrançois et al. 2005; Lefrançois & Domenici 2006). Hypoxia had a significant detrimental effect even at relatively mild hypoxia, i.e. ≤20% oxygen saturation in L. aurata (figure 3b) and ≤50% oxygen saturation in D. labrax. At these oxygen levels, fish showed a random directionality, i.e. the proportion of responses away: towards the starting stimulus response was not different from 50 : 50, suggesting a significant disorientation in the individuals tested. This suggests that D. labrax has a lower tolerance to hypoxia than L. aurata with respect to directionality and thus may be more vulnerable to predator attacks. While directionality was impaired in hypoxia, the subsequent escape trajectories (measured at the end of stage 2) were unaffected. Arguably, an early ‘mistake’ such as bending towards the predator may have ecological relevance, since the first milliseconds of the escape response may be crucial for surviving a predator attack.
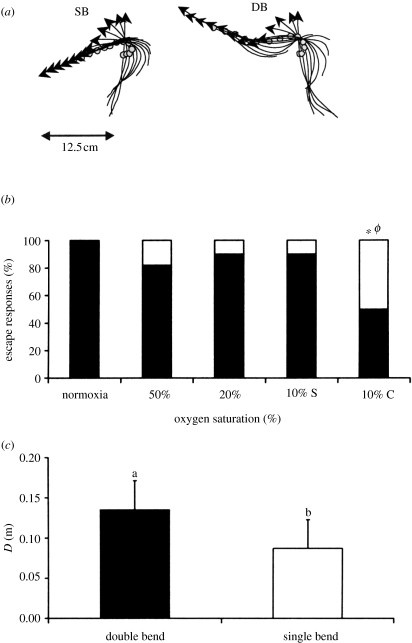
Work on three species present in the Mediterranean lagoon, showed that hypoxia does influence locomotor components of the escape response, although to a lesser extent than ‘non-locomotor’ performance (figure 2). The extent of the effect was species specific. Turning rate is a relevant measure of muscular performance and manoeuvrability, since high turning rates allow fishes to change direction more rapidly. Turning rates (measured during stage 1) were not affected by hypoxia (Lefrançois et al. 2005; Shingles et al. in preparation) as expected based on the idea of independency of anaerobic muscle performance from hypoxia (Beamish 1978). While no effect of hypoxia was found in the locomotor performance of sea bass (Lefrançois & Domenici 2006), the cumulative distance and the speed of L. aurata with no access to the surface were significantly affected (Lefrançois et al. 2005). This oxygen-dependent decrease in locomotor performance was attributed to a change in the escape kinematic type (figure 4). In hypoxia, when animal are denied access to ASR, Mugilidae showed a significantly higher proportion of escape responses lacking a stage 2 (i.e. single bend responses, SB; figure 4b; Lefrançois et al. 2005; Shingles et al. in preparation). Conversely, in normoxia, all responses consisted of escape responses that included stage 2 (i.e. double bend escapes, DB; figure 4). As in other species (Domenici & Blake 1991; Kasapi et al. 1993), single bend responses in golden grey mullet show lower locomotor performance than double bend responses (figure 4c). Lefrançois et al. (2005) suggested that lower locomotor performance in SB escape responses may be related to a lower energetic cost when compared with DB responses. Therefore, an increase in the proportion of SB responses with hypoxia may be the consequence of a trade-off between physiological exhaustion, requiring energy saving, and the need to escape from a predator attack. The glycogen reserves used for white muscle contractions can be highly depleted during hypoxic events in various teleost species (Zhou et al. 2000; Pichavant et al. 2002). Although swimming performance in golden grey mullet, but not in sea bass, was affected, golden grey mullet appear to have a higher responsiveness than sea bass. It appears therefore that, even in extreme oxygen conditions, golden grey mullet have a mechanism that allows them to escape, albeit using a slower response than in normoxia. This mechanism does not appear to be present in sea bass, as they either respond using high speed, or do not respond at all.
Figure 4.
Golden grey mullet (a) examples of tracings of a single bend (SB) and a double bend (DB) response, performed in hypoxia and normoxia, respectively. Midline and centre of mass (open circle) of the fish are shown at 10 ms intervals from the frame preceding the onset of the response. Arrows indicate the head. While the DB response shows a reversal in the direction of turning of the head (at frame six) and the fish return flip is complete, in SB the fish goes into a glide after frame six. (b) Percentage of DB responses (black bars) out of the total in normoxia and various hypoxic conditions. Ten per cent C indicates no access to the surface at 10% oxygen saturation. Asterisk indicates a significant difference from normoxia. ϕ indicates a significant difference between 10% S and 10% C. (c) The locomotor performance of SB and DB responses (D, distance covered in 80 ms). Values not sharing a common subscripts are significantly different. From Lefrançois et al. (2005).
These results demonstrate that hypoxia has a relevant role to play in prey escape performance. While locomotor performance is only affected in golden grey mullet at low oxygen saturation (i.e. 10% oxygen saturation), other variables which mainly depend on the fish sensory performance are impaired at higher oxygen level (e.g. directionality in golden grey mullet, figure 3b). Therefore, predator–prey relationships are likely to be affected at moderate to severe hypoxia. At moderate hypoxia, the potential increase of prey vulnerability mainly results from a decrease of responsiveness and an impairment of directionality in all the species studied. While most effects of hypoxia on escape response are likely to be due to systemic hypoxia, we cannot exclude behavioural effects due to decreased motivation to escape as a result of environmental hypoxia, since effects are detectable at a relatively high percentage of oxygen saturation (50% oxygen saturation affecting directionality in sea bass, Lefrancois & Domenici 2006).
4. Surfacing in hypoxia and its relation with predation risk
In many species of fish, ASR is used in order to survive at a very low oxygen level (Lewis 1970; Gee et al. 1978; Congleton 1980; Liem 1980; Kramer & Mehegan 1981; Kramer & McClure 1982; Kramer et al. 1983; Poulin et al. 1987; Chapman et al. 1994; Shingles et al. 2005). ASR is different from air-breathing (although both ASR and air-breathing imply surfacing), since it implies water breathing using the gills, while air-breathing requires an air-breathing organ, such as modified gills, mouth or gut (Kramer 1983b; Moyle & Cech 2000). In ASR, surfacing is triggered in hypoxia by the stimulation of O2 chemoreceptors located in the gills which are sensitive to both blood and water O2 levels (Shingles et al. 2005). During ASR, the snout and upper lip protrude above the water surface and fishes often hold air bubbles in the buccal cavity. This is thought to increase the O2 content of the water passing over the bubble and across the gills (Dickson Hoese 1985; Gee & Gee 1991; Chapman et al. 1994; Shingles et al. 2005).
ASR can be beneficial to fish but it can also incur some costs. The trade-offs associated by ASR are summarized in figure 5. ASR permits survival under hypoxic conditions which would otherwise be lethal (Lewis 1970; Kramer & Mehegan 1981) and has many other benefits such as the maintenance of growth rates (Weber & Kramer 1983; Stierhoff et al. 2003) and activity (Weber & Kramer 1983). In moderate hypoxia, ASR allows fish to have a higher food consumption when compared with fish that were denied access to the surface (Weber & Kramer 1983). As discussed above, ASR allows fish to show better escape performance than fish staying in the water column in hypoxia (Lefrancois et al. 2005). ASR also reduces the cost of ventilation compared to swimming in the water column in hypoxia, owing to the higher oxygen concentration in the surface water (Kramer 1983b). ASR can also be considered a long-term survival strategy in hypoxia as it can be integrated into other activities such as parental care (Reebs et al. 1984) and foraging and courtship (Kramer & Mehegan 1981).
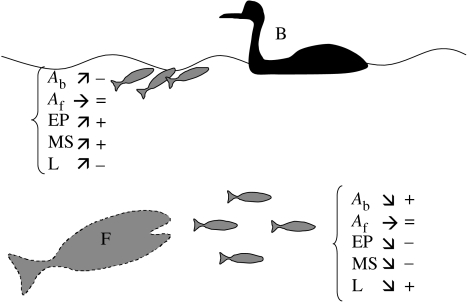
Figure 5.
Trade-offs in predator–prey risks for fish that perform ASR in hypoxia. AB indicates the probability of attack by aerial predators (B), Af, the probability of attacks by fish predators (F); EP, escape performance; MS, metabolic scope and the probability of survival related to the physiological effects of hypoxia, L, the cost of locomotion. Signs ↗, ↘ and → indicate a relative increase, decrease or no potential differences, respectively, when comparing surfacing versus staying in the water column. Piscivorous fish are represented in grey and dotted contour since they may not represent a threat in hypoxia. The sign ‘+’ indicates an advantage; ‘−’, a disadvantage; and ‘=’, no difference in relation to the fitness of the fish. Surfacing allows fish to avoid the negative effect of hypoxia on their metabolic scope and to have a high escape performance. On the other hand, surfacing increases the probability of being detected by aerial predators and it implies a higher cost of locomotion due to vertical excursions.
Surfacing, however, implies various costs (figure 5) including less time spent foraging than in normoxia, the loss of food to competitors and an increase in the energy expenditure during locomotion to and from the surface (Kramer & McClure 1981; Kramer & Braun 1983; Kramer 1987). Most notably, surfacing can lead to an increased risk of predation by forcing fish to leave shelter (Kramer 1987; Wolf & Kramer 1987) and move closer to the surface where they are more visible and more likely to be attacked by aquatic (e.g. air-breathing fish, Wolf & Kramer 1987) and aerial predators (Kramer et al. 1983). This trend was also observed in the field where low water O2 concentration in the early morning, following overnight respiration of aquatic vegetation, caused ASR activity in fish which led to a rise in the capture success of avian predators during feeding aggregation events (Kersten et al. 1991). In addition to increased visibility, fish are more prone to attacks from birds when at the surface as there is less water resistance to slow the speed of the strike and fish have less time to escape (Kramer et al. 1983). On the other hand, while vulnerability to various piscivorous predators (such as bird and air-breathing fish) may theoretically increase, feeding rates of water-breathing piscivorous fish decrease in hypoxia (Poulin et al. 1987; figure 5).
Kramer (1987) points out that air-breathing and ASR may differ in surfacing costs. In ASR fish, oxygen is stored in the blood rather than in a respiratory organ. As a consequence, in severe hypoxia, surfacing time tends to be longer and dive times shorter in ASR fish when compared with air-breathers (Kramer 1987). However, ASR fish usually start surfacing at a lower oxygen level than air-breathers. Therefore, Kramer (1983b, 1987) suggested that ASR fish may incur lower risks than air-breathers at moderate hypoxia, while air-breathers may have an advantage over ASR fish in extreme hypoxia.
Although driven by physiological constraints, there is evidence for elements of behavioural control over surfacing, as predator avoidance behaviour is aimed at minimizing the risk of predation (Kramer & Graham 1976; Shingles et al. 2005). Similarly, when faced with a perceived threat of predation, fish may decrease their activity level (Seghers 1974; Herbert & Wells 2001). Predation risk can inhibit surface use (figure 6a) and fish can show a reduction in surfacing rate (Kramer & Graham 1976; Kramer 1983a; Smith & Kramer 1986; Wolf & Kramer 1987; Herbert & Wells 2001) or delay its onset (Shingles et al. 2005). When fish do need to surface, they are known to show antipredator behaviours such as surfacing with irregularity, speed and synchrony (Kramer & Graham 1976). During synchronous surfacing, individuals rise in rapid succession or together to reduce the chance of contact between predator and any individual, and create confusion, in much the same way as schooling (Kramer & Graham 1976; Gee 1980; Chapman & Chapman 1994). Fish have also been noted to avoid the vicinity of the predator when surfacing, to decrease ASR frequency and increase mean depth (Kramer et al. 1983) and to change foraging site (Milinski & Heller 1978), as a compromise aimed at minimizing predator risk while avoiding the detrimental effect of hypoxia. Severe hypoxia may induce fish to leave cover such as patches of vegetation (Wolf & Kramer 1987; Kersten et al. 1991). Where available, fish choose to surface near shelters, as an alternative solution aimed at minimizing visibility to predators (Wolf & Kramer 1987; Shingles et al. 2005).
Figure 6.
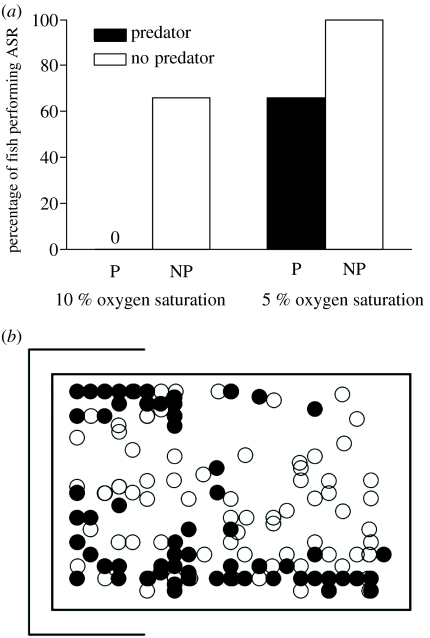
(a) Percentage of flathead grey mullet (M. cephalus) performing ASR in hypoxia (10 and 5% oxygen saturation) in the presence (P, black bars) and absence (NP, white bars) of a model bird predator. No fish performed ASR at 10% oxygen saturation in the presence of the model predator. Data from Shingles et al. 2005. (b) Location of the ASR episodes in hypoxia in the presence (black dots) and absence (white dots) of a predator model. Open rectangle on the left indicates a shelter area. In the presence of the predator model, fish preferred to perform ASR near the shelter area and near the walls (adapted from Shingles et al. 2005).
The trade-offs related to surfacing are even more complex once other environmental factors are considered, such as turbidity, time of the day, weather conditions, temperature, salinity and the presence of shelters. The ability of fish to adopt ASR behaviour can be affected by turbidity where fish are unable to establish if predators are present (Shingles et al. 2005). Shingles et al. (2005) found that when exposed to a model bird predator flown overhead, grey mullet delayed the onset of ASR to more extreme levels of hypoxia and when ASR began they chose to surface close to edges and under cover (figure 6b). Exposure to turbidity, however, abolished the delay of the onset of ASR in the presence of the model predator. Interestingly, fish in turbidity concurrent with severe hypoxia chose to perform ASR under areas of cover or close to edges, whether the predator was present or not (Shingles et al. 2005). This, presumably, is an adaptation to allow fish to perform ASR under cover when they are unable to establish whether there is a real predator threat. As pointed out by Kramer et al. (1983), variations in light levels, surface glare and rippling, water clarity and cover might affect both the absolute level and the gradient of risk associated with surfacing in the field. In reality, the situation invariably involves a trade-off between the benefits associated with surfacing and the danger of being more exposed to predators.
5. The effect of hypoxia on schooling behaviour
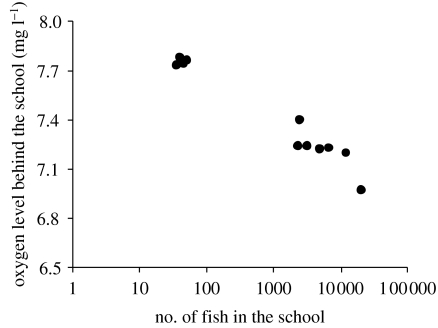
While many schooling species are pelagic (e.g. scombrids) and may not experience the hypoxic conditions caused by eutrophication in coastal areas, there are a number of coastal and lagoon fishes that are gregarious (e.g. Mugilidae) and may face hypoxic conditions seasonally. Other schooling species such as herring (Clupea harengus) may occur in areas with recurrent hypoxic events, such as in the Kattegat and some Norwegian Fjords (Dommassnes et al. 1994; Hognestad 1994; Domenici et al. 2002). In addition, previous fieldwork has shown that the oxygen level in a school may decrease along its axis of motion as a result of the oxygen consumption by the fish in the front of the school (McFarland & Moss 1967; Green & McFarland 1994; figure 7). Therefore, schooling itself may cause hypoxic conditions in the centre/back of a school. This phenomenon may be particularly relevant for large schools, and it may also limit the size of fish schools as modelled by Steffensen (1995).
Figure 7.
Oxygen level behind schools composed of different numbers of individuals of blacksmith (Chromis punctipinnis). Data from Green & McFarland (1994).
Schooling is considered to be mainly related to predator–prey interactions, be it for defence (e.g. Godin 1986; Magurran 1990; Pitcher & Parrish 1993; Domenici & Batty 1997; Crook 1999; Krause & Ruxton 2002) or as a feeding adaptation (see Pitcher & Parrish 1993, and Krause & Ruxton 2002, for reviews). Consequently, schooling behaviour is largely affected by the presence of predators and food. Indeed, schools are often formed and maintained as a response to predators or depending on hunger level (Pitcher & Parrish 1993; Hensor et al. 2003), e.g. in gregarious species such as certain cyprinids and Mugilidae, which move in and out of a school depending on the threat level (P. Domenici 2003, personal observations). School variables such as size, shape, and density can also be related to the presence of predators and food. The number of fish in a school of prey was shown to affect the predator attack rate and success (Major 1978; Krause & Godin 1995). Similarly, predator success increases with the number of fish in the predator school (Major 1978). School density was shown to increase in the presence of predators (Pitcher & Wyche 1983), although field densities of schools under attack may not be maximal, since they are lower than values found in laboratory conditions (Domenici et al. 2000b). School shape can vary depending on the presence/absence of predators (Abrahams & Colgan 1985). Predator formations were observed to take on specific shapes (Partridge et al. 1983), and schools of prey being threatened change shape depending on the behaviour of the predators (Pitcher & Wyche 1983).
While predators and prey are important factors in shaping school structure and dynamics, schooling behaviour is affected by a number of abiotic factors as well, including light level, weather conditions, water depth, temperature and oxygen (e.g. Glass et al. 1986; Scalabrin & Masse 1993; Weetman et al. 1999; Domenici et al. 2002). For example, light level has a direct effect on vision, which is one of the main sensory systems used for schooling (Pitcher & Parrish 1993). Therefore, it is not surprising that light level thresholds for schooling were found in various species (e.g. Glass et al. 1986; Miyazaki et al. 2000; Milne et al. 2005). Consequently, schools were observed to disperse at night (Glass et al. 1986). Recently, a potential mechanism that may prevent complete dispersion of schools at night was found in herring, i.e. the production of fast repetitive sound pulses at night (Wilson et al. 2004).
(a) School volume and spacing
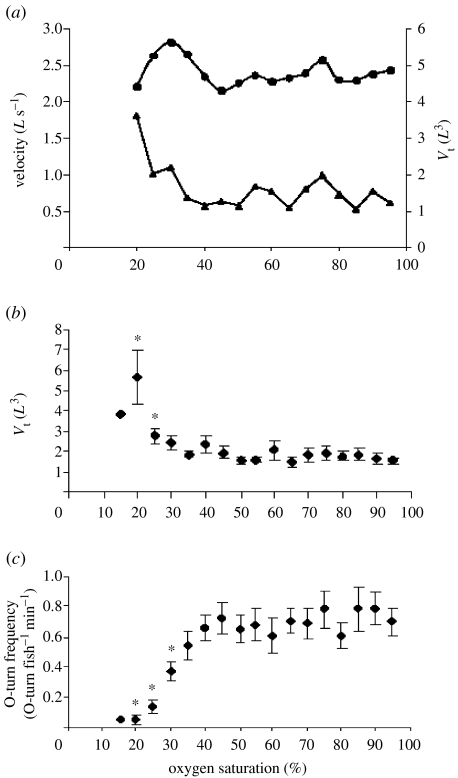
Various studies have shown that hypoxia is a determinant factor affecting the behaviour, structure and dynamics of fish schools (Moss & McFarland 1970; Isreal & Kimmel 1996; Domenici et al. 2000a, 2002). One of the main findings of these studies is that hypoxia induces an increase in school volume and therefore spacing between fish (figure 8a,b). Domenici et al. (2002) suggested that an increase in school volume may provide more oxygen available for each individual, thereby counteracting the limiting effect of hypoxia on schools. They indicate two potential ways through which schools subject to hypoxia may increase the oxygen available for rear fish through an increase in: (i) cross-sectional area (i.e. width and depth) of the school and (ii) school length, which may increase oxygen availability, as long as there is sufficient mixing of water mass within the school. As in Domenici et al. (2002), work was carried out in the laboratory, a larger school volume in such a confined set-up would not result in the avoidance of hypoxic conditions. However, the increased spacing between fish may be due to a behavioural reflex in hypoxia which, in nature, may alleviate the respiratory distress at the rear of the school, thereby decreasing the oxygen threshold for the break-up of a large school, as was observed by McFarland & Moss (1967). Laboratory data show an increase from a specific volume (volume of water per fish) of 1.5 L3 (1.5 length3) in normoxia to over 5 L3 at 20% oxygen saturation (figure 8b). Pitcher & Partridge (1979) suggest that a typical value of specific volume in herring and other schooling species should be around 1 L3. Unpublished field data (Batty et al. 2004, unpublished observations) show relatively low densities in herring schools in hypoxia as indicated by the loose spacing (nearest neighbour distance of 3.3 L) when compared with literature values reported in Pitcher & Partridge (1979) (i.e. 0.6–1.0 L).
Figure 8.
(a) Example of the effect of hypoxia on school volume (volume of water per fish) (Vt in length3) and speed (in length s−2) in a school of herring subject to progressive hypoxia. (b) The effect of oxygen saturation on the average herring school volume. (c) The effect of hypoxia on the frequency of O-turn manoeuvres in schools of herring. Adapted from Domenici et al. (2002). Asterisks represent significant differences from normoxia.
The rate of deoxygenation used in laboratory experiments may modulate the effect of hypoxia on schooling (Moss & McFarland 1970). Moss & McFarland (1970) found that a gradual decline (obtained within about 1.5 h) in oxygen level did not cause any changes in schooling behaviour (measured using a density index and a parallel orientation index) or swimming speed until near-lethal levels were reached (i.e. near 10% oxygen saturation). However, sudden decreases in oxygen level (about 0.5–1 mg O2 l−1 within a few seconds) induced by injecting hypoxic water caused changes in swimming speed within 20–30 s. Moss & McFarland (1970) suggest that such fast responses may be due to peripheral O2 sensitive receptors located in the gills, as described by Smatresk (1990). Such acute changes in oxygen levels are unlikely to occur in the wild within a given vertical and horizontal position in the water, because such temporal changes are likely to be of the order of hours (figure 9). However, acute changes in oxygen level occur when fish swim across a relatively steep oxygen gradient such as those found vertically in coastal areas and lagoons. As an example, Domenici et al. (2002) report a vertical oxygen gradient in the Kattegat Sea from 25% oxygen saturation to normoxia within 5 m. This would mean that a 25 cm fish swimming vertically at a cruising speed of 2 lengths s−1 would experience such an acute change in oxygen level within only 10 s.
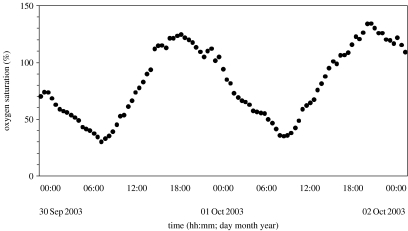
Figure 9.
The daily variation in oxygen level in Cabras Lagoon (western Sardinia, Italy) during the end of September 2003. Oxygen level goes from normoxia to hypoxia (about 30% oxygen saturation) within about 6 h.
Using progressive hypoxia (from normoxia to 25% oxygen saturation in 4 h), Domenici et al. (2002) showed that all the dimensions (i.e. X, Y and Z) of a school of herring increase in hypoxia. They suggest that the observed increase in school volume may result from either a behavioural mechanism that evolved as a response to hypoxia or lower sensory performance, similarly to the decreased performance observed in escape responses in hypoxia as discussed above. Isreali & Kimmel (1996) measured the effect of hypoxia on schooling behaviour in Carassius auratus, a freshwater species known as one of the least hypoxia sensitive fish due to alternative biochemical pathways (Shoubridge & Hochachka 1980). Fish were subjected to sublethal oxygen concentration, i.e. 1 mg O2 l−1 at 21°C. Isreali & Kimmel (1996) found that only one component of school structure, its horizontal dimension, increased in hypoxia. Similarly, results by Domenici et al. (2002) show that a school's horizontal spread (school area) increases significantly starting from 30% oxygen saturation, while school depth is significantly different from that in normoxia only at ≤20% oxygen saturation. It is therefore possible that the first effect of hypoxia may be to induce an increase in the horizontal spacing between fish. This may allow fish to keep some hydrodynamic advantages from following their neighbours (Liao 2007), while fish in different vertical planes would not experience such advantages (Weihs 1973b; Abrahams & Colgan 1985).
(b) School integrity and spontaneous activity
Hypoxia is known to have an effect on the spontaneous activity of various fish species. From a metabolic point of view, fish activity may be expected to reduce overall aerobic metabolic energy expenditure. This expectation is confirmed by work on a number of species (Metcalfe & Butler 1984; Fisher et al. 1992; Schurmann & Steffensen 1994). However, certain species show a temporary increase in activity when subjected to hypoxia, possibly as a behavioural ‘avoidance’ response (Dizon 1977; Bejda et al. 1987; Domenici et al. 2000a). As schooling structure and dynamics are largely affected by activity level and swimming speed (Pitcher & Partridge 1979), theoretically the change in school structure observed in hypoxia may be an indirect result of a change in activity. Work on herring showed that this was not the case. In herring, swimming speed peaks at around 30% oxygen saturation, after which speed starts decreasing (figure 8a). On the other hand, maximum school volume occurs at 20% (figure 8b). Therefore, in herring, the increase in school volume is relatively decoupled from the increase in swimming speed (Domenici et al. 2000a, 2002) although both are caused by hypoxia.
Herring is a species that can tolerate hypoxia (approx. 30% oxygen saturation in Dommasnes et al. 1994) for long periods of time. Domenici et al. (2000a) studied the effect of progressive hypoxia on school integrity and swimming speed in herring and found that swimming speed increased in hypoxia (around 15–34% oxygen saturation) to an extent that depended on the school's spontaneous activity prior to the hypoxia exposure. Following a peak in swimming speed, fish activity decreased until the school unity was disrupted (around 12–25% oxygen saturation). Similarly to swimming speed, school disruption occurred at higher oxygen levels in schools that had a higher spontaneous activity prior to the severe hypoxia. Fish that had higher activity may experience higher stress and exhaustion and their schools may therefore break-up at a higher oxygen level than those which had relatively low spontaneous activity. Respiratory distress may affect the sensory channels employed by fish for the coordination of schooling manoeuvres, such as lateral line and vision (Partridge & Pitcher 1980) and the fish's general awareness, resulting in the break-up of the school. Similarly, by affecting sensory performance, hypoxia may have detrimental effects in the synchronization of specific, fast antipredator manoeuvres such as fountain effects and skitter (Hall et al. 1986; Pitcher & Parrish 1993), which imply a high level of coordination to prevent collisions between neighbours.
(c) Schooling dynamics
Although individual positional preferences have been found in various species (Healy & Prieston 1973; Pitcher et al. 1982), there is evidence that individuals in a school perform a certain level of turn-over (Pitcher et al. 1982; Krause & Ruxton 2002). This may serve to expose each individual of the school to the various advantages and disadvantages related to each position. For example, front positions may confer feeding advantages (Krause 1993) but higher predation risk (Bumann et al. 1997) while back positions were suggested to confer hydrodynamic advantages (Herskin & Steffensen 1998).
Domenici et al. (2000a) hypothesized that shuffling rates may increase in hypoxia, as swimming activity increases. They hypothesized that rear fish may move to the front more frequently, since rear positions may experience reduced oxygen levels (Green & McFarland 1994). The effect of hypoxia on schooling dynamics, in terms of position shuffling, was studied by Domenici et al. (2002) in herring. They found that the leading fish had the tendency to perform a manoeuvre that would re-position them at the back of the school. This manoeuvre (termed ‘O-turn’) was performed on average about 0.8 per minute per individual in normoxia. O-turn frequency decreased dramatically in hypoxia, down to less than 0.1 manoeuvre per minute per fish (figure 8c). However, this decrease did not affect the time individual fish spent in leading positions. Therefore, while hypoxia had an effect on the internal dynamics in terms of ‘active’ manoeuvring by leading fish, the overall shuffling rate did not change. Shuffling rates may have been maintained constant by other mechanisms such as an increased rate of overtaking or falling back of individual fishes. Reduction in O- turn frequency could be due to their cost, which may not be supported during respiratory distress such as that caused by hypoxia.
(d) Trade-offs in schooling: the effect of biotic and abiotic factors
While schooling behaviour may confer a number of advantages, especially in terms of antipredator adaptations, but also in terms of foraging and energetics, the specific structure of each school and the behaviour of each individual in a school may be the result of a number of trade-offs. Figure 10 summarizes the changes induced in school structure by hypoxia and predator–prey interactions. School volume increases in hypoxia and various studies suggest that horizontal dimensions may be the first component of school volume to be affected (Isreali & Kimmel 1996; Domenici et al. 2002). There are a number of trade-offs to be taken into account when considering school volume (i.e. spacing between individual fish). In non-limiting oxygen conditions, spacing was suggested to be the result of two opposite forces corresponding to the main sensory modalities used in coordinating schools, i.e. attraction as driven by vision and repulsion as driven by the lateral line (Partridge & Pitcher 1980). Therefore, functionally, given spacing allows fish to keep track of one another without colliding. Small inter-individual distances are also advantageous in antipredator manoeuvres, as they may increase the confusion effect on the predator, and aid the synchronization of escape manoeuvres. Gray & Denton (1991) suggest that rapid escape manoeuvres in schools may be synchronized by fast sound pulses emitted by startled fish, which may startle neighbours at relatively close distances, of the order of one body length. While the pulse emitted by startled fish may be well above the auditory threshold at spacing found in hypoxia, sound pressure may be below the threshold required to trigger escape responses in neighbours and therefore a synchronized escape manoeuvre (Domenici et al. 2002). Similarly, increased distances between neighbours as observed in hypoxia, may decrease the energetic advantages of schooling such as those found by Herskin & Steffensen (1998). Optimal distances have been proposed for fish to take advantages of the vorticity created by fish swimming in front (Weihs 1973b). While no measurements of the energy dissipation of the vortices produced by fish in a school have been taken, increased horizontal distance between neighbours may affect the exploitation of the vorticity produced by fish in front. Therefore, while increased horizontal distance found in hypoxia may allow for a better oxygenation of the school, it may also incur in energetic and antipredator disadvantages.
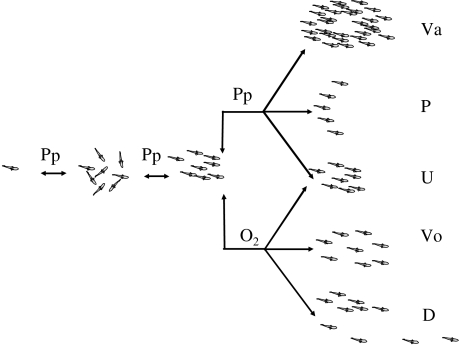
Figure 10.
The effect of hypoxia and predator–prey interactions on the aggregation behaviour of fish. From left to right, predator–prey (Pp) interactions may cause single fish to join in a group or vice versa, and they may affect the degree of polarization of a school and they may affect school speed (indicated by ‘U’) and shape (Pitcher & Parrish 1993). (Va) and (P) represent school shapes as described in Pitcher & Parrish 1993 (Va, ‘vacuole’ as a response to predator attacks) and Partridge et al. (1983) (P, ‘parabolic schools’ of predators), respectively. Oxygen level (O2) may affect school volume (Vo), it may cause schools to disrupt (D) and may affect school speed (U). Changes in speed do not imply changes in school shape.
While a larger volume may affect the hydrodynamic advantages of schooling, it is likely that large horizontal spacing will affect the hydrodynamic advantages to a lesser extent than large vertical spacing. This brings up the issue of trade-offs in school shape, identified by Abrahams & Colgan (1985). They tested the hypothesis that fish school shape may be the result of two conflicting forces: (i) schools should be relatively flat in order to maximize the hydrodynamic advantages of following the vorticity produced by fish in front and (ii) schools should be spread in depth, in order to maximize the visual fields of each individual, allowing for predator perception otherwise blocked by neighbours in the same plane. Abrahams & Colgan (1985) found that in the absence of predators, characin fish tend to form relatively flat schools, while they dispersed in depth when a predator was present. In hypoxia, the horizontal school dimension is the first variable to be affected (Isreali & Kimmel 1996), implying that maximization of energetic advantages may be preferred over maximization of visual fields for predator detection. However, this observation was made in laboratory conditions. It is unknown how fish schools would behave when facing a similar trade-off in natural conditions and in the presence of natural predators. Further field studies would be necessary to establish how fish deal with these potential trade-offs, since there is evidence that many of the behaviours induced by hypoxia (increase of inter-individual distances and increase in horizontal dimensions) are the opposite of those induced by predator presence.
Shuffling rate is the result of individual behaviour within the school, and it may represent the balance between the trade-offs associated to occupying different positions within a school. Front positions were suggested to be advantageous for feeding, while they imply higher predation risk and higher energetic costs (Bumann et al. 1997; Herskin & Steffensen 1998). In hypoxia, leading positions may be advantageous as leaders would not experience a further reduction in oxygen level due to the oxygen consumption of fish in the front (figure 7). However, hypoxia does not appear to affect the overall shuffling rates, although the ‘active’ reshuffling rate decreases (i.e. the occurrence of repositioning O-turn manoeuvres figure 8c; Domenici et al. 2002). Therefore, other factors may be more important in regulating shuffling rates, such as nutritional state, energetics and perceived risk of predation. For example, front positions are used by hungry fish as they offer better information about the location of food sources, while well-fed fish may take up less risky positions towards the back centre of the school (Krause 1993; Krause et al. 1998).
Schooling dynamics and school structure in field situations are influenced by a larger pool of factors than can be investigated in laboratory conditions, including biotic factors such as predation pressure and food distribution (figure 10). On the other hand, results from fieldwork are often confounded by the presence of a large number of varying physical characteristics, such as depth, light level, temperature and oxygen level. Work on the effect of physical factors on schooling behaviour often come from either laboratory or field studies. We believe that a full understanding of the behavioural and physiological processes at the basis of the dynamics of fish aggregations requires the integration of laboratory studies on the physiological and behavioural responses of fish to various controlled physical factors, with field studies that will test the predictions made on the basis of such laboratory studies.
6. Conclusions and future issues
By affecting escape behaviour, hypoxia may have a large effect on survival from predator attacks. Surfacing mitigates the negative effects of hypoxia on escape performance, but it also increases visibility to aerial predators. Fish deal with this situation by showing avoidance behaviour which ranges from decreasing surfacing frequency to surfacing near shelters (Kramer et al. 1983; Shingles et al. 2005). While schooling behaviour can also be affected by hypoxia, how would this alteration of schooling affect fish in terms of their fitness and survival? If we assume that schooling behaviour as observed in normoxia corresponds to an optimal solution in terms of school characteristics such as volume, spacing and shape, then any change in these characteristics may diminish the advantages of schooling, such as those related to antipredator behaviours and energetics. For example, increased spacing may result in less efficient synchronization of antipredator manoeuvres. Increase in volume may also increase the probability of detection by predators.
All of these effects of hypoxia need to be put into a specific environmental context. For example, in an environment where piscivorous fish are the main predators and hypoxia is ubiquitous, the deleterious effects of hypoxia on antipredator behaviours may be mitigated by the effect of hypoxia on the metabolic scope of fish predators, and therefore on their feeding rate. Work on fish–fish interactions shows that predation rates decrease in hypoxia (Breitburg et al. 1994; Shoji et al. 2005). In addition, recent work shows that prey fish may be more hypoxia-tolerant than their fish predator, and therefore hypoxic environment may have the potential to act as refuges from predators (Robb & Abrahams 2002, 2003). However, in certain situations, such as when hypoxia is limited to the deep layer, fish predators may stay in the upper layer and dart in to catch prey in hypoxia (Rahle & Nutzman 1994). Work using predators from different taxa, such as birds or hypoxia-tolerant organisms, such as jellyfish or even air-breathing fish, shows that hypoxia may increase prey mortality in fish (e.g. Wolf & Kramer 1987; Kersten et al. 1991; Shoji et al. 2005). Birds can learn to look for fish in hypoxic waters, where they perform ASR and become easier to catch (Kersten et al. 1991). It is also possible that certain marine mammals exploit similar situations, e.g. killer whales (Orcinus orca) attacking large schools of herring in northern Norwegian fjords (Domenici et al. 2000b).
While recent work shows that escape performance decreases in hypoxia, it is possible that in some cases mortality may also increase in hypoxia as a result of lower potential for sustained repetitive flights. While this scenario has been proposed by Beamish (1978), very little is known about the effect of hypoxia on sustained escape. The relative weight of these effects on evasive behaviour may be modulated by the structural complexity of the habitat, as this is a main determinant of the characteristics of predator–prey interactions in fish (Domenici 2003). Habitats with relatively high structural complexity may provide various refuges and hiding places to prey. In these habitats, predator–prey interactions are likely to be reduced to brief events, where a single successful escape manoeuvre may be sufficient to get out of sight of the predator. Here, the effect of hypoxia on escape performance such as responsiveness, directionality and kinematics may increase prey vulnerability. Relatively open habitats, however, provide few refuges for fish. In these cases, predator–prey interactions may imply relatively long chases, where the ability of prey to sustain flight would be fundamental for avoiding predation. Therefore, in structurally open habitats, hypoxia is likely to be even more deleterious to prey attacked by hypoxia-tolerant predators, as both escape performance and stamina may be limited. In summary, the effect of hypoxia on predator–prey interactions may depend on environmental conditions, as well as on the taxa and species involved. By acting differentially on different species/taxa of prey and predators depending on their tolerance of hypoxia (i.e. increasing predation rate in hypoxia-tolerant predators and decreasing it in hypoxia sensitive ones), oxygen fluctuations may have an important role in the establishment of fish communities.
While effects on both escape and schooling have been demonstrated, more work is needed in order to investigate the potential interactions among the effects of hypoxia on these two behaviours. Experiments aimed at studying whether hypoxia affects the coordination and effectiveness of escape manoeuvres in fish schools would be necessary for this purpose. In addition, insights into the mechanisms behind the effects of hypoxia on antipredator behaviours would increase the predictive power for generating possible future scenarios. For example, while escape responsiveness decreases in hypoxia, it is not clear whether this effect is due to ‘physiological limits’, i.e. an impairment of sensory performance or to ‘behavioural choice’ aimed at minimizing energetic expenses. A similar question can be addressed for the increase in school volume, i.e. it is not established whether such an increase is due to sensory impairment for the maintenance of shorter inter-individual distances or to a behavioural mechanism evolved to deal with the stress imposed by hypoxia. Behavioural choice was shown to modulate ASR by delaying its onset (Shingles et al. 2005). Experiments using variable predator risk could be used in order to establish the physiological limits imposed by hypoxia and the extent of behavioural choice in the hypoxia-related changes in antipredator behaviours.
Finally, the integration of laboratory and fieldwork will be necessary in order to allow us to put hypoxia-related effects within specific environmental contexts, as well as to generate predictive environmental scenarios. This would enhance our ability to predict community changes related to habitat degradation and the increasing trend of coastal hypoxia. Laboratory experiments focusing on the basis of the response on different species of predators and prey will need to be integrated with fieldwork in which other factors such as temperature and turbidity affect relevant aspects of fish physiology, i.e. their metabolic scope and sensory performance, respectively.
Acknowledgments
Financial support by the European Union, Directorate Fisheries, through contract QLRS- 2002-00799 (project ETHOFISH) is acknowledged. We also thank David McKenzie and an anonymous referee for their useful comments on an earlier draft.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Abrahams M.V, Colgan P. Risk of predation, hydrodynamic efficiency and their influence on school structure. Environ. Biol. Fishes. 1985;13:195–202. doi:10.1007/BF00000931 [Google Scholar]
- Barber J, Walker P, Svensson P.A. Behavioural responses to simulated avian predation in female three spined sticklebacks: the effect of experimental Schistocephalus solidus infections. Behaviour. 2004;141:1425–1440. doi:10.1163/1568539042948231 [Google Scholar]
- Batty R.S, Domenici P. Predator–prey relationships in fish and other aquatic vertebrates: kinematics and behaviour. In: Domenici P, Blake R.W, editors. Biomechanics in animal behaviour. BIOS Scientific Publishers Ltd; Oxford, UK: 2000. pp. 237–257. [Google Scholar]
- Beamish F.W.H. Respiration of fish with special emphasis on standard oxygen consumption, III. Influence of oxygen. Can. J. Zool. 1964;42:355–366. [Google Scholar]
- Beamish F.W.H. In: Swimming capacity. Fish physiology. Hoar W.S, Randall D.J, editors. vol. 7. Academic Press, Inc; New York, NY: 1978. pp. 101–187. [Google Scholar]
- Bejda A.J, Studholme A.L, Olla B.L. Behavioural responses of red hake, Urophycis chuss, to decreasing concentrations of dissolved oxygen. Environ. Biol. Fishes. 1987;19:611–621. doi:10.1007/BF00003227 [Google Scholar]
- Breitburg D.L, Steinberg N, DuBeau S, Cooksey C, Houde E.D. Effects of low dissolved oxygen on predation on estuarine fish larvae. Mar. Ecol. Prog. Ser. 1994;104:235–246. [Google Scholar]
- Breitburg D.L, Rose K.A, Cowan J.H. Linking water quality to larval survival: predation mortality of fish larvae in an oxygen-stratified water column. Mar. Ecol. Prog. Ser. 1999;178:39–54. [Google Scholar]
- Bumann D, Krause J, Rubenstein D. Mortality risk of spatial position in animal groups: the danger of being in the front. Behaviour. 1997;134:1063–1076. [Google Scholar]
- Bushnell P.G, Steffensen J.F, Johansen K. Oxygen consumption and swimming performance in hypoxia-acclimated rainbow trout Salmo gairdneri. J. Exp. Biol. 1984;113:225–235. [Google Scholar]
- Canfield J.G, Eaton R.C. Swimbladder acoustic pressure transduction initiates Mauthner-mediated escape. Nature. 1990;347:760–762. doi:10.1038/347760a0 [Google Scholar]
- Cech J.J, Rowell D.M, Glasgow J.S. Cardiovascular responses of the winter flounder Pseudopleuronectes americanus to hypoxia. Comp. Biochem. Physiol. A. 1977;57:123–125. doi:10.1016/0300-9629(77)90361-9 [Google Scholar]
- Chabot D, Dutil J.D. Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J. Fish Biol. 1999;55:472–491. doi:10.1111/j.1095-8649.1999.tb00693.x [Google Scholar]
- Chapman L.J, Chapman C.A. Observations on synchronous air breathing in Clarias liocephalus. Copeia. 1994;1994:246–249. doi:10.2307/1446696 [Google Scholar]
- Chapman L.J, Kaufman L.S, Chapman C.A, McKenzie F.E. Hypoxia tolerance in twelve species of East African cichlids: potential for low oxygen refugia in Lake Victoria. Conserv. Biol. 1994;9:1274–1287. doi: 10.1046/j.1523-1739.1995.9051262.x-i1. doi:10.1046/j.1523-1739.1995.9051274.x [DOI] [PubMed] [Google Scholar]
- Congleton J.L. Observations on the responses of some southern California tidepool fishes to nocturnal hypoxic stress. Comp. Biochem. Physiol. A. 1980;66:719–722. doi:10.1016/0300-9629(80)90026-2 [Google Scholar]
- Crook A.C. A quantitative analysis of the relationship between interspecific encounters, schooling behaviour and coloration in juvenile parrotfish (family Scaridae) Mar. Freshw. Behav. Physiol. 1999;33:1–19. [Google Scholar]
- Dalla Via D, Van den Thillart G, Cattani O, Cortesi P. Behavioural responses and biochemical correlates in Solea solea to gradual hypoxic exposure. Can. J. Zool. 1998;76:2108–2113. doi:10.1139/cjz-76-11-2108 [Google Scholar]
- Dean T.L, Richardson J. Responses of seven species of native freshwater fish and a shrimp to low levels of dissolved oxygen. N. Z. J. Mar. Freshwater Res. 1999;33:99–106. [Google Scholar]
- Dickson Hoese H. Jumping mullet: the internal diving bell hypothesis. Environ. Biol. Fishes. 1985;13:309–314. doi:10.1007/BF00002915 [Google Scholar]
- Dizon A.E. Effect of dissolved oxygen concentration and salinity on the swimming speed of two species of tuna. Fish. Bull. 1977;75:649–653. [Google Scholar]
- Domenici P. Scaling the locomotor performance in predator–prey interactions: from fish to killer whales. Comp. Physiol. Biochem. 2001;131:169–182. doi: 10.1016/s1095-6433(01)00465-2. doi:10.1016/S1095-6433(01)00465-2 [DOI] [PubMed] [Google Scholar]
- Domenici P. Habitat type, design and the swimming performance of fish. In: Bels V, Gasc J.P, Casinos A, editors. Vertebrate biomechanics and evolution. Bios Scientific Publishers; Oxford, UK: 2003. pp. 137–160. [Google Scholar]
- Domenici P, Batty R.S. Escape behaviour of solitary herring (Clupea harengus) and comparisons with schooling individuals. Mar. Biol. 1997;128:29–38. doi:10.1007/s002270050065 [Google Scholar]
- Domenici P, Blake R.W. The kinematics and performance of the escape response in the angelfish (Pterophyllum eimekei) J. Exp. Biol. 1991;156:187–205. [Google Scholar]
- Domenici P, Blake R.W. Escape trajectories in angelfish (Pterophyllum eimekei) J. Exp. Biol. 1993a;177:253–272. [Google Scholar]
- Domenici P, Blake R.W. The effect of size on the kinematics and performance of angelfish (Pterophyllum eimekei) escape responses. Can. J. Zool. 1993b;71:2319–2326. [Google Scholar]
- Domenici P, Blake R.W. The kinematics and performance of fish fast start swimming. J. Exp. Biol. 1997;200:1165–1178. doi: 10.1242/jeb.200.8.1165. [DOI] [PubMed] [Google Scholar]
- Domenici P, Steffensen J.F, Batty R.S. The effect of progressive hypoxia on swimming activity and schooling in Atlantic herring. J. Fish. Biol. 2000a;57:1526–1538. doi:10.1111/j.1095-8649.2000.tb02229.x [Google Scholar]
- Domenici P, Batty R.S, Simila T. Spacing of wild herring while encircled by killer whales. J. Fish Biol. 2000b;57:831–836. doi:10.1111/j.1095-8649.2000.tb00278.x [Google Scholar]
- Domenici P, Ferrari R.S, Steffensen J.F, Batty R.S. The effects of progressive hypoxia on school structure and dynamics in Atlantic herring Clupea harengus. Proc. R. Soc. B. 2002;269:2103–2111. doi: 10.1098/rspb.2002.2107. doi:10.1098/rspb.2002.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommasnes A, Rey F, Rottingen I. Reduced oxygen concentration in herring wintering areas. ICES J. Mar. Sci. 1994;51:63–69. doi:10.1006/jmsc.1994.1006 [Google Scholar]
- Druon J.N, Schrimpff W, Dobricic S, Stips A. Comparative assessment of large-scale marine eutrophication: North Sea area and Adriatic Sea as case studies. Mar. Ecol. Prog. Ser. 2004;272:1–23. [Google Scholar]
- Duthie G.G. The respiratory metabolism of temperature-adapted flatfish at rest and during swimming activity and the use of anaerobic metabolism at moderate swimming speeds. J. Exp. Biol. 1982;97:359–373. doi: 10.1242/jeb.97.1.359. [DOI] [PubMed] [Google Scholar]
- Eaton R.C, Hackett J.T. The role of Mauthner cells in fast-starts involving escape in teleost fish. In: Eaton R.C, editor. Neural mechanisms of startle behavior. Plenum Press; New York, NY: 1984. pp. 213–266. [Google Scholar]
- Eby L.A, Crowder L.B. Hypoxia-based habitat compression in the Neuse River Estuary: context-dependent shifts in behavioral avoidance thresholds. Can. J. Fish. Aquat. Sci. 2002;59:952–965. doi:10.1139/f02-067 [Google Scholar]
- Eby L.A, Crowder L.B, McClellan C.M, Peterson C.H, Powers M.J. Habitat degradation from intermittent hypoxia: impacts on demersal fishes. Mar. Ecol. Prog. Ser. 2005;291:249–261. [Google Scholar]
- Fisher P, Rademacher K, Kils U. In situ investigations on the respiration and behaviour of the eelpout Zoarces viviparus under short-terms hypoxia. Mar. Ecol. Prog. Ser. 1992;88:181–184. [Google Scholar]
- Foreman M.B, Eaton R.C. The direction change concept for reticulospinal control of goldfish escape. J. Neurosci. 1993;13:4101–4113. doi: 10.1523/JNEUROSCI.13-10-04101.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche R, Nilsson S. Cardiovascular responses to hypoxia in the Atlantic cod, Gadus morhua. J. Exp. Biol. 1989;48:153–160. [PubMed] [Google Scholar]
- Fry F.E. The effect of environmental factors on the physiology of fish. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. VI. Academic Press; New York, NY; London, UK: 1971. pp. 1–98. [Google Scholar]
- Fuiman L.A, Smith M.E, Malley V.N. Ontogeny of routine swimming speed and startle responses in red drum, with a comparison of responses to acoustic and visual stimuli. J. Fish Biol. 1999;55:215–226. [Google Scholar]
- Gee J.H. Respiratory patterns and antipredator responses in the central mudminnow, Umbra limi, a continuous, facultative, air-breathing fish. Can. J. Zool. 1980;58:819–827. [Google Scholar]
- Gee J.H, Gee P.A. Reaction of gobioid fishes to hypoxia: bouyancy control and aquatic surface respiration. Copeia. 1991;1991:17–28. doi:10.2307/1446244 [Google Scholar]
- Gee J.H, Tallman R.F, Smart H.J. Reactions of some great plains fishes to progressive hypoxia. Can. J. Zool. 1978;56:1962–1966. [Google Scholar]
- Gehrke P.C. Response surface analysis of teleost cardio-respiratory responses to temperature and dissolved oxygen. Comp. Biochem. Physiol. A. 1988;89:587–592. doi:10.1016/0300-9629(88)90837-7 [Google Scholar]
- Gilmour K.M. Gas exchange. In: Evans D.H, editor. The physiology of fishes. 2nd edn. CRC Press LLC; Boca Raton, FL: 1997. 101–128. [Google Scholar]
- Glass C.W, Wardle C.S, Mojsiewicz W.R. A light intensity threshold for schooling in the Atlantic mackerel, Scomber scombrus. J. Fish. Biol. 1986;29:71–81. doi:10.1111/j.1095-8649.1986.tb05000.x [Google Scholar]
- Godin J.G.J. Antipredator function of shoaling in teleost fishes: a selective review. Nat. Can. (Rev. Ecol. Syst.) 1986;113:241–250. [Google Scholar]
- Gray J.A.B, Denton E.J. Fast pressure pulses and communication between fish. J. Mar. Biol. Assoc. UK. 1991;71:83–106. [Google Scholar]
- Green D, McFarland W.N. Impact of foraging blacksmiths on constituents in the water column: implications on school behaviour and structure. In: Halverson W.L, Maender G.J, editors. The 4th California Islands Symp.: update on the status of resources. Santa Barbara Museum of Natural History; Santa Barbara, CA: 1994. pp. 97–102. [Google Scholar]
- Hagy J.D, Boynton W.R, Keefe C.W, Wood K.V. Hypoxia in Chesapeake Bay 1950-2001: long-term change in relation to nutrient loading and river flow. Estuaries. 2004;27:634–658. [Google Scholar]
- Hale M.E. Locomotor mechanics during early life history: effects of size and ontogeny on fast-start performance of salmonid fishes. J. Exp. Biol. 1999;202:1465–1479. doi: 10.1242/jeb.202.11.1465. [DOI] [PubMed] [Google Scholar]
- Hale M.E. S- and C-start escape responses of the muskellunge (Esox masquinongy) require alternative neuromotor mechanisms. J. Exp. Biol. 2002;205:2005–2016. doi: 10.1242/jeb.205.14.2005. [DOI] [PubMed] [Google Scholar]
- Hall S.J, Wardle C.S, Mac Lennan D.N. Predator evasion in a fish school- test of a model for the fountain effect. Mar. Biol. 1986;91:143–148. doi:10.1007/BF00397579 [Google Scholar]
- Healy M.C, Prieston R. The interrelationship among individuals in a fish school. Tech. Rep. Fish. Res. Bd. Can. 1973;389:1–15. [Google Scholar]
- Hensor E.M.A, Godin J.G.J, Hoare D.J, Krause J. Effects of nutritional state on the shoaling tendency of banded killifish, Fundulus diaphanus, in the field. Anim. Behav. 2003;65:663–669. doi:10.1006/anbe.2003.2075 [Google Scholar]
- Herbert N.A, Wells R.M.G. The aerobic physiology of the air breathing blue gourami, Trichogaster trichopterus, necessitates behavioural regulation of breath-hold limits during hypoxic stress and predatory challenge. J. Comp. Physiol. B. 2001;171:603–612. doi: 10.1007/s003600100211. doi:10.1007/s003600100211 [DOI] [PubMed] [Google Scholar]
- Herskin J, Steffensen J.F. Reduced tail beat frequency and oxygen consumption due to hydrodynamic interactions of schooling sea bass, Dicentrarchus labrax L. J. Fish Biol. 1998;53:366–376. doi:10.1111/j.1095-8649.1998.tb00986.x [Google Scholar]
- Hognestad P.T. The Lake Rossfjord herring (Clupea harengus L.) and its environment. ICES J. Mar. Sci. 1994;51:281–292. doi:10.1006/jmsc.1994.1029 [Google Scholar]
- Hugie D.M, Dill L.M. Fish and game: a game theoretic approach to habitat selection by predators and prey. J. Fish Biol. 1994;45:151–169. [Google Scholar]
- Israeli D, Kimmel E. Monitoring the behavior of hypoxia-stressed Carassius auratus using computer vision. Aquacult. Eng. 1996;15:423–440. doi:10.1016/S0144-8609(96)01009-6 [Google Scholar]
- Kasapi M.A, Domenici P, Blake R.W, Harper D.G. The kinematics and performance of the escape response in the knifefish Xenomystus nigri. Can. J. Zool. 1993;71:189–195. [Google Scholar]
- Kersten M, Britton R.H, Dugan P.J, Hafner H. Flock feeding and food intake in little egrets: the effects of prey distribution and behaviour. J. Anim. Ecol. 1991;60:241–252. doi:10.2307/5457 [Google Scholar]
- Kramer D.L. Aquatic surface respiration in the fishes of Panama: distribution in relation to risk of hypoxia. Environ. Biol. Fishes. 1983a;8:49–54. doi:10.1007/BF00004945 [Google Scholar]
- Kramer D.L. The evolutionary ecology of respiratory mode in fishes: an analysis based on the costs of breathing. Environ. Biol. Fishes. 1983b;9:145–158. doi:10.1007/BF00690859 [Google Scholar]
- Kramer D.L. Dissolved oxygen and fish behaviour. Environ. Biol. Fish. 1987;18:81–92. doi:10.1007/BF00002597 [Google Scholar]
- Kramer D.L, Braun E.A. Short term effects of food availability on air-breathing frequency in the fish Corydoras aeneus (Callichthyidae) Can. J. Zool. 1983;61:1964–1967. [Google Scholar]
- Kramer D.L, Graham J.B. Synchronous air breathing, a social component of respiration in fishes. Copeia. 1976;1976:689–697. doi:10.2307/1443450 [Google Scholar]
- Kramer D.L, McClure M. The transit cost of aerial respiration in the catfish Corydoras aeneus (Callichthydae) Physiol. Zool. 1981;54:189–194. [Google Scholar]
- Kramer D.L, McClure M. Aquatic surface respiration, a widespread adaptation to hypoxia in tropical freshwater fishes. Environ. Biol. Fishes. 1982;7:47–55. doi:10.1007/BF00011822 [Google Scholar]
- Kramer D.L, Mehegan J.P. Aquatic surface respiration, an adaptative response to hypoxia in the guppy, Poecilia reticulata (Pisces, Poeciliidae) Environ. Biol. Fishes. 1981;6:299–313. doi:10.1007/BF00005759 [Google Scholar]
- Kramer D.L, Manley D, Bourgeois R. The effect of respiratory mode and oxygen concentration on the risk of aerial predation in fishes. Can. J. Zool. 1983;61:653–665. [Google Scholar]
- Kramer D.L, Rangeley R.W, Chapman L.J. Habitat selection: patterns of spatial distribution from behavioural decisions. In: Godin J, editor. Behavioural ecology of teleost fishes. Oxford University Press; Oxford, UK: 1997. pp. 37–80. [Google Scholar]
- Krause J. The relationship between foraging and shoal position in a mixed shoal of roach (Rutilus rutilis) and chub (Leuciscus cephalus)—a field study. Oecologia. 1993;93:356–359. doi: 10.1007/BF00317878. doi:10.1007/BF00317878 [DOI] [PubMed] [Google Scholar]
- Krause J, Godin J.G.J. Predator preferences for attacking particular prey group sizes—consequences for predator hunting success and prey predation risk. Anim. Behav. 1995;50:465–473. doi:10.1006/anbe.1995.0260 [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Krause J, Reeves P, Hoare D. Positioning behaviour in roach shoals: the role of body length and nutritional state. Behaviour. 1998;135:1031–1039. [Google Scholar]
- Lefrançois C, Claireaux G. Influence of ambient oxygenation and temperature on metabolic scope and scope for heart rate of the sole (Solea solea) Mar. Ecol. Prog. Ser. 2003;259:273–284. [Google Scholar]
- Lefrançois C, Domenici P. Locomotor kinematics and responsiveness in the escape behaviour of European sea bass (Dicentrarchus labrax) exposed to hypoxia. Mar. Biol. 2006;149:969–977. doi:10.1007/s00227-006-0261-0 [Google Scholar]
- Lefrançois C, Shingles A, Domenici P. The effect of hypoxia on locomotor performance and behaviour during escape in the golden grey mullet (Liza aurata) J. Fish Biol. 2005;67:1–19. doi:10.1111/j.1095-8649.2005.00884.x [Google Scholar]
- Lewis W.M. Morphological adaptations of cyprinodontoids for inhabiting oxygen deficient waters. Copeia. 1970;1970:319–326. doi:10.2307/1441653 [Google Scholar]
- Liao J.C. A review of fish swimming mechanics and behavior in altered flows. Phil. Trans. R. Soc. B. 2007;362:1973–1993. doi: 10.1098/rstb.2007.2082. doi:10.1098/rstb.2007.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K.F. Air ventilation in advanced teloests: biomechanical and evolutionary aspects. In: Ali M.A, editor. Environmental physiology of fishes. Plenum Press; New York, NY: 1980. pp. 57–91. [Google Scholar]
- Magurran A.E. The adaptive significance of schooling as an anti-predator defence in fish. Ann. Zool. Fenn. 1990;27:51–66. [Google Scholar]
- Major P.F. Predator–prey interactions in two schooling fishes, Caranx ignobilis and Stolephorus purpureus. Anim. Behav. 1978;26:760–777. doi:10.1016/0003-3472(78)90142-2 [Google Scholar]
- Marvin D.E, Burton D.T. Cardiac and respiratory responses of rainbow trout, bluegills and brown bullhead catfish during rapid hypoxia and recovery under normoxic conditions. Comp. Biochem. Physiol. A. 1973;46:515–524. doi: 10.1016/0300-9629(73)90127-8. doi:10.1016/0300-9629(73)90127-8 [DOI] [PubMed] [Google Scholar]
- Matthews W.J, Hill L.G, Schellhaass S.C. Depth distribution of striped bass and other fish in Lake Texoma (Oklahoma–Texas) during summer stratification. Trans. Am. Fish. Soc. 1985;114:84–91. doi:10.1577/1548-8659(1985)114<84:DDOSBA>2.0.CO;2 [Google Scholar]
- McFarland W.N, Moss S.A. Internal behavior in fish schools. Science. 1967;156:260–262. doi: 10.1126/science.156.3772.260. doi:10.1126/science.156.3772.260 [DOI] [PubMed] [Google Scholar]
- Metcalfe J.D, Butler P.J. Changes in activity and ventilation in response to hypoxia in unrestrained, unoperated dogfish (Scyliorhinus canicula L.) J. Exp. Biol. 1984;180:153–162. doi: 10.1242/jeb.108.1.411. [DOI] [PubMed] [Google Scholar]
- Millinski M, Heller R. Influence of a predator on the optimal foraging behaviour of of sticklebacks (Gasterosteus aculeatus L.) Nature. 1978;275:642–644. doi:10.1038/275642a0 [Google Scholar]
- Milne S.W, Shuter B.J, Sprules W.G. The schooling and foraging ecology of lake herring (Coregonus artedi) in Lake Opeongo, Ontario, Canada. Can. J. Fish. Aquat. Sci. 2005;62:1210–1218. doi:10.1139/f05-030 [Google Scholar]
- Miyazaki T, Shiozawa S, Kogane T, Masuda R, Maruyama K, Tsukamoto K. Developmental changes of the light intensity threshold for school formation in the striped jack Pseudocaranx dentex. Mar. Ecol. Prog. Ser. 2000;192:267–275. [Google Scholar]
- Moss S.A, McFarland W.N. The influence of dissolved oxygen and carbon dioxide on fish schooling behaviour. Mar. Biol. 1970;5:100–107. doi:10.1007/BF00352592 [Google Scholar]
- Moyle P.B, Cech J.J. Prentice Hall; Upper Saddle River, NJ: 2000. Fishes. [Google Scholar]
- Neill W.H, Bryan J.D. Responses of fish to temperature and oxygen, and response integration through metabolic scope. In: Brune D.E, Tomasso J.R, editors. Aquaculture and water quality, advances in world aquaculture. The World Aquaculture Society; Baton Rouge, LA: 1991. pp. 30–57. [Google Scholar]
- Partridge B.L, Pitcher T.J. The sensory basis of fish schools: relative roles of lateral line and vision. J. Comp. Physiol. 1980;135:315–325. doi:10.1007/BF00657647 [Google Scholar]
- Partridge B.L, Johansson J, Kalish J. The structure of schools of giant bluefin tuna in Cape Cod Bay. Environ. Biol. Fishes. 1983;9:253–262. doi:10.1007/BF00692374 [Google Scholar]
- Pichavant K, Maxime V, Thebault M.T, Ollivier H, Garnier J.P, Bousquet B, Diouris M, Bœuf G, Nonnotte G. Effects of hypoxia and subsequent recovery on turbot Scophthalmus maximus: hormonal changes and anaerobic metabolism. Mar. Ecol. Prog. Ser. 2002;225:275–285. [Google Scholar]
- Pitcher T.J, Parrish J.K. Function of shoaling behaviour in teleosts. In: Pitcher T.J, editor. Behaviour of teleost fishes. Chapman and Hall; London, UK: 1993. pp. 363–439. [Google Scholar]
- Pitcher T.J, Partridge B.L. Fish school density and volume. Mar. Biol. 1979;54:383–394. doi:10.1007/BF00395444 [Google Scholar]
- Pitcher T.J, Wyche C.J. Predator avoidance behaviour of sand-eels schools: why schools seldom split? In: Noakes D.L.G, Lindquist B.G, Helfman G.S, Ward J.A, editors. Predators and prey in fishes. Junk; The Hague, The Netherlands: 1983. pp. 193–204. [Google Scholar]
- Pitcher T.J, Wyche C.J, Magurran A.E. Evidence for position preferences in schooling mackerel. Anim. Behav. 1982;30:932–934. doi:10.1016/S0003-3472(82)80170-X [Google Scholar]
- Plante S, Chabot D, Dutil J.D. Hypoxia tolerance in Atlantic cod. J. Fish Biol. 1998;53:1342–1356. doi:10.1111/j.1095-8649.1998.tb00253.x [Google Scholar]
- Poulin R, Wolf N, Kramer D.L. The effect of hypoxia on the vulnerability of guppies (Poecilia reticulata, Poeciliidae) to an aquatic predator (Astronotus ocellatus, Cichlidae) Environ. Biol. Fishes. 1987;20:285–292. [Google Scholar]
- Rabalais N.N, Turner R.E, Wiseman W.J. Gulf of Mexico hypoxia, aka “The dead zone”. Annu. Rev. Ecol. Syst. 2002;33:235–263. doi:10.1146/annurev.ecolsys.33.010802.150513 [Google Scholar]
- Rahel F.J, Nutzman N.W. Foraging in a lethal environment: fish predation in hypoxic waters of a stratified lake. Ecology. 1994;75:1246–1253. doi:10.2307/1937450 [Google Scholar]
- Randall D.J. Functional morphology of the heart in fishes. Am. Zool. 1968;8:179–189. doi: 10.1093/icb/8.2.179. [DOI] [PubMed] [Google Scholar]
- Randall D. The control of respiration and circulation in fish during exercise and hypoxia. J. Exp. Biol. 1982;100:275–288. [Google Scholar]
- Reebs S.G, Whoriskey F.G, Jr, FitzGerald G.L. Diel patterns of fanning activity, egg respiration, and the nocturnal behavior of male three-spined sticklebacks, Gasterosteus aculeatus L. (f. trachurus) Can. J. Zool. 1984;62:329–334. [Google Scholar]
- Robb T, Abrahams M.V. The influence of hypoxia on risk of predation and habitat choice by the fathead minnow, Pimephales promelas. Behav. Ecol. Sociobiol. 2002;52:25–30. doi:10.1007/s00265-002-0474-2 [Google Scholar]
- Robb T, Abrahams M.V. Variation in tolerance to hypoxia in a predator and prey species: an ecological advantage of being small. J. Fish Biol. 2003;62:1067–1081. doi:10.1046/j.1095-8649.2003.00097.x [Google Scholar]
- Rydberg L, Edler L, Floderus S, Granéli W. Interaction between supply of nutrients, primary production, sediment and oxygen consumption in SE Kattegat. Ambio. 1990;19:134–141. [Google Scholar]
- Sandberg E, Bonsdorff E. Effects of predation and oxygen deficiency on different age classes of the amphipod Monoporeia affinis. J. Sea Res. 1996;35:345–351. doi:10.1016/S1385-1101(96)90761-3 [Google Scholar]
- Satchell G.H. Cambridge University Press; Cambridge, UK; New York, NY; Melbourne, Australia: 1971. Circulation in fishes. [Google Scholar]
- Scalabrin C, Masse J. Acoustic detection of the spatial and temporal distribution of fish shoals in the Bay of Biscay. Aquat. Living Resour. 1993:269–283. doi:10.1051/alr:1993027 [Google Scholar]
- Schurmann H, Steffensen J.F. Spontaneous swimming activity of Atlantic cod Gadus morhua exposed to graded hypoxia at three temperatures. J. Exp. Biol. 1994;197:129–142. doi: 10.1242/jeb.197.1.129. [DOI] [PubMed] [Google Scholar]
- Seghers B.H. Geographic variation in the responses of guppies (Poecilia reticulata) to aerial predators. Oecologia. 1974;14:93–98. doi: 10.1007/BF00344900. doi:10.1007/BF00344900 [DOI] [PubMed] [Google Scholar]
- Shellart N.A.M, Wubbels R.J. The auditory and mechanosensory lateral line system. In: Evans D.H, editor. The physiology of fishes. CRC Marine Sciences Series; Boca Raton, FL: 1998. pp. 288–312. [Google Scholar]
- Shimps E.L, Rice J.A, Osborne J.A. Hypoxia tolerance in two juvenile estuary-dependent fishes. J. Exp. Mar. Biol. 2005;325:146–162. doi:10.1016/j.jembe.2005.04.026 [Google Scholar]
- Shingles A, McKenzie D.J, Claireaux G, Domenici P. Reflex cardioventilatory responses to hypoxia in the flathead grey mullet (Mugil cephalus) and their behavioural modulation by perceived threat of predation and water turbidity. Physiol. Biochem. Zool. 2005;78:744–755. doi: 10.1086/432143. doi:10.1086/432143 [DOI] [PubMed] [Google Scholar]
- Shingles, A., Standen, E. M., Lefrançois, C. & Domenici, P. In preparation. The effect of hypoxia on the escape response of the flathead grey mullet (Mugil cephalus) to visual stimulus.
- Shoji J, Masuda R, Yamashita Y, Tanaka M. Effect of low dissolved oxygen concentrations on behavior and predation rates on red sea bream Pagrus major larvae by the jellyfish Aurelia aurita and by juvenile Spanish mackerel Scomberomorus niphonius. Mar. Biol. 2005;147:863–868. doi:10.1007/s00227-005-1579-8 [Google Scholar]
- Shoubridge E.A, Hochachka P.W. Ethanol—novel end product of vertebrate anaerobic metabolism. Science. 1980;209:308–309. doi: 10.1126/science.7384807. doi:10.1126/science.7384807 [DOI] [PubMed] [Google Scholar]
- Sitko S, Honrubia V. Differential effects of ischemia on spontaneous and sinusoidal-evoked activity in the semicircular canal afferents in the bullfrog. Acta Otolaryngol. 1986;102:179–185. doi: 10.3109/00016488609108664. [DOI] [PubMed] [Google Scholar]
- Smatresk N.J. Chemoreceptor modulation of the endogenous respiratory rhythm in vertebrates. Am. J. Physiol. 1990;259:887–897. doi: 10.1152/ajpregu.1990.259.5.R887. [DOI] [PubMed] [Google Scholar]
- Smith K.J, Able K.W. Dissolved oxygen dynamics in salt marsh pools and its potential impacts on fish assemblages. Mar. Ecol. Prog. Ser. 2003;258:223–232. [Google Scholar]
- Smith R.J.F. Avoiding and deterring predators. In: Godin J, editor. Behavioural ecology of teleost fishes. Oxford University Press; Oxford, UK: 1997. pp. 163–190. [Google Scholar]
- Smith R.S, Kramer D.L. The effect of predation risk on the respiratory behaviour of the Florida gar (Lepisosteus platyrhincus) Can. J. Zool. 1986;64:2133–2136. [Google Scholar]
- Sollid J, De Angelis P, Gundersen K, Nilsson G.E. Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J. Exp. Biol. 2003;206:3667–3673. doi: 10.1242/jeb.00594. doi:10.1242/jeb.00594 [DOI] [PubMed] [Google Scholar]
- Steffensen J.F. Possible limitations of speed and size of swimming fish schools, based on oxygen consumption of herring, Clupea harengus, measured at different swimming speeds. J. Physiol. 1995;483:192. [Google Scholar]
- Steffensen J.F, Farrell A.P. Swimming performance, venous oxygen tension and cardiac performance of coronary-ligated rainbow trout, Oncorhynchus mykiss, exposed to progressive hypoxia. Comp. Biochem. Physiol. A. 1998;119:585–592. doi: 10.1016/s1095-6433(97)00470-4. [DOI] [PubMed] [Google Scholar]
- Stierhoff K.L, Targett T.E, Grecay P.A. Hypoxia tolerance of the mummichog: the role of access to the surface. J. Fish Biol. 2003;63:580–592. doi:10.1046/j.1095-8649.2003.00172.x [Google Scholar]
- Suthers I.M, Gee J.H. Role of hypoxia in limiting diel spring and summer distribution of juvenile yellow perch (Perca flavescens) in a prairie marsh. Can. J. Fish. Aquat. Sci. 1986;43:1562–1570. [Google Scholar]
- Thetmeyer H, Waller U, Black K.D, Inselmann S, Rosenthal H. Growth of European sea bass (Dicentrarchus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture. 1999;174:355–367. doi:10.1016/S0044-8486(99)00028-9 [Google Scholar]
- Van Den Thillart G, Dalla Via J, Vitali G, Cortesi P. Influence of long-term hypoxia exposure on the energy metabolism of Solea solea. I. Critical O2 levels for aerobic and anaerobic metabolism. Mar. Ecol. Prog. Ser. 1994;104:109–117. [Google Scholar]
- Wakeling J.M, Johnston I.A. Muscle power output limits fast-start performance in fish. J. Exp. Biol. 1998;201:1505–1526. doi: 10.1242/jeb.201.10.1505. [DOI] [PubMed] [Google Scholar]
- Wakeling J.M, Kemp K.M, Johnston I. The biomechanics of fast-starts during ontogeny in the common carp Cyprinus carpio. J. Exp. Biol. 1999;202:3057–3067. doi: 10.1242/jeb.202.22.3057. [DOI] [PubMed] [Google Scholar]
- Walker J.A, Ghalambor C.K, Griset O.L, McKenney D, Reznick D.N. Do faster starts increase the probability of evading predators? Funct. Ecol. 2005;19:808–815. doi:10.1111/j.1365-2435.2005.01033.x [Google Scholar]
- Wardle C.S. Limits of fish swimming speed. Nature. 1975;255:725–727. doi: 10.1038/255725a0. doi:10.1038/255725a0 [DOI] [PubMed] [Google Scholar]
- Webb P.W. The effect of size on the fast-start performance of rainbow trout Salmo gairdneri and a consideration of piscivorous predator–prey interaction. J. Exp. Biol. 1976;65:157–177. doi: 10.1242/jeb.65.1.157. [DOI] [PubMed] [Google Scholar]
- Webb P.W. Temperature effects on acceleration of rainbow trout Salmo gairdneri. J. Fish. Res. Bd Can. 1978;35:1417–1422. [Google Scholar]
- Webb P.W. Effect of body form and response threshold on the vulnerability of four species of teleost prey attacked by largemouth bass (Micropterus salmoides) Can. J. Fish. aquat. Sci. 1986;3:763–771. [Google Scholar]
- Webb P.W. Swimming. In: Evans D.H, editor. The physiology of fishes. CRC Marine Sciences Series; Boca Raton, FL: 1998. pp. 3–24. [Google Scholar]
- Webb P.W, Zhang H. The relationship between responsiveness and elusiveness of heat-shocked goldfish (Carassius auratus) to attacks by rainbow trout (Oncorhynchus mykiss) Can. J. Zool. 1994;72:423–426. [Google Scholar]
- Weber J.M, Kramer D.L. Effects of hypoxia and surface access on growth, mortality and behavior of juvenile guppies, Poecilia reticulata. Can. J. Fish. Aquat. Sci. 1983;40:1583–1588. [Google Scholar]
- Weetman D, Atkinson D, Chubb J.C. Water temperature influences the shoaling decisions of guppies Poecilia reticulata, under predation threat. Anim. Behav. 1999;58:735–741. doi: 10.1006/anbe.1999.1191. doi:10.1006/anbe.1999.1191 [DOI] [PubMed] [Google Scholar]
- Weihs D. The mechanism of rapid starting of slender fish. Biorheology. 1973a;10:343–350. doi: 10.3233/bir-1973-10308. [DOI] [PubMed] [Google Scholar]
- Weihs D. Hydromechanics and fish schooling. Nature. 1973b;241:290–291. doi:10.1038/241290a0 [Google Scholar]
- Wilson B, Batty R.S, Dill L.M. Pacific and Atlantic herring produce burst pulse sounds. Proc. R. Soc. B. 2004;271:S95–S97. doi: 10.1098/rsbl.2003.0107. doi:10.1098/rsbl.2003.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N.G, Kramer D.L. Use of cover and the need to breathe: the effects of hypoxia on vulnerability of dwarf gouramis to predatory snakeheads. Oecologia. 1987;73:127–132. doi: 10.1007/BF00376988. doi:10.1007/BF00376988 [DOI] [PubMed] [Google Scholar]
- Zhou B.S, Wu R.S.S, Randall D.J, Lam P.K.S, Ip Y.K, Chew S.F. Metabolic adjustments in the common carp during prolonged hypoxia. J. Fish Biol. 2000;57:1160–1171. doi:10.1111/j.1095-8649.2000.tb00478.x [Google Scholar]