Abstract
The mating system of eastern mosquito fish (Gambusia holbrooki) is dominated by male sexual coercion, where all matings are forced and females never appear to cooperate and actively avoid all attempts. Previous research has shown that male G. holbrooki offer a model system for examining the benefits of reversible thermal acclimation for reproductive success, but examining the benefits to female avoidance behaviour has been difficult. In this study, we examined the ability of non-male-deprived female G. holbrooki to avoid forced–coercive matings following acclimation to either 18 or 30°C for six weeks (12 h light : 12 h dark photoperiod). Thermal acclimation of burst and sustained swimming performance was also assessed, as these traits are likely to underlie their ability to avoid forced matings. There was no influence of thermal acclimation on the burst swimming performance of female G. holbrooki over the range 18–30°C; however, sustained swimming performance was significantly lower in the warm- than the cool-acclimation group. For mating behaviour, we tested the hypothesis that acclimation would enhance the ability of female G. holbrooki to avoid forced matings at their host acclimation temperature relative to females acclimated to another environment. However, our hypothesis was not supported. The rate of copulations was almost three times greater for females acclimated to 30°C than 18°C when tested at 30°C, indicating that they possess the ability to alter their avoidance behaviour to ‘allow’ more copulations in some environments. Coupled with previous studies, female G. holbrooki appear to have greater control on the outcome of coercive mating attempts than previously considered and can alter their propensity to receive forced matings following thermal acclimation. The significance of this change in female mating-avoidance behaviours with thermal acclimation remains to be explored.
Keywords: temperature, temperature acclimation, beneficial acclimation hypothesis, reproductive success, Gambusia holbrooki
1. Introduction
Biological processes are temperature-sensitive and the performance of all physiological traits is affected by changes in body temperature (Prosser 1979; Angilletta et al. 2003). In aquatic ectotherms, acute changes in body temperature can affect numerous factors that are likely to be critical for both survival and reproduction (Angilletta et al. 2003). Studies investigating the ecological consequences of temperature variation often use measures of whole-animal performance because it links lower-level physiological, morphological and biochemical traits with the behavioural performance and fitness of the organism (Arnold 1983; Bennett & Huey 1990; Garland & Losos 1994b). Despite the wide range of studies describing both the functional consequences of environmental temperature variation on whole-animal traits and their underlying mechanisms, few studies have examined the reproductive- and fitness-consequences of temperature-induced performance variation (Garland & Carter 1994a; Irschick & Garland 2001; Wilson 2005).
Seasonal temperature cycles may result in predictable and reversible modifications in physiological function (reversible acclimation), while variation in the thermal environment during ontogeny can lead to irreversible changes in the adult phenotype (irreversible acclimation; Piersma & Drent 2003; Angilletta et al. 2006). The ability to respond to long-term changes in environmental temperature by modifying underlying physiological state is a process known as thermal acclimation. The range of acclimation responses to seasonal changes in environmental temperature have been well studied; particularly, the functional responses of the locomotor system of fish (see Johnston & Temple (2002), for a review) and amphibians (Wilson et al. 2000). Many species of fish can modify their fast-start and sustained swimming performance following several weeks of exposure to a new thermal environment (Fry & Hart 1948; Beddow et al. 1995; Johnson & Bennett 1995). Temperature acclimation modifies the thermal optimum for various locomotory behaviours, allowing function at high performance levels across the range of environmental temperatures experienced during the year (see Johnston & Temple (2002) for review). The proximal mechanisms of thermal acclimation of locomotory performance includes changes in the proportion of muscle fibre types (Johnston & Lucking 1978; Hammill et al. 2004), the relative amounts of cellular organelles (Johnston & Maitland 1980) and altered expression of hundreds of genes (Gracey et al. 2004; Podrabsky & Somero 2004), including isoforms of class II myosins (Watabe 2002). Extensive changes in gene expression with thermal acclimation ultimately result in enhanced metabolic power and contractile function at the temperature of the new environment (Johnston et al. 1975, 1990; Wakeling et al. 2000; Rome & Swank 2001). Previous studies examining the ecological significance of seasonal acclimation responses have primarily used measures of locomotor performance owing to its presumed importance in predator–prey interactions. However, the link between locomotion and fitness is somewhat tenuous and is often assumed rather than tested (Angilletta et al. 2003), particularly for studies of reversible acclimation responses. Recent discussions have suggested that more direct correlates of fitness are required to investigate the adaptive benefits of acclimation responses, especially using traits closely associated with reproductive behaviour (Wilson & Franklin 2002; Johnston & Wilson 2005).
The mating behaviour of the eastern mosquito fish (Gambusia holbrooki) is ideal for examining questions related to the reproductive consequences of variation in body temperature (Wilson 2005). Mosquito fish possess one of the broadest reproductively active temperature ranges for any ectotherm and obtain copulations across a range of at least 14–38°C (Wilson 2005). The mating system of the eastern mosquito fish is dominated by male sexual coercion (Bisazza et al. 1989; McPeek 1992). Copulations only occur when males approach females with stealth and rapidly thrust their gonopodium (modified anal fin) into the female genital pore to release their spermatozoa (Farr 1989; McPeek 1992; Bisazza et al. 1996; Pilastro et al. 1997). Females are assumed to never cooperate but attempt to avoid mating by strategies including fleeing, lying against an object and adopting a position to thwart an attempt or attacking (Bisazza et al. 1996; Pilastro et al. 1997, 2003). Males, best able to compete against female avoidance, achieve the greatest number of copulations and increase their chances of producing more offspring (Cunningham & Birkhead 1998). In one of the first studies to examine the effects of temperature on reproductive behaviour, Wilson (2005) reported that temperature markedly affected both the propensity of male G. holbrooki to follow females and their ability to obtain coercive matings. Eastern mosquito fish also experience wide seasonal variation in temperature across their native and introduced distribution (Wilson & Johnston 2004). Hammill et al. (2004) reported that male G. holbrooki possessed the capacity to acclimate their maximum sustainable swimming performance over the range 18–30°C. In a recent study, Wilson et al. (2007) also reported that thermal acclimation influenced both the competitive ability of male G. holbrooki during aggressive interactions and their ability to obtain coercive matings across different thermal environments.
Although most studies of thermal acclimation have focused on its expression and underlying physiological mechanisms, research is increasingly becoming directed towards understanding the ecological consequences of these plastic modifications for interactions between both species and individuals within a species (e.g. sexual conflict; Miner et al. 2005). The thermal acclimation of male coercive mating ability suggests that female G. holbrooki may also respond to long-term changes in environmental temperature by acclimating their ability to avoid unwanted copulations (an acclimation ‘arms race’ between sexes). Clearly, females must receive at least some copulations for successful reproduction, but increased avoidance ability with acclimation would serve to reduce: (i) the number of copulations from males less adept at sneaky copulations (genetic benefits), (ii) physical damage to the female genital tract from the barbed male gonopodium (physical benefits), and (iii) the time/energetic costs associated with avoiding mating attempts (energetic benefits). In contrast with these expectations, Condon & Wilson (2006) found virgin female G. holbrooki acclimated to high temperatures actually received a greater number of copulations at their host acclimation temperature. Given that these females were virgins and required at least some initial matings to reproduce, the increased number of coercive matings for the warm-acclimated females may be due to their exposure to environmental conditions optimal for reproduction and their immediate requirement for sperm to reproduce (Condon & Wilson 2006). Although females were never observed to cooperate with coercive mating attempts, tacit compliance or a reduced keenness to escape violates the assumption that only the fittest males who are best able to ‘beat’ female avoidance strategies will achieve the most copulations. Thus, studies examining the benefits of thermal acclimation to the mating behaviour of female G. holbrooki may profit from examining the behaviour of non-male-deprived (experienced) females.
In this study, we examined the influence of thermal acclimation on the reproductive behaviour and swimming performance of experienced female G. holbrooki. As seasonal acclimation significantly influences mating behaviour, swimming performance and proportion of muscle fibre types of male G. holbrooki (Hammill et al. 2004; Wilson 2005), it is likely that whole-animal performance traits that underlie the ability of females to avoid unwanted coercive copulations may also be influenced by thermal variation. We hypothesized that acclimation would enhance the ability of sexually experienced female G. holbrooki to more effectively avoid coercive matings in a new thermal environment. As maximum swimming capacity probably underlies the ability of females to avoid unwanted coercive copulations, we also investigated the thermal acclimation of fast-start behaviour. Sustained swimming performance was also studied to provide a measure of aerobic ability. Finally, we also investigated the mating behaviour of female G. holbrooki over an extended period while being exposed to an environment where there was a high cost for receiving coercive matings (sex ratio of five males to one female). We predicted that over an extended period the cool-acclimated females would find it increasingly difficult to avoid unwanted copulations at a high test temperature, reflecting a reduction in aerobic metabolic capacity and sustained swimming performance.
2. Material and methods
Adult eastern mosquito fish (G. holbrooki) were collected using long-handled dip nets and box-traps in March 2005 from 18 Mile Swamp on North Stradbroke Island, Australia. Bait traps were checked every 20 min and fish were placed in aerated 100 l containers in which they were transported to the University of Queensland (4 h) at densities no greater than 0.5 fish per litre. In the laboratory, one male and one female (40 pairs) were introduced to 12 l plastic aquaria (31×22×15 cm deep) provided with gravel, a box filter and a small amount of Java moss weed (Vesicularia dubyana). One male was acclimated with each female to preclude any influence of male-deprivation on female mating behaviour (Pilastro et al. 2003). Aquaria were randomly assigned a thermal acclimation treatment (range: 18±0.5°C (N=18) or 30±0.5°C (N=18)) for six weeks. Temperature was maintained within a constant room temperature at 18±0.5°C and 30°C-treatment tanks were supplied with 55 W aquarium heaters (AquaOne, N2867) set at 30±0.5°C. Acclimation temperatures were chosen to approximate the maximum daily summer and winter conditions of North Stradbroke Island waterways and are within the natural range of G. holbrooki (Arthington & Lloyd 1989; Wilson 2005). Fish did not display any adverse reactions to acclimation or acute changes in temperature throughout this study and were fed daily with commercially supplied flakes (Wardleys, Sydney, Australia) or newly hatched brine shrimp.
Eighty adult male mosquito fish were maintained in 40 l aquaria at densities of 0.5 per litre at 26.0±0.5°C for one month prior to experiments. Since male mating success is size dependent (Bisazza & Marin 1995; Pilastro et al. 1997), males were divided into four size classes (20±0.5; 21±0.5; 22±0.5; 24±0.5 mm; (N=20 per group)). Total length of the fish was measured from still images captured by a Nikon digital camera and morphometric data obtained using the SigmaScan 5.0. At the termination of the experiment, mosquito fish were placed in mixed-sexed groups in the laboratory. This research was approved by the University of Queensland Animal Welfare and Ethics Committee (ZOO/ENT/485/04/ARC), and collections of fish were approved by the Queensland Department of Primary Industries (DPI) (PRM04626D).
After six weeks of acclimation, the mating behaviour and swimming performance of each female mosquito fish was examined at 18 and 30°C. Mating behaviour was initially examined and was followed by burst and then sustained swimming performance. Females were assessed at both their acclimation temperature and the alternate environment and the order of testing was randomized (18°C acclimated/18°C initial test temperature, N=13; 18°C/30°C, N=13; 30°C/18°C, N=18; 30°C/30°C, N=18). All mating trials at a single test temperature for each individual female were completed before changing to the next temperature. Acute changes in water temperature occurred within each individual's 12 l aquarium and the final temperature was reached at least 15 h prior to trials. Water temperature was gradually altered at a rate not exceeding 4°C h−1 (Wilson 2005). The initial test temperature remained the same for each individual in both the mating behaviour and swimming experiments. There was no significant difference between total female body length between acclimation treatments (two-way ANOVA; F=2.046, p=0.16; range: 28.3–35.7 mm) or initial test temperature groups (two-way ANOVA; F=0.419, p=0.523).
(a) Mating behaviour
Females were introduced into the observation tank 30 min prior to the start of an experiment to minimize stress. Each female was then exposed to a single male introduced into the observation aquarium and the behaviour of both the male and female was observed over a 10 min period. Data recording commenced when the male displayed sexual interest in the female or when 2 min had elapsed. Observation trials were repeated four times (with different males), 30 min apart, using one male from each of the four size groups at both test temperatures of 18 and 30°C. All males were rested for at least 24 h between trials. Observation tanks were of dimension 45×45×20 cm, containing aged tap water, gravel bed, aerator, a small quantity of java moss weed and a submersed aquarium heater (AquaOne 250 W). Black plastic was taped to the sides of each tank to reduce external stimuli. Test temperature of the aquaria was maintained by air temperature in a controlled temperature room at 18±0.5°C or using aquarium heaters set to 30±0.5°C.
During the 10 min observation period, female and male behaviours were recorded and entered into the behavioural software program Ethom v. 1.0 using a laptop computer (His-Te Shih, http://www.mbi.nsysu.edu.tw/~fiddler/ethom/intro_e.htm). The total time males spent following females, the number of coercive mating attempts made and the number of copulations were recorded for each individual female. Mating attempts were defined as when a male swum alongside a female and made a gonopodial swing greater than 90° from the lateral axis of the body towards the female. Total number of mating attempts included both successful copulations and unsuccessful mating attempts. Copulations were deemed to have occurred when a gonopodial swing was followed by the characteristic twisting motion made by the male following successful insertion to remove the barbed gonopodium from the female genital opening (Wilson 2005).
(b) Swimming performance
Burst swimming performance was assessed by filming at least five startle responses for each individual with a high speed digital camera at 18 and 30°C (N=13 for all acclimation groups at both 18 and 30°C except for N=10 for 18°C-acclimated tested initially at 18°C). Swimming responses were elicited by placing the fish into a swimming arena (0.3× 0.2×0.05 m deep glass aquarium) and tapping the side of the tank. Only burst swimming responses that consisted of a C-start reaction involving initial contraction of all muscle fibres on one side of the fish followed by one or more propulsive tail strokes were analysed (Eaton et al. 1977; Temple & Johnston 1998). C-start responses are used most often during escape from predators (Beddow et al. 1995) and only those from a stationary position with limited vertical movement were analysed. Swimming responses were filmed ventrally from 2 m away, capturing the image from a mirror at 45° beneath the glass bottomed arena and recorded using a high-speed 200 Hz digital camera (Redlake Imaging Cooperation, USA). The accompanying Redlake software package was used for analysis of the swimming sequences. Sequences were replayed frame-by-frame and the centre region of the head was digitized as it was the easiest and most consistent point of reference. Temperature was maintained by the flow of heated or cooled water from a controlled temperature water bath (Heto, CB 8-30E) through a submersed metal rod.
The first 40 ms of swimming sequences were analysed to determine the distance moved by each female between successive frames. Instantaneous measures of velocity were then calculated by differentiating distance data that was previously subjected to a three-point moving average filter (Walker 1998; Wilson & Franklin 1999). Measures of maximum burst swimming velocity were calculated from data obtained after the initial turning stage of a startle response (Ustart) and the first propulsive tail beat (Umax). The fastest of the swimming sequences analysed for each individual was used as a measure of their maximum burst swimming performance. All females were rested after initial mating behaviour observations for at least one week before swimming performance tests, and at least 48 h was given between burst recordings at each test temperature.
(i) Sustained swimming performance
The sustained swimming capacity of each individual female G. holbrooki was determined at both 18 and 30°C. Fish were assessed only once at each experimental temperature (N=13 for all acclimation groups at both 18 and 30°C except for N=10 for 18°C-acclimated tested initially at 18°C). Females were transferred to the test temperature at least 20 h before experiments begun and the order of test temperature for each individual remained the same as behavioural experiments. Sustained swimming capacity was measured in a swimming flume (Brett 1964) that consisted of a swimming chamber 15 cm long and 5.5 cm diameter. Fish were introduced into the flume at a water velocity of 16 cm s−1 and the current was increased by 3 cm s−1 every 3 min until the fish was fatigued, which was defined as when it was swept back by the current against the grid. Total time and the water velocity at exhaustion were recorded and used to calculate Ucrit using the equation (Brett 1964): Ucrit=Uf+((Tf/Ti)Ui), where Uf is the highest speed maintained for a 3 min interval; Tf is the time taken to exhaustion in the final speed interval; Ti is the time-interval length (3 min); and Ui is the speed increment.
(ii) Sustained mating behaviour
The sustained mating behaviour of female mosquito fish was assessed one month following the initial mating trials, with females kept at their host acclimation temperatures during this period. Experimental aquaria, data collection and the order of testing in thermal environments remained unchanged for each individual. One female was introduced to the observation aquaria 30 min before the experiment began and five males kept at 26±0.5°C were placed into the test arena. Once introduced to the tank the 10 min observation trial begun when one of the five males began following the female or after 2 min had elapsed. At the conclusion of the 10 min trial, the five males were left in the observation tank with the female. After 50 min had elapsed, mating behaviour data was collected again over a 10 min period. Fish were fed a small amount of newly hatched brine shrimp 30 min after the commencement of the initial observation.
(iii) Statistical analyses
All data were analysed using the statistical programming package R or SigmaStat v. 3.0. For behavioural data, the effects of acute change in temperature and acclimation group were analysed using a generalized linear model assuming a Poisson distribution (due to the inclusion of ‘count’ data). The behavioural data were not normally distributed but satisfied the assumptions of a Poisson distribution. Swimming performance data were normally distributed and the influence of temperature and acclimation treatment was tested using two-way ANOVAs. Post hoc Holm–Sidak multiple comparison testing was used to test for significant effects between test temperatures. Total body lengths of males and females between acclimation groups and initial test temperature were tested for significance using a two-way ANOVA. All results are presented as means ±s.e. Significance was taken at the level of p<0.05.
3. Results
(a) Mating behaviour
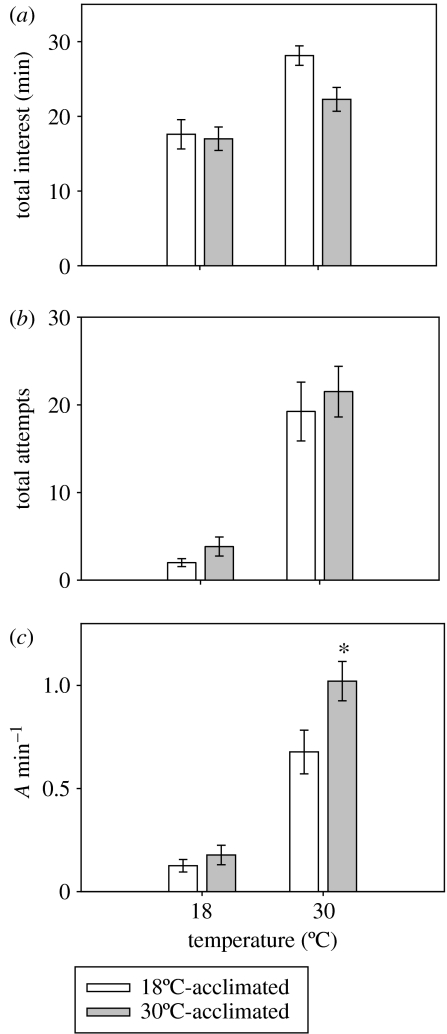
Both the total number of male sneaky-mating attempts and the total time males spent following females was significantly influenced by test temperature (F1,59=81.4; p<0.001; F1,59=19.7; p<0.001; figure 1a,b). However, no significant differences were detected in total following time or the total number of mating attempts between males tested with females acclimated to either 18 or 30°C (F1,59=0.98; p>0.05; F1,59=3.5; p>0.05; figure 1a,b). The total number of male sneaky-mating attempts per minute of time spent following females (A min−1) was also significantly influenced by test temperature (F1,59=47.6; p<0.001; figure 1c). However, 30°C-acclimated females were exposed to a significantly greater number of mating attempts per minute of male interest than the 18°C-acclimated females (F1,59=4.8; p>0.05; figure 1c). Total number of copulation attempts made by males towards 30°C-acclimated females was 1.02±0.10 A min−1 at 30°C, which was 50% greater than that experienced by the 18°C-acclimated females (0.68±0.15 A min−1). Thus, acclimation of female G. holbrooki to different thermal regimes seemed to alter the coercive mating interest of the males at high temperatures.
Figure 1.
Effect of temperature on the mating behaviour of male G. holbrooki when exposed to females acclimated to either 18 or 30°C for six weeks. (a) The total number of minutes males followed females during 40 min observation period, (b) the total number of coercive mating attempts, and (c) the total number of mating attempts made per minute of following females (A min−1). Data represent means ±s.e. Asterisk denotes a statistically significant difference between acclimation groups at the level of p<0.05.
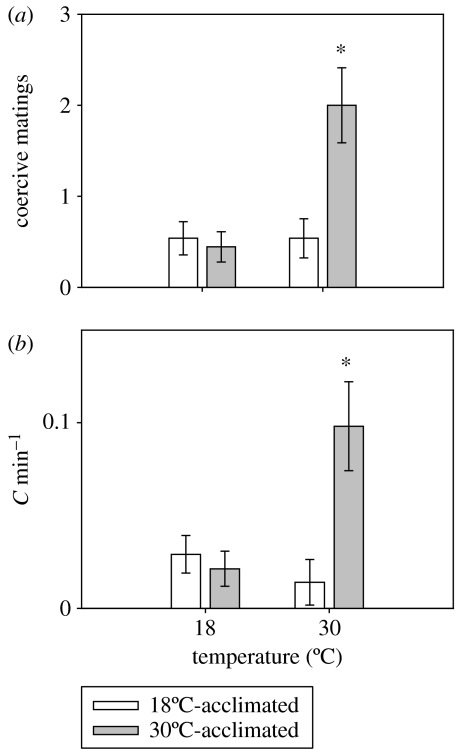
Test temperature significantly influenced the total number of copulations that female G. holbrooki received over the total combined observation period (F1,59=12.0; p<0.01; figure 2a), but this was due to the substantial increase in the number of copulations received by the 30°C-acclimated females. In addition, there was a significant interaction between test temperature and acclimation treatment for the total number of copulations received (F1,59=12.0; p<0.01), indicating acclimation influenced effect of temperature on copulation number. When tested at 30°C, warm-acclimated females received 2.0±0.1 copulations during the total observation period, which was significantly greater than that experienced by the cool-acclimated females (0.4±0.1; figure 2a). Similarly, the test temperature and the acclimation treatment both significantly influenced the total number of copulations received by the females per minute of male interest (A min−1) over the entire observation period (F1,60=6.4; p<0.05; F1,59=7.4; p<0.05; figure 2b). Warm-acclimated females received a total of 0.1±0.02 A min−1, which was approximately 10 times greater than that received by the cool-acclimated females (0.01±0.008 C min−1; figure 2b). For all females from both treatment groups, approximately 25% of all copulation attempts resulted in a coercive mating at the lowest test temperature, which was significantly higher than the avoidance ability observed at 30°C (less than 10% of attempted matings; F1,56=8.1; p<0.01). However, there was no influence of acclimation group on the avoidance ability (proportion of attempts resulting in copulations) of the female G. holbrooki (F1,56=0.001; p>0.05).
Figure 2.
Effect of temperature on the number of coercive matings received by female G. holbrooki acclimated to either 18 or 30°C for six weeks. (a) The total number of coercive matings, and (b) the number of matings made per minute of time males followed females (C min−1). Data represent means ±s.e. Asterisk denotes a statistically significant difference between acclimation groups at the level of p<0.05.
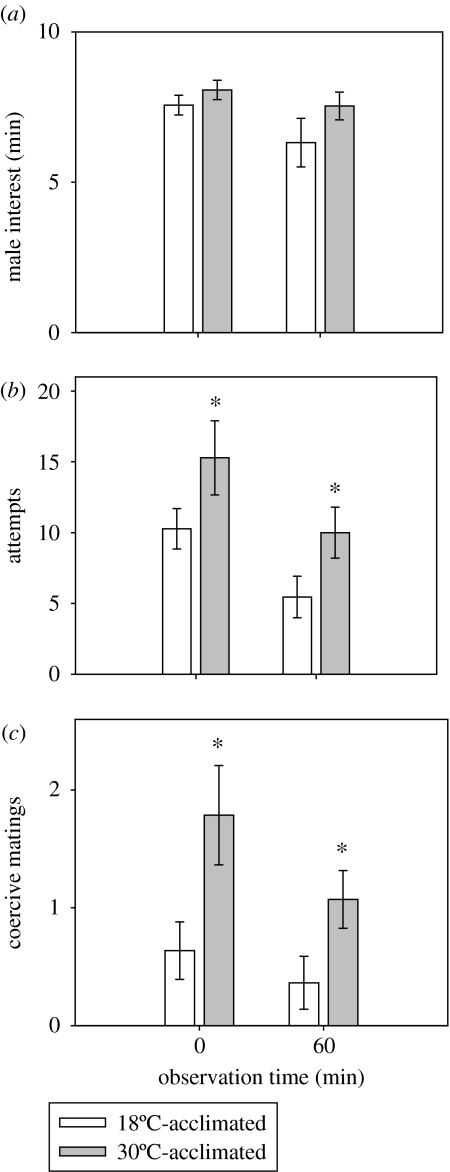
We also examined the mating behaviour of female G. holbrooki over an extended period in an environment where there was a high cost for receiving coercive matings. When tested at 30°C, we found no significant differences in total following time between males tested with females acclimated to either 18 or 30°C (F1,48=0.85; p>0.05; figure 3a). In contrast, there was a significant influence of female-acclimation group on the total number of coercive mating attempts made by male G. holbrooki (F1,48=6.9; p<0.05; figure 3b), with the warm-acclimated females experiencing approximately 50% more mating attempts during both observation periods. The total number of coercive mating attempts was lower 1 h after the males were initially introduced and decreased for both female-acclimation groups (F1,47=7.2; p<0.01; figure 3b). Similarly, warm-acclimated females received a greater number of coercive matings than the cool-acclimated females during both observation periods (F1,48=8.4; p<0.01; figure 3c). For example, the 30°C-acclimated females received 2.0±0.5 copulations during the initial 10 min observation period, which was more than double that experienced by the 18°C-acclimated females (0.7±0.2; figure 3c).
Figure 3.
Mating behaviour of female G. holbrooki when exposed to five males over a 60 min observation period with data collected for the first and last 10 min. (a) The total number of minutes males followed females during the observation period, (b) the total number of sneaky-mating attempts, and (c) the number of coercive matings made per minute of male following time (C min−1). Data represent means ±s.e. Asterisk denotes a statistically significant difference between acclimation groups at the level of p<0.05.
(b) Swimming performance
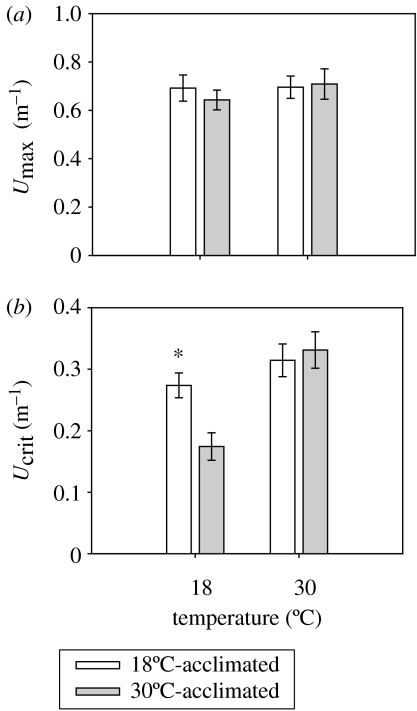
The effects of temperature on the burst and sustained swimming performance of female G. holbrooki were compared between individuals exposed to 18 and 30°C for six weeks. The maximum velocity attained by female G. holbrooki during the first full tail-beat following the initial C-start response (Umax) was not influenced by either acclimation treatment (F1,47=0.13; p>0.05; two-way ANOVA) or test temperature over the range studied (F1,46=0.51; p>0.05; two-way ANOVA; figure 4a). In contrast, the maximum sustainable swimming speed (Ucrit) of female G. holbrooki was significantly influenced by test temperature; indicating greater endurance at 18°C following acclimation to 18°C (F1,38=11.31; p<0.01; two-way ANOVA). In addition, there was a significant interaction between test temperature and acclimation treatment (F1,37=6.16; p<0.05; two-way ANOVA; figure 4b), indicating long-term exposure of the fish to different thermal regimes influenced the effects of temperature on performance. Although Ucrit was approximately 60% greater for the cool-acclimated females than the warm-acclimated fish when tested at 18°C, there was no difference between the acclimation groups when tested at 30°C.
Figure 4.
Effect of temperature on the swimming performance of female G. holbrooki exposed to either 18 or 30°C for six weeks. (a) The maximum burst swimming performance (Umax), and (b) the sustained swimming performance (Ucrit). Data represent means ±s.e. Asterisk denotes a statistically significant difference between acclimation groups at the level of p<0.05.
4. Discussion
Male mosquito fish do not display courting behaviours to females but use only a coercive mating strategy to achieve copulations. However, most coercive mating attempts are unsuccessful as females do not appear receptive to male sexual activity and efficiently avoid most attempts (Bisazza et al. 1989; McPeek 1992; Pilastro et al. 1997, 2003; Bisazza & Pilastro 2000). Although we found females were more efficient at avoiding coercive mating attempts at the highest test temperature, when averaged across both test temperatures females successfully avoided more than 80% of all coercive mating attempts. Based on these aspects of the mating system of G. holbrooki, we predicted that acclimation of female G. holbrooki to different thermal environments would increase their ability to avoid forced mating attempts at their host acclimation temperature. In contrast, we found that neither warm nor cool acclimation resulted in an increase in the ability of female G. holbrooki to avoid sneaky matings at their respective acclimation temperature. There was no difference in mating avoidance between acclimation groups when tested at 18°C. However, when females were tested at 30°C, warm-acclimated females were found to receive more ‘forced’ copulations and thus actually be poorer at avoiding male sneaky-matings than cool-acclimated females. The rate of copulations received by warm-acclimated females was three times greater than that of cool-acclimated females when both groups were tested at 30°C. This result was in direct contrast with our initial hypothesis that acclimation would produce an increase in sneaky-mating avoidance ability and thus a reduction in the number of copulations a female received.
An increase in the rate of coercive matings for warm-acclimated females is surprising given the wide-spread assumption that female mosquito fish always seek to avoid matings (Bisazza et al. 1989; McPeek 1992; Pilastro et al. 1997, 2003; Bisazza & Pilastro 2000) and there are probably genetic, physical and energetic benefits for greater control of the number of forced copulations received. Furthermore, we also found that warm-acclimated females received a greater number of coercive matings over an extended period while being exposed to an environment where there were probably high physical and energetic costs for receiving coercive matings (sex ratio of five males to one female). In contrast, we predicted that over an extended period the cool-acclimated females would find it increasingly difficult to avoid unwanted copulations at high test temperatures. Although Condon & Wilson (2006) also found warm acclimation increased the number of coercive copulations received by virgin female G. holbrooki at high temperatures, this was interpreted as a response by the virgin females to obtain initial sperm stores for reproduction in an optimal environment. Thus, it seems warm acclimation induces a change in the mating behaviour of both male-deprived and non-male-deprived female G. holbrooki that essentially modifies their propensity to avoid unwanted ‘forced’ copulations. In contrast, in a recent study of the benefits of thermal acclimation to the mating behaviour of male G. holbrooki, Wilson et al. (2007) found both sneaky-mating attempts and successful copulations were higher for males in both their cool- and warm-acclimated environment relative to males acutely transferred from the alternate acclimation temperature. This suggests that thermal acclimation provides a benefit for male mating performance across a range of thermal regimes; possibly linked to adaptive changes in the muscoskeletal system (Wilson 2005). A detailed kinematic analysis of forced copulations for both cool- and warm-acclimated fish may reveal subtle modifications in either male or female behaviour that are responsible for changes in copulation rate.
Although the ability of female G. holbrooki to avoid unwanted copulations is extremely efficient (approx. 80%), studies of females that are male-deprived for extended periods suggest that female avoidance behaviour is modified for a short period immediately after the reintroduction of males, increasing the probability of a successful copulation (Pilastro et al. 1997; Bisazza et al. 2001). As the length of male deprivation increased, the likelihood that females were inseminated in the 48 h following male reintroduction also increased, even though all mating attempts were coercive and females were never observed to cooperate (Pilastro et al. 1997). When deprived of males, female G. holbrooki possess reduced sperm stores and exhibit an increased tendency to associate with males (Bisazza et al. 2001; Pilastro et al. 2003). Additionally, the behaviour of non-deprived females that had just given birth was found to be indiscernible from male-deprived females when males were introduced to the observation tank (Bisazza et al. 2001). These studies suggest that female mosquito fish associate more often with males when sperm stores are reduced and when received sperm is likely to be used soon after in the fertilization of new ova (Bisazza et al. 2001). While females never appear to cooperate with mating attempts, results from our study along with previous research on virgin female G. holbrooki suggest females may play a greater role in the success of coercive mating attempts than previously considered. Females may modify their avoidance behaviour to increase the likelihood of a successful copulation. Although an increase in the number of forced matings may increase the immediate physical and energetic costs of reproduction for female mosquito fish, these costs may be outweighed by the potential genetic benefits that could occur through the promotion of greater sperm competition within the female reproductive tract.
While there was no apparent benefit to the ‘avoidance behaviour’ of female G. holbrooki with acclimation, females did possess the capacity to acclimate their sustained swimming performance across the test temperature range. Thermal acclimation of sustained and burst swimming ability has been demonstrated in a variety of fish (Fry & Hart 1948; Rome et al. 1985; Sisson & Sidell 1987; Beddow et al. 1995; Johnson & Bennett 1995; Temple & Johnston 1998) and amphibian taxa (Wilson et al. 2000). For example, Fry & Hart (1948) found that acclimation of goldfish (Carassius auratus) to cool or warm temperatures for several weeks increased their maximum cruising speeds at their acclimation temperatures relative to fish acclimated to other temperatures. Similarly, long-term exposure of male G. holbrooki to 18°C resulted in an Ucrit 20% higher than for 30°C-acclimated fish when tested at 18°C, and vice-versa when tested at 30°C. Similar to these previous studies, we found the Ucrit of cool-acclimated female G. holbrooki was approximately 60% greater at the test temperature of 18°C when compared with the warm-acclimation group, while no differences between the groups were detected at the highest test temperature. However, we found no influence of thermal acclimation on the burst swimming performance of female G. holbrooki when tested between 18 and 30°C; the relevant temperature range for mating behaviour. As burst swimming performance is likely to be a critical determinant of the ability of females to avoid unwanted copulations, the lack of alteration of this trait with acclimation suggests the changes in female mating behaviour with acclimation are the results of behavioural alterations rather than changes in physical capacity.
We found that the behaviour of the male G. holbrooki was largely independent of whether the males were tested with warm- or cool-acclimated females, suggesting acclimation of females to different thermal environments did not influence their attractiveness to males. There was no difference in the total number of mating attempts or total interest time between males tested with cool- or warm-acclimated females. However, males exposed to warm-acclimated females obtained more coercive matings per minute of following the females at 30°C than males exposed to the cool-acclimated females. This increase in mating attempts per minute of sexual interest was not great enough to explain an increase in the number of coercive copulations that the warm-acclimated females received at the high test temperature. Male reproductive behaviour was also influenced by test temperature. Within the time period that males were interested in sexual activity, male G. holbrooki attempted sneaky copulations at a rate of one attempt per minute at 30°C and attempted approximately 0.2 sneaky-matings per minute at 18°C, values similar to those previously reported (Wilson 2005). It has also been estimated that in wild populations male G. holbrooki make approximately one copulation attempt per minute, corresponding to several hundred throughout the day (Bisazza & Marin 1995).
Interest in the ecological consequences of phenotypic plasticity, and the more specific case of thermal acclimation, is rapidly expanding (Miner et al. 2005). The mating behaviour of the eastern mosquito fish offers a unique study system for examining questions directly related to the ecological consequences of phenotypic plasticity. In this study, we found that female G. holbrooki modify their mating behaviour following thermal acclimation so that the propensity to receive forced matings is greater for warm-acclimated females in the warmer environments. This contrasts with the alterations in the mating behaviour of male G. holbrooki with thermal acclimation, which results in improved coercive mating abilities of the males in their host acclimation environment (Wilson et al. 2007). Conflict between the sexes in mating behaviour with thermal acclimation offers a range of future questions addressing the ecological implications of phenotypic plasticity and their consequences for sexual interactions in nature.
Acknowledgments
R.S.W. thanks the Australian Research Council for financial support and Amanda Niehaus for logistical support. R.S.W. was supported by an ARC Postdoctoral Fellow at UQ. We also thank Mike Bennett for the loan of the high speed digital camera system.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Angilletta M.J, Jr, Wilson R.S, Navas C.A, James R.S. Evolution of thermal reaction norms. Trends Ecol. Evol. 2003;18:234–240. doi:10.1016/S0169-5347(03)00087-9 [Google Scholar]
- Angilletta M.J, Jr, Bennett A.F, Guderley H, Navas C.A, Seebacher F, Wilson R.S. Coadaptation: a unifying principle in evolutionary thermal biology. Physiol. Biochem. Zool. 2006;79:282–294. doi: 10.1086/499990. doi:10.1086/499990 [DOI] [PubMed] [Google Scholar]
- Arnold S.J. Morphology, performance and fitness. Am. Zool. 1983;23:347–361. [Google Scholar]
- Arthington A.H, Lloyd L.N. Introduced poeciliids in Australia and New Zealand. In: Meffe G.K, Snelson F.F Jr, editors. Ecology and evolution of live bearing fishes (Poeciliidae) Prentice Hall; New Jersey, NJ: 1989. pp. 333–348. [Google Scholar]
- Beddow T.A, Vanleeuwen J.L, Johnston I.A. Swimming kinematics of fast starts are altered by temperature-acclimation in the marine fish Myoxocephalus scorpius. J. Exp. Biol. 1995;198:203–208. doi: 10.1242/jeb.198.1.203. [DOI] [PubMed] [Google Scholar]
- Bennett A.F, Huey R.B. Studying the evolution of physiological performance. Oxford Surv. Evol. Biol. 1990;7:251–284. [Google Scholar]
- Bisazza A, Marin G. Sexual selection and sexual size dimorphism in the eastern mosquitofish Gambusia holbrooki (Pisces Poeciliidae) Ethol. Ecol. Evol. 1995;7:169–183. [Google Scholar]
- Bisazza A, Pilastro A. Variation of female preference for male coloration in the eastern mosquitofish Gambusia holbrooki. Behav. Genet. 2000;30:207–212. doi: 10.1023/a:1001914208075. doi:10.1023/A:1001914208075 [DOI] [PubMed] [Google Scholar]
- Bisazza A, Marconato A, Marin G. Male mate preferences in the mosquitofish Gambusia holbrooki. Ethology. 1989;83:335–343. [Google Scholar]
- Bisazza A, Pilastro A, Palazzi R, Marin G. Sexual behaviour of immature male eastern mosquitofish: a way to measure intensity of intra-sexual selection? J. Fish Biol. 1996;48:726–737. doi:10.1111/j.1095-8649.1996.tb01468.x [Google Scholar]
- Bisazza A, Vaccari G, Pilastro A. Female mate choice in a mating system dominated by male sexual coercion. Behav. Ecol. 2001;12:59–64. [Google Scholar]
- Brett J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish Res. Board Can. 1964;21:1183–1226. [Google Scholar]
- Condon C.H.L, Wilson R.S. Effect of thermal acclimation on female resistance to forced matings in the eastern mosquitofish. Anim. Behav. 2006;72:585–593. doi:10.1016/j.anbehav.2005.11.016 [Google Scholar]
- Cunningham E.J.A, Birkhead T.R. Sex roles and sexual selection. Anim. Behav. 1998;56:1311–1321. doi: 10.1006/anbe.1998.0953. doi:10.1006/anbe.1998.0953 [DOI] [PubMed] [Google Scholar]
- Eaton R.C, Bombardieri R.A, Meyer D.H. Mauthner initiated startle responses in teleost fish. J. Exp. Biol. 1977;66:65–81. doi: 10.1242/jeb.66.1.65. [DOI] [PubMed] [Google Scholar]
- Farr J.A. Sexual selection and secondary differentiation in poeciliids: determinants of male success and the evolution of female mate choice. In: Meffe G.K, Snelson F.F.J, editors. Ecology and evolution of live bearing fishes (Poeciliidae) Prentice Hall; New Jersey, NJ: 1989. pp. 91–124. [Google Scholar]
- Fry F.E.J, Hart J.S. Cruising speed of goldfish in relation to water temperature. J. Fish. Res. Board Can. 1948;7:175–199. [Google Scholar]
- Garland T, Jr, Carter P.A. Evolutionary physiology. Annu. Rev. Physiol. 1994a;56:576–621. doi: 10.1146/annurev.ph.56.030194.003051. doi:10.1146/annurev.ph.56.030194.003051 [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Losos J.B. Ecological morphology of locomotor performance in squamate reptiles. In: Wainwright P.C, Reilly S.M, editors. Ecological morphology—integrative organisimal biology. The University of Chicago Press; Chicago, IL: 1994b. pp. 240–302. [Google Scholar]
- Gracey A.Y, Fraser E.J, Li W.Z, Fang Y.X, Taylor R.R, Rogers J, Brass A, Cossins A.R. Coping with cold: an integrative, multitissue analysis of the transciptome of a poikilothermic vertebrate. Proc. Natl Acad. Sci. USA. 2004;101:16 970–16 975. doi: 10.1073/pnas.0403627101. doi:10.1073/pnas.0403627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammill E, Wilson R.S, Johnston I.A. Sustained swimming performance and muscle structure are altered by thermal acclimation in male mosquitofish. J. Therm. Biol. 2004;29:251–257. doi:10.1016/j.jtherbio.2004.04.002 [Google Scholar]
- Irschick D.J, Garland T., Jr Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annu. Rev. Ecol. Syst. 2001;32:367–396. doi:10.1146/annurev.ecolsys.32.081501.114048 [Google Scholar]
- Johnson T.P, Bennet A.F. The thermal acclimation of burst escape performance in fish: an integrated study of molecular and cellular physiology and organismal performance. J. Exp. Biol. 1995;198:2165–2175. doi: 10.1242/jeb.198.10.2165. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Lucking M. Temperature induced variation in the distribution of different muscle fibre type in the goldfish (Carassius auratus) J. Comp. Physiol. 1978;124:111–116. [Google Scholar]
- Johnston I.A, Maitland B. Temperature acclimation in crucian carp, (Carassius carasius L.) morphometric analyses of muscle fibre ultrastructure. J. Fish. Biol. 1980;17:113–125. doi:10.1111/j.1095-8649.1980.tb02746.x [Google Scholar]
- Johnston I.A, Temple G.K. Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J. Exp. Biol. 2002;205:2305–2322. doi: 10.1242/jeb.205.15.2305. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Wilson R.S. Temperature induced developmental plasticity. In: Warburton S.J, Burggren W.W, Pelster B, Reiber C.L, Spicer J, editors. Comparative developmental plasticity: contributions, tools and trends. Oxford University Press; Oxford, UK: 2005. [Google Scholar]
- Johnston I.A, Davison W, Goldspink G. Adaptations in Mg 2+ -activated myofibrillar ATPase activity induced by temperature acclimation. FEBS Lett. 1975;50:293–295. doi: 10.1016/0014-5793(75)80512-6. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Fleming J.R, Crockford T. Thermal acclimation and muscle contractile properties in cyprinid fish. Am. J. Physiol. 1990;259:R231–R236. doi: 10.1152/ajpregu.1990.259.2.R231. [DOI] [PubMed] [Google Scholar]
- McPeek M.A. Mechanisms of sexual selection operating on body size in the mosquitofish (Gambusia holbrooki) Behav. Ecol. 1992;3:1–12. doi:10.1093/beheco/3.1.1 [Google Scholar]
- Miner B.G, Sultan S.E, Morgan S.G, Padilla D.K, Relyea R.A. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005;20:685–692. doi: 10.1016/j.tree.2005.08.002. doi:10.1016/j.tree.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003;18:228–233. doi:10.1016/S0169-5347(03)00036-3 [Google Scholar]
- Pilastro A, Giacomello E, Bisazza A. Sexual selection for small size in male mosquitofish (Gambusia holbrooki) Proc. R. Soc. B. 1997;264:1125–1129. doi:10.1098/rspb.1997.0155 [Google Scholar]
- Pilastro A, Benetton S, Bisazza A. Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim. Behav. 2003;65:1161–1167. doi:10.1006/anbe.2003.2118 [Google Scholar]
- Podrabsky J.E, Somero G.N. Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in the annual killifish Austrofundulus limnaaeus. J. Exp. Biol. 2004;207:2237–2254. doi: 10.1242/jeb.01016. doi:10.1242/jeb.01016 [DOI] [PubMed] [Google Scholar]
- Prosser C.L. Wiley; New York, NY: 1979. Comparative animal physiology. [Google Scholar]
- Rome L.C, Swank D.M. The influence of thermal acclimation on power production during swimming I. In vivo simulation and length change pattern of scup red muscle. J. Exp. Biol. 2001;204:409–418. doi: 10.1242/jeb.204.3.409. [DOI] [PubMed] [Google Scholar]
- Rome L.C, Loughna P.T, Goldspink G. Temperature acclimation—improved sustained swimming performance in carp at low-temperatures. Science. 1985;228:194–196. doi: 10.1126/science.228.4696.194. doi:10.1126/science.228.4696.194 [DOI] [PubMed] [Google Scholar]
- Sisson J.E, Sidell B.D. Effect of thermal acclimation on muscle fibre recruitment of swimming striped bass (Morone saxatilis) Physiol. Zool. 1987;60:310–320. [Google Scholar]
- Temple G.K, Johnston I.A. Testing hypotheses concerning the phenotypic plasticity of escape performance in fish of the family Cottidae. J. Exp. Biol. 1998;201:317–331. doi: 10.1242/jeb.201.3.317. [DOI] [PubMed] [Google Scholar]
- Wakeling J.M, Cole N.J, Kemp K.M, Johnston I.A. The biomechanics and evolutionary significance of thermal acclimation in the common carp Cyprinus carpio. Am. J. Physiol. 2000;279:R3057–R3067. doi: 10.1152/ajpregu.2000.279.2.R657. [DOI] [PubMed] [Google Scholar]
- Walker J.A. Estimating velocities and accelerations of animal locomotion: a simulation experiment comparing numerical differential algorithms. J. Exp. Biol. 1998;201:981–995. [Google Scholar]
- Watabe S. Temperature plasticity of contractile properties in fish muscle. J. Exp. Biol. 2002;205:2231–2236. doi: 10.1242/jeb.205.15.2231. [DOI] [PubMed] [Google Scholar]
- Wilson R.S. Temperature influences the sneaky-mating and swimming performance of eastern mosquitofish (Gambusia holbrooki) Anim. Behav. 2005;70:1387–1394. doi:10.1016/j.anbehav.2004.12.024 [Google Scholar]
- Wilson R.S, Franklin C.E. Thermal acclimation of locomotor performance in tadpoles of the frog Limnodynastes peronii. J. Comp. Physiol. B. 1999;169:445–451. doi: 10.1007/s003600050241. doi:10.1007/s003600050241 [DOI] [PubMed] [Google Scholar]
- Wilson R.S, Franklin C.E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 2002;17:66–70. doi:10.1016/S0169-5347(01)02384-9 [Google Scholar]
- Wilson R.S, Johnston I.A. Combining studies of comparative physiology and behavioural ecology to test the adaptive benefits of thermal acclimation. Int. Cong. Ser. 2004;1275:201–208. doi:10.1016/j.ics.2004.08.078 [Google Scholar]
- Wilson R.S, James R.S, Johnston I.A. Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog Xenopus laevis. J. Comp. Physiol. B. 2000;170:117–124. doi: 10.1007/s003600050266. doi:10.1007/s003600050266 [DOI] [PubMed] [Google Scholar]
- Wilson R.S, Hammill E, Johnston I.A. Competition moderates the benefits of thermal acclimation to reproductive performance in male eastern mosquitofish. Proc. R. Soc. B. 2007;274:1199–1204. doi: 10.1098/rspb.2006.0401. doi:10.1098/rspb.2006.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]