Abstract
While foraging models of terrestrial mammals are concerned primarily with optimizing time/energy budgets, models of foraging behaviour in marine mammals have been primarily concerned with physiological constraints. This has historically centred on calculations of aerobic dive limits. However, other physiological limits are key to forming foraging behaviour, including digestive limitations to food intake and thermoregulation. The ability of an animal to consume sufficient prey to meet its energy requirements is partly determined by its ability to acquire prey (limited by available foraging time, diving capabilities and thermoregulatory costs) and process that prey (limited by maximum digestion capacity and the time devoted to digestion). Failure to consume sufficient prey will have feedback effects on foraging, thermoregulation and digestive capacity through several interacting avenues. Energy deficits will be met through catabolism of tissues, principally the hypodermal lipid layer. Depletion of this blubber layer can affect both buoyancy and gait, increasing the costs and decreasing the efficiency of subsequent foraging attempts. Depletion of the insulative blubber layer may also increase thermoregulatory costs, which will decrease the foraging abilities through higher metabolic overheads. Thus, an energy deficit may lead to a downward spiral of increased tissue catabolism to pay for increased energy costs. Conversely, the heat generated through digestion and foraging activity may help to offset thermoregulatory costs. Finally, the circulatory demands of diving, thermoregulation and digestion may be mutually incompatible. This may force animals to alter time budgets to balance these exclusive demands. Analysis of these interacting processes will lead to a greater understanding of the physiological constraints within which the foraging behaviour must operate.
Keywords: digestion, thermoregulation, foraging, diving, marine mammals, bioenergetics
1. Introduction
Scientists often refer to animals performing in an ‘optimal’ manner, either referring to how they allot their time to different behaviours, or the performance of their physiological systems. In truth, what an animal does and how it does it is the result of a series of compromises. Time is finite, and an animal rarely faces the luxury of having only a single objective to fulfil. Similarly, increasing the performance of a given physiological system will almost inevitably detract from other concurrent requirements. Like time, energy, blood and nutrients are also finite. With that in mind, an animal's observed foraging behaviour may only appear logical (or more importantly, predictable) if it is viewed as a concession to a number of concurrent demands.
Marine mammals face distinctive environmental conditions that can translate into unique physiological challenges. The following review examines the potential physiological constraints (or concurrent demands) to foraging behaviour in marine mammals. We present a novel framework that centres around three broad processes: prey acquisition; prey consumption; and thermoregulation. The framework allows us to review the effects and complex physiological interactions that shape the foraging behaviour at the individual level. We briefly review the scientific research that contributes to this paradigm, although the scope of the task precludes a complete synopsis of any single section. More importantly, we have placed past research in a novel larger context, and highlighted gaps in our current knowledge base that should be addressed in future studies. Our aim is to help direct future research with a view to improving quantitative foraging/energetic models.
There is a stark dichotomy in the general approaches to foraging models between scientists studying terrestrial mammals and those concentrating on marine mammals. Studies of terrestrial foraging have primarily approached the problem as an attempt by the animal to optimize energy and time in the acquisition of resources (for review, see Pyke 1984). This approach has resulted in a vast realm of theory that has largely been derived from initial optimal foraging models based on marginal value theorem (Charnov 1976; Stephens & Krebs 1986).
In contrast, studies of the foraging strategies of marine (diving) mammals have primarily focused on the physiological constraints that limit prey acquisition. In particular, these studies have been predominantly concerned with the way that oxygen reserves limit the time available below the surface or at depth to actively pursue and capture prey. The role of oxygen storage/usage in limiting foraging time is an overwhelmingly unique, almost defining, aspect of marine mammal foraging behaviour. Therefore, it should not be surprising that this constraint has been the focus of most of the research on marine mammal foraging. However, there are other physiological constraints that define foraging patterns in marine mammals which are often neglected. In this paper, we will discuss the interaction of three physiological processes that impose limitations on foraging patterns: the physiological demands of prey acquisition; prey processing; and thermoregulation.
Within these aforementioned physiological processes, we have defined three general types of potential conflict. First, there may be an absolute time restriction if the activities are mutually exclusive. Second, increased energy costs of one activity may also increase the costs (or decrease the efficiency) of another. Conversely, increased costs in one parameter may actually decrease the costs of another. Third, physiological changes required to maximize one process may be incompatible with other processes. Therefore, animals have to potentially prioritize among activities and/or manage with suboptimal performance in several aspects of their physiology.
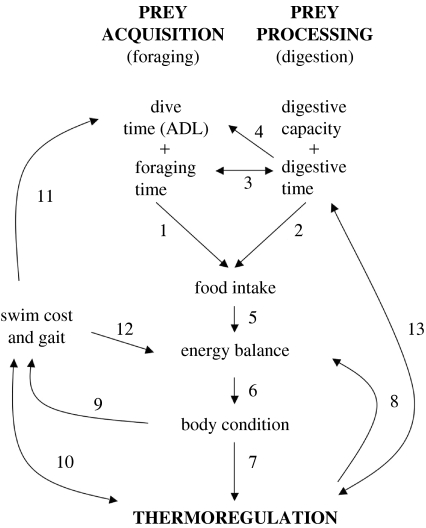
Certainly, the physiological interactions of digestion, thermoregulation and foraging are not unique to marine mammals. Marine mammals, after all, are anatomically and physiologically similar to terrestrial mammals in most respects. For example, while Williams et al. (2001) discerned allometric differences in the size of the small intestine between marine and terrestrial carnivores, there is no indication that the digestive physiology and biochemistry of marine mammals are fundamentally different from those of their terrestrial equivalents. And while marine mammals live in an environment with greater potential for heat loss (the specific heat capacity of water is 25 times that of air), many terrestrial mammals live in equally thermally challenging environments. However, the physiological adaptations used by marine mammals to maximize underwater foraging times potentially impact on their ability to digest prey and maintain thermoneutrality, and vice versa (figure 1). While these interactions may not represent over-riding physiological constraints to foraging behaviour in the same manner as the balanced usage of oxygen stores, we will illustrate how they are significant parameters that should be addressed in models of marine mammal foraging behaviour and energy budgets.
Figure 1.
Schematic of the interactions between the processes of prey acquisition, prey processing and thermoregulation.
- Prey acquisition is limited by the amount of time that an animal can spend foraging and the proportion of that foraging time which can be spent at depth pursuing prey. The latter is a function of the ADL, which is decreased by increased metabolic demands.
- Prey processing is limited by the animal's physiological maximum capacity for consumption and digestion and the amount of time that can be devoted to digest acquired prey.
- Foraging time and digestion time may be mutually exclusive activities, either due to behavioural or physiological constraints.
- Digestion will potentially decrease foraging efficiency by increasing metabolic overhead during dives, thereby decreasing ADLs.
- The amount and quality of food ingested will directly affect the animal's subsequent energy balance.
- Energy deficits will result in catabolism of body structures. In mammals, this will be the preferential usage of lipid reserves, leading to a decrease in body condition.
- If the lipid blubber layer is depleted too much, the animal will have to contend with increased thermoregulatory costs.
- Increased thermoregulatory costs will lead to an increased energy deficit.
- Changes in relative condition will also affect the animal's buoyancy and swimming biomechanics.
- Increased thermal costs will directly increase the total cost of locomotion. Additionally, the circulatory changes required for thermoregulation and diving may be physiologically in conflict. Conversely, the heat generated through muscular activity may serve to offset thermoregulatory costs.
- Changes in buoyancy and gait can increase the metabolic cost of locomotion and diving. This will serve to decrease the ADL, and subsequently decrease foraging efficiency.
- Increases in diving costs will also increase the amount of total energy that the animal requires.
- The HIF generated during digestion may help offset thermoregulatory demands. Conversely, circulatory demands of thermoregulation and digestion may be in conflict.
2. Limits on food intake
The ability of a marine mammal to meet its energy demands with sufficient prey is dependent on a number of limiting factors. In the framework of this paper, we have divided these factors into constraints on the processes of prey acquisition and processing.
Prey acquisition is limited by the amount of time that an animal can spend foraging and the proportion of that foraging time which can be spent at depth pursuing prey (figure 1 no. 1). Foraging time is impacted by the intrusion of other incompatible behaviours. As an obvious example, the time spent ashore (to nurse, mate, or for thermoregulatory considerations) directly decreases the potential foraging time. Within that potential foraging period, the amount of time that an animal can spend actively pursuing prey is also limited by the proportion of time it can spend at depth, minus the time ‘wasted’ diving to depth and recovering at the surface (inter- and post-dive intervals). These aspects of foraging efficiency are closely tied to the animal's aerobic dive limit (ADL), which is determined by physiological capacity and aerobic requirements (see §2a).
Food intake is further limited by aspects of prey processing. While additional prey may be available for capture, an animal can only consume a finite amount over a specific time, given the anatomical and biochemical limitations of the digestive system itself. The rate at which an animal can process prey is primarily limited by its consumption capacity (initial intake) and digestion time (passage rate through the system; figure 1 no. 2).
Finally, there are several avenues of interaction between prey acquisition and processing. There are indications that circulatory adjustments required to maximize diving and digestion may be, to a degree, incompatible (figure 1 no. 3; see §2c). Additionally, the metabolic requirements of digestion may impinge on the foraging efficiency by decreasing ADLs (figure 1 no. 4).
(a) Prey acquisition
The amount of time a marine mammal can spend at depth actively pursuing prey during a single dive, the frequency of those dives and the inter-dive and post-dive recovery periods are all related to its ADL. The ADL is defined as the amount of time that an animal can remain submerged without relying on anaerobic metabolism (i.e. without accumulating lactate; Kooyman et al. 1980, 1983). It is not that an animal is unable to dive beyond this physiological point, but there are important repercussions. Primarily, increased surface intervals are required to clear the circulating lactic acid levels from the blood. Aerobic diving limits are often viewed as a major determinant of diving ability (Costa et al. 2001); therefore, they represent a critical constraint to foraging behaviour (Castellini 1991). There is a growing body of literature that has examined the aspects of physiology which define an animal's ADL. Despite the obvious logistical difficulties in monitoring blood lactate levels in a diving marine mammal, such biochemical changes have been examined in both the laboratory and the field in several species (see review by Costa et al. 2001). As a generalization, it appears that marine mammals only rarely approach (let alone exceed) their ADLs when foraging in the wild.
In lieu of actual measures of ADL, several studies have relied upon the concept of calculated aerobic dive limits (cADL). This value is derived by estimating the animal's total internal oxygen stores (including haemoglobin stores in the blood, myoglobin stores in the muscle tissues and, in some cases, pulmonary stores in the lungs) and metabolism during the dive (the rate at which oxygen stores are consumed).
There are several problems with these estimates. First, they assume that all of the oxygen stores in the body are used completely (and evenly) before anaerobic metabolism begins. In truth, selective vasoconstriction will ensure that certain tissues will become hypoxic before all oxygen in the body is depleted. Indeed, low partial pressures are required before the substantial amounts of oxygen stored in myoglobin can be released from muscle stores (Davis et al. 2004). Second, there are very few direct measures of diving metabolic rate in marine mammals. These are usually restricted to captive animals and may not be comparable to animals foraging in the wild. As a result, there appears to be a discrepancy between cADLs and the behaviour of marine mammals in the wild, with some studies reporting a substantial proportion of observed diving bouts exceeding cADLs (Costa et al. 2001) and others reporting most foraging dives far below the cADL (Croll et al. 2001). Obviously, more reliable data would come from studies that have directly measured lactate levels in diving mammals, but the challenges in such research are considerable.
Physiologically based modelling of dive behaviour must not only take into account the behaviour observed during single foraging dives, but also explain the larger patterns of repeated dives with short surface intervals that are characteristic of most marine mammal foraging. This has led to a body of work that has produced models of optimal dive times (Houston & Carbone 1992) and (inversely) optimal breathing patterns (Kramer 1988). As succinctly stated by Green et al. (2005), foraging models of diving homeotherms, in a sense, have been primarily concerned with viewing oxygen rather than prey as the limited or patchy resource that defines behavioural patterns.
Our ability to test models of foraging behaviour and prey acquisition in marine mammals is further hampered by our general inability to differentiate between non-foraging, and successful and unsuccessful foraging dives. Although emerging technologies hold promise of actual measures of foraging success (e.g. Andrews 1998; Hooker et al. 2002; Austin et al. 2006), the current link between diving behaviour and actual foraging usually has to be inferred.
(b) Prey processing
Foraging models usually equate rates of prey acquisition with rates of energy and nutrient intake, i.e. prey processing. Rarely is there any consideration of the ability of the animal to process its food within a given time frame. Digestive constraints represent a finite limitation in the rate of energy intake. Digestive capacity (sometimes referred to as ‘consumption capacity’) is the amount of food that an animal can process over a sustained period. It is affected by the maturity of the animal, the size of its digestive system, the type of digestive system, the digestibility of prey, the flexibility of the digestive system to different prey types and availability, and levels and type of concurrent activity.
The importance of digestive capacity as a physiological bottleneck in the chain of resource acquisition and assimilation is not a novel concept (Kirkwood 1983; Karasov & Diamond 1988; Weiner 1992). The essential point is that an animal has a finite capacity to process prey items through its digestive system. The amount of food that an animal can eat per day is limited by two factors: the instantaneous content of the gastrointestinal tract (gut capacity); and the mean length of time taken by the food to pass through the tract (retention time). Digestive capacity is therefore maximized when gut capacity is maximized and retention time is minimized (Altmann 1998). The actual physiological bottleneck may be anywhere along this process, from initial mechanical and enzymatic breakdown in the stomach, through nutrient and water absorption in the intestines, and even rate of waste removal. Although some piscivorous marine mammals have relatively large intestines (Eastman & Coalson 1974; Williams et al. 2004a), their stomach capacity and retention times are similar to other mammals consuming similar diets (Krockenberger & Bryden 1994; Tollit et al. 2003; Trumble et al. 2003).
There are several ways that digestive capacity can impact foraging behaviour. First, as the energy density (or nutritional value) of individual prey items decreases, the animal must digest more prey within a time period to fulfil its energetic (or nutritional) requirements. At some point, the required level of food intake is greater than the digestive capacity of the animal. An alternate strategy (or an inevitable result) is for the animal to catabolize body tissues to fulfil energetic deficits. However, as will be discussed later, using this route to fulfil energy requirements has important implications regarding subsequent thermoregulatory and swimming costs (see §3a), let alone survival. Therefore, maximizing prey quality is a strategy not only for minimizing foraging time (and associated costs such as risk of predation), but also for ensuring energy balance before digestive capacity is reached. These considerations obviously apply to the net value of prey. For example, prey items that require longer foraging times (greater energetic expenditure) incur a direct energetic cost (i.e. overhead) which would effectively increase the minimum energy content that needs to be processed.
The frequency of feeding episodes is another way that digestive capacity can impact foraging behaviour. Most animals do not forage continuously; there is evidence that fragmented foraging periods may increase maximum intake levels (Zynel & Wunder 2002). However, this also means that animals must fulfil their daily energy requirements within a restricted period each day. Similarly, if animals are unable to forage every day (e.g. due to other required behaviours or lack of prey availability), then the amount of prey they have to consume to maintain overall energetic homeostasis must be proportionally increased. Restricted maximum foraging times will contribute to increased required food intake rates during the periods of foraging that approach the digestive capacity of an individual animal (see study by Rosen & Trites 2004 detailed below).
It is recognized that the digestive capacity has important ecological implications (Weiner 1992; Kersten & Visser 1996), including potentially explaining the proximate physiological cause of a metabolic ceiling (Peterson et al. 1990). However, most measures of digestive capacity are restricted to avian species (e.g. Zwarts et al. 1996; Kvist & Lindstrom 2003; Zharikov & Skilleter 2003; Van Gils et al. 2005), with only a few for carnivorous mammals (Kirkwood 1983; Zynel & Wunder 2002). This narrow focus on avian digestive capacity may be due to the fact that, in general, birds consume food which is abundant but high in indigestible material and thus requires long processing times (Bednekoff & Houston 1994), and that they have limited capacity for energy storage due to flight considerations. Therefore, many birds may live near the edge of their digestive capabilities.
Whatever the reason, there is a paucity of information on maximum digestive capacity among mammalian carnivores, and there exist only a few estimates for marine mammals. Kastelein published several reports of digestive capacity (measured as maximum sustained food intake) of captive marine mammals (Kastelein et al. 1990b, 1994, 1995). One paper (Kastelein et al. 1990a) reports the food intake of seven Steller sea lions (Eumetopias jubatus) (ranging in age from 1 to 16 years) as between 4 kg (the youngest) and 26 kg (the oldest male) after a day of fasting. However, this consumption was not sustained and the data are not available to determine the relationship between animal mass and digestive capacity.
In a study of young captive Steller sea lions, the digestive capacity was estimated by measuring the maximum food intake rates with varying levels of energy density of prey and foraging opportunities (Rosen & Trites 2004). The animals were normally maintained on herring at a ration equivalent to 5–7% of their own body mass. In the experiment, they were offered either high-energy herring or lower-energy capelin, free of human interference or performance demands.
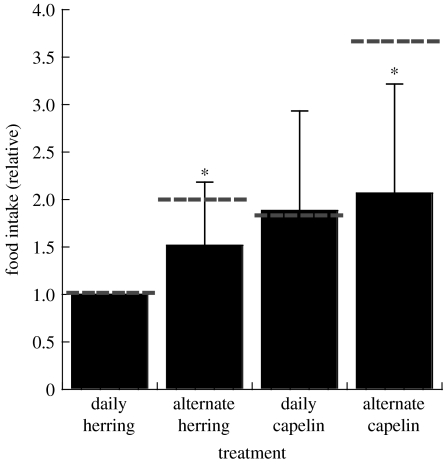
The primary aim of the experiment was to determine the capacity (and inclination) of these animals to increase food intake to compensate for differences in prey quality over short (5–10 days) periods of time. Based on differences in gross energy content, the sea lions would have had to consume 83% more capelin than herring. In fact, the sea lions consumed on average 89% more capelin during the trials when food was offered ad libitum for 7 h each day (figure 2).
Figure 2.
Food intake of captive Steller sea lions with different combinations of prey type and feeding opportunities. Average daily food intake (±s.d.) relative to the ‘baseline’ amount of fish consumed during the daily herring phase of the ‘satiation’ study. Dotted lines represent ‘expected’ values required to maintain an equivalent gross energy intake based on relative energy content of herring and capelin and daily or alternate day prey availability. Asterisk indicates significant differences between observed and expected consumption levels. Figure modified from Rosen & Trites (2004).
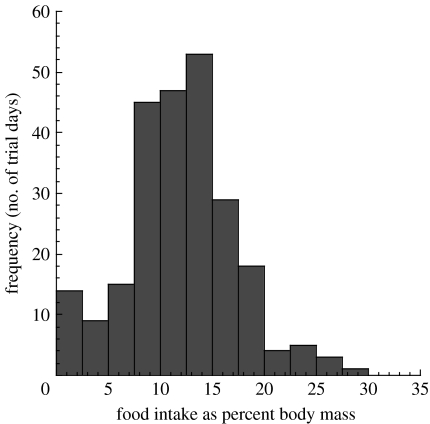
In order to ensure that the animal's digestive capacity was being measured, it was necessary to increase the rate of food intake required to fulfil energy requirements. Rather than increase the individual animal's daily energy requirements, the amount of time available to consume the required food was decreased. In one of the treatment combinations, the sea lions were offered food every other day (for 7 h), rather than daily. Theoretically, the sea lions should have consumed twice the amount of herring to maintain the same average intake levels over 2 days, and 236% more capelin, compared to trials when herring was offered every day. Although the sea lions increased their intake rates under these conditions, they appeared unable to consume sufficient prey to maintain their usual energy intake levels (figure 2). More interestingly, there appeared to be a maximum level of food intake equivalent to 16–20% of the animal's own body mass (figure 3). Behavioural observations appeared to confirm this physical limitation.
Figure 3.
Ad libitum food intake of captive Steller sea lions. The frequency of relative daily food intake (expressed as percentage of body mass) for five captive Steller sea lions in all the treatment combinations during ‘satiation’ trials (Rosen & Trites 2004).
Such rough estimates of maximum food intake can be integrated with data on available foraging times and ranges of prey energy densities to estimate a variety of parameters, including the minimum frequency and energy density of prey needed to sustain an animal. It can also be used to estimate minimum energy deficits that arise from scenarios where an animal is unable to ingest sufficient prey to meet its energy requirements. Unfortunately, estimates of maximum food intake are rarely integrated into models of individual foraging behaviour to set realistic endpoints to food intake variables.
Digestive capacity may be more problematic in younger animals, given their naturally higher metabolic rates due to growth. Larger body size also allows animals to fast longer, given that the metabolic requirements scale intraspecifically to body mass<1.0. A similar discrepancy of scaling exists between metabolic requirements and gut capacity, the latter of which scales isometrically with mass (Peters 1983; Schmidt-Nielsen 1984). This suggests that smaller animals have a smaller digestive capacity relative to energy requirements when compared with larger animals. It is unclear whether this scaling discrepancy exists on a developmental basis—although work has been done on the ontogeny of specific aspects of digestive physiology (e.g. Buddington & Diamond 1989; Debray et al. 2003), almost no work has been published on changes in maximum food intake with age. However, their relatively higher energy requirements imply that young animals are generally living on the edge, energetically speaking.
But what are the implications of these relationships, particularly in regard to foraging behaviour? First, younger animals may be under pressure for greater selectivity in prey types. Although many marine mammals are often categorized as ‘generalist’ feeders (often based solely on the observation of a varied diet), they may be more selective than is typically appreciated. The innate physiological limitation in food intake may require that animals bypass lower-quality prey resources and continue to ‘search’ for prey which can more readily fulfil their energetic requirements. Like many animals, this suggests that resource quality (gross nutritional value) may be as important as resource availability in driving prey selection. Whelan & Brown (2005) provide a review of the relationship between prey quality, prey selection and digestive constraints, but clearly more work needs to be done on the interactions between prey selection and digestive capacity in marine mammals.
(c) Trade-offs between prey acquisition and processing
There are at least three potential conflicts between prey acquisition (diving) and processing (digestion): time-budget restrictions; interactive energetic costs; and incompatible circulatory changes. In practice, it is very difficult to clearly differentiate these potential conflicts from observations of foraging behaviour alone, and the interactions between these demands are probably more complicated than we fully appreciate to date.
Time-budget restrictions may often be the result of physiological incompatibilities between diving and digestion, such that the time devoted to processing prey impinges upon time available for acquiring more prey. This disjunction may result from incompatible circulatory adjustments required to maximize the efficiency of diving and digestion (figure 1 no. 3). Given the scientific focus in marine mammal physiology on the adaptations they posses to maximize their diving ability, it is somewhat surprising that the results of these circulatory changes—specifically the potential for conflicting demands in circulatory patterns—have been largely overlooked.
Many early studies of marine mammal physiology specifically studied the ways that circulatory changes were employed to limit metabolism and, therefore, increase aerobic dive times. Irving et al. (1935) and Scholander (1940) helped define the classical ‘diving response’ which included the three pillars: apnea; bradycardia; and peripheral vasoconstriction. Scholander (1964) contended that vasoconstriction turned marine mammals into ‘heart, lung and brain machines’. Initial studies with restrained animals suggested an ‘absolute’ response of peripheral vaso-restriction and bradycardia (Scholander's ‘dive reflex’). Subsequent studies suggested that some of the previously observed ‘adaptations’ were by-products of the forced nature of the submergence. Later studies on free-diving animals suggested that the dive response was, in fact, graded to the length of the dive (Castellini 1991) and may not be apparent in shorter shallow dives (Kooyman 2002). Still, it would appear that the demands for peripheral vasoconstriction during longer foraging dives may be incompatible with the high rates of blood flow required for digestion and assimilation processes.
One of the most comprehensive studies on circulatory changes during marine mammal diving was conducted by Zapol et al. (1979). They studied changes in blood flow in a variety of systems in a Weddell seal (Leptonychotes weddelli) during forced submergence. Although changes in blood flow in the digestive system were not specifically measured, the overriding vasoconstriction observed throughout the seal—with the notable exception of the brain—adds weight to the theory that diving and digestion demand incompatible circulatory adjustments (figure 1 no. 3). This potential conflict is discussed further in §4c.
However, even if foraging dives are restricted to depths where there is no circulatory conflict between diving and digestion, there will be an inherent metabolic conflict (figure 1 no. 4). The various processes of digestion (mechanical and chemical breakdown, assimilation) are known to increase the rates of oxygen consumption (known as heat increment of feeding (HIF) or specific dynamic action; Blaxter 1989). Increased metabolism due to digestion will impact foraging ability by increasing metabolic overhead, thereby decreasing available aerobic dive time. At some point, the cost of digestion could possibly decrease the energetic efficiency of foraging to the point where it was no longer profitable.
The potential problems of incompatible circulatory demands and increased foraging costs have led to the (often assumed) disjunction between foraging and digestive activities. In the most extreme expression of this scenario, animals would concentrate solely on prey capture until their immediate gut capacity had been reached. At this point, the animal is forced to rest rather than continue foraging (Crocker et al. 1997). Drift dives, characterized by episodes of languid, non-powered ascents or descents (Crocker et al. 1997; Biuw et al. 2003) have been hypothesized to function as a period of inter-foraging digestion (as well as an opportunity to purge metabolic waste; Crocker et al. 1997).
Of course, this division between foraging and digestion does not necessitate that an animal should fill its stomach and then completely digest the contents before resuming foraging. A host of other factors (including prey availability, indirect costs of foraging, other required behaviours, flexibility in digestive capacity and processing times) help determine when an animal should switch between digestion and foraging. Some studies have clearly demonstrated that periodic foraging patterns increase maximum consumption capacity (Zynel & Wunder 2002).
The relationship between digestion and foraging is probably best characterized as a physiological ‘compromise’ in the sense that neither process is functioning at maximum efficiency. However, another rarely considered factor—the anatomical ability of marine mammals to consume their prey at depth—may necessitate a complete separation of digestion and foraging. Animals are ultimately limited in the size of the prey they can consume at depth by their gape size. Returning to the surface to consume prey alters foraging costs through increased energy and time costs, and potential increases in predation risk (e.g. killer whales, Orcinus orca). Nonetheless, it is common to observe seals coming to the surface to tear apart large fish such as salmon. The decision to forage on prey that has to be brought to the surface to be consumed represents another clear incompatibility between foraging and diving behaviour.
Sea otters (Enhydra lutris) process much of their prey at the surface. However, sea otters must also contend with a rather unique temporal conflict between foraging and thermoregulation. Rather than relying on a blubber layer, the sea otter's incredibly thick pelage serves as the primary thermal buffer (Morrison et al. 1974), aided at lower temperatures by vasoconstriction (Costa & Kooyman 1982). This results in two conflicting behavioural requirements. First, sea otters must consume tremendous amounts of food (equivalent to 20–25% of its own body mass daily), partly to maintain thermoneutrality, and therefore spend a large portion of their day foraging. However, to maintain the low thermal conductance of their pelage, they must also spend a large portion of their day grooming at the surface (Kenyon 1981). This grooming behaviour is also, in itself, a surprisingly energetically expensive behaviour (Yeates et al. 2005), adding to the animal's required daily food intake.
Clearly, the behavioural and physiological demands of digestion and foraging interact in a number of ways, not all of which are clearly understood. However, this complex relationship may prove central to constructing accurate bioenergetic and behavioural models of marine mammal foraging.
3. Consequences of insufficient food intake
Important to the discussion of physiological constraints to foraging are the subsequent effects of changes in body mass and composition which may result from an animal's inability to obtain energetically adequate levels of prey. Tissue catabolism is the direct result of an inability to obtain or process sufficient food to fulfil energy requirements. During certain periods, the usage of internal energy reserves is a predetermined strategy (i.e. part of their natural life history), but at other times, it is the result of unexpected energy deficits. To understand the consequences and limitations of this strategy, it is important to consider the subsequent effects on physiology, foraging costs and thermoregulatory capabilities.
(a) Changes in body composition
When an animal is unable to satisfy its energetic requirements from external sources (figure 1 no. 5), it must catabolize internal tissues. The anatomical source of mass loss can be almost as important as the degree of tissue loss. The preferential usage of either lipid or protein sources under certain conditions not only results in changes in relative body condition (figure 1 no. 6), but also reflects physiological ‘decisions’ resulting from numerous conflicting requirements and constraints.
Lipid is generally regarded as the preferred tissue for catabolism as it provides greater energy per mass (approx. 39 kJ g−1) than protein (approx. 18 kJ g−1; Schmidt-Nielsen 1997). Additionally, lipids provide greater amounts of metabolic water than protein (107 and 41 g per 100 g tissue, respectively) that may also be lacking from external sources. According to general theories of fasting physiology, an animal should primarily use the lipid stores to fulfil additional energy requirements, except for a small amount of protein catabolism required for gluconeogenesis for the central nervous system (Øritsland 1990). Only when lipid stores are depleted should substantial protein usage occur, since protein degradation is the proximate mechanism for death via organ failure (Cherel et al. 1992).
It is assumed that most mammals with predictable seasonal periods of food restriction (owing to either food scarcity or behavioural demands that preclude foraging) will display protein-sparing physiological adaptations. Certainly, there is a trend for mammals that experience such seasonal changes in energy balance (i.e. predicted periods when they must catabolize tissues) to have a substantial discrete hypodermal blubber layer in addition to lipid reserves situated around certain internal organs (e.g. Scholander et al. 1950; Pond 1978; Leader-Williams & Ricketts 1982; Adamczewski et al. 1995).
The blubber layer of marine mammals fulfils the (sometimes contrary) dual roles of insulation and energy reserve (except animals such as sea otters, polar bears (Ursus arctos) and, to a lesser extent, fur seals where pelage is the primary insulative organ). In field studies, the extent of subcutaneous lipid stores has been taken as an indication of a pinniped's energy state, health status or overall ‘condition’ (Renouf et al. 1993; Naess 1998; Pitcher et al. 2000; Tierney et al. 2001). The animal's lipid reserves are often expressed as a proportion of its total body mass, yielding a simple (or perhaps simplistic) body condition index.
However, the relative extent of a diving mammal's blubber reserves also affects the animal's thermoregulatory capabilities. Decreases in insulation due to tissue catabolism will potentially increase thermal costs (figure 1 no. 7), leading to further imbalances in the animal's energy budget (figure 1 no. 8), and eventually to further depletion of the lipid reserve. Depletion of the primary thermoregulatory organ can potentially lead to an escalating discrepancy between increasing energy requirements and decreasing energy reserves.
Energy requirements may also increase due to changes in buoyancy resulting from a reduction in lipid reserves. Changes in buoyancy directly affect the cost of diving (and even the animal's gait; figure 1 no. 9) which not only decreases foraging efficiency by decreasing ADLs but also potentially affects its energy balance (figure 1 no. 12) and, in turn, further depleting its lipid reserves.
Therefore, while primarily using lipids for fulfilling energy requirements (versus non-lipid tissues, primarily ‘core’ protein structures) is preferential from the perspective of maximizing energy yield per gram of tissue and sparing vital protein structures, there are additional considerations that may mitigate this strategy. Using greater core tissues will help maintain the thermal layer and preserve buoyancy, thereby limiting the cost of foraging (and limiting further energy deficits). However, while this strategy may save immediate locomotor and thermal costs, it may also have significant short- and long-term health costs.
There is a great deal of data on the patterns of tissue usage in pinnipeds. In general, they follow general mammalian fasting theory and primarily use lipids. Although there are a substantial number of studies, almost all are on phocid seals (see review by Castellini & Rea 1992). Few studies have been undertaken on otariid seals which generally have smaller relative lipid reserves than phocid seals. Studies of other marine mammals have concentrated on describing observed seasonal and developmental changes in lipid stores (Lockyer et al. 1985; Lockyer 1993; Naess 1998; Dunkin et al. 2005).
In general, phocid seals follow the mammalian strategy of employing protein-sparing metabolic strategies during natural fasts. Otariids appear to follow a similar strategy, although their relatively smaller lipid layers may preclude such extreme metabolic adjustments. Beauplet et al. (2003) found that 56% of mass loss was derived from lipids and only 10% from proteins in naturally fasting sub-Antarctic fur seal (Arctocephalus tropicalis) pups (the remainder being metabolic water). Similar results were reported for naturally fasting Antarctic fur seal pups (Arnould et al. 2001b) and adult males holding territories (Boyd & Duck 1991).
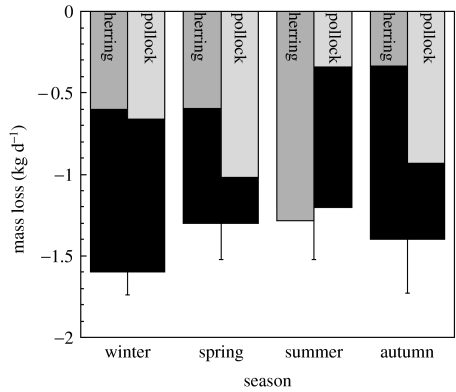
Further investigations have suggested that the pattern of tissue catabolism in marine mammals is more complicated, reflecting shifts in energetic priorities. In experimentally fasted/food-restricted captive Steller sea lions, approximately 60% of mass loss could be attributed to lipid sources (Rosen & Trites 2005; Kumagai et al. 2006). However, the relative rates of lipid versus protein tissue usage were dependent on both season and food quality (lipid/protein content). When on short-term restricted feedings, the proportion of total mass loss comprising lipid varied significantly between seasons, ranging from 24 to 107% (figure 4). In addition, a greater proportion of the total mass loss was derived from lipid stores while consuming a low-lipid/high-protein diet than while consuming a high-lipid/low-protein diet, except during the summer (a natural period of high growth) when the trend was substantially reversed. This variation may have been due to natural variation in energetic priorities, and may also reflect a seasonal ability to withstand unpredicted food shortages. Rea et al. (1999, 2007) suggested that captive Steller sea lions may not be capable of initiating metabolic adjustments that would serve to spare protein structures during certain seasons.
Figure 4.
Changes in Steller sea lion body mass and composition during food restriction. Mean ±s.d. of rate of total body mass loss (black bars, averaged across diets) during 9 days of restricted feeding of herring or pollock in seven captive Steller sea lions (n=7 for each season). Mass loss was significantly greater in the winter trials. The contribution of lipid loss to total mass loss is also shown separately for herring (grey bars) and pollock (white bars) diets. There was a significant season×diet interaction—more of the mass loss was derived from lipid stores while consuming pollock than while consuming herring, except in the summer when the pattern was reversed. More than 100% lipid loss in the herring group in the summer suggests that they were gaining lean body mass while losing overall body mass. Figure from data in Kumagai et al. (2006).
Another consideration with respect to patterns of tissue catabolism is that the depletion of lipid stores may be further constrained by the morphological function they serve in swimming. In cetaceans, blubber functions not only in buoyancy control, but also in streamlining and as structural components (Pabst et al. 1999). This can lead to preferential lipid usage at different sites of the body. For example, in harbour porpoises (Phocoena phocoena), the thorax appears to be the primary site for lipid deposition and depletion (Koopman et al. 2002). Although substantial lipid masses are located at the tailstock, these do not appear to be depleted during periods of undernutrition. This suggests that the blubber at the tailstock is required for maintaining locomotory capabilities and streamlining.
(b) Changes in swimming costs
As previously described, most studies of marine mammals indicate that they primarily lose lipid mass rather than protein mass during periods of negative energy balance. Two potential results of such a strategy would be a decrease in thermoregulatory capacity (figure 1 no. 7; see §3c) and an alteration in the animal's buoyancy (figure 1 no. 9). While there has been substantial research on the energetic consequences of the former, only recently has consideration been given to the latter.
It has been well documented that buoyancy affects diving performance (Lovvorn & Jones 1991). Therefore, it is not surprising that there is also evidence that buoyancy influences behavioural decisions about swimming in marine mammals. Efficient locomotion is particularly important for breath-holding divers, as increased locomotor costs will not only incur direct energetic costs (figure 1 no. 12) but also limit dive time (and decrease foraging efficiency) through increased oxygen depletion rates (figure 1 no. 11; Skrovan et al. 1999; Sato et al. 2003).
Change in gait (active strokes or gliding) is one strategy that animals use to optimize locomotion. Selection of a specific gait depends partly on the relative buoyancy of the animal in the medium (Williams 2001). An animal's buoyancy naturally decreases with water depth, largely through compression of pulmonary air stores (Williams et al. 2000), a physical characteristic that many divers appear to take advantage of, as evident through changes in gait with depth and individual density. However, the energetic consequences of changes in buoyancy induced by changes in lipid stores are not clear. Most work to date has concentrated upon changes in diving behaviour with changes in buoyancy.
A common characteristic in marine mammal swimming biomechanics is alternating between active stroking and gliding, during either ascent or descent portions of their dives. The use of gliding behaviour during diving has become loosely categorized as ‘drift diving’. As an example, right whales (Eubalaena spp.) must use strong strokes at the surface to counteract their positive buoyancy, but they can conversely take advantage of this buoyancy to power glides on their ascent (Nowacek et al. 2001). Sperm whales (Physeter macrocephalus) and bottlenose dolphins (Tursiops truncatus) also glide more during portions of dives when buoyancy aids their movement (due to natural changes in buoyancy with depth), and whales that glide more during ascent glide less during descent (and vice versa; Skrovan et al. 1999; Miller et al. 2004).
The plasticity of this change in diving behaviour is evident by comparing swimming strategies of individuals with different body densities. Sato et al. (2003) observed that fatter Weddell seals descended with a stroke-and-glide method, while thinner individuals prolonged their gliding phase. Gliding appeared to be a more efficient locomotor technique, as surface intervals between dives were less for gliding than for stroking animals.
In grey seals (Halichoerus grypus), body fat (hence density) was demonstrated to be related to dive characteristics—although all animals were negatively buoyant, those with higher fat mass (relatively more buoyant) had slower descent rates (Beck et al. 2000). These findings would suggest a cost to greater lipid stores, except that the more buoyant seals also had slower ascent rates, contrary to predictions. Similarly, in a study of northern elephant seals (Mirounga angustirostris) which had their buoyancies experimentally altered, less buoyant individuals had faster descent rates, but there was no relationship between buoyancy and rate of ascent (suggesting that ascents were all powered; Webb et al. 1998). In contrast, Biuw et al. (2003) found that fatter southern elephant seal (Mirounga leonina) pups had higher ascent and slower descent rates during the drift portion of dives. They even constructed a model that reasonably predicted measured lipid content by dive profiles alone. Some, but not all, male New Zealand fur seals (Arctocephalus forsteri) have been observed to use drift dives—the ones that did were mostly larger males (Page et al. 2005).
Changes in buoyancy are not just a by-product of changes in body composition. A number of diving vertebrates use lung capacity to alter buoyancy to maximize dive performance (e.g. Lovvorn & Jones 1991; Sato et al. 2002; Hays et al. 2004; Miller et al. 2004; Wilson & Zimmer 2004). However, other physiological considerations (e.g. nitrogen narcosis, decompression sickness, oxygen toxicity) may limit the amount of air or time during which this strategy may be used (Moore & Early 2004; Hooker et al. 2005).
Again, it should be noted that actual changes in the cost of diving with changes in body composition (through either changes in buoyancy or gait) has rarely been measured. In theory, increases in the cost of diving and swimming through direct energetic cost (figure 1. no. 12) and increased metabolic overhead for aerobic dives (figure 1 no. 11) can have substantial impacts on observed short- and long-term foraging behaviour.
(c) Changes in thermoregulatory capacity
The previously discussed tendency for marine mammals to preferentially metabolize stored fat to offset energy deficits can potentially reduce valuable insulation to the point of increasing the energetic cost of thermoregulation (figure 1. no. 7). This can contribute to the onset of a downward spiral of reduced body condition and increased energy deficit, until thermal balance can no longer be maintained (figure 1. no. 8).
The challenge of balancing the usage of the blubber layer for thermoregulation and for an energy source is especially relevant to young animals which, given their higher surface area to volume ratio compared with larger adult animals, would be expected to have higher rates of heat loss in water. Additionally, maintaining thermal neutrality may be a relatively greater energetic burden to juveniles which are also faced with the energetic costs of anabolism required to continue growing and complete ontogeny. As juveniles mature, their ability to thermoregulate also influences the energetic costs associated with the development of swimming and diving skills necessary for their primarily aquatic existence (Donohue et al. 2000).
The extent to which changes in body condition affect thermoregulatory abilities and costs has been directly measured primarily in pinnipeds, and mostly in young animals. This focus is partly due to logistical concerns, and also due to the fact that this age class potentially faces the greatest thermal challenge owing to high surface-to-volume ratios and high energetic requirements for growth.
Much work on the thermal effects of changes in blubber reserves on energy requirements has been completed on phocid seal pups (e.g. Irving & Hart 1957; Worthy 1991; Muelbert & Bowen 1993). Phocid pups typically undergo a long post-weaning fast on land. Besides facing the immediate paradox of having to use blubber as an energy source yet conserve it as an insulative layer, ‘weaners’ must also consider the future impact that blubber depletion would have on survivorship during subsequent foraging trips. For example, northern elephant seal pups exhibit differing patterns of energy usage depending on body mass and body composition at the end of weaning. Fatter pups use more fat and spare proportionally more protein than leaner pups. This individual variation in energy usage in elephant seal pups may ensure that leaner pups become fatter (as a proportion of total mass) by maintaining a higher level of protein catabolism, thus enabling all pups to have sufficient lipid stores for thermoregulation during their first foraging trip (Noren et al. 2003; Noren & Mangel 2004). Northern elephant seal pups that have sufficient lipid stores at the end of the post-weaning fast were shown to be able to remain thermally neutral in 4°C water (Noren 2002).
Similar results have been shown for harbour seal pups through energetic modelling exercises (Harding et al. 2005). Model results suggest that the cost of thermoregulation varies dramatically with body size. Smaller pups (17–32 kg) were estimated to require more than three times the extra energy to compensate for heat loss over the winter months as compared with larger (heavier) seals. The model predicts that the first year of winter pup survival is strongly related to pup masses measured in autumn, and that pups which do not reach at least 26 kg are likely to suffer the effects of cold stress at the onset of winter water temperatures (Harding et al. 2005).
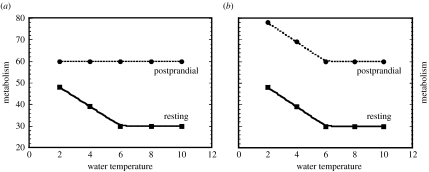
Fewer studies have measured how body condition affects the thermal capabilities of otariids. A study of Antarctic fur seals (Arctocephalus gazella) found that both pups and yearlings displayed elevated resting metabolic rates in cold water (0.6°C); in fact, the lower critical temperature for both age groups was estimated to be surprisingly high, 14.4°C. Both total body mass and percentage of lipid mass predicted thermoregulatory abilities in both age groups (Rutishauser et al. 2004).
While examining the ontogeny of thermoregulatory capabilities in northern fur seal (Callorhinus ursinus) pups, Donohue et al. (2000) noted that whole-body metabolic rates of post-moult pups were equivalent to those of smaller pre-moult pups measured in water. In other words, the mass-specific metabolic rates of pre-moult pups were higher than post-moult pups. This observation, coupled with stable body temperatures in post-moult pups, was thought to reflect increased thermoregulatory capabilities of post-moult pups compared with the greater thermal costs to pre-moult pups across a wide range of water temperatures (5–20°C). Decreasing mass-specific metabolic rates as the pups matured suggested energetic savings due to larger body size, increased lipid stores (from 14–18 to 34–39% as pups mature) and insulation provided by the post-moult pelage. Similarly, the dependence of sub-Antarctic fur seals on their pelage for reducing thermoregulatory costs is evident in the decrease in field metabolic rate from pre- to post-moult pups, along with the corresponding increases in lipid reserves (from 10.4 to 37.1%, respectively; Beauplet et al. 2003).
There are fewer direct measures of changes in thermoregulatory capabilities with changes in lipid mass in cetaceans. On an interspecific basis, small cetaceans from temperate climes have greater lipid reserves than more tropical species (Worthy & Edwards 1990). Loss of lipid in the former may have greater energetic consequences, given their colder environment (and hence greater potential for heat loss), although tropical species may actually live closer to their lower critical temperatures (Hampton & Whittow 1976). Emaciated bottlenose dolphins display a significant rise in the conductivity of their blubber layer, suggesting that the lipid content (quality) of the blubber layer changed rather than the actual depth (quantity; Dunkin et al. 2005).
Bioenergetic modelling has provided a vehicle to examine some of the constraints on thermoregulation that are otherwise difficult to collect on free-ranging animals (Hokkanen 1990; Boily 1995; Kvadsheim et al. 1997). For example, Roscow (2001) modelled the degree of heat loss in Steller sea lions under a variety of aquatic and terrestrial environmental conditions, from which costs of thermoregulation were estimated for animals over a range of body sizes (50–1000 kg) and body conditions (gauged by blubber depth, 1–5 cm). In general, under all environmental conditions, as the thickness of the blubber layer decreased the energy required for thermoregulation increased, so that sea lions with poor body condition (1 cm in blubber depth) experienced the highest thermoregulatory costs. For juvenile and adult sea lions (100 and 500 kg, respectively) at rest, critical blubber depths for three water temperatures (−2, 4 and 8°C) were modelled. Critical blubber depth (below which a thermoregulatory cost is incurred) was 3 cm for adults, when modelled with average winter water temperatures (4°C), and 2 cm with summer water temperatures (8°C). Juvenile sea lions were predicted to need slightly thicker blubber (3.5 cm) to avoid thermal costs at these water temperatures. In cold water (−2°C), critical blubber depth was never attained for either juveniles or adults with up to 5 cm of blubber depth. In air (0°C), critical blubber depth was approximately the same (between 1.25 and 1.5 cm) for juveniles and adults. Like many models, the results are limited by quality of physiological data currently available for this species, and further studies are required to refine the sensitivity of the model (e.g. Hoopes et al. 2004)
Despite the uncertainties involved in modelling thermoregulation, the depletion of a marine mammal's lipid reserves can have serious thermoregulatory consequences. To survive in the face of limited food intake, the animal must adopt a series of physiological and behavioural responses that limit or reverse the continuing cycle of depletion of insulation and resulting increasing energy deficit.
4. Energy and circulatory trade-offs between foraging and thermoregulation
The effect of depletion of the blubber layer on thermoregulatory costs only becomes important when it impinges on the minimum insulative layer required for thermal neutrality. The thickness of this minimum layer is difficult to ascertain, as it relies on many physiological and environmental factors, many of which have not been adequately explored. In fact, few measures of thermoneutral zones (or even lower critical temperatures) exist for marine mammals (Liao 1990; Williams et al. 1993; Hansen et al. 1995). Comparisons are complicated by the observation that pinnipeds have two effective lower critical temperatures—one on land and another in the water—that can differ substantially (Irving & Hart 1957), and the impact of activity and digestion on ‘effective’ thermoneutral zones (see below).
Although some species have relatively ‘skimpy’ lipid reserves, others have significantly more than required for thermoregulation alone, what Watts et al. (1993) coined ‘thermoregulation below the zone of irrelevance’. As an extreme example, Hokkanen (1990) observed that the blubber layer of the bowhead whale (Balaena mysticetus) was sufficient to maintain thermoneutrality in liquid nitrogen. However, whether a marine mammal has ‘overabundant’ insulation or not, there are significant interactions between thermoregulation and foraging that must be considered.
For those marine mammals that also live in the terrestrial environment, individuals must often balance the costs and benefits of thermoregulation and foraging behaviour. Pinnipeds must find a compromise between remaining on land where thermal costs may be lower than in the water (but at the expense of receiving no energy input), and spending time in the water foraging where they have greater opportunities to overcome energy debts through food intake (but may expend more energy in thermoregulation). An extreme example of the spatial discord between thermoregulatory and foraging considerations may be demonstrated by seasonal baleen whale migration from productive high-latitude feeding grounds to low-latitude breeding grounds. This movement has traditionally been explained as a required disjunction between areas optimal for foraging and those for thermoregulation in young calves (Brodie 1975), although the hypothesis is not universally accepted (Corkeron & Connor 1999).
Thermal considerations may modify short-term foraging behaviour. For example, potential heat loss increases with swimming speed due to increased costs of convective heat loss (Hoopes et al. 2004), and changes in water temperature with depth will increase the thermal gradient that the animal must combat.
It is important to note that thermoregulation and foraging are not always conflicting energetic demands. For pinnipeds, decreasing air temperatures and increasing precipitation and wind will result in environmental conditions under which thermal costs are lower in the water than hauled out on land. Additionally, the hypometabolism exhibited during diving may help offset the costs of thermoregulation (Costello & Whittow 1975; Castellini et al. 1992; Hurley & Costa 2001; Hastie et al. 2007).
A great deal of heat is also generated through muscular activity during behaviours such as diving and swimming. Similarly, heat is produced during the mechanical breakdown and biochemical absorption of food. Thus, swimming and digestion create heat ‘by-products’ that can potentially be used to offset costs of thermoregulation rather than simply expended as metabolic ‘waste’ (figure 1 no. 10 and no. 13).
In addition to energy trade-offs, physiological adjustments in circulation may be inconsistent between thermoregulation, foraging and digestion. For example, vasoconstriction that promotes diving capabilities may limit heat dissipation to the skin surface, thereby impinging upon thermoregulatory capabilities (figure 1 no. 10). Similarly, vasoconstriction during diving that limits blood flow to the digestive system will decrease the animal's ability to simultaneously process and acquire prey (figure 1 no. 3).
(a) Substitution of heat from activity
The idea that animals should minimize their energetic waste partly led to the suggestion that animals should divert ‘excess’ heat produced from activity to substitute for the heat required for thermoregulation (figure 1 no. 10). The importance of heat generated by muscular activity for offsetting thermoregulatory costs has been demonstrated in a range of homeotherms (Webster & Weathers 1990; Bevan & Butler 1992; Zerba & Walsberg 1992; Girardier et al. 1995; de Leeuw et al. 1998; McNamara et al. 2004; Kaseloo & Lovvorn 2006), including semi-aquatic mammals (Kruuk et al. 1994; MacArthur & Campbell 1994; Campbell & MacArthur 1998; Campbell et al. 2000). Studies have suggested that the level of thermal substitution varies with the levels of activity (heat production) and the rates of potential heat loss (Williams 1986; Hind & Gurney 1997; Kaseloo & Lovvorn 2005).
The benefits of using heat from muscular activity to offset thermoregulatory costs are only beneficial if the animal is not thermal neutral. Within the thermoneutral zone, the energy produced for basic metabolic processes is (by definition) sufficient to maintain core body temperature. The thermal balance (net heat flow) is a product of the external environment, the insulative properties of the animal and the level of internal heat generation. While the high thermal conductivity and heat capacity of water present unique challenges to marine mammals, their hypodermal blubber layer (or in the case of sea otters and some fur seals, their exceedingly dense pelage) usually provides sufficient insulation to limit heat loss during ‘normal’ physiological and environmental conditions (§3c).
Thermal substitution of heat from activity may not be possible in many cases. There is a tendency for some diving marine mammals to actually decrease body temperature during diving (Scholander et al. 1942; Kooyman et al. 1981). Whittow (1987) suggested that the observed decreases in core temperature indicated that metabolic production during dives was actually lower than at rest (although see Kooyman et al. 1980; Hill et al. 1987). This hypothesis is supported by the studies that have observed decreases in metabolic rate during diving (Costello & Whittow 1975; Castellini et al. 1992; Hurley & Costa 2001; Sparling & Fedak 2004; Hastie et al. 2007). A lack of excess heat production would obviously make any type of thermal substitution less probable.
However, hypometabolism during diving is not universal among marine mammals (and often only exhibited in deep long dives), and core temperatures have not always been observed to decline with submergence time. Increases in body temperatures during diving and swimming have been observed in a number of pinnipeds (McGinnis & Southworth 1967; Ohata et al. 1972) and cetaceans (Hampton et al. 1971; Whittow et al. 1974; Hampton & Whittow 1976). It has even been suggested that small cetaceans can only maintain internal body temperatures through the substitution of heat generated through activity (Parry 1949; Hampton & Whittow 1976).
Unfortunately, changes in core temperatures cannot definitively indicate whether thermal substitution is occurring, as it only indicates the end product of the combined demands of environmental load, internal heat production and dissipation ability. Studies that document changes in total metabolic costs across different (internal and external) thermal conditions would provide more convincing evidence.
(b) Substitution of heat from digestion
HIF is the increase in the rate of oxygen consumption (and resulting metabolic heat production) that follows the ingestion of a meal. This phenomenon is thought to result from both the mechanical and biochemical processes of digestion (Blaxter 1989). HIF has been quantified in numerous species consuming a variety of meal types, including several species of marine mammals (for review, see Rosen & Trites 1997). HIF can comprise a significant proportion (more than 20%) of gross energy intake.
It has long been suggested that homeotherms might be able to use the increase in heat production from digestion to offset concurrent thermoregulatory costs (figure 1. no. 13; Rubner 1902; Kleiber 1975; Lavigne et al. 1982). Unfortunately, empirical evidence to support this hypothesis is equivocal (Robbins 1993; Rosen & Trites 2003). While some studies of terrestrial mammals have provided evidence that HIF can at least partially substitute for thermoregulatory costs (Simek 1975; Masman et al. 1988; Chappell et al. 1997; Jensen et al. 1999), others have indicated little or no interaction between these two bioenergetic parameters (figure 5; Klaassen et al. 1989; Campbell et al. 2000).
Figure 5.
Theoretical interactions between HIF and thermoregulatory costs. (a) In one scenario (thermal substitution), the total cost of metabolism due to digestion and thermoregulation remains constant across environmental temperatures, even below the animal's lower critical temperature. (b) In an alternate scenario (thermal independence) total metabolism increases as temperature decreases due to increased thermoregulatory costs regardless of digestive heat production.
Several variables may affect the likelihood of thermal substitution occurring. First, more heat produced through digestion will mean a greater source and greater chance of detecting thermal substitution. In general, high protein diets will induce greater HIF (Jobling 1983), which has also been demonstrated among marine mammals (Rosen & Trites 1997). Second, a higher potential for heat loss (such as through increased thermal gradient, conductivity of the medium) should result in greater thermal substitution. Therefore, one might expect that the tendency for substitution would be greatest for aquatic mammals, given their potential for losing heat to their environment. However, while there is evidence to support thermal substitution among some aquatic mammals (Costa & Kooyman 1984), other experiments have failed to support this hypothesis (MacArthur & Campbell 1994; Campbell et al. 2000; Rosen & Trites 2003; Williams et al. 2004b). The apparent inconsistency in results between studies that have and have not reported thermal substitution raises interesting questions for comparative physiologists. Uncertainty whether thermoregulation and HIF are cumulative or offsetting costs also presents a potentially large source of error when calculating energy budgets or constructing bioenergetic models.
The aforementioned studies all measured the interaction between thermoregulation and digestion through changes in total energy production (figure 5). An alternative avenue for an energetic interaction between digestion and thermoregulation is when HIF decreases thermoregulatory costs by helping to maintain core temperatures (irrespective of changes in total metabolism). Several studies have demonstrated the combined effects of digestion and environmental temperature on both maintaining body temperature and reducing shivering thermogenesis (Costa & Kooyman 1984; Maloney et al. 1999; Rashotte et al. 1999). This includes a strategy of delayed digestion to maximize the substitution effects of HIF during the most thermally challenging periods (Rashotte et al. 1999). However, other studies of the effect of HIF on maintaining body temperature have yielded mixed results (MacArthur & Campbell 1994; Koh & MacLeod 1999).
A variation of this strategy is demonstrated in aquatic species such as mink (Mustela vison) and muskrats (Ondatra zibethicus) that actively forage for brief bouts in cold water before retreating to a more moderate clime to consume and digest their meals (MacArthur 1979; Williams 1986). The thermal benefit of digestion thus comes from a potential build-up of heat prior to entering the water (MacArthur & Campbell 1994). These animals appear to use digestion to offset the effects of a later thermal challenge by creating a ‘thermal buffer’ to protect core temperatures, in a manner that does not affect total metabolism (MacArthur & Campbell 1994). Obviously, many marine mammals do not, or cannot, use a strategy of minimized thermal exposure, since they are obligatorily aquatic for at least part of the year.
MacArthur (1989) questioned whether the reliance on HIF for thermoregulation is efficient in light of alternative behavioural and physiological adaptations that are specifically aimed at maintaining thermoneutrality and coping with thermal demands. HIF may be an inefficient thermoregulatory mechanism compared to other mechanisms under conditions, where the requirements to maintain thermoneutrality are minimal. However, as previously demonstrated, environmental and physiological conditions can result in higher thermoregulatory demands that could require additional metabolic processes. Regardless, future studies of thermal substitution should try to include measures of both oxygen consumption and core body temperatures across a variety of environmental temperatures. In addition, studies may be more fruitful by investigating the conditions that define the extent of substitution which occurs in an individual animal rather than classifying species into those that do or do not exhibit thermal substitution.
(c) Circulatory conflicts
Vasoconstriction is observed during long dives in marine mammals and serves primarily to maintain blood pressure in light of bradycardia. Vasoconstriction also results in certain circulatory changes that may impact thermoregulation while foraging. This includes hypometabolism in certain tissues and decreased core temperatures in very long dives. This section speculates on the potential circulatory conflicts between thermoregulation and diving, and the potential limitation placed on foraging behaviour by over-riding thermoregulatory concerns.
In small cetaceans (e.g. dolphins), the primary thermoregulatory concern during activity appears to be promoting heat dissipation. Surface vessel systems at peripheral sites (flippers and flukes) assist in heat dissipation during exercise and cooled returning blood is used to cool temperature-sensitive internal organs. However, vasoconstriction associated with diving physiology may limit heat dissipation abilities (Whittow 1987). This may partly explain the increases in body temperature observed during swimming in some species (Hampton et al. 1971; Whittow et al. 1974; Hampton & Whittow 1976).
Several studies have examined how bottlenose dolphins manage the seemingly incongruent demands of increased circulation for heat dissipation from exercise and vasoconstriction to maximize dive times (figure 1 no. 10). These animals decrease heat flow to thermal windows during submergence and increase blood flow to peripheral sites after exercise, presumably in an effort to maximize heat dissipation rates (Noren et al. 1999). These changes in heat flow were mirrored by changes in heart rate (Williams et al. 1999). These studies suggest that the majority of heat dissipation is deferred until animals surface, thereby maximizing the oxygen-sparing mechanisms associated with diving. However, this deferment of heat dissipation is limited by the animal's ability to withstand the resulting increase in core temperatures during dives, as demonstrated in studies with (restrained) harbour seals (Phoca vitulina) (Hammel et al. 1977) and ducks (Johansen 1964).
In large cetaceans, the potential build-up of metabolic heat from muscular exercise would be particularly critical (Hokkanen 1990). The sheer allometry of exceedingly low surface area to volume ratios makes internal build-up of critical internal temperatures much more probable. Passive heat loss would be negligible in large cetaceans (Innes 1986); therefore, a greater emphasis would be placed on circulatory transport of excess heat to the surface (Hokkanen 1990). Large cetaceans have demonstrated the capacity to minimize heat build-up even during intense surface travel (Brodie & Paasche 1985), but it is unclear how circulatory changes during diving might interfere with this ability. It is also unclear whether all large cetaceans actually generate the anticipated amounts of heat in the manner of smaller cetaceans. Anatomical streamlining, adjustments in buoyancy and decreased relative speeds may prevent higher metabolic rates and the resulting thermal loads. In some cases, whales even possess particular circulatory adaptations designed to minimize heat loss during foraging (Heyning & Mead 1997).
In contrast to cetaceans, most studies of the thermoregulatory mechanisms of pinnipeds have investigated their methods for minimizing heat loss. This focus derives from their generally smaller body size (therefore, higher surface area to volume ratio) and tendency for polar distributions. Additionally, although the heat capacity of water is greater than air, the potential heat loss while hauled out in the winter is often greater than in the water (due to lower temperatures and higher convective heat loss).
Decreases in skin temperatures observed during periods of swimming and diving imply a change in circulation in agreement with vasoconstriction required to maintain blood pressure during bradycardia in many diving vertebrates (Boyd 2000). Vasoconstriction away from flippers—a major avenue of heat dissipation—serves both to maintain body temperature and to restrict aerobic metabolism, both of which extend foraging times (Gallivan & Ronald 1979; Willis et al. 2005). Although overheating can be a serious problem while on land for pinnipeds, the need for dumping heat during foraging has rarely been addressed. This is partly due to the smaller size of most pinnipeds and possibly due to their generally lower levels of heat generation during swimming and diving compared with cetaceans.
While it appears that the circulatory adjustments required to maximize dive times and minimize thermoregulatory costs in pinnipeds are complementary, this relationship is finite. The same level of bradycardia that minimizes heat loss from the flippers and skin also prevents transfer of muscle-generated heat to the core of the animal, potentially leading to hypothermia. Some pinnipeds may reduce the energy required to maintain body temperatures by reducing their defined core region (another by-product of vasoconstriction) or by lowering their defended body temperature set-point. However, such adjustments will only delay hypothermia. Therefore, longer dives may be restricted not by the ADL but by the animal's ability to maintain viable core body temperatures.
5. Summary
Mathematical optimal foraging models have been used to explore time-budgeting and diving behaviour of marine mammals in consideration of energy requirements and prey availability, distribution and quality (Boyd 1999; Arnould et al. 2001a; Thompson & Fedak 2001; Houston et al. 2003; Mori et al. 2005). Recently, models have begun to consider the full dynamic process of foraging (from behaviour through energy allocation), by individual marine mammals in variable environments (e.g. Dall & Boyd 2002; Matthiopoulos et al. 2005; Frid et al. 2006). This paper delineates and describes some of the physiological interactions that partly formulate foraging behaviour. Inclusion of these concepts may aid in the refinement of predictive foraging models. This paper also highlights areas where research is lacking, as an aid to defining and stimulating future research in these areas.
This paper specifically examined how the foraging behaviour of marine mammals may be physiologically limited by demands of prey acquisition (diving), prey processing (digestion) and thermoregulation. The ability of an animal to consume sufficient prey to meet its energy requirements is partly determined by its foraging abilities (e.g. available foraging time and diving capabilities) and digestive capacity (e.g. time devoted to digestion, maximum consumption capacity). Failure to consume sufficient prey will further impact foraging, thermoregulatory and digestive capacity through several interacting avenues. Catabolism of tissues to maintain energy balance will cause changes in the cost of thermoregulation and diving/swimming. A downward spiral of increased tissue catabolism to pay for increased energy costs may result. Conversely, the heat generated through digestion and foraging activity may help offset thermoregulatory costs. Finally, there may be trade-offs between the circulatory demands of diving, thermoregulation and digestion.
Observed foraging behaviour is an integration of a multitude of competing demands on an animal. While it is difficult to model or conduct research on all of these parameters, we can concentrate upon those that we deem most important. Physiological processes alone cannot explain diving behaviour, but they represent finite limits to an animal's abilities. For example, as noted by Castellini (1991), ‘while metabolic limits define the outer boundaries of diving, behavioural patterns place the diving animal at certain points inside that window’.
Acknowledgments
The authors would like to thank G. Worthy and D. Thompson for their useful comments and discussion, and Brian Fadely and an anonymous reviewer for their helpful suggestions. This work was partly based on research funded by the North Pacific Marine Science Foundation to the North Pacific Universities Marine Mammal Research Consortium.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Adamczewski J.Z, Flood P.F, Gunn A. Body composition of muskoxen (Ovibus moschatus) and its estimation from condition index and mass measurements. Can. J. Zool. 1995;73:2021–2034. [Google Scholar]
- Altmann S.A. University of Chicago Press; Chicago, IL: 1998. Foraging for survival: yearling baboons in Africa. [Google Scholar]
- Andrews R.D. Remotely releasable instruments for monitoring the foraging behaviour of pinnipeds. Mar. Ecol. Prog. Ser. 1998;175:289–294. [Google Scholar]
- Arnould J.P.Y, Boyd I.L, Rawlins D.R, Hindell M.A. Variation in maternal provisioning by lactating Antarctic fur seals (Arctocephalus gazella): response to experimental manipulation in pup demand. Behav. Ecol. Sociobiol. 2001a;50:461–466. doi:10.1007/s002650100386 [Google Scholar]
- Arnould J.P.Y, Green J.A, Rawlins D.R. Fasting metabolism in Antarctic fur seal (Arctocephalus gazella) pups. Comp. Biochem. Physiol. A. 2001b;129:829–841. doi: 10.1016/s1095-6433(01)00339-7. doi:10.1016/S1095-6433(01)00339-7 [DOI] [PubMed] [Google Scholar]
- Austin D, Bowen W.D, McMillan J.I, Boness D.J. Stomach temperature telemetry reveals temporal patters of foraging success in a free-ranging marine mammal. J. Anim. Ecol. 2006;75:408–420. doi: 10.1111/j.1365-2656.2006.01057.x. doi:10.1111/j.1365-2656.2006.01057.x [DOI] [PubMed] [Google Scholar]
- Beauplet G, Guinet C, Arnould J.P.Y. Body composition changes, metabolic fuel use, and energy expenditure during extended fasting in subantarctic fur seal (Arctocephalus tropicalis) pups at Amsterdam island. Physiol. Biochem. Zool. 2003;76:262–270. doi: 10.1086/367951. doi:10.1086/367951 [DOI] [PubMed] [Google Scholar]
- Beck C.A, Bowen W.D, Iverson S.J. Seasonal changes in buoyancy and diving behaviour of adult grey seals. J. Exp. Biol. 2000;203:2323–2330. doi: 10.1242/jeb.203.15.2323. [DOI] [PubMed] [Google Scholar]
- Bednekoff P.A, Houston A.I. Avian daily foraging patterns: effects of digestive constraints and variability. Evol. Ecol. 1994;8:36–52. doi:10.1007/BF01237664 [Google Scholar]
- Bevan R.M, Butler P.J. The effects of temperature on the oxygen consumption, heart rate, and deep body temperature during diving in the tufted duck Aythya fuligula. J. Exp. Biol. 1992;163:139–151. [Google Scholar]
- Biuw M, McConnell B, Bradshaw C.J.A, Burton H, Fedak M. Blubber and buoyancy: monitoring the body condition of free-ranging seals using simple dive characteristics. J. Exp. Biol. 2003;206:3405–3423. doi: 10.1242/jeb.00583. doi:10.1242/jeb.00583 [DOI] [PubMed] [Google Scholar]
- Blaxter K. Cambridge University Press; Cambridge, UK: 1989. Energy metabolism in animals and man. [Google Scholar]
- Boily P. Theoretical heat flux in water and habitat selection of phocid seals and beluga whales during the annual molt. J. Theor. Biol. 1995;172:235–244. doi:10.1006/jtbi.1995.0020 [Google Scholar]
- Boyd I.L. Foraging and provisioning in Antarctic fur seals: interannual variability in time–energy budgets. Behav. Ecol. 1999;10:198–208. doi:10.1093/beheco/10.2.198 [Google Scholar]
- Boyd I.L. Skin temperatures during free-ranging swimming and diving in Antarctic fur seals. J. Exp. Biol. 2000;203:1907–1914. doi: 10.1242/jeb.203.12.1907. [DOI] [PubMed] [Google Scholar]
- Boyd I.L, Duck C.D. Mass changes and metabolism in territorial male Antarctic seals (Arctocephalus gazella) Physiol. Zool. 1991;64:375–392. [Google Scholar]
- Brodie P.F. Cetacean energetics, an overview of intraspecific size variation. Ecology. 1975;56:152–161. doi:10.2307/1935307 [Google Scholar]
- Brodie P, Paasche A. Thermoregulation and energetics of fin and sei whales based on postmortem, stratified temperature measurements. Can. J. Zool. 1985;63:2267–2269. [Google Scholar]
- Buddington R.K, Diamond J.M. Ontogenetic development of intestinal nutrient transporters. Annu. Rev. Physiol. 1989;51:601–619. doi: 10.1146/annurev.ph.51.030189.003125. doi:10.1146/annurev.ph.51.030189.003125 [DOI] [PubMed] [Google Scholar]
- Campbell K.L, MacArthur R.A. Nutrition and the energetic tactics of muskrats (Ondatra zibethicus): morphological and metabolic adjustments to seasonal shifts in diet quality. Can. J. Zool. 1998;76:163–174. doi:10.1139/cjz-76-1-163 [Google Scholar]
- Campbell K.L, McIntyre I.W, MacArthur R.A. Postprandial heat increment does not substitute for active thermogenesis in cold-challenged star-nosed moles (Condylura cristata) J. Exp. Biol. 2000;203:301–310. doi: 10.1242/jeb.203.2.301. [DOI] [PubMed] [Google Scholar]
- Castellini M.A. The biology of diving mammals: behavioral, physiological, and biochemical limits. Adv. Comp. Environ. Physiol. 1991;8:105–134. [Google Scholar]
- Castellini M.A, Rea L.D. The biochemistry of natural fasting at its limits. Experientia. 1992;48:575–582. doi: 10.1007/BF01920242. doi:10.1007/BF01920242 [DOI] [PubMed] [Google Scholar]
- Castellini M.A, Kooyman G.L, Ponganis P.J. Metabolic rates of freely diving Weddell seals: correlations with oxygen stores, swim velocity and diving duration. J. Exp. Biol. 1992;165:181–194. doi: 10.1242/jeb.165.1.181. [DOI] [PubMed] [Google Scholar]
- Chappell M.A, Bachman G.C, Hammond K.A. The heat increment of feeding in house wren chicks: magnitude, duration, and substitution for thermostatic costs. J. Comp. Physiol. B. 1997;167:313–318. doi:10.1007/s003600050079 [Google Scholar]
- Charnov E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. doi:10.1016/0040-5809(76)90040-X [DOI] [PubMed] [Google Scholar]
- Cherel Y, Heitz A, Calgari C, Robin J.-P, Le Maho Y. Relationships between lipid availability and protein utilization during prolonged fasting. J. Comp. Physiol. B. 1992;162:305–313. doi: 10.1007/BF00260757. doi:10.1007/BF00260757 [DOI] [PubMed] [Google Scholar]
- Corkeron P.J, Connor R.C. Why do baleen whales migrate? Mar. Mamm. Sci. 1999;15:1228–1245. doi:10.1111/j.1748-7692.1999.tb00887.x [Google Scholar]
- Costa D.P, Kooyman G.L. Oxygen consumption, thermoregulation, and the effect of fur oiling and washing on the sea otter, Enhydra lutris. Can. J. Zool. 1982;60:2761–2767. [Google Scholar]
- Costa D.P, Kooyman G.L. Contribution of specific dynamic action to heat balance and thermoregulation in the sea otter Enhydra lutris. Physiol. Zool. 1984;57:199–203. [Google Scholar]
- Costa D.P, Gales N.J, Goebel M.E. Aerobic dive limit: how often does it occur in nature? Comp. Biochem. Physiol. A. 2001;129:771–783. doi: 10.1016/s1095-6433(01)00346-4. doi:10.1016/S1095-6433(01)00346-4 [DOI] [PubMed] [Google Scholar]
- Costello R.R, Whittow G.C. Oxygen cost of swimming in a trained California sea lion. Comp. Biochem. Physiol. A. 1975;50:645–647. doi: 10.1016/0300-9629(75)90120-6. doi:10.1016/0300-9629(75)90120-6 [DOI] [PubMed] [Google Scholar]
- Crocker D.E, LeBoeuf B.J, Costa D.P. Drift diving in female northern elephant seals: implications for food processing. Can. J. Zool. 1997;75:27–39. [Google Scholar]
- Croll D.A, Acevedo-Gutierrez A, Tershy B.R, Urban-Ramirez J. The diving behavior of blue and fin whales: is dive duration shorter than expected based on oxygen stores? Comp. Biochem. Physiol. A. 2001;129:797–809. doi: 10.1016/s1095-6433(01)00348-8. [DOI] [PubMed] [Google Scholar]
- Dall S.R.X, Boyd I.L. Provisioning under the risk of starvation. Evol. Ecol. Res. 2002;4:883–896. [Google Scholar]
- Davis R.W, Polasek L, Watson R, Fuson A, Williams T.M, Kanatous S.B. The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comp. Biochem. Physiol. A. 2004;138:263–268. doi: 10.1016/j.cbpb.2004.05.003. doi:10.1016/j.cbpb.2004.05.003 [DOI] [PubMed] [Google Scholar]
- de Leeuw J.J, Butler P.J, Woakes A.J, Zegwaard F. Body cooling and its energetic implications for feeding and diving of tufted ducks. Physiol. Zool. 1998;71:720–730. doi: 10.1086/516003. [DOI] [PubMed] [Google Scholar]
- Debray L, Le Huerou-Luron I, Gidenne T, Fortun-Lamothe L. Digestive tract development in rabbit according to the dietary energetic source: correlation between whole tract digestion, pancreatic and intestinal enzymatic activities. Comp. Biochem. Physiol. A. 2003;135:443–455. doi: 10.1016/s1095-6433(03)00112-0. doi:10.1016/S1095-6433(03)00112-0 [DOI] [PubMed] [Google Scholar]
- Donohue M.J, Costa D.P, Goebel M.E, Baker J.D. The ontogeny of metabolic rate and thermoregulatory capabilities of northern fur seal, Callorhinus ursinus, pups in air and water. J. Exp. Biol. 2000;203:1003–1016. doi: 10.1242/jeb.203.6.1003. [DOI] [PubMed] [Google Scholar]
- Dunkin R.C, McLellan W.A, Blum J.E, Pabst D.A. The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J. Exp. Biol. 2005;208:1469–1480. doi: 10.1242/jeb.01559. doi:10.1242/jeb.01559 [DOI] [PubMed] [Google Scholar]
- Eastman J.T, Coalson R.E. The digestive system of the Weddell seal Leptonychotes weddelli—a review. In: Harrison R.J, editor. Functional anatomy of marine mammals. vol. 2. Academic Press; London, UK: 1974. pp. 253–320. [Google Scholar]
- Frid A, Baker G.G, Dill L.M. Do resource declines increase predation rates on North Pacific harbor seals? A behavior-based plausibility model. Mar. Ecol. Prog. Ser. 2006;312:265–275. [Google Scholar]
- Gallivan G.J, Ronald K. Temperature regulation in freely diving harp seals (Phoca groenlandica) Can. J. Zool. 1979;57:2256–2263. doi: 10.1139/z79-293. [DOI] [PubMed] [Google Scholar]
- Girardier L, Clark M.G, Seydoux J. Thermogenesis associated with spontaneous activity: an important component of thermoregulatory needs in rats. J. Physiol. 1995;488:779–787. doi: 10.1113/jphysiol.1995.sp021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.A, Halsey L.G, Butler P.J. To what extent is the foraging behaviour of aquatic birds constrained by their physiology? Physiol. Biochem. Zool. 2005;78:766–781. doi: 10.1086/432423. doi:10.1086/432423 [DOI] [PubMed] [Google Scholar]
- Hammel H.T, Elsner R.W, Heller H.C, Maggert J.A, Bainton C.R. Thermoregulatory responses to altering hypothalamic temperature in the harbor seal. Am. J. Physiol. 1977;232:R18–R26. doi: 10.1152/ajpregu.1977.232.1.R18. [DOI] [PubMed] [Google Scholar]
- Hampton I.F.G, Whittow G.C. Body temperature and heat exchange in the Hawaiian spinner dolphin, Stenella longirostris. Comp. Biochem. Physiol. A. 1976;55:195–197. doi: 10.1016/0300-9629(76)90092-x. doi:10.1016/0300-9629(76)90092-X [DOI] [PubMed] [Google Scholar]
- Hampton I.F.G, Whittow G.C, Szekerczes J, Rutherford S. Heat transfer and body temperature in the Atlantic bottlenosed dolphin, Tursiops truncatus. Int. J. Biochim. Bionet. 1971;15:247–253. doi: 10.1007/BF01803907. [DOI] [PubMed] [Google Scholar]
- Hansen S, Lavigne D.M, Innes S. Energy metabolism and thermoregulation in juvenile harbor seals (Phoca vitulina) in air. Physiol. Zool. 1995;68:290–315. [Google Scholar]
- Harding K.C, Fujiwara M, Axberg Y, Harkonen T. Mass-dependent energetics and survival in Harbour seal pups. Funct. Ecol. 2005;19:129–135. doi:10.1111/j.0269-8463.2005.00945.x [Google Scholar]
- Hastie G.D, Rosen D.A.S, Trites A.W. Reductions in oxygen consumption during dives and estimated submergence limitations of Steller sea lions (Eumetopias jubatus) Mar. Mamm. Sci. 2007;23:272–286. doi:10.1111/j.1748-7692.2007.00118.x [Google Scholar]
- Hays G.C, Metcalfe J.D, Walne A.W. The implications of lung-regulated buoyancy control for dive depth and duration. Ecology. 2004;85:1137–1145. [Google Scholar]
- Heyning J.E, Mead J.G. Thermoregulation in the mouths of feeding gray whales. Science. 1997;278:1138–1139. doi: 10.1126/science.278.5340.1138. doi:10.1126/science.278.5340.1138 [DOI] [PubMed] [Google Scholar]
- Hill R.D, et al. Heart rate and body temperature during free diving of Weddell seals. Am. J. Physiol. 1987;253:R344–R351. doi: 10.1152/ajpregu.1987.253.2.R344. [DOI] [PubMed] [Google Scholar]
- Hind A.T, Gurney W.S.C. The metabolic cost of swimming in marine homeotherms. J. Exp. Biol. 1997;200:531–542. doi: 10.1242/jeb.200.3.531. [DOI] [PubMed] [Google Scholar]
- Hokkanen J.E.I. Temperature regulation of marine mammals. J. Theor. Biol. 1990;145:465–486. doi: 10.1016/s0022-5193(05)80482-5. doi:10.1016/S0022-5193(05)80482-5 [DOI] [PubMed] [Google Scholar]
- Hooker S.K, Boyd I.L, Jessopp M, Cox O, Blackwell J, Boveng P.L, Bengston J.L. Monitoring the prey-field of marine predators: combining digital imaging with datalogging tags. Mar. Mamm. Sci. 2002;18:680–697. doi:10.1111/j.1748-7692.2002.tb01066.x [Google Scholar]
- Hooker S.K, Miller P.J.O, Johnson M.P, Cox O.P, Boyd I.L. Ascent exhalations of Antarctic fur seals: a behavioural adaptation for breath-hold diving? Proc. R. Soc. B. 2005;272:355–363. doi: 10.1098/rspb.2004.2964. doi:10.1098/rspb.2004.2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes L.A, Rea L.D, Rosen D.A.S, Worthy G.A.J. Symp. Comparative Nutrition Society, Hickory Corners, MI, 14–19 July 2004. Comparative Nutrition Society; Silver Spring, MD: 2004. Effects of body condition on resting metabolism in captive and free-ranging Steller sea lions (Eumetopias jubatus) pp. 79–82. [Google Scholar]
- Houston A.I, Carbone C. The optimal allocation of time during the diving cycle. Behav. Ecol. 1992;3:255–265. doi:10.1093/beheco/3.3.255 [Google Scholar]
- Houston A.I, McNamara J.M, Heron J.E, Zoltan B. The effect of foraging parameters on the probability that a dive is successful. Proc. R. Soc. B. 2003;270:2451–2455. doi: 10.1098/rspb.2003.2540. doi:10.1098/rspb.2003.2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J.A, Costa D.P. Standard metabolic rate at the surface and during trained submersions in adult California sea lions (Zalophus californianus) J. Exp. Biol. 2001;204:3273–3281. doi: 10.1242/jeb.204.19.3273. [DOI] [PubMed] [Google Scholar]
- Innes S. How fast should a dead whale cool? Can. J. Zool. 1986;64:2064–2065. [Google Scholar]
- Irving L, Hart J.S. The metabolism and insulation of seals as bare-skinned mammals in cold water. Can. J. Zool. 1957;35:497–511. [Google Scholar]
- Irving L, Solandt O.M, Solandt D.Y. The respiratory metabolism of the seal and its adjustment to diving. J. Cell. Comp. Physiol. 1935;7:137–151. doi:10.1002/jcp.1030070109 [Google Scholar]
- Jensen P.G, Pekins P.J, Holter J.B. Compensatory effect of the heat increment of feeding on thermoregulation costs of white-tailed deer fawns in winter. Can. J. Zool. 1999;77:1474–1485. doi:10.1139/cjz-77-9-1474 [Google Scholar]
- Jobling M. Towards an explanation of specific dynamic action (SDA) J. Fish. Biol. 1983;23:549–555. doi:10.1111/j.1095-8649.1983.tb02934.x [Google Scholar]
- Johansen K. Regional distribution of circulating blood during submersion asphyxia in the duck. Acta Physiol. Scand. 1964;62:1–9. doi: 10.1111/j.1748-1716.1964.tb03945.x. [DOI] [PubMed] [Google Scholar]
- Karasov W.H, Diamond J. Interplay between physiology and ecology in digestion. BioScience. 1988;38:602–611. doi:10.2307/1310825 [Google Scholar]
- Kaseloo P.A, Lovvorn J.R. Effects of surface activity patterns and dive depth on thermal substitution in fasted and fed lesser scaup (Aythya affinis) ducks. Can. J. Zool. 2005;83:301–311. doi:10.1139/z05-012 [Google Scholar]
- Kaseloo P.A, Lovvorn J.R. Substitution of heat from exercise and digestion by ducks diving for mussels at varying depths and temperatures. J. Comp. Physiol. B. 2006;176:265–275. doi: 10.1007/s00360-005-0047-6. doi:10.1007/s00360-005-0047-6 [DOI] [PubMed] [Google Scholar]
- Kastelein R.A, Ford J, Berghout E, Wiepkema P.R, van Boxsel M. Food consumption, growth and reproduction of belugas (Delphinapterus leucas) in human care. Aquat. Mammal. 1994;20:81–87. [Google Scholar]
- Kastelein R.A, Kershaw J, Berghout E, Wiepkema P.R. The food consumption of South American sea lions (Otaria flavescens) Aquat. Mammal. 1995;21:43–53. [Google Scholar]
- Kastelein R.A, Vaughan N, Wiepkema P.R. The food consumption of Steller sea lions (Eumetopias jubatus) Aquat. Mammal. 1990a;15:137–144. [Google Scholar]
- Kastelein R.A, Wiepkema P.R, Vaughan N. The food consumption of grey seals (Halichoerus grypus) in human care. Aquat. Mammal. 1990b;15:171–180. [Google Scholar]
- Kenyon K.W. In: Sea otter—Enhydra lutris. Handbook of marine mammals. Ridgway S.H, Harrison R.J, editors. vol. 1. Academic Press; London, UK: 1981. pp. 209–223. [Google Scholar]
- Kersten M, Visser W. The rate of food processing in the oystercatcher: food intake and energy expenditure constrained by a digestive bottleneck. Funct. Ecol. 1996;10:440–448. doi:10.2307/2389936 [Google Scholar]
- Kirkwood J.K. A limit to metabolisable energy intake in mammals and birds. Comp. Biochem. Physiol. A. 1983;75:1–3. doi: 10.1016/0300-9629(83)90033-6. doi:10.1016/0300-9629(83)90033-6 [DOI] [PubMed] [Google Scholar]
- Klaassen M, Bech C, Slagsvold G. Basal metabolic rate and thermal conductance in Arctic tern chicks and the effect of heat increment of feeding on thermoregulatory expenses. Ardea. 1989;77:193–200. [Google Scholar]
- Kleiber M. Robert E. Krieger Publisher Co; New York, NY: 1975. The fire of life: an introduction to animal energetics. [Google Scholar]
- Koh K, MacLeod M.G. Effects of ambient temperature on heat increment of feeding and energy retention in growing broilers maintained at different food intakes. Br. Poultry Sci. 1999;40:511–516. doi: 10.1080/00071669987287. doi:10.1080/00071669987287 [DOI] [PubMed] [Google Scholar]
- Koopman H.N, Pabst D.A, McLellan W.A, Dillaman R.M, Read A.J. Changes in blubber distribution and morphology associated with starvation in the harbor porpoise (Phocoena phocoena): evidence for regional differences in blubber structure and function. Physiol. Biochem. Zool. 2002;75:498–512. doi: 10.1086/342799. doi:10.1086/342799 [DOI] [PubMed] [Google Scholar]
- Kooyman G.L. Diving physiology. In: Perrin W.F, Wursig B, Thewissen J.G.M, editors. Encyclopedia of marine mammals. Academic Press; San Diego, CA: 2002. pp. 339–344. [Google Scholar]
- Kooyman G.L, Wahrenbrock E.A, Castellini M.A, Davis R.W, Sinnett E.E. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: evidence of preferred pathways from blood chemistry and behavior. J. Comp. Physiol. A. 1980;138:335–346. [Google Scholar]
- Kooyman G.L, Castellini M.A, Davis R.W. Physiology of diving in marine mammals. Annu. Rev. Physiol. 1981;43:343–356. doi: 10.1146/annurev.ph.43.030181.002015. doi:10.1146/annurev.ph.43.030181.002015 [DOI] [PubMed] [Google Scholar]
- Kooyman G.L, Castellini M.A, Davis R.W, Maue R.A. Aerobic diving limits of immature Weddell seals. J. Comp. Physiol. 1983;151:171–174. [Google Scholar]
- Kramer D.L. The behavioral ecology of air breathing by aquatic mammals. Can. J. Zool. 1988;66:89–94. [Google Scholar]
- Krockenberger M.B, Bryden M.M. Rate of passage through the alimentary tract of southern elephant seals (Mirounga leonina) (Carnovira: Phocidae) J. Zool. (Lond.) 1994;234:229–237. [Google Scholar]
- Kruuk H, Balharry E, Taylor P.T. Oxygen consumption of the Eurasian otter Lutra lutra in relation to water temperature. Physiol. Zool. 1994;67:1174–1185. [Google Scholar]
- Kumagai S, Rosen D.A.S, Trites A.W. Body mass and composition responses to short-term low energy intake are seasonally dependent in Steller sea lions (Eumetopias jubatus) J. Comp. Physiol. B. 2006;176:589–598. doi: 10.1007/s00360-006-0082-y. doi:10.1007/s00360-006-0082-y [DOI] [PubMed] [Google Scholar]
- Kvadsheim P.H, Gotaas A.R.L, Folkow L.P, Blix A.S. An experimental validation of heat loss models for marine mammals. J. Theor. Biol. 1997;184:15–23. doi:10.1006/jtbi.1996.0256 [Google Scholar]
- Kvist A, Lindstrom A. Gluttony in migratory waders—unprecedented energy assimilation rates in vertebrates. Oikos. 2003;103:397–402. doi:10.1034/j.1600-0706.2003.12259.x [Google Scholar]
- Lavigne D.M, Barchard W, Innes S, Øritsland N.A. Pinniped bioenergetics. Mammals in the seas. vol. IV. FAO; Rome, Italy: 1982. pp. 191–235. [Google Scholar]
- Leader-Williams N, Ricketts C. Seasonal and sexual patterns of growth and condition of reindeer introduced into South Georgia. Oikos. 1982;38:27–39. doi:10.2307/3544564 [Google Scholar]
- Liao, J. A. 1990 An investigation of the effect of water temperature on the metabolic rate of the California sea lion (Zalophus californianus). MSc thesis Dept. of Biology, p. 55: University of California at Santa Cruz.
- Lockyer C. Seasonal changes in body fat condition of northeast Atlantic pilot whales, and their biological significance. In: Donovan G.P, Lockyer C.H, Martin A.R, editors. Biology of northern hemisphere pilot whales. vol. special issue 14. International Whaling Commission; Cambridge, UK: 1993. pp. 325–350. [Google Scholar]
- Lockyer C.H, McConnell L.C, Waters T.D. Body condition in terms of anatomical and biochemical assessment of body fat in north Atlantic fin and sei whales. Can. J. Zool. 1985;63:2328–2338. [Google Scholar]
- Lovvorn J.R, Jones D.R. Effects of body size, body-fat, and change in pressure with depth on buoyancy and costs of diving in ducks (Aythya spp.) Can. J. Zool. 1991;69:2879–2887. [Google Scholar]
- MacArthur R.A. Seasonal patterns of body temperature and activity in free-ranging muskrats (Ondatra zibethicus) Can. J. Zool. 1979;57:25–33. [Google Scholar]
- MacArthur R.A. Aquatic mammals in cold. In: Wang L.C.H, editor. Advances in comparative and environmental physiology. vol. 4. Springer; Berlin, Germany: 1989. pp. 289–325. [Google Scholar]
- MacArthur R.A, Campbell K.L. Heat increment of feeding and its thermoregulatory benefit in the muskrat (Ondatra zibethicus) J. Comp. Physiol. B. 1994;164:141–146. doi: 10.1007/BF00301656. doi:10.1007/BF00301656 [DOI] [PubMed] [Google Scholar]
- Maloney S.K, Bronner G.N, Buffenstein R. Thermoregulation in the Angolan free-tailed bat Mops condylurus: a small mammal that uses hot roosts. Physiol. Biochem. Zool. 1999;72:385–396. doi: 10.1086/316677. doi:10.1086/316677 [DOI] [PubMed] [Google Scholar]
- Masman D, Daan S, Dietz M. Heat increment of feeding in the kestrel, Falco tinnunculus, and its natural seasonal variation. In: Bech C, Reinertsen R.E, editors. Physiology of cold adaptation in birds. Plenum Press; New York, NY: 1988. pp. 123–135. [Google Scholar]
- Matthiopoulos, J., Thompson, D. & Boyd, I. L. 2005 Implications of varying food distributions for fitness in Steller sea lions. Contract report for U.S. National Marine Fisheries Service, Steller Sea Lion Research Initiative contract number NA17FX1431. St Andrews, UK: Sea Mammal Research Unit, University of St Andrews.
- McGinnis S.M, Southworth T.P. Body temperature fluctuations in the northern elephant seal. J. Mammal. 1967;48:484–485. doi:10.2307/1377793 [Google Scholar]
- McNamara J.M, Ekman J, Houston A.I. The effect of thermoregulatory substitution on optimal energy reserves of small birds in winter. Oikos. 2004;105:192–196. doi:10.1111/j.0030-1299.2004.12188.x [Google Scholar]
- Miller P.J.O, Johnson M.P, Tyack P.L, Terray E.A. Swimming gaits, passive drag and buoyancy of diving sperm whales Physeter macrocephalus. J. Exp. Biol. 2004;207:1953–1967. doi: 10.1242/jeb.00993. doi:10.1242/jeb.00993 [DOI] [PubMed] [Google Scholar]
- Moore M.J, Early G.A. Cumulative sperm whale bone damage and the bends. Science. 2004;306:2215. doi: 10.1126/science.1105452. doi:10.1126/science.1105452 [DOI] [PubMed] [Google Scholar]
- Mori Y, Watanabe Y, Mitani Y, Sato K, Cameron M.F, Naito Y. A comparison of prey richness estimates for Weddell seals using diving profiles and image data. Mar. Ecol. Prog. Ser. 2005;295:257–263. [Google Scholar]
- Morrison P, Rosenmann M, Estes J.A. Metabolism and thermoregulation in the sea otter. Physiol. Zool. 1974;47:218–229. [Google Scholar]
- Muelbert M.M.C, Bowen W.D. Duration of lactation and postweaning changes in mass and body composition of harbour seal, Phoca vitulina, pups. Can. J. Zool. 1993;71:1405–1414. [Google Scholar]
- Naess A. Seasonal variation in body condition and muscular lipid contents in Northeast Atlantic minke whale Balaenoptera acutorosrata. Sarsia. 1998;83:211–218. [Google Scholar]
- Noren D.P. Thermoregulation of weaned northern elephant seal (Mirounga angustirostris) pups in air and water. Physiol. Biochem. Zool. 2002;75:513–523. doi: 10.1086/342254. doi:10.1086/342254 [DOI] [PubMed] [Google Scholar]
- Noren D.P, Mangel M. Energy reserve allocation in fasting northern elephant seal pups: inter-relationships between body condition and fasting duration. Funct. Ecol. 2004;18:233–242. doi:10.1111/j.0269-8463.2004.00840.x [Google Scholar]
- Noren D.P, Williams T.M, Berry P, Butler E. Thermoregulation during swimming and diving in bottlenose dolphins, Tursiops truncatus. J. Comp. Physiol. B. 1999;169:93–99. doi: 10.1007/s003600050198. doi:10.1007/s003600050198 [DOI] [PubMed] [Google Scholar]
- Noren D.P, Crocker D.E, Williams T.M, Costa D.P. Energy reserve utilization in northern elephant seal (Mirounga angustirostris) pups during the postweaning fast: size does matter. J. Comp. Physiol. B. 2003;173:443–454. doi: 10.1007/s00360-003-0353-9. doi:10.1007/s00360-003-0353-9 [DOI] [PubMed] [Google Scholar]
- Nowacek D.P, Johnson M.P, Tyack P.L, Shorter K.A, McLellan W.A, Pabst D.A. Buoyant balaenids: the ups and downs of buoyancy in right whales. Proc. R. Soc. B. 2001;268:1811–1816. doi: 10.1098/rspb.2001.1730. doi:10.1098/rspb.2001.1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata C.A, Matsuura D, Whittow G.C, Tinker S.W. Diurnal rhythm of body temperature in the Hawaiian monk seal (Monachus schauinslandi) Pac. Sci. 1972;26:117–120. [Google Scholar]
- Øritsland N.A. Starvation survival and body composition in mammals with particular reference to Homo sapiens. Bull. Math. Biol. 1990;52:643–655. doi: 10.1016/s0092-8240(05)80371-4. doi:10.1016/S0092-8240(05)80371-4 [DOI] [PubMed] [Google Scholar]
- Pabst D.A, Rommel S.A, McLennan W.A. The functional morphology of marine mammals. In: Reynolds J.E, Rommel S.A, editors. Biology of marine mammals. Smithsonian Institute Press; Washington, DC: 1999. pp. 15–72. [Google Scholar]
- Page B, McKenzie J, Hindell M.A, Goldsworthy S.D. Drift dives by male New Zealand fur seals (Arctocephalus forsteri) Can. J. Zool. 2005;83:293–300. doi:10.1139/z05-013 [Google Scholar]
- Parry D.A. The structure of whale blubber and a discussion of its thermal properties. Q. J. Micro. Sci. 1949;90:13–26. [PubMed] [Google Scholar]
- Peters R.H. Cambridge University Press; Cambridge, UK: 1983. The ecological implications of body size. [Google Scholar]
- Peterson C.C, Nagy K.A, Diamond J. Sustained metabolic scope. Proc. Natl Acad. Sci. USA. 1990;8:2324–2328. doi: 10.1073/pnas.87.6.2324. doi:10.1073/pnas.87.6.2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher K.W, Calkins D.G, Pendleton G.W. Steller sea lion condition indices. Mar. Mamm. Sci. 2000;16:427–436. doi:10.1111/j.1748-7692.2000.tb00934.x [Google Scholar]
- Pond C.M. Morphological aspects and ecological and mechanical consequences of fat deposition in wild vertebrates. Annu. Rev. Ecol. Syst. 1978;9:519–570. doi:10.1146/annurev.es.09.110178.002511 [Google Scholar]
- Pyke G.H. Optimal foraging theory—a critical review. Annu. Rev. Ecol. Syst. 1984;15:523–575. doi:10.1146/annurev.es.15.110184.002515 [Google Scholar]
- Rashotte M.E, Saarela S, Henderson R.P, Hohtola E. Shivering and digestion-related thermogenesis in pigeons during dark phase. Am. J. Physiol. 1999;227:R1579–R1587. doi: 10.1152/ajpregu.1999.277.6.R1579. [DOI] [PubMed] [Google Scholar]
- Rea, L. D., Rosen, D. A. S. & Trites, A. W. 1999 Seasonal differences in adaptation to prolonged fasting in juvenile Steller sea lions (Eumetopias jubatus). In Federation of American societies of experimental biology, vol. 13, pp. A740. Washington, DC.
- Rea L.D, Rosen D.A.S, Trites A.W. Utilization of stored energy reserves during fasting varies by age and season in Steller sea lions. Can. J. Zool. 2007;85:190–200. [Google Scholar]
- Renouf D, Gales R, Noseworthy E. Seasonal variation in energy intake and condition of harp seals: is there a harp seal morph? Problems for bioenergetic modelling. J. Zool. (Lond.) 1993;230:513–528. [Google Scholar]
- Robbins C.T. Academic Press; San Diego, CA: 1993. Wildlife feeding and nutrition. [Google Scholar]
- Roscow, E. 2001 Thermoregulation in Steller sea lions: a modelling approach. MSc thesis. College Station, TX: Texas A&M University.
- Rosen D.A.S, Trites A.W. Heat increment of feeding in Steller sea lions, Eumetopias jubatus. Comp. Biochem. Physiol. A. 1997;118:877–881. doi: 10.1016/s0300-9629(97)00039-x. doi:10.1016/S0300-9629(97)00039-X [DOI] [PubMed] [Google Scholar]
- Rosen D.A.S, Trites A.W. No evidence for bioenergetic interaction between digestion and thermoregulation in Steller sea lions, Eumetopias jubatus. Physiol. Biochem. Zool. 2003;76:899–906. doi: 10.1086/378140. doi:10.1086/378140 [DOI] [PubMed] [Google Scholar]
- Rosen D.A.S, Trites A.W. Satiation and compensation for short-term changes in food quality and availability in young Steller sea lions (Eumetopias jubatus) Can. J. Zool. 2004;82:1061–1069. doi:10.1139/z04-082 [Google Scholar]
- Rosen D.A.S, Trites A.W. Examining the potential for nutritional stress in Steller sea lions: physiological effects of prey composition. J. Comp. Physiol. B. 2005;175:265–273. doi: 10.1007/s00360-005-0481-5. doi:10.1007/s00360-005-0481-5 [DOI] [PubMed] [Google Scholar]
- Rubner M. Franz Deuticke; Leipzig, Germany: 1902. Die Gesetz des Energieverbrauchs bei der Ernahrung. [Google Scholar]
- Rutishauser M.R, Costa D.P, Goebel M.E, Williams T.M. Ecological implications of body composition and thermal capabilities in young Antarctic fur seals (Arctocephalus gazella) Physiol. Biochem. Zool. 2004;77:669–681. doi: 10.1086/421749. doi:10.1086/421749 [DOI] [PubMed] [Google Scholar]
- Sato K, Naito Y, Kato A, Niizuma Y, Watanuki Y, Charrassin J.B, Bost C.A, Handrich Y, Le Maho Y. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 2002;205:1189–1197. doi: 10.1242/jeb.205.9.1189. [DOI] [PubMed] [Google Scholar]
- Sato K, Mitani Y, Cameron M.F, Siniff D.B, Naito Y. Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J. Exp. Biol. 2003;206:1461–1470. doi: 10.1242/jeb.00265. doi:10.1242/jeb.00265 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, UK: 1984. Scaling: why is animal size so important? [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, UK: 1997. Animal physiology: adaptation and environment. [Google Scholar]
- Scholander P.F. Hvalradets Skrifter-scientific results of marine biological research. Det Norske Videnskaps-Akademi i Oslo; Oslo, Norway: 1940. Experimental investigations on the respiratory function in diving mammals and birds. [Google Scholar]
- Scholander P.F. Animals in aquatic environments: diving mammals and birds. In: Dill D.B, editor. Handbook of physiology. Section 4. Adaptation to the environment. Williams & Wilkins; Baltimore, MD: 1964. pp. 729–739. [Google Scholar]
- Scholander P.F, Irving L, Grinnell S.W. On the temperature and metabolism of the seal during diving. J. Cell Comp. Physiol. 1942;19:67–78. doi:10.1002/jcp.1030190107 [Google Scholar]
- Scholander P.F, Walters V, Hock R, Irving L. Body insulation of some Arctic and tropical mammals and birds. Biol. Bull. 1950;99:225–236. doi: 10.2307/1538740. doi:10.2307/1538740 [DOI] [PubMed] [Google Scholar]
- Simek V. Specific dynamic action of a high-protein diet and its significance for thermoregulation in the golden hamster. Physiologia Bohemoslovaca. 1975;24:421–424. [PubMed] [Google Scholar]
- Skrovan R.C, Williams T.M, Berry P.S, Moore P.W, Davis R.W. The diving physiology of bottlenose dolphins (Tursiops truncatus)—II. Biomechanics and changes in buoyancy at depth. J. Exp. Biol. 1999;202:2749–2761. doi: 10.1242/jeb.202.20.2749. [DOI] [PubMed] [Google Scholar]
- Sparling C.E, Fedak M. Metabolic rates of captive grey seals during voluntary diving. J. Exp. Biol. 2004;207:1615–1624. doi: 10.1242/jeb.00952. doi:10.1242/jeb.00952 [DOI] [PubMed] [Google Scholar]
- Stephens D.W, Krebs J.R. Princeton University Press; Princeton, NJ: 1986. Foraging theory. [Google Scholar]
- Thompson D, Fedak M.A. How long should a dive last? A simple model of foraging decisions by breath-hold divers in a patchy environment. Anim. Behav. 2001;61:287–296. doi:10.1006/anbe.2000.1539 [Google Scholar]
- Tierney M, Hindell M.A, Lea M.-A, Tollit D. A comparison of techniques used to estimate body condition of southern elephant seals (Mirounga leonina) Wildl. Res. 2001;28:581–588. doi:10.1071/WR00066 [Google Scholar]
- Tollit D.J, Wong M, Winship A.J, Rosen D.A.S, Trites A.W. Quantifying errors associated with using prey skeletal structures from fecal samples to determine the diet of the Steller sea lion (Eumetopias jubatus) Mar. Mamm. Sci. 2003;19:724–744. doi:10.1111/j.1748-7692.2003.tb01127.x [Google Scholar]
- Trumble S.J, Barboza P.S, Castellini M.A. Digestive constraints on an aquatic carnivore: effects of feeding frequency and prey composition on harbor seals. J. Comp. Physiol. B. 2003;173:501–509. doi: 10.1007/s00360-003-0358-4. doi:10.1007/s00360-003-0358-4 [DOI] [PubMed] [Google Scholar]
- Van Gils J.A, De Rooij S.R, Van Belle J, Van der Meer J, Dekinga A, Piersma T, Drent R. Digestive bottleneck affects foraging decisions in red knots Calidris canutus. I. Prey choice. J. Anim. Ecol. 2005;74:105–119. doi:10.1111/j.1365-2656.2004.00903.x [Google Scholar]
- Watts P, Hansen P, Lavigne D.M. Models of heat loss by marine mammals: thermoregulation below the zone of irrelevance. J. Theor. Biol. 1993;163:505–525. doi:10.1006/jtbi.1993.1135 [Google Scholar]
- Webb P.M, Crocker D.E, Blackwell S.B, Costa D.P, Le Boeuf B.J. Effects of buoyancy on the diving behavior of northern elephant seals. J. Exp. Biol. 1998;201:2349–2358. doi: 10.1242/jeb.201.16.2349. [DOI] [PubMed] [Google Scholar]
- Webster M.D, Weathers W.W. Heat produced as a by-product of foraging activity contributes to thermoregulation by verdins, Auriparus flaviceps. Physiol. Zool. 1990;63:777–794. [Google Scholar]
- Weiner J. Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. Trends Ecol. Evol. 1992;7:384–388. doi: 10.1016/0169-5347(92)90009-Z. doi:10.1016/0169-5347(92)90009-Z [DOI] [PubMed] [Google Scholar]
- Whelan C.J, Brown J.S. Optimal foraging and gut restraints: reconciling two schools of thought. Oikos. 2005;110:481–496. doi:10.1111/j.0030-1299.2005.13387.x [Google Scholar]
- Whittow G.C. Thermoregulatory adaptations in marine mammals: interacting effects of exercise and body mass. A review. Mar. Mamm. Sci. 1987;3:220–241. doi:10.1111/j.1748-7692.1987.tb00165.x [Google Scholar]
- Whittow G.C, Hampton I.F.G, Matsuura D.T, Ohata C.A, Smith R.M, Allen J.F. Body temperature of three species of whales. J. Mammal. 1974;55:653–656. doi:10.2307/1379555 [PubMed] [Google Scholar]
- Williams T.M. Thermoregulation of the North American mink during rest and activity in the aquatic environment. Physiol. Zool. 1986;59:293–305. [Google Scholar]
- Williams T.M, Davis R.W, Fuiman L.A, Francis J, Le Boeuf B.L, Horning M, Calambokidis J, Croll D.A. Sink or swim: strategies for cost-efficient diving by marine mammals. Science. 2000;288:133–136. doi: 10.1126/science.288.5463.133. doi:10.1126/science.288.5463.133 [DOI] [PubMed] [Google Scholar]
- Williams T.M. Intermittent swimming by mammals: a strategy for increasing energetic efficiency during diving. Am. Zool. 2001;41:166–176. doi:10.1668/0003-1569(2001)041[0166:ISBMAS]2.0.CO;2 [Google Scholar]
- Williams T.M, Friedl W.A, Haun J.A. The physiology of bottlenose dolphins (Tursiops truncatus): heart rate, metabolic rate and plasma lactate concentration during exercise. J. Exp. Biol. 1993;179:31–46. doi: 10.1242/jeb.179.1.31. [DOI] [PubMed] [Google Scholar]
- Williams T.M, Noren D.P, Berry P, Estes J.A, Allison C, Kirtland J. The diving physiology of bottlenose dolphins (Tursiops truncatus). III. Thermoregulation at depth. J. Exp. Biol. 1999;202:2763–2769. doi: 10.1242/jeb.202.20.2763. [DOI] [PubMed] [Google Scholar]
- Williams T.M, Haun J, Davis R.W, Fuiman L.A, Kohin S. A killer appetite: metabolic consequences of carnivory in marine mammals. Comp. Biochem. Physiol. A. 2001;129:785–796. doi: 10.1016/s1095-6433(01)00347-6. doi:10.1016/S1095-6433(01)00347-6 [DOI] [PubMed] [Google Scholar]
- Williams T.M, Estes J.A, Doak D.F, Springer A.M. Killer appetites: assessing the role of predators in ecological communities. Ecology. 2004a;85:3373–3384. [Google Scholar]
- Williams T.M, Fuiman L.A, Horning M, Davis R.W. The cost of foraging by a marine predator, the Weddell seal Leptonychotes weddellii: pricing by the stroke. J. Exp. Biol. 2004b;207:973–982. doi: 10.1242/jeb.00822. doi:10.1242/jeb.00822 [DOI] [PubMed] [Google Scholar]
- Willis K, Horning M, Rosen D.A.S, Trites A.W. Spatial variation of heat flux in swimming Steller sea lions: evidence for the importance of the body trunk in thermoregulation. J. Exp. Mar. Biol. Ecol. 2005;315:147–162. doi:10.1016/j.jembe.2004.09.019 [Google Scholar]
- Wilson R.P, Zimmer I. Inspiration by Magellanic penguins: reduced swimming effort when under pressure. Mar. Ecol. Prog. Ser. 2004;278:303–307. [Google Scholar]
- Worthy G.A.J. Insulation and thermal balance of fasting harp and grey seal pups. Comp. Biochem. Physiol. A. 1991;100:845–851. doi: 10.1016/0300-9629(91)90302-s. doi:10.1016/0300-9629(91)90302-S [DOI] [PubMed] [Google Scholar]
- Worthy G.A.J, Edwards E.F. Morphometric and biochemical factors affecting heat loss in a small temperate cetacean (Phocoena phocoena) and a small tropical cetacean (Stenella attenuata) Physiol. Zool. 1990;63:432–442. [Google Scholar]
- Yeates, L., Williams, T. M. & Tinker, M. T. 2005 Energy and thermal balance in male California sea otters (Enhydra lutris nereis): the effect of territoriality on daily energy expenditure. In 16th Biennial Conf. on the Biology of Marine Mammals, pp. 311. San Diego, CA.
- Zapol W.M, Liggins G.C, Schneider R.C, Qvist J, Snider M.T, Creasy R.K, Hochachka P.W. Regional blood flow during simulated diving in the conscious Weddell seal. J. Appl. Physiol. 1979;47:968–973. doi: 10.1152/jappl.1979.47.5.968. [DOI] [PubMed] [Google Scholar]
- Zerba E, Walsberg G.E. Exercise-generated heat contributes to thermoregulation by Gambel's quail in the cold. J. Exp. Biol. 1992;171:409–422. [Google Scholar]
- Zharikov Y, Skilleter G.A. Nonbreeding eastern curlews Numenius madagascariensis do not increase the rate of intake or digestive efficiency before long-distance migration because of an apparent digestive constraint. Physiol. Biochem. Zool. 2003;76:704–715. doi: 10.1086/376427. doi:10.1086/376427 [DOI] [PubMed] [Google Scholar]
- Zwarts L, Ens B.J, GossCustard J.D, Hulscher J.B, Kersten M. Why oystercatchers Haematopus ostralegus cannot meet their daily energy requirements in a single low water period. Ardea. 1996;84A:269–290. [Google Scholar]
- Zynel C.A, Wunder B.A. Limits to food intake by the prairie vole: effects of time for digestion. Funct. Ecol. 2002;16:58–66. doi:10.1046/j.0269-8463.2001.00601.x [Google Scholar]