Abstract
Southern elephant seals, Mirounga leonina, undertake large-scale oceanic movements to access favourable foraging areas. Successful foraging areas of elephant seals from the Kerguelen Islands are investigated here in relation to oceanographic parameters. Movements and diving activity of the seals as well as oceanographic data were collected through a new generation of satellite relayed devices measuring and transmitting locations, pressure, temperature and salinity. For the first time, we have associated foraging behaviour, determined by high increased sinuosity in tracks, and dive density (i.e. number of dives performed per kilometre covered), and changes in body condition, determined by variations in drift rate obtained from drift dives, to identify the oceanographic conditions of successful foraging zones for this species. Two main sectors, one close to the Antarctic continent and the other along the Polar Front (PF), where both foraging activity and body condition increase, seem to be of particular interest for the seals. Within these regions, some seals tended to focus their foraging activity on zones with particular temperature signatures. Along the Antarctic continent, some seals targeted colder waters on the sea bottom during benthic dives, while at the PF the favourable zones tended to be warmer. The possible negative effect of colder waters in Antarctic on the swimming performances of potential fish or squid prey could explain the behaviour of elephant seals in these zones, while warmer waters within the PF could correspond to the optimal conditions for potential myctophid prey of elephant seals.
Keywords: Mirounga leonina, marine ecology, foraging behaviour, drift dives, temperature profiles, satellite telemetry
1. Introduction
The survival and reproduction of animals depend on foraging success. Thus, we would predict the foraging behaviour of animals to change in response to changes in prey distribution. A foraging animal should increase its searching effort in areas where resources are plentiful rather than in areas where resources are scarce (optimal foraging theory; see McArthur & Pianka 1966). In a heterogeneous patchy environment, like oceans, foraging predators adjust their behaviour in relation to the environmental structure (Charnov 1976). Therefore, they have to make decisions about where to feed, when to feed and what to feed on. More precisely, the foraging success of predators is ultimately determined by the spatial and temporal occurrence of regions of oceanic productivity (Lea & Dubroca 2003) and their ability to locate and effectively exploit these patchily distributed resources.
For the last 20 years, satellite telemetry has been a very useful tool for wildlife monitoring and management (Fancy et al. 1988; Keating et al. 1991), especially to investigate the marine ecology of seabirds (e.g. Weimerskirch et al. 1993; Guinet et al. 1997) and marine mammals (e.g. Bonadonna et al. 2001; Matthiopoulos et al. 2004). Nowadays, technology not only enables animals to be located at sea but also provides important information on their foraging behaviour (Guinet et al. 1997; Bonadonna et al. 2001). However, the deployment of time–depth recorders, with limited data storage, is characterized by the conflicting demands of high resolution (frequent sampling) and a long duration of records (infrequent sampling). Recently, the Sea Mammal Research Unit at St Andrews (Scotland) has developed a new Argos, conductivity–temperature–depth (CTD) satellite relayed logger, for investigating the diving behaviour of marine mammals and for recording simultaneously the hydrological parameters of the water mass explored by the predator. This approach allows a direct comparison between the foraging behaviour and the environmental conditions encountered by the animals at the same period of time. Furthermore, the direct transmission of information by satellite enables a nearly real-time data analysis (Martin et al. 1998). This is particularly useful when investigating the foraging behaviour of species remaining at sea for long periods of time and for which retrieving the unit is problematic.
Southern elephant seals (Mirounga leonina) are major predators in the sub-Antarctic and Antarctic marine ecosystems (Boyd & Arnbom 1991; McConnell et al. 1992). After moulting in summer, they spend about eight months at sea before coming back ashore to breed in spring. During this period, their mobility and diving capabilities enable them to travel large distances (McConnell et al. 1992), reach depths greater than 1500 m, and stay submerged for up to 77 min. Dives are punctuated by periods of only 2–3 min at the surface (McConnell et al. 1992) and this performance can be maintained for weeks. All these factors provide southern elephant seals with the potential to have a major impact over a large range of habitats in the Southern Ocean. The movements of post-moulting southern elephant seals from Macquarie Island, South Georgia and Peninsula Valdés (Boyd & Arnbom 1991; Hindell et al. 1991; McConnell et al. 1992; Campagna et al. 1995; McConnell & Fedak 1996) as well as Northern Hemisphere congeners from Año Nuevo Point and San Miguel Island (Le Boeuf et al. 1988, 1992; Stewart 1997) have been described to some extent. However, little is known about how they are affected by their oceanographic environment and, particularly, by the temperature of the water masses that are explored. In contrast to the previous sites, even less is known for post-moulting southern elephant seals of the Kerguelen Islands, for which the distribution and movements at sea have only been studied since 2003.
In addition to their travelling and foraging dives, elephant seals regularly perform dives during which they spend a large proportion of time drifting passively through the water column (Webb et al. 1998; Biuw et al. 2003). The rate of vertical change in depth during these ‘drift’ dives is largely a result of the body condition. While lean tissue is denser than seawater, lipid tissue is less dense and animals with a large proportion of lipid will therefore be more buoyant (Webb et al. 1998). Thus, fatter seals show higher (more positive or less negative) drift rates when compared with leaner seals. The lungs also probably play a role in the buoyancy of marine mammals that inhale before diving (Webb et al. 1998). However, phocids exhale before diving. As all marine mammals have strengthened airways which cause a graded compression of the lung with increasing depth, with the remaining air moving from the compliant alveoli into the rigid, non-exchanging upper airways (Kooyman et al. 1970; Denison & Kooyman 1973), exhalation helps to promote alveolar collapse at relatively shallow depths (30–50 m; Kooyman et al. 1972; Falke et al. 1985). This prevents gas exchange and is thought to help avoid decompression sickness. Thus, beyond a 50 m depth, the lungs are unlikely to affect the buoyancy of elephant seals, which is therefore primarily determined by body composition. Over a given time, an increase in the drift rate can be considered as an index of a successful foraging activity (Biuw et al. 2003).
The aim of this study is to monitor simultaneously the position, foraging searching activity and changes in body condition of these animals, and to relate this information to water temperature and bathymetry, which are key physical parameters of their oceanic habitat.
2. Material and methods
(a) Deployment of device
During two consecutive summers in 2002–2003 and 2003–2004, 12 satellite relayed data loggers (SRLDs) from the Sea Mammal Research Unit (University of St Andrews, Scotland), collecting and transmitting locations, pressure, temperature and salinity (except in 2002 when only location and depth were recorded), were deployed at Kerguelen Islands. Seven juvenile male and five adult female elephant seals were equipped after moulting in summer.
All seals were caught with a canvas head-bag and anaesthetized with a combination of 1 : 1 tiletamine and zolazepam (Zoletil 100) injected intravenously. The recorders were glued onto the head of seals, using beds of quick-setting epoxy (Araldite AW 2101), after the hair was cleaned with acetone. Units stayed in place until the next moult during which they fell off.
(b) Data features
Data were collected every 5 s, but the limited Argos data channel does not allow all records to be transmitted. A systematic method to schedule the transmission of an unbiased sample of the stored records was used. We used the algorithm of Fedak et al. (2001) to compress dive profile information from time–depth records, selecting the four time–depth points where the dive trajectory changes most rapidly. Each profile was reconstructed by joining the selected points with straight lines. A temperature profile was collected for each dive by recording 12 points from the maximum depth to the surface, and 2–4 temperature profiles were transmitted each day.
(c) Smoothing of trajectories
Locations are determined during satellite uplinks by the Argos system. However, the raw track presents numerous outliers and a post-processing of locations is necessary. A location class (LC) is provided for each location by the Argos system: when more than three uplinks are available, a quality flag is computed (flag 0, 1, 2 or 3), whereas the flag A is given when only three uplinks are available and the flag B is given when only two uplinks are available. The lower the number of uplinks, the more uncertain is the location accuracy. The marine mammals' underwater habit results in a high proportion of locations of non-guaranteed accuracy (A and B). Approximately 50% of elephant seal data are B-classed and approximately 30% are A-classed, so it is impossible to reject locations on the basis of an LC criterion.
Two possible locations are provided by the Argos system for each point. The algorithm which chooses the true location between them can sometimes fail, thus the first correction step is to replace outliers by their homologous points. Then, unrealistic satellite locations are rejected using a forward/backward averaging filter (McConnell et al. 1992), based on the assumption that seals rarely travel at speeds higher than 3 m s−1 (Lea & Dubroca 2003). Finally, a 24 h running mean is applied to locations, and the locations are resampled at regular 3 h intervals for practical analysis.
(d) Temperature section
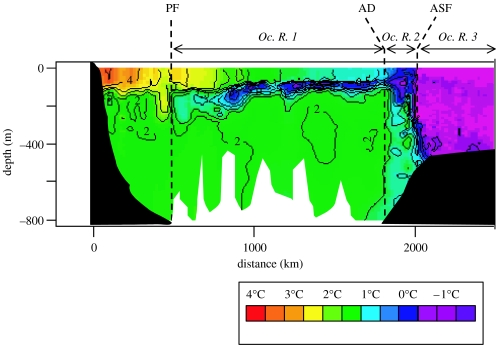
In our study, temperature–depth sections were carried out directly from data recorded by elephant seals. Briefly, temperature data were interpolated along the main foraging axis from every temperature profiles recorded at sea. The temperature data had 0.01°C accuracy and 0.001°C resolution, which were sufficient to determine in which hydrothermal structure each individual foraged. These sections allowed a fine-scale description of the hydrological fronts, and the corresponding oceanographic regions can therefore be clearly identified along each seal transect (Jacobs 1991; Park et al. 1993, 1998a,b; Belkin & Gordon 1996; figure 1). Distinctions are made between the oceanographic region no. 1 (Oc. R. 1) located between the Polar Front (PF) and the Antarctic Divergence (AD), the oceanographic region no. 2 (Oc. R. 2) located between the AD and the Antarctic Slope Front (ASF), and the oceanographic region no. 3 (Oc. R. 3) located between the ASF and the Antarctic continent (figure 1).
Figure 1.
Vertical temperature section obtained for one individual between Kerguelen and Antarctica. Position of the hydrological fronts is represented (PF, Polar Front; AD, Antarctic Divergence; ASF, Antarctic Slope Front). The fronts delimit three different oceanographic regions (Oc. R.).
(e) Sinuosity and dive density describing foraging searching activity
Along each path, the cosine of the angle formed by three consecutive location points was calculated. To obtain angles in radians, we applied the inverse cosine function and obtained results varying between 0 (straight ahead=0°) and π radian (about-turn=180°). The daily average of this variation was calculated and used as an index of the path sinuosity when seals were at sea.
Dive density is expressed as the number of dives per kilometre and was also calculated as a daily average. The distance was measured between each pair of successive locations and summed to give the distance travelled per day. The number of dives each day was calculated and divided by the distance travelled per day to give the daily dive density.
The association of these two parameters is used to describe the foraging searching activity of elephant seals.
(f) Selection of drift dives and benthic dives
Among over 50 000 individual dive records collected, dive identification was conducted with a program written using the software R (Ihaka & Gentleman 1996). Six distinct dive types are defined on the basis of the general shape of the dive profile. The main parameters used to sort the dive profiles are the slopes of the interpolated profile and the distance between the tie points used to draw the dive profile. Among the six profile categories identified, drift and square dives (Hindell et al. 1991) are particularly interesting for our study. The latter are often considered as benthic dives, but the comparison of the dive depth with bathymetry at the corresponding location clearly indicates that square dives were not always benthic dives. Therefore, benthic dives are defined according to the diving depth and the corresponding ETOPO 5 database (5′ latitude–longitude resolution) bathymetry.
The program could not categorize approximately 5% of dives into any category because they were just above the threshold determined and therefore classed them in a special category. A visual examination of this category was then necessary to identify potential misclassified drift dives. The shape of the putative drift segment was examined by fitting different regression lines through all inflection points (represented by the depth according to the time) or excluding the first or the last inflection point (see fig. 1 in Biuw et al. 2003). The drift rate (in m s−1) was calculated as the slope coefficient (positive or negative) of the best-fitting regression line. When the drift segment selected was just composed of two inflection points, the drift rate was calculated as the slope coefficient of the line connecting these two points. Only dives where the depth of the shallowest inflection point was at least 10 m and where the drift phase represented more than 40% of the total duration were selected (Biuw et al. 2003). The daily average of drift rate was calculated. Changes in drift rate are therefore observed over successive days.
Positive changes in drift rate were used to confirm that foraging searching activity was successful.
(g) Determination of successful foraging zones and physical characteristics
To identify successful foraging zones, we proceeded in several steps.
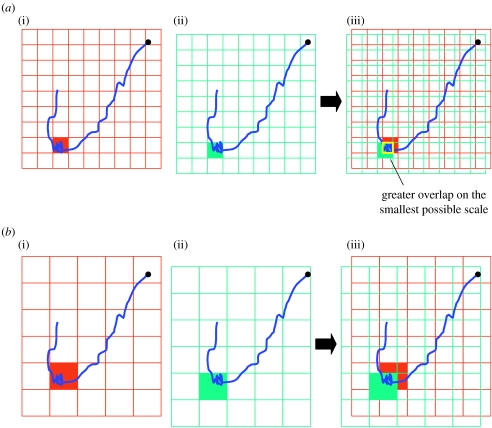
First, we assess the best scale at which the foraging searching activity could be identified using the sinuosity index of the track and the dive density. Thus, within a range of grid scales (0.1°, 0.5°, 1°, 2°, 3°, 4° and 5°)(figure 2a,b), the sectors where the foraging searching activity was most intensive were characterized (figure 2a,b(i)). Then, for the same range of scale, the sectors where changes in drift rate were positive (i.e. suggesting an area of successful foraging) were identified (figure 2a,b(ii)). The best positioning of the grid was conducted independently on the foraging searching activity (sinuosity and dive density) and on positive rate of drift dive (i.e. successful foraging) using an automated R procedure (function ‘ascgen’ within the package ‘adehabitat’) according to the changes in these parameters along the trips. Therefore, when grids obtained at the same scale were superimposed (figure 2a,b(iii)), the overlap was not necessarily perfect.
Figure 2.
Representation of the methodology used to determine the scale (a, scale 1; b, scale 2) for which successful foraging zones are described more precisely. A hypothetical track is represented in blue. Kerguelen is represented by the black dot. (i) Selection of the sectors where the foraging searching activity is most intensive (red area). (ii) Selection of the sectors where the drift rates increased (green area). (iii) Superimposition of the grids and determination of greatest overlap on the smallest possible scale. In this example, the scale 1 is selected.
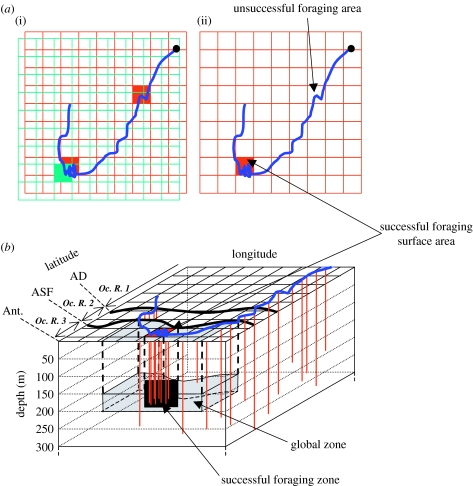
The successful foraging surface areas were thus defined as the grid cells where the foraging searching activity was most intensive, only when these cells were partially or entirely associated with the cells characterized by an increasing drift rates (figure 3a,b(iii)), i.e. where body condition improved.
Figure 3.
Representation of the methodology used to determine successful foraging zone. (a) The successful foraging surface areas were defined as the sectors where the foraging searching activity was most intensive (red area), only when these sectors were partially or entirely associated with a sector where the drift rates increased (green area). (b) Within successful surface areas identified, the water layer where the mean maximum depth of dives occurred was determined. The successful foraging zones were thus defined in three dimensions. A hypothetical track is represented in blue. Diving activity is represented by red strokes. AD, Antarctic Divergence; ASF, Antarctic Slope Front; Ant., Antarctica; Oc. R., oceanographic region.
Among the whole range of scale tested, the best scale to characterize the successful foraging areas was the scale allowing the greatest overlap on the smallest possible scale between the two grids in areas of increased foraging searching activity and foraging success (figure 2a,b(iii)).
Finally, water column was divided into sections every 50 m. Within successful surface areas identified, the water layer where the mean maximum depth of dives occurred was determined. The successful foraging zones were thus defined in three dimensions (figure 3b).
Concerning the physical characteristics of these three-dimensional zones, we compared the average temperatures observed within each successful foraging zone with the average temperature obtained for the same oceanic region at an identical depth range along the track of elephant seals (figure 3b).
(h) Statistical analysis
Determination of the foraging searching activity depends on the combination of two parameters (sinuosity and dive density). As each individual can affect the result of the relationship between these parameters (according to the record duration), we used a generalized linear mixed model (GLMM) with the variable ‘individual’ considered as a random variable to test this relation. Fn,d.f. is the result of the test, where n is the sample size and d.f. is the degree of freedom.
All the tests were computed with the software R (Ihaka & Gentleman 1996).
3. Results
(a) Trip duration and morphology of seals
Recording duration was 20.7±9.6 weeks (145±67 days) on average for all individuals. During this time, the seals covered an average distance of 5283±2779 km, with a range of 889–8356 km. Two individuals for which recording duration was very low (less than 30 days) were removed from the analysis. Therefore, all the results have been computed for 10 animals. Body mass and body length of animals equipped did not differ between sexes (males mean body mass=372.9±69.5 kg, n=5 and females' mean body mass=339.7±43.0 kg, n=5, U=8, p=0.42; males' mean body length=2.6±0.2 m and females' mean body length=2.4±0.1 m, U=5.5, p=0.17).
(b) Dive density and changes in sinuosity
Daily dive density was strongly related to the daily sinuosity index of individual animals (GLMM, F10,8=18.18, p<0.001). Therefore, we considered the foraging searching activity to be high, when both these parameters exceeded a threshold (5 dives per km per day and π/15 for dive density and sinuosity, respectively).
The foraging trips could be broadly described by several distinct phases. Animals shifted between phases of straight line, travelling with a low diving rate per kilometre per day, and phases of high sinuosity and dive density.
(c) Drift phase
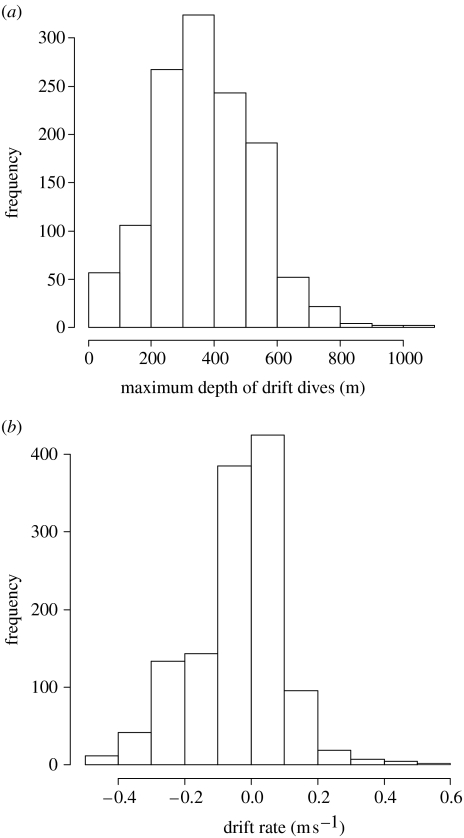
A total of 53 628 dives with a complete dive profile were recorded. Of these, 1270 dives were assigned to the drift dive category, with the number of dives extracted for each seal varying from 31 to 221. Most of the dives extracted (715 dives) showed a negative slope (depth according to time) during the drift segment (hereafter referred to as ‘negative’ dives), while 555 dives showed a positive slope (‘positive’ dives). The mean maximum dive depth of drift dives across all seals (i.e. the deepest depth attained during a given dive) was 350.1±151.0 and 396.0±160.7 m for negative and positive dives, respectively, and the maximum dive depths recorded among all individuals varied from 16 to 1006 m. However, only 4.25% of drift dives occurred shallower than 100 m. Frequency of the maximum depth of drift dives is presented in figure 4a. Moreover, 82.6% of drift rates are between −0.2 and 0.2 m s−1, and therefore close to neutral (which equals 0 m s−1; figure 4b).
Figure 4.
(a) Histogram of the maximum depth of drift dives. (b) Histogram of the drift rates.
As observed for dive density and sinuosity, trips could be divided into successive distinct phases during which animals showed cycles of decrease and increase in drift rate.
(d) Successful foraging zones
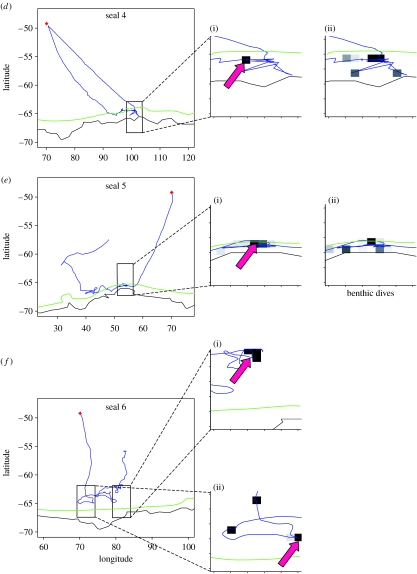
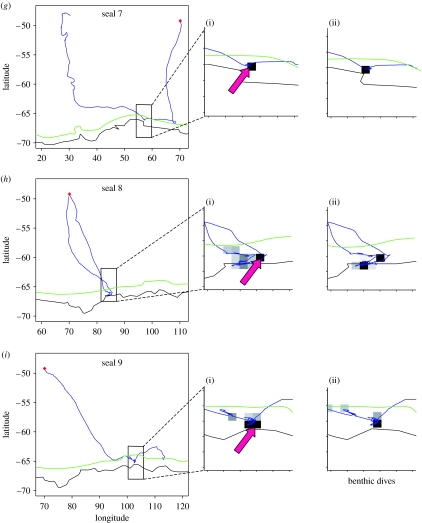
Although there was a degree of individual variability, a general pattern of foraging strategies with regard to time can be identified. Considering that most of the trips are incomplete due to the failure of devices, only two distinct phases by trip are distinguished. The first phase lasted for 5.8±2.2 weeks on average and consisted of most seals of travelling in a straight line while showing a low diving rate per kilometre. Moreover, most of the animals showed a gradual decrease in drift rate (i.e. an inferred decrease in body condition) over this period. The second phase is characterized by successive periods of increase and decrease in sinuosity and diving density. The cycles of increase and decrease in drift rate were not always correlated to changes in foraging searching activity. Only the areas where the changes in foraging searching activity were most strongly associated with an increasing drift rate were defined as successful foraging areas. In order to be as precise as possible, the strongest association was determined on the smallest possible scale; in this study, at a 0.5° scale (cf. §2 and figure 2a,b(iii)). Only the most favourable areas were selected corresponding to the sectors (defined by the 0.5° grid) where the greatest changes in foraging searching activity associated with a positive change in drift rate were observed. The depth of the water layer preferentially frequented by the seals within these areas is presented in table 1, while location of the successful foraging surface areas is presented in figure 5a–j(ii).
Table 1.
Physical characteristics (temperature) of successful foraging zones.
| seal | range of depth targeted within successful foraging area (m) | temperature (mean±s.d.) (°C) within successful foraging zone, temp. 1 | temperature (mean±s.d.) (°C) within global zone, temp. 2 | t-test between temp. 1 and temp. 2 |
|---|---|---|---|---|
| 1 | 500–550 | −0.56±0.71 | −0.16±0.89 | t=3.28, p=0.001 |
| 2 | 400–450 | 2.71±0.16 | 2.43±0.28 | t=−8.99, p<0.001 |
| 3 | 500–550 | — | — | — |
| 4 | 500–550 | — | — | — |
| 5 | 500–550 | −0.31±0.48 | −0.14±0.88 | t=1.46, p=0.15 |
| 6 | 200–250 | 2.63±0.08 | 2.21±0.64 | t=10.31, p<0.001 |
| 1.90±0.05 | t=7.95, p<0.001 | |||
| 7 | 200–250 | −1.25±0.07 | −0.49±1.04 | t=8.82, p<0.001 |
| 8 | 300–350 | −1.96±0.13 | −1.76±0.45 | t=7.64, p<0.001 |
| 9 | 300–350 | −1.83±0.04 | −1.84±0.05 | t=0.89, p=0.37 |
| 10 | 400–450 | −0.97±0.68 | −0.72±0.77 | t=−1.00, p=0.34 |
Figure 5.
Movements of 10 post-moulting elephant seals (a–j) tracked from Kerguelen Islands (blue). Enlarged sectors focus on the portion of tracks where foraging searching activity is greatest. (i) Red arrows focus on successful foraging surface areas. (ii) Intensive benthic activity is represented by dark square. Position of the Kerguelen Islands is marked by a red dot. The border of the Antarctic continent is represented by a black line. The limit of the Antarctic shelf (isobath 500 m) is represented by a green line.
(e) Oceanographic characteristics of successful foraging zones
All elephant seals, except one, reached the Antarctic shelf (seals 1, 3–5, 7–10) or the shelf edge (seal 6) (figure 5). One individual (seal 2) remained in pelagic waters along the PF to the east of the Kerguelen Islands. Thus, most seals crossed the ASF and spent time in the oceanographic region no. 3. Within this region, the temperature of the water layer targeted by diving elephant seals was significantly colder in successful foraging zones when compared with the water temperature observed at the same depth range along the track of the animals within the same oceanographic region but where potential foraging was unsuccessful (table 1). Moreover, most of the successful foraging activity took place benthically on the Antarctic shelf, as showed by the strong occurrence of benthic dives in this region (figure 5a–j(ii)).
The pelagic seal (seal 2; figure 5) moved regularly between the north and the south of the PF during its trip, but the successful foraging zone was located within the PF. The water layer targeted in this successful foraging zone was warmer when compared with the global zone in the PF visited by this elephant seal (table 1).
4. Discussion
In this study, we have identified the favourable foraging zones for free-ranging elephant seals and described some of the physical features of these zones. Previous studies have described the foraging behaviour of this species (Hindell et al. 1991; Jonker & Bester 1998; Van den Hoff et al. 2002) and others have proposed the use of drift dives to estimate the body condition of elephant seals at sea (Crocker et al. 1997; Webb et al. 1998; Biuw et al. 2003). Ours is the first, to our knowledge, to combine these two approaches to identify more precisely the feeding zones of an important predator of the Southern Ocean. Moreover, it is probably the first time that the three-dimensional feeding zones are described by the sea temperature collected simultaneously by the foraging elephant seals.
(a) Methodological comments
Not surprisingly, the two parameters chosen to describe the foraging searching activity, i.e. dive density and sinuosity, are strongly correlated. However, the correspondence between foraging searching activity and positive changes in body condition (i.e. successful foraging) is not always clear. In several sectors, elephant seals have shown a positive increase in their drift rate with no change in their foraging searching activity or vice versa. This discrepancy may result from a certain degree of error in drift dives identification. Firstly, the low number of inflection points selected to represent dive profiles could be insufficient to identify clearly the dive shape. However, Fedak et al. (2001) have shown that this procedure was the best trade-off between the fidelity of the profile returned, the energy cost of computation and the energy cost of transmitting the data. Secondly, to be certain of identifying the drift dives correctly, swim speed data might be necessary, and even though Biuw et al. (2003) have shown that identification of drift dives based on the dive shape only is acceptable using the parameters beforehand defined, it is probable that some errors in drift dives identification persist in our analysis. However, more than the use of isolated drift dive, we were using the trends in drift rate calculated over several days and the misclassification of few drift dives is unlikely to have completely modified the observed trends. Furthermore, the changes in the trend of drift rate through the foraging trip of the elephant seals are ecologically consistent with the changes in the behaviour observed in our study (i.e. travelling versus foraging).
On the other hand, although the drift rate of elephant seals will be determined largely by the proportions of lipid and lean tissue, it will also be affected by a variety of external characteristics, such as seawater density (i.e. salinity and temperature), and internal physiological and behavioural factors, such as residual air in the lungs or surface area and body volume. Indeed, with foraging trips lasting several months, surface area and body volume are likely to change significantly over the course of the trip. This is particularly true in the case of juvenile animals that grow continuously during the time spent at sea. These changes will notably influence the buoyancy and may lead to errors in the interpretation of changes in drift rates. However, Biuw et al. (2003) showed that variations in surface area have a greater influence towards the extremes of drift rate, whereas these errors should approach zero when the buoyancy approaches neutral. In our study, most drift rates are close to neutral and therefore the influence of the variations on surface area and body volume is limited. Secondly, residual air in the lungs can have a significant influence on drift rates. However, this influence is only important for the shallowest dives, where air in the lungs remains uncompressed. In this study, more than 95% of dives occur deeper than 100 m. Therefore, it is unlikely that residual air in the lungs strongly influences the results. Thirdly, we have no results regarding the effect of seawater density on drift rates but, according to Biuw et al. (2003), the effect of change in seawater density is very small (one order of magnitude less) than the effect associated with the variations in body density (seawater density range represented approximately 2.5% of the range of body densities of seals). We therefore assume that seawater density had a marginal effect on the changes in drift rates observed in our study.
The characterization of successful foraging zones is based on the overlap between changes in foraging searching activity and improvement in body condition. However, this overlap is not always perfect, because a spatial lag regularly takes place between these two parameters. One explanation for this lag could be that the ingestion of a prey item may not necessarily be followed by an immediate positive change in drift rate. Indeed, even if lipids from prey may be assimilated into the blubber tissue relatively rapidly, the excretion of residual materiel as faeces, which would maintain the body at a lower buoyancy until they are excreted, would take approximately 10–20 h (Biuw et al. 2003). However, as changes in both foraging searching activity and body condition were sampled at 1 day intervals in our study, changes occurring with a frequency lower than 24 h such as the assimilation–excretion delay should be essentially filtered out and therefore should not explain the temporal and spatial lags observed between foraging searching activity and increase in drift rate.
Rather, this could be more probably explained by the heterogeneous distribution of the resources. Some sectors of the Southern Ocean are not always necessarily profitable for the seals and high foraging searching activity is not always rewarded with prey capture. Furthermore, opportunistic feeding is also possible during the transit phase to the feeding grounds (McConnell et al. 1992). Elephant seals could catch prey encountered by chance without necessarily modifying their trajectory or their diving behaviour and thus improve their body condition occasionally along a straight line track. In short, our method does not guarantee an exhaustive identification of the successful foraging areas, but most of them, and probably the most biologically significant, are detected. Moreover, we believe that the combination of the information we have on one hand about the foraging searching behaviour and on the other about the changes in drift rate provides one of the best and most complete proxies available of successful foraging by elephant seals according to the parameters measured.
(b) Foraging in the vicinity of Antarctic continent
Although southern elephant seals range throughout the Southern Ocean, their feeding activity is confined to particular areas. As found for adult southern elephant seals from Heard Island (Slip & Burton 1993), most of the animals studied here migrate to the Antarctic continental shelf in winter. This area is highly productive due to the movement of water masses and changes in winds, particularly across the AD (Park et al. 1998a,b). Indeed, the development of primary producers and primary consumers, which are at the base of the global food web (Gage & Tyler 1991), is favoured by the circulation of such water masses. Moreover, in the Antarctic region, sea-ice extent during winter strongly affects the global ecosystem. Previously (Bailleul et al. 2007), we observed that elephant seals from Kerguelen, as found for elephant seals from the South Shetland Islands (Bornemann et al. 2000), were clearly influenced by the extent of sea-ice. Loeb et al. (1997) showed that krill reproduction and survival are significantly affected by the extent and duration of the ice cover. Although the diet of elephant seals is principally composed of cephalopods and fishes (Slip 1995) and although such prey in the Southern Ocean are scarce, it is probable that krill is a major food source for many species in the Antarctic ecosystem, including those that southern elephant seals consume (Rodhouse 1989). As for krill (Priddle et al. 1988), oceanographic features are likely to control the distribution of cephalopods (Piatkowski et al. 1991; Rodhouse et al. 1992) and fishes (Claireaux et al. 1995).
Close to the Antarctic continent, several elephant seals targeted the sea floor of the Antarctic shelf, particularly in areas with the coldest bottom waters. The influence exerted by water temperature on most biochemical and physiological processes makes it one of the most important physical factors in the environment of an aquatic organism (Reynolds & Casterlin 1979). Claireaux & Lagardère (1999) stated that the European sea bass (Dicentrarchus labrax) can sense very small differences in temperature. Moreover, in another study, Claireaux et al. (1995) have shown that swimming activity of Atlantic cod (Gadus morhua) decreases in colder water. Even though a temperature change of 0.5°C, as observed between successful and unsuccessful foraging areas in our study, probably would not significantly affect physiological performance in temperate species, we argue that such a small temperature difference might be physiologically significant at the very low water temperatures of the Antarctic shelf.
Therefore, if elephant seals' prey exhibit reduced activity in colder water, we can hypothesize, though more information on the physiology of prey species from this region is needed to confirm a such hypothesis, that seals target colder areas to facilitate the capture of prey that would be more lethargic and therefore easier to capture. However, three seals did not target the colder part of their foraging area, which could be explained by different factors. Firstly, as found for seabirds (Fauchald et al. 2000) and fur seals (Guinet et al. 2001; Lea & Dubroca 2003), although foraging activity is often significantly related to oceanographic conditions, it is probable that these relationships changed with the spatial scale investigated. Therefore, the scale used in this study (0.5° latitude and longitude) was probably unsuitable to detect fine-scale environmental conditions targeted by some individuals within their feeding areas. Also, it is possible that important variables, which have consistent effects on individuals but vary among sites, were not considered and therefore confound correlations between animals and particular variables. For instance, it is possible that it is not temperature per se that determines the foraging zones of predators, but rather abrupt gradients of temperature (thermoclines) at frontal areas (Guinet et al. 2001) where prey are concentrated by gradients in water movement. Another possibility is that younger, less experienced animals have not yet learnt to find or use the best zones within their potential foraging range (Field et al. 2001).
(c) Foraging at the Polar Front
For the trip orientated towards the east of the Kerguelen Islands, the analysis reveals one particularly favourable sector for feeding activity within the PF defined by the 4°C surface isotherm (Park et al. 1993). Previous studies have shown that adult female elephant seals also explore the PF areas (Hindell et al. 1991; Field et al. 2001). Southern Ocean fronts, and particularly the PF region, are known for their high biological production and are the main foraging areas of several species of seabird and marine mammal (e.g. Guinet et al. 1997; Charrassin & Bost 2001). The PF region has been recognized as a concentration area for several species of myctophid fish (Sabourenkov 1991). Some myctophid species, such as Electrona carlsbergi, are found in the diet of elephant seals of Heard Island, located near the Kerguelen Islands (Slip 1995). It is probable that the diet of the Kerguelen seals foraging along the PF differs from those foraging at the Antarctic continent, and that their diet contains myctophid fish like their conspecifics of Heard Island.
The control of metabolic activity by temperature conditions is generally considered central for fishes to optimize the use of their ecosystem in time and space (Magnuson et al. 1979). Therefore, we may argue that the warmer water layer targeted by the seal could correspond with the optimal conditions for its prey. For example, a 3°C average temperature at 200 m depth between 49° S and 50.50° S corresponds to the optimal temperature for Krefftichtys anderssoni, a myctophid fish located in this area (Hulley 1981). Although salinity data were not examined here, oceanic basins are by no means homogeneous water masses, but are vertically stratified in layers. Each layer is characterized by a relative homogeneity of temperature and salinity. To find their prey in a specific layer, which could correspond to the optimal zone for fishes, it is conceivable that elephant seals use their high sensitivity to salinity differences during a vertical dive to sample several layers and to choose the one that tastes right (Dehnhardt 2002).
(d) Conclusion
The frontal zones, which are major contributors to the production of the Southern Ocean, play a key role in the foraging activity of elephant seals. Recent data from Kerguelen seem to confirm that the Antarctic shelf and, to a lesser extent, the PF are particularly interesting for this species. It is probable that elephant seals concentrate their foraging searching activity in the most productive parts of the Southern Ocean at a large scale to obtain abundant feeding resources that are highly predictable. This contrasts with the open reaches of the Southern Ocean where concentrations of prey are both spatially and temporally highly variable and may be associated with unpredictable hydrographic conditions (El-Sayed 1988). At a finer scale, within the frontal zones, the sectors targeted by the seals present specific temperature and bathymetry conditions, which are probably decisive for the local distribution of prey. Our study contributes to the knowledge in marine ecology of a top predator in the sub-Antarctic ecosystem.
Acknowledgments
This work was supported by Institut Paul Emile Victor (IPEV) and the Territoire des Terres Australes et Antarctiques Françaises (TAAF) and the Region Poitou-Charentes. This study was also supported by the ‘Centre National d'Etudes Spatiales’ (CNES—programme Tosca) and the group CORIOLIS–MERCATOR. We would like to thank the members of the 53rd, 54th and 55th research missions at Kerguelen Island and the IPEV logistical staff for their assistance in the field. We wish particularly to thank C. McMahon, M. Hindell and Y.-H. Park. Thanks are extended to P. Gaspard and J. Vanderstraeten for their assistance in the smoothing of tracks. We also thank all the members of the ‘Centre d'Etudes Biologiques de Chizé’ for their assistance in the small daily problems. Many thanks to reviewers for this manuscript revision.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Bailleul F, Charrassin J.-B, Ezraty R, Girard-Ardhuin F, McMahon C.R, Field I.C, Guinet C. Southern elephant seals from Kerguelen Islands confronted by Antarctic sea ice. Changes in movements and in diving behaviour. Deep Sea Res. II. 2007;54:343–355. doi:10.1016/j.dsr2.2006.11.005 [Google Scholar]
- Belkin I.G, Gordon A.L. Southern Ocean fronts from the Greenwich meridian to Tasmania. J. Geophys. Res. 1996;101:3675–3696. doi:10.1029/95JC02750 [Google Scholar]
- Biuw M, McConnell B.J, Bradshaw C.J.A, Burton H.R, Fedak M.A. Blubber and buoyancy: monitoring the body condition of free-ranging seals using simple dive characteristics. J. Exp. Biol. 2003;206:3405–3423. doi: 10.1242/jeb.00583. doi:10.1242/jeb.00583 [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Lea M.A, Dehorter O, Guinet C. Foraging ground fidelity and route-choice tactics of a marine predator: the Antarctic fur seal Arctocephalus gazella. Mar. Ecol. Prog. Ser. 2001;223:287–297. [Google Scholar]
- Bornemann H, Kreyscher M, Ramdohr S, Martin T, Carlini A, Sellmann L, Plötz J. Southern elephant seal movements and Antarctic sea ice. Antarct. Sci. 2000;12:3–15. [Google Scholar]
- Boyd I.L, Arnbom T. Diving behaviour in relation to water temperature in the southern elephant seal: foraging implications. Polar Biol. 1991;11:259–266. doi:10.1007/BF00238460 [Google Scholar]
- Campagna C, Le Boeuf B.J, Blackwell S.B, Crocker D.E, Quintana F. Diving behaviour and foraging location of female southern elephant seals from Patagonia. J. Zool. Lond. 1995;236:55–71. [Google Scholar]
- Charnov E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. doi:10.1016/0040-5809(76)90040-X [DOI] [PubMed] [Google Scholar]
- Charrassin J.B, Bost C.A. Utilisation of the oceanic habitat by king penguins over the annual cycle. Mar. Ecol. Prog. Ser. 2001;221:285–297. [Google Scholar]
- Claireaux G, Lagardère J.-P. Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. J. Sea Res. 1999;42:157–168. doi:10.1016/S1385-1101(99)00019-2 [Google Scholar]
- Claireaux G, Webber D.M, Kerr S.R, Boutilier R.G. Physiology and behaviour of free-swimming atlantic cod (Gadus morhua) facing fluctuating temperature conditions. J. Exp. Biol. 1995;198:49–60. doi: 10.1242/jeb.198.1.49. [DOI] [PubMed] [Google Scholar]
- Crocker D.E, Le Boeuf B.J, Costa D.P. Drift diving in female northern elephant seals: implications for food processing. Can. J. Zool. 1997;75:27–39. [Google Scholar]
- Dehnhardt G. Sensory systems. In: Rus Hoelzel A, editor. Marine mammal biology—an evolutionary approach. Blackwell Science Ltd; Oxford, UK: 2002. pp. 116–141. [Google Scholar]
- Denison D.M, Kooyman G.L. The structure and function of the small airways in pinniped and sea otter lungs. Resp. Physiol. 1973;17:1–10. doi: 10.1016/0034-5687(73)90105-9. doi:10.1016/0034-5687(73)90105-9 [DOI] [PubMed] [Google Scholar]
- El-Sayed S.Z. Seasonal and interannual variabilities in Antarctic phytoplankton with reference to krill distribution. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 101–119. [Google Scholar]
- Falke K.J, Hill R.D, Qvist J, Schneider R.C, Guppy M, Liggins G.C, Hochachka P.W, Elliott R.E, Zapol W.M. Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science. 1985;229:556–558. doi: 10.1126/science.4023700. doi:10.1126/science.4023700 [DOI] [PubMed] [Google Scholar]
- Fancy S.G, Pank L.F, Douglas D.C, Curby C.H, Garner G.W, Amstrup S.C, Regelin W.L. Satellite telemetry: a new tool for wildlife research and management. Fish Wildl. Serv. Resource Pub. 1988;172:54. [Google Scholar]
- Fauchald P, Erikstad K.E, Skarsfjord H. Scale-dependant predator–prey interactions: the hierarchical spatial distribution of seabirds and prey. Ecology. 2000;81:773–783. doi:10.2307/177376 [Google Scholar]
- Fedak M.A, Lovell P, Grant S.M. Two approaches to compressing and interpreting time–depth information as collected by time–depth recorders and satellite-linked data recorders. Mar. Mammal. Sci. 2001;17:94–110. doi:10.1111/j.1748-7692.2001.tb00982.x [Google Scholar]
- Field I, Hindell M.A, Slip D.J, Michael K.J. Foraging strategies of southern elephant seals (Mirounga leonina) in relation to frontal zones and water masses. Antarct. Sci. 2001;13:371–379. doi:10.1017/S0954102001000529 [Google Scholar]
- Gage J.D, Tyler P.A. The development of deep-sea biology, the physical environment and methods of study. In: Gage J.D, Tyler P.A, editors. Deep-sea biology: a natural history of organisms at the deep-sea floor, part I. Cambridge University Press; Cambridge, UK: 1991. pp. 9–31. [Google Scholar]
- Guinet C, Koudil M, Bost C.-A, Durbec J.P, Georges J.Y, Mouchot M.C, Jouventin P. Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago, a study during three austral summers. Mar. Ecol. Prog. Ser. 1997;150:11–20. [Google Scholar]
- Guinet C, Dubroca L, Lea M.A, Goldsworthy S, Cherel Y, Duhamel G, Bonadonna F, Donnay J.P. Spatial distribution of foraging in female Antarctic fur seals Arctocephalus gazella in relation to oceanographic variables: a scale-dependent approach using geographic information systems. Mar. Ecol. Prog. Ser. 2001;219:251–264. [Google Scholar]
- Hindell M.A, Slip D.J, Burton H.R. The diving behaviour of adult male and female southern elephant seals, Mirounga leonina (Pinnipedia: Phocidae) Aust. J. Zool. 1991;39:595–619. doi:10.1071/ZO9910595 [Google Scholar]
- Hulley P.A. Results of the research cruise of FRV ‘Walter Herwig’ to South America. LVIII. Family Myctophidae (Osteichtyes Myctophiformes) Arch. Fisch. Wiss. 1981;31:1–300. [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J. Comp. Graph. Stat. 1996;5:299–314. doi:10.2307/1390807 [Google Scholar]
- Jacobs S.S. On the nature and significance of the Antarctic Slope Front. J. Mar. Chem. 1991;35:9–24. [Google Scholar]
- Jonker F.C, Bester M.N. Seasonal movements and foraging areas of adult southern female elephant seals, Mirounga leonina, from Marion Island. Antarct. Sci. 1998;10:21–30. [Google Scholar]
- Keating K.A, Brewster W.G, Key C.H. Satellite telemetry: performance of animal-tracking systems. J. Wildl. Manage. 1991;55:160–171. [Google Scholar]
- Kooyman G.L, Hammond D.D, Schroeder J.P. Bronchograms and tracheograms of seals under pressure. Science. 1970;169:82–84. doi: 10.1126/science.169.3940.82. doi:10.1126/science.169.3940.82 [DOI] [PubMed] [Google Scholar]
- Kooyman G.L, Schroeder J.P, Denison D.M, Hammond D.D, Wright J.M, Bergman W.P. Blood N2 tensions of seals during simulated deep dives. Am. J. Physiol. 1972;223:1016–1020. doi: 10.1152/ajplegacy.1972.223.5.1016. [DOI] [PubMed] [Google Scholar]
- Le Boeuf B.J, Costa D.P, Huntley A.C, Feldkamp S.D. Continuous, deep diving in female northern elephant seals, Mirounga angustirostris. Can. J. Zool. 1988;66:446–458. [Google Scholar]
- Le Boeuf B.J, Naito Y, Asaga T, Crocker D.E, Costa D.P. Swim speed in a female northern elephant seal: metabolic and foraging implications. Can. J. Zool. 1992;70:786–795. [Google Scholar]
- Lea M.A, Dubroca L. Fine-scale linkages between the diving behaviour of Antarctic fur seals and oceanographic features in the southern Indian Ocean. ICES J. Mar. Sci. 2003;60:990–1002. doi:10.1016/S1054-3139(03)00101-2 [Google Scholar]
- Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. doi:10.1038/43174 [Google Scholar]
- Magnuson J.J, Crowder L.B, Medrick P.A. Temperature as an ecological resource. Am. Zool. 1979;19:331–334. [Google Scholar]
- Martin A.R, Smith T.G, Cox O.P. Dive form and function in belugas Delphinapterus leucas of the eastern Canadian High Arctic. Polar Biol. 1998;20:218–228. doi:10.1007/s003000050299 [Google Scholar]
- Matthiopoulos J, McConnell B.J, Duck C.D, Fedak M.A. Using satellite telemetry and aerial counts to estimate space use by grey seals around the British Isles. J. Appl. Ecol. 2004;41:476–491. doi:10.1111/j.0021-8901.2004.00911.x [Google Scholar]
- McArthur R.H, Pianka E.R. On optimal use of a patchy environment. Am. Nat. 1966;100:603–609. doi:10.1086/282454 [Google Scholar]
- McConnell B.J, Fedak M.A. Movements of southern elephant seals. Can. J. Zool. 1996;74:1485–1496. [Google Scholar]
- McConnell B.J, Chambers C, Fedak M.A. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct. Sci. 1992;4:393–398. [Google Scholar]
- Park Y.H, Gamberoni L, Charriaud E. Frontal structure, water masses, and circulation in the Crozet Basin. J. Geophys. Res. 1993;98:12 361–12 385. [Google Scholar]
- Park Y.H, Charriaud E, Fieux M. Thermohaline structure of the Antarctic surface water/winter water in the Indian sector of the Southern Ocean. J. Mar. Syst. 1998a;17:5–23. doi:10.1016/S0924-7963(98)00026-8 [Google Scholar]
- Park Y.H, Charriaud E, Ruiz Pino D, Jeandel C. Seasonal and interannual variability of the mixed layer properties and steric height at station KERFIX, southwest of Kerguelen. J. Mar. Syst. 1998b;17:571–586. doi:10.1016/S0924-7963(98)00065-7 [Google Scholar]
- Piatkowski U, Rodhouse P.G, Duhamel G. Occurrence of the cephalopod Martialia hyadesi (Teuthoidea: Ommastrephidae) at the Kerguelen Islands in the Indian Ocean sector of the Southern Ocean. Polar Biol. 1991;11:273–275. doi:10.1007/BF00238462 [Google Scholar]
- Priddle J, Croxall J.P, Everson I, Heywood R.B, Murphy E.J, Prince P.A, Sear C.B. Large-scale fluctuations in distribution and abundance of krill—a discussion of possible causes. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 169–182. [Google Scholar]
- Reynolds W.W, Casterlin M.E. Behavioral thermoregulation and the ‘final preferendum’ paradigm. Am. Zool. 1979;19:211–224. [Google Scholar]
- Rodhouse P.G. Cephalopods in the diet of wandering albatrosses and sea-surface temperatures at the sub-Antarctic Front. Sci. Mar. 1989;53:277–281. [Google Scholar]
- Rodhouse P.G, Arnbom T.R, Fedak M.A, Yeatman J, Murray A.W.A. Cephalopod prey of the southern elephant seal, Mirounga leonina L. Can. J. Zool. 1992;70:1007–1015. [Google Scholar]
- Sabourenkov, E. N. 1991 Mesopelagic fish of the Southern Ocean—summary results of recent Soviet studies. Selected Scientific Papers. Scientific Committee Conservation Antarctic Living Resources (CCAMLR) 1990, Hobart, Tasmania, pp. 433–457.
- Slip D.J. The diet of southern elephant seals (Mirounga leonina) from Heard Island. Can. J. Zool. 1995;73:1519–1528. [Google Scholar]
- Slip, D. J. & Burton, H. R. 1993 Movements and diving behaviour of southern elephant seals from Heard Island. In Abstracts of the Tenth Biennial Conference on the Biology of Marine Mammals Galveston, Texas, November 11–15, p. 100.
- Stewart B.S. Ontogeny of differential migration and sexual segregation in Northern elephant seals. J. Mammal. 1997;78:1101–1116. doi:10.2307/1383053 [Google Scholar]
- Van den Hoff J, Burton H.R, Hindell M.A, Sumner M.D, McMahon C.R. Migrations and foraging of juvenile southern elephant seals from Macquarie Island within CCAMLR managed areas. Antarct. Sci. 2002;14:134–145. doi:10.1017/S095410200200069X [Google Scholar]
- Webb P.M, Crocker D.E, Blackwell S.B, Costa D.P, Le Boeuf B.J. Effect of Buoyancy on the diving behavior of northern elephant seals. J. Exp. Biol. 1998;201:2349–2358. doi: 10.1242/jeb.201.16.2349. [DOI] [PubMed] [Google Scholar]
- Weimerskirch H, Salamolard M, Sarrazin F, Jouventin P. Foraging strategy of wandering albatrosses through the breeding season: a study using satellite telemetry. Auk. 1993;110:325–342. [Google Scholar]