Abstract
For diving endotherms, modelling costs of locomotion as a function of prey dispersion requires estimates of the costs of diving to different depths. One approach is to estimate the physical costs of locomotion (Pmech) with biomechanical models and to convert those estimates to chemical energy needs by an aerobic efficiency (η=Pmech/Vo2) based on oxygen consumption (Vo2) in captive animals. Variations in η with temperature depend partly on thermal substitution, whereby heat from the inefficiency of exercising muscles or the heat increment of feeding (HIF) can substitute for thermogenesis. However, measurements of substitution have ranged from lack of detection to nearly complete use of exercise heat or HIF. This inconsistency may reflect (i) problems in methods of calculating substitution, (ii) confounding mechanisms of thermoregulatory control, or (iii) varying conditions that affect heat balance and allow substitution to be expressed. At present, understanding of how heat generation is regulated, and how heat is transported among tissues during exercise, digestion, thermal challenge and breath holding, is inadequate for predicting substitution and aerobic efficiencies without direct measurements for conditions of interest. Confirming that work rates during exercise are generally conserved, and identifying temperatures at those work rates below which shivering begins, may allow better prediction of aerobic efficiencies for ecological models.
Keywords: aerobic efficiency, diving energetics, exercise heat, heat increment of feeding, thermal substitution, thermoregulation
1. Introduction
Estimating the food requirements of animals, and how foraging profitability changes with prey dispersion, are important to understanding constraints on where the animals can live and how much viable habitat is available (Lovvorn & Gillingham 1996; Luo et al. 2001; Fryxell et al. 2004). For diving endotherms, modelling costs of locomotion as a function of prey dispersion requires estimates of the costs of diving to different depths for varying durations. Heat loss to water is often substantial; however, much heat can be produced by the inefficiency of exercising muscles and the processing of food, thereby reducing the need for shivering thermogenesis. This energy savings, termed thermal substitution, varies with water temperature, exercise level, and the type, amount and timing of food eaten; it is also quite difficult to quantify in actively diving animals. As a result, and for reasons not well understood, measurements of thermal substitution have varied widely with experimental conditions. Despite its ecological importance, past studies of substitution have yielded little ability to predict its magnitude under different circumstances and its effects on the overall efficiency of energy use during foraging. In light of these needs, this paper reviews current concepts, methods and results regarding heat balance, thermal substitution and aerobic efficiency in diving endotherms. In regard to modelling dive costs, a central question will be, ‘Can we estimate the amount of shivering thermogenesis required to maintain body temperature, after accounting for substitution of heat from exercise and digestion?’
2. Alternative methods for measuring dive costs
In animals that normally dive to depths of less than a few metres, total dive costs (including thermoregulation) can be measured directly on captive animals in dive tanks via respirometry (e.g. MacArthur & Krause 1989; Kruuk et al. 1994; de Leeuw 1996; references in Kaseloo & Lovvorn 2005). The buoyancy of air in the respiratory system, and both the buoyancy and insulative value of the air layer in fur or plumage, decrease dramatically as the air is compressed with increasing depth, especially in the top 5–10 m (Lovvorn & Jones 1991; Wilson et al. 1992). Owing to changes in work against buoyancy with depth, and increased heat loss as air layers are more compressed, measurements in shallow tanks or for animals swimming only horizontally may not capture patterns or total costs of diving to deeper depths (cf. Croll & McLaren 1993 versus Lovvorn et al. 2004; Culik et al. 1994 versus Sato et al. 2002; Schmid et al. 1995 versus Enstipp et al. 2001 and Watanuki et al. 2005). A number of aquatic endotherms dive to depths of tens to even hundreds of metres (Schreer & Kovacs 1997), and respirometry on free-ranging animals is seldom possible except when individuals can be restricted to surfacing in a small chamber (Williams et al. 2004). Doubly labelled water measurements can be made on animals in the field (Nagy et al. 1984; Butler et al. 2004), but integrate costs of all activities (including non-locomotor costs) over periods of approximately a day, and are specific to the conditions during measurements; thus, they provide limited ability to predict dive costs for different patterns of prey dispersion.
Heart-rate microloggers have much promise for estimating variable dive costs in the field (Butler et al. 2004). However, heart-rate data must be calibrated to measurements of energy expenditure during exercise by captive animals, often for very different dive depths, temperatures, fluid media (air versus water) or even types of locomotion (treadmill walking versus diving; Hawkins et al. 2000; Froget et al. 2004; Guillemette et al. 2004). In extrapolating such calibrations to deep breath-hold dives, studies are needed of how correlations between heart rate and energy use are affected by diving responses of bradycardia, selective vasoconstriction and anaerobic metabolism (Williams et al. 1991; Kooyman et al. 1992; Bevan et al. 1997; Enstipp et al. 2001). Correlations between heart rate and oxygen consumption (Vo2) are not the same for exercise versus thermoregulation (Froget et al. 2002), and heart rate may not change with increased Vo2 due to the heat increment of feeding (HIF; McPhee et al. 2003). Also, heart-rate loggers on free-ranging animals must be retrieved for downloading data, which can prevent their use during non-breeding periods when some species may be difficult or impossible to recapture.
3. Biomechanics, aerobic efficiencies and substitution
Yet another approach for estimating dive costs is to calculate the physical power required for locomotion based on biomechanical models. Such models can account for varying dive depths, swim speeds, propulsive modes (e.g. wings versus feet) and durations of different dive phases (descent, activities at the bottom and ascent; Lovvorn et al. 1991, 1999, 2004; Lovvorn & Liggins 2002). These mechanical costs are then converted to chemical energy (food) requirements by an aerobic efficiency η, defined as mechanical power output divided by aerobic power input (Lovvorn 1994; Lovvorn & Gillingham 1996). This method has also been widely used in models of aerial flight (Pennycuick 1989). Calculation of η requires measuring Vo2 and estimating mechanical power output (Pmech) during exercise in captive animals (Kaseloo & Lovvorn 2005) so that
| (3.1) |
Values of η subsume a number of component efficiencies: (i) the efficiency of oxygen delivery to muscles by the respiratory and circulatory systems, (ii) the efficiency of muscles in doing contractile work, (iii) the efficiency of contractile work in moving fluid as opposed to accelerating and deforming tissues in the propulsive limbs, and (iv) the Froude efficiency or the fraction of work done to move fluid that yields useful thrust as opposed to being dissipated in the water as heat (Daniel 1991). Although heat dissipated in moving fluid (item iv) is ultimately derived from Vo2, it does not increase the heat content of the animal as do items i–iii; thus, internal heat generation is not a direct or simple function of overall aerobic efficiency.
Since the deepest dive tanks available are only approximately 10 m deep (see Enstipp et al. 2001), and control of temperature in these tanks is limited, conditions for determining η often differ from those in the field. It is generally believed that η stays relatively constant over the range of work rates exhibited by free-ranging animals, or conversely that the animals regulate their work levels to stay within limited ranges of power output and efficiency (Pennycuick 1991; Lovvorn et al. 1999; Lovvorn 2001; but see Lichtwark & Wilson 2005). Once identified, such ranges might be useful in predicting aerobic power output and efficiency for varied combinations of buoyant resistance and swim speed (see Banister & Jackson 1967; Lovvorn et al. 2004). However, because η depends on aerobic power measured as Vo2 (equation (3.1)), η will change if more energy is required for thermoregulation at colder temperatures. The amount of additional thermogenesis by shivering that is needed at a lower temperature depends on how much heat is already being generated by exercise. If Qshiv is the ‘residual thermogenesis’, or the cost of shivering to generate heat above that supplied by substitution, then the value of η at the colder temperature (ηcold) could be expressed as
| (3.2) |
where Vo2-sub is Vo2 during exercise within the range of substitution.
For field applications, we would like to estimate ηcold without measuring Vo2 under conditions of temperature, buoyancy and compression of insulative air layers that are often infeasible to duplicate in dive-tank studies. By equation (3.2), calculating ηcold requires estimating residual thermogenesis Qshiv at a given temperature and exercise level. If suitable methods are available (see §8), Qshiv might be calculated from estimates of heat lost and the amount of heat generated that could replace heat lost. Alternatively, if direct measurements or estimates of heat balance are not possible, respirometry might yield values of Qshiv. As temperature decreases during a given level of exercise, there may be a threshold in temperature below which substitution of heat from exercise is exceeded and shivering begins, as revealed by increases in Vo2 (McArdle et al. 1984). By this threshold concept, heat from digestion (HIF) would be expected to lower the temperature for onset of residual thermogenesis. In animals with an insulative air layer in fur or plumage, compression of the air layer at depth would raise the temperature threshold. In later sections, methods of estimating heat balance, and the potential for identifying thresholds of thermal substitution with respirometry, will be discussed in more detail.
4. Why are measurements of substitution inconsistent?
Despite its intuitive importance, experimental results regarding thermal substitution have been variable. HIF, also known as specific dynamic action, is the heat produced by the digestion, absorption or processing of food. For substitution of HIF, findings have ranged from lack of detection through partial or even complete use of HIF for thermoregulation (references in Appendix A; see Rosen & Trites 2003). For heat from exercise, estimated substitution has ranged from none to apparently complete (Appendix B), although the validity of specific estimates is questionable (see §5). Despite a number of studies on birds, substitution of exercise heat in mammals has seldom been measured directly (Appendix B), but rather inferred based on patterns of body temperature (Costa & Kooyman 1982; Williams 1986; MacArthur 1989). For large marine mammals, exercise heat may often exceed heat loss (Williams et al. 1999, 2004) so that aerobic efficiency changes little due to varying substitution; however, even in large mammals such as dolphins, this balance may depend on body size (McGinnis et al. 1972). In birds and smaller mammals for which variations in substitution can be important, what factors account for the inconsistent patterns?
Studies of thermal substitution of the heat increment of feeding (HIF) in endotherms, based on energy consumption.
| species | substitution | reference |
|---|---|---|
| birds | ||
| mallard (Anas platyrhynchos) | present | Kaseloo & Lovvorn (2003) |
| kestrel (Falco tinnunculus) | present | Masman et al. (1989) |
| Japanese quail (Coturnix coturnix) | present | Marjoniemi (2000) |
| Arctic tern (Sterna paradisaea) | none | Klaassen et al. (1989) |
| pigeon (Columba livia) | present | Rashotte et al. (1999) |
| tawny owl (Strix aluco) | present | Bech & Præsteng (2004) |
| house wren (Troglodytes aedon) | present | Chappell et al. (1997) |
| mammals | ||
| short -tailed shrew (Blarina brevicauda) | present | Hindle et al. (2003) |
| star-nosed mole (Condylura cristata) | none | Campbell et al. (2000) |
| golden hamster (Mesocricetus auratus) | present | Šimek (1975) |
| muskrat (Ondatra zibethicus) | present | MacArthur & Campbell (1994) |
| Steller sea lion (Eumetopias jubatus) | none | Rosen & Trites (2003) |
| white-tailed deer (Odocoileus virginianus) | present | Jensen et al. (1999) |
Studies of thermal substitution of heat produced by exercise in endotherms, based on energy consumption.
| species | substitution | reference |
|---|---|---|
| birds | ||
| tufted duck (Aythya fuligula) | present | Bevan & Butler (1992a) |
| lesser scaup (Aythya affinis) | present | Kaseloo & Lovvorn (2005) |
| Japanese quail (Coturnix coturnix) | present | Nomoto et al. (1983) |
| Gambel's quail (Callipepla gambelii) | present | Zerba & Walsberg (1992) |
| knot (Calidris canutus) | present | Bruinzeel & Piersma (1998) |
| ruby-throated hummingbird (Archilochus colubris) | present | Chai et al. (1998) |
| verdin (Auriparus flaviceps) | present | Webster & Weathers (1990) |
| dipper (Cinclus cinclus) | none | Bryant et al. (1985) |
| white-crowned sparrow (Zonotrichia leucophrys) | present | Ketterson & King (1977) and Paladino & King (1984) |
| dark-eyed junco (Junco hyemalis) and yellow-eyed junco (J. phaeonotus) | present | Weathers & Sullivan (1993) |
| house finch (Carpodacus mexicanus) | present | Zerba et al. (1999) |
| common chaffinch (Fringilla coelebs) | present | Pohl (1969) |
| common redpoll (Carduelis flammea) | present | Pohl & West (1973) |
| mammals | ||
| white rat (Rattus norvegicus) | present | Hart & Jansky (1963) |
| Australian water rat (Hydromys chrysogaster) | present | Dawson & Fanning (1981) |
I will explore three aspects that might contribute to variable results from studies of thermal substitution in smaller endotherms: (i) problems in methods of calculating substitution, (ii) confounding mechanisms of thermoregulatory control, and (iii) varying conditions that affect heat balance and allow substitution to be expressed.
5. Methods for calculating thermal substitution
A major problem in studying thermal substitution is that it must usually be measured indirectly by methods with definite shortcomings. For substitution of HIF, the usual approach is to measure resting metabolic rate (RMR) via oxygen consumption in fasted animals at thermoneutral and sub-thermoneutral temperatures, and then to make the same measurements in fed animals (references in Appendix A). If the increase in Vo2 from thermoneutral to sub-thermoneutral conditions is less in fed animals, then the difference is attributed to substitution of HIF which reduces the need for shivering at the cold temperature in the fed animals. Any differences in heat storage between fasted and fed animals must be accounted for, which is usually done by implanting a deep-body temperature sensor in the abdomen. However, due to regional heterothermy (see §8), measurements of heat storage based on sensors in the abdomen may be inadequate for detecting low levels of substitution of HIF.
Owing to differences in intermediary metabolism, foods with high protein content tend to have high HIF, whereas foods containing mainly lipid or carbohydrate have lower HIF (Blaxter 1989). The HIF usually increases as food intake exceeds that needed for maintenance metabolism, with the excess being used in anabolic processes which have lower efficiency (greater heat production) than catabolic processes (Blaxter 1989; Robbins 1993). Since the potential for substitution depends strongly on the magnitude of HIF, the above measurement approach works well for species that eat high-protein foods in large meals (e.g. Costa & Kooyman 1984; Markussen et al. 1994; Hawkins et al. 1997). However, for species that normally eat low-protein foods in smaller intermittent meals, peaks of HIF and thermal substitution tend to be low relative to the typically high variance in Vo2 (Kaseloo & Lovvorn 2003, 2005). Thus, the substitution of HIF may be occurring, but the signal-to-noise ratio might be low for certain diets and consumption patterns.
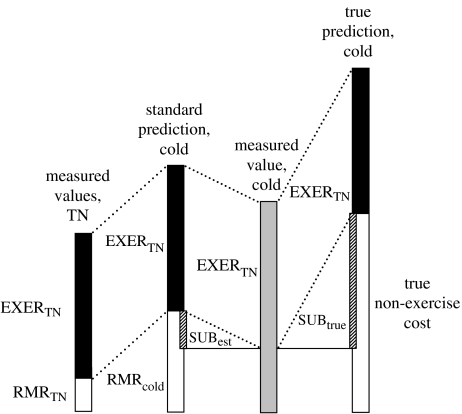
For measuring substitution of heat from exercise (figure 1), Vo2 is measured at rest (RMR) and during exercise at thermoneutral temperature, and again under sub-thermoneutral conditions of either cold temperature or high convection (wind). The cost of exercise alone is estimated by subtracting RMR from total Vo2 during exercise under thermoneutral conditions (Stainsby et al. 1980). By the standard method, the sum of RMR under cold conditions (RMRcold) plus the cost of exercise alone under thermoneutral conditions (EXERTN) is used to estimate the total cost of exercise under cold conditions (‘standard prediction, cold’ in figure 1). The latter estimate is then compared to the actual measurement of total cost during exercise under the cold conditions (‘measured value, cold’ in figure 1). Any decrease in the measured value compared to the standard prediction of total cost is attributed to substitution (cross-hatched area on second bar in figure 1; references in Appendix B). Substitution is presumed to have lowered that part of RMRcold that was due to shivering.
Figure 1.
Graphical depiction of the standard method of calculating thermal substitution of exercise heat, and demonstration of how this standard method can underestimate substitution. For explanation refer to §5 of text. RMRTN, resting metabolic rate at thermoneutral temperature; EXERTN, cost of exercise above RMR at thermoneutral temperature; RMRcold, RMR at cold temperature; SUBest, substitution of exercise heat as estimated by standard method; SUBtrue, true substitution of exercise heat.
Although similar problems apply to terrestrial animals, this measurement approach has particular shortcomings when applied to diving birds (cf. Bevan & Butler 1992a; de Leeuw et al. 1998; Kaseloo & Lovvorn 2005). The standard method assumes that the thermoregulation cost of a bird only partly submerged while resting on the water surface at the cold temperature (the conditions for RMRcold, figure 1) is the same as for a bird completely submerged and experiencing high convection while swimming underwater. However, heat loss to water is increased several fold by complete submergence as opposed to floating on the surface (de Vries & van Eerden 1995; Banta et al. 2004), and convection during active underwater swimming can enhance this effect exponentially (Boutilier et al. 1977; Fish 1983). Consequently, the ‘True non-exercise cost’ (see fourth bar in figure 1) of a bird swimming underwater at the cold temperature is much larger than RMRcold. This difference means that the sum of EXERTN and RMRcold (standard prediction, cold) greatly underestimates the sum of EXERTN and the true non-exercise cost while moving underwater at the cold temperature (‘true prediction, cold’, figure 1). Thus, the standard estimate of reduction in cost due to substitution, SUBest, is much smaller than the true reduction in cost, SUBtrue (compare cross-hatched areas in second and fourth bars in figure 1).
Owing to this methodological shortcoming, Vo2 comparisons do not always indicate when and how much substitution is occurring, and will often underestimate the magnitude of energy savings. If there is no increase at all in the total cost of diving between thermoneutral and colder temperatures, it can be assumed that costs of thermoregulation are either negligible or entirely met by substitution and incur no additional cost over maintenance and locomotion. However, we have no measure of thermogenic costs for a non-exercising bird that is submerged and experiencing high convection. Consequently, if the measured value of total dive cost is higher at the cold temperature than at thermoneutrality, it does not mean that less than 100% of exercise heat was used for substitution, as the increase in Vo2 might have been much greater if substitution had not occurred. In other words, we cannot know the total amount or fraction of exercise heat that is used for substitution, without knowing the total heat loss (and shivering cost to replace that loss) from a submerged, inactive bird experiencing convection at the cold temperature.
6. Mechanisms of thermoregulatory control
Potentially important in predicting patterns of substitution are the strategies and priorities of thermoregulatory control. If substitution is less than complete, a possible reason is that deep-body sensors that detect increases in internal heat do not trigger decreases in shivering, because those sensors are overridden by sensors in the skin which are more sensitive to cooler peripheral temperatures (cf. Bullard & Rapp 1970; Jessen 1990; Østnes & Bech 1997, 1998). To my knowledge, no studies of thermal substitution relative to temperatures of different body regions have been done, so the viability of this explanation is unknown. More will be said about control of thermogenesis by peripheral versus deep-body sensors in §8g.
Thermoregulatory control strategies unique to breath-hold diving in cold water may confound studies of substitution, by causing dramatic regional changes in body temperature and lags in thermogenic responses. In some diving birds, the temperature of inactive tissues can drop substantially during long dives, in extreme cases by 10°C or more. This phenomenon has been interpreted as active downregulation of temperature via vasoconstriction, to decrease the oxygen demand of those tissues (Bevan et al. 1997; Handrich et al. 1997). By assuming a Q10 of 3 and an average temperature drop of 2.4°C for the entire body over a dive bout, Bevan et al. (2002) estimated for gentoo penguins (Pygoscelis papua) that the maximum duration of a dive fuelled by aerobic metabolism could be extended by 38 s, and that all dives they recorded for free-ranging penguins would be within that limit. The question of whether temperatures in peripheral body tissues are actively reduced or simply allowed to fall passively was considered irrelevant, as cooling would decrease metabolic rate regardless of cause (Bevan et al. 2002).
However, for modelling costs of thermoregulation as they affect aerobic efficiency during dives, understanding whether changes in tissue temperature are regulated or passive is critical. For example, if cooling is only passive, patterns of heat balance and distribution might differ among animals of different body size, which have different work rates and heat generation relative to body volume and surface area, and might stay submerged for shorter periods at shallower depths with less compression of the insulative air layer. In very large birds, measurements of temperature at a range of body sites in freely diving emperor penguins (Aptenodytes forsteri) did not indicate metabolic suppression of the abdominal organs as a means of decreasing oxygen demand during dives (Ponganis et al. 2003). High temperatures were maintained in the body core, while there were decreases in forelimb, hindlimb, anterior abdomen, subcutaneous and sub-feather temperatures. These patterns indicated preservation of core temperature and cooling of peripheral tissues by either vasoconstriction, decreased insulation of the compressed air layer in feathers, or convection of heat to the water.
Regarding these mechanisms, the extent and importance of voluntary peripheral vasoconstriction has not been directly demonstrated in freely diving birds. In unrestrained redhead ducks (Aythya americana), diving to shallow depths with access to the water surface, the heart rate, arterial blood pressure and blood flow distribution were the same as during surface swimming without breath holding (Stephenson & Jones 1992); similar results were obtained for rhinoceros auklets (Cerorhinca monocerata; Stephenson et al. 1992). Dive depths and durations in these experiments were quite small, and both diving ducks and auklets showed marked cardiovascular responses when prevented from surfacing. Thus, although diving birds are certainly capable of such responses, it is unclear how much free-ranging birds use peripheral vasoconstriction during deeper dives.
Lower tissue temperature also decreases the temperature gradient that drives heat loss to water, especially while insulative air layers are compressed by hydrostatic pressure. Consequently, even without regulated vasoconstriction, smaller endotherms such as birds may let the temperature of certain tissues fall passively during dives and later restore that temperature upon resurfacing. This delayed response would lower thermogenic costs due to more favourable temperature gradients and thicker insulative air layers at the surface (MacArthur 1989; Wilson & Grémillet 1996). Moreover, shivering at the surface could be done by large locomotor muscles that were unavailable for shivering during exercise (Hong & Nadel 1979; Nomoto & Nomoto-Kozawa 1985; Rautenberg 1989; Hohtola et al. 1998; Kvadsheim et al. 2005; see §8e below).
In short, the degree to which cooling is passive versus actively regulated in different body regions during dives, and the magnitude of energy savings due to reduced heat gradients versus temperature-induced metabolic depression, are poorly known (Hochachka 1988; Culik et al. 1996; Boyd 2000; Ponganis et al. 2001; Kvadsheim et al. 2005). However, from the standpoint of thermal substitution, it is perhaps important that the time course and consistency of thermoregulatory patterns might differ between these two mechanisms. For example, vasoconstriction to regulate cooling for metabolic depression might occur rather quickly and consistently among taxa, whereas passive cooling might vary substantially among species of different body size and thermal inertia (e.g. large penguins versus small alcids). If passive cooling is an important mechanism for reduced body temperature, high variability among body sizes and insulative types (air versus blubber) might confound attempts to identify common conditions for expression of substitution in diving endotherms.
7. Heat balance and expression of thermal substitution
Another possible reason for varying assessments of the importance of substitution is that experiments may not create conditions of heat balance that allow substitution to be expressed. In addition to potential effects of control mechanisms mentioned in §8, the onset and magnitude of thermal substitution may depend on several factors: (i) heat loss must be high enough to create opportunity for substitution, (ii) heat generated by exercise or digestion must be great enough to offset heat loss and thereby reduce the need for shivering, (iii) meal size or protein content must be high enough to produce appreciable HIF, and (iv) heat from either exercise or digestion may satisfy thermogenic demands and thereby reduce need for the other (Kaseloo & Lovvorn 2006).
For heat from digestion, some experiments that seem to meet these criteria have not detected substitution (see Rosen & Trites 2003), perhaps owing to deficiencies in measurement methods or confounding mechanisms of thermoregulatory control (see §§5–6). Distinguishing these other factors from effects of overall heat balance requires estimates of heat generation versus heat loss under varying conditions. Section 8 reviews methods that have been used to estimate heat balance in diving endotherms, and their potential value in identifying patterns of substitution.
8. Approaches to measuring heat generation and loss
(a) Direct calorimetry
Total heat loss from body parts such as the feet of diving birds can be determined from changes in water temperature in a small water bath (Kilgore & Schmidt-Nielsen 1975; Midtgård 1980). Heat flux across unfeathered legs and feet may be an important component of overall heat balance. However, measurements for limbs immobilized in a small water bath generally do not reflect the high convection experienced by propulsors during swimming, or perhaps the circulatory patterns in active limbs. Consequently, resulting values may yield erroneous estimates of heat loss during locomotion.
(b) Heat flux across intact or excised integument
Two related approaches to estimating heat loss of diving endotherms involve (i) measuring heat flux across fat layers, skin, and fur or feathers in either living animals or excised tissue (Øritsland 1970; Frisch et al. 1974; Kooyman et al. 1976; review in Dunkin et al. 2005) or (ii) placing heat-flux sensors on the integument surface of captive or free-ranging animals (Kasting et al. 1989; Noren et al. 1999; Willis & Horning 2005). For animals with an insulative air layer in fur or plumage, a major challenge in such methods is maintaining the air layer in a natural state, without the constant cleaning, preening and ptilomotion exhibited by live birds and some marine mammals (Nolet & Kruuk 1989).
Heat-flux measurements on animals without insulative air layers can be relatively straightforward, but establishing the proper context for applying a few measurements to the animal's entire surface area is not. Depending on the intensity of the ‘dive response’, breath-hold diving can result in peripheral vasoconstriction and selective perfusion of different tissues including organs, muscles, fat and skin (Fish 1979; Stephenson & Jones 1992). As a result, integument (fat and skin) that overlies active locomotor muscles may experience different internal boundary conditions for heat flux than integument that overlies organs or muscles that are selectively ischaemic during a dive (e.g. pectoral muscles in a foot-propelled diver; Cooper et al. 1959; Luecke et al. 1975; Bevan & Butler 1992b; Østnes & Bech 1998). Although the conductivity of subcutaneous fat may decrease due to peripheral vasoconstriction during dives, during resting the conductivity of living blubber in seals submerged in cold water exceeded that of dead blubber by about 50% (Hart & Irving 1959). Perfusion and vasoconstriction in some tissues may cycle over time to avoid adverse effects, and blood from cooled extremities may be periodically flushed back to the body core and vice versa; thus, temporal variations may be important to heat flux at a given site (Johansen & Millard 1973; Hong & Nadel 1979; Johansen & Bech 1983; Cherepanova et al. 1993; Østnes & Bech 1998; but see Davis & Kanatous 1999). Moreover, both the thickness of subcutaneous fat, and the type and thickness of fur or plumage, can vary widely among body areas (Øritsland 1970; Evans & Moen 1975; Beck & Smith 1995), and different parts of the body surface can experience very different convective regimes during oscillatory stroking at different speeds (Liu et al. 1996; Wolfgang et al. 1999). For body surfaces exposed to the air after dives, thermal imaging can be used to assess regional variations of surface temperature and resulting heat flux (Hill et al. 1980; Williams et al. 1999). However, such methods cannot be used underwater, when factors affecting surface temperature and heat flux in swimming animals are quite different.
Consequently, before heat-flux measurements for only a few locations on the body can be used to estimate total heat loss, it should be shown that heat flux in those few locations is representative and over what portions of the body they are representative. Nevertheless, it is possible that some of these issues can be neglected in deriving useful values, or at least in assessing whether more detailed studies are justified. For birds and mammals with insulative air layers, such analyses will require data on the thickness of the air layer over different areas of the body, especially in species which depress their feather layer just before diving (cf. Grémillet et al. 1998). To date, no study has adequately evaluated the assumptions required to translate heat flux at selected locations into estimates of total heat loss from an actively diving animal.
(c) Heat loss estimated from physical and theoretical models
Given the difficulty of extrapolating a few heat-flux measurements to the entire body, a number of researchers have used taxidermic mounts stretched over heated metal casts to estimate overall heat balance in a range of terrestrial microclimates. Results have been mixed, often with unacceptably large errors (Walsberg & Wolf 1996; Dzialowski 2005). In water, such physical models have the same problems as heat-flux sensors in duplicating the insulative air layers that are so carefully maintained by live birds and some mammals. This approach has been little used for aquatic endotherms (Williams 1986) and not for diving birds or large diving mammals.
For endotherms that lack an insulative air layer in fur or plumage, total heat loss has also been estimated by theoretical models of heat flux through internal tissue layers and across the interface between integument and water (Luecke et al. 1975; Watts et al. 1993; Boily 1995; Hind & Gurney 1997). Estimates from such models have seldom been validated by empirical measurements, especially for actively swimming animals (for an exception, see Hind & Gurney 1997). In one case, all input parameters were measured, and estimates of total heat loss were compared with Vo2 of inactive seals submerged in cold water. Large errors appeared to result from the fact that the blubber envelope and body core (mainly muscles and internal organs) were not concentrically positioned cylinders as they were in the model; heat flux was overestimated unless input data were based on direct morphometric measurements of the core (Kvadsheim et al. 1997). If one further considers the dive responses of bradycardia and peripheral vasoconstriction, the error in these models is potentially large and currently unassessed. Despite recent advances for horizontal swimming (Hind & Gurney 1997), such models need substantial validation and refinement before being used routinely to estimate thermal substitution or additional thermogenesis required in deep-diving animals.
(d) Respiratory heat loss
Evaporative heat loss in expired air may also be important to the heat balance of diving endotherms. When resting, both penguins and seals can decrease heat loss substantially by counter-current exchange of water and heat in nasal sinuses (Murrish 1973; Huntley et al. 1984). At low temperatures in air, several bird species have also altered their ventilation rates while increasing oxygen extraction rates to decrease respiratory heat loss (review in Johansen & Bech 1983; Brent et al. 1984; Stahel & Nicol 1988). Perhaps due to a combination of such mechanisms, respiratory heat loss in an inactive harp seal (Phoca groenlandica) submerged in cold water was only 1.8% of total metabolic heat production (Gallivan & Ronald 1979). Data for birds resting in air suggest that values may be higher in birds (Murrish 1973; Baudinette et al. 1986). Respiratory heat loss increases during exercise and can vary appreciably with ambient temperature and seasonality of insulation (Folkow & Mercer 1986). Effects of these factors on heat loss in air expired after exercise during breath-hold dives have not been investigated. Lacking such studies, it is not possible at present to make reliable predictions of respiratory heat loss in actively diving endotherms.
(e) Activity and heat generation by muscle
If total heat generation and loss are so difficult to quantify, might the onset and costs of residual thermogenesis (equation (3.2)) be determined from the activity of selected muscle groups? For shivering, groups of skeletal muscles appear to be recruited sequentially depending on muscle size and function. For example, in pigeons (Columba livia) and house finches (Carpodacus mexicanus) with large pectoral muscles for flight and only small leg muscles, pectoral muscles were the first to start shivering as ambient temperature declined during resting, with much lower temperature thresholds for shivering in leg muscles (Nomoto & Nomoto-Kozawa 1985; Carey et al. 1989). In domestic chickens (Gallus domesticus), which seldom fly and have much larger leg muscles, shivering started first in the leg muscles and began in pectoral muscles only at lower temperatures (Aulie & Tøien 1988). However, these patterns were for inactive birds, and exercise is believed to prevent shivering in active skeletal muscles while shivering can continue in muscles not being used for locomotion (Hong & Nadel 1979; Nomoto & Nomoto-Kozawa 1985; Rautenberg 1989; Hohtola et al. 1998). Thus, the first and primary muscles to be used for shivering in a resting animal would probably not be used for shivering during normal locomotion, when other muscles would be recruited instead. If alternative muscle groups important to shivering during exercise could be identified, their activity could perhaps be monitored in free-ranging animals to indicate the onset of residual thermogenesis. However, in diving animals, much of thermogenesis probably occurs after resurfacing (Wilson & Grémillet 1996) when large locomotor muscles may be available for shivering.
In terms of generating heat, the efficiency of producing mechanical power from chemical substrates by vertebrate skeletal muscle is approximately 25% (Taylor 1980). However, the efficiency of muscle contraction (and therefore heat produced) can vary appreciably with the mix of fibre types, muscle temperature, contraction speed and load (Holmer & Bergh 1974; Goldspink 1981; Pennycuick 1991). Substantial research would be needed to develop reliable estimates of total heat generation based on the activity of selected muscles.
(f) Heat increment of feeding
Although aspects such as peristalsis and enhanced blood flow play a role, HIF appears to result mostly from intermediary metabolism (Blaxter 1989). Heat increments for carbohydrate, fat and protein have been measured for a variety of endotherms at intake rates both above and below maintenance (Blaxter 1989), and conversion efficiencies for carbohydrate and fat are well understood and predictable (Schulz 1978; McDonald et al. 1981; Livesey 1984). However, carbohydrates and lipids have very low HIF compared with proteins, and HIF from protein metabolism varies appreciably with a range of factors including amino acid content of the food and amino acid needs of the animal (Krebs 1964; Buttery & Boorman 1976; Kielanowski 1976). Larger meals yield more HIF, and HIF usually increases as energy balance becomes more positive and more nutrients are used in anabolic rather than catabolic processes (Blaxter 1989; Robbins 1993). For birds that eat bivalves, crushing of shells in the gizzard might contribute to HIF (de Leeuw et al. 1998), but this effect was minimal when directly investigated (Piersma et al. 2003). Heating of foods obtained from cold water may also be a large fraction of HIF (Wilson & Culik 1991), although results have been mixed regarding the importance of heating food versus metabolic processing (Hawkins et al. 1997). Given all the factors affecting HIF of high-protein foods obtained from cold water, determining effects of HIF will in most cases require direct measurements with captive animals, using typical foods and meal sizes consumed on natural schedules. Simultaneous substitution of HIF and exercise heat has seldom been examined (Poehlman & Horton 1989; Kaseloo & Lovvorn 2006), but such studies are needed to understand patterns in actively foraging animals.
(g) Temperatures linked to thermogenic control
For deploying thermistors and microloggers on free-ranging animals, it is desirable to identify a few key temperature measurements that would integrate effects of heat generation and heat loss, and thereby indicate the degree of thermogenesis needed to restore body temperature after dives. For endotherms in air, much effort has been made to quantify the increase in whole-body Vo2 resulting from change in temperature of various tissues (in W kg−1 °C−1; Simon et al. 1986; Jessen 1990). A shortcoming of many of these studies was that the temperatures of particular tissues (skin, spinal cord, hypothalamus, lower gut, etc.) were manipulated independently while others were held constant. This approach eliminated important feedbacks from other sensors, and elicited responses quite different (and often inappropriate) from those under normal conditions of environmental cooling (Simon et al. 1986; Mercer & Simon 1987). These earlier manipulative studies indicated that deep-body sensors were more important than sensors in the skin for controlling thermogenesis, although it was recognized that the overall metabolic response reflected inputs from multiple temperature sensors from the skin, spinal cord, skeletal muscle and elsewhere throughout the body (Simon et al. 1986; Kuhnen & Jessen 1988; Jessen 1990).
In more recent studies under conditions of natural cold exposure, total metabolism (Vo2) in adult pigeons and ducklings responded to short-term changes in skin temperature well before changes in deep-body temperature occurred (Østnes & Bech 1997, 1998). In humans whose body temperature had dropped during submersion in cold water, shivering stopped as skin temperatures levelled off during rewarming, but well before deep-body temperatures had increased to normal values (Bullard & Rapp 1970). These results suggest that at the time-scale of one or a series of dives, skin temperature should be a better predictor of shivering thermogenesis. In birds, however, there can be large variations in skin temperature among areas of the body, such as bare skin versus feathered skin that either does or does not overlie active skeletal muscle; these areas in turn have differing influence on thermogenic responses (Necker 1977; Østnes & Bech 1997, 1998). Moreover, metabolic response to changes in skin temperature can have a dynamic component, where the magnitude of change in Vo2 (W kg−1 °C−1) depends on the rate of change in skin temperature (Kuhnen & Jessen 1988). Study of correlations between Vo2 and the magnitude and rates of change in skin temperature might identify a few key sites for measurements in the field. However, such research has not been done in diving endotherms.
9. Is thermal substitution predictable?
The above review suggests that our understanding of how heat generation is regulated, and how heat is transported among tissues during exercise, digestion and thermal challenge, is inadequate to predict the transition between thermal substitution and residual thermogenesis, and thus effects of thermoregulation on aerobic efficiencies (equation (3.2)). The situation is especially complicated in breath-hold divers. These animals may slow their heart rate and restrict blood flow to certain tissues while underwater and then re-perfuse these cooled tissues between dives, perhaps causing rapid reversals among tissues in relative temperature and metabolic activity. In the foreseeable future, it appears that estimates of aerobic efficiencies for modelling dive costs will continue to depend on Vo2 in captive animals under as relevant conditions as possible. Measurements should be made for endotherms swimming over the range of usual speeds for typical dive durations, at ambient field temperatures, eating realistic meals at usual intervals (Kaseloo & Lovvorn 2006). For shallow divers, realistic dive depths should be used to the extent possible (de Leeuw 1996; Enstipp et al. 2001). For deeper divers, work is needed to verify key assumptions that allow extrapolation of measurements in shallow tanks to deeper dives.
10. Extrapolation of aerobic efficiencies
(a) Preferred work rates and aerobic efficiencies
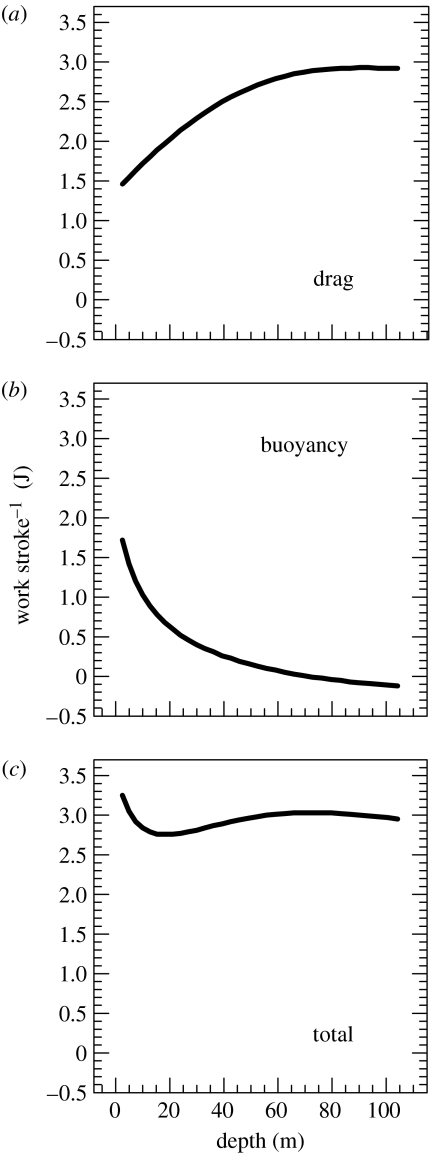
For deep-diving animals, given our dependence on measuring Vo2 and aerobic efficiencies in shallow tanks, what principles might aid in extrapolating such data to the field? Recent work suggests that diving birds regulate their swim speeds and stroke rates to maintain relatively constant work per stroke (Lovvorn et al. 2004; Watanuki et al. 2005). As the buoyancy of air spaces declined with increasing compression during descent, wing-propelled Brünnich's guillemots (Uria lomvia) gradually increased their speed and associated drag to maintain relatively constant work per stroke, as estimated from biomechanical models (figure 2). Unlike the guillemots, foot-propelled European shags (Phalacrocorax aristotelis) decreased their stroke frequencies as buoyancy decreased during descent; however, they too appeared to maintain relatively constant work per stroke, as indicated by heave acceleration perpendicular to the direction of motion (Watanuki et al. 2005). Based on work with isolated muscles and respirometry of humans peddling ergometers at different speeds and resistance (Hill 1950; Gibbs & Gibson 1972; Gaesser & Brooks 1975; Goldspink 1981; di Prampero 1989), it has long been inferred that muscles are adapted to function most efficiently over certain ranges of contraction speeds and loads (Pennycuick 1991; Lovvorn et al. 1999; Lovvorn 2001). Animals should avoid exercising outside these ranges owing to rapid declines in efficiency. Such constraints might be especially important for breath-hold divers, for which efficient use of oxygen is critical.
Figure 2.
Estimated work per stroke against (a) drag, (b) buoyancy, and (c) both combined by a Brünnich's guillemot descending to 105 m, based on time-depth and accelerometer data from a micrologger deployed on a free-ranging guillemot, estimated volumes of air in the respiratory system and plumage, and drag measurements for a frozen specimen at different speeds (Lovvorn et al. 2004).
It is also apparent, however, that the locomotor systems of animals can accommodate quite variable demands. For example, guillemots (Uria spp.) stroke their wings at approximately 8.7 Hz during flight in air, but at 1.9–2.8 Hz when swimming underwater in a medium 800 times denser than air (Pennycuick 1987). Mechanisms for accommodating such different conditions, and the consequences for exercise efficiency, are an active area of research (Boggs & Dial 1993; Biewener et al. 1998; Sokoloff et al. 1998; Biewener & Gillis 1999). Although there may be clear optimal conditions for achieving maximum power and efficiency, muscle can potentially achieve high levels of both over a range of conditions (Lichtwark & Wilson 2005). Low temperature reduces the efficiency of muscle contraction in the short term (Holmer & Bergh 1974; Swoap et al. 1993); and in the long term, functional and biochemical adaptations of muscles to chronic shivering may reduce their efficiency for exercise (Schaeffer et al. 2005). Thus, optimal work rates and efficiencies may change with water temperature. Moreover, given the potential for substituting the heat of inefficiency for thermoregulation, lower efficiencies at higher contraction speeds or greater loads might have reduced impacts on total costs at cold temperatures (Hind & Gurney 1997; Chai et al. 1998).
Nevertheless, a number of studies have shown that endotherms often choose to swim at certain speeds (Ponganis et al. 1990; Culik et al. 1991; Williams et al. 1993; Schmid et al. 1995; Allers & Culik 1997; Bethge et al. 1997; Pfeiffer & Culik 1998; Lovvorn et al. 2004; Watanuki et al. 2005). Work rates at these voluntary speeds presumably correspond to maximum aerobic efficiencies. If diving endotherms do seek a narrow range of efficiencies, measurements on captive animals might identify associated work rates for different body sizes and locomotor modes. These efficiencies might then be extrapolated to similar work rates estimated from biomechanical models for different conditions. For example, the aerobic efficiency at a given work rate during horizontal swimming, when there is higher speed against drag but little work against buoyancy, might be extrapolated to the same total work rate during descent at lower speed but with higher work against buoyancy (Banister & Jackson 1967).
(b) Caveats to extrapolation
Even if animals of comparable size tend to limit themselves to similar ranges of work rates, the same aerobic efficiencies cannot be assumed among animals with different swimming modes or types of insulation (Fish 1993, 1996). In analogous experiments (table 1), great cormorants (Phalacrocorax carbo) weighed 10% less and had RMR in air 18% lower than Adélie penguins (Pygoscelis adeliae). However, in water, RMR of the cormorants was 68% higher at 13°C than for the penguins at 4°C, and total costs of underwater swimming were 135% higher in the cormorants (table 1). Cormorants have partially wettable plumage that allows higher heat loss to water (Wilson & Grémillet 1996), and they also swim by foot propulsion which is generally less efficient than wing propulsion (Fish 1996; Lovvorn & Liggins 2002). Thus, the striking difference in swimming cost apparently resulted from both greater heat loss and lower propulsive efficiency in cormorants, which would lower their overall aerobic efficiency relative to penguins (see also Enstipp et al. 2005 regarding dive costs in cormorants and penguins). These studies and others show that aerobic efficiencies may differ appreciably between locomotor modes, even within the same species (Williams 1989). The overall significance of different locomotor efficiencies depends partly on thermal substitution, as more heat produced by less efficient locomotion may allow greater reductions in shivering (Hind & Gurney 1997; Chai et al. 1998). At present, we have no way to predict these interactions and must rely on empirical measurements at relevant temperatures.
Table 1.
Comparison of the metabolic costs of Adélie penguins and great cormorants resting (RMR) in air and water, and swimming horizontally underwater in a shallow tank.
A further limitation to extrapolating aerobic efficiencies (η, see §3) among locomotor modes is that values of η (mechanical power output/aerobic power input) are specific to the models used to estimate mechanical work. If a different model is used that differs in estimated mechanical cost, the new estimate must be divided by the original aerobic cost measurement to generate a new value of η that is specific to the different model (equation (3.1)). Although timely ecological applications may require use of standard values, practitioners should always be aware that values of η depend strongly on how well different models for the same or different swimming modes estimate mechanical work (see table 3 in Stephenson et al. 1989). For example, if one model accounts for 80% of the mechanical work while another accounts for only 70%, the calculated aerobic efficiency in the former case would be appreciably higher, despite using the same measurement of aerobic cost (Vo2).
(c) Cost of transport
To avoid uncertainties in the relative adequacy of mechanical work estimates (see §10b), it has been suggested that minimum cost of transport (COTmin) is a more standardized variable for prediction of swimming costs (Fish 1996, 2000). Cost of transport (J kg−1 m−1) is defined as the metabolic energy required to transport a unit mass a unit distance, and does not require biomechanical models for prediction. Animals are expected to select speeds that minimize COT.
Some species do choose to swim at speeds near their COTmin (Culik et al. 1991; Williams et al. 1993; Bethge et al. 1997). However, birds and mammals in fluids often travel slower than the ‘optimum’ speed (Schmid et al. 1995; Allers & Culik 1997; Fish et al. 1997; Pennycuick 1997; Pfeiffer & Culik 1998; Luna-Jorquera & Culik 2000). In some cases, COT does not change much between the slower speed and the apparent optimum; for example, in the study of great cormorants described in §10b (Schmid et al. 1995), COTmin was unchanged over speeds of 1.5–2.1 m s−1. However, power output increased by 23% over the same range and the birds preferred to swim at 1.5 m s−1. If the ability of muscles to generate power is not limiting, and COT does not change over this range of speeds, the birds should swim at 2.1 m s−1 because they cover the same distance at the same cost in less time. However, for steady cruising, the higher power at faster speeds might not be sustainable. The fact that the birds do not swim at the higher speed suggests a limit to acceptable inefficiency or power output, so that exercise becomes too inefficient or unsustainable if the work against drag increases above a threshold that occurs around 1.5 m s−1. These findings caution against using allometric or other broad patterns of COTmin to predict the swimming speed of a given species under particular conditions, without knowing aerobic efficiencies at different speeds (see also Pennycuick 1997).
11. Future prospects
Acquiring captive animals, obtaining adequate facilities and equipment, and conducting respirometry are costly and time-consuming. Consequently, for estimating foraging profitability and the extent of viable habitat, better ability to predict aerobic efficiencies under a range of conditions is very important to modelling based on biomechanical estimates. Advances are being made in understanding and estimating the mechanical costs of different swimming modes (Skrovan et al. 1999; Lovvorn & Liggins 2002; Johansson 2003). Improved electronic devices for free-ranging animals are providing more and better data (Sato et al. 2002; Watanuki et al. 2005), which can be used in developing mechanical models applicable to animals that cannot be recaptured to retrieve loggers (Lovvorn et al. 2004). At present, however, there are few known relationships for predicting aerobic efficiencies needed to convert those mechanical estimates to food requirements, and costly empirical measurements on captive animals seem essential. Moving towards predictive capability will require developing ways to measure and estimate thermal substitution, and its effects on aerobic efficiencies at depths not possible in dive tanks.
To help focus future efforts based on respirometry, I propose the following approach to predicting aerobic efficiencies for varying dive depths and water temperatures:
Assess the need to consider thermoregulation costs by seeking combinations of exercise levels and temperatures below which substitution can no longer replace lost heat and shivering begins.
At temperatures below the limits to substitution, assume that thermoregulation costs are additive to those of exercise. In other words, in calculating ηcold, aerobic power would include the cost of exercise within the range of substitution, plus any shivering (equation (3.2)).
Extrapolate the resulting aerobic efficiencies to varying depths and temperatures by assuming that work rates and aerobic power are conserved. In other words, work against buoyancy is included as part of the total work rate, and residual shivering depends on temperature independent of that total work rate.
By this construct, identifying the threshold temperature for onset of residual thermogenesis during exercise (equation (3.2)) would be akin to identifying the lower critical temperature of the thermoneutral zone for resting animals. That there is often a clear transition between substitution and shivering during exercise is yet to be determined (see McArdle et al. 1984); however, the lower critical temperature in a resting animal is usually quite recognizable, and similar principles may apply. Moreover, this construct does not yet deal with effects on heat loss of the compression of insulative air layers with depth, a topic we know very little about (see Grémillet et al. 1998). However, if the above approach can serve as a testable framework, it may facilitate progress towards merging biomechanics and physiology to determine energetic constraints on viable foraging conditions.
Acknowledgments
I thank P. Domenici, G. Claireaux and D. J. McKenzie for inviting me to participate in this symposium, and P. A. Kaseloo for many valuable discussions about the nature and measurement of thermal substitution. Financial support was provided by U.S. National Science Foundation grants OPP-9813979 and ARC-0454454, and by the California Department of Fish and Game's Oil Spill Response Trust Fund through the Oiled Wildlife Care Network at the Wildlife Health Centre, School of Veterinary Medicine, University of California, Davis.
Appendix A
Appendix B
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Allers D, Culik B.M. Energy requirements of beavers (Castor canadensis) swimming underwater. Physiol. Zool. 1997;70:456–463. doi: 10.1086/515852. [DOI] [PubMed] [Google Scholar]
- Aulie A, Tøien Ø. Threshold for shivering in aerobic and anaerobic muscles in bantam cocks and incubating hens. J. Comp. Physiol. B. 1988;158:431–435. doi: 10.1007/BF00691140. doi:10.1007/BF00691140 [DOI] [PubMed] [Google Scholar]
- Banister E.W, Jackson R.C. The effect of speed and load changes on oxygen intake for equivalent power outputs during bicycle ergometry. Int. Z. Angew. Physiol. 1967;24:284–290. doi: 10.1007/BF00698204. [DOI] [PubMed] [Google Scholar]
- Banta M.R, Lynott A.J, VanSant M.J, Bakken G.S. Partitioning heat loss from mallard ducklings swimming on the air–water interface. J. Exp. Biol. 2004;207:4551–4557. doi: 10.1242/jeb.01313. doi:10.1242/jeb.01313 [DOI] [PubMed] [Google Scholar]
- Baudinette R.V, Gill P, O'Driscoll M. Energetics of the little penguin, Eudyptula minor: temperature regulation, the calorigenic effect of food, and moulting. Aust. J. Zool. 1986;34:35–45. doi:10.1071/ZO9860035 [Google Scholar]
- Bech C, Præsteng K.E. Thermoregulatory use of heat increment of feeding in the tawny owl (Strix aluco) J. Therm. Biol. 2004;29:649–654. doi:10.1016/j.jtherbio.2004.08.034 [Google Scholar]
- Beck G.G, Smith T.G. Distribution of blubber in the northwest Atlantic harp seal, Phoca groenlandica. Can. J. Zool. 1995;73:1991–1998. [Google Scholar]
- Bethge P, Nicol S, Culik B.M, Wilson R.P. Diving behaviour and energetics in breeding little penguins (Eudyptula minor) J. Zool. Lond. 1997;242:483–502. [Google Scholar]
- Bevan R.M, Butler P.J. The effects of temperature on the oxygen consumption, heart rate and deep body temperature during diving in the tufted duck Aythya fuligula. J. Exp. Biol. 1992a;163:139–151. [Google Scholar]
- Bevan R.M, Butler P.J. Cardiac output and blood flow distribution during swimming and voluntary diving of the tufted duck (Aythya fuligula) J. Exp. Biol. 1992b;168:199–217. [Google Scholar]
- Bevan R.M, Boyd I.L, Butler P.J, Reid K, Woakes A.J, Croxall J.P. Heart rates and abdominal temperatures of free-ranging South Georgian shags, Phalacrocorax georgianus. J. Exp. Biol. 1997;200:661–675. doi: 10.1242/jeb.200.4.661. [DOI] [PubMed] [Google Scholar]
- Bevan R.M, Butler P.J, Woakes A.J, Boyd I.L. The energetics of gentoo penguins, Pygoscelis papua, during the breeding season. Funct. Ecol. 2002;16:175–190. doi:10.1046/j.1365-2435.2002.00622.x [Google Scholar]
- Biewener A.A, Gillis G.B. Dynamics of muscle function during locomotion: accommodating variable conditions. J. Exp. Biol. 1999;202:3387–3396. doi: 10.1242/jeb.202.23.3387. [DOI] [PubMed] [Google Scholar]
- Biewener A.A, Corning W.R, Tobalske B.W. In vivo pectoralis muscle force-length behavior during level flight in pigeons (Columba livia) J. Exp. Biol. 1998;201:3293–3307. doi: 10.1242/jeb.201.24.3293. [DOI] [PubMed] [Google Scholar]
- Blaxter K.M. Cambridge University Press; Cambridge, MA: 1989. Energy metabolism in animals and man. [Google Scholar]
- Boggs D.F, Dial K.P. Neuromuscular organization and regional EMG activity of the pectoralis in the pigeon. J. Morphol. 1993;218:43–57. doi: 10.1002/jmor.1052180104. doi:10.1002/jmor.1052180104 [DOI] [PubMed] [Google Scholar]
- Boily P. Theoretical heat flux in water and habitat selection of phocid seals and beluga whales during the annual molt. J. Theor. Biol. 1995;172:235–244. doi:10.1006/jtbi.1995.0020 [Google Scholar]
- Boutilier C, Bougues L, Timbal J. Experimental study of convective heat transfer coefficient for the human body. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977;42:93–100. doi: 10.1152/jappl.1977.42.1.93. [DOI] [PubMed] [Google Scholar]
- Boyd I.L. Skin temperatures during free-ranging swimming and diving in Antarctic fur seals. J. Exp. Biol. 2000;203:1907–1914. doi: 10.1242/jeb.203.12.1907. [DOI] [PubMed] [Google Scholar]
- Brent R, Pedersen P.F, Bech C, Johansen K. Lung ventilation and temperature regulation in the European coot Fulica atra. Physiol. Zool. 1984;57:19–25. [Google Scholar]
- Bruinzeel L.W, Piersma T. Cost reduction in the cold: heat generated by terrestrial locomotion partly substitutes for thermoregulation costs in knot Calidris canutus. Ibis. 1998;140:323–328. [Google Scholar]
- Bryant D.M, Hails C.J, Prys-Jones R. Energy expenditure by free-living dippers (Cinclus cinclus) in winter. Condor. 1985;87:177–186. doi:10.2307/1366880 [Google Scholar]
- Bullard R.W, Rapp G.M. Problems of body heat loss in water immersion. Aerosp. Med. 1970;41:1269–1277. [PubMed] [Google Scholar]
- Butler P.J, Green J.A, Boyd I.L, Speakman J.R. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 2004;18:168–183. doi:10.1111/j.0269-8463.2004.00821.x [Google Scholar]
- Buttery P.J, Boorman K.N. The energetic efficiency of amino acid metabolism. In: Cole D.J.A, Boorman K.N, Buttery P.J, Lewis D, Neale R.J, Swan H, editors. Protein metabolism and nutrition. Butterworths; Boston, MA: 1976. pp. 197–206. [Google Scholar]
- Campbell K.L, McIntyre I.W, MacArthur R.A. Postprandial heat increment does not substitute for active thermogenesis in cold-challenged star-nosed moles (Condylura cristata) J. Exp. Biol. 2000;203:301–310. doi: 10.1242/jeb.203.2.301. [DOI] [PubMed] [Google Scholar]
- Carey C, Johnston R.M, Bekoff A. Thermal thresholds for recruitment of muscles during shivering in winter acclimatized house finches. In: Mercer J.B, editor. Thermal physiology. Elsevier; Amsterdam, The Netherlands: 1989. pp. 685–690. [Google Scholar]
- Chai P, Chang A.C, Dudley R. Flight thermogenesis and energy conservation in hovering hummingbirds. J. Exp. Biol. 1998;201:963–968. doi: 10.1242/jeb.201.7.963. [DOI] [PubMed] [Google Scholar]
- Chappell M.A, Bachman G.C, Hammond K.A. The heat increment of feeding in house wren chicks: magnitude, duration, and substitution for thermostatic costs. J. Comp. Physiol. B. 1997;167:313–318. doi:10.1007/s003600050079 [Google Scholar]
- Cherepanova V, Neshumova T, Elsner R. Muscle blood flow in diving mammals. Comp. Biochem. Physiol. A. 1993;106:1–6. doi: 10.1016/0300-9629(93)90029-4. doi:10.1016/0300-9629(93)90029-4 [DOI] [PubMed] [Google Scholar]
- Cooper T, Randall W.C, Hertzman A.B. Vascular convection of heat from active muscle to overlying skin. J. Appl. Physiol. 1959;14:207–211. doi: 10.1152/jappl.1959.14.2.207. [DOI] [PubMed] [Google Scholar]
- Costa D.P, Kooyman G.L. Oxygen consumption, thermoregulation, and the effect of fur oiling and washing on the sea otter, Enhydra lutris. Can. J. Zool. 1982;60:2761–2767. [Google Scholar]
- Costa D.P, Kooyman G.L. Contribution of specific dynamic action to heat balance and thermoregulation in the sea otter Enhydra lutris. Physiol. Zool. 1984;57:199–203. [Google Scholar]
- Croll D.A, McLaren E. Diving metabolism and thermoregulation in common and thick-billed murres. J. Comp. Physiol. B. 1993;163:160–166. doi: 10.1007/BF00263602. doi:10.1007/BF00263602 [DOI] [PubMed] [Google Scholar]
- Culik B.M, Wilson R.P, Dannfeld R, Adelung D, Spairani H.J, Coco Coria N.R. Pygoscelid penguins in a swim canal. Polar Biol. 1991;11:277–282. doi:10.1007/BF00238463 [Google Scholar]
- Culik B.M, Wilson R.P, Bannasch R. Under-water swimming at low energetic cost by pygoscelid penguins. J. Exp. Biol. 1994;197:65–78. doi: 10.1242/jeb.197.1.65. [DOI] [PubMed] [Google Scholar]
- Culik B.M, Pütz K, Wilson R.P, Bost C.A, Le Maho Y, Verselin J.-L. Core temperature variability in diving king penguins (Aptenodytes patagonicus): a preliminary report. Polar Biol. 1996;16:371–378. [Google Scholar]
- Daniel T.L. Efficiency in aquatic locomotion: limitations from single cells to animals. In: Blake R.W, editor. Efficiency and economy in animal physiology. Cambridge University Press; Cambridge, MA: 1991. pp. 83–95. [Google Scholar]
- Davis R.W, Kanatous S.B. Convective oxygen transport and tissue oxygen consumption in Weddell seals during aerobic dives. J. Exp. Biol. 1999;202:1091–1113. doi: 10.1242/jeb.202.9.1091. [DOI] [PubMed] [Google Scholar]
- Dawson T.J, Fanning F.D. Thermal and energetic problems of semiaquatic mammals: a study of the Australian water rat, including comparisons with the platypus. Physiol. Zool. 1981;54:285–296. [Google Scholar]
- de Leeuw J.J. Diving costs as a component of daily energy budgets of aquatic birds and mammals: generalizing the inclusion of dive-recovery costs demonstrated in tufted ducks. Can. J. Zool. 1996;71:720–730. [Google Scholar]
- de Leeuw J.J, Butler P.J, Woakes A.J, Zegward F. Body cooling and its energetic implications for feeding and diving in tufted ducks. Physiol. Zool. 1998;71:720–730. doi: 10.1086/516003. [DOI] [PubMed] [Google Scholar]
- di Prampero H.P.E. Shortening speed and muscular efficiency: from frog sartorius to exercising man. In: Wieser W, Gnaiger E, editors. Energy transformations in cells and organisms. Georg Thieme Verlag Stuttgart; New York, NY: 1989. pp. 46–53. [Google Scholar]
- de Vries J, van Eerden M.R. Thermal conductance in aquatic birds in relation to the degree of water contact, body mass, and body fat: energetic implications of living in a strong cooling environment. Physiol. Zool. 1995;68:1143–1163. [Google Scholar]
- Dunkin R.C, McLellan W.A, Blum J.E, Pabst D.A. The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J. Exp. Biol. 2005;208:1469–1480. doi: 10.1242/jeb.01559. doi:10.1242/jeb.01559 [DOI] [PubMed] [Google Scholar]
- Dzialowski E.M. Use of operative temperature and standard operative temperature models in thermal biology. J. Therm. Biol. 2005;30:317–334. doi:10.1016/j.jtherbio.2005.01.005 [Google Scholar]
- Enstipp M.R, Andrews R.D, Jones D.R. The effects of depth on the cardiac and behavioural responses of double-crested cormorants (Phalacrocorax auritus) during voluntary diving. J. Exp. Biol. 2001;204:4081–4092. doi: 10.1242/jeb.204.23.4081. [DOI] [PubMed] [Google Scholar]
- Enstipp M.R, Grémillet D, Lorentsen S.-H. Energetic costs of diving and thermal status in European shags (Phalacrocorax aristotelis) J. Exp. Biol. 2005;208:3451–3461. doi: 10.1242/jeb.01791. doi:10.1242/jeb.01791 [DOI] [PubMed] [Google Scholar]
- Evans K.E, Moen A.N. Thermal exchange between sharp-tailed grouse (Pedioecetes phasianellus) and their winter environment. Condor. 1975;77:160–168. doi:10.2307/1365786 [Google Scholar]
- Fish F.E. Thermoregulation in the muskrat (Ondatra zibethicus): the use of regional heterothermia. Comp. Biochem. Physiol. A. 1979;64:391–397. doi:10.1016/0300-9629(79)90459-6 [Google Scholar]
- Fish F.E. Metabolic effects of swimming velocity and water temperature in the muskrat (Ondatra zibethicus) Comp. Biochem. Physiol. A. 1983;75:397–400. doi: 10.1016/0300-9629(83)90100-7. doi:10.1016/0300-9629(83)90100-7 [DOI] [PubMed] [Google Scholar]
- Fish F.E. Influence of hydrodynamic design and propulsive mode on mammalian swimming energetics. Aust. J. Zool. 1993;42:79–101. doi:10.1071/ZO9940079 [Google Scholar]
- Fish F.E. Transition from drag-based to lift-based propulsion in mammalian swimming. Am. Zool. 1996;36:628–641. [Google Scholar]
- Fish F.E. Biomechanics and energetics in aquatic and semiaquatic mammals: platypus to whale. Physiol. Biochem. Zool. 2000;73:683–698. doi: 10.1086/318108. doi:10.1086/318108 [DOI] [PubMed] [Google Scholar]
- Fish F.E, Baudinette R.V, Frappell P.B, Sarre M.P. Energetics of swimming by the platypus Ornithorhynchus anatinus: metabolic effort associated with rowing. J. Exp. Biol. 1997;200:2647–2652. doi: 10.1242/jeb.200.20.2647. [DOI] [PubMed] [Google Scholar]
- Folkow L.P, Mercer J.B. Partition of heat loss in resting and exercising winter- and summer-insulated reindeer. Am. J. Physiol. 1986;251:R32–R40. doi: 10.1152/ajpregu.1986.251.1.R32. [DOI] [PubMed] [Google Scholar]
- Frisch J, Øritsland N.A, Krog J. Insulation of furs in water. Comp. Biochem. Physiol. A. 1974;47:403–410. doi: 10.1016/0300-9629(74)90002-4. doi:10.1016/0300-9629(74)90002-4 [DOI] [PubMed] [Google Scholar]
- Froget G, Handrich Y, Le Maho Y, Rouanet J.-L, Woakes A.J, Butler P.J. The heart rate/oxygen consumption relationship during cold exposure of the king penguin: a comparison with that during exercise. J. Exp. Biol. 2002;205:2511–2517. doi: 10.1242/jeb.205.16.2511. [DOI] [PubMed] [Google Scholar]
- Froget G, Butler P.J, Woakes A.J, Fahlman A, Kuntz G, Le Maho Y, Handrich Y. Heart rate and energetics of free-ranging king penguins (Aptenodytes patagonicus) J. Exp. Biol. 2004;207:3917–3926. doi: 10.1242/jeb.01232. doi:10.1242/jeb.01232 [DOI] [PubMed] [Google Scholar]
- Fryxell J.M, Wilmshurst J.F, Sinclair A.R.E. Predictive models of movement by Serengeti grazers. Ecology. 2004;85:2429–2435. [Google Scholar]
- Gaesser G.A, Brooks G.A. Muscular efficiency during steady-state exercise: effects of speed and work rate. J. Appl. Physiol. 1975;38:1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- Gallivan G.J, Ronald K. Temperature regulation in freely diving harp seals (Phoca groenlandica) Can. J. Zool. 1979;57:2256–2263. doi: 10.1139/z79-293. [DOI] [PubMed] [Google Scholar]
- Gibbs C.L, Gibson W.R. Energy production of rat soleus muscle. Am. J. Physiol. 1972;223:864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- Goldspink G. The use of muscles during flying, swimming, and running from the point of view of energy saving. Symp. Zool. Soc. Lond. 1981;48:219–238. [Google Scholar]
- Grémillet D, Tuschy I, Kierspel M. Body temperature and insulation in diving great cormorants and European shags. Funct. Ecol. 1998;12:386–394. doi:10.1046/j.1365-2435.1998.00199.x [Google Scholar]
- Guillemette M, Woakes A.J, Henaux V, Grandbois J.-M, Butler P.J. The effect of depth on the diving behaviour of common eiders. Can. J. Zool. 2004;82:1818–1826. doi:10.1139/z04-180 [Google Scholar]
- Handrich Y, Bevan R.M, Charrassin J.-B, Butler P.J, Pütz K, Woakes A.J, Lage J, Le Maho Y. Hypothermia in foraging king penguins. Nature. 1997;388:64–67. doi:10.1038/40392 [Google Scholar]
- Hart J.S, Irving L. The energetics of harbor seals in air and water with special consideration of seasonal change. Can. J. Zool. 1959;37:447–457. [Google Scholar]
- Hart J.S, Jansky L. Thermogenesis due to exercise and cold in warm- and cold-acclimated rats. Can. J. Biochem. Physiol. 1963;41:629–634. [PubMed] [Google Scholar]
- Hawkins P.A.J, Butler P.J, Woakes A.J, Gabrielsen G.W. Heat increment of feeding in Brünnich's guillemot Uria lomvia. J. Exp. Biol. 1997;200:1757–1763. doi: 10.1242/jeb.200.12.1757. [DOI] [PubMed] [Google Scholar]
- Hawkins P.A.J, Butler P.J, Woakes A.J, Speakman J.R. Estimation of the rate of oxygen consumption of the common eider duck (Somateria mollissima), with some measurements of heart rate during voluntary dives. J. Exp. Biol. 2000;203:2819–2832. doi: 10.1242/jeb.203.18.2819. [DOI] [PubMed] [Google Scholar]
- Hill A.V. The dimensions of animals and their muscular dynamics. Sci. Prog. 1950;38:209–230. [Google Scholar]
- Hill R.W, Beaver D.L, Veighte J.H. Body surface temperatures and thermoregulation in the black-capped chickadee (Parus atricapillus) Physiol. Zool. 1980;53:305–321. [Google Scholar]
- Hind A.T, Gurney W.S.C. The metabolic cost of swimming in marine homeotherms. J. Exp. Biol. 1997;200:531–542. doi: 10.1242/jeb.200.3.531. [DOI] [PubMed] [Google Scholar]
- Hindle A.G, McIntyre I.W, Campbell K.L, MacArthur R.A. The heat increment of feeding and its thermoregulatory implications in the short-tailed shrew (Blarina brevicauda) Can. J. Zool. 2003;81:1445–1453. doi:10.1139/z03-137 [Google Scholar]
- Hochachka P.W. Metabolic suppression and oxygen availability. Can. J. Zool. 1988;66:152–158. [Google Scholar]
- Hohtola E, Henderson R.P, Rashotte M.E. Shivering thermogenesis in the pigeon: the effects of activity, diurnal factors, and feeding state. Am. J. Physiol. 1998;275:R1553–R1562. doi: 10.1152/ajpregu.1998.275.5.R1553. [DOI] [PubMed] [Google Scholar]
- Holmer I, Bergh U. Metabolic and thermal response to swimming in water at various temperatures. J. Appl. Physiol. 1974;37:702–705. doi: 10.1152/jappl.1974.37.5.702. [DOI] [PubMed] [Google Scholar]
- Hong S.-L, Nadel E.R. Thermogenic control during exercise in a cold environment. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979;47:1084–1089. doi: 10.1152/jappl.1979.47.5.1084. [DOI] [PubMed] [Google Scholar]
- Huntley A.C, Costa D.P, Rubin R.D. The contribution of nasal countercurrent heat exchange to water balance in the northern elephant seal, Mirounga angustirostris. J. Exp. Biol. 1984;113:447–454. doi: 10.1242/jeb.113.1.447. [DOI] [PubMed] [Google Scholar]
- Jensen P.G, Pekins P.J, Holter J.B. Compensatory effect of the heat increment of feeding on thermoregulation costs of white-tailed deer fawns in winter. Can. J. Zool. 1999;77:1474–1485. doi:10.1139/cjz-77-9-1474 [Google Scholar]
- Jessen C. Thermal afferents in the control of body temperature. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. Pergamon Press; New York, NY: 1990. pp. 153–183. [Google Scholar]
- Johansen K, Millard R.W. Vascular responses to temperature in the foot of the giant fulmar, Macronectes giganteus. J. Comp. Physiol. 1973;85:47–64. doi:10.1007/BF00694140 [Google Scholar]
- Johansen K, Bech C. Heat conservation during cold exposure in birds (vasomotor and respiratory implications) Polar Res. 1983;1:259–268. [Google Scholar]
- Johansson L.C. Indirect estimates of wing-propulsion forces in horizontally diving Atlantic puffins (Fratercula arctica L.) Can. J. Zool. 2003;81:816–822. doi:10.1139/z03-058 [Google Scholar]
- Kaseloo P.A, Lovvorn J.R. Heat increment of feeding and thermal substitution in mallard ducks feeding voluntarily on grain. J. Comp. Physiol. B. 2003;173:207–213. doi: 10.1007/s00360-002-0321-9. [DOI] [PubMed] [Google Scholar]
- Kaseloo P.A, Lovvorn J.R. Effects of surface activity patterns and dive depth on thermal substitution in fasted and fed lesser scaup ducks. Can. J. Zool. 2005;83:301–311. doi:10.1139/z05-012 [Google Scholar]
- Kaseloo P.A, Lovvorn J.R. Substitution of heat from exercise and digestion by ducks diving for mussels at varying depths and temperatures. J. Comp. Physiol. B. 2006;176:265–275. doi: 10.1007/s00360-005-0047-6. doi:10.1007/s00360-005-0047-6 [DOI] [PubMed] [Google Scholar]
- Kasting N.W, Adderley S.A.L, Safford T, Hewlett K.G. Thermoregulation in beluga (Delphinapterus leucas) and killer (Orcinus orca) whales. Physiol. Zool. 1989;62:687–701. [Google Scholar]
- Ketterson E.D, King J.R. Metabolic and behavioral responses to fasting in the white-crowned sparrow (Zonotrichia leucophrys gambelii) Physiol. Zool. 1977;50:115–129. [Google Scholar]
- Kielanowski J. Energy cost of protein deposition. In: Cole D.J.A, Boorman K.N, Buttery P.J, Lewis D, Neale R.J, Swan H, editors. Protein metabolism and nutrition. Butterworths; Boston, MA: 1976. pp. 207–215. [Google Scholar]
- Kilgore D.L, Schmidt-Nielsen K. Heat loss from ducks' feet immersed in cold water. Condor. 1975;77:475–478. doi:10.2307/1366094 [Google Scholar]
- Klaassen M, Bech C, Slagsvold G. Basal metabolic rate and thermal conductance in arctic tern chicks and the effect of heat increment of feeding on thermoregulatory expenses. Ardea. 1989;77:193–200. [Google Scholar]
- Kooyman G.L, Gentry R.L, Bergman W.P, Hammel H.T. Heat loss in penguins during immersion and compression. Comp. Biochem. Physiol. A. 1976;54:75–80. doi: 10.1016/s0300-9629(76)80074-6. doi:10.1016/S0300-9629(76)80074-6 [DOI] [PubMed] [Google Scholar]
- Kooyman G.L, Ponganis P.J, Castellini M.A, Ponganis E.P, Ponganis K.V, Thorson P.H, Eckert S.A, LeMaho Y. Heart rates and swim speeds of emperor penguins diving under sea ice. J. Exp. Biol. 1992;165:161–180. doi: 10.1242/jeb.165.1.161. [DOI] [PubMed] [Google Scholar]
- Krebs H.A. The metabolic fate of amino acids. In: Munro H.N, Allison J.B, editors. Mammalian protein metabolism. Academic Press; New York, NY: 1964. pp. 125–176. [Google Scholar]
- Kruuk H, Balharry E, Taylor P.T. Oxygen consumption of the Eurasian otter Lutra lutra in relation to water temperature. Physiol. Zool. 1994;67:1174–1185. [Google Scholar]
- Kuhnen G, Jessen C. The metabolic response to skin temperature. Pflügers Archiv. 1988;412:402–408. doi: 10.1007/BF01907559. doi:10.1007/BF01907559 [DOI] [PubMed] [Google Scholar]
- Kvadsheim P.H, Gotaas A.R.L, Folkow L.P, Blix A.S. An experimental validation of heat loss models for marine mammals. J. Theor. Biol. 1997;184:15–23. doi:10.1006/jtbi.1996.0256 [Google Scholar]
- Kvadsheim P.H, Folkow L.P, Blix A.S. Inhibition of shivering in hypothermic seals during diving. Am. J. Physiol. 2005;289:R326–R331. doi: 10.1152/ajpregu.00708.2004. [DOI] [PubMed] [Google Scholar]
- Lichtwark G.A, Wilson A.M. Effects of series elasticity and activation conditions on muscle power output and efficiency. J. Exp. Biol. 2005;208:2845–2853. doi: 10.1242/jeb.01710. doi:10.1242/jeb.01710 [DOI] [PubMed] [Google Scholar]
- Liu H, Wassersug R.J, Kawachi K. A computational fluid dynamics study of tadpole swimming. J. Exp. Biol. 1996;199:1245–1260. doi: 10.1242/jeb.199.6.1245. [DOI] [PubMed] [Google Scholar]
- Livesey G. The energy equivalents of ATP and the energy values of food proteins and fats. Br. J. Nutr. 1984;51:15–28. doi: 10.1079/bjn19840005. doi:10.1079/BJN19840005 [DOI] [PubMed] [Google Scholar]
- Lovvorn J.R. Biomechanics and foraging profitability: an approach to assessing trophic needs and impacts of diving ducks. Hydrobiologia. 1994;279–280:223–233. doi:10.1007/BF00027856 [Google Scholar]
- Lovvorn J.R. Upstroke thrust, drag effects, and stroke-glide cycles in wing-propelled swimming by birds. Am. Zool. 2001;41:154–165. doi:10.1668/0003-1569(2001)041[0154:UTDEAS]2.0.CO;2 [Google Scholar]
- Lovvorn J.R, Gillingham M.P. Food dispersion and foraging energetics: a mechanistic synthesis for field studies of avian benthivores. Ecology. 1996;77:435–451. doi:10.2307/2265620 [Google Scholar]
- Lovvorn J.R, Jones D.R. Effects of body size, body fat, and change in pressure with depth on buoyancy and costs of diving in ducks (Aythya spp.) Can. J. Zool. 1991;69:2879–2887. [Google Scholar]
- Lovvorn J.R, Liggins G.A. Interactions of body shape, body size and stroke-acceleration patterns in costs of underwater swimming by birds. Funct. Ecol. 2002;16:106–112. doi:10.1046/j.0269-8463.2001.00604.x [Google Scholar]
- Lovvorn J.R, Jones D.R, Blake R.W. Mechanics of underwater locomotion in diving ducks: drag, buoyancy and acceleration in a size gradient of species. J. Exp. Biol. 1991;159:89–108. [Google Scholar]
- Lovvorn J.R, Croll D.A, Liggins G.A. Mechanical versus physiological determinants of swimming speeds in diving Brünnich's guillemots. J. Exp. Biol. 1999;202:1741–1752. doi: 10.1242/jeb.202.13.1741. [DOI] [PubMed] [Google Scholar]
- Lovvorn J.R, Watanuki Y, Kato A, Naito Y, Liggins G.A. Stroke patterns and regulation of swim speed and energy cost in free-ranging Brünnich's guillemots. J. Exp. Biol. 2004;207:4679–4695. doi: 10.1242/jeb.01331. doi:10.1242/jeb.01331 [DOI] [PubMed] [Google Scholar]
- Luecke R.H, Natarajan V, South F.E. A mathematical biothermal model of the California sea lion. J. Therm. Biol. 1975;1:35–45. doi:10.1016/0306-4565(75)90009-1 [Google Scholar]
- Luna-Jorquera G, Culik B.M. Metabolic rates of swimming Humboldt penguins. Mar. Ecol. Prog. Ser. 2000;203:301–309. [Google Scholar]
- Luo J, Hartman K.J, Brandt S.B, Cerco C.F, Rippetoe T.H. A spatially-explicit approach for estimating carrying capacity: an application for the Atlantic menhaden (Brevoortia tyrannus) in Chesapeake Bay. Estuaries. 2001;24:545–556. doi:10.2307/1353256 [Google Scholar]
- MacArthur R.A. Aquatic mammals in cold. Adv. Comp. Environ. Physiol. 1989;4:289–325. [Google Scholar]
- MacArthur R.A, Campbell K.L. Heat increment of feeding and its thermoregulatory benefit in the muskrat (Ondatra zibethicus) J. Comp. Physiol. B. 1994;164:141–146. doi: 10.1007/BF00301656. doi:10.1007/BF00301656 [DOI] [PubMed] [Google Scholar]
- MacArthur R.A, Krause R.E. Energy requirements of freely diving muskrats (Ondatra zibethicus) Can. J. Zool. 1989;67:2194–2200. [Google Scholar]
- Marjoniemi K. The effect of short-term fasting on shivering thermogenesis in Japanese quail chicks (Coturnix coturnix japonica): indications for a significant role of diet-induced/growth related thermogenesis. J. Therm. Biol. 2000;25:459–465. doi: 10.1016/s0306-4565(00)00011-5. doi:10.1016/S0306-4565(00)00011-5 [DOI] [PubMed] [Google Scholar]
- Markussen N.H, Ryg M, Øritsland N.A. The effect of feeding on the metabolic rate in harbour seals (Phoca vitulina) J. Comp. Physiol. B. 1994;164:89–93. doi: 10.1007/BF00301648. doi:10.1007/BF00301648 [DOI] [PubMed] [Google Scholar]
- Masman D, Daan S, Dietz M. Heat increment of feeding in the kestrel, Falco tinnunculus, and its natural seasonal variation. In: Bech C, Reinertsen R.E, editors. Physiology of cold adaptation in birds. Plenum Press; New York, NY: 1989. pp. 123–135. [Google Scholar]
- McArdle W.D, Magel J.R, Spina R.J, Gergley T.J, Toner M.M. Thermal adjustment to cold-water exposure in exercising men and women. Am. J. Physiol. Respir. Environ. Exerc. Physiol. 1984;56:1572–1577. doi: 10.1152/jappl.1984.56.6.1572. [DOI] [PubMed] [Google Scholar]
- McDonald P, Edwards R.A, Greenhalgh J.F.D. 3rd edn. Longman; London, UK: 1981. Animal nutrition. [Google Scholar]
- McGinnis S.M, Whittow G.C, Ohata C.A, Huber H. Body heat dissipation and conservation in two species of dolphins. Comp. Biochem. Physiol. A. 1972;43:417–423. doi: 10.1016/0300-9629(72)90200-9. doi:10.1016/0300-9629(72)90200-9 [DOI] [PubMed] [Google Scholar]
- McPhee J.M, Rosen D.A.S, Andrews R.D, Trites A.W. Predicting metabolic rate from heart rate in juvenile Steller sea lions Eumetopias jubatus. J. Exp. Biol. 2003;206:1941–1951. doi: 10.1242/jeb.00369. doi:10.1242/jeb.00369 [DOI] [PubMed] [Google Scholar]
- Mercer J.B, Simon E. Appropriate and inappropriate hypothalamic cold thermosensitivity in willow ptarmigan. Acta Physiol. Scand. 1987;131:73–80. doi: 10.1111/j.1748-1716.1987.tb08207.x. [DOI] [PubMed] [Google Scholar]
- Midtgård U. Heat loss from the feet of mallards Anas platyrhynchos and arterio-venous heat exchange in the Rete tibiotarsale. Ibis. 1980;122:354–359. [Google Scholar]
- Murrish D.E. Respiratory heat and water exchange in penguins. Respir. Physiol. 1973;19:271–278. doi: 10.1016/0034-5687(73)90030-3. doi:10.1016/0034-5687(73)90030-3 [DOI] [PubMed] [Google Scholar]
- Nagy K.A, Siegfried W.R, Wilson R.P. Energy utilization by free-ranging jackass penguins, Spheniscus demersus. Ecology. 1984;65:1648–1655. doi:10.2307/1939143 [Google Scholar]
- Necker R. Thermal sensitivity of different skin areas in pigeons. J. Comp. Physiol. 1977;116:239–246. doi:10.1007/BF00605405 [Google Scholar]
- Nolet B.A, Kruuk H. Grooming and resting of otters Lutra lutra in a marine habitat. J. Zool. Lond. 1989;218:433–440. [Google Scholar]
- Nomoto S, Nomoto-Kozawa E. EMG activity in pectoral and femoral muscles during spinal cord cooling in exercising pigeons. Pflügers Arch. 1985;404:337–341. doi: 10.1007/BF00585345. doi:10.1007/BF00585345 [DOI] [PubMed] [Google Scholar]
- Nomoto S, Rautenberg W, Iriki M. Temperature regulation during exercise in the Japanese quail (Coturnix coturnix japonica) J. Comp. Physiol. B. 1983;149:519–525. doi:10.1007/BF00690011 [Google Scholar]
- Noren D.P, Williams T.M, Berry P, Butler E. Thermoregulaton during swimming and diving in bottlenose dolphins, Tursiops truncatus. J. Comp. Physiol. B. 1999;169:93–99. doi: 10.1007/s003600050198. doi:10.1007/s003600050198 [DOI] [PubMed] [Google Scholar]
- Øritsland N.A. Temperature regulation of the polar bear (Thalarctos maritimus) Comp. Biochem. Physiol. 1970;37:225–233. doi:10.1016/0010-406X(70)90547-5 [Google Scholar]
- Østnes J.E, Bech C. Ontogeny of deep-body cold sensitivity in Pekin ducklings Anas platyrhynchos. J. Comp. Physiol. B. 1997;167:241–248. doi:10.1007/s003600050070 [Google Scholar]
- Østnes J.E, Bech C. Thermal control of metabolic cold defence in pigeons Columba livia. J. Exp. Biol. 1998;201:793–803. doi: 10.1242/jeb.201.6.793. [DOI] [PubMed] [Google Scholar]
- Paladino F.V, King J.R. Thermoregulation and oxygen consumption during terrestrial locomotion by white-crowned sparrows Zonotrichia leucophrys gambelii. Physiol. Zool. 1984;57:226–236. [Google Scholar]
- Pennycuick C.J. Flight of seabirds. In: Croxall J.P, editor. Seabirds: feeding ecology and role in marine ecosystems. Cambridge University Press; Cambridge, MA: 1987. pp. 43–62. [Google Scholar]
- Pennycuick C.J. Oxford University Press; Oxford, UK: 1989. Bird flight performance: a practical calculation manual. [Google Scholar]
- Pennycuick C.J. Adapting skeletal muscle to be efficient. In: Blake R.W, editor. Efficiency and economy in animal physiology. Cambridge University Press; Cambridge, MA: 1991. pp. 33–42. [Google Scholar]
- Pennycuick C.J. Actual and ‘optimum’ flight speeds: field data reassessed. J. Exp. Biol. 1997;200:2355–2361. doi: 10.1242/jeb.200.17.2355. [DOI] [PubMed] [Google Scholar]
- Pfeiffer P, Culik B.M. Energy metabolism of underwater swimming in river-otters (Lutra lutra L.) J. Comp. Physiol. B. 1998;168:143–148. doi: 10.1007/s003600050130. doi:10.1007/s003600050130 [DOI] [PubMed] [Google Scholar]
- Piersma T, Dekinga A, van Gils J.A, Achterkamp B, Visser G.H. Cost-benefit analysis of mollusc eating in a shorebird. I. Foraging and processing costs estimated by the doubly labeled water method. J. Exp. Biol. 2003;206:3361–3368. doi: 10.1242/jeb.00545. doi:10.1242/jeb.00545 [DOI] [PubMed] [Google Scholar]
- Poehlman E.T, Horton E.S. The impact of food intake and exercise on energy expenditure. Nutr. Rev. 1989;47:129–137. doi: 10.1111/j.1753-4887.1989.tb02817.x. [DOI] [PubMed] [Google Scholar]
- Pohl H. Some factors influencing the metabolic response to cold in birds. Fed. Proc. 1969;28:1059–1064. [PubMed] [Google Scholar]
- Pohl H, West G.C. Daily and seasonal variation in metabolic response to cold during rest and forced exercise in the common redpoll. Comp. Biochem. Physiol. A. 1973;45:851–867. doi: 10.1016/0300-9629(73)90088-1. doi:10.1016/0300-9629(73)90088-1 [DOI] [PubMed] [Google Scholar]
- Ponganis P.J, Ponganis E.P, Ponganis K.V, Kooyman G.L, Gentry R.L. Swimming velocities in otariids. Can. J. Zool. 1990;68:2105–2112. [Google Scholar]
- Ponganis P.J, Van Dam R.P, Knower T, Levenson D.H. Temperature regulation in emperor penguins foraging under sea ice. Comp. Biochem. Physiol. A. 2001;129:811–820. doi: 10.1016/s1095-6433(01)00349-x. doi:10.1016/S1095-6433(01)00349-X [DOI] [PubMed] [Google Scholar]
- Ponganis P.J, Van Dam R.P, Levenson D.H, Knower T, Ponganis K.V, Marshall G. Regional heterothermy and conservation of core temperature in emperor penguins diving under sea ice. Comp. Biochem. Physiol. A. 2003;135:477–487. doi: 10.1016/s1095-6433(03)00133-8. doi:10.1016/S1095-6433(03)00133-8 [DOI] [PubMed] [Google Scholar]
- Rashotte M.E, Saarela S, Henderson R.P, Hohtola E. Shivering and digestion-related thermogenesis in pigeons during dark phase. Am. J. Physiol. 1999;277:R1579–R1587. doi: 10.1152/ajpregu.1999.277.6.R1579. [DOI] [PubMed] [Google Scholar]
- Rautenberg W. Shivering thermogenesis and its interaction with other autonomic controlled systems. In: Rautenberg W, editor. Living in the cold II. John Libbey Eurotext; London, UK: 1989. pp. 409–418. [Google Scholar]
- Robbins C.T. 2nd edn. Academic Press; San Diego, CA: 1993. Wildlife feeding and nutrition. [Google Scholar]
- Rosen D.A.S, Trites A.W. No evidence for bioenergetic interaction between digestion and thermoregulation in Steller sea lions Eumetopias jubatus. Physiol. Biochem. Zool. 2003;76:899–906. doi: 10.1086/378140. doi:10.1086/378140 [DOI] [PubMed] [Google Scholar]
- Sato K, Naito Y, Kato A, Niizuma Y, Watanuki Y, Charrassin J.B, Bost C.-A, Handrich Y, Le Maho Y. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 2002;205:1189–1197. doi: 10.1242/jeb.205.9.1189. [DOI] [PubMed] [Google Scholar]
- Schaeffer P.J, Villarin J.J, Pierotti D.J, Kelly D.P, Lindstedt S.L. Cost of transport is increased after cold exposure in Monodelphis domestica: training for inefficiency. J. Exp. Biol. 2005;208:3159–3167. doi: 10.1242/jeb.01703. doi:10.1242/jeb.01703 [DOI] [PubMed] [Google Scholar]
- Schmid D, Grémillet D.J.H, Culik B.M. Energetics of underwater swimming in the great cormorant (Phalacrocorax carbo sinensis) Mar. Biol. 1995;123:875–881. doi:10.1007/BF00349133 [Google Scholar]
- Schreer J.F, Kovacs K.M. Allometry of diving capacity in air-breathing vertebrates. Can. J. Zool. 1997;75:339–358. [Google Scholar]
- Schulz A.R. Simulation of energy metabolism in the simple-stomached animal. Br. J. Nutr. 1978;39:235–254. doi: 10.1079/bjn19780034. doi:10.1079/BJN19780034 [DOI] [PubMed] [Google Scholar]
- Šimek V. Specific dynamic action of a high-protein diet and its significance for thermoregulation in the golden hamster. Physiol. Bohemoslov. 1975;24:421–424. [PubMed] [Google Scholar]
- Simon E, Pierau F.-K, Taylor D.C.M. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol. Rev. 1986;66:235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- Skrovan R.C, Williams T.M, Berry P.S, Moore P.W, Davis R.W. The diving physiology of bottlenose dolphins (Tursiops truncatus). II. Biomechanics and changes in buoyancy at depth. J. Exp. Biol. 1999;202:2749–2761. doi: 10.1242/jeb.202.20.2749. [DOI] [PubMed] [Google Scholar]
- Sokoloff A.J, Ryan J.M, Valerie E, Wilson D.S, Goslow G.E. Neuromuscular organization of avian flight muscle: morphology and contractile properties of motor units in the pectoralis (pars thoracicus) of pigeon (Columba livia) J. Morphol. 1998;236:179–208. doi: 10.1002/(SICI)1097-4687(199806)236:3<179::AID-JMOR3>3.0.CO;2-Z. doi:10.1002/(SICI)1097-4687(199806)236:3<179::AID-JMOR3>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Stahel C.D, Nicol S.C. Ventilation and oxygen extraction in the little penguin (Eudyptula minor), at different temperatures in air and water. Respir. Physiol. 1988;71:387–398. doi: 10.1016/0034-5687(88)90030-8. doi:10.1016/0034-5687(88)90030-8 [DOI] [PubMed] [Google Scholar]
- Stainsby W.N, Gladden L.B, Barclay J.K, Wilson B.A. Exercise efficiency: validity of base-line subtractions. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980;48:518–522. doi: 10.1152/jappl.1980.48.3.518. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Jones D.R. Blood flow distribution in submerged and surface-swimming ducks. J. Exp. Biol. 1992;166:285–296. doi: 10.1242/jeb.166.1.285. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Lovvorn J.R, Heieis M.R.A, Jones D.R, Blake R.W. A hydromechanical estimate of the power requirements of diving and surface swimming in lesser scaup (Aythya affinis) J. Exp. Biol. 1989;147:507–519. [Google Scholar]
- Stephenson R, Hedrick M.S, Jones D.R. Cardiovascular responses to diving and involuntary submergence in the rhinoceros auklet (Cerorhinca monocerata Pallas) Can. J. Zool. 1992;70:2303–2310. [Google Scholar]
- Swoap S.J, Johnson T.P, Josephson R.K, Bennett A.F. Temperature, muscle power output and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J. Exp. Biol. 1993;174:185–197. [Google Scholar]
- Taylor C.R. Mechanical efficiency of terrestrial locomotion: a useful concept? In: Elder H.Y, Trueman E.R, editors. Aspects of animal movement. Cambridge University Press; Cambridge, MA: 1980. pp. 235–244. [Google Scholar]
- Walsberg G.E, Wolf B.O. An appraisal of operative temperature mounts as tools for studies of ecological energetics. Physiol. Zool. 1996;69:658–681. [Google Scholar]
- Watanuki Y, Takahashi A, Daunt F, Wanless S, Harris M, Sato K, Naito Y. Regulation of stroke and glide in a foot-propelled avian diver. J. Exp. Biol. 2005;208:2207–2216. doi: 10.1242/jeb.01639. doi:10.1242/jeb.01639 [DOI] [PubMed] [Google Scholar]
- Watts P.W, Hansen S, Lavigne D.M. Models of heat loss by marine mammals: thermoregulation below the zone of irrelevance. J. Theor. Biol. 1993;163:505–525. doi:10.1006/jtbi.1993.1135 [Google Scholar]
- Weathers W.W, Sullivan K.A. Seasonal patterns of time and energy allocation by birds. Physiol. Zool. 1993;66:511–536. [Google Scholar]
- Webster M.D, Weathers W.W. Heat produced as a by-product of foraging activity contributes to thermoregulation by verdins, Auriparus flaviceps. Physiol. Zool. 1990;63:777–794. [Google Scholar]
- Williams T.M. Thermoregulation of the North American mink during rest and activity in the aquatic environment. Physiol. Zool. 1986;59:293–305. [Google Scholar]
- Williams T.M. Swimming by sea otters: adaptations for low energetic cost locomotion. J. Comp. Physiol. A. 1989;164:815–824. doi: 10.1007/BF00616753. doi:10.1007/BF00616753 [DOI] [PubMed] [Google Scholar]
- Williams T.M, Kooyman G.L, Croll D.A. The effect of submergence on heart rate and oxygen consumption of swimming seals and sea lions. J. Comp. Physiol. 1991;160:637–644. doi: 10.1007/BF00571261. [DOI] [PubMed] [Google Scholar]
- Williams T.M, Friedl W.A, Haun J.E, Chun N.K. Balancing power and speed in bottlenose dolphins (Tursiops truncatus) Symp. Zool. Soc. Lond. 1993;66:383–394. [Google Scholar]
- Williams T.M, Noren D, Berry P, Estes J.A, Allison C, Kirtland J. The diving physiology of bottlenose dolphins (Tursiops truncatus). III. Thermoregulation at depth. J. Exp. Biol. 1999;202:2763–2769. doi: 10.1242/jeb.202.20.2763. [DOI] [PubMed] [Google Scholar]
- Williams T.M, Fuiman L.A, Horning M, Davis R.W. The cost of foraging by a marine predator, the Weddell seal Leptonychotes weddellii: pricing by the stroke. J. Exp. Biol. 2004;207:973–982. doi: 10.1242/jeb.00822. doi:10.1242/jeb.00822 [DOI] [PubMed] [Google Scholar]
- Willis K, Horning M. A novel approach to measuring heat flux in swimming animals. J. Exp. Mar. Biol. Ecol. 2005;315:147–162. doi:10.1016/j.jembe.2004.09.019 [Google Scholar]
- Wilson R.P, Culik B.M. The cost of a hot meal: facultative specific dynamic action may ensure temperature homeostasis in post-ingestive endotherms. Comp. Biochem. Physiol. A. 1991;100:151–154. doi: 10.1016/0300-9629(91)90198-l. doi:10.1016/0300-9629(91)90198-L [DOI] [PubMed] [Google Scholar]
- Wilson R.P, Grémillet D. Body temperatures of free-living African penguins (Spheniscus demersus) and bank cormorants (Phalacrocorax neglectus) J. Exp. Biol. 1996;199:2215–2223. doi: 10.1242/jeb.199.10.2215. [DOI] [PubMed] [Google Scholar]
- Wilson R.P, Hustler K, Ryan P.G, Burger A.E, Nöldeke E.C. Diving birds in cold water: do Archimedes and Boyle determine energetic costs? Am. Nat. 1992;140:179–200. doi:10.1086/285409 [Google Scholar]
- Wolfgang M.J, Anderson J.M, Grosenbaugh M.A, Yue D.K.P, Triantafyllou M.S. Near-body flow dynamics in swimming fish. J. Exp. Biol. 1999;202:2303–2327. doi: 10.1242/jeb.202.17.2303. [DOI] [PubMed] [Google Scholar]
- Zerba E, Walsberg G.E. Exercise-generated heat contributes to thermoregulation by Gambel's quail in the cold. J. Exp. Biol. 1992;171:409–422. [Google Scholar]
- Zerba E, Dana A.N, Lucia M.A. The influence of wind and locomotor activity on surface temperature and energy expenditure of the eastern house finch (Carpodacus mexicanus) during cold stress. Physiol. Biochem. Zool. 1999;72:265–276. doi: 10.1086/316665. doi:10.1086/316665 [DOI] [PubMed] [Google Scholar]